Abstract
Indo-1 and fluo-3 imaging techniques were used to investigate the role of gap junctions in the changes in cytosolic calcium concentrations ([Ca2+]i) induced by several receptor agonists. Subpopulations of confluent cultured astrocytes from the rat striatum were superfused with submaximal concentrations of endothelin-1 (Et1) and the α1-adrenergic and muscarinic receptor agonists, methoxamine and carbachol, respectively.
Combined binding and autoradiographic studies indicated that all striatal astrocytes possess binding sites for Et1. In contrast, α1-adrenergic and muscarinic binding sites were found to be heterogeneously distributed. In agreement with these findings, Et1 induced fast calcium responses in all cells while only subsets of striatal astrocytes responded to the application of either methoxamine or carbachol.
Halothane, heptanol and octanol, which are commonly used as gap junction inhibitors, drastically reduced the amplitude of Et1-induced calcium responses. In contrast, 18-α-glycyrrhetinic acid (αGA) used at a concentration known to block gap junction permeability in astrocytes had no significant effect on the amplitude of these calcium responses.
As demonstrated by quantitative and topological analysis, Et1 application similarly increased [Ca2+]i levels in all astrocytes in both the absence and presence of αGA.
In control conditions, subpopulations of cells responding to methoxamine or carbachol exhibited two main types of calcium responses which differed in their shape and kinetic characteristics. In the presence of αGA the number of cells responding to these receptor agonists was significantly reduced. Indeed, responses characterized by their long latency, slow rise time and weak amplitude disappeared in the presence of αGA while responses with short latency and fast rise time were preserved.
These results indicate that permeable gap junction channels tend to attenuate the pharmacological and functional heterogeneity of populations of astrocytes, while their inhibition restricts calcium responses in astrocytes expressing high densities of transmitter receptors coupled to phospholipase C.
Knowledge about the properties of brain glial cells has progressed greatly during the last decade. This is particularly the case for astrocytes which were first considered as passive bystanders of neurones but which are now thought to contribute in several ways to brain function (see Travis, 1994). Therefore, the earliest concept that the different classes of glial cells are relatively homogeneous is thus challenged. Indeed, several studies indicate that the properties of astrocytes may differ from one brain structure to another. For instance, neurones exhibit a larger number of neurites when grown on astrocytes from the same brain structure than on astrocytes from other cerebral regions (Denis-Donini, Glowinski & Prochiantz, 1984). In addition, as estimated by the formation of second messengers in response to the activation of neurotransmitter receptors, differences in the presence of several types of receptors can be observed among various brain structures (see Glowinski, Marin, Tence, Stella, Giaume & Prémont, 1994). Besides this regional heterogeneity of astrocytic populations, electrophysiological recordings of individual cells have demonstrated astrocytic heterogeneity within a defined structure. Indeed, cultured astrocytes derived from a given brain area may be characterized by their distinct phenotypes as indicated by ionic channel expression (see Sontheimer & Ritchie, 1995) and differences in their responses to the application of neurotransmitters (see Hösli & Hösli, 1993). Such individual differences were also observed in combined receptor binding studies and calcium imaging experiments undertaken for determining the distribution of α1-adrenergic receptors in cultured astrocytes from the rat cerebral cortex (Lerea & McCarthy, 1989; Shao, Porter & McCarthy, 1994).
Although, astrocytes are devoid of regenerative action potentials and synaptic communication, these cells have the capacity to exchange signals. Indeed, changes in intracellular calcium concentration ([Ca2+]i) can be induced in astrocytes by a great variety of signals (Verkhratsky & Kettenmann, 1996) and these cells display a network organization since they are highly connected by gap junction channels (see Giaume & McCarthy, 1996). The combination of these two properties provides the basis for inter-astrocytic communication. Initial evidence for such a long-range signalling pathway was obtained by the observation of propagating intercellular calcium ‘waves’ in astrocytes (Cornell-Bell, Finkbeiner, Cooper & Smith, 1990; Charles, Merrill, Dirksen & Sanderson, 1991). This finding suggests that intercellular calcium signalling (ICS) represents a form of cell excitability enabling astrocytes to communicate between each other and to interact with surrounding neurones (see Smith, 1994). This statement based upon experiments performed with cultured cells, was further supported by the demonstration of propagating calcium waves in freshly excised CNS tissue (Newman & Zahs, 1997). Furthermore, astrocytic calcium waves can be induced by nerve activity in organotypic cultures (Dani, Chernjavsky & Smith, 1992) and astrocytic calcium waves can activate surrounding neurones in cocultures (Nedergaard, 1994; Parpura, Basarsky, Liu, Jeftinija & Haydon, 1994) and in accute brain slices (Bezzi et al. 1998).
The pharmacological heterogeneity of astrocytes indicating the existence of a certain degree of cell individuality and the network organization allowing the synchronization of intracellular events occurring in groups of astrocytes could be considered as antagonistic properties. However, these features could be interdependent since the permeability of gap junctions can be regulated by neurotransmitters (see Giaume & MacCarthy, 1996). The present study was undertaken to further understand the role of gap junctional communication in ICS between astrocytes. For this purpose, we have compared the calcium responses of populations of communicating and non-communicating striatal astrocytes induced by superfusion of subpopulations of these cells with several receptor agonists.
METHODS
Cell cultures
Pregnant OFA rats (IFFA Credo, Lyon, France) were killed by prolonged exposure to a rising concentration of carbon dioxide. Embryos were rapidly removed from the uteri and placed into phosphate-buffered saline (PBS) supplemented with glucose (33 mM). Primary cultures were prepared as previously described (see Venance, Stella, Glowinski & Giaume, 1997). Briefly, striata were dissected from day 16 rat embryos, and then mechanically dissociated using a flame-narrowed Pasteur pipette in PBS-glucose solution. Cells were plated on poly-L-ornithine-coated (6 μg ml−1) 35 mm diameter culture dishes (1.5 × 106 cells per dish) (NUNC, Roskilde, Denmark) for binding assays and on glass slides (3 × 106 cells per slide; dimensions, 45 mm × 70 mm) (Rouvier-Gassalem, Paris, France) previously coated with poly-L-ornithine (15 μg ml−1) and natural mouse laminin (2 μg ml−1) for calcium imaging experiments. The culture medium consisted of a 1:1 mixture of minimal essential medium and F-12 nutrient (Gibco, Gaithersburg, MD, USA) supplemented with (mM): glutamine, 2; NaHCO3, 13; Hepes, 20; glucose, 33; antibiotics (50 i.u. ml−1 penicillin and 50 i.u. ml−1 streptomycin) and 5 % Nu-serum (Collaborative Research, Bedford, MA, USA). Cells were incubated at 37°C for 21–25 days in a humidified atmosphere of 95 % air and 5 % CO2. The culture medium was changed once a week. On day 8, cytosine arabinoside (2 μM) was added for 60 h to prevent the proliferation of fibroblasts and microglial cells. Following 21 days in culture, more than 95 % of the cells stained positive for glial fibrillary acid protein (GFAP) by indirect immunofluorescence (ICN Biochemicals, Costa Mesa, CA, USA). Replating of the cells was achieved as follows: 18 to 21 day confluent astrocytes were exposed for 10 min to a serum-free culture medium containing 1 ml of 0.25 % trypsin and 1 ml of Versene solution (1/5000). Detached cells were collected with an Eppendorf cone, and the trypsin action was stopped by addition of 10 % fetal calf serum. After a 10 min centrifugation (120 g), the pellet was resupended in 1 ml of culture medium and cells were replated at low density (5 × 105 cells per slide; dimensions 45 mm × 70 mm). Replated astrocytes were stored in the incubator and used 2–4 days later.
