Abstract
Arousal from sleep is an important protective mechanism that is depressed by repeated episodes of hypoxia. We aimed to determine how rapidly arousal depression occurs during repeated hypoxia and to determine if the depression is sleep state specific.
Three successive 12 h overnight sleep recordings were performed in six newborn lambs instrumented to record sleep state, blood pressure, heart rate and blood gases. The first (control) and third (recovery) nights were baseline studies (inspired oxygen fraction, FI,O2 = 0.21) to determine the spontaneous arousal probability. During the second (test) study night, lambs were exposed to a 60 s episode of isocapnic hypoxia (FI,O2 = 0.10; inspired carbon dioxide fraction, FI,CO2 = 0.03) during every epoch of sleep.
During quiet sleep (QS), the probability of arousing to hypoxia (56 %) remained significantly higher than the probability of arousing spontaneously (18 %) throughout the repeated hypoxic exposures (χ2 = 81.5, P < 0.001). By contrast, during active sleep (AS) arousal rapidly became depressed with repetition of the hypoxic stimulus; the probability of arousal in hypoxia (52 %) was significantly higher than the probability of spontaneous arousal (12 %) during the first ten hypoxic exposures (χ2 = 18.2, P < 0.001), but there was no difference thereafter.
We conclude that, when repeated, moderate hypoxia very rapidly becomes ineffective as an arousing stimulus in AS, but not in QS. These results suggest that the arousal mechanism is particularly vulnerable to failure during AS.
Arousal from sleep in response to respiratory stresses is considered to be a key protective mechanism that is essential for re-acquisition of respiratory reflexes and other protective responses that are depressed or lost during sleep (Phillipson & Sullivan, 1978). Among the respiratory stresses that can lead to arousal, hypoxia is particularly powerful in a range of species, including man, and over a range of ages encompassing newborn life to adulthood (Phillipson, Sullivan, Read, Murphy & Kozar, 1978; Henderson-Smart & Read, 1979b; Jeffery & Read, 1980; Neubauer, Santiago & Edelman, 1981; Berthon-Jones & Sullivan, 1982; Fewell & Baker, 1987). Yet the arousal response to hypoxia appears to be defective in infants at risk for sudden infant death syndrome (SIDS) (McCulloch, Brouillette, Guzzetta & Hunt, 1982) and in adults with obstructive sleep apnoea (OSA), who can experience profound oxyhaemoglobin desaturation before awakening during obstructive episodes (Davies & Stradling, 1993).
Repetitive hypoxaemia is a prominent feature of OSA in the adult (Davies & Stradling, 1993), and possibly a feature of SIDS. Postmortem examinations (Naeye, 1974; Rognum & Saugstad, 1991) and prospective monitoring (Kahn et al. 1992) suggest that the majority of SIDS victims experience repeated hypoxaemic episodes during sleep. Significantly, arousal mechanisms in general, and paradoxically, the arousing effects of hypoxia itself, may be impaired or even abolished by chronic exposure to hypoxia. Thus, though hypotension is a potent arousing stimulus under conditions of normoxia in lambs (Horne, Berger, Bowes & Walker, 1989) it fails to be arousing in lambs exposed to continuous mild hypoxia (Walker, Carroll, de Preu & Horne, 1993). Likewise, repeated hypoxia induced by episodic exposure to low inspired oxygen (Fewell & Konduri, 1989) or to airway obstruction (Fewell, Williams, Szabo & Taylor, 1988; Brooks, Horner, Kimoff, Kozar, Render-Teixeira & Phillipson, 1997) delays arousal from sleep and exaggerates the extent of arterial oxygen desaturation.
Two major issues remain unresolved concerning the depression of the arousal mechanism and as a consequence, the potential for severe hypoxaemia or death to occur during an episode of hypoxia during sleep. The first of these issues, how rapidly arousal depression might occur with repetition of the hypoxic stress, is important as the duration of apnoea can lengthen with progression of sleep and repetition of OSA episodes (Charbonneau, Marin, Olha, Kimoff, Levy & Cosio, 1994). Moreover, sudden death in infants may occur soon after the onset of sleep (Hori, 1987). While rapid depression of the arousal mechanism may be implicated in such situations (Walker et al. 1993), how quickly exposure to hypoxia might lead to arousal depression is not known. Previous studies have not examined the early changes in arousability following the onset of repeated hypoxia, focusing instead upon the changes occurring after much longer test periods ranging from over 24 h (Fewell et al. 1988; Fewell & Konduri, 1989) up to 1–2 weeks (Brooks et al. 1997).
The second issue is which of the two sleep states, active sleep (AS) or quiet sleep (QS), may be more prone to arousal failure. QS might be considered a vulnerable state as human infants do not always arouse during hypoxic challenges (Davidson Ward, Bautista & Keens, 1992). In some animal studies the depressant effects of hypoxia upon arousal occur in both sleep states (Fewell & Konduri, 1989; Brooks et al. 1997) whereas others have shown that arousal depression is confined to the AS state (Walker et al. 1993; Fewell et al. 1988). If arousal failure was to occur only in AS, this would suggest that AS is a particularly vulnerable sleep state, as it is characterized by reduced lung volume, irregular breathing pattern and diminished intercostal muscle function, factors that exaggerate the vulnerability to hypoxaemia (Henderson-Smart & Read, 1979a). Human respiratory studies are in accord with the view that AS is the more vulnerable of the two sleep states, as OSA occurs predominantly during AS in the newborn (Kahn et al. 1992) and the desaturations associated with OSA are more severe during this state in the adult (Sullivan & Issa, 1980; Charbonneau et al. 1994).
