Abstract
Intracellular recordings were made from neurones in slices of rat striatum in vitro.
The forty-nine neurones studied were immunoreactive for choline acetyltransferase and had the electrophysiological characteristics typical of large aspiny interneurones.
Focal stimulation of the slice elicited a hyperpolarizing inhibitory postsynaptic potential in thirty-five neurones. This IPSP lasted 0.5–1 s and reversed polarity at a membrane potential which was dependent on the logarithm of the extracellular potassium concentration.
The IPSP was reversibly blocked by scopolamine and methoctramine, which has some selectivity for the M2 subtype of muscarinic receptor. It was unaffected by 6-cyano-7-nitroquinoxaline-2,3-dione (10 μM), DL-2-amino-phosphonovaleric acid (30 μM) and bicuculline (30 μM).
Exogenous acetylcholine and muscarine also hyperpolarized the neurones, and this was blocked by methoctramine but not by pirenzepine, which is an M1 receptor-selective antagonist.
The findings demonstrate that muscarinic IPSPs occur in the central nervous system. The IPSP may mediate an ‘autoinhibition’ of striatal cholinergic neurone activity.
It has long been known that muscarinic cholinergic transmission in the striatum is essential for normal voluntary movement and, despite their many drawbacks, anticholinergic drugs have played a significant role in the therapy of Parkinson's disease. The striatum has a high content of acetylcholine (ACh) and muscarinic receptors (Weiner, Levey & Brann, 1990; Hersch, Gutekunst, Rees, Heilman & Levey, 1994; reviewed by Graybiel, 1990; Graybiel, Aosaki, Flaherty & Kimura, 1994); indeed, it was the uneven distribution of the degradative enzyme acetylcholinesterase which provided the first indication of existence of striatal compartmentalization (Graybiel, 1990). In contrast, there is less information regarding the cellular actions of ACh in the striatum. The most widely described action of exogenous muscarinic agonists is presynaptic inhibition of the release of both excitatory and inhibitory transmitters (glutamic acid and γ-aminobutyric acid, respectively) (Dodt & Misgeld, 1986; Sugita, Uchimura, Jiang & North, 1991). Only one direct effect of ACh has been reported on the membrane properties of the principal (medium spiny) striatal neurones; this is an altered voltage dependence of a transient potassium current (Akins, Surmeier & Kitai, 1990).
The ACh-containing interneurones themselves comprise less than 5 % of all striatal neurones (Phelps, Houser & Vaughn, 1985; Graybiel, 1990) and only relatively recently has it been possible to distinguish them from the principal neurones during the course of intracellular recording studies in vitro (Kawaguchi, 1993; Kawaguchi, Wilson, Augood & Emson, 1995). These experiments have described the membrane properties of the cholinergic neurones, most notably the long-lasting after-hyperpolarization which follows an action potential (Kawaguchi, 1993). They have also shown that exogenous muscarinic agonists can inhibit voltage-dependent calcium currents (Yan & Surmeier, 1996) and this may contribute to the inhibition of acetylcholine release observed earlier with muscarinic agonists (James & Cubeddu, 1987). However, those experiments at the cellular level have not directly addressed the functional role of endogenous, synaptically released ACh in the striatum. In the present work, intracellular recording from identified cholinergic neurones was combined with focal stimulation elsewhere in the striatum. This led to the finding of cholinergic IPSPs on the cholinergic interneurones themselves.
METHODS
Wistar rats were used (150–250 g), as described previously (Calabresi et al. 1997). They were deeply anaesthetized with halothane and killed by severing the major blood vessels in the chest and the brains quickly removed. Corticostriatal coronal slices (200–300 μm) were prepared from tissue blocks of the brain with a Vibratome. A single slice was submerged in continuously flowing Krebs solution (35°C, 2–3 ml min−1) gassed with 95 % O2-5 % CO2. Exchange of the solution in the chamber, as when drugs were applied, took 90 s. The composition of the control solution was (mM): 126 NaCl, 2.5 KCl, 1.2 MgCl2, 1.2 NaH2PO4, 2.4 CaCl2, 11 glucose and 25 NaHCO3. In some experiments, the slice surface was visualized with a × 40 water immersion objective, and the larger striatal cells were selected. Intracellular recordings were made with glass microelectrodes containing KCl (1 or 2 M) usually with biocytin (2–4 %) (resistance, 30–60 MΩ). An Axoclamp-2A amplifier was used either in current-clamp or in single-electrode voltage-clamp mode (switching frequency 3 kHz with headstage voltage monitoring). Electrical stimulation was with bipolar electrodes (100 μs pulses) with tips 300–600 μm from that of the recording electrode.
