Abstract
Rats habituate to repeated exposure to homotypic stressors. The present studies were designed to define how altered frequency of exposure to a stressor affects the development of habituation and how this habituation is reflected in alterations in basal expression and responsiveness of hypothalamic corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP) messenger and heteronuclear RNA (hnRNA).
Rats were exposed to a 60 min period of restraint stress every 7th day, every 3rd day, alternate days or daily for 2 weeks and their response to a final episode of stress on day 15 was compared with that of a control group of unstressed rats.
The response of plasma corticosterone to the final stressor on day 15 was diminished in animals which had been stressed on only two previous occasions, 7 days apart, and diminished further with increasing frequency of previous stressors until it failed to respond at all in animals stressed daily.
The pattern of CRH hnRNA and mRNA responses were similar, decreasing with increasing frequency of exposure to the stressor, while AVP mRNA responses increased in response to repeated stress.
The gradual emergence of increased AVP transcription at a time of diminishing CRH response suggests that repeated stress results in a specific facilitation of AVP gene expression, perhaps by impairment of corticosterone feedback.
Corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP) are synthesized in the parvocellular neurons of the paraventricular nucleus (PVN) (Whitnall, Smyth & Gainer, 1987) and are co-secreted from the nerve terminals in the external zone of the median eminence into the portal circulation as major regulators of pituitary adrenocorticotrophic hormone (ACTH) secretion during stress (Linton, Tilders, Hodgkinson, Berkenbosch, Vermes & Lowry, 1985; Antoni, 1993). AVP is only a weak ACTH secretagogue on its own and also acts synergistically with CRH (Gillies, Linton & Lowry, 1982; Scaccianoce, Muscolo, Cigliana, Navarra, Nicolai & Angelucci, 1991) to regulate ACTH secretion. There are two subpopulations of CRH neurons in the parvocellular division of the PVN, those in which CRH co-exists with AVP and those that do not express or only express undetectable levels of AVP under normal conditions (Whitnall et al. 1987). The ratio of AVP-positive to AVP-negative cells in the PVN increases in response to repeated stress (De Goeij, Kvetnansky, Whitnall, Jezova, Berkenbosch & Tilders, 1991; De Goeij, Jezova & Tilders, 1992b; Bartanusz, Jezova, Bertini, Tilders, Aubry & Kiss, 1993a).
In response to acute stress there is a rapid increase in levels of both CRH mRNA (Lightman & Young, 1988; Harbuz & Lightman, 1989; Lightman & Young 1989; Harbuz, Jessop, Lightman & Chowdrey, 1994; Makino, Smith & Gold, 1995; Imaki et al. 1995; Ma, Levy & Lightman, 1997a) and AVP mRNA (Lightman & Young, 1988; Bartanusz, Auby, Jezova, Baffi & Kiss, 1993b; Herman, 1995; Ma et al. 1997a) in the parvocellular division of the PVN. There have also been some studies, which have usually utilized quite severe stressors, which have shown increased CRH mRNA after repeated or chronic stress (Lightman & Young, 1989; Bartanusz et al. 1993a; Herman, Adams & Prewitt, 1995; Makino et al. 1995; Gomez, Lahmame, de Kloet & Armario, 1996). On the other hand repeated or chronic stress may result in an adaptation or desensitization of the hypothalamic-pituitary-adrenal (HPA) axis to the homotypic stressor whereby the response to subsequent stresses is reduced and may even be abolished (Kant, Lue, Anderson & Mougey, 1987; Hashimoto, Suemaru, Takao, Sugawara, Makino & Ota, 1988; Lightman & Harbuz, 1993; Ma, Levy & Lightman, 1997b). Previous studies in our laboratory have demonstrated that levels of CRH mRNA in the PVN fail to respond to restraint stress after repeated exposure to daily restraint for 12 days (Lightman et al. 1993) or actually decrease during chronic inflammatory stress (Harbuz, Rees, Eckland, Jessop, Brewerton & Lightman, 1992; Chowdrey et al. 1995). Chronic osmotic stimulation also results in a decrease in CRH mRNA in the PVN associated with the reduction of ACTH responsiveness (Aguilera, Lightman & Kiss, 1993; Lightman & Harbuz, 1993). A recent study showed that chronic social stress can also downregulate CRH mRNA levels in the PVN in conjunction with impaired corticosterone responses to stress in non-responsive subordinates (Albeck et al. 1997).
There is now increasing evidence that AVP has an important role in repeated and chronic stress protocols (Hashimoto et al. 1988; Whitnall, 1989; Scaccianoce et al. 1991; De Goeij et al. 1991, 1992b; Chowdrey et al. 1995; Makino et al. 1995). Transient activation of CRH neurons in the PVN results in a long-lasting increase in AVP co-expression (Schmidt, Janszen, Binnekade & Tilders, 1997). Repeated stress has been shown to result in a recruitment of CRH neurons to produce AVP that may represent a physiological response to increased functional demand (De Goeij et al. 1991; Bartanusz et al. 1993a). There are, however, two reports that AVP mRNA levels in the parvocellular PVN did not change significantly in response to 30 days of repeated stress or 14 days of chronic social stress (Herman et al. 1995; Albeck et al. 1997). These data suggest that the individual responses of CRH and AVP transcription in the parvocellular neurons of the PVN in response to chronic or repeated stress are stressor specific. Since repeated or chronic stress-induced adaptive changes in CRH neurons of the PVN need time to develop, the number or frequency of repetitions of the repeated stress may also be important parameters governing the development of adaptive changes in the CRH neuron. To investigate this possibility we have studied CRH and AVP gene transcription in the parvocellular PVN in response to different frequencies of exposure to a homotypic stressor.
