Abstract
The role of K+ channels and membrane potential in myoblast fusion was evaluated by examining resting membrane potential and timing of expression of K+ currents at three stages of differentiation of human myogenic cells: undifferentiated myoblasts, fusion-competent myoblasts (FCMBs), and freshly formed myotubes.
Two K+ currents contribute to a hyperpolarization of myoblasts prior to fusion: IK(NI), a non-inactivating delayed rectifier, and IK(IR), an inward rectifier.
IK(NI) density is low in undifferentiated myoblasts, increases in FCMBs and declines in myotubes. On the other hand, IK(IR) is expressed in 28 % of the FCMBs and in all myotubes.
IK(IR) is reversibly blocked by Ba2+ or Cs+.
Cells expressing IK(IR) have resting membrane potentials of −65 mV. A block by Ba2+ or Cs+ induces a depolarization to a voltage determined by IK(NI) (−32 mV).
Cs+ and Ba2+ ions reduce myoblast fusion.
It is hypothesized that the IK(IR)-mediated hyperpolarization allows FCMBs to recruit Na+, K+ and T-type Ca2+ channels which are present in these cells and would otherwise be inactivated. FCMBs, rendered thereby capable of firing action potentials, could amplify depolarizing signals and may accelerate fusion.
Myoblast fusion is essential to skeletal muscle growth during embryonic, fetal and postnatal life. In mature muscle, myoblast fusion allows skeletal muscle repair via the activation of muscle satellite cells (Mauro, 1961; Campion, 1984). Insight into the mechanisms responsible for fusion between myoblasts, or between myoblasts and myotubes, is important in view of the prospect of myoblast transplantation for the treatment of muscle diseases or for treating other disorders by ex vivo somatic gene therapy.
Myoblast fusion may be preceded or accompanied by a major change in membrane electrical properties. Indeed, the resting potential of myogenic cells in different species changes from approximately −10 mV in myoblasts to values ranging between −55 and −70 mV in myotubes (Fischbach, Nameroff & Nelson, 1971; Ritchie & Fambrough, 1975; Spector & Prives, 1977; Hamann et al. 1994). It has therefore been hypothesized that a hyperpolarization may render myoblasts more sensitive to putative depolarizing signals which could then promote cell fusion (Entwistle, Zalin, Bevan & Warner, 1988).
The goal of this paper is to explore, in human myoblasts, the mechanisms underlying the hyperpolarization associated with fusion. The hyperpolarization preceding fusion would presumably be due to the expression of new potassium conductances in myoblasts that are about to fuse. However, precise indications of the types of potassium currents involved and the timing of expression is still a matter of debate and may also depend on the species studied. In chick embryonic myoblasts, it has been reported that an inward rectifier potassium current is involved in the early phase of the fusion-linked hyperpolarization, from −15 to −27 mV (Shin, Park, Kwon, Chung & Kang, 1997). In human postnatal myoblasts, however, an early hyperpolarization of similar magnitude is due to the expression of a non-inactivating delayed rectifier K+ current and not to an inward rectifier potassium current (Bernheim, Liu, Hamann, Haenggeli, Fischer-Lougheed & Bader, 1996).
In the present study, we take advantage of several properties of human myoblast clonal cultures (Baroffio, Aubry, Kaelin, Krause, Hamann & Bader, 1993) to evaluate the precise temporal relationship between K+ current expressions, changes in membrane potential, and myoblast fusion. As clonal cultures are composed of strictly pure myogenic cells, the assessment of their resting potential is not biased by the presence of non-muscle cells. Simple changes of culture medium composition allow us either to grow and maintain human myogenic cells as ‘undifferentiated’ (proliferating) myoblasts for several weeks, or to induce fusion. Moreover, the events occurring at the onset of fusion can be investigated, using a preparation of ‘fusion-competent’ myoblasts, that is cells cultured at a low density in the fusion-promoting medium. Due to their low density plating, these cells remain mononucleated, but are expected to proceed through the differentiating steps preceding fusion. Thus, it is possible to study different myogenic cell populations at defined stages of differentiation.
Our results indicate that the setting of the resting potential of fusion-competent myoblasts involves the sequential expression of two different potassium currents. A non-inactivating delayed rectifier potassium current, IK(NI), is expressed first and hyperpolarizes fusion-competent myoblasts to an intermediate resting potential of approximately −32 mV. Then, slightly before fusion, the expression of an inward rectifier potassium current, IK(IR), causes fusion-competent myoblasts to hyperpolarize to approximately −65 mV. Our results also suggest that the expression of functional KIR channels is required for the physiological process of human myoblast fusion to take place.
METHODS
Dissociation and culture procedures
Samples of human skeletal muscle (436 ± 107 mg) were obtained during corrective orthopaedic surgery of six patients (7 months to 13 years old) without any known neuromuscular disease. Biopsies of muscles were obtained in accordance with the guidelines of the ethical committee of the University Hospital of Geneva, Switzerland (written informed consent was obtained from patients or their legal guardians).
Clonal cultures were prepared from satellite cells as described previously (Baroffio et al. 1993). Briefly, muscle biopsies were minced and incubated for 1 h in a solution containing 0.5 mg ml−1 trypsin. The suspension was centrifuged and resuspended several times in a wash medium to remove muscular debris (Ham's F-10 with 15 % fetal calf serum). Red blood cells were lysed with Tris-ammonium chloride buffer. Using a micropipette, single satellite cells were then manually collected (clonal culture) and cultured in proliferation medium (Ham's F-10 nutrient medium with 15 % fetal calf serum, 0.5 mg ml−1 bovine serum albumin, 0.5 mg ml−1 fetuin, 10 ng ml−1 epidermal growth factor, 0.39 mg ml−1 dexamethasone, 0.18 mg ml−1 insulin, and 0.1 g ml−1 gentamicin; Ham, St Clair, Blau & Webster, 1989). The satellite cells divide actively in proliferation medium and their progeny can be kept for several months. When nearly confluent, myoblasts were replated at a lower density. Myotube formation is induced by replacing the proliferation medium with differentiation medium which promotes myoblast fusion (Dulbecco's modified Eagle's medium (DMEM) with 0.5 mg ml−1 bovine serum albumin, 10 ng ml−1 epidermal growth factor, 10 g ml−1 insulin, and 1 g ml−1 gentamicin; St Clair, Meyer, Demarest & Ham, 1992). Half of the culture medium was changed 3 times a week.
