Abstract
Patch-clamp recordings were made from rat cerebellar granule cells in primary culture. In cells pre-exposed to concanavalin A (ConA) to remove kainate receptor desensitization, concentration-response data for kainate showed two components. The EC50 value for the high-affinity component (4 μM) was consistent with activation of kainate-type channels. ConA enhanced the apparent potency of the kainate receptor ligand SYM 2081 by 100-fold.
In ConA-treated granule cells, currents evoked by 10 μM kainate were not significantly reduced by the AMPA receptor antagonist GYKI 53655, nor were these currents significantly reduced by the co-application of 100 μM AMPA. Currents activated by low concentrations of kainate in the presence of AMPA were completely inhibited by 10 μM La3+.
Single-cell reverse transcriptase-polymerase chain reaction (RT-PCR) analysis indicated that granule cells express both unedited (Q) and edited (R) versions of GluR5, with the majority of the GluR5 transcripts being unedited. In contrast, GluR6(R) was detected in seven cells and GluR6(Q) was detected in one granule cell.
Whole-cell current-voltage curves for kainate-type currents in granule cells were measured and the ratio of the slope conductances at +40 mV and −40 mV was used as an index of rectification. The mean +40 mV/-40 mV ratio determined from thirty-six granule cells was 1.3 ± 0.1. Spectral density analysis of kainate-evoked whole-cell current noise gave values for the apparent single-channel conductance, γnoise, that were on average about 1 pS.
To compare further the properties of recombinant kainate channels with the native kainate-type channels in granule cells, we determined EC50 and γnoise values for SYM 2081 in stable cell lines expressing either GluR6(R) or GluR6(R) and KA2. Co-expression of KA2 with GluR6(R) shifts the EC50 and γnoise values determined for SYM 2081 closer to the values typically found for native kainate-type channels in granule cells.
The results demonstrate that cerebellar granule cells in culture express functional kainate-type channels and that in most cells these channels show properties that are similar to those determined for heteromeric channels formed from GluR6(R) and KA2. However, the results also suggest that different granule cells express different repertoires of kainate-type channels with different, and perhaps variable, subunit composition.
The ionotropic glutamate receptors (GluRs) can be divided into three subtypes based on their selectivity for agonists: N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and kainate. Considerable evidence indicates that GluRs are oligomeric assemblies of individual subunit proteins, and five different gene products have been identified which are known to form kainate-type channels (GluR5, GluR6, GluR7, KA1 and KA2; Hollmann & Heinemann, 1994; Bettler & Mülle, 1995). Some kainate receptor subunits can form homomeric channels (GluR5 and GluR6), whereas others (KA1 and KA2) do not appear to form functional homomeric channels, but their coassembly with either GluR5 or GluR6 results in channels with unique properties (Herb, Burnashev, Werner, Sakmann, Wisden & Seeburg, 1992; Howe, 1996; Swanson, Feldmeyer, Kaneda & Cull-Candy, 1996). Additional functional diversity of kainate-type channels can result from RNA editing (Sommer, Köhler, Sprengel & Seeburg, 1991; Köhler, Burnashev, Sakmann & Seeburg, 1993; Swanson et al. 1996).
In comparison to NMDA- and AMPA-type channels, relatively little is known about the properties and role of kainate-type channels in central neurons, despite the widespread expression of kainate receptor subunits in brain (Wisden & Seeburg, 1993; Bahn, Volk & Wisden, 1994). Native kainate-type channels were first identified in dorsal root ganglion neurons (Huettner, 1990), and subsequent studies have characterized the properties of kainate-type channels in cultured hippocampal neurons (Lerma, Paternain, Naranjo & Mellström, 1993; Paternain, Morales & Lerma, 1995; Wilding & Huettner, 1997). Recent evidence supports the involvement of kainate-type channels in synaptic transmission in the hippocampus (Castillo, Malenka & Nicoll, 1997; Clarke et al. 1997; Rodriguez-Moreno, Herreras & Lerma, 1997; Vignes & Collingridge, 1997). Kainate-type channels have also been identified in rat trigeminal ganglion neurons, and the biophysical characteristics of these channels, along with reverse transcriptase-polymerase chain reaction (RT-PCR) studies, suggest that they may be heteromeric assemblies containing KA2 and the edited (R) version of GluR5 (Sahara, Noro, Iida, Soma & Nakamura, 1997).
The properties of GluR channels in cultured cerebellar granule cell neurons have been the focus of extensive investigation (Cull-Candy, Howe & Ogden, 1988; Howe, Cull-Candy & Colquhoun, 1991; Wyllie, Traynelis & Cull-Candy, 1993). In situ hybridization studies have shown that granule cells express mRNAs encoding the kainate-type subunits GluR5, GluR6 and KA2 (Bettler et al. 1990; Egebjerg, Bettler, Hermans-Borgmeyer & Heinemann, 1991; Herb et al. 1992; Bahn et al. 1994), suggesting that granule cells express kainate-type channels. However, the EC50 values determined previously for kainate in granule cells (and outside-out patches from these neurons) are more consistent with kainate activation of AMPA-type channels (Traynelis & Cull-Candy, 1991; Wyllie et al. 1993). Because many kainate-type channels are known to exhibit rapid and complete desensitization (Herb et al. 1992; Lerma et al. 1993), kainate-type responses may have been missed in earlier studies, where the agonist applications were relatively slow and kainate receptor desensitization was intact.
In the present study, we have isolated and characterized kainate-type channels in cerebellar granule cells by removing kainate receptor desensitization with concanavalin A (ConA) and applying kainate, or the kainate receptor ligand SYM 2081 (Zhou et al. 1997), in the presence of AMPA or the AMPA receptor antagonist GYKI 53655. Our results indicate that granule cells express channels with a high affinity for kainate which show properties consistent with the known properties of channels formed from the kainate-type subunits GluR5, GluR6 and KA2.
METHODS
Cerebellar cultures
Rat pups (aged 5–15 days) were anaesthetized with ether and decapitated, and primary cultures of cerebellar granule cells were prepared as described previously (Cull-Candy et al. 1988). Cells were dissociated without enzymes and plated onto acid-washed coverslips coated with poly-L-lysine (100 μg ml−1 for 1 h). The resulting cultures were kept in Dulbecco's modified Eagle's medium containing 25 μM KCl and 10% fetal bovine serum.
Patch-clamp recording
Whole-cell patch-clamp recordings were made from granule cells maintained in culture for 2–6 days. Granule cells were identified by their small size and capacitance (2–5 pF). Patch pipettes had initial resistances of 8–15 MΩ. Currents were recorded with an EPC 9 amplifier (HEKA, Lambrecht, Germany) controlled with software (Pulse, HEKA) run on a Macintosh Quadra 800 computer. Series resistance compensation was not employed. The series resistance (R) and the cell capacitance (C) were determined electronically by subtracting the capacitive currents at the onset and offset of a 10 mV voltage step. The cancellation of the whole-cell transients was virtually complete in all cases. Analog signals were low-pass filtered at 10 kHz (Bessel-type, −3 dB) and were stored on videotape with a VR10b digital data recorder (Instrutech, Great Neck, NY, USA) and a VCR at a sampling rate of 94 kHz.
The pipette solution contained (mM): CsCl, 150; CaCl2, 0.5; Na2EGTA, 5; and K-Hepes, 10. Spermine (100 μM, Sigma) was included in the pipette solution in most experiments. The extracellular solution contained (mM): NaCl, 150; KCl, 2.5; CaCl2, 1; and Na-Hepes, 10. The pH of the pipette and extracellular solutions was adjusted to 7.2 with dilute HCl. dl-2-Amino-5-phosphonovaleric acid (dl-APV, 100 μM; RBI) was included in the extracellular solution to block NMDA receptors. Stock solutions of kainate (Sigma), AMPA (RBI), dl-APV, SYM 2081 ((2S,4R)-4-methylglutamate; Symphony Pharmaceuticals), and GYKI 53655 (1(4-aminophenyl)-3-methylcarbamyl-4-methyl-7,8-methylenedioxy-3,4-dihydro-5H-2,3-benzodiazepine; Eli Lilly) were prepared and stored at −20°C. Frozen aliquots of the stock solutions were diluted to final concentration in extracellular solution on the day they were used. Agonists and antagonists were applied by local superfusion. Unless otherwise noted, coverslips of cells were pre-exposed to 10–25 μM ConA (Sigma) for 20 min to remove desensitization of kainate channels. All recordings were performed at room temperature (20–22°C).
Single-cell RT-PCR and analysis of GluR5 and GluR6 editing
Whole-cell patch-clamp recordings were established on granule cells in short-term primary culture and the cytoplasm was extracted from the cell by applying suction to the back of the recording pipette. The extracted cytoplasm was expelled into a siliconized 0.5 ml thin-walled microcentrifuge tube and the tubes were quickly immersed in a dry ice-ethanol bath. First-strand cDNA was synthesized from the cytoplasmic contents of single cells (in 6–7 μl of pipette solution) with Superscript II reverse transcriptase (Life Technologies) using oligo(dT) and random hexamer primers (10 pmol) according to manufacturer's recommendations. The entire volume of the single-cell reverse transcription reactions was used for PCR amplification, consisting of one cycle of 94°C for 2 min; thirty cycles of: 94°C for 30 s, 58°C or 60°C for 30 s, 72°C for 30 s; and one cycle of 72°C for 10 min. The reaction volume was 50 μl and contained 10 pmol of each primer pair. The primers used for PCR amplification of GluR5 and GluR6 are given in Table 1 of Belcher & Howe (1997). Controls included mock cDNA synthesis (no reverse transcriptase), samples treated with RNase A, and mock RT-PCR reactions in which template was excluded. For all samples analysed, no detectable product was generated from any of the negative control reactions.
The extent of RNA editing at the Q/R site of GluR5 and GluR6 was determined using a protocol based on differential digestion of RT-PCR products with the restriction endonuclease BbvI (Belcher & Howe, 1997). The GluR5 and GluR6 RT-PCR products amplified from single granule cells (10 μl of a 50 μl reaction) were digested for 4–6 h at 37°C with 4–6 U of BbvI (New England Biolabs, Beverly, MA, USA). The digestion products were fractionated on polyacrylamide gels and the molar ratios of edited and unedited products were determined by densitometric analysis. To determine the extent of editing at the two GluR6 sites in the first transmembrane segment (TMI), as well as the Q/R site, the entire population of GluR6 RT-PCR products generated from single granule cells was directly sequenced with 5′-end-labelled primers. The proportions of unedited and edited transcripts were then estimated by comparing the signal intensities of the ddA and ddG lanes on the sequencing gel.
To assess the sensitivity of comparisons based on the intensity of sequencing terminations, known amounts of the cDNAs encoding the Q and R versions of GluR6 were mixed at ratios of 1:10, 1:1 and 10:1. Aliquots of the samples containing 1 ng of DNA were amplified by PCR and analysed by DNA sequencing. Not only were ddA and ddG terminations detected in each sample, but densitometric estimates of the percentages of Q- and R-encoding products agreed within 5% of the known percentages for each dilution.
