Abstract
A high density of angiotensin II receptors was observed in the rat carotid body by in vitro autoradiography employing 125I-[Sar1,Ile8]-angiotensin II as radioligand. Displacement studies demonstrated that the receptors were of the AT1 subtype.
The binding pattern indicated that the AT1 receptors occurred over clumps of glomus cells, the principal chemoreceptor cell of the carotid body. Selective lesions of the sympathetic or afferent innervation of the carotid body had little effect on the density of receptor binding, demonstrating that the majority of AT1 receptors were intrinsic to the glomus cells.
To determine the direct effect of angiotensin II on chemoreceptor function, without the confounding effects of the vasoconstrictor action of angiotensin II, carotid sinus nerve activity was recorded from the isolated carotid body in vitro. The carotid body was superfused with Tyrode solution saturated with carbogen (95% O2, 5% CO2), maintained at 36 °C, and multi-unit nerve activity recorded with a suction electrode.
Angiotensin II elicited a dose-dependent excitation of carotid sinus nerve activity (maximum increase of 36 ± 11% with 10 nm angiotensin II) with a threshold concentration of 1 nm. The response was blocked by the addition of an AT1 receptor antagonist, losartan (1 μm), but not by the addition of an AT2 receptor antagonist, PD123319 (1 μm).
In approximately 50% of experiments the excitation was preceded by an inhibition of activity (maximum decrease of 24 ± 8% with 10 nm angiotensin II). This inhibitory response was markedly attenuated by losartan but not affected by PD123319.
These observations demonstrate that angiotensin II, acting through AT1 receptors located on glomus cells in the carotid body, can directly alter carotid chemoreceptor afferent activity. This provides a means whereby humoral information about fluid and electrolyte homeostasis might influence control of cardiorespiratory function.
The octapeptide angiotensin II (Ang II) is well characterized as a circulating humoral and tissue-based regulator of fluid and electrolyte homeostasis and cardiovascular function. These actions occur through effects on many tissues, including kidney, adrenal gland, vasculature and brain (Timmermans et al. 1993).
The role of Ang II in the response to altered respiratory function is less well studied. Many studies indicate that systemic Ang II levels are increased during hypoxia (Rose, Kimmel, Godine, Kaiser & Carey, 1983; Honig, 1989; Marshall, 1994), and that Ang II may be involved in the renal and cardiovascular responses to hypoxia (Marshall & Metcalfe, 1990; Louwerse & Marshall, 1992; Neylon, Marshall & Johns, 1996). An effect on central pathways regulating respiration, acid-base balance and hence ion concentration has also been proposed (Ohtake & Jennings, 1993). There is also one report of an excitatory effect of Ang II on carotid chemoreceptor activity in the anaesthetized cat, although this was suggested to be a secondary response to local, vasoconstriction-induced hypoxia (McQueen, 1981).
Whilst the carotid body is predominantly involved in sensing blood gas composition and inducing compensatory reflex alterations in respiratory and cardiovascular control, it is clear that these reflexes also involve alterations in fluid and electrolyte homeostasis (Honig, 1989). One of the initial responses to hypoxia involves a reduction in extracellular plasma volume with consequent concentration of haemoglobin and an increase in the oxygen carrying capacity of blood (Assmussen & Nielsen, 1945; Honig, 1989). In addition, carotid body activity is altered by changes in blood osmolality (Gallego, Eyzaguirre & Monti-Bloch, 1979). It is therefore quite likely that Ang II might interact with the carotid body to influence chemoreceptor reflex function.
Recent evidence indicates that many vasoactive peptides interact directly with the carotid body, independently of alterations in blood flow. These include atrial natriuretic peptide and endothelin (Wang, He, Stensaas, Dinger & Fidone, 1991; McQueen, Dashwood, Cobb, Bond, Marr & Spyer, 1995). Atrial natriuretic peptide, in many instances a physiological antagonist of Ang II, is synthesized in the carotid body and modulates the response of the carotid body to hypoxia (Wang et al. 1991).