METHODS
Adult male Sprague-Dawley rats weighing 200–250 g were group-housed and exposed to a 12 h light-dark cycle for 5 days prior to study. Food and water were available ad libitum. All experiments were carried out between 09.00 h and 13.00 h. Stress consisted of restraint in plastic restraint cages for 60 min following which the animals were either killed immediately by decapitation or returned to their home cages. All procedures were carried out in accordance with UK Home Office regulations.
There were fourteen experimental groups (see Table 1). The first group (n = 7) consisted of unhandled control animals (UC) which were killed without any treatment on day 15. The second control group (n = 7) was handled daily for 14 days (HC) and was killed on day 15. The third group (n = 6) was restrained on alternate days for 14 days (AD) and was killed on day 15 without a final period of restraint. The fourth control group (n = 6) was restrained daily for 14 days (D) and was killed on day 15 without a final period of restraint. The fifth and the sixth groups were restrained on day 15 only (S). The seventh and the eighth groups were restrained once every 7 days and killed after a final restraint on day 15 (7S). The ninth and the tenth groups were restrained every third day for 15 days and killed after a final restraint on day 16 (3S). The eleventh and the twelfth groups were restrained on alternate days for 14 days and killed after a final restraint on day 15 (ADS). The last two groups of rats were restrained daily for 14 days and killed after a final restraint on day 15 (DS). All rats in groups 5, 7, 9, 11 and 13 were restrained for 60 min (n = 6) on the last day and killed by decapitation immediately. All rats in groups 6, 8, 10, 12 and 14 were restrained for 60 min and then returned to their home cages, and killed 3 h (n = 6) after the onset of the last 60 min of restraint at the same time. All rats from different groups were killed on day 15 or day 16 at the same time.
Table 1.
Arginine vasopressin and CRH gene transcription responses to varied frequencies of repeated stress in rats
| Day | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
| No stress on day 15 | 1 | Unhandled control (UC) | — | — | — | — | — | — | — | — | — | — | — | — | — | — | killed | |
| 2 | Daily handled control (HC) | × | × | × | × | × | × | × | × | × | × | × | × | × | × | killed | ||
| 3 | Alternate day stress (AD) | s | — | s | — | s | — | s | — | s | — | s | — | s | — | killed | ||
| 4 | Daily stress (D) | s | s | s | s | s | s | s | s | s | s | s | s | s | s | killed | ||
| Stress on day 15 | 5, 6 | Single acute stress (S) | — | — | — | — | — | — | — | — | — | — | — | — | — | — | s, killed | |
| 7, 8 | Stress every 7th day (7S) | s | — | — | — | — | — | — | s | — | — | — | — | — | — | s, killed | ||
| 9, 10 | Stress every 3rd day (3S) | s | — | — | s | — | — | s | — | — | s | — | — | s | — | — | s, killed | |
| 11, 12 | Alternate day stress (ADS) | s | — | s | — | s | — | s | — | s | — | s | — | s | — | s, killed | ||
| 13, 14 | Daily stress (DS) | s | s | s | s | s | s | s | s | s | s | s | s | s | s | s, killed | ||
s, stress applied by restraint in plastic restraint cages; ×, control handling.
Animals were killed by decapitation. Trunk blood was collected into heparinized tubes, and the brains removed and frozen on dry ice. Sections of 12 μm were cut through the medial parvocellular subdivision of the PVN, thaw mounted on twice-gelatin-coated slides and stored at −80°C.
In situ hybridization histochemistry
In situ hybridization was performed as previously described (Lightman & Young, 1988). Briefly, prior to hybridization, sections were air dried at room temperature and fixed with 4 % formaldehyde for 5 min at room temperature, washed 3 times with phosphate-buffered saline (PBS), and then incubated in 0.25 % acetic anhydride in 0.1 M triethanolamine-0.9 % NaCl (pH 8.0) for 10 min at room temperature. Sections were transferred through 70 % (1 min), 80 % (1 min), 95 % (2 min) and 100 % ethanol (1 min); 100 % chloroform (5 min); and 100 % (1 min) and 95 % ethanol (1 min); and dried. All control and experimental sections were hybridized in the same hybridization reaction.
The rat CRH intron (CRHin) probe (kindly supplied by Dr B. Thompson, University of Michigan, Ann Arbor, MI, USA), was generated from a 530 base pair (bp) pvuII fragment of the CRH gene subcloned into pGEM-3 (Promega, Madison, WI, USA) and linearized by XbaI. The rat CRH (CRHex 2) cDNA (B. Thompson) was a 770 bp BamHI fragment subcloned in pGEM-3Z (Promega), linearized by Hind III. The 35S-cRNA probes for CRHin and CRH exon (CRHex) were produced as previously described (Ma et al. 1997a). Sections were hybridized at 55°C with 1 × 106 c.p.m. labelled CRHin or CRHex probe per slide containing two sections. Then non-specifically hybridized probe was removed by washing 2 times with 2 × saline-sodium citrate buffer (SSC; 1 × SSC = 0.15 M NaCl, 0.015 M sodium citrate, pH 7.0) containing 50 % formamide at 50°C, incubated with ribonuclease A for 30 min at 37°C, followed by washing 2 times with 2 × SSC containing 50 % formamide at 50°C and 2 times with 2 × SSC at room temperature for 10 min. Finally, slides were dipped in 70 % ethanol and air dried.