Immunoprecipitation analysis
To compare further the properties of the native kainate-type channels in granule cells with those of recombinant kainate-type channels, experiments were also performed on HEK 293 cell lines that stably express either GluR6(R) or GluR6(R) and KA2 in a tetracycline-responsive manner (Howe, Skyrabin, Belcher, Zerillo & Schmauss, 1995; Howe, 1996). Immunoprecipitation analysis was performed to characterize the heteromeric interactions between GluR6 and KA2 in the GluR6(R)-KA2-expressing cells. For this analysis, the cells were grown in Eagle's minimal essential medium with Earle's salts in the presence of G418 (0.5 mg ml−1; Life Technologies), hygromycin B (200 U ml−1; Calbiochem) and tetracycline (2 μg ml−1; Sigma). The expression of GluR6 and KA2 was induced by removing tetracycline from the media. Plates of 70–80% confluent cells were harvested and GluR subunit proteins solubilized as described previously (Ripellino, Neve & Howe, 1998). The extraction conditions used result in the solubilization of 85–90% of the GluR6 and KA2 proteins. Detergent extracts (100–150 μg protein) were precleared by incubating with immobilized protein A (Repligen, Needham, MA, USA) for 30 min at 4°C, separated by centrifugation, and GluR subunit-specific antibodies were added to the supernatants. Polyclonal anti-GluR6/7 and anti-KA2 antisera were purchased from Upstate Biotechnologies Inc. (Lake Placid, NY, USA) and used at a concentration of 2 μg ml−1, which was sufficient to immunoprecipitate all of the specific antigen. Immunoprecipitations, Western blots, and densitometric comparisons were performed as described in Ripellino et al. (1998).
Analysis of patch-clamp results
For the analysis of agonist-evoked currents, signals were replayed from videotape and digitized at the original sampling rate of 94 kHz. The record was low-pass filtered with a digital Gaussian filter at 2 kHz (-3 dB) and ‘compressed’ by a factor of 10 to generate a new file where the sampling rate was 9.4 kHz. Concentration-response data were generated by measuring the steady-state amplitude of agonist-evoked currents (from the digitized records) at a membrane potential, Vm, of −80 mV. The concentration-response data were fitted with Hill-type equations:
| (1) |
where I is the steady-state current at a given concentration of agonist, [A]; and Imax, EC50, and nH are parameters to be estimated from the fit. A formally equivalent equation was used to estimate IC50 values for GYKI 53655, where the current values at each inhibitor concentration were normalized to the control amplitude. In some cases, agonist concentration-response data were fitted with equations consisting of the sum of two Hill-type components.
Current-voltage (I-V) relationships for agonist-evoked currents were obtained by performing 500 ms voltage ramps (-80 mV to +80 mV) during steady-state currents (responses to four ramps were averaged for each curve). The I-V curves for kainate-type currents measured in the presence of AMPA were obtained by subtracting the I-V curve during application of 100 μM AMPA from the corresponding curve during the concurrent application of AMPA and kainate or SYM 2081. Difference currents were sampled at 1 kHz. The small size of the kainate-evoked currents in most granule cells meant that errors as small as 2–3 pA in the subtraction procedure used to obtain the difference currents could result in significant errors in reversal potential, Vrev, determinations. These errors in turn could lead to significant errors in estimates of channel rectification from measurements of the ratio of the current amplitudes. Rather than adjust the I-V curves such that Vrev was zero (Köhler et al. 1993), the I-V curves were fitted with fourth-order polynomial functions and these fits used to generate slope conductance vs. potential (G-V) relationships for each cell. The slope conductances do not depend on Vrev, and the ratio of the slope conductances at +40 and −40 mV (+40 mV/-40 mV ratio) was calculated for each cell and used as an index of rectification. To minimize unequal weighting of the data due to the increased current variance at large driving force, the standard deviation (σ) was calculated for contiguous ten point sections and the squared deviation for each data point was weighted during the least-squares minimization by its appropriate 1/σ value. The first derivative of the polynomial fit was evaluated at +40 mV and −40 mV to calculate the +40 mV/-40 mV rectification ratio.
The net one-sided spectral density, G(f), for agonist-evoked current noise was obtained as described in detail in Howe (1996). For kainate-type currents evoked in the presence of AMPA, the background spectrum was taken as the spectrum during application of 100 μM AMPA. Net spectra were fitted with one Lorentzian, or the sum of two Lorentzian components, and the current variance was calculated from the fit as:
| (2) |
where G(0)i and fci are the zero-frequency asymptote and half-power frequency of the ith component, respectively. The apparent single-channel current was taken to be: inoise= VarI/I, where I is the mean steady-state agonist-evoked current at Vm= −80 mV; and the apparent single-channel conductance, γnoise= i/(Vm - Vrev), was calculated assuming the reversal potential, Vrev, was 0 mV. The results of noise analysis of whole-cell currents recorded when τ= RC was greater than 200 μs were discarded to minimize distortion of the results by RC filtering.
Mean (±s.e.m.) values are given for the various parameters determined unless indicated otherwise.
RESULTS
Pretreatment with ConA reveals a high-affinity kainate current
The whole-cell patch-clamp technique was used to determine if kainate-type channels are expressed in rat cerebellar granule cells in primary culture. All the results reported here were obtained in the presence of the competitive NMDA-receptor antagonist dl-APV (100 μM) to prevent activation of NMDA receptors. While kainate can also activate AMPA-type channels, pre-incubation of cells with the lectin concanavalin A (ConA) facilitates the detection of kainate-type currents by selectively reducing fast desensitization of these channels (see, for example: Huettner, 1990; Partin, Patneau, Winters, Mayer & Buonanno, 1993; Wong & Mayer, 1993). We therefore compared the potency of kainate in granule cells that were or were not exposed to ConA.
In the absence of exposure to ConA, applications of 10 μM kainate rarely gave measurable whole-cell currents in granule cells, and responses were not seen at kainate concentrations below 10 μM (Fig. 1A). The currents evoked by higher concentrations of kainate showed little, if any, desensitization, and concentration-response data were obtained by measuring the steady-state currents evoked at −80 mV. Figure 1Ab shows a typical concentration- response curve obtained from a granule cell that was not exposed to ConA. The Hill-type fit to the results gave an EC50 value of 46 μM. Similar measurements from six granule cells gave mean values for the EC50 and Hill coefficient (nH) of 55 ± 5 μM and 1.7 ± 0.2, respectively.
Figure 1. Pretreatment of cerebellar granule cells with ConA reveals high-affinity kainate-type currents.
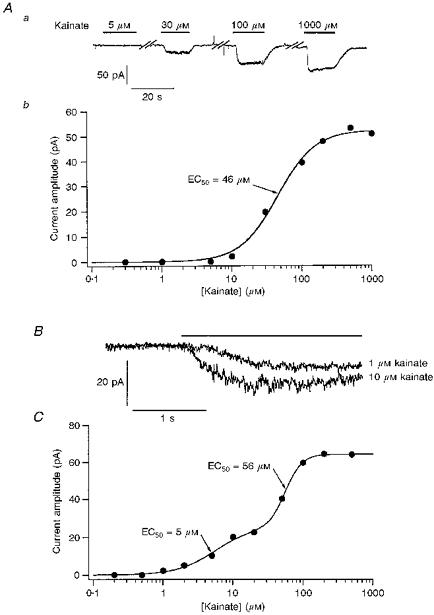
Aa, whole-cell currents evoked at −80 mV by increasing concentrations of kainate in a cerebellar granule cell not exposed to ConA. Note the lack of a response to 5 μM kainate. Breaks in the record are indicated by the diagonal bars. Ab, concentration-response curve for steady-state currents evoked by kainate from the same granule cell as in Aa. The Hill-type fit to the data gave an EC50 of 46 μM. B, whole-cell currents evoked at −80 mV by 1 and 10 μM kainate in a granule cell pre-exposed to ConA. Kainate applications are denoted by the bar above the traces. C, concentration-response curve for kainate in a granule cell that was pretreated with ConA (different cell from that in B). The smooth line shows the two-component Hill-type fit to the results which gave EC50 values of 5 and 56 μM for the high- and low-affinity components, respectively.
Whereas 10 μM kainate rarely evoked whole-cell currents in untreated cells, concentrations of kainate of 10 μM or less evoked measurable currents in the majority of granule cells (78 out of 105) in cultures pre-exposed to ConA (10–25 μM for 20 min). The whole-cell currents evoked in a ConA-treated cell by 1 and 10 μM kainate are shown in Fig. 1B. The whole-cell currents evoked at −80 mV by 10 μM kainate in ConA-treated cells ranged from 4 to 85 pA. Inspection of frequency histograms of the current amplitudes indicated that the values were log-normally distributed. A Gaussian fit to the log-amplitude results gave a mean whole-cell current value of 14 pA (anti-log(mean ±s.d.): 5–47 pA, n = 59). The capacitance values measured for the cells were typically very similar (2–3 pF) and expressing the data as current densities (pA pF−1) did not substantially reduce cell-to-cell variability nor alter the log-normal nature of the distribution.
To better assess the effect of ConA treatment on the apparent affinity of kainate for GluR channels in granule cells, concentration-response data were obtained over a wide range of kainate concentrations. Figure 1C shows the results obtained from a ConA-treated granule cell with kainate concentrations ranging from 0.2 to 500 μM. The results were fitted with the sum of two Hill-type components with respective EC50 values of 5 and 56 μM. Two similar components were detected in each of six cells tested (10–12 concentrations per cell; 0.1 or 0.2 μM to 0.5 or 1 mM kainate). The mean EC50 and nH values were 3.9 ± 0.7 μM and 1.8 ± 0.4, respectively, for the high-affinity component, and 120 ± 30 μM and 2.3 ± 0.4, respectively, for the low-affinity component.
Studies with the AMPA receptor antagonist GYKI 53655
Currents through AMPA-type channels are selectively reduced by 2,3-benzodiazepines (Donevan & Rogawski, 1993; Zorumski, Yamada, Price & Olney, 1993), and GYKI 53655 is one of the most selective of such non-competitive AMPA receptor antagonists currently available (Paternain et al. 1995; Wilding & Huettner, 1995; Bleakman et al. 1996). If the kainate-evoked currents in granule cells not exposed to ConA result primarily from non-desensitizing activation of AMPA-type channels, then these currents should be inhibited by GYKI 53655. Figure 2A shows whole-cell currents evoked in a granule cell (not exposed to ConA) with 200 μM kainate in the absence and presence of GYKI 53655. Inhibition of the currents is apparent with 1 μM GYKI 53655 and is virtually complete at a GYKI 53655 concentration of 100 μM. Kainate and GYKI 53655 were applied simultaneously and the fade in the currents reflects the slow kinetics of 2,3-benzodiazepine inhibition (Donevan & Rogawski, 1993). The concentration- response curve for the steady-state inhibition produced by GYKI 53655 in the same granule cell is shown in Fig. 2B. The fit to the results gave an IC50 value of 8.2 μM, which was close to the mean value obtained (8.1 ± 2.3 μM, n = 5 cells).