The aim of this study was to examine whether Ang II receptors occur in the carotid body and, if so, what effect Ang II has on carotid chemoreceptor activity.
METHODS
All experiments were performed in accordance with the Australian National Health and Medical Research Council code of practice for the care and use of animals for scientific purposes and were approved by the Animal Experimentation Ethics Committee of the Howard Florey Institute.
Tissues
Male Sprague-Dawley rats (300-350 g) were anaesthetized with halothane and decapitated. The carotid bifurcation was removed bilaterally and optimal cutting temperature compound (OCT, TissueTek, Sakura Finetek, Torrance, CA, USA) injected into the common carotid artery to fill all major vessels. The tissue was immersed in OCT and frozen in isopentane on dry ice. Tissues were stored at -70°C for less than 1 month prior to sectioning.
In vitro autoradiography
Sections (10-20 μm) of carotid body were cut in a cryostat for in vitro autoradiographic localization of Ang II receptor binding as previously described (Song, Allen, Paxinos & Mendelsohn, 1992). Following pre-incubation, sections were incubated in phosphate buffered saline containing 125I-[Sar1,Ile8]-Ang II (90 pm) for 1 h, washed in cold buffer and exposed to X-ray film for 7-21 days (Agfa-Scopix CR3B, Agfa-Gevaert, Belgium). The receptor subtype was determined by incubating consecutive, serial sections with the AT1 receptor antagonist losartan (10 μm; DuP753, Dupont), the AT2 receptor antagonist PD123319 (10 μm; Parke-Davis) or Ang II amide (1 μm; Hypertensin, Ciba). These concentrations of antagonists have been demonstrated to bind specifically to their respective receptor subtype without cross displacement (Song et al. 1992).
Binding densities were determined using an MCID image analysis system (Imaging Research Inc., St Catharines, Ontario, Canada). Quantification of densities was performed by comparing the optical densities of the film images with those of radioactivity standards which were exposed with the tissue sections. For receptor subtype analysis, binding densities were measured in three sections for each treatment (i.e. 125I-[Sar1,Ile8]-Ang II alone or in the presence of Ang II amide, losartan or PD123319) from each of three animals. The data for each animal were averaged and the means for each treatment compared by ANOVA followed by Fisher's LSD test with individual error set at 0.05.
Denervation of the carotid body
Male Sprague-Dawley rats (250-300 g) were anaesthetized with sodium pentobarbitone (60 mg kg−1, administered intraperitoneally). In four rats, the superior cervical ganglion was identified through a mid-line ventral incision. The common carotid artery was gently retracted medially without interrupting flow. The sympathetic trunk and superior cervical ganglion could then be identified dorsal to the carotid bifurcation. The trunk and nerve branches coursing ventrally from the ganglion were cut, with care being taken not to damage overlying tissue, particularly the carotid body. Once freed, the ganglion was cut as far centrally as possible and removed. The contralateral superior cervical ganglion was exposed but not sectioned and this carotid body served as the sham-lesioned control.
In a further four rats the carotid body region was identified through a lateral incision. This enabled the glossopharyngeal nerve to be identified at the central end of the field of view. The carotid sinus nerve was identified at its junction with the glossopharyngeal nerve and sectioned. An attempt was made to cut selectively the afferent axons without affecting the parasympathetic innervation which travels in a branch of the vagus nerve, joining the carotid sinus nerve closer to the carotid body. However, given the size constraints and the difficult nature of this surgery this selectivity might not have been achieved reliably. The contralateral side was exposed but the nerves were not identified or isolated and this side served as the sham-lesioned control.
Following recovery from surgery all animals appeared healthy and gained weight normally. Ptosis was obvious on the ipsilateral side of the animals which received superior cervical ganglion lesions. Rats were left for 7 days, to allow for degeneration of the neural inputs to the carotid body, and then treated as described above for localization of Ang II receptor binding.
