Abstract
Whole-cell patch-clamp recordings were made from rat striatal cholinergic interneurones in slices of brain tissue in vitro. In the absence of ATP in the electrode solution, these neurones were found to gradually hyperpolarize through the induction of an outward current at −60 mV. This outward current and the resultant hyperpolarization were blocked by the sulphonylureas tolbutamide and glibenclamide and by the photorelease of caged ATP within neurones.
This ATP-sensitive outward current was not observed when 2 mM ATP was present in the electrode solution. Under these conditions, 500 μM diazoxide was found to induce an outward current that was blocked by tolbutamide.
Using permeabilized patch recordings, neurones were shown to hyperpolarize in response to glucose deprivation or metabolic poisoning with sodium azide (NaN3). The resultant hyperpolarization was blocked by tolbutamide.
In cell-attached recordings, metabolic inhibition with 1 mM NaN3 revealed the presence of a tolbutamide-sensitive channel exhibiting a unitary conductance of 44.1 pS.
Reverse transcription followed by the polymerase chain reaction using cytoplasm from single cholinergic interneurones demonstrated the expression of the ATP-sensitive potassium (KATP) channel subunits Kir6.1 and SUR1 but not Kir6.2 or SUR2.
It is concluded that cholinergic interneurones within the rat striatum exhibit a KATP channel current and that this channel is formed from Kir6.1 and SUR1 subunits.
ATP-sensitive potassium (KATP) channels are present in many types of excitable cell and are believed to provide a link between excitability and metabolic status (Ashcroft & Ashcroft, 1990). In addition to their regulation by intracellular ATP, various pharmacological agents can also modulate KATP channel activity. For example, potassium channel openers such as diazoxide can potentiate channel activity (Kozlowski, Hales & Ashford, 1989) whilst numerous agents can reduce channel activity. These agents include the antidiabetic sulphonylureas (Sturgess, Cook, Hales & Ashford, 1985), imidazoline compounds (Lee, Ozanne, Rowe, Hales & Ashford, 1994b) and the anorectic agent ciclazindol (Lee et al. 1996).
Recent studies have shown that these channels are formed by the molecular interaction between an inwardly rectifying K+ channel subunit (Kir 6.1 or Kir 6.2; Inagaki et al. 1995b; Sakura, Ämmälä, Smith, Gribble & Ashcroft, 1995) and a high affinity receptor for the sulphonylureas (SUR1 or SUR2; Inagaki et al. 1995a). When expressed in isolation, these proteins are unable to form molecules which recapitulate the properties of native KATP channels. However, either channel subunit may couple with each sulphonylurea receptor to produce functional KATP channels.
Within the central nervous system (CNS) these channels have been identified in several types of neurone where they may provide a protective role in anoxia and/or hypoglycaemia since their activation is believed to cause membrane hyperpolarization with consequent reduction in energy demand (Ashcroft & Ashcroft, 1990). With regard to this, we have recently described the presence of a sulphonylurea-sensitive current in large striatal cholinergic interneurones (Lee, Brownhill & Richardson, 1997). Within the striatum, these neurones are known to be relatively resistant to hypoxia and hypoglycaemia whilst striatal medium spiny projection neurones are particularly vulnerable (Francis & Pulsinelli, 1982). Consequently, in the present study, we have sought to address the molecular and pharmacological properties of this current and to determine whether this current may perform a protective role under aglycaemic conditions. Some of these findings have been communicated to the British Pharmacological Society (Lee, Brownhill, Dixon, Freeman & Richardson, 1997).
METHODS
Preparation
For the purposes of this study 14- to 28-day-old male Sprague-Dawley rats were mainly used although 7- to 8-week-old animals were also employed to confirm results when necessary. Animals, all weighing less than 500 g, were killed by cervical dislocation, brains removed and 300 μm coronal slices containing the striatum were prepared in ice-cold physiological saline using a vibratome. A Zeiss Axioskop microscope (Carl Zeiss Ltd, Welwyn Garden City, UK) fitted with a × 64 water-immersion objective lens together with gradient contrast optics (Luigs & Neumann, Ratingen, Germany) was used to view slices. Light in the infrared range (> 740 nm) was used in conjunction with a contrast-enhancing Newvicon camera (Hamamatsu, Hamamatsu City, Japan) to resolve individual neurones better within slices (see Stuart, Dodt & Sakmann, 1993).
Recording and analysis
Recording electrodes were pulled from borosilicate glass capillaries and when filled with electrolyte had resistances of 8–12 MΩ for isolated patch experiments, and 2–5 MΩ for whole-cell recording. Electrophysiological signals were detected using an Axopatch-1D patch-clamp amplifier and were recorded onto digital audio tape for later figure production. Following formation of the whole-cell configuration, series resistance was partially compensated using the amplifier, and cellular conductance was continually monitored via the injection of hyperpolarizing current (current-clamp mode; −100 pA in amplitude, 300 ms in duration at 0.1 Hz) or voltage (voltage-clamp mode; −10 mV in amplitude, 300 ms duration at 0.1 Hz). Membrane signals were filtered at 1 kHz for whole-cell experiments and at 500 Hz for single channel recordings. These data were digitized at 5 kHz through a Digidata 1200B A/D converter using pCLAMP 6.0 software (Axon instruments Inc., Foster City, CA, USA). In voltage-clamp recordings, neurones were clamped at −60 mV and current-voltage relationships were examined using a voltage ramp protocol between −140 and −40 mV (20 mV s−1).
Concentration-response data for tolbutamide were fitted according to the relationship:
| (1) |
where I is the amplitude of the current in the presence of the test concentration of tolbutamide, IC is the control-current amplitude measured as the mean of pre- and post-drug current amplitude, [tolbutamide] is the test concentration of tolbutamide, IC50 is the half-maximal effective inhibitory concentration of tolbutamide and nH is the Hill coefficient. To assess whether a particular procedure led to a significant change in the magnitude of the current under study, data were subjected to a Student's t test. Electrophysiological records used for illustrative purposes were replayed into a chart recorder (Gould 2400S) which had a nominal frequency response of 140 Hz. All data in the text and figures are presented as mean values ±s.e.m. unless stated otherwise.