In this study we conducted experiments to address these unresolved issues concerning arousal during sleep. We first aimed to establish how rapidly arousal might be depressed with repetition of a hypoxic stress. Our second aim was to resolve the uncertainty that exists regarding the relative sensitivity of the arousal mechanism to depression by hypoxia in AS and QS. We addressed these two issues by examining the time course of changes in the arousal responses of lambs exposed to repeated, short episodes of hypoxia during overnight sleep.
METHODS
All surgical and experimental protocols were carried out in accordance with the guidelines established by the National Health and Medical Research Council of Australia and with the approval of the Standing Committee in Ethics in Animal Experimentation of Monash University. At the completion of the study, the lambs were killed with a lethal dose of anaesthetic (150 mg kg−1 sodium pentobarbitone, i.v.).
Animals
Newborn Border Leicester × Merino lambs (4.6 ± 0.3 kg, mean ±s.e.m.; n = 6) were separated from their ewes 12–48 h after birth, housed with another lamb and taught to feed unaided (Lamb Milk Replacer, Veanavite, Shepparton, Australia).
Surgical preparation
Each lamb was instrumented (3–6 days old) using sterile surgical techniques. Anaesthesia was induced with 2 % halothane in 60 % oxygen and nitrous oxide delivered via a face mask, and after intubation the lamb was mechanically ventilated. Non-occlusive catheters (Tygon, o.d. 1.5 mm) were inserted into an axillary artery for blood sampling and vascular pressure measurements. To assess behavioural state, paired Teflon-coated stainless steel wire (Medwire, NY, USA) electrodes were implanted on the parietal cortex to record the electrocorticogram (ECoG), at the inner and outer canthus of the right eye to record the electro-oculogram (EOG) and in the dorsal musculature of the neck to record the nuchal electromyogram (EMGn). All electrodes were referenced to a single electrode sewn into the subcutaneous tissue of the scalp.
A fenestrated tracheostomy tube (i.d. 5.0 mm; Shiley Inc., Irvine, CA, USA) was implanted in the trachea. When capped, the fenestrated tube allowed the animal to breathe normally via the upper airway. During studies, an inner cannula was placed in the tracheostomy tube which blocked the fenestration leading to the upper airway and allowed the animal to breathe through an external circuit, allowing for rapid changes in inspired gas mixtures.
Experimental procedure
Lambs were allowed a minimum of 72 h recovery from surgery before they were studied in controlled temperature conditions (22-25°C). The lamb's cage was partitioned so that the lamb was able to stand, lie down, move forward and backward and feed freely, but was not able to turn around. The arterial catheter was connected to a calibrated strain gauge manometer (Cobe CDX III, Cobe Laboratories, Lakewood, CO, USA). Arterial pressure was referenced to the mid-thoracic level when the animal was lying down. A pulse oximeter probe was placed around the (shaved) tail to measure arterial oxygen saturation (Sp,O2, Nellcor N200, Nellcor Inc., Hayward, CA, USA). The strain gauge manometer, pulse oximeter and the electrodes (ECoG, EOG and EMGn) were connected to a signal conditioner (Cyberamp 380, Axon Instruments). Arterial pressure and Sp,O2 were low-pass filtered at 100 Hz. The electrophysiological signals were filtered at 30–100 Hz for the EMGn, and 0.3–40 Hz for the EOG and the ECoG. All signals were continuously displayed on a thermal chart recorder (model 7758A, Hewlett-Packard, Waltham, MA, USA). Data were also intermittently stored on a personal computer (486 DX/50) at a sampling rate of 200 Hz, using an analog-digital converting board (ADAC 4801A, ADAC, Woburn, MA, USA) and acquisition software (CVSOFT Data Acquisition and Analysis Software, Odessa Computer Systems, Calgary, Canada).
Each study consisted of three successive 12 h overnight sleep recordings, performed on sequential control, test and recovery nights, and conducted between 20.00 and 08.00 h. Duplicate studies were conducted in four of six lambs, after allowing 4–7 days recovery, for a total of ten studies over an age range of 7–21 days (14 ± 1.4 days). The lambs tolerated the brief episodes of moderate hypoxia without distress, showed no lasting effects from the test procedure (as evidenced by their normal sleep on the night subsequent to the test night), and grew and behaved normally throughout the study. During the first (control) and third (recovery) nights of the study, lambs were randomly assigned to breathe room air either via the upper airway, or, alternatively, via a breathing circuit; the random assignment was used to control for possible effects of the breathing circuit on sleep and arousability. A bias flow of 20 l min−1 was used in the breathing circuit and a positive end-expiratory pressure of 2 cmH2O was applied to preserve lung inflation while the upper airway was bypassed. During the second (test) night of the study, the lambs were exposed to repeated isocapnic hypoxia by administering a step change in inspired gas mixture (inspired oxygen fraction, FI,O2 = 0.10 and inspired carbon dioxide fraction, FI,CO2 = 0.03 in N2, via the breathing circuit) during every AS and QS epoch. The stimulus was presented at the beginning of each epoch after allowing a 30 s baseline period for the epoch to be clearly established, and maintained for 60 s.