Calcium imaging experiments
For fluo-3 experiments, astrocytes were loaded with 12 μM fluo-3 AM (Molecular Probes, Eugene, CA, USA) for 45 min at 37°C. Excitation was performed at 488 nm while emission was recorded at 526 nm. Since fluo-3 cannot provide direct information about steady-state [Ca2+]i levels and cannot produce ratio images, this probe was used only to determine the distribution of subpopulations of astrocytes responding to receptor agonists. Measurements of [Ca2+]i in cultured striatal astrocytes were achieved under dual emission microfluorimetry using the cell-permeant fluorescent calcium probe indo-1 AM (Sigma, St Louis, MO, USA) as previously described (Delumeau, Tence, Marin, Cordier, Glowinski & Prémont, 1992). Cells were loaded for 1 h in the presence of indo-1 AM (12 μM) in a cell incubator. Following loading, the glass slide was placed in a perfusion chamber and cells were excited using a 75 W xenon light, filtered at 340 nm with a 10 nm wide interference filter. Excitation and emission spectra were separated by a 380 nm dichroic long-pass filter and emission spectra were then divided into two halves by a dichroic long-pass filter mounted on an inverted microscope (Nikon, Tokyo, Japan). Two discriminant bands were selected from the two halves at 400–410 and 470–480 nm, and both fluorescent images were digitized (8 video frames per image). The camera dark noise was subtracted from the recorded crude image using an image processing system (Hamamatsu Ltd, Hamamatsu City, Japan).
[Ca2+]i was calculated from the fluorescence ratio (R) measured at 400–410 and 470–480 nm according to the equation described by Grynkiewicz, Poenie & Tsien (1985):
where the KD of indo-1 AM for ionized calcium is 250 nM, F480f is the fluorescence of free indo-1 AM, F480b is the fluorescence of the probe bound to calcium. Rmin and Rmax were determined in the presence of ionomycin (5 μM), with 2 mM EGTA or 1.1 mM CaCl2, respectively. Ratios were estimated at 1 or 3 s intervals in the cell bodies of individual astrocytes. All measurements were given as ratios which ranged from 0.01 to 1.50 corresponding to maximal estimated [Ca2+]i values up to 2000 nM.
After loading, the glass slide supporting the astrocytes was placed in a perfusing chamber in which the renewal rate of the solution was 6 ml min−1. In addition, the superfusion of astrocytes was performed using a multichannel cell perfusion device driven by a hydrolic micromanipulator, allowing a complete change of the medium in < 200 ms on a cellular field containing about 100 confluent astrocytes (Delumeau et al. 1992). Focal applications of receptor agonists were carried out by applying a pressure pulse (276 kPa, 20 ms) with a pneumatic Pico pump (PV800, World Precision Instruments, New Haven, CT, USA) connected to a patch-clamp pipette filled with the external solution containing the tested compound. These local applications for single cell stimulation since when gap junctions were inhibited these applications were followed by an increase in [Ca2+]i only in the targeted astrocyte and in very few of its neighbours (Giaume & McCarthy, 1996; Venance et al. 1997).
Immunocytochemistry
Cultured cells were fixed for 10 min with 2 % paraformaldehyde. Astrocytes were then permeabilized with Tween 20 (0.05 %, pH 7.5) and identified using a monoclonal mouse anti-GFAP antibody diluted to 1/200 which was revealed with a rhodamine-labelled goat anti-mouse IgG (1/400) secondary antibody (Jackson Immunresearch Laboratory, Baltimore, MD, USA).
Autoradiographic studies
Binding assays were performed on striatal astrocytes cultured for 3 weeks in 35 mm Petri dishes as described above. Intact cells were then incubated with: 3[125I] iodotyrosyl)-endothelin ([125I] Et, specific activity 2000 Ci mmol−1 used at a concentration of 16 pM, from Amersham, Bucks, UK) for endothelin B receptors, β-([125I] iodo-4-hydroxyphenyl)-ethyl-aminomethyl-tetralone ([125I] HEAT-2, specific activity 2200 Ci mmol−1 used at a concentration of 44 pM, from NEN Dupont de Nemours, Belgium) for α1-adrenergic receptors, and L-4,4′-[3H]-quinuclinyl benzylate ([3H] QNB, specific activity 45.4 Ci mmol−1 used at a concentration of 61 pM, from NEN Dupont de Nemours) for muscarinic receptors. Incubations were carried out in Krebs-phosphate buffer solution containing (mM): NaCl, 120; KCl, 4.8; CaCl2, 1.2; MgSO4, 1.2; NaH2PO4, 15.6; glucose, 33; supplemented with 0.2 % bovine serum albumin, 30 μg ml−1 bacitracin, 10 μg ml−1 leupeptin and 1.1 mM ascorbic acid. Incubation times were 45 min for [125I] Et1 and [3H] QNB, and 30 min for [125I] HEAT-2. Following five washes at 4°C and rapid drying, cells were fixed at room temperature (20-22°C) by exposure for 3 h to an atmosphere saturated with paraformaldehyde vapour and then covered with an Ilford K5 emulsion (Ilford, UK). Cells were exposed at room temperature for 10 or 30 days for the iodinated or tritiated ligands, respectively, and then developed with Kodak D19 developer (Kodak, New Haven, CT, USA). Labelled cells were then examined and photographed with a microscope under bright-field illumination (Olympus BH-2).
Solutions and chemicals
All experiments were performed at room temperature (20–22°C) in a standard solution containing (mM): NaCl, 145; KCl, 5.5; CaCl2, 1.1; MgCl2, 0.9; glucose, 20; Hepes, 20 (Calbiochem, San Diego, CA, USA), adjusted to pH 7.3. Drugs used were purchased from Sigma, except Et1 which was from Neosystem (Strasbourg, France).
Statistical analysis
Data were expressed as means ±s.e.m. and statistical significance was established by a one-way analysis of variance (ANOVA) followed by non-parametric tests.