The AVP exonic probe was a 48-base oligonucleotide complementary to part of the exonic mRNA sequence coding for AVP. The probes were labelled with 35S-dATP as previously described (Ma et al. 1997a). In brief, sections were hybridized overnight at 37°C with 3 × 105 c.p.m. AVP probe per two sections, then washed in four 15 min rinses of 1 × SSC on a shaking waterbath (55°C), followed by two 30 min rinses in 1 × SSC at room temperature. Finally, slides were dipped in 70 % ethanol and air dried.
Plasma corticosterone assays
Total plasma corticosterone was determined in plasma (5 μl diluted in 500 μl buffer) by radioimmunoassay as described previously (Harbuz et al. 1994). 125I-labelled corticosterone was used as the tracer with a specific activity of 2–3 mCi mg−1. Corticosterone antibody was a kind gift of Dr G. Makara, Hungarian Academy of Science, Budapest, Hungary. The sensitivity of the assay was 25 ng ml−1.
Analysis and quantification
For quantification of CRH hnRNA and CRH mRNA in the PVN, the sections and 14C-labelled standards of known radioactivity (American Radiochemical, St Louis, MO, USA) were placed in X-ray cassettes and then exposed to Hyperfilm MP autoradiography film (Amersham International plc, Amersham, UK) for 3 days (CRHex) or 15 days (CRHin). For cellular localization of AVP and CRH hybrids, the slides were subsequently dipped in nuclear emulsion (K-5; Ilford, Mobberley, Cheshire, UK) and exposed for appropriate times (AVPex, 9 days; CRHin, 35 days; CRHex, 12 days). The slides were counterstained with Cresyl Violet acetate (Sigma). The optical density of autoradiographic images was measured by using a computerized image analysis system as described (Harbuz et al. 1994). In brief, the autoradiographic images of the probe bound to parvocellular CRH mRNA and CRH hnRNA, together with 14C-labelled standards, were measured using a computerized image analysis system (Image 1.22, developed by W. Rasband, National Institutes of Health, Bethesda, MD, USA) on an Apple Mac IICi computer. The optical densities were obtained in two consecutive sections per rat, the average value for each rat was used to calculate group means, and the results are presented as mean percentage change from unhandled control with s.e.m. Analysis of changes in the prevalence of AVP mRNA in the medial parvocellular and posterior magnocellular subdivisions of the PVN was carried out in the Cresyl Violet counterstained sections using a × 40 objective with brightfield condenser as described by Herman (1995). Briefly, the medial parvocellular AVP neurons in the PVN were differentiated histologically from magnocellular neurons on the basis of their overall size, their relatively low level of AVP expression and their small, dense-staining nuclei. Magnocellular cells in the medial parvocellular subdivision were characterized by large numbers of superimposed silver grains and their large size. The neurons in the medial parvocellular subdivision of the PVN were considered to be positive for AVP mRNA if overlying grain densities were at least 3 times that of background, measured in the vicinity of the labelled cells. The relative levels of AVP mRNA were quantified in the medial parvocellular and posterior magnocellular subdivisions of PVN using computerized densitometry as described above on counterstained coronal sections, subtracting background from the proximity of the measured cells. The grain density measurements for parvocellular AVP mRNA were made over individual cells identified as parvocellular, and scattered magnocellular cells in the medial parvocellular subdivision of the PVN were excluded from the analysis on the basis of their histological appearance. For each animal, at least four paraventricular nuclei on two sections were assessed, and the average value of each section for each rat was used to calculate group means. The results are presented as mean percentage change from unhandled control with s.e.m.
Statistical analysis was performed by one-way ANOVA followed by Fisher's protected least significant difference (PLSD) test to assess statistical significance between control and experimental groups at each time point. P < 0.05 was considered to be statistically significant.
RESULTS
The changes of plasma corticosterone
Daily handling, alternate day stress or daily stress had no effect on basal levels of corticosterone. Acute restraint stress in naive rats resulted in an increase in plasma corticosterone to 908.7 % of unhandled control at 1 h. The effect of previous exposure to this same stress can be seen in Fig. 1, which shows a diminution in responsiveness which increases as the frequency of previous exposure to stress increases. Thus rats that had only been exposed to the stressor on two occasions, the 1st and the 8th day previously, showed 664.8 % of the unhandled control response. Rats exposed to stress every 3 days showed 363.5 % of the unhandled control response, rats exposed to stress on alternate days showed 263.5 % of the unhandled control response and finally rats exposed to daily stress showed no significant response at all.
Figure 1. Corticosterone response in each experimental group.