QS was defined by the presence of high voltage, low frequency waves on the ECoG, absence of eye movements and reduced EMGn activity compared with the awake state. Arousal from this state was characterized by a change in the ECoG to low voltage, high frequency waves, increased EMGn activity, and opening of the eyes (Fig. 1). AS was defined by the presence of low voltage, high frequency waves on the ECoG, the presence of rapid eye movements and the absence of EMGn tone. Arousal was characterized by a return of tonic activity in the EMGn (Fig. 1).
Figure 1. Arousal from sleep in response to hypoxia.
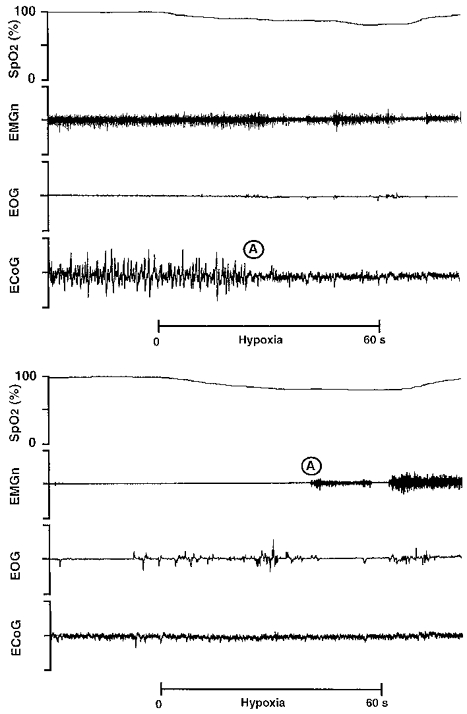
Physiological recording in a sleeping lamb during a period of quiet sleep (upper panel) and active sleep (lower panel), illustrating arousal responses to acute hypoxia. Sp,O2, pulse oxygen saturation; EMGn, nuchal electromyogram; EOG, electro-oculogram; ECoG, electrocorticogram. The 60 s hypoxic stimulus, induced by rapidly changing the air in the inspiratory line to a gas mixture of 10 % O2 and 3 % CO2 in N2, is denoted by the bar. Arousal, signified by a change in the ECoG and/or the EMGn, is shown at A.
Arterial blood was sampled for analysis of pH, arterial partial pressure of carbon dioxide (Pa,CO2), arterial partial pressure of oxygen (Pa,O2) and base excess (ABL500 Radiometer, Copenhagen, Denmark) during periods of normoxia and hypoxia for both sleep states.
Data analysis
The effects of the study on sleep were quantified by determining the number and duration of AS and QS epochs, and total sleep time for the control, test and recovery nights. Probability analysis was employed (Horne et al. 1989; Horne, Berger, de Preu & Walker, 1991) to quantify the arousing effect of the hypoxic stimulus. The probability of arousal during hypoxia in each sleep state was calculated as the percentage of tests in which arousal occurred during the 60 s stimulus. The probability of spontaneous arousal for each state was determined for the control and recovery nights by calculating the percentage of spontaneous arousals that occurred between 30 and 90 s of each epoch to match the period in which the hypoxic stimuli were presented. Differences between the probability of arousal in hypoxia and the probability of spontaneous arousal, the probability of arousal in hypoxia for sequential groups of ten hypoxia exposures, the probability of spontaneous arousal on the control and recovery nights, and the probability of spontaneous arousal with or without the breathing circuit were tested using the χ2 test. Data were stratified (Horne et al. 1989, 1991) to prevent bias arising from differences in the number of sleep epochs between animals.
Mean arterial blood pressure, heart rate and Sp,O2 values were calculated from digitized data (CVSOFT Data Acquisition and Analysis Software, Odessa Computer Systems, Calgary, Canada) for the 30 s normoxic period prior to the hypoxic stimulus and for approximately 10 s during hypoxia immediately prior to arousal. For tests which did not result in an arousal, values were calculated over 10 s prior to the 30 s point of the hypoxic exposure, which corresponded to the average arousal time. For each lamb, determinations of Sp,O2 were corrected for the time delay due to the breathing circuit (1 s), the oximeter response time and the circulation time to the tail (range, 6–9 s; mean ±s.e.m., 8 ± 1 s).
All cardiovascular, blood gas and acid-base data were averaged to give one value for each animal where duplicate studies were conducted. Data from the six lambs were then averaged and presented as means ±s.e.m. A two-way analysis of variance for repeated measures was used to test differences between sleep states and between normoxic and hypoxic periods for the cardiovascular, blood gas and acid-base data. For each sleep state, a one-way analysis of variance for repeated measures was used to detect differences in the number of sleep epochs, epoch length, and total sleep time across the three study nights. Any differences detected by the analyses of variance were isolated by a Student-Newman- Keuls test. In all tests, P < 0.05 was considered statistically significant.
RESULTS
In AS the imposition of repetitive hypoxia did not alter epoch number, epoch length or total sleep time over all three study nights (Table 1). In QS, while the number of epochs remained constant, epoch length and total sleep time were reduced on the test night (P < 0.05). There were no differences in epoch number, epoch length or total sleep time according to whether the upper airway was intact or bypassed, nor between the control and recovery nights in either sleep state. Accordingly, for each state the probability of spontaneous arousal on the control and recovery nights was pooled for comparison with arousal in response to hypoxia. The analysis included the first forty sleep epochs obtained on each of the three study nights; too few animals were exposed to a greater number of hypoxic tests to undertake probability analysis further.
Table 1.
