Abstract
Molecular mechanisms underlying maturation of the central respiratory rhythm are largely unknown. Previously, we found that brain-derived neurotrophic factor (BDNF) is required for expression of normal breathing behaviour in newborn mice, raising the possibility that maturation of central respiratory output is dependent on BDNF.
Respiratory activity was recorded in vitro from cervical ventral roots (C1 or C4) using the isolated brainstem–spinal cord preparation from postnatal day (P) 0.5–2.0 and P4.5 wild-type mice and mice lacking functional bdnf alleles.
Loss of one or both bdnf alleles resulted in an approximately 50 % depression of central respiratory frequency compared with wild-type controls. In addition, respiratory cycle length variability was 214 % higher in bdnf null (bdnf−/−) animals compared with controls at P4.5. In contrast, respiratory burst duration was unaffected by bdnf gene mutation.
These derangements of central respiratory rhythm paralleled the ventilatory depression and irregular breathing characteristic of bdnf mutants in vivo, indicating that central deficits can largely account for the abnormalities in resting ventilation produced by genetic loss of BDNF. BDNF is thus the first growth factor identified that is required for normal development of the central respiratory rhythm, including the stabilization of central respiratory output that occurs after birth.
The respiratory rhythm is produced by a distributed network of neurons located in the lower brainstem (for review, see Bianchi et al. 1995; Ramirez & Richter, 1996). During perinatal development the respiratory network undergoes marked maturational changes, which include alterations in the morphological, biochemical and electrophysiological properties of individual neurons (Denavit-Saubiéet al. 1994), as well as enhancement of synaptic interactions (Paton & Richter, 1995b). These alterations are critical for maturation of normal respiratory behaviour, and derangements of brainstem development have been implicated in human developmental disorders of breathing (Kinney et al. 1995). However, although developmental changes in central respiratory neurons have been described (Greer et al. 1992; Paton & Richter, 1995a; Di Pasquale et al. 1996), underlying molecular mechanisms are not understood.
Recent studies from our laboratory demonstrated that brain-derived neurotrophic factor (BDNF), a member of the neurotrophin class of growth factors, is required for development of normal breathing behaviour in mice (Erickson et al. 1996a). Specifically, newborn mice lacking functional bdnf alleles exhibit markedly depressed and irregular resting ventilation and increased incidence of apnoeas (Erickson et al. 1996a). Moreover, bdnf null mutants (bdnf−/−) lack a population of BDNF-dependent primary sensory neurons that constitute the afferent pathway between carotid body chemoreceptors and the brainstem (Hertzberg et al. 1994; Erickson et al. 1996a). These cells provide a powerful tonic excitatory drive to respiration (Dejours, 1962) that is absent in bdnf−/− mice (Erickson et al. 1996a). However, animals lacking BDNF exhibit a more severe respiratory depression than wild-type animals in which chemoafferent input is transiently suppressed (Erickson et al. 1996a), indicating that other changes, in addition to the lack of chemoafferent drive alone, probably account for the respiratory phenotype in bdnf null mutants. For example, chemoafferent cell loss could derange central respiratory output by depriving the central respiratory network of important developmental cues.
BDNF could also act directly to regulate maturation of respiratory neurons, either by supporting neuronal survival (Schwartz et al. 1997) or by regulating processes that underlie normal synaptic function, such as dendritic growth (McAllister et al. 1997) and enhancement of synaptic innervation density (Causing et al. 1997) or chemical neurotransmission (Leßmann et al. 1994). In fact, functional maturation of respiration-related central neurons has been associated with dendritic morphogenesis (Kalia et al. 1993; Denavit-Saubiéet al. 1994) and increased synaptic efficacy (Paton & Richter, 1995b) during early postnatal development. The possibility that BDNF can directly regulate such processes is supported by the observation that TrkB, the high-affinity receptor for BDNF, exhibits a widespread distribution in the brainstem (Yan et al. 1997).