After recording, slices were fixed in 4 % paraformaldehyde in 0.1 M phosphate buffered saline (PBS) overnight at 4°C, incubated in PBS containing sucrose (30 %) for 3 h, frozen and cryostat sectioned at 40 μm, and then incubated overnight in fluorescein isothiocyanate conjugated to avidin (diluted 1:200 in PBS containing 0.1 % Triton X-100). Washed and glycerol-mounted sections were observed with epifluorescence. Sections with an identified large aspiny neurone were further processed by incubation with a rat choline acetyltransferase monoclonal antibody (Boehringer; 1:250 in PBS containing 10 % normal goat serum and 2 % bovine serum albumin) for 3 h. Immunoreactivity was detected after exposure to goat anti-rabbit IgG (Sigma; 1:50) conjugated to tetramethylrhodamine isothiocyanate and avidin-conjugated fluorescein isothiocyanate (1:200) for 2 h. After washing, the sections were mounted on slides with glycerol in PBS (1:3). With appropriate filters, immunoreactive neurones were seen in red and biocytin-positive cells in yellow-green.
RESULTS
Large aspiny cholinergic interneurones were identified by morphological, histochemical and electrophysiological criteria. They comprised 21 of 427 cells when electrodes were placed into the striatum without visual control, the remaining neurones having morphological and electrophysiological characteristics of spiny neurones; with visual placement of the recording electrode, a further twenty-eight cholinergic neurones were obtained. The distinguishing features of these neurones (Kawaguchi, Wilson & Emson, 1989; Wilson, Chang & Kitai, 1990; Jiang & North, 1991; Kawaguchi, 1993; Kawaguchi et al. 1995; Calabresi, Pisani, Mercuri & Bernardi, 1996; Calabresi et al. 1997) were (i) expression of choline acetyltransferase immunoreactivity (Fig. 1); (ii) large somata (25–55 μm) with three to five primary dendrites bearing no spines (Fig. 1); (iii) low resting membrane potential (−60 ± 3 mV, n = 49 cells; this and other values are means ± standard error of mean) and high input resistance (195 ± 55 MΩ, n = 45) compared with other striatal neurones; (iv) marked accommodation of action potential discharge; and (v) prominent caesium-sensitive decline in hyperpolarizing electrotonic potential indicate of the cation current Ih. All forty-nine neurones showing the electrophysiological characteristics typical of cholinergic interneurones were found to be positive for choline acetyltransferase immunoreactivity.
Figure 1. Identification of cholinergic interneurones after intracellular recording.
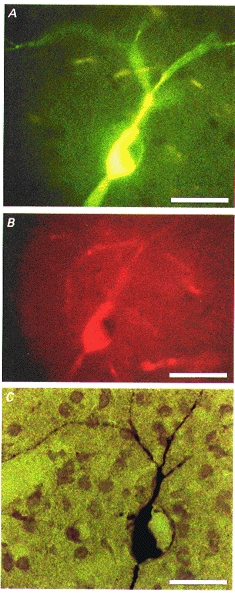
A, the cell was injected with biocytin and visualized by fluorescein isothiocyanate-conjugated avidin. Note the large body and the roundish unstained nucleus. B, choline acetyltransferase immunoreactivity visualized by tetramethylrhodamine isothiocyanate-conjugated secondary antibody. C, permanent staining after incubation with avidin-biotin-peroxidase complex and diaminobenzidine. The three photomicrographs show the same cell. Note the beaded aspinous dendrites. Scale bar, 25 μm.