Fusion-competent myoblasts that remain mononucleated were obtained by plating undifferentiated myoblasts at a very low density (10 000 cells per 35 mm culture dish) in differentiation medium for 1–3 days. Under these conditions myoblasts are in a more mature state but, as physical contact is impeded by low density plating, most myoblasts are prevented from fusing. The relationship between cell capacitance and nuclei number was studied in these myoblasts and indicated that fusion-competent myoblasts were always mononucleated when their capacitance was < 50 pF (Krause, Hamann, Bader, Liu, Baroffio & Bernheim, 1995).
Electrophysiological recordings
Whole-cell configuration of the patch-clamp technique was used to measure ionic currents (Hamill, Marty, Neher, Sakmann & Sigworth, 1981). Signals were recorded with an Axopatch 200A amplifier. The same amplifier in current-clamp mode was used to measure resting membrane potential. The pipette resistances were 2–5 MΩ (compensation between 30 and 70 % was used). Capacitance of the cells was obtained by direct reading of the whole-cell capacitance potentiometer of the Axopatch 200A amplifier. Leak current subtraction procedures are discussed in the figure legends. Currents were recorded at 20–22°C, low-pass-filtered at 1 kHz, and sampled at 5 kHz. To obtain spherical cells which were more easily patched, cells were treated with 0.05 % trypsin and replated 1–2 h before recording.
Solutions and materials
Whole-cell recording
The extracellular solution was composed of (mM): NMG-Cl, 100; KCl, 5; MgCl2, 3; Hepes, 5; NaOH, 50; acetic acid, 50; and glucose, 8. The pH was adjusted to 7.4 with NMG. The intracellular (pipette) solution was composed of (mM): KCl, 110; NaCl, 5; MgCl2, 1; Hepes, 5; BAPTA, 20; and glucose, 5. The pH was adjusted to 7.4 with KOH.
Trypsin (from bovine pancreas) was from Boehringer Mannheim. Ham's F-10, DMEM and gentamicin were from Gibco. Bovine serum albumin, dexamethasone, fetuin, insulin, tetrodotoxin and Hepes were from Sigma. Epidermal growth factor was from Collaborative Research. Fetal calf serum was from Ready System or Inotech. NaCl, KCl, MgCl2, CaCl2, BaCl2, KOH and sodium acetate were from Merck. CsCl, BAPTA and N-methyl-D-glucamine (NMG) were from Fluka.
Fusion index
The fusion index is defined as the number of nuclei in myotubes divided by the total number of nuclei counted. Cultures were fixed for 5 min at −20°C with 100 % methanol, and stained with Haematoxylin. Nuclei were counted in twenty randomly chosen microscope fields in two separate cultures. One microscope field usually contains between 100 and 150 nuclei. In Student's t tests, n refers to the number of microscope fields counted.
Fusion block
Cs+ (10 mM) was added to the differentiation medium. Half of the medium was changed every 2 days. Batches of cells were fixed at 36 h, and 3 and 5 days, and their fusion index determined.
Statistics
Results are expressed as means ±s.e.m. Significant differences between results were assumed when P < 0.05.
RESULTS
Evolution of the resting potential during the differentiation of myogenic cells
Figure 1A illustrates the resting potential of undifferentiated myoblasts, fusion-competent myoblasts and freshly formed myotubes. A marked hyperpolarization is observed as human myogenic cells differentiate. The resting potential of undifferentiated myoblasts is low (−8 ± 1 mV; n = 218) (Hamann et al. 1994), and that of freshly formed myotubes (approximately 6 nuclei) is hyperpolarized (−65 ± 1 mV; n = 71). An interesting observation illustrated in Fig. 1A is that there are two distinct cell populations of fusion-competent myoblasts with respect to resting membrane potential. The first population has a mean resting potential of −32 ± 1 mV (n = 94), a potential which lies about half-way between the resting potential of undifferentiated myoblasts (−8 ± 1 mV) and that of freshly formed myotubes (−65 ± 1 mV). The second population has a mean resting potential of −64 ± 2 mV (n = 37; filled column), a value which does not differ from that of freshly formed myotubes (P = 0.7). We will refer to this second population as hyperpolarized fusion-competent myoblasts.
Figure 1. Resting potential, non-inactivating (IK(NI)) and inward rectifier (IK(IR)) potassium current expression at various stages of differentiation of myogenic cells.