The present study was undertaken, therefore, to determine whether BDNF is required for functional maturation of the central respiratory rhythm. To approach this issue we compared development of central respiratory output in newborn wild-type mice and mice lacking one or both functional bdnf alleles, using the in vitro brainstem–spinal cord preparation (Suzue, 1984). This preparation permits recording of motor output from the intact respiratory network, under controlled environmental conditions, in the absence of afferent influences from peripheral or suprapontine structures. We also analysed central respiratory output in mice lacking neurotrophin-4 (NT-4) which, like BDNF, acts through the TrkB receptor. Our data indicate that BDNF, but not NT-4, is critical for functional maturation of the central respiratory rhythm.
METHODS
Animals
bdnf heterozygous and nt-4 homozygous mutant mice (Conover et al. 1995) were obtained from Regeneron Pharmaceuticals, Inc. (Tarrytown, NY, USA) and used for breeding. The day of birth was designated P0.5. The genotype at the bdnf locus was determined for each animal by polymerase chain reaction, using the following primers: 5′ primer, 5′-CATACTTCGGTTGCATG-3′; 3′ primer, 5′-GATCACTGTCACACACGCTCA-3′; Neo 3′ primer, 5′-ATGGAAGGATTGGAGCTA-3′. Altogether, 221 neonatal mice derived from 36 litters were used. All electrophysiological recordings, data collection and analysis were conducted by an investigator blinded to the genotype of the mice.
brainstem–spinal cord preparation
The experiments were performed using isolated brainstem–spinal cord preparations (Suzue, 1984). The animals were deeply anaesthetized with ether, and the head and vertebral column rapidly isolated under a dissecting microscope. The preparation was then placed in a dissection chamber filled with an ice-cold artificial cerebrospinal fluid solution (for composition, see below) continuously aerated with a gas mixture of 95 % O2 and 5 % CO2. After a craniotomy, the cerebrum was removed by transection at the pontine level rostral to the entry of the Vth cranial nerves, and the cerebellum, skull base and vertebral bodies were also removed. Total dissection time averaged 9.8 ± 0.54 min. The isolated brainstem–spinal cord preparation was transferred to a recording chamber (volume of 100 ml) and pinned down along the lateral edge, ventral surface upward, on Sylgard resin. The preparation was superfused continuously with artificial cerebrospinal fluid composed of (mM): 130 NaCl, 5.4 KCl, 0.8 KH2PO4, 26 NaHCO3, 1.0 MgCl2, 0.8 CaCl2 and 30 glucose (pH = 7.2 as measured in perfusate samples; Jacquin et al. 1996) at 24–25°C and directly oxygenated (95 % O2 and 5 % CO2). After stabilizing for 45–60 min, the bath temperature was raised to 26–27°C before recording.
Recordings
Activity was recorded extracellularly with a suction electrode applied to the proximal cut end of either the C1 ventral root (50 cases) or the C4 ventral root (171 cases), which contains phrenic motor axons. The suction electrode was constructed of polyethylene tubing (PE-190) flame-pulled to have a tip tightly fitting to the recorded root (diameter, 50–100 μm). The electrical signals were differentially amplified (AM502 Differential Amplifier, Tektronix, Inc., Beaverton, OR, USA) with a bandwidth of 1 Hz to 3 kHz, before display on an oscilloscope (Tektronix 5103N). The data were assessed for two age groups: (1) during the first 48 h after birth (P0.5–2.0), and (2) during the fifth postnatal day (P4.5). Data for the first two postnatal days were combined, as there were no significant differences among these preparations.
In forty-nine preparations no respiratory activity was detected during the 30 min recording period, although slow bursting activity of spinal origin was present (see Results). The incidence of such preparations was similar among bdnf−/− (n = 10), bdnf+/− (n = 28) and bdnf+/+ (n = 11) genotypes, and was higher in P0.5–2.0 (40 of 154) than in P4.5 (9 of 57) preparations. These preparations were not included in the present study.