Figure 2. Kainate-evoked currents in granule cells not pre-exposed to ConA are blocked by the AMPA-receptor antagonist GYKI 53655.
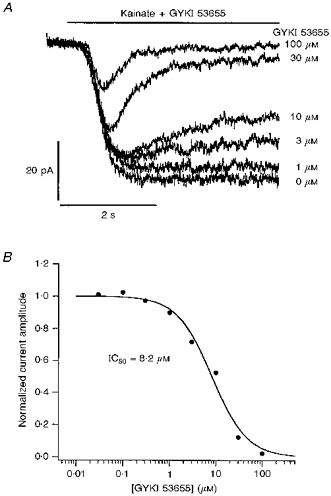
A, whole-cell currents evoked by 200 μM kainate in a granule cell held at −80 mV in the presence of increasing concentrations of GYKI 53655. The cell was not pretreated with ConA. Portions of the continuous record are displayed so that the onsets of the applications (bar above the traces) are aligned. Kainate and GYKI 53655 were applied simultaneously; the time-dependent fade of the currents reflects the slow kinetics of the GYKI 53655 inhibition. Concentrations of GYKI 53655 are shown to the right of each trace. B, concentration-response curve for GYKI 53655 inhibition of steady-state currents evoked by 200 μM kainate (same cell as in A). The fit to the results (smooth curve) gave an IC50 of 8.2 μM.
Whereas 100 μM GYKI 53655 produced near-maximal inhibition of kainate-evoked currents in cells not pre-exposed to ConA, this concentration of the AMPA receptor blocker had little effect on currents evoked by 10 μM kainate in ConA-treated granule cells (Fig. 3A), and the mean percentage change in the amplitude of these currents was not significantly different from zero (-12 ± 6%, n = 9 cells). In four of the cells, the currents evoked by 100 μM kainate were also measured in the absence and presence of 100 μM GYKI 53655. The results from these cells are shown in Fig. 3B. While the co-application of GYKI 53655 had little effect on the currents evoked by 10 μM kainate, the currents evoked by 100 μM kainate were reduced significantly (72 ± 3%). Additionally, the currents evoked by 100 μM kainate in the presence of GYKI 53655 were similar in size to the currents evoked in the same cells by 10 μM kainate (with or without the antagonist), suggesting that the residual currents seen in the presence of 100 μM GYKI 53655 were due to activation of kainate-type channels.
Figure 3. High-affinity kainate-type currents in ConA-treated granule cells are resistant to block by GYKI 53655.
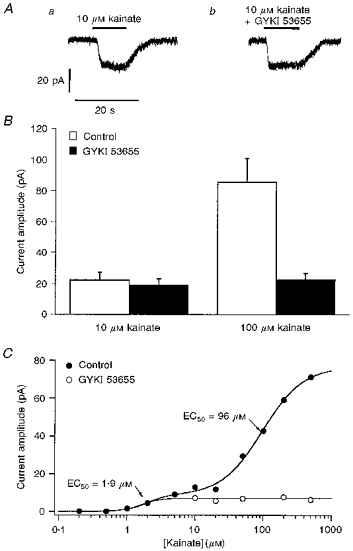
A, whole-cell currents evoked at −80 mV by 10 μM kainate in the absence (a) and presence (b) of 100 μM GYKI 53655 in a ConA-treated granule cell. B, bar-graph showing the mean steady-state amplitude of whole-cell currents evoked in 4 ConA-treated granule cells by 10 μM kainate (left) and 100 μM kainate (right) in both the absence (control, □) and presence of 100 μM GYKI 53655 (▪). Error bars indicate s.e.m. The currents elicited by 10 μM kainate were little affected by the non-competitive AMPA receptor antagonist, whereas the currents evoked by 100 μM kainate were reduced in size to amplitudes similar to those seen with 10 μM kainate. C, kainate concentration-response data from a granule cell pretreated with ConA in the absence (control, •) and presence (^) of 100 μM GYKI 53655. The two-component fit to the results obtained in the absence of GYKI 53655 gave EC50 values of 1.9 and 96 μM. GYKI 53655 blocks the low-affinity, but not the high-affinity, component.
Concentration-response data obtained from one cell in the absence and presence of 100 μM GYKI 53655 are shown in Fig. 3C. The results in the absence of the antagonist are fitted well with the sum of two Hill-type components with respective EC50 values of 1.9 and 96 μM. The lower-affinity component is absent in the presence of 100 μM GYKI 53655, and the data can be fitted by a single Hill-type component with an EC50 of 1.6 μM. Similar results were obtained from five ConA-treated granule cells; the single-component fits to the results obtained in the presence of 100 μM GYKI 53655 gave a mean EC50 value for kainate of 2.4 ± 0.8 μM (nH= 1.8 ± 0.4).
AMPA does not inhibit the high-affinity kainate currents
If, as the above results suggest, the currents evoked in ConA-treated granule cells by kainate concentrations of 10 μM and below are primarily the result of activation of kainate-type channels, then concentrations of AMPA sufficient to occupy the majority of AMPA-type channels in the cells should have little effect on these currents. Applications of 100 μM AMPA produced measurable currents in seventy-six out of eight-six granule cells examined. Pooled concentration-response data for AMPA from eleven cells gave an EC50 of 12 μM. The steady-state amplitude of currents evoked by 100 μM AMPA was typically smaller (≤ 50%) than the AMPA receptor component of kainate-evoked currents in the same cells. This result suggests that 100 μM AMPA produced substantial steady-state desensitization of AMPA-type channels, although the speed of our solution changes was insufficient to detect it. Thus, 100 μM AMPA would be expected to interfere with kainate activation of AMPA-type channels both by competing for occupancy of the receptors and by promoting receptor desensitization (Lerma et al. 1993).
In ConA-treated granule cells that gave responses to 10 μM kainate alone, twenty-three out of twenty-eight cells also showed responses to 10 μM kainate when kainate was co-applied with 100 μM AMPA (Fig. 4A). In each of these cells, the amplitude of the steady-state currents evoked by 10 μM kainate was little affected by the presence of AMPA. The ratio of the steady-state currents evoked in the same cell by 10 μM kainate alone and by 10 μM kainate during the concurrent application of 100 μM AMPA was 1.0 ± 0.1 (n = 23). However, the whole-cell currents evoked by 100 μM kainate were reduced significantly in the presence of 100 μM AMPA (mean reduction, 56 ± 5%). As was the case for the results obtained with 100 μM GYKI 53655, the amplitude of the residual current evoked by 100 μM kainate was similar to the amplitude of the currents evoked in the same cell by 10 μM kainate.
Figure 4. Kainate-type currents in ConA-treated granule cells are insensitive to the concurrent application of AMPA and GYKI 53655.
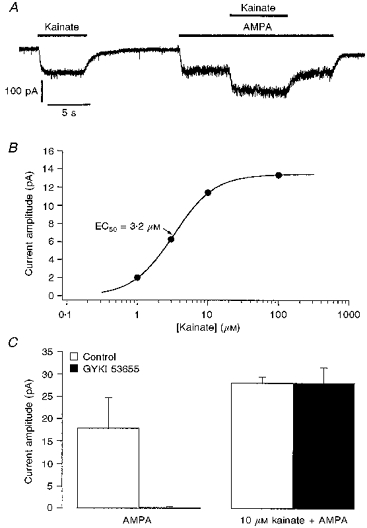
A, whole-cell currents evoked at −80 mV by 10 μM kainate alone, and by 10 μM kainate in the presence of 100 μM AMPA, in a granule cell pretreated with ConA. Agonist applications are indicated by bars above the record. B, concentration-response curve for currents evoked by kainate in the presence of 100 μM AMPA in a ConA-treated granule cell (different cell from that in A). The Hill-type fit to the data gave an EC50 value of 3.2 μM. C, bar-graph showing the mean amplitude of currents evoked by 100 μM AMPA (left) and by 10 μM kainate during the concurrent application of 100 μM AMPA (right). The respective currents were measured in each of 5 granule cells in the absence (control, □) and presence of 100 μM GYKI 53655 (▪). Applications of GYKI 53655 that virtually abolished currents evoked by AMPA had no effect on the currents evoked by 10 μM kainate. Error bars indicate the s.e.m.
The kainate EC50 values obtained in the presence of 100 μM AMPA were similar to those obtained for the high-affinity component detected in two-component fits in the absence of competing ligands, as well as to the corresponding values measured in 100 μM GYKI 53655. Concentration-response data from one cell are shown in Fig. 4B. The mean EC50 obtained for kainate in the presence of 100 μM AMPA was 3.1 ± 0.7 μM, and the mean nH value was 1.6 ± 0.2 (n = 8 cells, 4–6 kainate concentrations per cell).
Figure 4C shows that 100 μM GYKI 53655 had no effect on responses to 10 μM kainate that were evoked in the presence of 100 μM AMPA (mean change, −1 ± 13%; n = 5 cells), whereas the currents evoked in the same cells by 100 μM AMPA were completely inhibited (99 ± 1%).
High-affinity kainate currents are blocked by lanthanum
Previous studies have shown that La3+ inhibits kainate-type channels at concentrations that potentiate kainate activation of AMPA-type channels (Huettner, 1991; Wilding & Huettner, 1997). In our experiments, we found that currents evoked in ConA-treated granule cells by 10 μM kainate were significantly reduced by 10 μM La3+ (Fig. 5A). On average, the amplitude of the steady-state current evoked by 10 μM kainate was reduced by 84 ± 5% (n = 9 cells). In the same cells, 10 μM La3+ decreased the amplitude of the responses evoked by 100 μM kainate by 42 ± 11%. Applications of 10 μM La3+ had no significant effect on currents evoked by 100 μM AMPA, but virtually abolished the currents elicited in the same cells by 10 μM kainate in the presence of AMPA, reducing the kainate-activated currents by 91 ± 6% (Fig. 5B, n = 6). In the presence of AMPA, La3+ reduced responses to 100 μM kainate by 67 ± 14%.
Figure 5. Kainate-type currents are blocked by lanthanum.
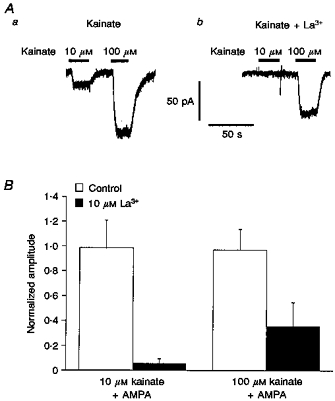
A, whole-cell currents evoked in a ConA-treated granule cell by kainate (10 and 100 μM) in the absence (a) and presence (b) of 10 μM La3+. Agonist applications are indicated by the bars above the traces. B, bar-graph showing the mean amplitude of the currents evoked by 10 μM kainate (left) and 100 μM kainate (right) in the absence (control, □) and presence (▪) of 10 μM La3+. The respective currents were measured in 6 granule cells during the concurrent application of 100 μM AMPA and were normalized to the size of the current evoked in each cell by 10 μM kainate alone. Error bars indicate s.e.m.