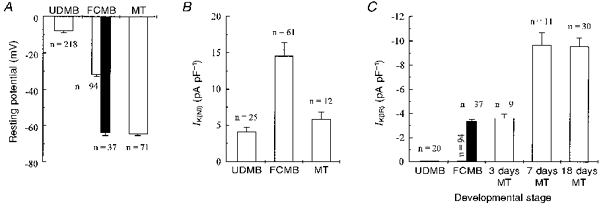
A, resting potential in undifferentiated myoblasts (UDMB), fusion-competent myoblasts (FCMB) without IK(IR) (open column) and fusion-competent myoblasts expressing IK(IR) (filled column), and myotubes (MT). B, IK(NI) density in these different types of myogenic cells. IK(NI) was evaluated at the end of a steady holding voltage at +40 mV lasting 3 min. Leak current at +40 mV was estimated by linear extrapolation from the leak current recorded at −80, −70 and −60 mV, and subtracted. Cell capacitances were: UDMB = 14 ± 2 pF; FCMB = 25 ± 1 pF; MT = 150 ± 22 pF. C, IK(IR) density in undifferentiated myoblasts (UDMB), fusion-competent myoblasts (FCMB; 37 cells out of 131 expressed IK(IR) and the density of the current in these cells was not different from that recorded in 3 day-differentiated myotubes), and myotubes (MT; 3 days, 7 days, and 18 days in differentiation medium; all cells expressed IK(IR)). IK(IR) was evaluated during a step to −140 mV from a holding potential at −60 mV. Leak current was evaluated either by linear extrapolation from the leak current recorded at −40, −50 and −60 mV or by adding 500 μM Ba2+ to the external solution, and subtracted. Cell capacitances were: UDMB = 16 ± 1 pF; FCMB without IK(IR) = 24 ± 1 pF; FCMB with IK(IR) = 26 ± 2 pF; 3 day-differentiated MT = 62 ± 7 pF; 7 day-differentiated MT = 192 ± 13 pF; 18 day-differentiated MT = 160 ± 16 pF.
The hyperpolarization from −8 to −32 mV is mainly linked to the expression of a non-inactivating delayed rectifier type potassium current, IK(NI) (Bernheim et al. 1996), which activates at voltages more depolarized than −40 mV and is therefore able to hyperpolarize the myoblasts to approximately −32 mV. IK(NI) is already detectable, but at a very low density, in undifferentiated myoblasts. Its expression increases by more than threefold in fusion-competent myoblasts, and then decreases in myotubes (Fig. 1B). Blocking IK(NI) depolarizes fusion-competent myoblasts to about the resting potential of undifferentiated myoblasts (Bernheim et al. 1996), demonstrating that this current is indeed responsible for the resting potential of fusion-competent myoblasts. The hyperpolarization from −32 mV (fusion-competent myoblasts) to −65 mV (hyperpolarized fusion-competent myoblasts and myotubes) cannot be due to IK(NI) as, at this potential, IK(NI) is not activated. We will show that this second hyperpolarization is due to the expression of an inward rectifier potassium current, IK(IR).
In Fig. 1C, it can be seen that IK(IR) is absent in undifferentiated myoblasts, as well as in the population of fusion-competent myoblasts which have a mean resting potential of −32 ± 1 mV. On the other hand, hyperpolarized fusion-competent myoblasts (filled column) and freshly formed myotubes express IK(IR). We observed that all tested myotubes expressed large IK(IR) and that the current density increased with time in culture to reach a plateau. The majority of fusion-competent myoblasts (72 %) do not express IK(IR). It should be noted, however, that in the fusion-competent myoblasts expressing IK(IR) (28 %; filled column), the KIR current density (-3.4 ± 0.8 pA pF−1; n = 37) did not differ (P = 0.9) from that of freshly formed myotubes (-3.6 ± 0.4 pA pF−1; n = 9). This may explain why the hyperpolarized fusion-competent myoblasts have the same resting potential as myotubes.
Characterization of the inward rectifier potassium current
Before assessing the precise contribution of IK(IR) to the resting potential of human myogenic cells, we confirmed that the current we named IK(IR) was indeed inwardly rectifying and mainly permeable to potassium ions, and that it could be blocked by Ba2+ and Cs+ ions, all characteristic properties of this type of current (Hille, 1992).
Figure 2 illustrates whole-cell current traces recorded in fusion-competent myoblasts. Figure 2A shows current traces recorded in a hyperpolarized fusion-competent myoblast which expressed a hyperpolarization-activated inward current, whereas Fig. 2B shows current traces recorded in a cell belonging to the larger population of fusion-competent myoblasts (72 %) which do not express such a current. The voltage protocol used in Fig. 2A and B is described in Fig. 2C (holding potential at −60 mV; voltage steps lasting 400 ms to potentials between −40 and −140 mV). Figure 2D represents the current-voltage relationship of peak currents illustrated in Fig. 2A. It can be seen that, when the myoblast is depolarized to −40 mV, there is only a small outward current, whereas a substantial inward current is present when the cell is hyperpolarized to voltages more negative than −80 mV.
Figure 2. Whole-cell properties of the inward rectifier potassium current (IK(IR)).
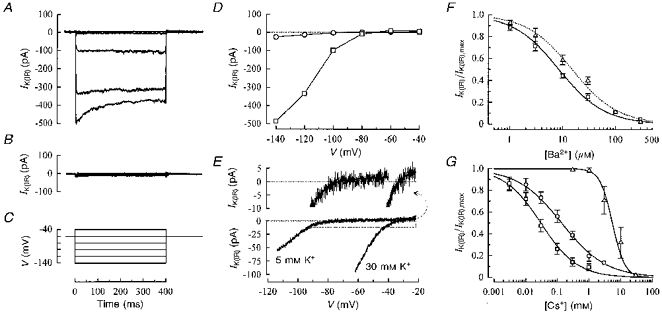
To determine the main charge carriers of the hyperpolarization-activated current, voltage-clamped ramps were applied to a fusion-competent myoblast in two different extracellular potassium concentrations (Fig. 2E). The accurate reversal potential of the hyperpolarization-activated current was obtained by fitting the current traces with a theoretical equation (enlargement shown in upper panel of Fig. 2E; see figure legend for the equation used). In 5 mM , the reversal potential of the current was −72 mV (EK = −78 mV in these recording conditions) and the current-voltage relationship showed a strong inward rectification around that value, a property which is expected for an inward rectifier potassium current (Hagiwara, Miyazaki & Rosenthal, 1976). When was raised to 30 mM (EK = −33 mV), both the reversal potential and the inward rectification were shifted to a more depolarized potential (-33 mV). Similar results were observed with freshly formed human myotubes (data not shown).