Intrastriatal electrical stimulation evoked excitatory depolarizing synaptic potentials which resulted from activation of glutamate receptors and GABAA receptors, because these could be fully blocked by a combination of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μM), DL-2-amino-phosphonovaleric acid (APV, 30 μM) and bicuculline (30 μM) (Fig. 2A) (Kawaguchi et al. 1989; Wilson et al. 1990; Jiang & North, 1991; Calabresi et al. 1997). In thirty-five of forty-nine cholinergic neurones this depolarizing synaptic potential was followed by a slower hyperpolarization (IPSP) (Fig. 2A). At −60 mV, this ranged from 2 to 16 mV in amplitude and from 450 to 800 ms in duration depending on the strength of the stimulus. In general, the same stimulus strength which elicited the fast depolarizing potential was used to evoke the IPSP; it is possible that a greater proportion of cells would have shown IPSPs if either the stimulus strength was increased or the proper stimulation site was found. The IPSP resulted from an increase in membrane potassium conductance because it reversed polarity at −105 ± 2 mV (n = 5), −90 ± 4 mV (n = 3) and −75 ± 4 mV (n = 3) in 2.5, 5 and 7.5 mM extracellular potassium (Fig. 2B and D), respectively; these values agree well with those expected from the Nernst equation. IPSPs were reversibly blocked by the potassium channel blocker barium (500 μM, n = 3) (Fig. 2C).
Figure 2. Cholinergic IPSPs in striatal interneurones.
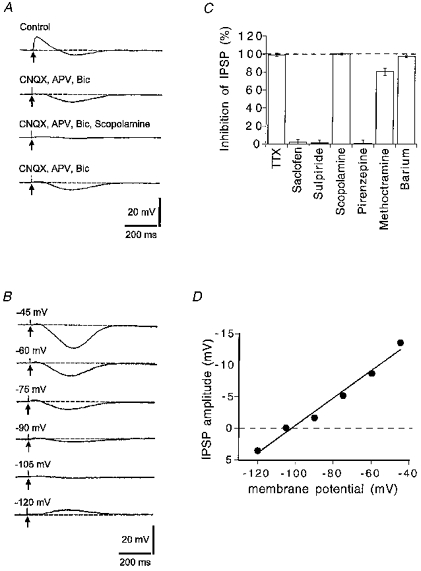
A, a single pulse stimulus (arrows) evoked a fast depolarizing synaptic potential followed by a slower hyperpolarizing synaptic potential (IPSP). The depolarization was blocked by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μM), DL-2-amino-phosphonovaleric acid (APV, 50 μM) and bicuculline (Bic, 30 μM) (5 min). The IPSP was blocked by further addition of scopolamine (1 μM) for 5 min, and this reversed after washing (80 min). Resting potential, −60 mV. B, the IPSP reversed polarity at about −105 mV. The membrane potential was set to the value indicated beside each trace (mV) prior to eliciting the IPSP with a single pulse stimulus (arrows). C, summary of pharmacological experiments on IPSPs. Concentrations were tetrodotoxin (TTX), 1 μM; saclofen, 500 μM; sulpiride, 3 μM; scopolamine, 1 μM; pirenzepine, 100 nM; methoctramine, 200 nM; and barium, 500 μM; n > 3 in each case. D, the graph shows the relationship of the IPSP amplitude with the membrane potential of the recorded cell (data obtained from the cell shown in B).
The IPSP was completely blocked by tetrodotoxin (Fig. 2C), but it was unaffected by the GABAB receptor antagonist saclofen (500 μM, n = 4) or by the D2 dopamine receptor antagonist L-sulpiride (3 μM, n = 3) (Fig. 2C). The IPSP was reversibly blocked by the muscarinic receptor antagonists scopolamine (1 μM, n = 9) and methoctramine (200 nM, n = 4), which has some selectivity for M2 receptors (Fig. 2C); however, pirenzepine (100 nM, n = 4), which at this concentration would block M1 receptors, had no effect (Fig. 2C). These experiments indicate that intrastriatal stimulation releases endogenous ACh from presynaptic nerves, which hyperpolarizes cholinergic interneurones by activating muscarinic receptors located on their somato-dendritic region.