Single cell RT-PCR (reverse transcriptase-polymerase chain reaction)
The cytoplasm from five large cells was gently aspirated under visual control into the recording electrode until at least 40%of the somatic cytoplasm had been collected. The electrode was then withdrawn from the cell to form an outside-out patch which prevented contamination when subsequently withdrawn from the slice. The contents of the electrode were forced into a microtube and the RNA reverse transcribed using an anchored oligo dT primer and 200 Units of MMLV reverse transcriptase (BRL) according to the manufacturer's recommendations. After 60 min at 37°C the cDNA was stored frozen at −20°C prior to processing. After amplification of the cDNA using Taq polymerase (A. K. Dixon, P. J. Richardson, K. Lee, N. Carter, D. R. Bentley & T. C. Freeman, unpublished results), the expression of specific genes was measured using primers designed to amplify products of between 150 and 250 base pairs in length, close to the 3′ ends of the mRNA transcripts.
The primers used were: for Kir6.1 (accession number D42145), forward primer (bases 1292–1311): GAGTGAACTGTCGCACCAGA; reverse primer (bases 1539–1520): CGATCACCAGAACTCAGCAA; Kir 6.2 (accession number X97041), forward primer (bases 787–804): TCCAACAGCCCGCTCTAC; reverse primer (bases 954–937): GATGGGGACAAAACGCTG; sulphonylurea receptor 1 (accession number L40624), forward primer (bases 4824–4842): TGAAGCAACTGCCTCCATC; reverse primer (bases 5005–4987): GAAGCTTTTCCGGCTTGTC; sulphonylurea receptor 2 (accession number D83598), forward primer (bases 4853–4872): ACCTGCTCCAGCACAAGAAT: reverse primer (bases 4997–4976): TCTCTTCATCACAATGACCAGG; choline acetyltransferase: forward primer (bases 2134–2155): TACTAAGCTCTGTTCCCATCCC; reverse primer (bases 2303–2285): ACCCAGGTTGCTTCCAAAC; glutamate decarboxylase (GAD 67, accession number X57573): forward primer (bases 2912–2933): ATCTTGCTTCAGTAGCCTTTGC; reverse primer (bases 3131–3110): TGTCTTCAAAAACACTTGTGGG; β-actin (accession number V01217): forward primer (bases 3527–3544): CATCCATGCCCTGAGTCC; reverse primer (bases 3736–3717): ACACCTCAAACCACTCCCAG. These polymerase chain reactions (PCRs) were run for 50 cycles of 92°C (denaturing, 2.5 min), 55°C (annealing, 1.5 min) and 72°C (extension, 1 min), followed by a final extension of 10 min at 72°C. The PCR products were separated on 2.5%agarose gels and the product sizes were as predicted from the sequences. Confirmation of the nature of some of these bands was achieved using restriction enzyme analysis after reamplification with the appropriate gene specific primers (Hha I or Eco RI for 1 h at 37°C). In control experiments on diluted brain cDNA, the Kir6.1, Kir6.2, SUR1 and SUR2 primer pairs were able to detect positive products of the predicted size using 0.1 pg of cDNA but not 0.01 pg (Fig. 8A). Furthermore, in experiments where the electrode was positioned next to a cell without seal formation or harvesting of the cytoplasmic contents, no PCR products were detected (n = 3).
Figure 8. Molecular identity of the KATP channel complex.
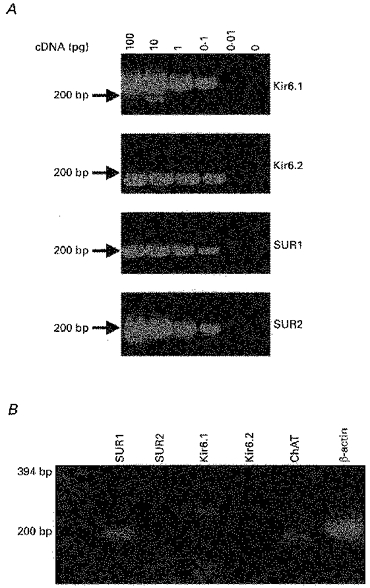
A, 2.5%agarose gel showing the sensitivity of the Kir6.1, Kir6.2, SUR1 and SUR2 primer pairs. B, using the cytoplasmic contents harvested from a single cholinergic neurone, the expression of ChAT, Kir6.1, SUR1 and β-actin was detected.
Solutions and drugs
The physiological saline contained (mM): 125.0 NaCl, 25.0 NaHCO3, 10.0 glucose, 2.5 KCl, 1.25 NaH2PO4, 2.0 CaCl2, 1.0 MgCl2; and was bubbled with a 95%O2-5%CO2 gas mixture. In some experiments, the glucose concentration was increased to 20 mM whilst in others, glucose was removed from the physiological saline. In some of these latter experiments, 10 mM sucrose was added to maintain osmolarity. The intracellular (pipette) solution was adjusted to pH 7.4 using KOH in all experiments. For conventional whole-cell recordings this solution comprised (mM): 120.0 potassium gluconate, 10.0 NaCl, 2.0 MgCl2, 0.5 K2EGTA, 10.0 Hepes, 2.0 Na2ATP, 0.1 Na2GTP. In some experiments, Na2ATP was removed from this solution, whilst in others Na2ATP was increased to 4 mM or replaced by 10 mM adenosine 5′-triphosphate, P3-(1-(4,5-dimethoxy-2-nitrophenyl)ethyl)ester (caged ATP). In cell-attached experiments, the electrode solution comprised (mM): 120.0 KCl, 1.0 MgCl2, 2.0 CaCl2, 10.0 Hepes; whilst in experiments utilizing the permeabilized patch recording technique, the electrode solution comprised (mM): 75.0 K2SO4, 55.0 KCl, 7.0 MgCl2, 10.0 Hepes; and 240 μg ml−1 Amphotericin B (Rae, Cooper, Gates & Watsky, 1991). In these latter experiments, current-clamp recordings were performed only when the input resistance had reached a stable level following establishment of a gigaohm seal (typically 30–50 min). Cells in which a sudden decrease in input resistance was observed during the permeabilization procedure were rejected.