Data acquisition and analysis
Respiratory activity was recorded in unprocessed form only and captured at a sampling rate of 1 kHz using an analog-to-digital converter (Lab Master DMA 100AX, Scientific Solutions, Solon, OH, USA) and AxoTape software (version 2.0.1, Axon Instruments). The mean recording time from each preparation was 24.3 ± 1.13 min. The central respiratory output was evaluated by averaging within each genotype: (1) mean respiratory frequency, (2) variability of instantaneous respiratory cycle length, calculated as the standard deviation divided by the mean, (3) maximal respiratory cycle length, and (4) respiratory burst duration. Subsequent data analysis, including measurements of instantaneous cycle length, was performed using AxoTape software.
Data are presented as means ±s.e.m. and were analysed by ANOVA and MANOVA followed by Scheffé‘s multiple comparison procedure. Student's unpaired t test was used to compare nt-4−/− preparations with wild-type controls. P < 0.05 was considered significant; n indicates the number of preparations.
RESULTS
Central respiratory output, recorded from spinal ventral roots in the isolated newborn brainstem–spinal cord preparation, consists of rhythmic discharges that are abolished after bulbospinal transection (Greer et al. 1992). In our experiments two types of discharges were observed concomitantly in 75 out of 172 preparations, regardless of genotype or recording site (C1 or C4). The first type of discharge, which was more frequently observed in the younger preparations, was characterized by bursts of relatively long duration (3–40 s), appearing every 5–20 min. These discharges persisted after transection at the bulbospinal level, indicating that they were not related to output from the medullary respiratory network (Perségol & Viala, 1994) and were therefore not included in the present analysis. The second type of activity, recorded in all 172 preparations, consisted of rhythmic discharges (Fig. 1A) typically lasting less than 1 s and was abolished after bulbospinal transection (n = 5; Fig. 1B). The results presented below are based on analysis of this central respiratory activity.
Figure 1. Influence of bdnf gene mutation on central respiratory output.
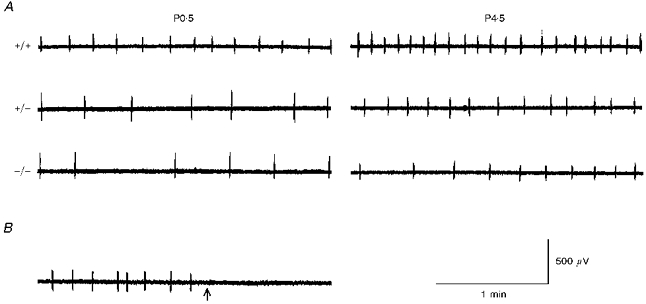
A, central respiratory activity was recorded in vitro on the first (P0.5) and fifth (P4.5) postnatal day from the C4 ventral root in wild-type (+/+), bdnf heterozygous (+/-) and bdnf knockout (-/-) brainstem–spinal cord preparations. A markedly lower frequency of respiratory discharges in bdnf mutants compared with wild-type controls was apparent at both ages tested. At both ages, the preparations of different genotypes were derived from littermates. B, bulbospinal transection (arrow) abolished respiratory activity recorded from the C4 ventral root. This recording was made from the same P0.5 wild-type preparation shown in A.
Respiratory frequency and cycle length variability in bdnf mutants
We found previously that mean respiratory frequency, measured by plethysmography in intact newborn bdnf−/− mice, was depressed by approximately 50 % compared with wild-type controls (Erickson et al. 1996a). Moreover, the cycle-to-cycle variability in frequency, a measure of breathing irregularity, was increased 2-fold in P4.5 bdnf−/− mice. To determine whether these deficits in respiratory behaviour could be accounted for by derangement of central respiratory output, we compared the frequency and variability of respiratory discharges recorded in isolated brainstem–spinal cord preparations from wild-type, bdnf+/− and bdnf−/− mice at various postnatal ages. At P0.5–2.0 the mean frequency of respiratory discharges recorded in bdnf−/− and bdnf+/− preparations was 46 and 63 %, respectively, of the wild-type control value (P < 0.01 compared with wild-type; Figs 1A and 2A). There was no significant difference between the hetero- and homozygous mutants (P = 0.14). This depression of respiratory frequency in the bdnf+/− and bdnf−/− mutants persisted with increasing age. Thus although the absolute respiratory frequency increased approximately 2-fold for each genotype, the values for bdnf−/− and bdnf+/− preparations remained 45 and 62 %, respectively, of the wild-type control value on P4.5 (Figs 1A and 2A).