The muscarinic receptor on the cholinergic interneurones was further characterized by applying exogenous agonists and antagonists. Muscarine induced a membrane hyperpolarization (Fig. 3A) or an outward current (Fig. 3C) in cells voltage clamped at −60 mV. This was concentration dependent (3 μM: 3 ± 1 mV (n = 4); 10 μM: 8 ± 2 mV (n = 6) and 55 ± 10 pA (n = 4); 30 μM: 10 ± 2 mV (n = 4) or 76 ± 15 pA (n = 3)), unaffected by tetrodotoxin (1 μM, n = 3) and reversed polarity at −106 ± 3.5 mV (n = 3; 2.5 mM potassium) (Fig. 3F). These effects of muscarine were mimicked by oxotremorine (100–500 nM, n = 5) (Fig. 3Ba) but not by the M1 receptor agonist 4-(m-chlorophenyl-carbamoyloxy)-2-butynyltrimethyl ammonium (McN-A-343; 1–10 μM, n = 4) (Fig. 3Bb), and they were blocked by methoctramine (100–300 nM, n = 5) (Fig. 3Eb) but not by pirenzepine (100 nM, n = 6) (Fig. 3E a). The acetylcholine esterase inhibitor neostigmine also elicited a concentration-dependent hyperpolarization (1 μM: 3 ± 1 mV (n = 4); 3 μM: 6 ± 2 mV (n = 4); 10 μM: 9 ± 3 mV (n = 3)) which was reversed by scopolamine (1 μM, n = 3) (Fig. 3D) and methoctramine (300 nM, n = 3), but not by pirenzepine (100 nM, n = 3). Together, these experiments suggest that ACh is released spontaneously within the tissue slice, and that its concentration rises sufficiently to activate the muscarinic receptors when its degradation is inhibited.
Figure 3. Effects of exogenous muscarinic agonists and antagonists on identified cholinergic neurones.
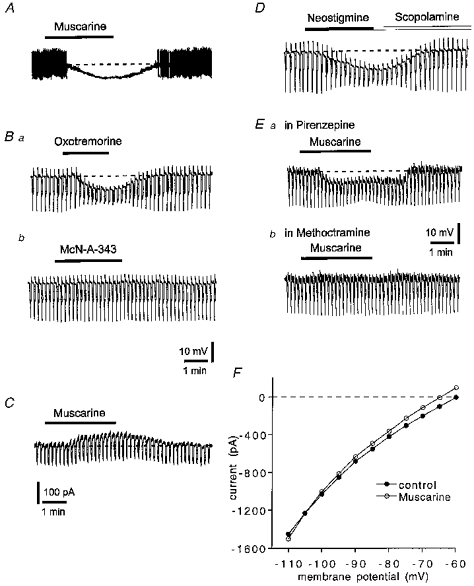
A, muscarine (10 μM) hyperpolarized a striatal cholinergic interneurone and inhibited action potential firing. Resting level is −55 mV (dashed line); full action potential height not captured by pen recorder. B, oxotremorine (300 nM) mimicked the action of muscarine (a) but McN-A343 (10 μM) did not (b). Holding potential, −60 mV. C, in a voltage-clamp experiment muscarine (10 μM) induced an outward current and increased membrane conductance. Holding potential, −60 mV. Downward deflections were induced by negative voltage steps (5 mV, 3 s). D, neostigmine (3 μM) hyperpolarized a cholinergic interneurone and this effect was reversed by scopolamine (1 μM). E, the hyperpolarization induced by muscarine (10 μM) was unaffected by pirenzepine (100 nM) (a) but blocked by methoctramine (300 nM) (b). Holding potential, −60 mV; same cell as A. Downward deflections in B, D and E are hyperpolarizing electrotonic potentials evoked by rectangular current pulses (200 pA, 3 s); their decline after the initial peak reflects the prominent Ih in these cells. F, current-voltage relationship of a cholinergic neurone before (•) and during 30 μM muscarine (^). The values were calculated by measuring the steady-state current generated by 3 s voltage steps of progressively increasing amplitude. Holding potential, −60 mV.