Throughout the course of an experiment, slices were continuously perfused at a rate of 3–4 ml min−1 with physiological saline by means of a gravity feed system. Tolbutamide and other drugs used in this study were applied by changing the solution which superfused the slice to one which contained the drug. In voltage-clamp recordings, 1 μM TTX, 50 μM D-2-amino-5-phosphovalerate (D-APV) and 5 μM 2,3-dihydroxy-6-nitro-7-sulphamoyl-benzo(F)quinoxalone (NBQX) were usually added to the physiological saline. All experiments were conducted at 32–35°C. In order to induce photolysis of caged ATP within neurones, UV light was directed onto neurones via the epifluorescence port of the microscope in a manner similar to that described previously (Lee & Boden, 1997).
All drugs were obtained from Sigma (Poole, UK) except NBQX and D-APV which were from Tocris Cookson (Bristol, UK) and caged ATP which was obtained from Molecular Probes (Eugene, OR, USA).
RESULTS
Identification of striatal cholinergic interneurones
This study was performed upon a total of 182 visually identified neurones present in striatal slices taken from 96 animals. Only neurones which were seen to have soma in excess of 30 μm in their longest axis were chosen for this study. Following formation of the whole-cell configuration, these neurones displayed electrophysiological characteristics similar to those previously attributed to cholinergic interneurones (Kawaguchi, 1992, 1993). For example, in current-clamp recordings, these neurones had a resting membrane potential of −58.6 ± 1.4 mV (n = 64) and often fired spontaneous action potentials at a rate of 2–5 Hz which were associated with a large after-hyperpolarization (24.1 ± 1.3 mV (n = 47); Fig. 1). Immediately after membrane breakthrough these neurones had an input resistance of 286.9 ± 23.2 MΩ (n = 47) and exhibited a characteristic reduction in apparent input resistance in response to hyperpolarizing current injection (Fig. 1; Jiang & North, 1991). These neurones were also found to be sensitive to the NK1 receptor agonist (Sar9, Met(O2)11) substance P (Sar9) which produced a 15.6 ± 2.8 mV (n = 20) depolarization when applied at a concentration of 100 nM (see Fig. 6).
Figure 1. Characterization of rat striatal cholinergic interneurones.
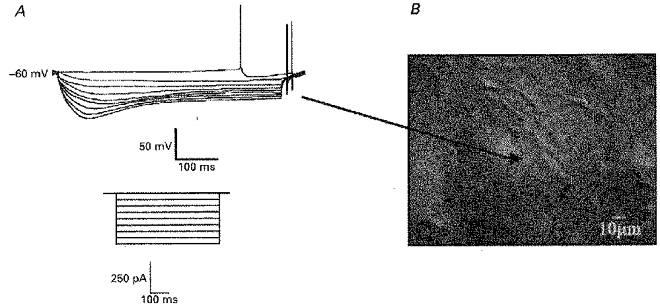
A, voltage response of a cholinergic interneurone to hyperpolarizing current injection. Inset depicts current protocol. B, picture of a section of the striatum as viewed under infrared optics illustrating the appearance of a cholinergic interneurone (denoted by arrow).
Figure 6. The effect of aglycaemia and metabolic inhibition of the electrical activity of cholinergic interneurones.
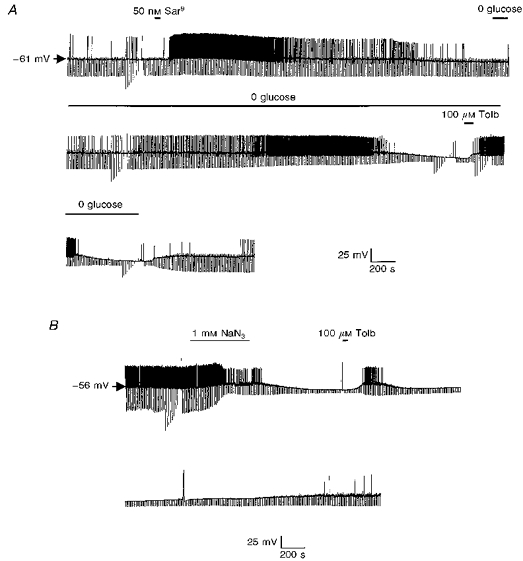
A, current-clamp recording using an amphotericin B-permeabilized patch. Application of the NK1 agonist Sar9 produced marked depolarization with associated increase in firing rate. Removal of glucose causes cellular hyperpolarization in a tolbutamide-sensitive manner. B, in this permeabilized patch recording metabolic inhibition with sodium azide (NaN3) hyperpolarized the cell and this was reversed by tolbutamide.
To confirm the identity of these neurones, in five cells, following electrophysiological identification, cytoplasm was harvested and processed for reverse transcription and cDNA amplification. The resultant cDNA was subjected to PCR analysis with primers specific for the enzyme choline acetyltransferase (ChAT). In each case, neurones identified electrophysiologically as cholinergic were positive for this enzyme (see Fig. 8).
Identification of KATP channel activity
When neurones were dialysed with an ATP-free solution and glucose removed from the physiological saline, they underwent a gradual hyperpolarization with cessation of action potential firing and an associated decrease in apparent input resistance. Within approximately 10 min of membrane breakthrough, these neurones hyperpolarized to a potential of −83.6 ± 3.2 mV (n = 42) and had an apparent input resistance of 123.5 ± 22.3 MΩ (n = 42, Fig. 2A).
Figure 2. The effect of dialysis with an ATP-free electrode solution.
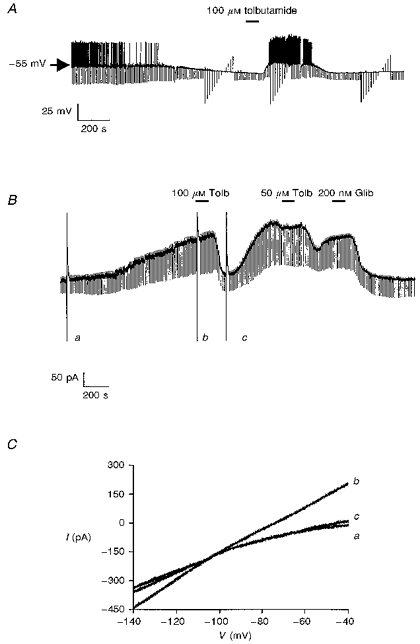
A, continuous whole-cell current-clamp recording demonstrating the effect of tolbutamide on the hyperpolarization resulting from dialysis with an ATP-free electrode solution. B, the effect of tolbutamide (Tolb) and glibenclamide (Glib) on the time-dependent outward current at a holding potential of −60 mV. The large deflections are the current responses to voltage ramps from −140 to −40 mV as depicted in C. C, expanded traces of the current responses to the voltage ramps shown in B. The letters beside these currents correspond to those shown in B.