Angiotensin AT1 receptor densities were measured in three to five consecutive sections from each denervated and control carotid body. These values were averaged and the means of denervated and control were compared using Student's paired t test.
In vitro electrophysiology
The activity of the carotid sinus nerve was recorded in vitro according to published methods (Kholwadwala & Donnelly, 1992). Male Sprague-Dawley rats (150-280 g) were anaesthetized with halothane and decapitated. The carotid bifurcation was removed rapidly and placed in ice-cold, Tyrode solution of composition (mm): 112 NaCl, 4.7 KCl, 2.2 CaCl2, 1.1 MgCl2, 42 sodium glutamate, 5 Hepes and 5.6 glucose; pH 7.4; saturated with carbogen (95% O2, 5% CO2). Using a dissecting microscope, the carotid body was identified and removed with a section of carotid sinus nerve attached. This was placed in carbogen-saturated Tyrode solution containing 0.1 mg ml−1 protease (type VIII, Sigma) and 0.2 mg ml−1 collagenase (type 1, Gibco BRL) at 37°C (pH 7.4) for 30 min. Following the enzymatic treatment, the carotid body was placed in a chamber and superfused with carbogen-saturated Tyrode solution (∼2 ml min−1). The chamber was contained in a temperature controlled bath (36°C) and the superfusion fluid was maintained at 36 ± 0.5°C at the chamber. The carotid body was pinned to the chamber and the carotid sinus nerve stripped of connective tissue. This resulted in the cut end of the nerve fanning out and enabled a suction electrode to be placed for recording. Suction electrodes were made from 2 mm glass pipettes, pulled in a vertical puller (Narishige, Tokyo), bumped against a glass rod to the required dimensions, and fire polished. With this method, multi-fibre recordings were reliably obtained. Nerve activity was amplified (1000-10000 times) and filtered (10 Hz to 3 kHz) (Dam 80 amplifier, World Precision Instruments Inc., Sarasota, FL, USA). Activity was displayed on an oscilloscope and recorded on videotape (Vetter model 400 Pcm, A. R. Vetter Co., Inc., Rebersburg, PA, USA). At the completion of the experiment, the nerve was cut and activity recorded to determine the baseline noise level. For subsequent analysis of spike activity the gate of a window discriminator was set to eliminate all of this baseline noise.
After a 15-20 min stabilization period, Ang II (0.1-100 nm, Auspep, Melbourne, Australia) was superfused for 2-3 min. A period of 30 min was then allowed for recovery before the stimulus was re-applied. Even after 30 min recovery the response to Ang II, particularly at the higher doses, was not always of similar magnitude to the initial response, suggesting tachyphylaxis was occurring. In addition, a gradual decrease in basal activity occurred over this time. Thus all data shown are from the initial application of Ang II only.
For the antagonist studies, Tyrode solution containing either losartan (1 μm, n = 5) or PD123319 (1 μm, n = 5) was superfused for 5 min after the initial stabilization period. Angiotensin II (10 nm) was then applied in Tyrode solution containing the antagonist for 3 min.
Analysis was performed off-line. Nerve activity was re-played through a window discriminator (Finntronics Inc., Orange, CT, USA) with the gate being set from the baseline noise level determined at the completion of the experiment. Spike activity which crossed the gate was counted and integrated into 10 s bins. These values were used for all further analysis. For each of the test periods, activity was counted for the 2 min period prior to the test and this was taken as the control activity. During the stimulus activity was counted for 40-60 s around the maximal increase and/or decrease in activity. The mean values of the control and stimulus periods were compared by Student's unpaired t test and the significance of any change determined for each individual experiment. The number of carotid bodies that demonstrated statistically significant changes for each dose of Ang II is presented as a fraction in Results.
Activity during the selected stimulus periods was also averaged and expressed as a percentage of the average control activity to enable comparison of signals containing different numbers of active fibres. The mean percentage change from control of all experiments at each dose was then compared by ANOVA followed by Fisher's LSD test with individual error set at 0.05. Where there was no detectable change in activity, for example during the superfusion of losartan, the activity at the time of changes observed in other experiments was measured.