Figure 2. Mutation of the bdnf gene results in decreased frequency and increased variability of respiratory discharges.
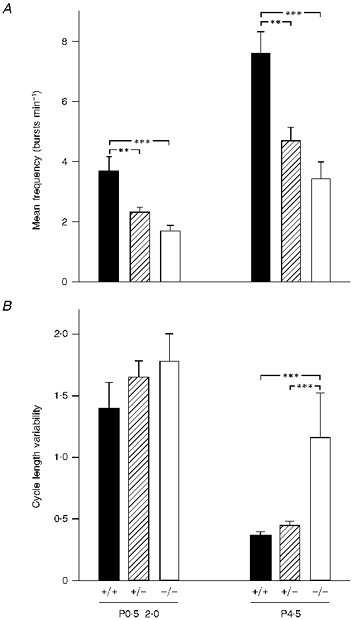
Mean frequency of respiratory discharges (A) and respiratory cycle length variability (B) in wild-type (+/+), bdnf heterozygous (+/-) and bdnf knockout (-/-) in vitro brainstem–spinal cord preparations recorded during the first 48 h after birth (P0.5–2.0) and on P4.5. Respiratory cycle length variability is expressed as a coefficient of variation (s.d./mean). The mean number of measured respiratory cycles was not significantly different among groups (P0.5–2.0: +/+, 90 ± 17.4; +/−, 59 ± 8.2; −/−, 57 ± 10.3; P4.5: +/+, 120 ± 12.8; +/−, 95 ± 9.8; −/−, 105 ± 21.7). The number of preparations in each group is as follows: P0.5–2.0: +/+, 27; +/−, 57; −/−, 30; P4.5: +/+, 15; +/−, 25; −/−, 8; **P < 0.01, ***P < 0.001.
Respiratory cycle length variability, based on a cycle-by-cycle analysis, demonstrated that the first two postnatal days were characterized by highly irregular central respiratory output, regardless of genotype. In fact, at this age, the differences in variability among bdnf−/−, bdnf+/−, and bdnf+/+ preparations were not significant (Fig. 2B). This observation is consistent with results obtained in vivo demonstrating that, during the first two days of life, all animals, regardless of genotype, exhibited irregular ventilation (Erickson et al. 1996a). By postnatal day 4.5, however, cycle length variability had decreased significantly in bdnf+/+ (P = 0.0132) and bdnf+/− (P = 0.00003) preparations, compared with the values at P0.5–2.0. In contrast, in preparations derived from bdnf−/− mice, although variability tended to decrease with age, the decrease was not statistically significant (MANOVA, P = 0.7177, Fig. 2B). Therefore, at P4.5, cycle length variability in bdnf−/− was 158 and 214 % higher, respectively, than in the bdnf+/− and wild-type control preparations (P < 0.001).
Maximal length of the respiratory cycle in bdnf mutants
In addition to depressed and irregular breathing, bdnf null mutants exhibit an increased occurrence of apnoeas, defined as respiratory pauses longer than 2–3 s (Erickson et al. 1996a). To determine whether central respiratory output in bdnf mutants exhibited a potential neural correlate of these apnoeic pauses, we compared the duration of the longest respiratory cycle observed during each recording session, among bdnf+/+, bdnf+/− and bdnf−/− preparations. This analysis revealed that the maximal length of the respiratory cycle was disproportionately and significantly greater in bdnf−/− compared with bdnf+/+ and bdnf+/− preparations at P4.5 (Fig. 3). In fact, the ratio of the maximal to mean cycle length was more than 3-fold higher in P4.5 bdnf knockout preparations compared with wild-type controls (9.8 ± 4.88 vs. 2.7 ± 0.33).
Figure 3. Maximal length of the respiratory cycle is disproportionately greater in bdnf null mutants compared with wild-type and heterozygous animals at P4.5.