Spontaneous sleep distribution data in overnight recordings
| Study night | Quiet sleep | Active sleep | |
|---|---|---|---|
| Number of epochs | Control | 32 ± 2 | 20 ± 2 |
| Test | 34 ± 3 | 18 ± 3 | |
| Recovery | 31 ± 3 | 21 ± 2 | |
| Epoch length (min) | Control | 6.0 ± 0.3 | 3.9 ± 0.4 |
| Test | 4.2 ± 0.3 * | 3.3 ± 0.2 | |
| Recovery | 5.8 ± 0.3 | 3.9 ± 0.2 | |
| Total sleep time (min) | Control | 187 ± 14 | 86 ± 14 |
| Test | 138 ± 8 * | 59 ± 8 | |
| Recovery | 175 ± 15 | 83 ± 9 |
Values are means ±s.e.m. (n = 10 studies).
P < 0.05, control vs. test night, recovery vs. test night.
When examined over the entire series of episodes of repetitive hypoxia, the probability of arousing from QS (56 %) was significantly higher than the overall probability of spontaneous arousal from QS (18 %, χ2 = 81.5, P < 0.001). A similar analysis in AS showed that the overall probability of arousal in hypoxia (27 %) was significantly higher than the spontaneous arousal probability (13 %, χ2 = 12.3, P < 0.001). When partitioned into sequential groups of ten hypoxia exposures, the pattern of arousal probability during hypoxia differed significantly between sleep states (Fig. 2). During QS, the probability of arousal in hypoxia remained significantly higher than the probability of spontaneous arousal throughout the sequence of hypoxia tests (P < 0.01). Conforming with this result, there were no differences in the probability of arousal in hypoxia across the sequential groups of ten hypoxia tests (P > 0.05). These results were in keeping with the reductions in epoch length and total sleep time that were found in QS (Table 1). By contrast, in AS only during the first ten hypoxia tests was the probability of arousal significantly higher than the probability of spontaneous arousal (χ2 = 18.2, P < 0.001). Subsequently, the probability of arousal in hypoxia decreased to equal the probability of spontaneous arousal. Furthermore, the probability of arousal during the first ten hypoxia exposures was significantly higher than the subsequent exposures; eleven to twenty exposures (χ2 = 6.1, P < 0.05); twenty-one to thirty exposures (χ2 = 12.9, P < 0.001); thirty-one to forty exposures (χ2 = 8.1, P < 0.01). The rapid depression of arousal in AS is consistent with the observation that epoch number, epoch length or total sleep time in AS was essentially unchanged by the testing procedure (Table 1).
Figure 2. Probability of arousal from sleep.
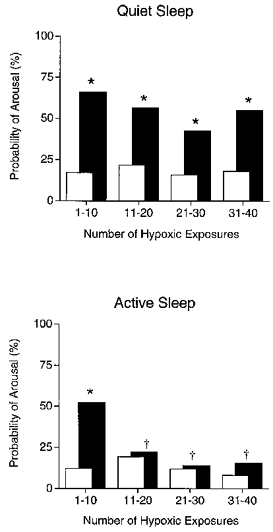
Probability of arousal from sleep during repetitive hypoxia (▪) and the probability of spontaneous arousal (□) during quiet sleep (upper panel) and active sleep (lower panel). Note that, with repeated exposure to hypoxia, lambs continued to arouse from quiet sleep whereas arousal was abolished after just 10 hypoxic exposures in active sleep. *P < 0.05, probability of arousal during hypoxia vs. probability of spontaneous arousal; †P < 0.05, probability of arousal during hypoxia vs. probability of arousal during hypoxia for the first 10 hypoxic exposures.
Blood pressure increased significantly during hypoxia, although the changes were minimal and equal in both sleep states (QS: 79 ± 5 mmHg during normoxia, 81 ± 5 mmHg during hypoxia, P < 0.05; AS: 77 ± 5 mmHg during normoxia, 80 ± 5 mmHg during hypoxia, P < 0.05). Heart rate did not change in response to hypoxia in QS (163 ± 6 beats min−1 during normoxia, 170 ± 7 beats min−1 during hypoxia), or AS (160 ± 8 beats min−1 during normoxia, 159 ± 5 beats min−1 during hypoxia).
Arterial blood gas analysis confirmed that Pa,O2 was significantly lower during periods of hypoxia in both sleep states (P < 0.05) compared with normoxia, whereas there were no differences in Pa,CO2, pH or base excess during normoxia and hypoxia (Table 2). There were no sleep state related differences of blood gases or pH in normoxia. Consistent with the poorer ventilatory response to hypoxia that is characteristic of AS in the lamb (Henderson-Smart & Read, 1979b), Pa,O2 reached lower values in AS (P < 0.05, Table 2).
Table 2.
Effects of hypoxia on respiratory variables during sleep in lambs
| Quiet sleep | Active sleep | |||
|---|---|---|---|---|
| Normoxia | Hypoxia | Normoxia | Hypoxia | |
| Pa,O2 (mmHg) | 93 ± 3 | 53 ± 2 * | 93 ± 2 | 46 ± 2 *† |
| Pa,CO2 (mmHg) | 42 ± 2 | 40 ± 2 | 42 ± 1 | 43 ± 2 |
| pH | 7.40 ± 0.01 | 7.41 ± 0.01 | 7.40 ± 0.01 | 7.39 ± 0.02 |
| Base excess (mmol l−1) | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 |
Values are means ±s.e.m., n = 6 lambs. Pa,CO2, arterial partial pressure of carbon dioxide; Pa,O2, arterial partial pressure of oxygen.