RESULTS
Autoradiographic localization of binding sites for Et1, α1-adrenergic or muscarinic ligands in rat striatal astrocytes
Binding experiments were performed with [125I] Et, [125I] HEAT-2 and [3H] QNB which are radioactive ligands specific for endothelin B, α1-adrenergic and muscarinic receptors, respectively. Non-specific binding for these ligands was determined in the presence of 0.1 μM Et1, 1 μM prazosin or 0.1 mM atropine, respectively (Fig. 1, insets). As estimated following a 45 min incubation, specific bindings for [125I] Et, [125I] HEAT-2 and [3H] QNB were 96, 58 and 35 % of total binding, respectively.
Figure 1. Autoradiographic visualization of the distribution of specific binding sites for Et, muscarinic and α1-adrenergic receptor ligands in confluent cultures of rat striatal astrocytes.
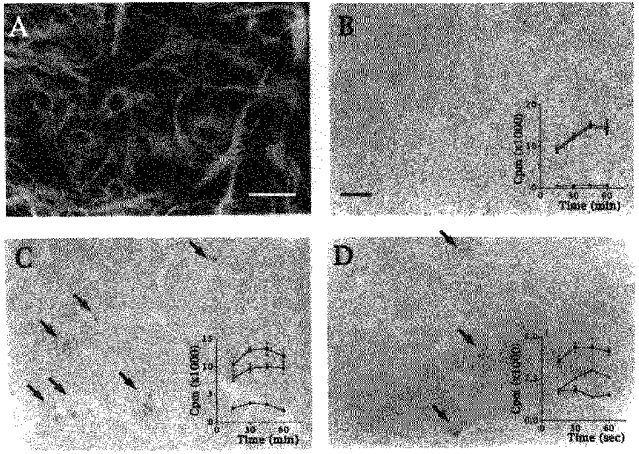
A, immunostaining with GFAP antibodies of 3-week-old astrocytes showing typical primary cultures used for autoradiographic binding assays. B-D, labelling of cultured astrocytes with [125I] Et (B), [3H] QNB (C) and [125I] HEAT-2 (D) indicating that binding sites for the endothelin receptor ligand are homogeneously distributed, while binding sites for muscarinic and α1-adrenergic receptor ligands are heterogeneously distributed in subsets of astrocytes and often observed in clusters (arrows). Insets, curves showing total (▪), non-specific (•) and specific (▵) binding of [125I] Et, [3H] QNB and [125I] HEAT-2 ligands which were used at concentrations of 1.6, 4.4 and 6.1 pM and either the presence or absence of Et1 (0.1 μM), atropine (0.1 mM) and prazosin (1 μM), respectively. Calibration bars indicate 100 μm in A and 120 μm in B (applies also to C and D).
[125I] Et binding sites were homogeneously distributed throughout the whole astrocytic population (Fig. 1B) while [3H] QNB (Fig. 1C) and [125I] HEAT-2 (Fig. 1D) binding sites were heterogeneously distributed and mainly detected in clusters (Fig. 1C and D, arrows).
Distribution and pattern of calcium responses induced by superfusion of Et1, methoxamine and carbachol in striatal astrocytes
Calcium imaging experiments were performed in confluent cultures of striatal astrocytes loaded with indo-1 AM. In resting cells, the fluorescence ratio of indo-1 emissions (F405/F480) was 0.01 ± 0.03 (n = 1154), which corresponded to a basal [Ca2+]i of 73 ± 13 nM.
Receptor stimulation in populations of confluent astrocytes was achieved with either 0.1 μM Et1, 0.1 mM methoxamine, an α1-adrenergic receptor agonist, or 1 mM carbachol, a mixed muscarinic and nicotinic receptor agonist which selectively stimulates muscarinic receptors in striatal astrocytes since these cells do not possess nicotinic receptors. These compounds were used at their maximal effective concentration for the stimulation of inositol phosphate formation and the associated increase in [Ca2+]i levels in cultured striatal astrocytes (Marin, Delumeau, Durieu-Trautmann, Le Nguyen, Prémont, Strosberg & Couraud, 1991; Delumeau et al. 1992). These receptor agonists were applied using a rapid superfusion system (see Methods) allowing the simultaneous pharmacological stimulation of more than 100 astrocytes. The analysis of calcium responses was carried out with the investigated microscopic field comprising about thirty to thirty-five cells. Thus, this experimental approach, referred as superfusion, allows for monitoring changes in [Ca2+]i and the behaviour of populations of cells which receive the agonist simultaneously. This protocol is distinct from the focal application of receptor agonists used in a previous study which resulted in the stimulation of single astrocytes (see Venance et al. 1997).
Superfusion with Et1 (0.1 μM) induced a rapid and large response in all superfused striatal astrocytes (Figs 2A and 5A). Short-term superfusions (5–20 s) typically evoked biphasic responses in all cells characterized by an initial spike followed by a sustained plateau which persisted even following the end of Et1 application. The mean amplitudes of the relative changes in fluorescence ratios (ΔF405/F480) were 0.68 ± 0.02 (n = 138) and 0.16 ± 0.01 (n = 138) for the peak and the plateau, respectively.
Figure 2. Pattern of calcium responses induced by Et1 and methoxamine in cultured striatal astrocytes.
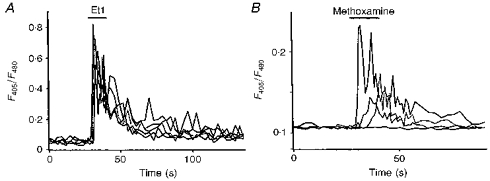
A, quantification of [Ca2+]i responses evoked by a brief application of 0.1 μM Et1 (horizontal bar) recorded in 5 astrocytes loaded with indo-1 AM which were selected from the same microscopic field. Note that all cells responded with similar kinetics and amplitudes. B, diversity of [Ca2+]i responses induced by a brief application of 0.1 mM methoxamine in 4 astrocytes from the same microscopic field. Note that the latency, rise time and amplitude of the responses are different for each responding cell.
Figure 5. Effect of αGA on calcium responses induced by Et1 and carbachol.
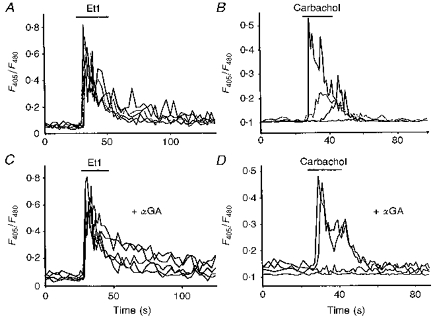
[Ca2+]i increases evoked by a short application (horizontal bar) of 0.1 μM Et1 (A) or 1 mM carbachol (B) recorded in astrocytes loaded with indo-1 AM. C and D, responses to the application of the same receptor agonists, respectively, recorded from astrocytes which have been previously treated for 5 min with 10 μM αGA. As shown in A, note that all cells responded with similar kinetics and amplitudes to application of Et1 (C), while delayed and slow increases were suppressed when carbachol-induced increases in [Ca2+]i were monitored in the presence of the uncoupler (D). In all cases, cells were recorded from the same microscopic field and originated from the same culture.