ConA reveals currents activated by the kainate-selective ligand SYM 2081
The glutamate analogue SYM 2081 produces rapidly desensitizing activation of kainate-type channels at nanomolar concentrations, whereas the EC50 for SYM 2081 activation of AMPA-type channels has been reported to be about 300 μM (Wilding & Huettner, 1997; Zhou et al. 1997). It was therefore of interest to compare the potency of SYM 2081 in cells that were and were not exposed to ConA. Figure 6A shows whole-cell currents evoked by increasing concentrations of SYM 2081 in a ConA-treated granule cell (Aa) and in a granule cell with intact kainate receptor desensitization (Ab). The concentration-response data obtained from the two cells are plotted in Fig. 6B. The fit to the data from the cell not exposed to ConA (^) gave an EC50 value of 216 μM. The fit to the results from the ConA-treated cell (•) gave an EC50 value of 0.95 μM. Because SYM 2081 concentrations of 10 μM and greater evoked measurable currents in some cells not exposed to ConA (suggesting activation of AMPA-type channels), the data from the ConA-treated cell were also fitted excluding the 10, 30 and 100 μM points (dashed curve). The fit to the results for SYM 2081 concentrations of 3 μM and less gave an EC50 value of 0.51 μM.
Figure 6. Pretreatment of granule cells with ConA reveals activation by SYM 2081 of high-affinity kainate-type currents.
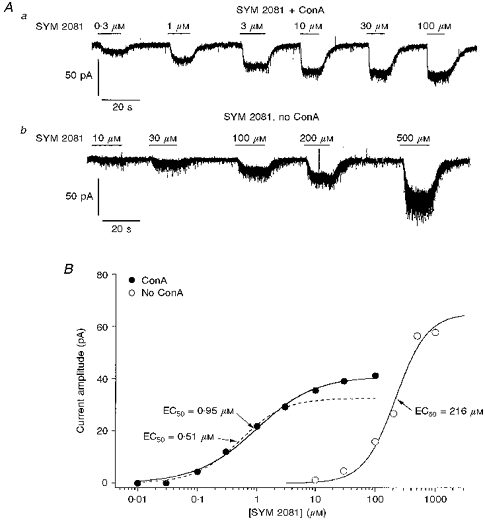
A, whole-cell currents evoked at −80 mV by increasing concentrations of SYM 2081 in 2 granule cells that either were (a) or were not (b) pretreated with ConA. B, concentration-response data from the complete set of results obtained for the cells in Aa and Ab: •, data from a cell exposed to ConA; ^, results from a granule cell not pretreated with ConA. Continuous lines are one-component Hill-type fits to each set of data. The fit to the results obtained from the cell pretreated with ConA gave an EC50 value of 0.95 μM, whereas the corresponding value obtained from the cell not exposed to ConA was 216 μM. The dashed line indicates the Hill-type fit that was obtained for the results from the ConA-treated granule cell when the data for SYM 2081 concentrations of 10 μM and above were excluded (EC50= 0.51 μM).
Analysis of concentration-response data for SYM 2081-activated currents in five granule cells that were not pretreated with ConA gave mean EC50 and nH values of 260 ± 51 μM and 1.6 ± 0.2, respectively. The mean EC50 and nH values obtained from eight ConA-treated granule cells were 3.3 ± 0.9 μM and 1.1 ± 0.3, respectively, if data for concentrations of 10–100 μM SYM 2081 were included; and the corresponding values if these values were excluded (as in Fig. 6B) were 1.7 ± 0.3 μM and 1.3 ± 0.3, respectively (n = 8).
Rectification behaviour of kainate-type currents in granule cells
In ConA-treated granule cells, I-V curves were measured for steady-state currents evoked by 10 μM kainate under conditions where kainate activation of AMPA receptors was minimal (i.e. in the presence of either 100 μM AMPA or 100 μM GYKI 53655). Both inwardly and outwardly rectifying currents were observed.
Current-voltage curves from two different granule cells are shown in Fig. 7A and B. The slope conductance vs. potential (G-V) relationships derived from the I-V curves in Fig. 7A and B are shown in Fig. 7C and D, respectively, and gave +40 mV/-40 mV ratios of 1.5 and 0.8, respectively. Values for the +40 mV/-40 mV ratio were measured for thirty-six granule cells. The mean +40 mV/-40 mV ratio was 1.3 ± 0.1, but the values varied substantially (ranging from 0.4 to 3.6). Current-voltage curves were also obtained during steady-state responses to applications of 10 μM SYM 2081 in five granule cells (in AMPA or GYKI 53655); the mean +40 mV/-40 mV ratio was 1.2 ± 0.1.
Figure 7. Rectification of kainate-type channels in granule cells.
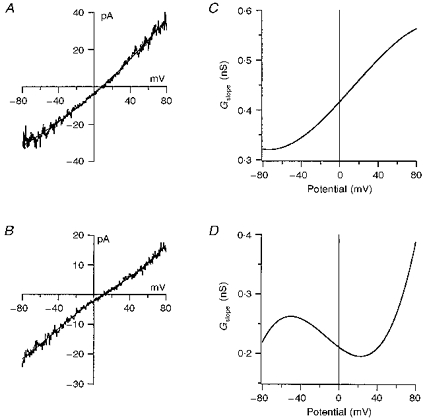
A and B, I-V relationships for steady-state currents evoked by 10 μM kainate in the presence of 100 μM AMPA from 2 different cerebellar granule cells. Currents were measured during 500 ms voltage ramps from −80 to +80 mV. The results are the difference currents obtained by subtracting the currents during application of 100 μM AMPA from the currents during the concurrent application of 100 μM AMPA and 10 μM kainate. The smooth curves are fourth-order polynomial fits to the results. C and D, the first derivative of the polynomial fits to the results in A and B, respectively. The values of the function at +40 and −40 mV were used to determine the ratio of the slope conductances at these membrane potentials.
Noise analysis of kainate-type currents in granule cells
Although the conductances of some types of recombinant kainate-type channels are too small to detect single-channel currents directly, previous studies have shown that differences in the apparent unitary conductance of these channels can be detected from spectral density analysis of agonist-evoked current noise (Howe, 1996; Swanson et al. 1996). To estimate the unitary conductance of the channels underlying the kainate-type currents in granule cells, and to compare their conductance with previous estimates of the conductance of native (Huettner, 1990; Sahara et al. 1997) and recombinant kainate-type channels, noise analysis of steady-state whole-cell current noise was performed.
While the whole-cell currents were small, the resolution in most recordings was sufficient to detect an increase in current noise during applications of 10 μM kainate. Noise spectra were obtained from currents activated by 10 μM kainate in forty-three granule cells where the kainate applications were made in the presence of either 100 μM GYKI 53655 or 100 μM AMPA, thus ensuring that the current fluctuations arose primarily from the gating of kainate-type channels. Similar data were obtained from seven cells with 10 μM SYM 2081.
Figure 8A shows a portion of the current evoked by 10 μM kainate in the presence of 100 μM AMPA. The AMPA application is associated with a clear increase in noise, and there is an additional increase in noise during kainate application. Figure 8B shows the power spectrum for the kainate-evoked noise from the same cell, where the background spectrum was taken during the steady-state AMPA-evoked current. The spectrum was fitted with the sum of two Lorentzian components with half-power frequencies, fc1 and fc2, of 45 and 202 Hz, respectively. The γnoise value obtained from the fit was 1.0 pS. The whole-cell current evoked by 10 μM SYM 2081 in another granule cell in the presence of 100 μM GYKI 53655 is shown in Fig. 8C. The power spectrum obtained from the same cell is shown in Fig. 8D. The spectrum was fitted adequately by a single Lorentzian with a half-power frequency of 55 Hz. The fit gave a γnoise value of 1.1 pS.
Figure 8. Apparent unitary conductance of kainate-type channels in granule cells.
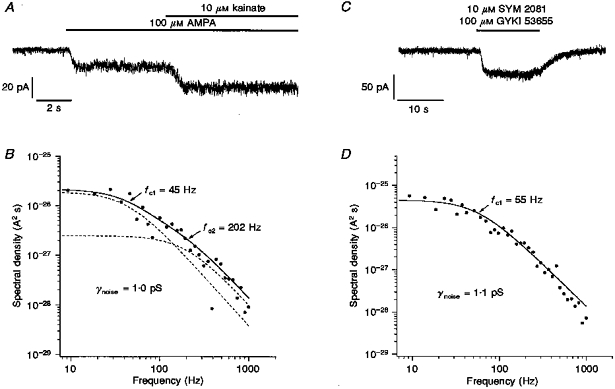
Whole-cell currents evoked at −80 mV by 10 μM kainate in the presence of 100 μM AMPA (A), and by 10 μM SYM 2081 in the presence of 100 μM GYKI 53655 (C) in 2 different ConA-treated granule cells. Agonist applications are indicated by the bars above the traces. In C, SYM 2081 and GYKI 53655 were applied simultaneously. B and D show the power spectra obtained from spectral density analysis of the current noise evoked by 10 μM kainate and 10 μM SYM 2081 during the responses shown in A and C, respectively. The power spectrum for the kainate-evoked noise was fitted (continuous curve) with the sum of two Lorentzian components with respective half-power frequencies of 45 and 202 Hz (dashed curves). The fit gave a γnoise value of 1.0 pS. The single Lorentzian fit to the SYM 2081 results gave a γnoise value of 1.1 pS.
Of the forty-three cells from which kainate spectra were obtained in the presence of AMPA or GYKI 53655, the power spectra from twenty-nine cells were adequately fitted by a single Lorentzian. The mean half-power frequency obtained from these spectra was 42 ± 4 Hz. In the remaining fourteen cells, two Lorentzian components were apparent, although the higher-frequency component was not especially prominent. The mean half-power frequencies for the two Lorentzian components were 28 ± 3 and 290 ± 32 Hz, and the mean ratio of the zero frequency asymptotes, G(0)1/G(0)2, was 33 ± 6. The γnoise values determined from the two Lorentzian spectra were somewhat, but not significantly, larger than the corresponding values obtained from the single Lorentzian fits (1.1 ± 0.3 pS and 0.75 ± 0.14 pS, respectively).
In the presence of 100 μM AMPA or 100 μM GYKI 53655, the mean γnoise estimated from the current fluctuations during the application of 10 μM SYM 2081 was 2.0 ± 0.6 pS (n = 7 cells). This value did not differ significantly from the value obtained with 10 μM kainate in the same cells (1.2 ± 0.3 pS). In three cells, the SYM 2081 spectra were best fitted with two Lorentzian components, whereas only a single component was resolved in the spectra from the other four cells. The mean γnoise values from the single- and two-Lorentzian fits did not differ significantly.