DISCUSSION
This is the first report of a muscarinic IPSP in the brain, although exogenous muscarinic agonists have been shown to increase potassium conductance in some other central neurones (Egan & North, 1986; reviewed by North, 1989a). Muscarinic IPSPs of similar time course and ionic mechanism are seen in other peripheral neurones and are generally considered to underlie the slowing of the heart in response to vagus nerve stimulation (reviewed by North, 1989a, b). The conductance activated by the released acetylcholine shows little rectification over the range of potentials around the equilibrium potential for potassium (EK) when normal extracellular potassium concentrations are used, as has been generally described (Hartzell, Kuffler, Stickgold & Yoshikami, 1977; Dodd & Horn, 1982; Egan & North, 1986). The pharmacological identification of the receptor through which ACh hyperpolarizes striatal interneurones is based largely on the effectiveness of methoctramine as an antagonist (Caulfield, 1993); this might be M2 or M4, and is consistent with the predominant expression of mRNA and protein for both these receptors in presumed interneurones (Weiner et al. 1990; Hersch et al. 1994).
The time course and ionic mechanism of this IPSP are fundamentally similar to that described in dopamine-, noradrenaline- and 5-hydroxytyptamine-containing neurones of the brain (reviewed by North, 1989b), thus indicating that the phenomenon of slow synaptic auto-inhibition can be extended also to central cholinergic neurones. In those cases, as for the action of muscarine in other cells, the receptors are coupled to potassium channels through the activation of a heterotrimeric G protein (see North, 1989a), suggesting that this mechanism of synaptic inhibition is fundamentally conserved in the brain whether the transmitter is acetylcholine, dopamine, noradrenaline, 5-hydroxytryptamine or γ-aminobutyric acid (acting at GABAB receptors) (North, 1989a).
It is thought that cholinergic interneurones integrate glutamatergic inputs arising from the cortex and thalamus with dopaminergic inputs originating from the substantia nigra (Kawaguchi et al. 1995; Calabresi et al. 1996). The axonal fields of cholinergic interneurones are very extensive, and they have much more widespread dendritic trees than do the projecting neurones (Graybiel, 1990; Wilson et al. 1990; Yan & Surmeier, 1996). These features have suggested the possibility that striatal cholinergic neurones may function as associative interneurones (Kawaguchi et al. 1995). Tonically active striatal neurones recorded in primates have electrophysiological properties which closely resemble the activity of identified cholinergic interneurones (Graybiel et al. 1994); they respond in a temporally correlated way after behavioural conditioning and modulate the activity of other striatal neurones during learning. The cholinergic interneurones could change the activity of spiny projecting neurones via pre- and postsynaptic mechanisms (Akins et al. 1990; Sugita et al. 1991; Howe & Surmeier, 1995). In addition, the cholinergic auto-inhibition brought about by the inhibitory synaptic potential described here is likely to play a crucial role in co-ordinated changes of striatal activity. Anticholinergic drugs have been widely used to ameliorate Parkinsonian symptoms by reducing the striatal cholinergic tone, but they can cause cognitive deficits. Our results suggest that muscarinic agonists selective for M2 receptors might limit the release of endogenous ACh not only by calcium current inhibition (Yan & Surmeier, 1996) but also by direct hyperpolarization of cholinergic interneurones.
Acknowledgments
This work was supported by a Biomed Grant (BMH 4–97-2215) to P.C. and by a Network Grant (Italian MURST - Cofinanziamento 1997) to P.C. We thank M. Tolu for excellent technical assistance.