In contrast to Et1, calcium responses evoked by superfusion of either methoxamine (Fig. 2B) or carbachol (Fig. 5B) were heterogeneous. Firstly, a significant proportion of astrocytes did not respond to the α1-adrenergic (42 %, n = 364) and muscarinic (31 %, n = 743) receptor agonists. Secondly, with both receptor agonists, two types of [Ca2+]i responses were observed: (i) rapid responses with short latency and relatively high amplitude, although lower than those obtained with Et1 (relative change in fluorescence ratios: 0.28 ± 0.07 (n = 101) and 0.25 ± 0.04 (n = 353) for methoxamine and carbachol, respectively); (ii) delayed responses with slow rise time and low amplitude, (relative change in fluorescence ratios: 0.15 ± 0.03 (n = 105) and 0.13 ± 0.03 (n = 159) for methoxamine and carbachol, respectively). Whatever the type of response and the receptor agonist used, no plateau could be observed. The heterogeneity of calcium responses to methoxamine or carbachol superfusions was not related to a difference in the basal levels of [Ca2+]i. Indeed, the initial fluorescence ratios were identical not only in cells responding with either a short latency and high amplitude (0.09 ± 0.03, n = 455) or a long latency and low amplitude (0.09 ± 0.03, n = 262), but also in unresponsive cells (0.10 ± 0.03, n = 372) (these data were not significantly different: P > 0.5, statistical analysis conducted by one-way ANOVA followed by the Bonferroni test).
This functional astrocytic heterogeneity in response to methoxamine or carbachol was further demonstrated by examining the effects of two successive brief applications of each receptor agonist on the same subpopulation of confluent astrocytes. In these experiments, fluo-3 was preferred to indo-1 because this probe exhibits a higher increase in fluorescence emission intensity for low enhancement of [Ca2+]i. As illustrated in Fig. 3, only subpopulations of the superfused astrocytes responded to methoxamine (0.1 mM) during the first seconds (< 10 s) of the drug application (Fig. 3B). Following washing, [Ca2+]i returned to basal levels in all responding astrocytes (3 to 5 min) (not shown). Fourteen minutes later, the second superfusion with methoxamine induced calcium responses (Fig. 3C) which mainly occurred in the same subpopulations of cells (Fig. 3D). Similar observations were made in three other trials performed with either methoxamine or carbachol (1 mM). As mentioned above, most (> 95 %) of the cells present in the investigated microscopic field responded simultaneously to Et1 (0.1 μM, n = 4).
Figure 3. Cellular distribution of calcium responses induced by methoxamine superfusion of a population of confluent striatal astrocytes.
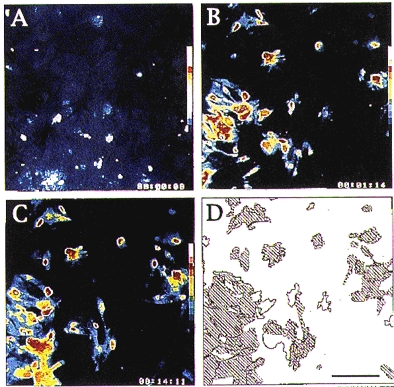
A-C, pseudocolour sequence of images showing the changes in [Ca2+]i evoked in the same field of astrocytes loaded with fluo-3 AM. A, basal [Ca2+]i monitored 1 min before the first application of 0.1 mM methoxamine, this image was taken as the background fluorescence of the investigated microscopic field and was subtracted from images obtained thereafter. B, increase in [Ca2+]i monitored 4 s after the beginning of the first brief (10 s) superfusion with 0.1 mM methoxamine. C, distribution of [Ca2+]i responses monitored 3 s after the beginning of the second methoxamine superfusion performed 13 min after the first (shown in B). D, drawing representing the areas in which changes in [Ca2+]i occurred in the course of the 2 successive methoxamine superfusions as shown in B and C. Shaded areas illustrate the localization of overlapping subsets of responding astrocytes (calibration bar, 200 μm).
Effects of gap junction blockers on Et1-induced calcium responses
Uncouplers of gap junctions were used to determine the role of gap junction permeability in the functional heterogeneity observed in response to a pharmacological stimulation of populations of confluent astrocytes. Long chain alcohols such as octanol (Giaume, Marin, Cordier, Glowinski & Prémont, 1991) or heptanol (Venance, Cordier, Monge, Zalc, Glowinski & Giaume, 1995a) and the volatile anaesthetic, halothane (Mantz, Cordier & Giaume, 1993), which inhibit astrocytic junctional permeability, were first selected for this purpose. Experiments were performed with Et1 (0.1 μM), since, as described above, the superfusion of this peptide increases [Ca2+]i in all astrocytes with a similar pattern of response. Halothane (2 mM), octanol (0.6 mM) and heptanol (1 mM) had no significant effect on basal [Ca2+]i levels (Fig. 4D) but these compounds drastically inhibited the calcium responses induced by the peptide. The extent of inhibition was 44, 63 and 63 % for halothane (n = 99), octanol (n = 52) and heptanol (n = 36), respectively (Fig. 4C and D). Therefore, probably due to interferences with some of the initiating events (receptor itself, phospholipase C (PLC), inositol 1,4,5-trisphosphate (IP3)-sensitive intracellular calcium stores, protein kinase C (PKC) and/or basal cytosolic calcium concentration) of the calcium signalling process (Jensen, Lindroth, Sjölander & Eintrei, 1994; Franks & Lieb, 1994; Deutsch, Williams & Yule, 1995), these uncoupling agents could not be used in further experiments.
Figure 4. Comparative effects of 18-α-glycyrrhetinic acid (αGA) and other commonly used uncoupling agents on basal [Ca2+]i and on the amplitude of calcium responses induced by Et1.