Single-cell RT-PCR analysis of GluR5 and GluR6 editing
Previous studies have shown that granule cells express mRNA encoding GluR5 and GluR6 (Bettler et al. 1990; Egebjerg et al. 1991; Bahn et al. 1994). Both the rectification behaviour and apparent unitary conductance of channels containing GluR5 and GluR6 are influenced by RNA editing at the Q/R site common to both subunits (Köhler et al. 1993; Swanson et al. 1996). We have recently shown that both Q and R versions of GluR5 and GluR6 are present in RNA from populations of acutely isolated granule cells (Belcher & Howe, 1997). It was therefore of interest to determine whether this reflected the presence of unedited and edited variants in individual cells. Because our population results indicated that editing changes during early development, the cells were obtained at postnatal (P) days which would maximize detection of both unedited and edited cDNAs (GluR5, P15; GluR6, P5-P10).
Whole-cell recordings were obtained from granule cells in short-term primary culture, and the cytoplasmic contents were harvested and used as a template for RT-PCR amplification with GluR5- or GluR6-specific primers. The ratios of unedited and edited GluR5 products were determined from analysis of the restriction fragments obtained upon BbvI digestion (Belcher & Howe, 1997). The data obtained for GluR5 from three individual granule cells are shown in Fig. 9A. In each cell the majority of GluR5 transcripts are unedited, although some product arising from edited RNA is also detected. Sufficient GluR5-specific product for reliable analysis was amplified from eight granule cells (from P15 rats). The mean percentage of edited GluR5 transcripts was 24 ± 5%.
Figure 9. RT-PCR analysis of GluR5 and GluR6 editing in single cerebellar granule cells.
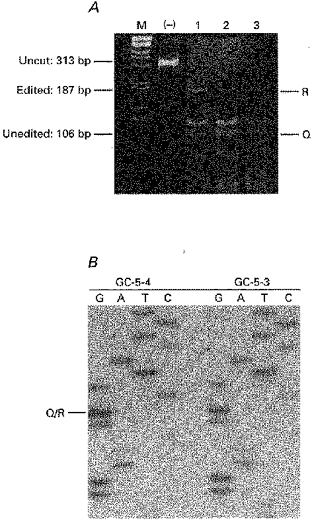
A, GluR5 RT-PCR amplification products from 3 granule cells. The samples were separated on a 6% polyacrylamide gel that was stained with ethidium bromide and photographed. The lane marked (-) contains undigested RT-PCR products. Lanes 1–3 show RT-PCR products from each cell after digestion with BbvI. The sizes of the uncut GluR5 product (313 bp) and the edited (R, 187 bp) and unedited (Q, 106 bp) restriction fragments are indicated at the sides of each panel. The lane labelled M contains a 1 kb DNA ladder (Life Sciences) as a size standard. B, sequence analysis of GluR6-specific RT-PCR products obtained from 2 granule cells (GC-5–4 and GC-5-3). The position of the Q/R site is indicated. Note that for both cells a termination signal is only detected in the ddG lane.
In addition to the Q/R site in the putative pore-forming region, GluR6 contains two sites in TMI that are also subject to RNA editing (Köhler et al. 1993). To analyse editing at the three sites in GluR6, in each of eight granule cells (from P5-P10 rats) the population of GluR6 RT-PCR products was directly sequenced with 5′-end-labelled primers. The proportions of unedited and edited transcripts were then estimated by comparing the signal intensities of the ddA and ddG lanes on the sequencing gel. Two examples of this analysis for the Q/R site in GluR6 are shown in Fig. 9B. In each case there is a strong termination in the ddG lane and no detectable termination in the ddA lane, suggesting that the vast majority of GluR6 mRNA in each granule cell was edited at the Q/R site. In seven of the eight cells analysed, the GluR6 products appeared to be completely edited at all three sites, whereas the reaction products from the remaining cell were edited at both TMI sites and unedited at the Q/R site. At each editing site analysed, specific terminations for both ddA and ddG were never detected. Analysis of samples containing known amounts of the cDNAs encoding GluR6(Q) and GluR6(R) indicated that we could have detected either cDNA if it represented at least 10% of the RT-PCR product from a given cell (see Methods).
Kainate and SYM 2081 activation of GluR6(R) and GluR6(R)/KA2 channels
In addition to GluR5 and GluR6, cerebellar granule cells also express KA2. Co-expression of KA2 with GluR6 results in channels with unique properties (Herb et al. 1992; Howe, 1996; Swanson et al. 1996), and there is evidence for heteromeric interactions between GluR6 and KA2 in cerebellum (Ripellino et al. 1998). Characterization of heteromeric GluR6/KA2 channels is therefore relevant to understanding the subunit composition of native kainate-type channels in granule cells. This characterization is complicated, however, by the possible expression of both homomeric and heteromeric channels in cells co-transfected with GluR6 and KA2. Clonal HEK 293 cells that stably express GluR6(R) and KA2 were therefore analysed to determine to what extent the cells express channels that are heteromeric assemblies containing both subunits.
Western blots of GluR6 and KA2 protein in the GluR6(R)-KA2 stable transfectants are shown in Fig. 10A. Equal amounts of protein from parallel cultures in which kainate subunit expression was suppressed with tetracycline (not induced, NI), or in which expression was induced by removing tetracycline from the media (induced, I), were electrophoresed in adjacent lanes of the same gel. The background expression of GluR6 is substantial in the presence of tetracycline, whereas relatively little KA2 expression is seen in the non-induced cells and the induction of expression obtained with KA2 is consequently more robust (Fig. 10A). At 5–6 days post-induction, quantitative densitometric analysis gave a mean GluR6:KA2 ratio of 1.5 ± 0.2 (n = 4) in the cell line studied here. At these post-induction times, it was possible to immunoprecipitate the vast majority of either subunit protein with antibody directed against the other. The results of one such immunoprecipitation experiment are shown in Fig. 10B. Detergent extracts of total cellular protein were immunoprecipitated with anti-KA2 (left) or anti-GluR6/7 (right) antibody, and aliquots of the detergent extracts and the postimmunoprecipitate supernatants and pellets were immunoblotted with anti-GluR6/7 (left) or anti-KA2 (right). The nearly complete depletion of immunoreactivity from the supernatant in both cases suggests that the vast majority of GluR6 and KA2 proteins are present in heteromeric assemblies. Similar results were obtained in three additional experiments.
Figure 10. GluR6 and KA2 protein expression and agonist affinity in HEK 293 cells stably expressing both subunits.
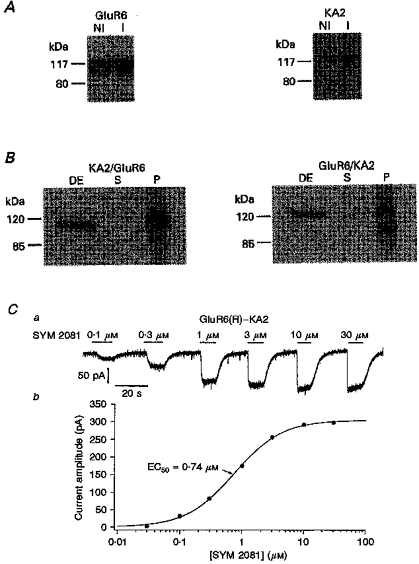
A, Western blots of total cellular protein from HEK 293 cells stably expressing the GluR6 and KA2 subunits. The immunoblots were probed with anti-GluR6/7 (left) or anti-KA2 (right) antibody. The cells were harvested before (NI) and 5 days after (I) inducing expression of GluR6 and KA2 by removing tetracycline from the media. Note that the background expression of KA2 in the presence of tetracycline is lower than the corresponding expression of GluR6, and that the subsequent induction of expression is greater. B, detergent extracts of total cellular protein from cells stably expressing GluR6-KA2 were immunoprecipitated with anti-KA2 (left) or anti-GluR6/7 (right) antibody. Aliquots of the detergent extracts (DE) and postimmunoprecipitate supernatants (S) and pellets (P) were subjected to SDS-PAGE and immunoblotted with anti-GluR6/7 (left) or anti-KA2 (right) antibody. The positions of 120 and 85 kDa molecular weight standards are shown. C, whole-cell currents (a) evoked at −80 mV by increasing concentrations of SYM 2081, and the resultant concentration-response data (b), in a ConA-treated HEK 293 cell stably expressing the GluR6(R) and KA2 subunits. SYM 2081 applications are indicated by bars above the current traces. The fit to the results gave an EC50 value of 0.74 μM.
The co-immunoprecipitation results might be misleading if functional plasmalemmal receptors comprise only a small fraction of the total cellular receptor pool. We therefore determined the extent to which AMPA, which activates GluR6/KA2 channels but not GluR6 homomers (Egebjerg et al. 1991; Herb et al. 1992), would compete with kainate for activation of the functional channels present in the cells expressing both GluR6(R) and KA2. Previous results indicate that the EC50 value for kainate in cells expressing GluR6(R) alone is about 0.5 μM, whereas the corresponding values for kainate and AMPA in GluR6(R)-KA2-expressing cells are about 1.5 and 250 μM, respectively (Howe, 1996). Therefore, 3 mM AMPA should effectively prevent activation of GluR6(R)/KA2 channels by 0.5 μM kainate, but not interfere with kainate activation of any homomeric GluR6(R) channels present. As expected from previous work (Egebjerg et al. 1991), applications of 3 mM AMPA did not produce detectable currents in three cells expressing GluR6(R) alone and did not reduce currents evoked by 0.5 μM kainate. In contrast, 3 mM AMPA produced currents in each of the six GluR6(R)-KA2-expressing cells studied. Applications of 0.5 μM kainate gave clear inward currents when applied alone in the same cells (52.1 ± 16.0 pA, at −80 mV), but the currents were markedly reduced (97 ± 3%) when the applications were repeated in the presence of 3 mM AMPA. The results show that the expression of homomeric GluR6 channels is minimal in our stable GluR6(R)-KA2-transfected cells.
Kainate displays 3-fold lower affinity for GluR6/KA2 heteromers than GluR6 homomeric channels. It was therefore of interest to determine whether SYM 2081 also discriminates between these two types of recombinant channels. Whole-cell currents evoked at −80 mV by SYM 2081 in a HEK 293 cell stably expressing the GluR6(R) and KA2 subunits are shown in Fig. 10Ca. The fit to the concentration-response curve (Fig. 10Cb) gave an EC50 value of 0.74 μM. The mean EC50 and nH values determined for SYM 2081 in five cells expressing both GluR6(R) and KA2 were 0.58 ± 0.12 μM and 1.2 ± 0.04, respectively. The corresponding values determined in GluR6(R)-expressing cells were 0.22 ± 0.02 μM and 1.6 ± 0.4, respectively (n = 4). Thus, heteromeric GluR6(R)/KA2 channels show about 3-fold lower affinity for SYM 2081 than homomeric GluR6(R) channels. In addition, the co-expression of KA2 results in a shallower dependence on agonist concentration, as evidenced by the lower nH value in the cells expressing GluR6(R)/KA2 heteromeric channels. Both of these results are similar to those reported previously for kainate (Howe, 1996).