Bath application of the sulphonylurea tolbutamide at this point rapidly (within 2–8 min) depolarized neurones (by 17.5 ± 1.9 mV (n = 15)) with a concomittant increase in apparent input resistance (to 301.9 ± 3.1 MΩ (n = 15)) and a return of action potential firing (Fig. 2A). These effects of tolbutamide were reversible on washout. As previously reported, the ability of tolbutamide to depolarize these cells and to increase input resistance persisted after treatment of the slice with 1 μM TTX (control: 13.1 ± 2.5 mV depolarization; 1 μM TTX: 14.2 ± 2.1 mV depolarization (n = 5), not shown; Lee, Brownhill & Richardson, 1997). Similarly, these actions of tolbutamide were unaffected when the slice was treated with a Ca2+-free physiological saline solution which contained no CaCl2 and 10 mM MgCl2, (control: 18.3 ± 2.1 mV depolarization; Ca2+-free saline: 17.9 ± 1.9 mV depolarization (n = 4), not shown).
To determine the nature of the sulphonylurea-sensitive current(s) responsible for these effects, neurones were voltage clamped at −60 mV. At this potential, an outward current of 172.3 ± 28.3 pA (n = 18) was observed to develop over time when ATP was omitted from the electrode solution (Fig. 2B). The development of this outward current exhibited a similar time course to the hyperpolarization seen in current-clamp recordings and was associated with an increase in cellular conductance (from 3.7 ± 0.5 to 6.7 ± 0.7 nS as assessed by 10 mV hyperpolarizing step commands (n = 5)) and had a reversal potential of −99.5 ± 2.9 mV (as assessed by ramp command potentials (n = 8)) when 2.5 mM K+ was present in the extracellular solution (Fig. 2C). In three further experiments, the physiological saline was modified to contain 5 mM KCl and under these conditions, this time-dependent current reversed polarity at −83.4 ± 2.1 mV. This change in reversal potential with change in extracellular K+ concentration is close to that expected from the Nernst equation for a potassium-selective current.
Once this time-dependent current had reached its peak magnitude, bath application of either tolbutamide (100 μM) or glibenclamide (200 nM) was found to inhibit this current (Fig. 2B). However, although the inhibitory effects of tolbutamide were reversible on washout, the inhibition produced by glibenclamide was not reversed during the time course of these experiments.
In contrast, when 2 mM Na2ATP was added to the electrode solution, no sulphonylurea-sensitive hyperpolarization was observed to develop with time in current-clamp recordings (n = 25). Under these conditions, bath application of 500 μM diazoxide hyperpolarized all cholinergic interneurones tested (by 10.1 ± 1.9 mV (n = 12), Fig. 3A) in a manner that was unaffected by treatment of the slice with 1 μM TTX (n = 5) but was blocked by co-application of tolbutamide (200 μM, n = 3, not shown).
Figure 3. The effect of diazoxide on cholinergic interneurones.
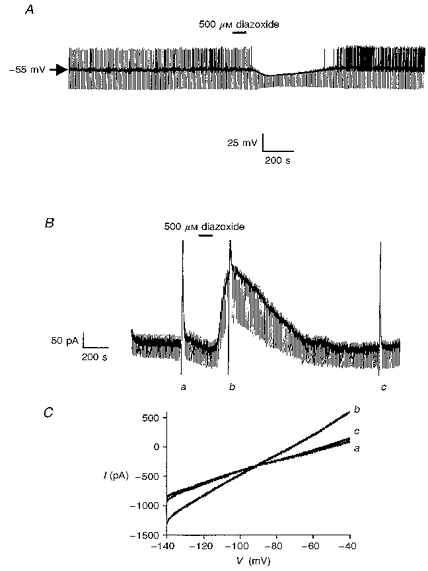
A, in the presence of 2 mM ATP in the electrode, diazoxide induces a membrane hyperpolarization with associated decrease in input resistance. B, in a neurone voltage clamped at −60 mV, diazoxide induces an outward current. The large deflections are the current responses to voltage ramps from −140 to −40 mV as depicted in C. C, expanded traces of the current responses to the voltage ramps shown in B. The letters beside these currents correspond to those shown in B.
Similarly, with 2 mM Na2ATP in the electrode solution, no time-dependent outward current was observed in neurones voltage clamped at −60 mV. However, bath application of diazoxide induced an outward current, 139.3 ± 32.4 pA in magnitude (n = 14, Fig. 3B), which was associated with an increase in cellular conductance (from 3.2 ± 0.6 to 5.9 ± 0.5 nS as assessed by 10 mV hyperpolarizing step commands (n = 5)) and had a reversal potential of −97.2 ± 3.4 mV with 2.5 mM K+ present in the extracellular solution (n = 5, Fig. 3C). In contrast, when diazoxide was applied concomitantly with 200 μM tolbutamide, this outward current was not seen to develop (n = 3, not shown).
To demonstrate that the sulphonylurea-sensitive current observed in the absence of intracellular ATP arose due to the activation of a KATP channel current following dialysis of ATP from the neurone, a number of voltage-clamp recordings were performed using 10 mM caged ATP in the electrode solution. With this internal solution, a sulphonylurea-sensitive outward current 109.3 ± 23.3 pA (n = 7) in magnitude at −60 mV was seen to develop with time. Once this current had fully developed, UV light was directed onto the neurone under visual control to initiate the release of free ATP inside the neurones. This process led to the rapid inhibition of this current (by 93.2 ± 3.2%(n = 7); Fig. 4) for the duration of the period of UV illumination. Furthermore, these effects were reversed following cessation of the UV photoillumination period (1 min). In contrast, in the absence of caged ATP in the electrode solution, exposure to UV light for a similar length of time had no effect on the sulphonylurea-sensitive current at −60 mV (n = 5, not shown).
Figure 4.