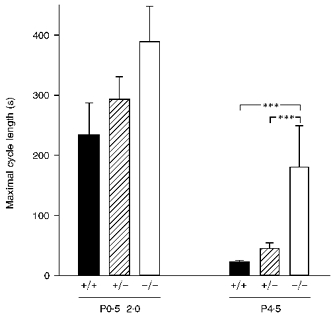
Mean values of maximal respiratory cycle length in P0.5–2.0 and P4.5 in vitro brainstem–spinal cord preparations (+/+ wild-type, +/−bdnf heterozygous, and −/−bdnf knockout). At P0.5–2.0 there were no statistically significant differences among genotypes, whereas at P4.5 there was a significantly longer (***P < 0.001) maximal cycle length in bdnf knockout mice. The number of preparations in each group is the same as in Fig. 2.
Respiratory burst duration in bdnf mutants
Ramirez et al. (1996) demonstrated that the duration of respiratory bursts recorded from the transverse brainstem slice preparation of mice tends to increase with age, indicating that burst duration is one locus at which developmental mechanisms act to regulate postnatal maturation of central respiratory output. To determine whether the absence of BDNF alters this maturational process, we compared respiratory burst duration in bdnf mutants and wild-type littermates on P0.5–2.0 and P4.5. Within each genotype, respiratory burst duration was significantly longer in P4.5 brainstems (bdnf+/+, 713.6 ± 84.14 ms; bdnf+/−, 695.0 ± 36.42 ms; bdnf−/−, 695.5 ± 72.69 ms) compared with P0.5–2.0 preparations (bdnf+/+, 517.0 ± 39.48 ms; bdnf+/−, 468.9 ± 17.60 ms; bdnf−/−, 448.0 ± 22.23 ms). However, no significant differences in burst duration were observed among the various genotypes at either age tested.
Central respiratory output in neurotrophin-4 knockout mice
NT-4, like BDNF, is required for survival of subsets of visceral sensory neurons in vitro and in vivo (Erickson et al. 1996a). However, in contrast to bdnf null mutants, mice lacking functional nt-4 alleles exhibit normal respiratory behaviour (Erickson et al. 1996b). This could indicate that central respiratory output is normal in nt-4 null mutants, or, alternatively, that compensatory mechanisms, such as increased peripheral drive, overcome any potential central deficit. To distinguish between these possibilities, we compared respiratory activity generated in brainstem–spinal cord preparations derived from P1.5 nt-4−/− mice and wild-type controls. No significant differences in mean respiratory frequency, cycle length variability or burst duration were observed (Table 1).
Table 1.
Characteristics of central respiratory output in nt-4 knockout mice (nt-4−/−) compared with wild-type mice at P1.5
| Genotype | n | Mean frequency (bursts min−1) | Respiratory cycle length variability † | Burst duration (ms) |
|---|---|---|---|---|
| Wild-type | 4 | 4.00 ± 0.26 | 1.02 ± 0.13 | 456 ± 88.0 |
| nt-4−/− | 6 | 3.69 ± 0.64 ‡ | 1.24 ± 0.16 ‡ | 432 ± 60.0 ‡ |
Respiratory cycle length variability is expressed as a coefficient of variation (s.d./mean); mean number of measured cycles was not significantly different between groups (wild-type, 56.8 ± 8.7; nt-4−/−, 74.0 ± 18.1).
Not significantly different from wild-type.
DISCUSSION
The current study demonstrates that disruption of the bdnf gene results in selective defects in central respiratory output. Specifically, discharge frequency and cycle length variability are significantly altered by loss of BDNF, whereas burst duration is unchanged. BDNF is therefore the first growth factor identified that is required for normal development of the central respiratory rhythm. Moreover, the fact that differences in respiratory frequency between wild-type animals and bdnf mutants were apparent as early as the first postnatal day suggests that BDNF is required for functional maturation of the central respiratory network before birth.