Agonist-specific differences in γnoise have been reported for certain types of recombinant AMPA-type channels (Swanson, Kamboj & Cull-Candy, 1997). We therefore compared the characteristics of the current fluctuations seen with kainate and SYM 2081 in our stable GluR6(R)- and GluR6(R)-KA2-expressing cells. The mean γnoise value obtained for GluR6(R) channels with 10 μM kainate was similar to values reported previously (0.25 ± 0.04 pS, n = 14). Estimates of γnoise obtained in three cells with 10 μM SYM 2081 gave a mean value of 0.20 ± 0.06 pS and were close to the values obtained in the same cells with 10 μM kainate. The mean γnoise values determined with 10 μM kainate and 10 μM SYM 2081 in six GluR6(R)-KA2-expressing cells were 0.74 ± 0.10 and 0.54 ± 0.04 pS, respectively. Power spectra obtained with both agonists contained two Lorentzian components with similar half-power frequencies.
The γnoise values we have obtained for the GluR6(R)/KA2 channels studied here are similar to those reported previously under conditions where the relative expression of the two subunits was unknown (Howe, 1996; Swanson et al. 1996). If GluR channels are pentameric, then the relative abundance of GluR6 and KA2 in the clonal line examined suggests that, on average, the channels have a GluR6:KA2 stoichiometry of approximately 3:2. We have found no evidence for agonist-specific differences in γnoise for either homomeric or heteromeric kainate-type channels.
DISCUSSION
Identification of kainate-type channels in granule cells
One of our main findings is that exposure of granule cells to ConA reveals a component of steady-state currents that are activated by low concentrations of kainate. Several lines of evidence support the conclusion that the channels underlying these currents are of the kainate subtype.
Firstly, many studies of both native and recombinant channels have shown that ConA selectively removes desensitization of kainate-type channels (see, for example: Huettner, 1990; Partin et al. 1993; Wong & Mayer, 1993), and the EC50 value (about 4 μM) for the high-affinity component of kainate-activated currents revealed by ConA treament is consistent with activation of kainate-type channels. In contrast, the EC50 value of 120 μM determined for the low-affinity component detected in ConA-treated granule cells is consistent with kainate activation of AMPA receptor channels, as is the EC50 determined for kainate in the absence of ConA treatment. In addition, ConA exposure also revealed currents activated by concentrations of SYM 2081 likely to be selective for kainate-type channels. The much greater EC50 value determined for SYM 2081 in cells not exposed to ConA (260 μM) is consistent with its known affinity at AMPA-type channels (Zhou et al. 1997).
Secondly, the non-competitive AMPA-selective antagonist GYKI 53655 had no effect on the high-affinity component of kainate-activated currents detected in ConA-treated granule cells at concentrations that completely abolished the low-affinity component. Paternain et al. (1995) report an IC50 value of 450 μM for GYKI 53655 inhibition of kainate-type currents in hippocampal neurons. The IC50 value of 8 μM we determined for GYKI 53655 inhibition of kainate-activated currents in the absence of ConA exposure is consistent with inhibition of AMPA-type channels, although it is larger than the values of about 1 μM determined for AMPA-type channels in cultured hippocampal neurons, neocortical neurons and Purkinje cells (Paternain et al. 1995; Wilding & Huettner, 1995; Bleakman et al. 1996), and is closer to the values found for recombinant AMPA-type channels (Bleakman et al. 1996; Partin & Mayer, 1996).
Thirdly, the coapplication of 100 μM AMPA reduced the amplitude of steady-state currents evoked by concentrations of kainate sufficient to activate AMPA-type channels, but had little or no effect on currents evoked by kainate concentrations of 10 μM or less. Importantly, 100 μM GYKI 53655 had no effect on the amplitude of currents evoked by 10 μM kainate in the presence of 100 μM AMPA, while the AMPA responses in the same cells were blocked completely.
At high concentrations, AMPA has been shown to activate some native and recombinant kainate-type channels (Huettner, 1990; Herb et al. 1992; Sommer, Burnashev, Verdoorn, Keinänen, Sakmann & Seeburg, 1992; Howe, 1996). However, the currents evoked by 10 μM kainate in the absence and presence of 100 μM AMPA were similar in size, and the EC50 values obtained for kainate in the presence of either 100 μM AMPA or 100 μM GYKI 53655 were similar to the EC50 values determined for the high-affinity kainate component detected in the absence of AMPA-selective ligands. These results support the conclusion that neither AMPA nor GYKI 53655 interfered with kainate activation of kainate-type channels in granule cells.
Finally, currents evoked by low concentrations of kainate in the presence of 100 μM AMPA were completely blocked by 10 μM La3+, which distinguishes between AMPA- and kainate-type channels (Huettner, 1991; Wilding & Huettner, 1997). Applications of La3+ that abolished responses to 10 μM kainate did not reduce steady-state currents evoked by 100 μM AMPA. Indeed, low concentrations of La3+ have been reported to enhance AMPA-type currents (Reichling & MacDermott, 1991; Wilding & Huettner, 1997), an effect which may account for the incomplete inhibition by La3+ of currents evoked by 100 μM kainate in the presence of 100 μM AMPA.
In total, the results support the conclusion that cerebellar granule cells in culture express kainate-type ion channels and that their steady-state properties can be studied by minimizing kainate-receptor desensitization with ConA and applying low concentrations of kainate in the presence of 100 μM GYKI 53655 or AMPA.
Comparison with native kainate-type channels in other cells
Exposure to ConA does not influence the rectification properties or unitary conductance of native (Huettner, 1990) or recombinant kainate-type channels (cf. Howe, 1996; Swanson et al. 1996). In most studies, ConA also did not alter agonist EC50 values (Huettner, 1990; Wong, Mayer, Jane & Watkins, 1994), although modest increases in apparent affinity have been reported (Sahara et al. 1997; Wilding & Huettner, 1997). Thus, it is unlikely that the properties of the channels studied here, other than desensitization, were influenced substantially by ConA.
The EC50 value determined for kainate in the presence of 100 μM AMPA or 100 μM GYKI 53655 was about 3 μM. The apparent affinity of kainate for the native channels in cerebellar granule cells is therefore higher than its affinity for kainate-type channels in dorsal root ganglion neurons (EC50= 15 μM; Huettner, 1990) or embryonic hippocampal neurons (EC50= 22 μM; Lerma et al. 1993). The kainate EC50 value in granule cells is also less than the values of 7 μM reported for kainate activation of ConA-treated channels in cultured hippocampal (Wilding & Huettner, 1997) or trigeminal ganglion neurons (Sahara et al. 1997), and is closest to the value of 5 μM found for kainate-type channels in an oligodendrocyte cell line (Patneau, Wright, Winters, Mayer & Gallo, 1994). Interestingly, this glial cell line expresses predominantly mRNAs encoding the GluR6 and KA2 subunits and does not express detectable levels of GluR5 transcripts. Previous studies of recombinant channels indicate that the EC50 value for kainate activation of homomeric GluR6 channels is about 30-fold less than the corresponding value for homomeric GluR5 channels (Egebjerg et al. 1991; Sommer et al. 1992).
The γnoise values we obtained were typically below the mean values reported for kainate-type channels in dorsal root ganglion neurons (2–4 pS; Huettner, 1990). Sahara et al. (1997) reported a mean γnoise of 1.6 pS for kainate-type currents in cultured trigeminal ganglion neurons, which is close to most of the values in granule cells. The properties of the channels in trigeminal neurons, together with analysis of RNA extracted from the trigeminal ganglia, suggest that the kainate-type channels in these cells are heteromeric assemblies containing GluR5(R) and KA2.
Expression of GluR5 and GluR6 mRNAs in single granule cells
Both Q and R versions of GluR5 and GluR6 are present in populations of purified granule cells acutely isolated from P1 to P15 rats (Belcher & Howe, 1997). Our present results indicate that individual granule cells in short-term culture express both Q and R versions of GluR5. The ratio of Q- to R-encoding GluR5 transcripts was similar in each cell studied and the mean ratio (76 ± 5%) is reasonably close to the corresponding ratio determined at P15 from populations of acutely isolated cells (87 ± 4%; Belcher & Howe, 1997). In contrast, only edited versions of GluR6 were detected in seven out of eight individual granule cells. These latter results differ from those obtained from single hippocampal neurons in culture, where unedited versions predominate (Ruano, Lambolez, Rossier, Paternain & Lerma, 1995).
Our finding that most granule cells express GluR6(R) is consistent with the predominance of R-encoding transcripts in populations of granule cells at P7-P15 (63–86%; Belcher & Howe, 1997). However, in seven out of eight individual cells, only transcripts fully edited at each of the three sites in GluR6 were detected. In acutely isolated granule cells, the proportion of GluR6 transcripts edited at both TMI sites increased from only 39% at P7 to 74% at P15, as determined from sequence analysis of cloned cDNAs. It therefore appears that editing of the TMI sites in GluR6, and perhaps also Q/R editing, increases in vitro.
Possible subunit composition of kainate-type channels in granule cells
The presence in granule cells of kainate-type channels containing the GluR6 and KA2 subunits is supported by both immunocytochemical and immunoprecipitation studies (Ripellino et al. 1998, and references therein). Our present and previous RT-PCR results indicate that most granule cells express predominantly unedited GluR5 and edited GluR6 transcripts, and that GluR6 transcripts are 3- to 4-fold more abundant than those encoding GluR5 (Belcher & Howe, 1997). The available evidence therefore suggests that the native channels in most granule cells are probably composed of the GluR6(R), KA2 and GluR5(Q) subunits, and that at least some of the channels are heteromeric assemblies containing both GluR6 and KA2.
The agonist affinities determined here are consistent with the expression of GluR6/KA2 heteromeric channels in granule cells. For both kainate and SYM 2081, the EC50 values for the kainate-type channels in granule cells are about twice the EC50 values for activation of heteromeric GluR6(R)/KA2 channels (Howe, 1996; this paper). By comparison, the agonist EC50 values determined in granule cells are 5–6 times the corresponding values for homomeric GluR6(R) channels, and the kainate EC50 is about 10-fold less than for activation of homomeric GluR5 channels (34 μM; Sommer et al. 1992).
Although there was significant cell-to-cell variability, the biophysical properties of the kainate-type channels in most granule cells are also consistent with the expression of channels containing both GluR6(R) and KA2. The mean +40 mV/-40 mV ratio for the kainate-type currents in granule cells of 1.3 is close to the corresponding value of about 1.4 determined in our stable GluR6(R)-KA2-expressing cells (K. E. Pemberton & J. R. Howe, unpublished observations). The γnoise values we typically obtained from granule cells are greater than those reported for homomeric channels formed from edited GluR5 or GluR6 subunits (≤ 250 pS) and are similar to the values reported for recombinant channels containing GluR6(R) and KA2 (Howe, 1996; Swanson et al. 1996). The γnoise values measured in granule cells were typically below those found for homomeric or heteromeric channels formed from unedited GluR5 or GluR6 subunits and KA2 (3–6 pS; Swanson et al. 1996); and recombinant channels formed from these subunits showed marked inward rectification (Herb et al. 1992; Köhler et al. 1993; Kamboj, Swanson & Cull-Candy, 1995; Bähring, Bowie, Benveniste & Mayer, 1997).