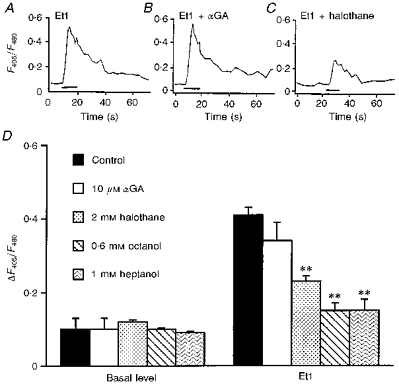
A-C, typical calcium responses induced by 0.1 μM Et1 in control conditions (A) and in the presence of either 10 μM αGA (B) or 2 mM halothane (C). All traces were averaged from 7 individual recordings performed in the same microscopic field of astrocytes loaded with indo-1 AM. D, histogram illustrating the basal [Ca2+]i levels (left-hand side) and Et1-evoked increases in [Ca2+]i (right-hand side). αGA had no effect on the Et1-evoked increase in [Ca2+]i whereas in the presence of 2 mM halothane, 0.6 mM octanol or 1 mM heptanol the Et1-evoked increases in [Ca2+]i were drastically reduced. In contrast, none of these uncouplers have any significant effect on the basal [Ca2+]i. Data are averaged from 36–279 cells. Statistical analysis was conducted by one-way ANOVA, followed by post hoc Dunnett's multiple comparison test. Significance was established at P < 0.01 (**), all other data were not significantly different (P > 0.5). In D the vertical scale refers to relative changes in the fluorescence ratios (ΔF405/F480) of indo-1 emissions compared with the basal level.
Additional experiments were thus performed with 18-α-glycyrrhetinic acid (αGA) (Davidson, Baumgarten & Harley, 1986) since, as previously observed, when used at micromolar concentration, this aglycone derivative of glycyrrhizic acid induces a reversible inhibition of dye diffusion through gap junctions in C6 glioma cells (Goldberg, Bechberger & Naus, 1995) and cultured astrocytes (Venance, Piomelli, Glowinski & Giaume, 1995b; Tabernero, Giaume & Medina, 1996). Treatment of striatal astrocytes with αGA (10 μM) modified neither the pattern (Fig. 4B) nor the mean amplitude (Fig. 4D) of the Et1 calcium responses.
More precise quantification of the Et1-induced response was then achieved by measuring the proportion of responsive cells and the latency, rise time and amplitude of the increases in [Ca2+]i levels in either the absence or presence of αGA (10 μM). Confirming results described above, in control conditions Et1-evoked calcium responses were detected in most recorded astrocytes (93 %, n = 83). This proportion of responsive cells was not significantly changed in the presence of αGA (90 %, n = 69) (Fig. 5A and C). As illustrated in Fig. 6A and D, these responses were characterized by their homogeneity since similar latencies and rise times were measured in both the absence (1.4 ± 0.2 s (n = 81) and 2.1 ± 0.3 s (n = 71), respectively) or presence (2.1 ± 0.3 s (n = 81) and 2.6 ± 0.2 s (n = 71), respectively) of αGA. Nevertheless, in agreement with previous observations (Venance et al. 1997), variations in the amplitude of the Et1-induced [Ca2+]i responses were observed from one cell to another in control conditions. These fluctuations in amplitude were also monitored when gap junction permeability was inhibited by αGA.
Figure 6. Quantitative analysis of calcium responses to Et1, carbachol and methoxamine recorded from communicating and non-communicating confluent striatal astrocytes.
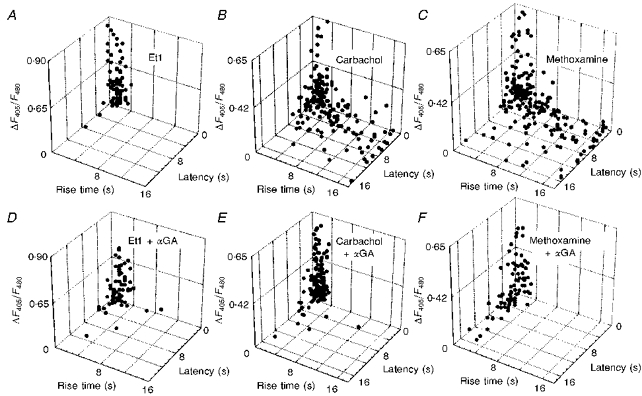
Receptor agonist-induced calcium responses were plotted using 3 parameters: amplitude, latency and rise time of the increases in [Ca2+]i. These responses were recorded from astrocytes perfused with 0.1 μM Et1 (A and D), 1 mM carbachol (B and E) and 0.1 mM methoxamine (C and F), in either the absence (A-C) or presence (D-F) of 10 μM αGA. The vertical scale refers to relative changes in the fluorescence ratios (ΔF405/F480) of indo-1 emissions compared with the basal level.
Blockade of gap junctions selectively abolishes the delayed and slow rising calcium responses evoked by methoxamine and carbachol
As already indicated and contrasting with the Et1 calcium responses, in control conditions, the methoxamine and carbachol calcium responses were characterized by their great heterogeneity (Fig. 6B and C, respectively). This did not occur any more in the presence of αGA since all responses were similar, exhibiting a short latency and a rapid onset (Fig. 6E and F). However, in the absence of αGA, as observed with Et1, a great variability occurred in the amplitude of the methoxamine- and carbachol-evoked responses (Fig. 6B and C, respectively). This observation, which is in agreement with previous reports (see Finkbeiner, 1993), was also made in the presence of αGA (Fig. 6E and F).
The quantitative analysis of [Ca2+]i changes generated by either carbachol or methoxamine indicated that a subclass of responses was selectively suppressed in the presence of the uncoupler. Indeed, in control conditions, only 70 % (n = 364) and 60 % (n = 743) of the investigated astrocytes responded to the superfusion of these two receptor agonists, respectively. These percentages were reduced to 41 % (n = 222) and 38 % (n = 469) respectively, when gap junction channels were blocked.
Analysis of the patterns of the α1-adrenergic and muscarinic responses revealed that only those which had a typical shape of a full calcium response evoked by a submaximal concentration of each receptor agonist were not affected by αGA. According to these observations, methoxamine and carbachol primarily induced fast kinetic calcium responses which were defined as ‘primary’ responses. Frequency histogram analysis indicated that these responses were identical to those observed with Et1 in both the absence and presence of αGA. These primary responses were characterized by a mean latency and rise time of less than 2 s and 3 s, respectively. The rise times of calcium responses evoked by superfusion of carbachol or methoxamine, and the latency of carbachol-induced calcium increases were found to be extremely different (P < 0.0001, statistical analysis conducted with Student's unpaired t test) when recordings were compared in the absence (rise time: 3.86 ± 0.22 ms (n = 270) and 6.79 ± 0.36 ms (n = 230) for carbachol and methoxamine, respectively; latency: 2.92 ± 0.19 ms (n = 274) for carbachol) and in the presence of αGA (rise time; 2.37 ± 0.11 ms (n = 131) and 2.84 ± 0.26 ms (n = 83) for carbachol and methoxamine, respectively; latency: 2.01 ± 0.12 ms (n = 132) for carbachol). In contrast, the difference in latency of calcium responses evoked by superfusion of methoxamine was found to be not significant (P > 0.05) when compared in the absence (4.86 ± 0.24 ms, n = 230) and in the presence of αGA (5.00 ± 0.38 ms, n = 82). This observation must be considered in light of a previous study showing that the latency between application of an α1-adrenergic agonist and the cell response depends on the density of α1-adrenergic receptors in cultured astrocytes (Shao et al. 1994). Accordingly, in the present study primary responses induced by methoxamine could be delayed due to the fact that some cells express less α1-adrenergic receptors. However, the number of receptors in these cells was sufficiently high to generate a full calcium response which was not suppressed by αGA.