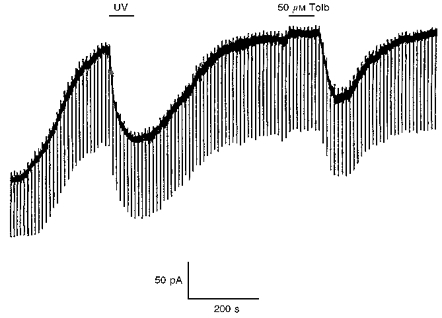
UV photolysis of caged ATP inhibits the outward sulphonylurea-sensitive current in a cholinergic interneurone voltage clamped at −60 mV.
Pharmacology of the KATP channel current
The results presented so far demonstrate the presence of a KATP channel current similar to that reported in a wide variety of tissues including central neurones (Ashcroft & Ashcroft, 1990). However, in contrast to peripheral tissues, there is a paucity of data presently available on the pharmacological properties of KATP channel currents within central neurones. Consequently, in the present study, we examined the effects of several KATP channel modulators on this current as documented in Fig. 5.
Figure 5. Pharmacology of the sulphonylurea-sensitive current.
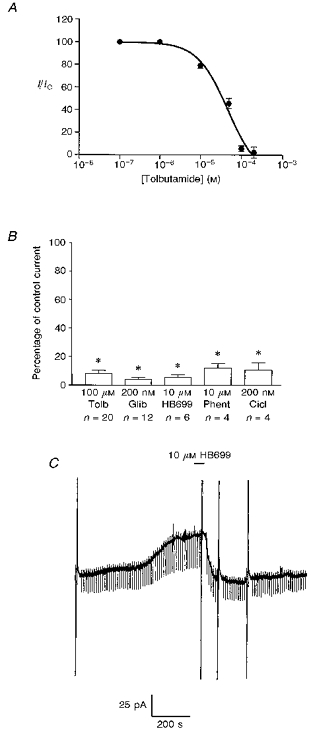
A, concentration-response curve for tolbutamide. All points are means of three to six separate experiments. Vertical lines indicate s.e.m.; where no lines are apparent, the error was within the size of the symbol. The associated line is the line of best fit to eqn (1). B, mean data illustrating the pharmacology of the sulphonylurea-sensitive current at −60 mV. The effect of each agent was compared with the control response obtained in the same neurone. HB699 (meglitinide); Phent, phentolamine; Cicl, ciclazindol. Error bars indicate s.e.m. and n shows the number of cells tested for each condition * Significantly less than the control response (100 %), P < 0.05. C, the effect of HB699 in a cell voltage clamped at −60 mV. The large vertical deflections are current responses to voltage ramps.
Firstly, we examined the concentration dependence of tolbutamide to inhibit this whole-cell KATP channel current at −60 mV. As shown in Fig. 5, this relationship could be fitted to eqn (1) (Fig. 5A), which gave an IC50 value of 23.6 ± 0.8 μM and an associated Hill coefficient of 1.2 ± 0.2.
In addition to tolbutamide and the second generation sulphonylurea glibenclamide (Fig. 2), the non-sulphonylurea structural component of glibenclamide, HB699 or meglitinide (Lee Ozanne, Hales & Ashford, 1994a), also inhibited this current in a poorly reversible manner (Fig. 5C). Similarly, the anorectic compound ciclazindol (Lee et al. 1996) inhibited this current in a manner that was not readily reversible on continued washing as did the imidazoline compound phentolamine (Fig. 5; Lee et al. 1994b).
In addition to these inhibitors of KATP channel activity, several potassium channel openers were also tested on these neurones in the presence of 2 mM ATP in the electrode solution. Although 500 μM diazoxide was found to induce an outward current at −60 mV, neither pinacidil (500 μM, n = 4) or levcromakalim (500 μM, n = 4) were found to exert similar effects.
Physiological role
In several regions of the CNS, KATP channels have been reported to perform a cytoprotective role in hypoxic and/or hypoglycaemic conditions (e.g. Trapp & Ballanyi, 1995). Consequently, in the next series of experiments, we employed the perforated-patch technique to assess the sequence of events which occur in these neurones in response to aglycaemia. Using this technique, neurones were found to exhibit a similar resting potential (-55.2 ± 2.4 mV (n = 8)) and input resistance (325.2 ± 45.3 MΩ (n = 8)) to that reported using conventional whole-cell recording techniques. Similarly, the NK1 receptor agonist Sar9 was found to depolarize these neurones to a similar extent as that observed with conventional techniques (100 nM produced a 12.6 ± 3.2 mV depolarization (n = 4); Fig. 6).
Under normoglycaemic conditions, bath application of 100 μM tolbutamide was found to induce a small increase in firing frequency which was not associated with any significant increase in apparent input resistance (not shown). However, following removal of glucose from the extracellular solution, after several minutes, neurones were observed to undergo an initial increase in firing frequency followed by a dramatic hyperpolarization (to −78.4 ± 4.5 mV (n = 5)) with an associated reduction in apparent input resistance (to 176 ± 19.5 MΩ (n = 5)). This hyperpolarization was reversed either by application of tolbutamide (100 μM, which decreased the resting membrane potential to −53.4 ± 3.2 mV and increased the apparent input resistance to 289.4 ± 23.2 MΩ (n = 4)), or by re-introduction of glucose to the bath solution (which reduced the resting membrane potential to −51.2 ± 4.2 mV and increased the apparent input resistance to 302.4 ± 26.2 MΩ (n = 4)).
Similarly, it was also possible to induce membrane hyperpolarization in these neurones by metabolic inhibition with NaN3 (Fig. 6B). In this instance, bath application of 1 mM NaN3 produced a rapid hyperpolarization (to −81.4 ± 4.2 mV (n = 3)) which was immediately preceded by a transient increase in action potential firing. Once again 100 μM tolbutamide was found to reverse this hyperpolarization (to −53.2 ± 2.2 mV (n = 3)) and initiate a return to action potential firing.
Single channel properties
To address the unitary properties of the channel responsible for this current, cell-attached patch recordings were performed. As observed under current-clamp conditions, at rest under normal conditions, these interneurones were found to frequently exhibit action potential firing activity (Fig. 7A). However, following metabolic inhibition with 1 mM NaN3 this pattern of activity was replaced by the opening of a small channel, 3.8 ± 0.3 pA in amplitude (at 0 mV pipette potential), with an open probability of 0.07 ± 0.02 (n = 8). The channel was observed to reverse polarity at a pipette potential between 80 and 100 mV (Fig. 7B) and exhibited a unitary conductance of 41.4 ± 0.8 pS (as judged over the linear part of the I-V relationship (n = 8)). The activity of this channel was unaffected by membrane voltage (Fig. 7B) but was completely inhibited by bath application of 100 μM tolbutamide (Fig. 7A).