These results all indicate that the hyperpolarization-activated current of fusion-competent myoblasts is mainly carried by potassium ions and that the inward rectification of this current is linked to EK, a characteristic feature of IK(IR).
Ba2+ and Cs+ are known to block inward rectifier potassium currents. Figure 2F illustrates that increasing concentrations of Ba2+ inhibit IK(IR) in fusion-competent myoblasts (□). IK(IR) amplitude was measured during voltage steps to −140 mV in various Ba2+ concentrations and normalized to control IK(IR) amplitude. Figure 2F also shows that a similar result was observed in freshly formed myotubes (▵). Unlike the block by Ba2+, the block by Cs+ is strongly voltage dependent. Figure 2G illustrates the inhibition of IK(IR) by Cs+ in fusion-competent myoblasts at three different potentials (-140, −100 and −60 mV). All Ba2+ and Cs+ effects were reversible (data not shown).
Taken together, these results indicate that the IK(IR) we observe in hyperpolarized fusion-competent myoblasts resembles that described in other cell types. In addition, the characteristics of IK(IR) are similar in fusion-competent myoblasts and in freshly formed myotubes. It is worth mentioning that the effect of IK(IR) on the membrane potential in the voltage range between −32 mV (fusion-competent myoblasts) and −64 mV (hyperpolarized fusion-competent myoblasts), is due to the small outward current carried by IK(IR) in this voltage range (see Fig. 2D and E).
Contribution of IK(IR) to the resting potential of fusion-competent myoblasts and freshly formed myotubes
As mentioned earlier, the resting potential analysis of fusion-competent myoblasts subdivided these into two subpopulations according to their expression of IK(IR). The resting potential of fusion-competent myoblasts with IK(IR) is significantly (P < 0.01) more hyperpolarized (−64 ± 2 mV; n = 37) than that of the subpopulation without IK(IR) (−32 ± 1 mV; n = 94), and has the same value as that of freshly formed myotubes (−65 ± 1 mV; P = 0.7).
The precise contribution of IK(IR) to the resting potential of myogenic cells was further analysed in fusion-competent myoblasts expressing IK(IR) and in freshly formed myotubes, which all expressed IK(IR). Ba2+ and Cs+ were tested, as these ions inhibit IK(IR) without affecting IK(NI), the other potassium current involved in the resting potential of these cells (data not shown). Figure 3A shows that, in the presence of increasing concentrations of Ba2+, hyperpolarized fusion-competent myoblasts (main panel) and myotubes (inset) depolarize in a dose-dependent manner up to a value of −35 to −40 mV. This voltage range corresponds to the beginning of the activation zone of IK(NI). IK(NI) prevents any further depolarization by Ba2+ at the concentrations used. A similar result was obtained using increasing concentrations of Cs+ to block IK(IR) (Fig. 3B). As the Cs+ block depends upon the membrane potential (see Fig. 2G), it is not surprising that millimolar concentrations of Cs+ had to be used to depolarize the cells from −70 to near −40 mV (the Kd for Cs+ is 5.5 mM at a potential of −60 mV).
Figure 3. Contribution of IK(IR) to the resting potential of fusion-competent myoblasts and myotubes.
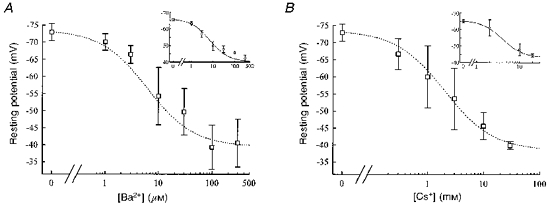
Membrane resting potential of fusion-competent myoblasts during the progressive block of IK(IR) with Ba2+ (A; n = 3 or more) and Cs+ (B; n = 3 or more). Insets: similar experiment done with myotubes (Ba2+, n = 5; Cs+, n = 4). The resting potentials of fusion-competent myoblasts and myotubes are depolarized by increasing concentrations of Ba2+ and Cs+. Dotted lines were drawn by eye.
Therefore, we conclude that the membrane potential change illustrated in Fig. 1A, from −32 mV (myoblasts which did not exhibit IK(IR)) to −65 mV (myoblasts and myotubes exhibiting IK(IR)), is due to the expression of IK(IR).
The inward rectifier potassium current is involved in the process of myoblast fusion
Our results suggest that the expression of IK(IR) is an event that precedes myoblast fusion. As IK(IR) can be suppressed by Cs+ and Ba2+, we tested the effect of these two ions on the fusion process.
In control conditions, myoblast fusion was complete within 3 days (Fig. 4A, ^). The top panel of Fig. 4B shows that large myotubes have formed in a control culture after 3 days in differentiation medium. In sister cultures treated with 10 mM Cs+, it can be seen that Cs+ drastically reduced myoblast fusion to 3 % of the control at 3 days (Fig. 4A, ▵). The bottom panel of Fig. 4B illustrates the absence of fusion in these conditions. The culture consists exclusively of mononucleated myoblasts which appear normal from a morphological point of view. To exclude a possible toxic effect of Cs+ on the cells, Cs+ was removed from the culture medium after 3 days and fusion was evaluated 2 days later. Figure 4A (▵, Wash) shows that fusion proceeded normally after Cs+ removal. If cultures were kept longer in the presence of Cs+ they maintained low fusion rates even after 5 days (Fig. 4A, □). From these observations, we conclude that the inhibition of fusion by Cs+ is not a result of a toxic effect of this ion, and that IK(IR) is essential for normal myoblast fusion to occur. The inhibition of myoblast fusion by Cs+ was observed in three separate experiments.
Figure 4. Myoblast fusion is inhibited by Cs+, and the effect is reversible.