As gap junctions are permeable to small molecules, the delayed and slow responses induced by methoxamine and carbachol were considered to result from the intercellular diffusion of calcium signalling molecules, probably IP3 and/or Ca2+ (Saez, Connor, Spray & Bennett, 1989). In agreement with this interpretation, this category of responses was always recorded from astrocytes directly in contact with cells from which ‘primary’ responses were monitored. These delayed and slow [Ca2+]i increases were defined as ‘secondary’ responses, since they were unlikely to be mediated by the stimulation of membrane receptors as indicated by their suppression by αGA.
Classification of responses as ‘primary’ and ‘secondary’ was carried out from a total of 1751 cells recorded in twenty-eight independent experiments. As illustrated in Fig. 7, in control conditions, cells characterized by a secondary response induced by either methoxamine or carbachol represented 24 ± 4 % (n = 128) and 29 ± 12 % (n = 157), respectively, of the total proportion of recorded astrocytes. As already indicated, these secondary responses were selectively eliminated by αGA, while the number of non-responsive cells was increased by an equivalent amount.
Figure 7. Selective suppression of 1 class of methoxamine- and carbachol-evoked calcium responses following inhibition of gap junction permeability.
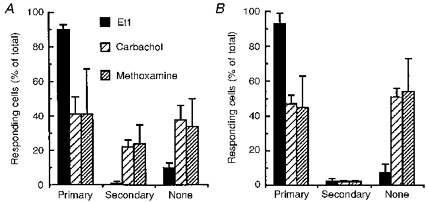
Calcium responses to the perfusion of the 3 receptor agonists were monitored in astrocytes loaded with indo-1 AM. When no significant change in [Ca2+]i (relative change in fluorescence ratio < 0.05) was recorded, astrocytes were considered as non-responding cells (None). Calcium responses were separated into 2 groups depending on the kinetics of the change in [Ca2+]i: ‘primary’ responses were defined by a latency shorter than 2 s and a rise time faster than 3 s while these values were higher in secondary responses. This classification was performed in either the absence (A) or presence (B) of 10 μM αGA. The analysis of these 2 types of response indicated that the inhibition of gap junctional communication results in the selective disappearance of ‘secondary’ responses which were only monitored following applications of carbachol and methoxamine. Histograms were established from a total number of 910 and 763 measurements in A and B, respectively.
The participation of gap junction permeability in the secondary responses was further confirmed by performing focal applications of receptor agonists and by counting the number of responding cells exhibiting an increase in [Ca2+]i for each trial. Indeed, the pattern and amplitude of the increase in [Ca2+]i evoked in target cells by the focal application of either the α1-adrenergic or the muscarinic receptor agonists (Venance et al. 1997) were similar to those of primary response shown in Figs 2B and 5D. Moreover, with these receptor agonists, the percentage of responding cells following these focal applications were identical to those found in superfusion experiment performed in the presence of αGA (Table 1). As also shown in this table, single cell pharmacological stimulations indicated that almost all astrocytes responded to Et1 while only subpopulations of astrocytes responded to methoxamine or carbachol. Interestingly, as shown with methoxamine, the percentage of responding cells was in the same range as that observed in confluent cells treated with αGA when the superfusion was performed on striatal astrocytes replated at a low density, i.e. without physical contact (Table 1).
Table 1.
Comparison of the percentage of responding (increase in [Ca2+]i) confluent or dissociated astrocytes stimulated by superfusion or focal application of receptor agonists
| Cells responding to receptor agonist | |||||
|---|---|---|---|---|---|
| Method of application | Cells tested | Treatment | 1 mM carbachol (%) | 0.1 mM methoxamine (%) | 0.1 μM Et1 (%) |
| Superfusion | Confluent | Control | 60 (743) | 70 (364) | 93 (83) |
| Confluent | 10 μM αGA | 38 (469) | 41 (222) | 90 (69) | |
| Dissociated | Control | n.d. | 32 (41) | n.d. | |
| Focal | Confluent | Control | 43 (42) | 51 (35) | 98 (11) |
Numbers in parentheses indicate the number of cells which have been investigated in each condition. n.d., not determined
DISCUSSION
We have recently reported that the ability of a receptor agonist to induce intercellular calcium waves in cultured astrocytes depends on its potential to stimulate PLC and to evoke the formation of inositol phosphate derivatives (Venance et al. 1997). Indeed, when applied focally on a single astrocyte, the two potent activators of PLC, Et1 and glutamate, were shown to trigger propagating calcium waves through the astrocytic network. In contrast, α1-adrenergic and muscarinic receptor agonists, which weakly activate PLC, did not induce propagating ICS but only calcium increases in few surrounding astrocytes. Although it is well documented that the inhibition of junctional permeability blocks the propagation of calcium waves (Finkbeiner, 1992; Enkvist & McCarthy, 1992; Venance et al. 1995b), the regulation of gap junctional communication is probably not limited to their role in controlling such propagating calcium waves. In fact, gap junctions might also participate in intercellular exchange of calcium signalling molecules which do not lead to a long-range propagating process. In the present study, this property was investigated mainly on populations of confluent astrocytes by superfusion rather that by focal application of receptor agonists. This difference in experimental protocol permits us to follow calcium changes in populations of confluent astrocytes that are simultaneously exposed to the receptor agonist, whereas the focal applications used previously (Venance et al. 1997) resulted in the pharmacological stimulation of single cells.
According to combined binding and autoradiographic studies, [125I] Et binding sites were shown to be distributed homogeneously in all cultured astrocytes from the rat striatum. This observation agrees with results obtained by other authors who reported that endothelin receptors are expressed in most astrocytes from explant cultures of the rat cerebellum, brainstem and spinal cord (see Hösli & Hösli, 1993). In addition, our data indicate that most striatal astrocytes possess functional endothelin receptors since simultaneous and identical calcium responses were observed in more than 90 % of the astrocytes when these cells were exposed to Et1. In contrast to the homogenous distribution of the endothelin receptors, individual differences in the expression of several neurotransmitter receptors may be seen among astrocytes originating from the same brain region (Shao & McCarthy, 1993). Indeed, subsets of cortical astrocytes were shown to be sensitive to several pharmacological agents, including α1-adrenergic and muscarinic receptor agonists (McCarthy & Slam, 1991). Our study indicates that such heterogeneity exists in striatal astrocytes. For example, binding sites for [125I] HEAT-2 and [3H] QNB, which are selective ligands for α1-adrenergic and muscarinic receptors, respectively, were found to be distributed heterogeneouly and often present in clusters. In addition, calcium responses were observed only in subpopulations of cells when groups of striatal astrocytes were briefly superfused with a maximal effective concentration of either methoxamine or carbachol. Therefore, our results confirm that cultured astrocytes from a given brain structure may differ in their phenotype. This heterogeneity explains why striatal astrocytes can respond in different ways, both qualitatively and quantitatively, when they are simultaneously exposed to various receptor agonists.