RESULTS
Ang II receptor distribution
A high density of specific Ang II receptor binding occurs in the rat carotid body (Fig. 1). Displacement studies indicate that the Ang II receptor in the carotid body is predominantly of the AT1 subtype although there may be a small proportion of AT2 receptor binding (Fig. 2). All further discussion refers to the distribution of AT1 receptors only as displacement was performed with losartan. The residual binding following displacement with losartan is negligible.
Figure 1. Angiotensin AT1 receptor distribution in the rat carotid body and carotid bifurcation.
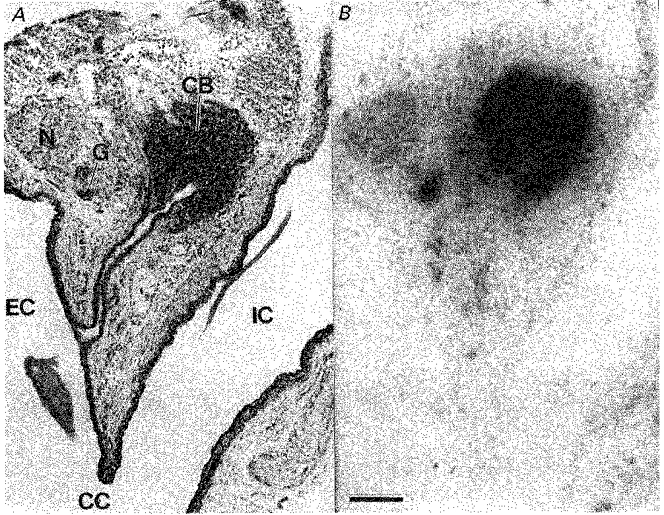
A, a haematoxylin- and eosin-stained 20 μm section of the carotid bifurcation region. B, an X-ray film depicting binding of 125I-[Sar1,Ile8]-Ang II to the section adjacent to that in A. Black silver deposits in B indicate the presence of Ang II AT1 receptors. The binding is totally displaced by 10 μm losartan or 1 μm Ang II amide. The calibration bar represents 250 μm. CB, carotid body; CC, common carotid artery; EC, external carotid artery; IC, internal carotid artery; G, isolated clump of ganglion cells; N, sympathetic nerve bundle arising from the superior cervical ganglion.
Figure 2. Density of Ang II AT1 and AT2 receptor subtypes in the rat carotid body.
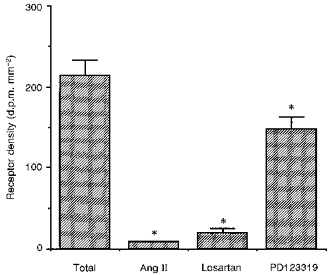
Bar graph demonstrating the density of 125I-[Sar1,Ile8]-Ang II binding to the rat carotid body alone and in the presence of 1 μm Ang II amide, 10 μm losartan or 10 μm PD123319. The asterisk denotes a significant difference in receptor density compared to total binding (P < 0.05).
AT1 receptors in the carotid body are distributed in clumps characteristic of the organization of glomus cell clusters. Superior cervical ganglionectomy did not alter the density of receptor binding (Fig. 3), demonstrating that these receptors are not located on the terminals of sympathetic postganglionic neurons. Carotid sinus nerve lesions resulted in a small (15%), but consistent and statistically significant reduction in receptor binding (Fig. 3). Overall, the localization studies demonstrate that the majority of Ang II AT1 receptors occur on glomus cells in the carotid body.
Figure 3. Effect of selective nerve lesions on Ang II receptor density in the rat carotid body.