The abnormalities in central respiratory output that we observed in bdnf−/− brainstem–spinal cord preparations in vitro reflected faithfully the respiratory phenotype of bdnf null mutants in vivo (Erickson et al. 1996a). For example, although the absolute frequency of respiratory output in the in vitro preparation is lower than ventilatory frequency in vivo, bdnf null mutants nonetheless exhibited a further 54–55 % depression in central respiratory frequency compared with wild-type littermates, paralleling the ventilatory depression observed in vivo (Erickson et al. 1996a). Similarly, the bdnf null mutation resulted in markedly higher respiratory cycle length variability at P4.5 in vitro, as in vivo (Erickson et al. 1996a). These data indicate, therefore, that the depression and irregularity of breathing observed in bdnf null mutants in vivo can probably be accounted for by deficits in central respiratory output. Thus BDNF appears to be required both for development of normal respiratory frequency and for the stabilization of central respiratory output that normally occur during the perinatal period (Jansen & Chernick, 1991; Di Pasquale et al. 1996). In addition, bdnf−/− preparations exhibited disproportionately longer pauses between respiratory bursts compared with wild-type controls. We speculate that these pauses could predispose the bdnf−/− animals to more frequent apnoeas, as is seen in vivo (Erickson et al. 1996a).
Interestingly, central respiratory output was significantly depressed in both hetero- and homozygous preparations, indicating that both bdnf alleles are required for development of a normal central respiratory frequency. However, breathing frequency in vivo was unchanged in heterozygous animals compared with wild-type controls (Erickson et al. 1996a). This probably reflects the fact that, in vivo, excitatory drives, such as peripheral chemoafferent input, which are absent in the isolated preparation in vitro, are able to compensate for depressed activity of the brainstem respiratory network. Thus even with a reduced central respiratory output, and partial reduction in chemoafferent cell numbers (Erickson et al. 1996a), heterozygotes are able to sustain a relatively normal respiratory frequency. Respiratory cycle length variability, on the other hand, was the same in heterozygous and wild-type preparations on P4.5, indicating that in contrast to mean respiratory frequency, one bdnf allele is sufficient to support maturation of this specific parameter.
One likely mechanism by which BDNF promotes development and stabilization of normal respiratory rhythm is by supporting survival of peripheral chemoafferent neurons that provide sensory input to central respiratory structures (Hertzberg et al. 1994; Erickson et al. 1996a). We speculate that loss of this afferent input in bdnf knockout mice (Erickson et al. 1996a), in addition to eliminating ventilatory drive, may derange development of central respiratory neurons. In other systems, for example, afferent inputs to central neural structures play a critical role in development of postsynaptic target cells (cf. Guillery, 1973). Moreover, loss of chemoafferent input after birth is known to have a destabilizing influence on respiration (Hofer, 1984). It is also possible, of course, that development of the respiratory network is indirectly influenced by other, still unidentified inputs, which may be affected in bdnf null mutants.
Another possibility is that BDNF has direct effects, either developmental or acute, on neurons and/or synaptic function within the medullary respiratory central pattern generator (Smith et al. 1991). In the hippocampus, for example, exogenous BDNF can reverse the effects of the bdnf null mutation on synaptic function, indicating that BDNF is required for normal transmission but not for development of underlying circuitry (Patterson et al. 1996). Loss of BDNF could also affect neurons in the ventrolateral portion of the caudal pontine reticular formation, which provide excitatory drive to the medullary respiratory central pattern generator (Borday et al. 1997). Elimination of these cells in Krox-20 mutant mice resulted in a 2.5-fold slower respiratory frequency compared with wild-type controls (Jacquin et al. 1996), mimicking, to some extent, the bdnf null phenotype. Although BDNF is not required for survival of spinal cord or brainstem motoneurons (Conover et al. 1995), it is also possible that loss of BDNF leads to functional changes in phrenic motoneurons (Gonzalez & Collins, 1997). The fact that central respiratory output was normal in NT-4 mutants indicates either that NT-4 is not required for development of the respiratory controller or that its absence is compensated for by another factor.
In summary, our data indicate that BDNF is required for normal development of central respiratory output. However, not all aspects of central respiratory output exhibit the same requirement for BDNF. For example, whereas both bdnf alleles are required for development of normal respiratory frequency, one allele is sufficient for normal cycle length variability. Whether this quantitative difference in BDNF dependence reflects distinct mechanisms or targets of BDNF action remains to be defined. Burst duration, on the other hand, was unaffected by disruption of the bdnf gene, indicating that BDNF is probably only one of several factors responsible for maturation of central respiratory output as a whole.