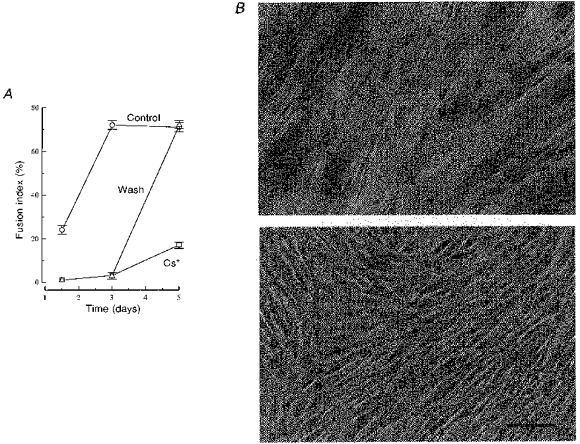
A, fusion was induced with differentiation medium and assessed after 36 h, and 3 and 5 days. ^, fusion in control conditions. In sister cultures, 10 mM Cs+ was added to the differentiation medium either during the entire experiment (□) or for a period of 3 days (▵). The upper panel in B shows Haematoxylin-stained culture of myotubes after 3 days in differentiation medium. The lower panel shows a sister culture after the same time in differentiation medium containing 10 mM Cs+ (only myoblasts are present). From a morphological point of view, the myoblasts appear perfectly normal despite the presence of 10 mM Cs+. Scale bar represents 40 μm.
A similar inhibition of fusion was obtained using Ba2+ instead of Cs+ except that the magnitude of the effect was smaller. Exposure to 30, 100 and 300 μM Ba2+ decreased fusion significantly by 5, 24 and 38 %, respectively. Saturation was reached at 300 μM, as 500 μM Ba2+ reduced myoblast fusion to the same extent. Ba2+ and Cs+ are both able to completely block IK(IR) (and depolarize the cells to similar resting potentials) and therefore a similar effect on fusion was anticipated. There are, however, major differences between the experiments testing the effect of Ba2+ on membrane potential and those testing its effect on fusion. Electrophysiological recording is performed in simple salt solutions with artificial buffers and over short time periods. On the other hand, fusion is examined over several days in a complex culture medium, and in the presence of phosphate and of a HCO3−/CO2 buffer. We attribute the diminished effect of Ba2+ on fusion, as compared with Cs+, to a lower than expected ion concentration, due to precipitation of a fraction of the total Ba2+ added, as we consistently observed white precipitates in the dishes exposed to Ba2+ for the period required to study the effect on fusion. This precipitate was never seen with Cs+. Among other possibilities, the presence of phosphate in the culture medium may have contributed to the precipitation of Ba2+, as barium phosphates are poorly soluble. Higher concentrations of Ba2+ could not be used in an attempt to eliminate compounds precipitating with Ba2+, as the cells did not survive well at concentrations above 500 μM, and the amount of precipitate increased.
To confirm the hypothesis of a reduction of Ba2+ concentration due to a precipitate, we did the following experiment. IK(IR) was recorded from small myotubes exposed for 24 h to the fusion-promoting culture medium in the absence (control) or in the presence of 100 μM Ba2+. Recording was done in the culture medium to which the cells had been exposed for 24 h. We found that IK(IR) current density was reduced by 52 % in the cells that were incubated with Ba2+, with respect to control (4.6 ± 0.4 versus 9.6 ± 1.7 pA pF−1; n = 4 in each condition). This effect was unambiguously due to a blockade of the current, as an extensive superfusion with a medium devoid of Ba2+ restored IK(IR) to a value similar to that found in control cells.
Figure 2F shows that a true free Ba2+ concentration of 100 μM should reduce IK(IR) by about 90 % (and not 52 %). Conversely, in simple salt solutions, a 52 % reduction (as observed in the culture medium) occurs for a concentration of approximately 10 μM Ba2+. Thus, this bioassay on myotubes indicates that nearly 90 % of the Ba2+ initially added to the culture medium was not in solution. This result confirms our suspicion that a fraction of Ba2+ was precipitating in the culture medium, and explains the reduced effect of Ba2+ on fusion when compared with Cs+.
Knowing that 90 % of the 100 μM Ba2+ is precipitated in the culture medium, it was of interest to compare the efficiency of fusion block by free Ba2+ with that by Cs+. On the basis of Fig. 3A and B, it can be seen that, in terms of effects on resting potential, 10 μM Ba2+ produces an effect similar to that seen with 3 mM Cs+. Our data on the fusion index at different Cs+ concentrations can be fitted with a Hill equation (Kd = 4.3 mM and Hill coefficient = 2.9). Using this relationship, we can estimate that 3 mM Cs+ would inhibit fusion by 27 %. This value is not statistically different from the 24 % inhibition seen with 100 μM Ba2+ (of which only 10 % remained free in solution). We conclude that the effect of Ba2+on fusion, like that of Cs+, confirms that interfering with the functioning of IK(IR) reduces fusion. Moreover, when the actual free Ba2+ concentration is taken into account, Ba2+ appears to block myoblast fusion as efficiently as Cs+.
DISCUSSION
The goal of this study was to examine the electrophysiological properties of human myoblasts shortly before they fuse, with three specific objectives. A first objective was to describe the changes, if any, of the membrane potential as myoblasts become fusion-competent. A second objective was to determine the mechanisms responsible for this putative change. Finally, we wanted to know whether these electrophysiological events were important for the fusion process. This study was facilitated by the availability of primary myogenic cell cultures at various differentiation stages, namely: (i) undifferentiated myoblasts, (ii) fusion-competent myoblasts that are prevented from fusing, and (iii) myotubes.