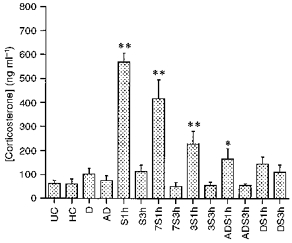
For explanation of the experimental groups see Table 1. Values are presented as means +s.e.m.**P < 0.01 compared with UC; *P < 0.05 compared with UC (one-way ANOVA followed by Fisher's PLSD test).
The changes of CRH hnRNA and CRH mRNA in the PVN
Expression of CRH hnRNA in the PVN under resting conditions was extremely low, but a signal could be observed in the parvocellular subdivision of the PVN compared with the neighbouring section hybridized with sense probe (Fig. 2A and B). Basal levels of CRH hnRNA or mRNA were unaffected by daily handling, alternate day stress for 14 days or daily stress for 14 days (Figs 4 and 5). In rats which were previously naive or exposed to the stress on two previous occasions 7 days apart, the levels of CRH hnRNA in the PVN significantly increased at 60 min (Fig. 2C) (P < 0.01vs. unhandled control) while the level of CRH mRNA increased at 3 h (P < 0.05vs. unhandled control) after the onset of the 60 min period of restraint (Figs 4 and 5). In rats which had been exposed to repeated restraint every 3rd day or alternate days the rise in CRH hnRNA (Fig. 2D and E) (P < 0.05vs. unhandled control) and mRNA levels showed a diminished response to the last period of stress. The response of both CRH hnRNA and CRH mRNA to restraint disappeared in rats which had been exposed to daily repeated restraint for 14 days (Figs 2F, 4 and 5). Corticosterone concentrations were highly positively correlated with CRH hnRNA (r = 0.955, P < 0.0001) but are not correlated with CRH mRNA levels (r = 0.13, P = 0.66).
Figure 2. Effect of acute and repeated restraint on CRH hnRNA levels in the PVN.
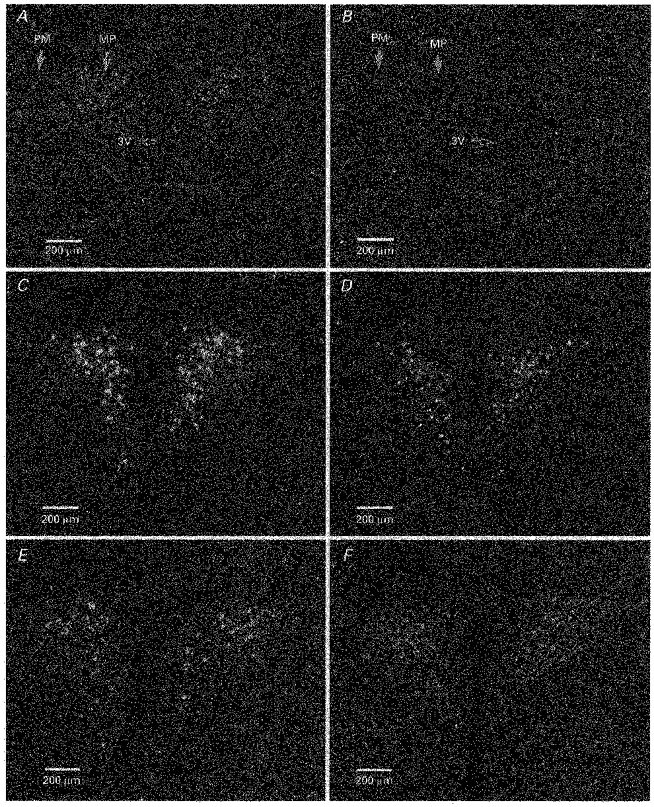
Dark-field photographs taken through the medial parvocellular subdivision of the PVN showing CRH hnRNA signals under normal resting conditions (unhandled control) (A), 60 min after the onset of single acute restraint (C), and 60 min after the onset of last period of restraint in repeatedly stressed rats (D, once every 3 days; E, alternate days; F, daily). No signal was observed in the parvocellular PVN hybridized with sense probe (B). 3V, 3rd ventricle; PM, posterior magnocellular subdivision of the PVN; MP, parvocellular subdivision of the PVN.
Figure 4. The levels of CRH hnRNA in the PVN in response to acute and repeated stress.

**P < 0.01 compared with UC, *P < 0.05 compared with UC. The results are presented as percentage means +s.e.m. change from UC (one-way ANOVA followed by Fisher's PLSD test).
Figure 5. The levels of CRH mRNA in the PVN in response to acute and repeated stress.

*P < 0.05 compared with UC. The results are presented as percentage means +s.e.m. change from UC (one-way ANOVA followed by Fisher's PLSD test).
The changes of parvocellular AVP mRNA in the PVN
Basal expression of AVP mRNA in the parvocellular neurons of the PVN was very low under normal unstressed conditions (Fig. 3A and B) and was unaffected by daily handling, alternate day stress or daily stress (Fig. 6). The level of AVP mRNA in the naive rats increased 3 h after the onset of 60 min acute restraint (Fig. 3C). The levels of parvocellular AVP mRNA increased 3 h after the onset of the last period of restraint in all repeatedly stressed groups and the amplitude of increased parvocellular AVP mRNA levels progressively increased with the increased frequency of the previous episodes of restraint, although the difference between S3h and either ADS3h or DS3h did not reach significant difference (Figs 3D,E and F, and 6). Parvocellular AVP mRNA levels were negatively correlated with corticosterone concentrations (r = −0.42, P = 0.14) and not correlated with CRH hnRNA (r = −0.02, P = 0.95) or CRH mRNA (r = 0.34, P = 0.24).