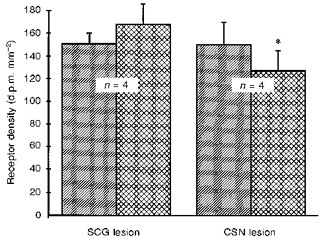
Bar graph showing the effect of superior cervical ganglionectomy (SCG lesion) or carotid sinus nerve section (CSN lesion) on AT1 receptor binding in the rat carotid body. Hatched bars represent the sham-lesioned contralateral carotid body which served as control. Cross-hatched bars are the lesioned carotid bodies. The asterisk denotes a significant difference from control (P < 0.05).
In the tissue surrounding the carotid body, dense AT1 receptor binding is also observed overlying small collections of ganglion cells (Fig. 1). These may be sympathetic postganglionic cells from part of the superior cervical ganglion wrapping around the carotid body. Alternatively, and more likely, they may be parasympathetic postganglionic neurons whose axons innervate the carotid body and neighbouring tissue. No further effort has been made in this study to determine the nature of these AT1 receptor-expressing neurons.
A low density of specific AT1 receptor binding is also observed in some sympathetic nerve bundles arising from the superior cervical ganglion (Fig. 1). As described previously (Castren, Kurihara, Gutkind & Saavedra, 1987), neurons within the superior cervical ganglion also possess a moderate density of AT1 receptors. It should be noted that the density of Ang II receptors in the superior cervical ganglion is considerably less than that of the sporadic clusters of ganglion cells mentioned above.
Effect of Ang II on chemoreceptor afferent activity of the rat carotid body
Superfusion of the carotid body in vitro with Ang II-containing solutions resulted in a dose-dependent increase in carotid sinus nerve activity (Fig. 4). Significant excitation (P < 0.05, Student's paired t test) was observed in 1/5 preparations with 10 and 100 pm Ang II, 5/8 preparations with 1 nm Ang II, 5/7 preparations with 10 nm Ang II and 7/9 preparations with 100 nm Ang II. The greatest mean increase observed was 36 ± 11% with a dose of 10 nm (Fig. 5). Considerable variation was, however, observed in the magnitude of individual responses, probably reflecting variation in the proportion of responsive axons in each multi-unit recording. The range of significant responses was 15-99% with 1 nm Ang II, 24-73% with 10 nm Ang II and 8-70% with 100 nm Ang II. Analysis of the response of all experiments demonstrated significant excitation of carotid sinus nerve activity with 1, 10 and 100 nm Ang II (P < 0.05, ANOVA followed by Fisher's LSD test; Fig. 5). The maximal increase occurred 123 ± 3 s after the solution reached the chamber. This time tended to decrease with increasing concentrations of Ang II. Preliminary experiments, in which the chamber concentration of Ang II was measured by radioimmunoassay (data not shown), indicated that at the flow rate employed, the concentration of Ang II reached its theoretical maximum value approximately 120 s after reaching the bath.
Figure 4. Carotid sinus nerve activity during application of Ang II (1 nm) to the carotid body in vitro.
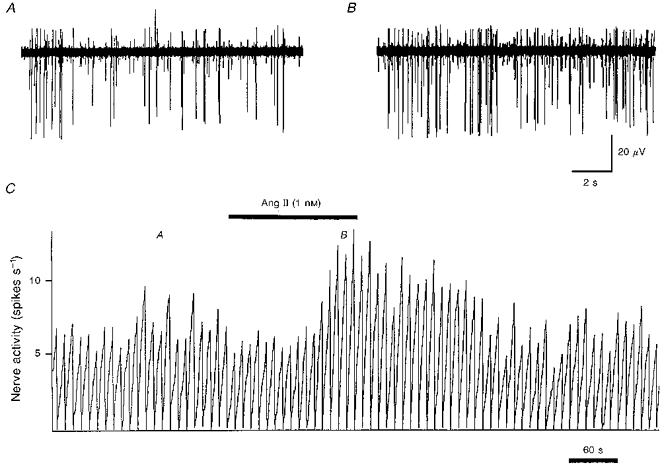
An example of the change in carotid sinus nerve activity in response to superfusion of the carotid body in vitro with 1 nm Ang II in Tyrode solution. Raw multi-unit activity is shown before (A) and during (B) superfusion with Ang II. The integrated spike activity (10 s bins) is shown in C. The times of the recordings shown in A and B are denoted by the respective letters in C. It can be observed, particularly with the largest spike, that the increase in multi-unit activity is due to an increase in the rate of firing, and not just recruitment of new neurons.