P < 0.05, normoxia vs. hypoxia
P < 0.05, quiet sleep vs. active sleep.
Continuous pulse oximetry showed that for both sleep states there were no differences in the level of hypoxaemia in epochs which resulted in an arousal response (measured at the point of arousal) and those in which there was no arousal (measured after 30 s of hypoxia). During QS, Sp,O2 was 83 ± 2 % during tests that resulted in arousal and 82 ± 2 % during tests that did not. During AS, Sp,O2 was 78 ± 2 % during tests that resulted in arousal and 80 ± 2 % during tests that did not. In addition, the Sp,O2 during hypoxia did not differ with repeated exposure. Values during QS were: one to ten exposures, 80 ± 2 %; eleven to twenty exposures, 82 ± 3 %; twenty-one to thirty exposures, 85 ± 2 %; thirty-one to forty exposures, 83 ± 2 %. During AS, the values were: one to ten exposures, 79 ± 2 %; eleven to twenty exposures, 78 ± 4 %; twenty-one to thirty exposures, 80 ± 3 %; thirty-one to forty exposures, 76 ± 3 %. In epochs that did not result in an arousal, Sp,O2 (measured after 60 s of hypoxia) was lower in AS (65 ± 4 %) compared with QS (73 ± 4 %; P < 0.05).
DISCUSSION
Our study has revealed important new features about the effectiveness of hypoxia as an arousing stimulus in sleep. Though moderate hypoxia can act acutely as a powerful arousing stimulus, with repetition it becomes ineffective. The loss of the arousing effect of hypoxia is very rapid in onset, occurring after as few as ten episodes of hypoxia. The rapid abolition of arousal is confined to AS; by contrast, moderate hypoxia remains a powerful arousing stimulus in QS. As we argue below, the abolition of arousal by repetitive hypoxia during AS appears to result from depression of specific neural processes subserving the arousal mechanism in this sleep state, rather than a generalized depression that affects all behavioural states.
We approached the study of arousal from sleep by employing a probability analysis that compares the probability of arousing in response to an imposed stimulus (test) with the probability of arousing spontaneously from sleep (Horne et al. 1989, 1991). By comparing test and spontaneous arousal probabilities, this type of analysis takes account of the fact that spontaneous arousals do occur, and that these contaminate the ‘true’ probability of arousal being induced by the stimulus under study. Clearly, this contamination can be substantial if the sleep epoch length is short or the time over which the stimulus is applied is long. Moreover, it raises uncertainty about the conclusions of previous studies examining arousal responses to repetitive stimuli in which spontaneous arousal was not taken into account (Fewell et al. 1988; Fewell & Konduri, 1989). Our form of analysis allows us to not only determine the true effects of an arousing stimulus, but also allows us to control for temporal changes in arousability such as might occur overnight.
Under the conditions of our study, the probability of the lambs arousing spontaneously during the period in which the stimulus was applied remained constant throughout the night at levels of approximately 18 % in QS and 13 % in AS. In QS, the probability of arousing in response to hypoxia ranged from 43–66 % throughout the study, substantially greater than the spontaneous probability of arousal. In AS the probability of arousal was also substantially greater than the spontaneous probability of arousal, but only during the first ten tests when it averaged 52 %. Subsequently, hypoxia was no longer an arousing influence as the probability of arousal in AS declined to equal the spontaneous probability and remained at this level (17 %) throughout the rest of the night.
The arousal depression we observed during AS cannot be attributed to variation in the hypoxic stimulus, as this was constant throughout the study. Nor is it likely that the depression is due to disruption of sleep (Bowes, Woolf, Sullivan & Phillipson, 1980; Brooks et al. 1997) as in our studies, in AS, epoch number, epoch length and total sleep time were unaffected by the imposition of hypoxic testing. Moreover, the mild disruption of QS was not associated with any diminution in arousal in response to hypoxia. Our study suggests that hypoxia itself, not necessarily coincident with sleep disruption, can independently depress arousal.
Our study differs from the single published study of arousal from sleep under conditions of repetitive hypoxia in the important respect that it has revealed the rapid onset of arousal depression. In the study of Fewell & Konduri (1989), arousal deficits signified by delayed arousal and lower arterial saturation at arousal were found after 100 repeated exposures in studies spanning up to 38 h; the time of onset of arousal depression was not determined. In our study, an arousal decrement was apparent after just ten episodes of hypoxia each of 1 min duration, i.e. after as little as 10 min of accumulated hypoxia.
Our findings also differ from those of Fewell & Konduri (1989) in that we observed an arousal decrement in AS, but not in QS, whereas they observed decrements in both sleep states. The most obvious difference between the two studies is that a severe level of hypoxia (FI,O2 = 0.05) was used by Fewell & Konduri (1989), so that arterial oxygen saturation was reduced to very low levels (38 ± 11 %, mean ±s.d.) sufficient to induce cerebral hypoxia (Fewell & Baker, 1987). It is possible therefore that the animals experienced global cerebral depression that impaired their overall level of arousability regardless of the state of sleep or wakefulness. By contrast, animals in our study were exposed to milder hypoxia and experienced selective depression of arousability in AS. Our findings are consistent with the presence of a physiological threshold for depression of the arousal response that is more commonly exceeded in AS, perhaps due to the poorer ventilatory response in this state. In our study, Sp,O2 levels at the end of the 60 s test period averaged 65 % in AS and 73 % in QS, suggesting that the threshold for arousal depression may be as high as 70 % arterial oxygen saturation. Further studies of arousal in which lambs are exposed to hypoxaemic levels less than 70 % arterial oxygen saturations during QS are needed to examine this possibility.