Figure 3. Effect of acute and repeated restraint on AVP mRNA levels in the parvocellular neurons of the hypothalamic PVN.
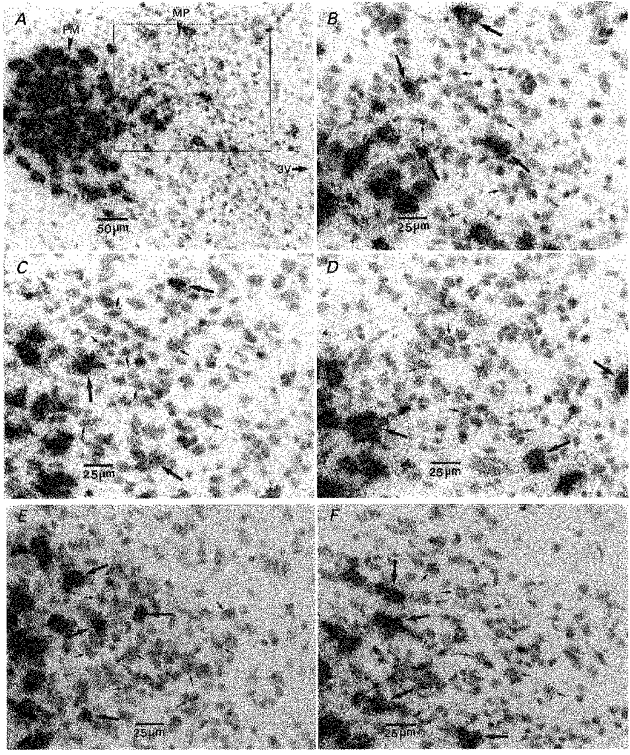
Bright-field photographs taken through the parvocellular subdivision of the PVN showing signals under normal resting conditions (unhandled control) (A and B), 3 h after the onset of single acute restraint (C), and 3 h after the onset of last period of restraint in repeatedly stressed rats (D, once every 3 days; E, alternate days; F, daily). 3V, 3rd ventricle; PM, posterior magnocellular subdivision of the PVN; MP, medial parvocellular subdivision of the PVN; small arrows, parvocellular neurons; large arrows, magnocellular neurons.
Figure 6. The levels of AVP mRNA in the parvocellular subdivision of PVN (parvoAVP mRNA) in response to acute and repeated stress.

**P < 0.01 compared with UC, *P < 0.05 compared with UC. The results are presented as percentage means +s.e.m. change from UC (one-way ANOVA followed by Fisher's PLSD test).
Magnocellular AVP mRNA in the posterior magnocellular subdivision of the PVN was measured throughout the study. No change in magnocellular AVP mRNA was found at any time point.
DISCUSSION
Repeated stress can result in habituation of corticosterone responses to the same (homotypic) stressor. This has been described for restraint stress (Armario, Hidalgo & Giralt, 1988; Hashimoto et al. 1988; Hauger, Lorang, Irwin & Aguilera, 1990), water immersion (De Boer, Koopmans, Slangen & Van Der Gugten, 1990) and foot shock (Kant et al. 1987). The desensitization of corticosterone responsiveness to repeated restraint may in part be associated with the desensitization of the pituitary ACTH response and downregulation of the anterior pituitary CRH receptors (Hauger et al. 1990) but exposure of chronically stressed rats to a different (heterotypic) stressor induces greater and more rapid increases in plasma ACTH and corticosterone (Vernikos, Dallman, Bonner, Katzen & Shinsako, 1982; Hashimoto et al. 1988). This suggests that a centrally mediated mechanism can selectively modulate the response to different incoming signals.
We have now investigated whether the frequency of exposure to a stressor has a direct effect on the response, and we have chosen a specific stress protocol - restraint - which is known to show habituation.
Our data confirm that the levels of corticosterone and of CRH hnRNA and mRNA increase significantly in response to acute restraint stress. These increases became progressively smaller as the frequency of previous exposure to the homotypic stressor increased such that finally the response to restraint disappeared altogether after rats had been restrained daily for 14 days. In contrast the response of AVP gene transcription in the parvocellular PVN is maintained, and indeed the levels of AVP mRNA in the parvocellular PVN progressively increased in response to restraint with increased frequency of previous exposures to this stress. The upregulation of parvocellular AVP gene transcription in this experimental model is consistent with previous studies showing that parvocellular AVP mRNA levels rise significantly after repeated immobilization (Bartanusz et al. 1993a; Makino et al. 1995; Ma et al. 1997b) or repeated foot shock (Sawchenko, Arias & Mortrud, 1993). These data suggest that AVP synthesized in the parvocellular PVN plays a more important role in maintaining HPA axis activity under conditions of chronic stress and may be involved in the relative resistance of the HPA axis to adaptation in response to such repeated stress (Scaccianoce et al. 1991), and even in the increased responsiveness to heterotypic stressors (Hashimoto et al. 1988).