References
- Akins PT, Surmeier DJ, Kitai ST. Muscarinc modulation of a transient K+ conductance in rat neostriatal neurons. Nature. 1990;344:240–242. doi: 10.1038/344240a0. 10.1038/344240a0. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Ascone CM, Centonze D, Pisani A, Sancesario G, D'Angelo V, Bernardi G. Opposite membrane potential changes induced by glucose deprivation in striatal spiny neurons and in large aspiny interneurons. Journal of Neuroscience. 1997;17:1940–1949. doi: 10.1523/JNEUROSCI.17-06-01940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. The corticostriatal projection: from synaptic plasticity to dysfunctions of the basal ganglia. Trends in Neurosciences. 1996;19:19–24. doi: 10.1016/0166-2236(96)81862-5. [DOI] [PubMed] [Google Scholar]
- Caulfield MP. Muscarinic receptors: characterization, coupling and function. Pharmacology and Therapeutics. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- Dodd J, Horn JP. Muscarinic inhibition of sympathetic C neurones in the bullfrog. The Journal of Physiology. 1982;334:271–291. doi: 10.1113/jphysiol.1983.sp014494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodt HU, Misgeld U. Muscarinic slow excitation and muscarinic inhibition of synaptic transmission in the rat neostriatum. The Journal of Physiology. 1986;380:593–608. doi: 10.1113/jphysiol.1986.sp016304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan TM, North RA. Acetylcholine hyperpolarizes central neurones by acting on an M2 muscarinic receptor. Nature. 1986;319:405–407. doi: 10.1038/319405a0. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends in Neurosciences. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. 10.1016/0166-2236(90)90104-I. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty A, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- Hartzell HC, Kuffler SW, Stickgold R, Yoshikami D. Synaptic exctiation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. The Journal of Physiology. 1977;271:817–846. doi: 10.1113/jphysiol.1977.sp012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI. Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. Journal of Neuroscience. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe AR, Surmeier DJ. Muscarinic receptors modulate N-, P-, and L-type Ca2+ currents in rat striatal neurons through parallel pathways. Journal of Neuroscience. 1995;15:458–469. doi: 10.1523/JNEUROSCI.15-01-00458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MK, Cubeddu LX. Pharmacological characterization and functional role of muscarinic autoreceptors in the rabbit striatum. Journal of Pharmacology and Experimental Therapeutics. 1987;240:203–215. [PubMed] [Google Scholar]
- Jiang ZG, North RA. Membrane properties and synaptic responses of rat striatal neurones in vitro. Journal of Physiology. 1991;443:533–553. doi: 10.1113/jphysiol.1991.sp018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. Journal of Neuroscience. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends in Neurosciences. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. Journal of Neurophysiology. 1989;62:1052–1069. doi: 10.1152/jn.1989.62.5.1052. [DOI] [PubMed] [Google Scholar]
- North RA. Muscarinic cholinergic receptor regulation of ion channels. In: Brown JH, editor. Muscarinic Receptor Subtypes. Clifton, NJ, USA: Humana; 1989a. pp. 341–373. [Google Scholar]
- North RA. Drug receptors and the inhibition of nerve cells. British Journal of Pharmacology. 1989b;19:13–28. doi: 10.1111/j.1476-5381.1989.tb16855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps PE, Houser CR, Vaughn J. Immunocytochemical localization of choline acetyltransferase within the rat neostriatum: a correlated light and electron microscopic study of cholinergic neurons and synapses. Journal of Comparative Neurology. 1985;238:286–307. doi: 10.1002/cne.902380305. [DOI] [PubMed] [Google Scholar]
- Sugita S, Uchimura N, Jiang Z-G, North RA. Distinct muscarinic receptors inhibit release of γ-aminobutyric acid and excitatory amino acids in mammalian brain. Proceedings of the National Academy of Sciences of the USA. 1991;88:2608–2611. doi: 10.1073/pnas.88.6.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proceedings of the National Academy of Sciences of the USA. 1990;87:7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat striatum. Journal of Neuroscience. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Surmeier DJ. Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-limited G-protein pathway. Journal of Neuroscience. 1996;16:2592–2604. doi: 10.1523/JNEUROSCI.16-08-02592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