Our results suggests that two types of potassium currents underlie a hyperpolarization that precedes fusion. Firstly, a non-inactivating potassium current (IK(NI)) present in all fusion-competent myoblasts causes an initial hyperpolarization to approximately −32 mV. Subsequently, an inwardly rectifying potassium current (IK(IR)) is expressed and hyperpolarizes the membrane potential to approximately −65 mV. The expression of IK(IR) probably occurs just before fusion as it is present in only 28 % of the fusion-competent myoblasts, but is present in all freshly formed myotubes. IK(IR) has the voltage-dependent and pharmacological properties of classical inward rectifiers.
Blocking of IK(IR) both depolarizes myogenic cells and inhibits fusion, suggesting that the hyperpolarization or some other process linked to the presence of functional KIR channels is required for the fusion process to occur.
IK(IR) in human myogenic cells
The existence of a potassium conductance which allows mainly an inward flux of potassium ions (below EK) and little outward flux (above EK) was first described almost half a century ago (Katz, 1949). Since then, inwardly rectifying channels have been characterized in many mammalian cells, including muscle cells (Matsuda & Stanfield, 1989).
The hyperpolarization-activated potassium current of human fusion-competent myoblasts and myotubes has many characteristics similar to IK(IR) studied in other preparations (Hille, 1992). The reversal potential of the current is around EK and it is shifted to more depolarized values when the extracellular potassium concentration is increased. Furthermore, the voltage dependence of gating depends strongly on the extracellular potassium concentration and, as expected, follows EK. Finally, the current is blocked by extracellular Ba2+ and Cs+ ions.
IK(IR) and membrane potential
We have shown (Fig. 2E, enlargement) that, despite its strong inward rectification, IK(IR) is able to carry some outward current at voltages more depolarized than EK. In fact, we found that this small potassium current leaving the cell through KIR channels is sufficient to explain the hyperpolarization of myogenic cells. This was demonstrated by using two well-known blockers of IK(IR), Ba2+ and Cs+ (Hille, 1992). Both ions produced a concentration-dependent inhibition of IK(IR) and, associated with this inhibition, a depolarization of the myoblasts up to about −40 mV, when IK(IR) was fully blocked. Ba2+ and Cs+ did not depolarize the cells to zero potential as, at the concentrations used, these ions do not affect IK(NI), the other potassium current involved in the resting potential of these cells (Bernheim et al. 1996).
Unfortunately, we could not test the contribution of IK(IR) to the resting potential of the cells in the absence of IK(NI) as no specific blocker of IK(NI) is yet available. For the same reason, we could not test the specific role of IK(NI) in the presence of IK(IR). Thus, from these results, we conclude that IK(IR) is responsible for at least the more hyperpolarized part (-32 to −65 mV) of the resting membrane potential of hyperpolarized fusion-competent myoblasts and of myotubes.
Timing of the expression of the potassium currents in myogenic cells
Undifferentiated myoblasts are very depolarized cells. IK(NI) is detectable at very low density in these cells, but IK(IR) is not. On the other hand, all fusion-competent myoblasts express IK(NI) at a high density, and 28 % of these express IK(IR). The fusion-competent myoblasts that express IK(IR) are as hyperpolarized as myotubes. Interestingly, in contrast to the density of IK(IR), which increases and thereafter remains stable in ageing myotubes, the expression of IK(NI) is transient: the current density reaches a peak in fusion-competent myoblasts and then decreases after fusion.
Taken together, these results suggest that, when the process of myoblast fusion is initiated, IK(NI) is expressed first. This drives the membrane potential of fusion-competent myoblasts to about −32 mV, i.e. in the vicinity of the potential which activates IK(NI). Following this, IK(IR) is expressed and brings the cell to its final resting potential. We do not yet know whether all fusion-competent myoblasts must express IK(IR) before they fuse, but we favour this hypothesis for two reasons. Firstly, all freshly formed myotubes expressed IK(IR), indicating that this current must be expressed either just before or immediately after fusion. Secondly, we clearly found a subpopulation of hyperpolarized fusion-competent myoblasts expressing IK(IR).
Specificity of Cs+ on myoblast fusion and possible roles of IK(IR) that are not linked to membrane potential
We observed that Cs+ nearly completely suppresses human myoblast fusion. Ba2+ also reduced fusion with similar efficiency, but, due to precipitation in the culture medium, a sufficiently high free Ba2+ concentration could not be reached in order to achieve a full block of fusion (see Results section).
A cytotoxic effect of Cs+ (10 mM) is unlikely as cell death was not observed, even when Cs+ was applied to cultures for several days. In addition, a fully normal fusion process occurred after removal of Cs+. Moreover, an even higher concentration of Cs+ (20 mM) has been previously used on embryonic chick myoblasts, and it was found that fusion was simply delayed but thereafter proceeded normally despite the presence of Cs+ (Santini, Bonincontro, Cametti & Indovina, 1988). In human cultures, only minimal escape occurs in the continuous presence of Cs+ (see Fig. 4A, □). It is worth mentioning that in embryonic chick myoblast cultures, 20 mM Cs+ impeded modifications of the lipid composition of the plasma membrane that normally occur during fusion (Santini, Indovina, Cantafora & Blotta, 1990). This could be a consequence of the inability of the cells to rapidly generate the appropriate signals for controlling their membrane lipid composition upon IK(IR) blockade.
It is also possible that KIR channels are involved in the fusion process by mechanisms other than solely their role in regulating membrane potential. One possibility is a mechanical interaction with the cytoskeleton. The whole-cell characteristics of IK(IR) (marked inward rectification, and insensitivity to GTP or ATP: data not shown) suggest that it may belong to the Kir 2.0 family (Nichols & Lopatin, 1997), and, interestingly, two members of this subfamily have been reported to bind to PSD-95, a cytoskeletal protein (Cohen, Brenman, Snyder & Bredt, 1996). A reorganization of the cytoskeleton has to occur during fusion and such an interaction may play a role in the process. However, the interaction between the KIR channel and the cytoskeleton would have to be modulated by the activity of the channel, as blockade of the current by Cs+ or Ba2+ inhibits fusion.