Acknowledgments
This work was supported by United States Public Health Service grants (NHLBI and CHHD) to D. M. K. The authors gratefully acknowledge the support of Dr Guillermo Pilar's laboratory in providing the electrophysiological equipment used in this study. In particular, we acknowledge the generous help of Dr Pilar and Luis Polo-Parada in fabricating the recording set-up. We also thank Dr Lynn Landmesser for helping us with recording techniques. We thank Regeneron Pharmaceuticals, Inc. for supplying the founding breeder animals required for this study. We also acknowledge the expert work of Roseann Brady, who did the PCR genotyping.
References
- Bianchi AL, Denavit-Saubié M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiological Reviews. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Borday V, Kato F, Champagnat J. A ventral pontine pathway promotes rhythmic activity in the medulla of neonate mice. NeuroReport. 1997;8:3679–3683. doi: 10.1097/00001756-199712010-00005. [DOI] [PubMed] [Google Scholar]
- Causing CG, Gloster A, Aloyz R, Bamji SX, Chang E, Fawcett J, Kuchel G, Miller FD. Synaptic innervation density is regulated by neuron-derived BDNF. Neuron. 1997;18:257–267. doi: 10.1016/s0896-6273(00)80266-4. [DOI] [PubMed] [Google Scholar]
- Conover JC, Erickson JT, Katz DM, Bianchi LM, Poueymirou WT, McClain J, Pan L, Helgren M, Ip NY, Boland P, Friedman B, Wiegand S, Vejsada R, Kato AC, DeChiara TM, Yancopoulos GD. Neuronal deficits, not involving motor neurons, in mice lacking BDNF and/or NT4. Nature. 1995;375:235–238. doi: 10.1038/375235a0. [DOI] [PubMed] [Google Scholar]
- Dejours P. Chemoreflexes in breathing. Physiological Reviews. 1962;42:335–358. doi: 10.1152/physrev.1962.42.3.335. [DOI] [PubMed] [Google Scholar]
- Denavit-Saubié M, Kalia M, Pierrefiche O, Schweitzer P, Foutz AS, Champagnat J. Maturation of brainstem neurons involved in respiratory rhythmogenesis: biochemical, bioelectrical and morphological properties. Biology of the Neonate. 1994;65:171–175. doi: 10.1159/000244048. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Tell F, Monteau R, Hilaire G. Perinatal developmental changes in respiratory activity of medullary and spinal neurons: an in vitro study on fetal and newborn rats. Brain Research.Developmental Brain Research. 1996;91:121–130. doi: 10.1016/0165-3806(95)00170-0. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Conover JC, Borday V, Champagnat J, Barbacid M, Yancopoulos G, Katz DM. Mice lacking brain-derived neurotrophic factor exhibit visceral sensory neuron losses distinct from mice lacking NT4 and display a severe developmental deficit in control of breathing. Journal of Neuroscience. 1996a;16:5361–5371. doi: 10.1523/JNEUROSCI.16-17-05361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Conover JC, Borday V, Champagnat J, Katz DM. BDNF-, but not NT4-knockout mice are deficient in dopaminergic visceral sensory neurons and display severe developmental deficits in control of breathing. Society for Neuroscience Abstracts. 1996b;22:991. doi: 10.1523/JNEUROSCI.16-17-05361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M, Collins WF. Modulation of motoneuron excitability by brain-derived neurotrophic factor. Journal of Neurophysiology. 1997;77:502–506. doi: 10.1152/jn.1997.77.1.502. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL. Respiratory and locomotor patterns generated in the fetal rat brainstem–spinal cord in vitro. Journal of Neurophysiology. 1992;67:996–999. doi: 10.1152/jn.1992.67.4.996. [DOI] [PubMed] [Google Scholar]
- Guillery RW. Quantitative studies of transneuronal atrophy in the dorsal lateral geniculate nucleus of cats and kittens. Journal of Comparative Neurology. 1973;149:423–437. doi: 10.1002/cne.901490403. [DOI] [PubMed] [Google Scholar]