Figure 7. Single channel characteristics of the tolbutamide-sensitive channel activated by metabolic inhibition with NaN3.
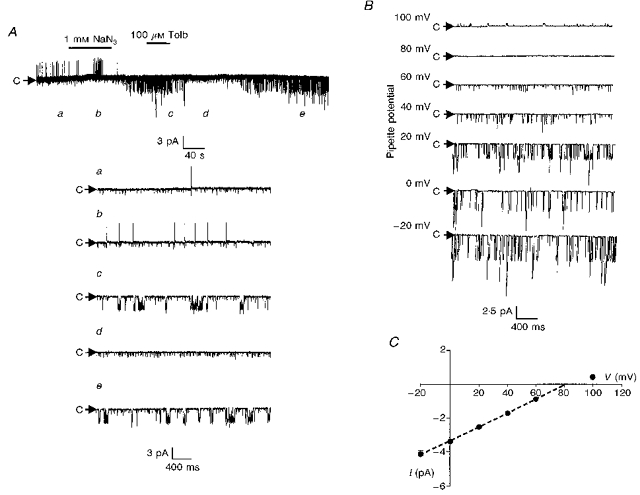
A, cell-attached recording made at a pipette potential of 0 mV. Bath application of NaN3 induced the activity of several 3.2 pA channels which were inhibited by tolbutamide. The letters refer to the panels shown on the expanded timescale in the lower part of the figure. Note the presence of a smaller channel that was active throughout the recording and was unaffected by tolbutamide. B, cell-attached recording of the NaN3-activated channel at different pipette potentials. C, current-voltage relationship revealed a unitary conductance of 41.4 ± 0.8 pS in the linear part of the plot (denoted by the dashed line). Each point is the mean of between three and five observations.
Single cell RT-PCR studies
In five cells which were identified electrophysiologically as cholinergic, the cytoplasm was harvested and subjected to reverse transcription. The resulting cDNA was subsquently amplified and subjected to PCR analysis using primer oligonucleotides specific for the cytoplasmic marker gene β-actin and the enzyme ChAT. In all cells identified as cholinergic, mRNA encoding the enzyme ChAT was detected (n = 5;Fig. 8B) whilst the GABAergic cell marker GAD67 was not. Furthermore, in those cells which were identified as cholinergic and which were shown to express ChAT mRNA, the KATP channel subunits Kir6.1 and SUR1 were detected whilst Kir6.2 and SUR2 were not (Fig. 8B). The Kir6.1 and SUR1 PCR products from two cells were gel purified and re-amplified using the appropriate primer pairs. Subsequent digestion with the restriction enzyme Hha I (SUR1) or Eco RI (Kir6.1) confirmed their identity (not shown).
In contrast, in five tolbutamide-insensitive non-cholinergic (i.e. medium spiny projection neurones) cells, although the expression of β-actin and GAD67 was detected, neither ChAT nor the four KATP channel subunits were expressed (n = 5;not shown).
DISCUSSION
Characterization of cholinergic interneurones in the rat striatum
At least six types of neurone have been identified within the striatum based on structural and neurochemical analysis (Kawaguchi, Wilson, Augood & Emson, 1995). Two of these six subtypes of neurone combine to form the GABAergic medium spiny projection neurones which are the principle neurones of the striatum and account for over 90%of all striatal neurones.
The remaining 10%of striatal neurones are formed by four types of aspiny interneurone. Of these interneurones, the cholinergic interneurones account for 1–2%of the total neuronal population. Despite their small number, these neurones possess extensive dendritic and axonal fields and are believed to exert a profound modulatory influence upon the medium spiny neurone and hence basal ganglia activity (Di Chiara, Morelli & Consolo, 1994).
In the present study, we have exploited several characteristics exhibited by these cells in order to distinguish them from other striatal subtypes. It is well documented that these neurones have the largest somatic size of all striatal neurones (typically in excess of 30 μm in the longest axis; Kawaguchi et al. 1995). Consequently, we have used the ability to identify neurones visually within brain slices in order to record selectively from these morphologically distinct cells.
These neurones also demonstrate a number of electrophysiological and pharmacological characteristics which distinguish them from other striatal subtypes. For example, these neurones demonstrate a relatively depolarized resting membrane potential and are often seen to fire spontaneous action potentials at rest which are followed by a relatively large and prolonged after-hyperpolarization (Kawaguchi, 1992, 1993). Under resting conditions, these neurones exhibit a large input resistance and display a prominent sag in the voltage response to hyperpolarizing current injection due to the presence of a hyperpolarization-activated cation conductance (IH) (Jiang & North, 1991). Pharmacologically, cholinergic interneurones are readily depolarized by the tachykinin substance P (Aosaki & Kawaguchi, 1996) and together with the somatostatin-containing interneurones are known to exclusively express tachykinin NK1 receptors within the striatum (Kaneko, Shigemoto, Nakanishi & Mizuno, 1993). Thus we have been able to use these electrophysiological and pharmacological criteria to confirm the nature of these visually identified neurones. Finally, to confirm the cholinergic nature of these cells, five were examined for the expression of choline acetyltransferase (ChAT), the marker enzyme for cholinergic cells, using the technique of single cell RT-PCR. All cells identified by this technique as cholinergic on the basis of the above criteria were found to be positive for ChAT and thus, by definition, were cholinergic.
Properties of the KATP channel current
The principle findings of the present study are that cholinergic interneurones within the rat striatum express a KATP channel current. Using whole-cell recordings, dialysis of cholinergic neurones with a solution containing no ATP was found to hyperpolarize these neurones through the activation of a sulphonylurea-sensitive outward current which had a reversal potential close to the calculated equilibrium potential for potassium ions (approximately −100 mV). This current was not observed when ATP was present in the electrode solution suggesting that the outward sulphonylurea-sensitive current observed in this study is also ATP sensitive.
This hypothesis is supported by the results obtained using caged ATP in the electrode solution. Prior to photoillumination, this compound is able to partially inhibit KATP channel activity (Nichols, Niggli & Lederer, 1991). However, when exposed to light of the appropriate wavelength, this compound undergoes a photochemical reaction with the liberation of free ATP. Consequently, the ability of UV photoillumination to reverse the sulphonylurea-sensitive outward current under these conditions suggests that the current responsible for this is modulated by the freshly liberated ATP.