Hypothesis on the functional link between IK(IR), hyperpolarization and myoblast fusion
Membrane hyperpolarization is clearly a consequence of IK(IR) expression in myogenic cells. How could hyperpolarization be linked with the fusion process? It is possible that some proteins involved in cell-cell interactions, for example, cell adhesion molecules (Mege et al. 1992) and connexins (Mege, Goudou, Giaume, Nicolet & Rieger, 1994), may require a transmembrane electrical gradient in order to be correctly oriented in the membrane to favour cell fusion.
Another possibility is that hyperpolarized myoblasts may become more sensitive to extracellular signals, which facilitate fusion. One appealing hypothesis is that IK(IR) expression and the hyperpolarization it produces could allow bursts of action potentials to be initiated in myogenic cells. Indeed, human myoblasts express voltage-dependent sodium and potassium currents, which inactivate at depolarized voltages (Hamann et al. 1994; Widmer, Hamann, Baroffio, Bijlenga & Bader, 1995). In fusion-competent myoblasts hyperpolarized by IK(IR), these currents would recover from inactivation and the cells could then fire short duration action potentials in the presence of depolarizing signals.
The presence of IK(IR) in fusion-competent myoblasts may therefore lead to an amplification of depolarizing signals acting upon them. The action potentials may, in turn, contribute to the intracellular increase in Ca2+ concentration which is essential for fusion (Shainberg, Yagil & Yaffe, 1969; Przybylski, MacBride & Kirby, 1989). For this to occur, however, a voltage-gated calcium current should be present other than the L-type current, as this current is not required for fusion of primary cultures of human myoblasts (Bernheim et al. 1996). We have detected the presence of a T-type Ca2+ current in 38 % of the fusion-competent myoblasts that express IK(IR). As T-channels have to be activated from a hyperpolarized potential to be operational, a hyperpolarization is thus a prerequisite for the involvement of T-channels in the fusion process. These T-channels will be fully described in a future publication but it is important to mention, in the present context, that their steady-state inactivation properties are consistent with a substantial removal of inactivation by the presence of IK(IR) (50 % of the maximum T-current is available at −65 mV). T-channels, besides being possibly involved in calcium entry, could themselves contribute to a bursting activity of fusion-competent myoblasts, as their current is known to promote an oscillatory behaviour in neurones (Jahnsen & Llinas, 1984).
Based on the present knowledge of ionic channel expression in human myogenic cells at the onset of fusion, we propose the following model. Through the sequential expression of IK(NI) and IK(IR), fusion-competent myoblasts hyperpolarize to a voltage range at which sodium, potassium and T-type calcium channels can all recover from inactivation. This could enable fusion-competent myoblasts to amplify depolarizing signals by producing bursts of action potentials. On the basis of previous results, we propose that one of the putative depolarizing signals could be cholinergic. Indeed, human myogenic cells synthesize and release an ACh-like compound (Hamann et al. 1995), which could depolarize fusion-competent myoblasts by acting on nicotinic receptors that are expressed in these cells at a high density (Krause et al. 1995). During bursts of action potentials, intracellular Ca2+ concentration would increase due partly to the activation of T-type calcium channels. A large synchronized release of ACh could also promote entry of Ca2+ into the cell through ACh channels (Krause et al. 1995; Constantin, Cognard & Raymond, 1996). Intracellular Ca2+ may then activate mechanisms promoting fusion and may also stimulate those bursting cells to release more signalling molecules (including ACh) into the extracellular space. These molecules could activate surrounding cells as well as the bursting cells themselves, by an autocrine mechanism. Termination of the bursting activity could occur when the intracellular Ca2+ concentration has reached a level sufficient to activate the Ca2+-dependent K+ channels present in myogenic cells (Hamann et al. 1994). The cells expressing IK(IR) could therefore constitute a ‘nucleation centre’ for fusion, promoting the differentiation of neighbouring myoblasts.
Acknowledgments
We thank M. Berti and C. Castelbou for their excellent technical assistance with cell culture and Professor Fritz Baumann for his helpful comments on the manuscript. This work was supported by the Fonds National Suisse pour la Recherche Scientifique (31–43′597.95 and 31–46′893.96), by the Fondation Suisse pour la Recherche sur les Maladies Musculaires, and by the Sir Jules Thorn Overseas Trust.