In agreement with results of binding studies, the high synchronicity and similarity of the calcium increases induced by Et1 indicated that responses were evoked simultaneously from all astrocytes. In contrast, changes in [Ca2+]i induced by either methoxamine or carbachol varied not only in their distribution but also in their shape. Although differences always occurred in the maximal amplitude of these responses (see Finkbeiner, 1993), two main types of calcium responses could be distinguished in responsive cells when groups of confluent astrocytes were exposed briefly to either methoxamine or carbachol. In half of the cells, the increase in [Ca2+]i appeared following a short delay and developed rapidly (primary responses) while in others, the responses were characterized by their long latency, slow rise time and small amplitude (secondary responses). A higher density of α1-adrenergic or muscarinic receptors in cells exhibiting ‘primary’ responses compared with those exhibiting secondary responses could explain this difference. In support of the existence of distinctive phenotypes between these two types of responsive cells is the finding that delayed and slow calcium responses were suppressed selectively in the presence of the uncoupling agent αGA. It appears that cells exhibiting primary responses could correspond to those in which clusters of binding sites were observed on autoradiograms. The suppression by αGA in the cells demonstrating secondary responses strongly suggests that signalling calcium molecules (IP3 and/or Ca2+) that originate from cells exhibiting primary responses may diffuse to adjacent cells through open gap junction channels. In support of this statement, it was found that the respective proportion of responding cells were similar whether the α1-adrenergic or the muscarinic receptor agonists were superfused over a subpopulation of confluent αGA-treated astrocytes or focally ejected from a pipette on single cells. This functional heterogeneity observed under αGA is supported by the observation indicating that the proportion of responding cells is similar when methoxamine superfusions are performed on astrocytes cultured at low density and do not communicate through gap junctions (Table 1).
In conclusion, when receptors coupled to PLC are distributed heterogeneously in a confluent population of astrocytes, the gap junctional communication between these cells, which bear a high density of neurotransmitter receptors, and other cells, having only few (or lacking) receptors, might lead the latter cells to respond to neuronal signalling molecules. Thus permeable gap junctions may partially attenuate the pharmacological heterogeneity existing among astrocytes. This property can be unmasked by the inhibition of gap junction permeability. As indicated, several neurotransmitters may regulate the permeability of gap junctions (see Giaume & McCarthy, 1996), so depending on which neurons are active, the astrocytic network may change over time in size and topological organization. Consequently, astrocyte sensitivity to a given neurotransmitter or neuromodulator will depend not only on the density of its membrane receptors but also on the state of their gap junction permeability at the time of stimulation.
In future studies, it will be important to determine whether the ‘pharmacological’ heterogeneity demonstrated in striatal cultured astrocytes exists in vivo. Already there is some evidence indicating that this could be the case for the α1-adrenergic receptors. Binding experiments performed with [125I] HEAT on freshly isolated mature astrocytes have indicated that only a subpopulation of cells from the rat brainstem possesses α1-adrenergic receptors (Shao & Sutin, 1992). Moreover, a great disparity in the proportion of calcium responses to an α1-adrenergic receptor agonist was observed in hippocampal astrocytes when these responses were monitored in either brain slices or acutely isolated cells (Duffy & MacVicar, 1995). Indeed, 85–90 % of astrocytes in brain slices, which exhibited a high level of dye-coupling (Konietzo & Müller, 1994) responded to phenylephrine while this was only the case for 5 % of the dissociated cells. It cannot be excluded that the isolation procedure is partly responsible for this observation. Nevertheless, this important difference could indicate that the α1-adrenergic receptors may be present only on a small subset of astrocytes in intact slices, and that calcium responses are transmitted through gap junctions to coupled neighbouring cells.
Acknowledgments
The authors wish to thank Dr Brigitte Lessaffre who participated in some of the preliminary experiments, and Professor Kenna Peusner and Christian Naus who made helpful comments on the manuscript.
References
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Lodi Rizzini B, Pozzan T, Voltera A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Charles AC. Glial-neuron intercellular signalling. Developmental Neuroscience. 1994;16:196–206. doi: 10.1159/000112107. [DOI] [PubMed] [Google Scholar]
- Charles AC, Merrill JE, Dirksen ER, Sanderson MJ. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991;6:983–992. doi: 10.1016/0896-6273(91)90238-u. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocytes networks. Neuron. 1992;8:429–440. doi: 10.1016/0896-6273(92)90271-e. 10.1016/0896-6273(92)90271-E. [DOI] [PubMed] [Google Scholar]
- Davidson JS, Baumgarten I, Harley EH. Reversible inhibition of intercellular junctional communication by glycyrrhetinic acid. Biochemical and Biophysical Research Communications. 1986;134:29–36. doi: 10.1016/0006-291x(86)90522-x. [DOI] [PubMed] [Google Scholar]
- Delumeau JC, Tence M, Marin P, Cordier J, Glowinski J, Prémont J. Synergistic regulation of cytosolic Ca2+ concentration by adenosine and α1-adrenergic agonists in mouse striatal astrocytes. European Journal of Neuroscience. 1992;3:539–550. doi: 10.1111/j.1460-9568.1991.tb00841.x. [DOI] [PubMed] [Google Scholar]
- Denis-Donini S, Glowinski J, Prochiantz A. Glial heterogeneity may define the three-dimensional shape of mouse mesencephalic neurons. Nature. 1984;307:641–643. doi: 10.1038/307641a0. [DOI] [PubMed] [Google Scholar]
- Deutsch DE, Williams JA, Yule DI. Halothane and octanol block Ca2+ oscillations in pancreatic acini by multiple mechanisms. American Journal of Physiology. 1995;32:G779–788. doi: 10.1152/ajpgi.1995.269.5.G779. [DOI] [PubMed] [Google Scholar]