These findings are also supported by the results obtained from the molecular analysis of gene expression in these neurones. Using single cell RT-PCR, we have demonstrated the expression of Kir6.1 and SUR1 subunits in these neurones. In contrast, no evidence for the expression of Kir6.2 or SUR2 subunits was detected suggesting that the KATP channel current under study is formed exclusively by the combination of Kir6.1 and SUR1 subunits. Although this combination of KATP channel subunits has not previously been reported in the CNS, Kir6.1 is known to be ubiquitously expressed in a wide variety of tissues including brain (Inagaki et al. 1995b) whilst SUR1 is thought to be the predominant sulphonylurea receptor isoform expressed in the CNS (Karschin, Ecke, Ashcroft & Karschin, 1997).
Pharmacology of the KATP channel current
Under voltage-clamp conditions, tolbutamide was found to inhibit the KATP channel current present in the striatal cholinergic interneurones with an IC50 of 23 μM. This value is close to that reported for KATP channel inhibition in a number of peripheral tissues including cardiac tissue and panceatic β cells (Ashcroft & Ashcroft, 1990).
Similarly, this current was sensitive to the second generation sulphonylurea glibenclamide and the non-sulphonylurea moiety of glibenclamide, meglitinide. The ability of these agents to profoundly inhibit the KATP channel current at nanomolar and low micromolar concentrations, respectively, and the irreversibilty of these agents is akin to that seen for both the native and cloned β cell KATP channel complex (Kir6.2 and SUR1; Lee et al. 1994a; Gribble, Ashfield, Ämmälä & Ashcroft, 1997). Furthermore, this current displays a similar sensitivity to the imidazoline KATP channel inhibitor phentolamine and the anorectic agent ciclazindol as that seen for the β cell KATP channel (Lee et al. 1994b, 1996). Thus it would appear that the KATP channel complex present in these neurones is pharmacologically similar to the complex present in the β cell.
This observation is supported by the response of this current to KATP channel openers. As in the β cell, this current is readily activated by diazoxide but is less sensitive to pinacidil and levcromakalim. In agreement with this, recent studies have shown that the SUR2 sulphonylurea receptor isoform is more sensitive to the KATP channel openers but less sensitive to inhibition by glibenclamide (Inagaki et al. 1996; Isomoto et al. 1996).
Physiological role of the KATP channel current
It is well established that whilst striatal medium spiny projection neurones are extremely vulnerable to ischaemic conditions, striatal interneurones, including cholinergic interneurones, are relatively resistant to such insults (Francis & Pulsinelli, 1982; Chesselet, Gonzales, Lin, Polsky & Jin, 1990). In relation to this, in the present study we demonstrate that metabolic inhibition and aglycaemic episodes hyperpolarize cholinergic interneurones through the activation of a KATP channel current. Thus it is tempting to speculate that this current may perform a protective role under conditions of metabolic deprivation as has been postulated for KATP channel currents elsewhere in the CNS (Ashcroft & Ashcroft, 1990).
In addition to these observations, we have shown using the permeabilized patch technique that tolbutamide is able to increase action potential firing in metabolically intact cholinergic interneurones. This finding suggests that KATP channel activity contributes to the resting membrane properties of these neurones. This is supported by previous studies in which the sulphonylureas were shown to stimulate striatal acetylcholine release under resting conditions (Lee, Brownhill & Richardson, 1997). Thus, it would appear that KATP channel activity may also perform an important role in the modulation of the electrical activity of these neurones and as such, may be an important site of modulation by neurotransmitters.
In a recent study, Calabresi et al. (1997) report that striatal interneurones hyperpolarize in response to aglycaemic episodes through the activation of a potassium current that is insensitive to the sulphonylureas tolbutamide and glipizide. It is difficult to put these findings into the context of the present study although it is, of course, possible that cholinergic interneurones express additional types of potassium channel that are activated in aglycaemia. However, if this were the case, it is unclear why these were not seen in the present study. Alternatively, since this former study was performed on only a small number of interneurones, it is possible that those tested for sulphonylurea sensitivity were not cholinergic and that other interneurones in the striatum respond to anoxic and/or aglycaemic conditions by the activation of other types of channel.
Previous studies have shown at the single channel level that striatal medium spiny neurones also exhibit KATP channel activity (Schwanstecher & Bassen, 1997). However, it appears that these channels do not contribute to the resting potential of these cells under normal resting conditions and do not afford cytoprotective effects during aglycaemic periods (Calabresi et al. 1997). The reasons why these channels do not appear to protect medium spiny neurones remains to be resolved and with regard to this, it will be interesting to determine the molecular nature of the KATP channel in these neurones. In relation to this, the unitary conductance of the observed channel in the present report (44 pS) is considerably smaller than that reported in medium spiny neurones (70 pS) under similar conditions (Schwanstecher & Panten, 1994). Furthermore, Karschin et al. (1997) have recently shown that mRNA for Kir6.2 is the most abundantly expressed isoform in the rat striatum. Thus it is possible that the KATP channel complex present in medium spiny projection neurones is different from that present in cholinergic interneurones and may not offer the same level of cytoprotection under conditions of metabolic deprivation.