Taken together, these studies indicate that AS is particularly sensitive to hypoxia in the lamb, with arousal decrements occurring after as few as ten hypoxic episodes. QS by contrast appears to be an order of magnitude less sensitive to depression, as arousal depression occurs only after as many as 100 hypoxic episodes, possibly in association with global depression. Thus, in settings in which there are repeated hypoxic episodes, such as in episodic sleep apnoea, an arousal decrement may appear first in AS. Subsequently, with loss of arousal in AS and, as a consequence, the development of progressively more severe arterial oxygen desaturation in successive episodes in this state, arousal decrements may also develop in QS. At present it is unclear whether the human infant might experience similar arousal depression with repeated hypoxia, as testing has been confined to the QS state and the number of repeated challenges have been limited to two (Davidson Ward et al. 1992). Insofar as arousal in response to hypoxia in QS occurs in 30–80 % of trials in infants (see review in Davidson Ward et al. 1992), a rate that is similar to that which we observe in the lamb, the possibility exists that the infant may share a similar arousal mechanism and also a similar propensity for depression with repeated hypoxia.
The mechanisms responsible for the loss of arousability associated with repetitive or prolonged hypoxia are complex and remain to be elucidated. For example, earlier studies have identified a role for chemoreceptors (Bowes, Townsend, Kozar, Bromley & Phillipson, 1981; Fewell, Kondo, Dascalu & Filyk, 1989) and baroreceptors (Horne et al. 1989, 1991) as sources of powerful arousing inputs to the reticular activating system. However it is unlikely that adaptations of the chemoreceptors and baroreceptors explain the arousal decrement in AS that we have observed. It is well established that carotid body activity is not diminished by sustained hypoxia over periods substantially longer than the short period within which arousal was abolished in our study. Thus, carotid sinus nerve activity was unaffected in newborn rats exposed to chronic hypoxia for 5–10 weeks (Eden & Hanson, 1987). There is also indirect evidence to suggest that the carotid body continues to function during repetitive episodic hypoxia. Thus, hypertension develops in the rat exposed to repeated, episodic hypoxia if the carotid bodies are intact, but not after chemodenervation (Fletcher, Lesske, Behm, Miller, Stauss & Unger, 1992). In that study, baroreflexes could be elicited following the treatment, so that baroreceptor inputs are also unaffected by repeated, episodic hypoxia.
Declines in input from the thoracic mechanoreceptor may contribute to our findings. These receptors normally have a powerful arousing influence when breathing is stimulated (Yasuma, Kozar, Kimoff, Bradley & Phillipson, 1991). However the ventilatory response is lost during repeated hypoxia (Worthington, Hedner & Sullivan, 1993), just as it is with prolonged hypoxia (Rigatto, 1984; Weil, 1994) so it is possible that the important contribution of thoracic mechanoreceptors to arousal may be lost with repetition of the hypoxic stimulus. As it has been proposed that mechanoreceptors are not critical to arousal in active sleep (Baker & Fewell, 1987), further studies are needed to define their role in the loss of arousal responses that we have observed in this sleep state.
The arousal decrement we observed shares several common features with the response decrement occurring with habituation to sound (Sharpless & Jasper, 1956). For example, arousal in response to repeated sound stimulation may be lost rapidly, within as few as six repetitions, similar to our observations. Secondly, as with habituation to sound (Sharpless & Jasper, 1956), loss of the arousal response to hypoxia is not explained by receptor adaptation nor by global depression of the central nervous system. Peripheral chemoreceptor activity continues with prolongation of hypoxia (Eden & Hanson, 1987) and responsiveness to carbon dioxide remains after the ventilatory response to hypoxia disappears (Long, Lobchuk & Anthonisen, 1994). Thirdly, just as habituation to sound can persist well beyond the period of stimulation (Sharpless & Jasper, 1956), hypotension-induced arousal is lost for at least an hour after a period of prolonged hypoxia (Walker et al. 1993). Finally, just as habituation is abolished by a new stimulus, the arousal response that is lost after repeated hypoxia is restored by coincident exposure to carbon dioxide (Fewell & Konduri, 1988).
Though habituation is an appropriate response to an innocuous stimulus or safe environment, it may be inappropriate and life-threatening in conditions of hypoxia, particularly in AS. The newborn is susceptible to developing more severe hypoxaemia in AS because of reduced lung volume, irregular breathing pattern and diminished intercostal muscle function (Henderson-Smart & Read, 1979a). Adults experience more severe desaturations during sleep apnoea in rapid eye movement sleep (REM, equivalent to AS in the newborn) than in non-REM (or QS) (Sullivan & Issa, 1980). In keeping with this phenomenon, the results of our study suggest that impairment of arousal would exaggerate the extent of desaturation during apnoea and expose them to life-threatening hypoxaemia.