Figure 5. Change in carotid sinus nerve activity in response to carotid body superfusion with Ang II.
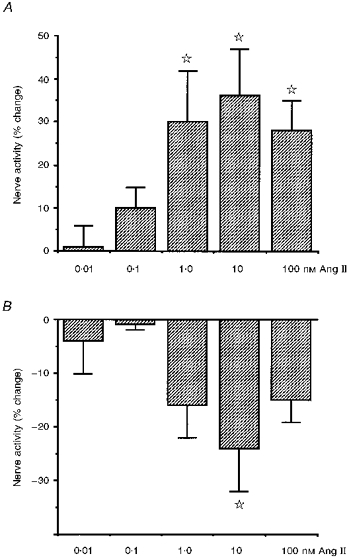
Histogram demonstrating mean ±s.e.m. changes in multi-unit carotid sinus nerve activity in response to various doses of Ang II applied to the carotid body in vitro. A, mean increases in activity which occurred in the majority of experiments 123 ± 3 s after exposure to Ang II. B, mean decreases in activity which preceded the excitation in approximately 50% of experiments at a time of 50 ± 5 s after exposure to Ang II. Activity is expressed as a percentage change from the preceding control period in each case. The stars denote that the mean is significantly different from control (P < 0.05, ANOVA followed by Fisher's LSD test).
In many experiments an initial inhibition of carotid sinus nerve activity preceding the excitation was also observed (Fig. 5). Significant inhibition (P < 0.05, Student's paired t test) was observed in 1/5 preparations with 10 pm Ang II, 0/5 preparations with 100 pm Ang II, 4/8 preparations with 1 nm Ang II, 4/7 preparations with 10 nm Ang II and 4/9 preparations with 100 nm Ang II. Whilst the decrease was apparent in several individual experiments between 1 and 100 nm, the change for all samples only reached significance for 10 nm (P < 0.05, ANOVA with Fisher's LSD test; Fig. 5). At this dose activity decreased 24 ± 8% from control. The maximal decrease occurred 50 ± 5 s after the solution reached the chamber.
To examine the effect of subtype-specific Ang II receptor antagonists on the response to Ang II, the 10 nm dose of Ang II was chosen because a clear biphasic effect was observed with this concentration. Superfusion with either losartan or PD123319, at a dose of 1 μm, had no significant effect on basal nerve activity (activity after superfusion for 4 min with losartan was -11 ± 6% and with PD123319 was -8 ± 6%). A statistically significant excitatory response to Ang II was observed in 5/7 control experiments, 4/5 tests with PD123319 superfusion but only 1/5 tests with losartan superfusion. Comparison of the means demonstrated that the excitatory response to Ang II was markedly attenuated by losartan (P < 0.05; Fig. 6). Statistically significant inhibitory responses to Ang II were observed in 4/7 control experiments, 3/5 tests with PD123319 superfusion, but only 1/5 tests with losartan superfusion. Whilst the inhibitory response to Ang II appeared to be blocked by losartan, this did not attain significance, presumably due to the small sample size (Fig. 6). The response to Ang II during superfusion with PD123319 was not different from the response to Ang II alone. Overall, these results indicate that the carotid body chemoreceptor response to Ang II is mediated by the AT1 receptor.
Figure 6. The effect of AT1 and AT2 receptor antagonists on the changes in chemoreceptor activity in response to superfusion with 10 nm Ang II.