References
- Aosaki T, Kawaguchi Y. Actions of substance P on rat neostriatal neurons in vitro. Journal of Neuroscience. 1996;16:5141–5153. doi: 10.1523/JNEUROSCI.16-16-05141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft SJH, Ashcroft FM. Properties and functions of ATP-sensitive K+ channels. Cellular Signalling. 1990;2:197–214. doi: 10.1016/0898-6568(90)90048-f. 10.1016/0898-6568(90)90048-F. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Ascone CM, Centonze D, Pisani D, Sancesario G, D'Angelo V, Bernardi G. Opposite membrane potential changes induced by glucose deprivation in striatal spiny neurons and in large aspiny interneurons. Journal of Neuroscience. 1997;17:1940–1949. doi: 10.1523/JNEUROSCI.17-06-01940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesselet M-F, Gonzales C, Lin C-S, Polsky K, Jin B-K. Ischemic damage in the striatum of adult gerbils: Relative sparing of somatostatinergic and cholinergic interneurons contrasts with loss of efferent neurons. Experimental Neurology. 1990;110:209–218. doi: 10.1016/0014-4886(90)90032-n. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/ NMDA interactions. Trends in Neurosciences. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Francis A, Pulsinelli W. The response of GABAergic and cholinergic neurones to transient cerebral ischemia. Brain Research. 1982;243:271–278. doi: 10.1016/0006-8993(82)90250-5. 10.1016/0006-8993(82)90250-5. [DOI] [PubMed] [Google Scholar]
- Gribble FM, Ashfield R, Ämmälä C, Ashcroft FM. Properties of cloned ATP-sensitive K+ currents expressed in Xenopus oocytes. The Journal of Physiology. 1997;498:87–98. doi: 10.1113/jphysiol.1997.sp021843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: An inward rectifier subunit plus the sulfonylurea receptor. Science. 1995a;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, Wang C-Z, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. 10.1016/S0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Tsuura Y, Namba N, Masuda K, Gonoi T, Horie M, Mizuta M, Seino S. Cloning and functional characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat tissues including pancreatic islets, pituitary, skeletal muscle and heart. Journal of Biological Chemistry. 1995b;270:5691–5694. doi: 10.1074/jbc.270.11.5691. 10.1074/jbc.270.11.5691. [DOI] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, Matsuzawa Y, Kurachi Y. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. Journal of Biological Chemistry. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- Jiang Z-G, North RA. Membrane properties and synaptic responses of rat striatal neurones in vitro. The Journal of Physiology. 1991;443:533–553. doi: 10.1113/jphysiol.1991.sp018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Substance P receptor-immunoreactive neurons in the rat neostriatum are segregated into somatostinergic and cholinergic aspiny neurons. Brain Research. 1993;631:297–303. doi: 10.1016/0006-8993(93)91548-7. 10.1016/0006-8993(93)91548-7. [DOI] [PubMed] [Google Scholar]
- Karschin C, Ecke C, Ashcroft FM, Karschin A. Overlapping distribution of KATP channel-forming Kir6.2 subunit and the sulfonylurea receptor SUR1 in rodent brain. FEBS Letters. 1997;401:59–64. doi: 10.1016/s0014-5793(96)01438-x. 10.1016/S0014-5793(96)01438-X. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Large aspiny cells in the matrix of the rat neostriatum in vitro: Physiological identification, relation to the compartments and exitatory postsynaptic currents. Journal of Neurophysiology. 1992;67:1669–1682. doi: 10.1152/jn.1992.67.6.1669. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. Journal of Neuroscience. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological chracterization. Trends in Neurosciences. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kozlowski RZ, Hales CN, Ashford MLJ. Dual effects of diazoxide on ATP-K+ currents recorded from an insulin-secreting cell line. British Journal of Pharmacology. 1989;97:1039–1050. doi: 10.1111/j.1476-5381.1989.tb12560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Boden PR. Characterization of the inward current induced by metabotropic glutamate receptor stimulation in rat ventromedial hypothalamic neurones. The Journal of Physiology. 1997;504:649–663. doi: 10.1111/j.1469-7793.1997.649bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Brownhill V, Dixon AK, Freeman TC, Richardson PJ. Characterisation of a KATP channel current in rat striatal cholinergic interneurones. British Journal of Pharmacology. 1997;122:288P.. [Google Scholar]
- Lee K, Brownhill V, Richardson PJ. Antidiabetic sulphonylureas stimulate acetylcholine release from striatal cholinergic interneurones through inhibition of KATP channel activity. Journal of Neurochemistry. 1997;69:1774–1776. doi: 10.1046/j.1471-4159.1997.69041774.x. [DOI] [PubMed] [Google Scholar]
- Lee K, Khan RN, Rowe ICM, Ozanne SE, Hall AC, Papadakis E, Hales CN, Ashford MLJ. Ciclazindol inhibits ATP-sensitive K+ channels and stimulates insulin secretion in CRI-G1 insulin-secreting cells. Molecular Pharmacology. 1996;49:715–720. [PubMed] [Google Scholar]
- Lee K, Ozanne SE, Hales CN, Ashford MLJ. Mg2+ dependent inhibition of KATP channels by sulphonylureas in CRI-G1 insulin secreting cells. British Journal of Pharmacology. 1994a;111:632–640. doi: 10.1111/j.1476-5381.1994.tb14783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Ozanne SE, Rowe ICM, Hales CN, Ashford MLJ. The effects of trypsin on ATP-sensitive potassium channel properties and sulfonylurea receptors in the CRI-G1 insulin-secreting cell line. Molecular Pharmacology. 1994b;45:176–185. [PubMed] [Google Scholar]
- Nichols CG, Niggli E, Lederer WJ. Modulation of ATP-sensitive potassium channel activity by flash-photolysis of ‘caged-ATP’ in rat heart cells. Pflügers Archiv. 1991;415:510–512. doi: 10.1007/BF00373635. [DOI] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. Journal of Neuroscience Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Sakura H, Ämmälä C, Smith PA, Gribble FM, Ashcroft FM. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel expressed in pancreatic β cells, brain, heart and skeletal muscle. FEBS Letters. 1995;377:338–344. doi: 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- Schwanstecher C, Bassen D. KATP channel on the somata of spiny neurones in rat caudate nucleus: regulation by drugs and nucleotides. British Journal of Pharmacology. 1997;121:193–198. doi: 10.1038/sj.bjp.0701120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanstecher C, Panten U. Identification of an ATP-sensitive K+ channel in spiny neurons of rat caudate nucleus. Pflügers Archiv. 1994;427:187–189. doi: 10.1007/BF00585961. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Dodt H-U, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflügers Archiv. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Sturgess NC, Hales CN, Cook DL, Ashford MLJ. The sulphonylurea receptor may be an ATP-sensitive K+ channel. Lancet. 1985;ii:474–475. doi: 10.1016/s0140-6736(85)90403-9. [DOI] [PubMed] [Google Scholar]
- Trapp S, Ballanyi K. KATP channel mediation of anoxia-induced outward current in rat dorsal vagal neurons in vitro. Journal of Physiology. 1995;487:37–50. doi: 10.1113/jphysiol.1995.sp020859. [DOI] [PMC free article] [PubMed] [Google Scholar]