The characteristics of the kainate-type channels in granule cells are therefore at odds with a number of possible subunit combinations and are consistent with the expression of heteromeric GluR6(R)/KA2 channels. The biophysical properties of the granule cell channels are also consistent with the properties of GluR5(R)/KA2 channels, but our mRNA studies suggest that GluR5(R) is present at low relative abundance. Although similar, the mean EC50 and γnoise values found in granule cells are somewhat larger than the corresponding values for GluR6(R)/KA2 channels. Both deviations are consistent with the contribution of GluR5(Q) subunits (which could be present in either homomeric or heteromeric assemblies) to the native channels in granule cells.
Variation in channel phenotype
While the mean EC50, +40 mV/-40 mV ratio and γnoise values obtained in granule cells are consistent with the expression of channels composed of GluR6(R) and KA2, and possibly GluR5(Q), the above comparisons ignore the substantial variability observed. The +40 mV/-40 mV ratios from different granule cells varied more than 6-fold, and the γnoise values varied more than 15-fold. If this variation resulted primarily from true cell-to-cell variation in channel phenotype, then conclusions based on mean values would obviously not be very meaningful. It is therefore important to consider how much of the observed variation is likely to be attributable to experimental error.
The contribution of random error can be estimated from the variation seen for repeated measures on the same cell. Two measurements of the +40 mV/-40 mV ratio were obtained from sixteen cells. The coefficient of variation for the difference between the repeated measures was 0.35, suggesting that the variation arising from random error should be about 4-fold (which is similar to that seen for +40 mV/-40 mV ratios in our GluR6(R)-transfected cells after scaling the currents to give similar signal-to-noise ratios). Repeated measures of γnoise made in nineteen cells gave a coefficient of variation for the difference values of 0.25, suggesting that random error should result in about 3-fold variation in γnoise. The predicted variation, while large in both cases, is therefore less than that observed. In addition, one-way analysis of variance performed on the repeated measures of the +40 mV/-40 mV ratios and γnoise indicated that there was statistically significant cell-to-cell variation, with about 40% of the possible comparisons for each parameter being significant at the P < 0.05 level.
The above analysis does not, however, take into account cell-to-cell variation arising from systematic error. For example, some of the variation in γnoise values might reflect cell-to-cell differences in resolution. However, the similar γnoise values obtained from power spectra in which one or two Lorentzians were resolved suggests that missing high-frequency variance is unlikely to have contributed substantially to the variation in γnoise values. Differences in intracellular polyamine concentration are unlikely to have affected the +40 mV/-40 mV ratios we determined, because 100 μM spermine was present in the pipette solution, we always waited 2–3 min before making the measurements, and the cells are very small. Systematic errors could also be introduced by the subtraction procedure used to obtain the difference currents. However, we found no correlation between the size of the mean currents (at −80 mV) and the +40 mV/-40 mV ratios determined, as might be expected if this were true.
We therefore believe that some of the observed variation reflects true cell-to-cell variation in channel phenotype. This may reflect differences in the developmental state of granule cells cultured from young animals, since we have found developmental changes in editing of GluR5 and GluR6, as well as in KA2 expression (Belcher & Howe, 1997; Ripellino et al. 1998). For example, some granule cells may express channels containing primarily unedited GluR5 or GluR6 subunits, whereas channels in other cells may contain primarily edited subunits. If this were true, then there should be a strong negative correlation between the +40 mV/-40 mV ratios and γnoise values obtained for individual cells. The results from thirty-six granule cells in which both γnoise and the +40 mV/-40 mV ratio were measured are shown in Fig. 11A. There is little indication that the two parameters are correlated. Regression analysis gave a correlation coefficient of −0.37, which was not statistically significant.
Figure 11. Relationship between γnoise and rectification ratio.
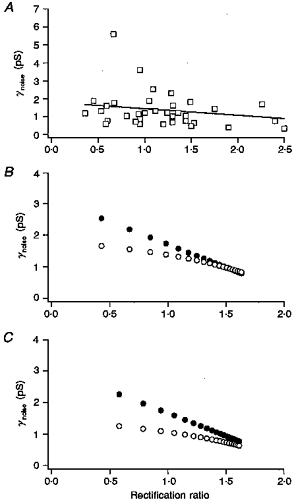
A, γnoise values determined for kainate-type currents that were obtained from 36 individual granule cells are plotted against the +40 mV/-40 mV ratio determined fom the same cell. The line shows regression analysis of the results. One point (3.6, 1.12 pS) is off scale to the right. B, values of the apparent unitary conductance (determined at −80 mV) and rectification ratio that are expected to be obtained from cells expressing different relative amounts of homomeric GluR6(R) and homomeric GluR5(Q) channels. The +40 mV/-40 mV ratios were calculated as the ratio of the whole-cell currents at these membrane potentials, which gives values similar to the ratio of slope conductances for currents that reverse near 0 mV. The respective values for γnoise and the +40 mV/-40 mV ratio were taken to be 0.25 pS and 2.0, respectively, for the GluR6(R) homomers, and 3 pS and 0.1, respectively, for the GluR5(Q) homomeric channels. •, results when it was assumed that the Po for each channel subtype was similarly low and the GluR6(R):GluR5(Q) ratio was varied from 2.5 to 50 in constant increments. ^, results when the Po values for GluR6(R) and GluR5(Q) channels were taken to be 0.2 and 0.5, respectively, and were included in calculation of γnoise. The GluR6(R):GluR5(Q) ratio was varied from 1 to 20. C, predicted relationship between γnoise and the +40 mV/-40 mV ratio for mixed populations of GluR6(R), GluR5(Q), and GluR6(R)/KA2 channels (γnoise= 0.6 pS, +40 mV/40 mV ratio = 1.5). •, results if it was assumed that the Po was low for each population. The ratio of GluR6(R) to GluR5(Q) homomers was varied from 1 to 50 and the ratio of GluR6(R) to GluR6(R)/KA2 channels was varied from 1 to 4 (in constant increments). ^, results when the Po for the 2 populations of GluR6(R)-containing channels was taken to be be 0.2 and the Po for the GluR5(Q) channels was taken to be 0.5. The GluR6(R):GluR5(Q) ratio was varied from 1 to 20.
It might be argued that this lack of correlation supports the view that the variation in each parameter arises largely from random error. However, the converse argument is hard to refute, namely that there was such a correlation but that it was obscured by scatter in the measurements. We therefore sought to determine what relationship between γnoise and the +40 mV/-40 mV ratio would be expected for cells expressing different populations of kainate-type channels. Figure 11B shows the predicted results if individual cells expressed a mixed population of homomeric GluR6(R) and homomeric GluR5(Q) channels, and if the relative proportion of the two types of channels varied from cell to cell. The relative change in +40 mV/-40 mV ratio exceeds the relative change in γnoise, reflecting the fact that γnoise scales with the square of the single-channel current. Figure 11C shows the results of similar calculations for cells expressing GluR6(R) and GluR5(Q) homomers, as well as GluR6(R)/KA2 heteromeric channels. The filled circles in Fig. 11B and C are the results obtained when it was assumed that the steady-state open probability, Po, was low for each channel type and could therefore be ignored in the calculation of γnoise, an assumption we made for our γnoise calculations in granule cells. While the assumption is valid (under similar conditions) for GluR6(R) and GluR6(R)/KA2 channels (Howe, 1996), as well as for some native kainate-type channels (Sahara et al. 1997), we could not verify that Po was always low in granule cells, because in many cells we could not reliably detect a noise increase at kainate concentrations that gave submaximal currents. Non-stationary fluctuation analysis of rapidly desensitizing currents through homomeric GluR6(Q) channels indicates that the peak Po can be as high as 0.85 (Traynelis & Wahl, 1997). If the steady-state Po of channels formed exclusively from Q-containing subunits was similarly high in ConA-treated cells, then γnoise might have been preferentially underestimated in cells expressing these channels. The open circles in Fig. 11B and C show the results if the Po values for the GluR6(R)-containing channels and the GluR5(Q) homomers are taken to be 0.2 and 0.5, respectively, and are included in the calculation of γnoise.
The results in Fig. 11 demonstrate that data similar to those found for granule cells are to be expected if individual cells express multiple populations of kainate-type channels in different relative ratios. Cell-to-cell variation in channel phenotype might also arise from differences in the average subunit stoichiometry of the heteromeric channels expressed in different cells.
Functional role of kainate-type channels in granule cells
While the involvement of AMPA and NMDA receptors in transmission at central synapses is well established, the role of kainate-type channels in brain function is less clear. Indeed, it might be argued that the small size of the kainate-type currents seen here belies a significant role of these channels in granule cells. However, given the small size of the cells, and consequently their high input resistance, small currents can produce substantial depolarizations (Cull-Candy et al. 1988). Also, recent fast-perfusion data in hippocampal neurons have shown that ConA actually suppresses the peak kainate-type currents recorded in these cells (Wilding & Huettner, 1997). Thus, the peak kainate-type currents in granule cells, had we been able to resolve them, may have been larger than the steady-state currents we recorded in the presence of ConA.
Recent evidence supports a role for postsynaptic kainate-type channels in mediating certain types of synaptic transmission in the hippocampus (Castillo et al. 1997; Vignes & Collingridge, 1997). In addition, there is evidence that presynaptic kainate receptors regulate transmitter release at hippocampal synapses (Chittajula, Vignes, Dev, Barnes, Collingridge & Henley, 1996; Clarke et al. 1997; Rodriques-Moreno et al. 1997). The expression of kainate-type subunit proteins in granule cells in the external germinal layer (Ripellino et al. 1998), long before the cells receive synaptic inputs, suggests the possibility that kainate-type channels may serve non-synaptic functions during early development. The availability of non-competitive AMPA receptor blockers like GYKI 53655, and ligands like SYM 2081 which promote kainate-receptor desensitization in the absence of ConA, should prove useful in evaluating the potential involvement of granule cell kainate-type channels in synaptic transmission, as well as their role in developing granule cells.
Acknowledgments
We wish to thank Stacey Irizarry and Steve Traynelis for helpful comments, and Tony Giordano (Symphony Pharmaceuticals) and David Leander (Eli Lilly) for kindly providing us with SYM 2081 and GYKI 53655, respectively. This work was supported by NIH grant NS-30996 (J. R. H.) and postdoctoral fellowships from the NIH (K. E. P.; T32-NS07136) and The Patrick and Catherine Weldon Donaghue Medical Research Foundation (S. M. B.).