- Duffy S, MacVicar BR. Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. Journal of Neuroscience. 1995;15:5535–5550. doi: 10.1523/JNEUROSCI.15-08-05535.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkvist MOK, McCarthy KD. Activation of protein kinase C blocks astroglial gap junction communication and inhibit the spread of calcium waves. Journal of Neurochemistry. 1992;59:519–526. doi: 10.1111/j.1471-4159.1992.tb09401.x. [DOI] [PubMed] [Google Scholar]
- Finkbeiner SM. Calcium waves in astrocytes - filling the gap. Neuron. 1992;8:1101–1108. doi: 10.1016/0896-6273(92)90131-v. 10.1016/0896-6273(92)90131-V. [DOI] [PubMed] [Google Scholar]
- Finkbeiner SM. Glial calcium. Glia. 1993;9:83–104. doi: 10.1002/glia.440090202. [DOI] [PubMed] [Google Scholar]
- Franks NP, Lieb WR. Molecular and cellular mechanism of general anesthetics. Nature. 1994;367:607–614. doi: 10.1038/367607a0. 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- Giaume C, McCarthy KD. Control of gap-junctional communication in astrocytic networks. Trends in Neurosciences. 1996;19:319–325. doi: 10.1016/0166-2236(96)10046-1. 10.1016/0166-2236(96)10046-1. [DOI] [PubMed] [Google Scholar]
- Giaume C, Marin P, Cordier J, Glowinski J, Prémont J. Adrenergic regulation of intercellular communications between cultured striatal astrocytes from the mouse. Proceedings of the National Academy of Sciences of the USA. 1991;88:5577–5581. doi: 10.1073/pnas.88.13.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowinski J, Marin P, Tence M, Stella N, Giaume C, Prémont J. Glial receptors and their intervention in astrocyto-astrocytic and astrocyto-neuronal interactions. Glia. 1994;11:201–208. doi: 10.1002/glia.440110214. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Bechberger JF, Naus CCG. A pre-loading method for evaluating gap junctional communication by fluorescent dye transfer. Biotechniques. 1995;18:492–498. [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescent properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hösli E, Hösli L. Receptors for neurotransmitters on astrocytes in the mammalian central nervous system. Progress in Neurobiology. 1993;40:477–506. doi: 10.1016/0301-0082(93)90019-o. 10.1016/0301-0082(93)90019-O. [DOI] [PubMed] [Google Scholar]
- Jensen G, Lindroth M, Sjlander A, Eintrei C. Propofol induces changes in the cytosolic free calcium concentration and the cytoskeletal organization of cultured human glial cells and primary embryonic rat brain cells. Anesthesiology. 1994;81:1200–1229. doi: 10.1097/00000542-199411000-00016. [DOI] [PubMed] [Google Scholar]
- Konietzo U, Mflller C. Astrocytic dye coupling in rat hippocampus: topography, developmental onset, and modulation by protein kinase C. Hippocampus. 1994;3:506–516. doi: 10.1002/hipo.450040313. [DOI] [PubMed] [Google Scholar]
- Lerea LS, McCarthy KD. Astroglial cells in vitro are heterogeneous with respect to expression of the alpha1-adrenergic receptors. Glia. 1989;2:135–147. doi: 10.1002/glia.440020302. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, Slam AK. Pharmacologically-distinct subsets of astroglia can be identified by their calcium responses to neuroligands. Neuroscience. 1991;2/3:325–333. doi: 10.1016/0306-4522(91)90330-q. [DOI] [PubMed] [Google Scholar]
- Mantz J, Cordier J, Giaume C. Effects of general anesthetics on intercellular communications mediated by gap junctions between astrocytes in primary culture. Anesthesiology. 1993;78:892–901. doi: 10.1097/00000542-199305000-00014. [DOI] [PubMed] [Google Scholar]
- Marin P, Delumeau JC, Durieu-Trautmann O, Le Nguyen D, Prémont J, Strosberg AD, Couraud PO. Are several G proteins involved in the different effect of endothelin-1 in mouse striatal astrocytes? Journal of Neurochemistry. 1991;56:1270–1275. doi: 10.1111/j.1471-4159.1991.tb11421.x. [DOI] [PubMed] [Google Scholar]
- Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- Newman EA, Zahs KR. Calcium waves in retinal glial cells. Science. 1997;275:844–847. doi: 10.1126/science.275.5301.844. 10.1126/science.275.5301.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Saez JC, Connor JA, Spray DC, Bennett MVL. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate and to calcium ions. Proceedings of the National Academy of Sciences of the USA. 1989;86:2708–2712. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, McCarthy KD. Regulation of astroglial responsiveness to neuroligands in primary culture. Neuroscience. 1993;55:991–1001. doi: 10.1016/0306-4522(93)90313-5. 10.1016/0306-4522(93)90313-5. [DOI] [PubMed] [Google Scholar]
- Shao Y, Porter JT, McCarthy KD. Neuroligand receptor heterogeneity among astroglia. Perspectives on Developmental Neurobiology. 1994;2:205–215. [PubMed] [Google Scholar]
- Shao Y, Sutin J. Expression of adrenergic receptors in individual astrocytes and motor neurons isolated from the adult rat brain. Glia. 1992;6:108–117. doi: 10.1002/glia.440060205. [DOI] [PubMed] [Google Scholar]
- Smith SJ. Neuromodulatory astrocytes. Current Biology. 1994;4:807–810. doi: 10.1016/s0960-9822(00)00178-0. [DOI] [PubMed] [Google Scholar]
- Sontheimer H, Ritchie M. Voltage-gated sodium and calcium channels. In: Kettenmann H, Ransom BR, editors. Neuroglia. Oxford: Oxford University Press; 1995. pp. 202–220. [Google Scholar]
- Tabernero A, Giaume C, Medina J-M. Endothelin-1 regulates glucose utilization in cultured astrocytes by controlling intercellular communication through gap junctions. Glia. 1996;16:187–195. doi: 10.1002/(SICI)1098-1136(199603)16:3<187::AID-GLIA1>3.0.CO;2-#. 10.1002/(SICI)1098-1136(199603)16:3<187::AID-GLIA1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Travis J. Glia: the brain's other cells. Science. 1994;266:970–972. doi: 10.1126/science.7973679. [DOI] [PubMed] [Google Scholar]
- Venance L, Cordier J, Monge M, Zalc B, Glowinski J, Giaume C. Homotypic and heterotypic coupling mediated by gap junctions during glial cell differentiation. European Journal of Neuroscience. 1995a;7:451–461. doi: 10.1111/j.1460-9568.1995.tb00341.x. [DOI] [PubMed] [Google Scholar]
- Venance L, Piomelli D, Glowinski J, Giaume C. Inhibition by anandamide of gap junctions and intercellular calcium signalling in striatal astrocytes. Nature. 1995b;376:590–594. doi: 10.1038/376590a0. 10.1038/376590a0. [DOI] [PubMed] [Google Scholar]
- Venance L, Stella N, Glowinski J, Giaume C. Mechanism involved in initiation and propagation of receptor-induced intercellular calcium signaling in cultured rat astrocytes. Journal of Neuroscience. 1997;17:1981–1992. doi: 10.1523/JNEUROSCI.17-06-01981.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Kettenmann H. Calcium signalling in glial cells. Trends in Neurosciences. 1996;19:346–352. doi: 10.1016/0166-2236(96)10048-5. 10.1016/0166-2236(96)10048-5. [DOI] [PubMed] [Google Scholar]