The significant increase of AVP transcription but not CRH transcription in the parvocellular PVN represents remarkable transcript-specific alteration in gene regulation and supports the hypothesis that parvocellular AVP and CRH gene expression, translation, storage and secretion in the PVN may have quite different control mechanisms (Herman, Wiegand & Watson, 1990; De Goeij et al. 1992b; Tilders, Schmidt & De Goeij, 1993; Kovács & Sawchenko, 1996; Schmidt et al. 1997). Repeated immobilization increased the proportion of CRH parvocellular neurons containing AVP from 50 % to 90 % and resulted in a twofold increase in the number of AVP-positive parvocellular neurons, while the number of CRH parvocellular neurons remained at a normal level (Bartanusz et al. 1993a). In addition, repeated exposure to immobilization once daily did not affect CRH stores but resulted in a progressive increase in the AVP stores and co-localization of AVP with CRH in the median eminence (De Goeij et al. 1991). In another study psychosocial stress was found to increase AVP content in the external zone of the median eminence to 160–190 % of that found in controls while CRH content in the external zone of the median eminence did not change (Tilders et al. 1993). Furthermore, AVP secretion from the CRH terminals of the external zone of the median eminence increased in response to repeated or chronic stress (De Goeij et al. 1992b; De Goeij, Binnekade & Tilders, 1992a; Tilders et al. 1993; Bartanusz et al. 1993a). It is also worth noting that transient activation of hypothalamic CRH neurons by a single stressor results in long-lasting increases in AVP co-expression irrespective of the nature of the stressor, which is in most cases not accompanied by changes of CRH (Schmidt et al. 1997). Repeated immobilization resulted in a significant increase of response of plasma corticosterone and ACTH to exogenous AVP, but not CRH (Hashimoto et al. 1988). Repeated immobilization also induced an increase in AVP binding affinity and AVP V1b receptor mRNA in the pituitary (Rabadan-Diehl, Lolait & Aguilera, 1995). In contrast, repeated immobilization results in downregulation of anterior pituitary CRH receptors and the concomitant reduction of corticotrope responsiveness to CRH, but not AVP (Hauger, Millan, Lorang, Harwood & Aguilera, 1988).
These differences in the regulation of AVP and CRH transcription are further emphasized by previous studies which have demonstrated that the response of the AVP gene in CRH neurons to corticosterone is more sensitive than that of the CRH gene (Makino et al. 1995; Ma, Levy & Lightman, 1997c), and that the glucocortocoid receptors in the PVN are downregulated in response to repeated stress (Makino et al. 1995; Herman et al. 1995). This suggests a complex regulatory mechanism by which repeated stress may temporarily impair corticosterone feedback and thus facilitate AVP gene expression in the CRH neurons.
Acknowledgments
The authors would like to thank Dr G. Aguilera for her support in preparation of this manuscript, and Dr K. Kondo for his help in measuring plasma corticosterone concentration. This work was supported by a grant from the Neuroendocrinology Charitable Trust (to X.-M. M).
References
- Aguilera G, Lightman SL, Kiss A. Regulation of the hypothalamic-pituitary-adrenal axis during water deprivation. Endocrinology. 1993;132:241–248. doi: 10.1210/endo.132.1.8380375. 10.1210/en.132.1.241. [DOI] [PubMed] [Google Scholar]
- Albeck DS, McKittrick CR, Blanchard DC, Blanchard RJ, Nikulina J, McEwen BS, Sakai RR. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. Journal of Neuroscience. 1997;17:4895–4903. doi: 10.1523/JNEUROSCI.17-12-04895.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni FA. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Frontiers in Neuroendocrinology. 1993;14:76–122. doi: 10.1006/frne.1993.1004. 10.1006/frne.1993.1004. [DOI] [PubMed] [Google Scholar]
- Armario A, Hidalgo J, Giralt M. Evidence that the pituitary-adrenal axis does not cross adapt to stressors: comparison to other physiological variables. Neuroendocrinology. 1988;47:263–267. doi: 10.1159/000124921. [DOI] [PubMed] [Google Scholar]
- Bartanusz V, Jezova D, Bertini LT, Tilders FJH, Aubry JM, Kiss JZ. Stress-induced increase in vasopressin and corticotropin-releasing factor expression in hypophysiotrophic paraventricular neurons. Endocrinology. 1993a;132:895–902. doi: 10.1210/endo.132.2.8425502. [DOI] [PubMed] [Google Scholar]
- Bartanusz V, Aubry J-M, Jezova D, Baffi J, Kiss JZ. Upregulation of vasopressin mRNA in paraventricular hypophysiotrophic neurons after acute immobilization stress. Neuroendocrinology. 1993b;58:625–629. doi: 10.1159/000126602. [DOI] [PubMed] [Google Scholar]