In summary, repetitive moderate hypoxia significantly depressed arousal from AS in response to the hypoxic stimulus itself in newborn lambs after only ten exposures. By contrast, repetitive hypoxia remained a powerful arousing stimulus in QS. The depressant effect of hypoxia upon arousal has similarities with the depression of ventilation that occurs during prolonged hypoxia, implying that there may be a similar, albeit as yet unknown, mechanism mediating the response in both situations. The sleep state-specific arousal depression suggests that the possibility of arousal failure is greater in AS than QS in the newborn. Thus the vulnerability of the newborn to developing hypoxaemia in AS coupled with our observations of a dramatic depression of arousal with repetitive hypoxia suggest that arousal failure in AS may have a role in SIDS.
Acknowledgments
This work was supported by the Sudden Infant Death Research Foundation, Victoria, Australia, the Monash Research Foundation for Mothers and Babies, the National Health and Medical Research Council of Australia, and the Alberta Heritage Foundation for Medical Research. The valuable technical support of Mrs Jennene Wild and Mr Vojta Brodecky is gratefully acknowledged.
References
- Baker SB, Fewell JE. Effect of hyperoxia on the arousal response to upper airway obstruction. Pediatric Research. 1987;21:116–120. doi: 10.1203/00006450-198702000-00002. [DOI] [PubMed] [Google Scholar]
- Berthon-Jones M, Sullivan CE. Ventilatory and arousal responses to hypoxia in sleeping humans. American Review of Respiratory Disease. 1982;125:632–639. doi: 10.1164/arrd.1982.125.6.632. [DOI] [PubMed] [Google Scholar]
- Bowes G, Townsend ER, Kozar LF, Bromley SM, Phillipson EA. Effect of carotid body denervation on arousal response to hypoxia in sleeping dogs. Journal of Applied Physiology. 1981;51:40–45. doi: 10.1152/jappl.1981.51.1.40. [DOI] [PubMed] [Google Scholar]
- Bowes G, Woolf GM, Sullivan CE, Phillipson EA. Effect of sleep fragmentation on ventilatory and arousal responses of sleeping dogs to respiratory stimuli. American Review of Respiratory Disease. 1980;122:899–908. doi: 10.1164/arrd.1980.122.6.899. [DOI] [PubMed] [Google Scholar]
- Brooks D, Horner RL, Kimoff J, Kozar LF, Render-Teixeira CL, Phillipson EA. Effect of obstructive sleep apnea versus sleep fragmentation on responses to airway occlusion. American Journal of Respiratory and Critical Care Medicine. 1997;155:1609–1617. doi: 10.1164/ajrccm.155.5.9154865. [DOI] [PubMed] [Google Scholar]
- Charbonneau M, Marin JM, Olha A, Kimoff RJ, Levy RD, Cosio MG. Changes in obstructive sleep apnea characteristics through the night. Chest. 1994;106:1695–1701. doi: 10.1378/chest.106.6.1695. [DOI] [PubMed] [Google Scholar]
- Davidson Ward SL, Bautista DB, Keens TG. Hypoxic arousal responses in normal infants. Pediatrics. 1992;89:860–864. [PubMed] [Google Scholar]
- Davies RJO, Stradling JR. Acute effects of obstructive sleep apnoea. British Journal of Anaesthesia. 1993;71:725–729. doi: 10.1093/bja/71.5.725. [DOI] [PubMed] [Google Scholar]
- Eden GJ, Hanson MA. Effects of chronic hypoxia from birth on the ventilatory response to acute hypoxia in the newborn rat. The Journal of Physiology. 1987;392:11–19. doi: 10.1113/jphysiol.1987.sp016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell JE, Baker SB. Arousal from sleep during rapidly developing hypoxemia in lambs. Pediatric Research. 1987;22:471–477. doi: 10.1203/00006450-198710000-00023. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Kondo CS, Dascalu V, Filyk SC. Influence of carotid denervation on the arousal and cardiopulmonary response to rapidly developing hypoxemia in lambs. Pediatric Research. 1989;25:473–477. doi: 10.1203/00006450-198905000-00009. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Konduri GG. Repeated exposure to rapidly developing hypoxemia influences the interaction between oxygen and carbon dioxide in initiating arousal from sleep in lambs. Pediatric Research. 1988;24:28–33. doi: 10.1203/00006450-198807000-00008. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Konduri GG. Influence of repeated exposure to rapidly developing hypoxaemia on the arousal and cardiopulmonary response to developing hypoxaemia in lambs. Journal of Developmental Physiology. 1989;11:77–82. [PubMed] [Google Scholar]
- Fewell JE, Williams BJ, Szabo JS, Taylor BJ. Influence of repeated upper airway obstruction on the arousal and cardiopulmonary response to upper airway obstruction in lambs. Pediatric Research. 1988;23:191–195. doi: 10.1203/00006450-198802000-00013. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Lesske J, Behm R, Miller CC, III, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. Journal of Applied Physiology. 1992;72:1978–1984. doi: 10.1152/jappl.1992.72.5.1978. [DOI] [PubMed] [Google Scholar]