- Hertzberg T, Fan G, Finley JCW, Erickson JT, Katz DM. BDNF supports mammalian chemoafferent neurons in vitro and following peripheral target removal in vivo. Developmental Biology. 1994;166:801–811. doi: 10.1006/dbio.1994.1358. 10.1006/dbio.1994.1358. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Lethal respiratory disturbance in neonatal rats after arterial chemoreceptor denervation. Life Sciences. 1984;34:489–496. doi: 10.1016/0024-3205(84)90505-8. 10.1016/0024-3205(84)90505-8. [DOI] [PubMed] [Google Scholar]
- Jacquin TD, Borday V, Schneider-Maunoury S, Topilko P, Ghilini G, Kato F, Charnay P, Champagnat J. Reorganization of pontine rhythmogenic neuronal networks in Krox-20 knockout mice. Neuron. 1996;17:747–758. doi: 10.1016/s0896-6273(00)80206-8. 10.1016/S0896-6273(00)80206-8. [DOI] [PubMed] [Google Scholar]
- Jansen AH, Chernick V. Fetal breathing and development of control of breathing. Journal of Applied Physiology. 1991;70:1431–1446. doi: 10.1152/jappl.1991.70.4.1431. [DOI] [PubMed] [Google Scholar]
- Kalia M, Schweitzer P, Champagnat J, Denavit-Saubié M. Two distinct phases characterize maturation of neurons in the nucleus of the tractus solitarius during early development: morphological and electrophysiological evidence. Journal of Comparative Neurology. 1993;327:37–47. doi: 10.1002/cne.903270104. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Filiano JJ, Sleeper LA, Mandell F, Valdes-Dapena M, White WF. Decreased muscarinic receptor binding in the arcuate nucleus in Sudden Infant Death Syndrome. Science. 1995;269:1446–1450. doi: 10.1126/science.7660131. [DOI] [PubMed] [Google Scholar]
- Leßmann V, Gottmann K, Heumann R. BDNF and NT-4/5 enhance glutamatergic synaptic transmission in cultured hippocampal neurons. NeuroReport. 1994;6:21–25. doi: 10.1097/00001756-199412300-00007. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Richter DW. Maturational changes in the respiratory rhythm generator of the mouse. Pflügers Archiv. 1995a;430:115–124. doi: 10.1007/BF00373846. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Richter DW. Role of fast inhibitory synaptic mechanisms in respiratory rhythm generation in the maturing mouse. The Journal of Physiology. 1995b;484:505–521. doi: 10.1113/jphysiol.1995.sp020682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TAS, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Perségol L, Viala D. Characteristics of slow bursting activities recorded in cervical ventral roots in the in vitro brainstem–spinal cord preparation of the neonatal rat. Somatosensory and Motor Research. 1994;11:57–64. doi: 10.3109/08990229409028857. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Quellmalz UJA, Richter DW. Postnatal changes in the mammalian respiratory network as revealed by the transverse brainstem slice of mice. The Journal of Physiology. 1996;491:799–812. doi: 10.1113/jphysiol.1996.sp021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Richter DW. The neuronal mechanisms of respiratory rhythm generation. Current Opinion in Neurobiology. 1996;6:817–825. doi: 10.1016/s0959-4388(96)80033-x. [DOI] [PubMed] [Google Scholar]
- Schwartz PM, Borghesani PR, Levy RL, Pomeroy SL, Segal RA. Abnormal cerebellar development and foliation in BDNF −/− mice reveals a role for neurotrophins in CNS patterning. Neuron. 1997;19:269–281. doi: 10.1016/s0896-6273(00)80938-1. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brainstem–spinal cord preparation of the neonatal rat. The Journal of Physiology. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Radeke MJ, Matheson CR, Talvenheimo J, Welcher AA, Feinstein SC. Immunocytochemical localization of TrkB in the central nervous system of the adult rat. Journal of Comparative Neurology. 1997;378:135–157. [PubMed] [Google Scholar]