References
- Bahn S, Volk B, Wisden W. Kainate receptor gene expression in the developing rat brain. Journal of Neuroscience. 1994;14:5525–5547. doi: 10.1523/JNEUROSCI.14-09-05525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähring R, Bowie D, Benveniste M, Mayer ML. Permeation and block of rat GluR6 glutamate receptor channels by internal and external polyamines. The Journal of Physiology. 1997;502:575–590. doi: 10.1111/j.1469-7793.1997.575bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher SM, Howe JR. Characterization of RNA editing of the glutamate-receptor subunits GluR5 and GluR6 in granule cells during cerebellar development. Molecular Brain Research. 1997;52:139–148. doi: 10.1016/s0169-328x(97)00252-0. 10.1016/S0169-328X(97)00231-3. [DOI] [PubMed] [Google Scholar]
- Bettler B, Boulter J, Hermans-Borgmeyer I, O'Shea-Greenfield A, Deneris E, Moll C, Borgmeyer U, Hollmann M, Heinemann S. Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron. 1990;5:583–595. doi: 10.1016/0896-6273(90)90213-y. [DOI] [PubMed] [Google Scholar]
- Bettler B, Mülle C. Review: neurotransmitter receptors. II. AMPA and kainate receptors. Neuropharmacology. 1995;34:123–139. doi: 10.1016/0028-3908(94)00141-e. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Ballyk BA, Schoepp DE, Palmer AJ, Bath CP, Sharpe EF, Woolley ML, Bufton HR, Kamboj RK, Tarnawa I, Lodge D. Activity of 2,3-benzodiazepines at native rat and recombinant human glutamate receptors in vitro: stereospecificity and selectivity profiles. Neuropharmacology. 1996;35:1689–1702. doi: 10.1016/s0028-3908(96)00156-6. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Malenka RC, Nicoll RA. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388:182–186. doi: 10.1038/40645. 10.1038/40645. [DOI] [PubMed] [Google Scholar]
- Chittajula E, Vignes M, Dev KK, Barnes JM, Collingridge GL, Henley JM. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature. 1996;379:78–81. doi: 10.1038/379078a0. [DOI] [PubMed] [Google Scholar]
- Clarke VRJ, Ballyk BA, Hoo KH, Mandelzys A, Pellizarri A, Bath CP, Thomas J, Sharpe EF, Davies CH, Ornstein PL, Schoepp DD, Kamboj RK, Collingridge GL, Lodge D, Bleakmann D. A hippocampal GluR5 kainate receptor regulating inhibitory synaptic tramsmission. Nature. 1997;389:599–603. doi: 10.1038/39315. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Howe JR, Ogden DC. Noise and single channels activated by excitatory amino acids in rat cerebellar granule neurones. The Journal of Physiology. 1988;400:189–222. doi: 10.1113/jphysiol.1988.sp017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donevan SD, Rogawski MA. GYKI 52466, a 2,3-benzodiazepine, is a highly selective, noncompetitive anatagonist of AMPA/kainate receptor responses. Neuron. 1993;10:51–59. doi: 10.1016/0896-6273(93)90241-i. 10.1016/0896-6273(93)90241-I. [DOI] [PubMed] [Google Scholar]
- Egebjerg J, Bettler B, Hermans-Borgmeyer I, Heinemann S. Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature. 1991;351:745–748. doi: 10.1038/351745a0. 10.1038/351745a0. [DOI] [PubMed] [Google Scholar]
- Herb A, Burnashev N, Werner P, Sakmann B, Wisden W, Seeburg PH. The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron. 1992;8:775–785. doi: 10.1016/0896-6273(92)90098-x. 10.1016/0896-6273(92)90098-X. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annual Review of Neuroscience. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Howe JR. Homomeric and heteromeric ion channels formed from the kainate-type subunits GluR6 and KA2 have very small, but different, unitary conductances. Journal of Neurophysiology. 1996;76:510–519. doi: 10.1152/jn.1996.76.1.510. [DOI] [PubMed] [Google Scholar]
- Howe JR, Cull-Candy SG, Colquhoun D. Currents through single glutamate receptor channels in outside-out patches from rat cerebellar granule cells. The Journal of Physiology. 1991;432:143–202. doi: 10.1113/jphysiol.1991.sp018381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe JR, Skryabin BV, Belcher SM, Zerillo CA, Schmauss C. The responsiveness of a tetracycline-sensitive expression system differs in different cell lines. Journal of Biological Chemistry. 1995;270:14168–14174. doi: 10.1074/jbc.270.23.14168. 10.1074/jbc.270.23.14168. [DOI] [PubMed] [Google Scholar]
- Huettner JE. Glutamate receptor channels in rat DRG neurons: Activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron. 1990;5:255–266. doi: 10.1016/0896-6273(90)90163-a. 10.1016/0896-6273(90)90163-A. [DOI] [PubMed] [Google Scholar]
- Huettner JE. Glutamate receptor channels in rat DRG neurons: selective inhibition by lanthanum and gadolinium. Society for Neuroscience Abstracts. 1991;17:1167. [Google Scholar]
- Kamboj SK, Swanson GT, Cull-Candy SG. Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. The Journal of Physiology. 1995;486:297–303. doi: 10.1113/jphysiol.1995.sp020812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler M, Burnashev N, Sakmann B, Seeburg PH. Determinants of Ca2+ permeability in both TM1 and TM2 of high affinity kainate receptor channels: diversity by RNA editing. Neuron. 1993;10:491–500. doi: 10.1016/0896-6273(93)90336-p. 10.1016/0896-6273(93)90336-P. [DOI] [PubMed] [Google Scholar]
- Lerma J, Paternain AV, Naranjo JR, Mellström B. Functional kainate-selective glutamate receptors in cultured hippocampal neurons. Proceedings of the National Academy of Sciences of the USA. 1993;90:11688–11692. doi: 10.1073/pnas.90.24.11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partin KM, Mayer ML. Negative allosteric modulation of wild-type and mutant AMPA receptors by GYKI 53655. Molecular Pharmacology. 1996;49:142–148. [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993;11:1069–1082. doi: 10.1016/0896-6273(93)90220-l. 10.1016/0896-6273(93)90220-L. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Morales M, Lerma J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron. 1995;14:185–189. doi: 10.1016/0896-6273(95)90253-8. 10.1016/0896-6273(95)90253-8. [DOI] [PubMed] [Google Scholar]
- Patneau DK, Wright PW, Winters C, Mayer ML, Gallo V. Glial cells of the oligodendrocyte lineage express both kainate- and AMPA-preferring subtypes of glutamate receptor. Neuron. 1994;12:357–371. doi: 10.1016/0896-6273(94)90277-1. 10.1016/0896-6273(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Reichling DB, MacDermott AB. Lanthanum actions on excitatory amino acid-gated currents and voltage-gated calcium currents in rat dorsal horn neurons. The Journal of Physiology. 1991;441:199–218. doi: 10.1113/jphysiol.1991.sp018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripellino JA, Neve RL, Howe JR. Expression and heteromeric interactions of non-NMDA glutamate-receptor subunits in the developing and adult cerebellum. Neuroscience. 1998;82:485–497. doi: 10.1016/s0306-4522(97)00296-0. 10.1016/S0306-4522(97)00296-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Herreras O, Lerma J. Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron. 1997;19:893–901. doi: 10.1016/s0896-6273(00)80970-8. 10.1016/S0896-6273(00)80970-8. [DOI] [PubMed] [Google Scholar]
- Ruano D, Lambolez B, Rossier J, Paternain AV, Lerma J. Kainate receptor subunits expressed in single cultured hippocampal neurons: molecular and functional variants by RNA editing. Neuron. 1995;14:1009–1017. doi: 10.1016/0896-6273(95)90339-9. 10.1016/0896-6273(95)90339-9. [DOI] [PubMed] [Google Scholar]
- Sahara Y, Noro N, Iida Y, Soma KL, Nakamura Y. Glutamate receptor subunits GluR5 and KA-2 are co-expressed in rat trigeminal ganglion neurons. Journal of Neuroscience. 1997;17:6611–6620. doi: 10.1523/JNEUROSCI.17-17-06611.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B, Burnashev N, Verdoorn TA, Keinänen K, Sakmann B, Seeburg PH. A glutamate receptor channel with high affinity for domoate and kainate. EMBO Journal. 1992;11:1651–1656. doi: 10.1002/j.1460-2075.1992.tb05211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B, Köhler M, Sprengel R, Seeburg PH. RNA editing controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Feldmeyer D, Kaneda M, Cull-Candy SG. Effect of editing and subunit co-assembly on single-channel properties of recombinant kainate receptors. The Journal of Physiology. 1996;492:129–142. doi: 10.1113/jphysiol.1996.sp021295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson GT, Kamboj SV, Cull-Candy SG. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. Journal of Neuroscience. 1997;17:58–69. doi: 10.1523/JNEUROSCI.17-01-00058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Cull-Candy SG. Pharmacological properties and H+ sensitivity of excitatory amino acid receptor channels in rat cerebellar granule neurones. The Journal of Physiology. 1991;433:727–763. doi: 10.1113/jphysiol.1991.sp018453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wahl P. Control of rat GluR6 glutamate receptor open probability by protein kinase A and calcineurin. The Journal of Physiology. 1997;503:513–531. doi: 10.1111/j.1469-7793.1997.513bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignes M, Collingridge GL. The synaptic activation of kainate receptors. Nature. 1997;388:179–182. doi: 10.1038/40639. [DOI] [PubMed] [Google Scholar]
- Wilding TJ, Huettner JE. Differential antagonism of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-preferring and kainate-preferring receptors by 2,3-benzodiazepines. Molecular Pharmacology. 1995;47:582–587. [PubMed] [Google Scholar]
- Wilding TJ, Huettner JE. Activation and desensitization of hippocampal kainate receptors. Journal of Neuroscience. 1997;17:2713–2721. doi: 10.1523/JNEUROSCI.17-08-02713.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Seeburg PH. A complex mosaic of high-affinity kainate receptors in rat brain. Journal of Neuroscience. 1993;13:3582–3598. doi: 10.1523/JNEUROSCI.13-08-03582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LA, Mayer ML. Differential modulation by cyclothiazide and concanavalin A of desensitization at native α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid- and kainate-preferring glutamate receptors. Molecular Pharmacology. 1993;44:504–510. [PubMed] [Google Scholar]
- Wong LA, Mayer ML, Jane DE, Watkins JC. Willardines differentiate agonist binding sites for kainate- versus AMPA-preferring glutamate receptors in DRG and hippocampal neurons. Journal of Neuroscience. 1994;14:3881–3897. doi: 10.1523/JNEUROSCI.14-06-03881.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie DJA, Traynelis SF, Cull-Candy SG. Evidence for more than one type of non-NMDA receptor in outside-out patches from the cerebellar granule cells of the rat. The Journal of Physiology. 1993;463:193–226. doi: 10.1113/jphysiol.1993.sp019591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L-M, Gu Z-Q, Costa AM, Yamada KA, Mansson PE, Giordano T, Skolnick P, Jones KA. (2S,4R)-4-methylglutamic acid (SYM 2081): a selective, high-affinity ligand for kainate receptors. Journal of Pharmacology and Experimental Therapeutics. 1997;281:422–427. [PubMed] [Google Scholar]
- Zorumski CF, Yamada KA, Price MT, Olney JW. A benzodiazepine recognition site associated with the non-NMDA glutamate receptor. Neuron. 1993;10:61–67. doi: 10.1016/0896-6273(93)90242-j. 10.1016/0896-6273(93)90242-J. [DOI] [PubMed] [Google Scholar]