- Chowdrey HS, Larsen PJ, Harbuz MS, Jessop DS, Aguilera G, Eckland DJ, Lightman SL. Evidence for arginine vasopressin as the primary activator of the HPA axis during adjuvant-induced arthritis. British Journal of Pharmacology. 1995;116:2417–2424. doi: 10.1111/j.1476-5381.1995.tb15089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer SF, Koopmans SJ, Slangen JL, Van der Gugten J. Plasma catecholamine, corticosterone, and glucose responses to repeated stress in rats: effect of interstressor interval length. Physiology and Behavior. 1990;47:1117–1124. doi: 10.1016/0031-9384(90)90361-7. 10.1016/0031-9384(90)90361-7. [DOI] [PubMed] [Google Scholar]
- De Goeij DCE, Binnekade R, Tilders FJH. Chronic stress enhances vasopressin but not corticotropin-releasing factor secretion during hypoglycemia. American Journal of Physiology. 1992a;263:E394–399. doi: 10.1152/ajpendo.1992.263.2.E394. [DOI] [PubMed] [Google Scholar]
- De Goeij DCE, Jezova D, Tilders FJH. Repeated stress enhances vasopressin synthesis in corticotropin releasing factor neurons in the paraventricular nucleus. Brain Research. 1992b;577:165–168. doi: 10.1016/0006-8993(92)90552-k. 10.1016/0006-8993(92)90552-K. [DOI] [PubMed] [Google Scholar]
- De Goeij DCE, Kvetnansky R, Whitnall MH, Jezova D, Berkenbosch F, Tilders FJH. Repeated stress-induced activation of corticotropin-releasing factor neurons enhances vasopressin stores and colocalization with corticotropin releasing factor neurons in the median eminence of rats. Neuroendocrinology. 1991;53:150–159. doi: 10.1159/000125712. [DOI] [PubMed] [Google Scholar]
- Gillies G, Linton EA, Lowry PF. Corticotropin-releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982;299:355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- Gomez F, Lahmame A, de Kloet ER, Armario A. Hypothalamic-pituitary-adrenal response to chronic stress in five inbred rat strains: Differential responses are mainly located at the adrenocortical level. Neuroendocrinology. 1996;63:327–337. doi: 10.1159/000126973. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Jessop DS, Lightman SL, Chowdrey HS. The effect of restraint or hypertonic saline stress on corticotropin-releasing factor, arginine vasopressin, and proenkephalin A mRNAs in the CFY, Sprague-Dawley and Wistar strains of rat. Brain Research. 1994;667:6–12. doi: 10.1016/0006-8993(94)91707-8. 10.1016/0006-8993(94)91707-8. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Lightman SL. Responses of hypothalamic and pituitary mRNA to physical and physiological stress in the rat. Journal of Endocrinology. 1989;122:705–711. doi: 10.1677/joe.0.1220705. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Rees RG, Eckland D, Jessop DS, Brewerton D, Lightman SL. Paradoxical responses of hypothalamic corticotropin-releasing factor (CRF) messenger ribonucleic acid (mRNA) and CRF-41 peptide and adenohypophysial proopiomelanocortin mRNA during chronic inflammatory stress. Endocrinology. 1992;130:1394–1400. doi: 10.1210/endo.130.3.1537299. 10.1210/en.130.3.1394. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Suemaru S, Takao T, Sugawara M, Makino S, Ota Z. Corticotropin-releasing hormone and pituitary adrenocortical responses in chronically stressed rats. Regulatory Peptides. 1988;23:117–126. doi: 10.1016/0167-0115(88)90019-5. 10.1016/0167-0115(88)90019-5. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Lorang M, Irwin M, Aguilera G. CRF receptor regulation and sensitization of ACTH responses to acute ether stress during chronic intermittent immobilization stress. Brain Research. 1990;532:34–40. doi: 10.1016/0006-8993(90)91738-3. 10.1016/0006-8993(90)91738-3. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Millan MA, Lorang M, Harwood JP, Aguilera G. Corticotropin-releasing factor receptors and pituitary adrenal responses during immobilization stress. Endocrinology. 1988;123:396–405. doi: 10.1210/endo-123-1-396. [DOI] [PubMed] [Google Scholar]
- Herman JP. In situ hybridization analysis of vasopressin gene transcription in the paraventricular and supraoptic nuclei of the rat: Regulation by stress and glucocorticoids. Journal of Comparative Neurology. 1995;363:15–27. doi: 10.1002/cne.903630103. [DOI] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Herman JP, Wiegand SJ, Watson SJ. Regulation of basal corticotropin-releasing hormone and arginine vasopressin messenger ribonucleic acid expression in the paraventricular nucleus: effects of selective hypothalamic deafferentations. Endocrinology. 1990;127:2408–2417. doi: 10.1210/endo-127-5-2408. [DOI] [PubMed] [Google Scholar]
- Imaki T, Wang X-Q, Shibasaki T, Yamada K, Harada S, Chikada N, Naruse M, Demura H. Stress-induced activation of neuronal activity and corticotropin-releasing factor gene expression in the paraventricular nucleus is modulated by glucocorticoid in rats. Journal of Clinical Investigation. 1995;96:231–238. doi: 10.1172/JCI118026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant GJ, Lue JR, Anderson SM, Mougey EH. Effects of chronic stress on plasma corticosterone, ACTH and prolactin. Physiology and Behavior. 1987;40:775–779. doi: 10.1016/0031-9384(87)90282-4. 10.1016/0031-9384(87)90282-4. [DOI] [PubMed] [Google Scholar]