References
- Baroffio A, Aubry JP, Kaelin A, Krause RM, Hamann M, Bader CR. Purification of human muscle satellite cells by flow cytometry. Muscle and Nerve. 1993;16:498–505. doi: 10.1002/mus.880160511. [DOI] [PubMed] [Google Scholar]
- Bernheim L, Liu J-H, Hamann M, Haenggeli CA, Fischer-Lougheed J, Bader CR. Contribution of a non-inactivating potassium current to the resting potential of fusion-competent human myoblasts. The Journal of Physiology. 1996;493:129–141. doi: 10.1113/jphysiol.1996.sp021369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campion DR. The muscle satellite cell: a review. International Review of Cytology. 1984;87:225–251. doi: 10.1016/s0074-7696(08)62444-4. [DOI] [PubMed] [Google Scholar]
- Cohen NA, Brenman JE, Snyder SH, Bredt DS. Binding of the inward rectifier K+ channel Kir 2.3 to PSD-95 is regulated by protein kinase A phosphorylation. Neuron. 1996;17:759–767. doi: 10.1016/s0896-6273(00)80207-x. [DOI] [PubMed] [Google Scholar]
- Constantin B, Cognard C, Raymond G. Myoblast fusion requires cytosolic calcium elevation but not activation of voltage-dependent calcium channels. Cell Calcium. 1996;19:365–374. doi: 10.1016/s0143-4160(96)90109-8. [DOI] [PubMed] [Google Scholar]
- Entwistle A, Zalin RJ, Bevan S, Warner AE. The control of chick myoblast fusion by ion channels operated by prostaglandins and acetylcholine. Journal of Cell Biology. 1988;106:1693–1702. doi: 10.1083/jcb.106.5.1693. 10.1083/jcb.106.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach GD, Nameroff M, Nelson PG. Electrical properties of chick skeletal muscle fibers developing in cell culture. Journal of Cellular Physiology. 1971;78:289–299. doi: 10.1002/jcp.1040780218. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Miyazaki S, Rosenthal NP. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. Journal of General Physiology. 1976;67:621–638. doi: 10.1085/jgp.67.6.621. 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham RG, St Clair JA, Blau HM, Webster C. Serum-free media for growth and differentiation of human muscle satellite cells. In: Kedes LE, Stockdale FE, editors. Cellular and Molecular Biology of Muscle Development. Liss, New York: 1989. pp. 905–914. [Google Scholar]
- Hamann M, Chamoin MC, Portalier P, Bernheim L, Baroffio A, Widmer H, Bader CR, Ternaux JP. Synthesis and release of an acetylcholine-like compound by human myoblasts and myotubes. The Journal of Physiology. 1995;489:791–803. doi: 10.1113/jphysiol.1995.sp021092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M, Widmer H, Baroffio A, Aubry JP, Krause RM, Kaelin A, Bader CR. Sodium and potassium currents in freshly isolated and in proliferating human muscle satellite cells. The Journal of Physiology. 1994;475:305–317. doi: 10.1113/jphysiol.1994.sp020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer Associates; 1992. [Google Scholar]
- Jahnsen H, Llinas R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. The Journal of Physiology. 1984;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. Les constantes électriques de la membrane du muscle. Archives des Sciences Physiologiques. 1949;2:285–299. [Google Scholar]
- Krause RM, Hamann M, Bader CR, Liu JH, Baroffio A, Bernheim L. Activation of nicotinic acetylcholine receptors increases the rate of fusion of cultured human myoblasts. The Journal of Physiology. 1995;489:779–790. doi: 10.1113/jphysiol.1995.sp021091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Stanfield PR. Single inwardly rectifying potassium channels in cultured muscle cells from rat and mouse. The Journal of Physiology. 1989;414:111–124. doi: 10.1113/jphysiol.1989.sp017679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A. Satellite cells of skeletal muscle fibers. Journal of Biophysical and Biochemical Cytology. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mege RM, Goudou D, Diaz C, Nicolet M, Garcia L, Geraud G, Rieger F. N-cadherin and N-CAM in myoblast fusion: compared localisation and effect of blockade by peptides and antibodies. Journal of Cell Science. 1992;103:897–906. doi: 10.1242/jcs.103.4.897. [DOI] [PubMed] [Google Scholar]
- Mege RM, Goudou D, Giaume C, Nicolet M, Rieger F. Is intercellular communication via gap junctions required for myoblast fusion? Cell Adhesion and Communication. 1994;2:329–343. doi: 10.3109/15419069409014208. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annual Review of Physiology. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- Przybylski RJ, MacBride RG, Kirby AC. Calcium regulation of skeletal myogenesis. I. Cell content critical to myotube formation. In Vitro Cellular and Developmental Biology. 1989;25:830–838. doi: 10.1007/BF02623667. [DOI] [PubMed] [Google Scholar]
- Ritchie AK, Fambrough DM. Electrophysiological properties of the membrane and acetylcholine receptor in developing rat and chick myotubes. Journal of General Physiology. 1975;66:327–355. doi: 10.1085/jgp.66.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair JA, Meyer S, Demarest SD, Ham RG. Improved medium with EGF and BSA for differentiated human skeletal muscle cells. Muscle and Nerve. 1992;15:774–779. doi: 10.1002/mus.880150705. [DOI] [PubMed] [Google Scholar]
- Santini MT, Bonincontro A, Cametti C, Indovina PL. Cesium ions delay membrane fusion of chick embryo myoblasts in vitro: a conductivity study. Biochimica et Biophysica Acta. 1988;945:56–64. doi: 10.1016/0005-2736(88)90362-8. [DOI] [PubMed] [Google Scholar]
- Santini MT, Indovina PL, Cantafora A, Blotta I. The cesium-induced delay in myoblast membrane fusion is accompanied by changes in isolated membrane lipids. Biochimica et Biophysica Acta. 1990;1023:298–304. doi: 10.1016/0005-2736(90)90426-o. [DOI] [PubMed] [Google Scholar]
- Shainberg A, Yagil G, Yaffe D. Control of myogenesis in vitro by Ca2+ concentration in nutritional medium. Experimental Cell Research. 1969;58:163–167. doi: 10.1016/0014-4827(69)90127-x. [DOI] [PubMed] [Google Scholar]
- Shin KS, Park JY, Kwon H, Chung CH, Kang MS. A possible role of inwardly rectifying K+ channels in chick myoblast differentiation. American Journal of Physiology. 1997;272:C894–900. doi: 10.1152/ajpcell.1997.272.3.C894. [DOI] [PubMed] [Google Scholar]
- Spector I, Prives JM. Development of electrophysiological and biochemical membrane properties during differentiation of embryonic skeletal muscle in culture. Proceedings of the National Academy of Sciences of the USA. 1977;74:5166–5170. doi: 10.1073/pnas.74.11.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer H, Hamann M, Baroffio A, Bijlenga P, Bader CR. Voltage-dependent potassium current precedes fusion of human muscle satellite cells (myoblasts) Journal of Cellular Physiology. 1995;162:52–63. doi: 10.1002/jcp.1041620108. [DOI] [PubMed] [Google Scholar]