- Henderson-Smart DJ, Read DJC. Reduced lung volume during behavioural active sleep in the newborn. Journal of Applied Physiology. 1979a;46:1081–1085. doi: 10.1152/jappl.1979.46.6.1081. [DOI] [PubMed] [Google Scholar]
- Henderson-Smart DJ, Read DJC. Ventilatory responses to hypoxaemia during sleep in the newborn. Journal of Developmental Physiology. 1979b;1:195–208. [PubMed] [Google Scholar]
- Hori CG. Pathology of sudden infant death syndrome. The American Journal of Forensic Medicine and Pathology. 1987;8:93–96. doi: 10.1097/00000433-198708020-00001. [DOI] [PubMed] [Google Scholar]
- Horne RSC, Berger PJ, Bowes G, Walker AM. Effect of sinoaortic denervation on arousal responses to hypotension in newborn lambs. American Journal of Physiology. 1989;256:H434–440. doi: 10.1152/ajpheart.1989.256.2.H434. [DOI] [PubMed] [Google Scholar]
- Horne RSC, Berger PJ, de Preu ND, Walker AM. Arousal responses to hypertension in lambs: effect of sinoaortic denervation. American Journal of Physiology. 1991;260:H1283–1289. doi: 10.1152/ajpheart.1991.260.4.H1283. [DOI] [PubMed] [Google Scholar]
- Jeffery HE, Read DJC. Ventilatory responses of newborn calves to progressive hypoxia in quiet and active sleep. Journal of Applied Physiology. 1980;48:892–895. doi: 10.1152/jappl.1980.48.5.892. [DOI] [PubMed] [Google Scholar]
- Kahn A, Groswasser J, Rebuffat E, Sottiaux M, Blum D, Foerster M, Franco P, Bochner A, Alexander M, Bachy A, Richard P, Verghote M, Le Polain D, Wayenberg JL. Sleep and cardiorespiratory characteristics of infant victims of sudden death: a prospective case-control study. Sleep. 1992;15:287–292. doi: 10.1093/sleep/15.4.287. [DOI] [PubMed] [Google Scholar]
- Long W, Lobchuk D, Anthonisen NR. Ventilatory response to CO2 and hypoxia after sustained hypoxia in awake cats. Journal of Applied Physiology. 1994;76:2262–2266. doi: 10.1152/jappl.1994.76.6.2262. [DOI] [PubMed] [Google Scholar]
- McCulloch K, Brouillette RT, Guzzetta AJ, Hunt CE. Arousal responses in near-miss sudden infant death syndrome and in normal infants. Journal of Pediatrics. 1982;101:911–917. doi: 10.1016/s0022-3476(82)80009-7. [DOI] [PubMed] [Google Scholar]
- Naeye RL. Hypoxaemia and the sudden infant death syndrome. Science. 1974;186:837–838. doi: 10.1126/science.186.4166.837. [DOI] [PubMed] [Google Scholar]
- Neubauer JA, Santiago TV, Edelman NH. Hypoxic arousal in intact and chemodenervated sleeping cats. Journal of Applied Physiology. 1981;51:1294–1299. doi: 10.1152/jappl.1981.51.5.1294. [DOI] [PubMed] [Google Scholar]
- Phillipson EA, Sullivan CE. Arousal: the forgotten response to respiratory stimuli. American Review of Respiratory Disease. 1978;118:807–809. doi: 10.1164/arrd.1978.118.5.807. [DOI] [PubMed] [Google Scholar]
- Phillipson EA, Sullivan CE, Read DJC, Murphy E, Kozar LF. Ventilatory and arousal responses to hypoxia in sleeping dogs. Journal of Applied Physiology. 1978;44:512–520. doi: 10.1152/jappl.1978.44.4.512. [DOI] [PubMed] [Google Scholar]
- Rigatto H. Control of ventilation in the newborn. Annual Review of Physiology. 1984;46:661–674. doi: 10.1146/annurev.ph.46.030184.003305. 10.1146/annurev.ph.46.030184.003305. [DOI] [PubMed] [Google Scholar]
- Rognum TO, Saugstad OD. Hypoxanthine levels in vitreous humor: evidence of hypoxia in most infants who died of sudden infant death syndrome. Pediatrics. 1991;87:306–310. [PubMed] [Google Scholar]
- Sharpless S, Jasper H. Habituation of the arousal reaction. Brain. 1956;79:655–680. doi: 10.1093/brain/79.4.655. [DOI] [PubMed] [Google Scholar]
- Sullivan CE, Issa FG. Pathophysiological mechanisms in obstructive sleep apnea. Sleep. 1980;3:235–246. doi: 10.1093/sleep/3.3-4.235. [DOI] [PubMed] [Google Scholar]
- Walker AM, Carroll J, de Preu ND, Horne RSC. Modification of arousal responses following hypoxia in newborn lambs. In: Walker AM, McMillen C, National SIDS Council, editors. Second SIDS International Conference and First SIDS Global Strategy Meeting. New York: Perinatology Press; 1993. pp. 183–188. [Google Scholar]
- Weil JV. Ventilatory response to CO2 and hypoxia after sustained hypoxia in awake cats (editorial) Journal of Applied Physiology. 1994;76:2251–2252. doi: 10.1152/jappl.1994.76.6.2251. [DOI] [PubMed] [Google Scholar]
- Worthington JM, Hedner JA, Sullivan CE. Depressed hypoxic ventilatory responsiveness after prolonged intermittent hypoxia in the rat. In: Walker AM, McMillen C, National SIDS Council, editors. Second SIDS International Conference and First SIDS Global Strategy Meeting. New York: Perinatology Press; 1993. pp. 238–242. [Google Scholar]
- Yasuma F, Kozar LF, Kimoff RJ, Bradley TG, Phillipson EA. Interaction of chemical and mechanical respiratory stimuli in the arousal response to hypoxia in sleeping dogs. American Review of Respiratory Disease. 1991;143:1274–1277. doi: 10.1164/ajrccm/143.6.1274. [DOI] [PubMed] [Google Scholar]