- Kovács KJ, Sawchenko PE. Regulation of stress-induced transcriptional changes in the hypothalamic neurosecretory neurons. Journal of Molecular Neuroscience. 1996;7:125–133. doi: 10.1007/BF02736792. [DOI] [PubMed] [Google Scholar]
- Leng G, Russell JA. Learning to cope with repeated stress. The Journal of Physiology. 1998;510:331. doi: 10.1111/j.1469-7793.1998.331bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman SL, Harbuz MS. Expression of corticotropin releasing factor mRNA in response to stress. In: Chadwick DJ, Marsh J, Ackill K, editors. Corticotropin-Releasing Factor. Chichester, UK: John Wiley & Sons; 1993. pp. 173–188. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Young WS., III Corticotropin-releasing factor, vasopressin and pro-opiomelanocortin mRNA responses to stress and opiates in the rat. The Journal of Physiology. 1988;403:511–523. doi: 10.1113/jphysiol.1988.sp017261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman SL, Young WS., III Influence of steroids on the hypothalamic corticotropin-releasing factor and preproenkephalin mRNA responses to stress. Proceedings of the National Academy of Sciences of the USA. 1989;86:4306–4310. doi: 10.1073/pnas.86.11.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton EA, Tilders FJH, Hodgkinson S, Berkenbosch F, Vermes I, Lowry PJ. Stress-induced secretion of adrenocorticotropin in rats is inhibited by administration of antisera to ovine corticotropin releasing factor and vasopressin. Endocrinology. 1985;116:966–970. doi: 10.1210/endo-116-3-966. [DOI] [PubMed] [Google Scholar]
- Ma X-M, Levy A, Lightman SL. Rapid changes in heteronuclear RNA for corticotropin-releasing hormone and arginine vasopressin in response to acute stress. Journal of Endocrinology. 1997a;152:81–89. doi: 10.1677/joe.0.1520081. [DOI] [PubMed] [Google Scholar]
- Ma X-M, Levy A, Lightman SL. Emergence of an isolated AVP response to stress following repeated restraint: a study of both AVP and CRH mRNA and hnRNA. Endocrinology. 1997b;138:4351–4357. doi: 10.1210/endo.138.10.5446. 10.1210/en.138.10.4351. [DOI] [PubMed] [Google Scholar]
- Ma X-M, Levy A, Lightman SL. Rapid changes of heteronuclear RNA for arginine vasopressin but not for corticotropin-releasing hormone in response to acute corticosterone administration. Journal of Neuroendocrinology. 1997c;9:723–728. doi: 10.1046/j.1365-2826.1997.00646.x. 10.1046/j.1365-2826.1997.00646.x. [DOI] [PubMed] [Google Scholar]
- Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136:3299–3309. doi: 10.1210/endo.136.8.7628364. 10.1210/en.136.8.3299. [DOI] [PubMed] [Google Scholar]
- Rabadan-Diehl C, Lolait SJ, Aguilera G. Regulation of pituitary vasopressin V1b receptor mRNA during stress in the rat. Journal of Neuroendocrinology. 1995;7:903–910. doi: 10.1111/j.1365-2826.1995.tb00734.x. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Arias CA, Mortrud MT. Local tetrodoxin blocks chronic stress effects on corticotropin-releasing factor and vasopressin messenger ribonucleic acid in hypophysiotropic neurons. Journal of Neuroendocrinology. 1993;5:341–348. doi: 10.1111/j.1365-2826.1993.tb00493.x. [DOI] [PubMed] [Google Scholar]
- Scaccianoce S, Muscolo LAA, Cigliana G, Navarra D, Nicolai R, Angelucci L. Evidence for a specific role of vasopressin in sustaining pituitary-adrenocortical stress response in the rat. Endocrinology. 1991;128:3138–3143. doi: 10.1210/endo-128-6-3138. [DOI] [PubMed] [Google Scholar]
- Schmidt ED, Janszen JW, Binnekade R, Tilders FJH. Transient suppression of resting corticosterone levels induces sustained increase of AVP stores in hypothalamic CRH-neurons of rats. Journal of Neuroendocrinology. 1997;9:69–77. doi: 10.1046/j.1365-2826.1997.00618.x. 10.1046/j.1365-2826.1997.00618.x. [DOI] [PubMed] [Google Scholar]
- Tilders FJH, Schmidt ED, De Goeij DCE. Phenotypic plasticity of CRF neurons during stress. Annals of the New York Academy of Sciences. 1993;697:39–52. doi: 10.1111/j.1749-6632.1993.tb49921.x. [DOI] [PubMed] [Google Scholar]
- Vernikos J, Dallman MF, Bonner C, Katzen A, Shinsako J. Pituitary-adrenal function in rats chronically exposed to cold. Endocrinology. 1982;110:413–420. doi: 10.1210/endo-110-2-413. [DOI] [PubMed] [Google Scholar]
- Whitnall MH. Stress selectively activates the vasopressin-containing subset of corticotropin-releasing hormone neurons. Neuroendocrinology. 1989;50:702–707. doi: 10.1159/000125302. [DOI] [PubMed] [Google Scholar]
- Whitnall MH, Smyth D, Gainer H. Vasopressin coexists in half of the corticotropin-releasing factor axons present in the external zone of the median eminence in normal rats. Neuroendocrinology. 1987;45:420–424. doi: 10.1159/000124768. [DOI] [PubMed] [Google Scholar]