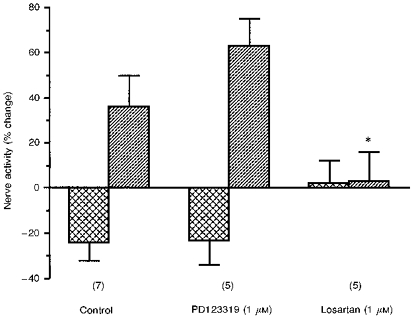
Bar graph indicating changes in carotid sinus nerve activity (means ±s.e.m.) in response to superfusion with 10 nm Ang II alone (control), or in the presence of 1 μm PD123319 or 1 μm losartan. The values depicted by the cross-hatched bars are for the initial inhibitory period whilst the hatched bars are for the later excitatory response. The numbers in parentheses indicate the number of experiments at each point. The asterisk denotes that the excitatory response to Ang II is significantly decreased in the presence of losartan (P < 0.05).
DISCUSSION
This study demonstrates that Ang II, acting through AT1 receptors, can alter the activity of carotid body chemoreceptor afferents. The majority of AT1 receptors occur on the glomus cells of the carotid body. Whilst the predominant effect of Ang II is excitation, an early inhibition is also observed. This is not the first study to demonstrate that Ang II can excite chemoreceptor afferent neurons. In experiments on the anaesthetized cat, McQueen (1981) demonstrated that Ang II has an excitatory action. The conclusion of that study was, however, that the effect was probably a secondary response following vasoconstriction-induced hypoxia. In the experiments described here the effect of vasoconstriction was removed.
In the dog, systemic administration of Ang II stimulates respiration (Potter & McCloskey, 1979; Ohtake & Jennings, 1993). This response is not affected by sinoaortic denervation, indicating that it occurs through a direct interaction with the central nervous system (Potter & McCloskey, 1979). Potter & McCloskey (1979) also recorded the activity of one chemoreceptor afferent fibre from one dog. This was not affected by systemic administration of Ang II. Whilst the inability to show chemoreceptor activation in the dog is in contrast with this study, it is hard to be conclusive on the basis of this limited data. The two most likely explanations are that this represents a species difference, or that possibly only a proportion of chemoreceptor afferents respond to Ang II. The latter possibility might explain why the multi-unit activity response to Ang II in this study was variable both between preparations and in magnitude. Even at the highest dose of Ang II (100 nm) only 7/9 preparations responded to Ang II. At this dose the magnitude of the excitatory response also varied, from 8 to 70%, suggesting that possibly the proportion of responsive units within the multi-unit recording might alter the magnitude of the response. Further studies will be required to resolve this issue.
The response to Ang II observed in this study is small in magnitude, being a mean 36% increase in multi-unit activity with a 10 nm dose. In comparison, application of chemoreceptor stimulants such as 0.2 M orthophosphate or sodium cyanide result in severalfold increases in activity (data not shown). This would suggest that Ang II modulates activity in the carotid body, rather than being a primary transmitter. Whilst beyond the scope of the current study, it is likely that Ang II potentiates transmitter release from the glomus cell and would thus modulate the carotid sinus nerve response to stimuli such as hypoxia. This will be the subject of further investigations. Precedent for this has already been set in the response to atrial natriuretic peptide, which has no effect on basal nerve activity, but dramatically attenuates the chemoreceptor response to hypoxia (Wang et al. 1991).
The density of AT1 receptor binding in the rat carotid body is relatively high. In comparison with other organs, the density observed here is similar to that found in the forebrain circumventricular organs, the sites of highest density in the rat brain, and higher than densities associated with vascular or cardiac smooth muscle. In fact the only sites of significantly greater density occur in the kidney and adrenal gland. Whilst receptor density does not necessarily equate with physiological importance, the observations presented here suggest that effects of Ang II on chemoreceptor activity should be examined in more detail.
The threshold concentration for a statistically significant increase in chemoreceptor activity in this study is 1 nm. However, consistent small increases in activity are observed with the 100 pm dose. Normal circulating levels of Ang II are around 100 pm. With physiological stimuli, such as water deprivation, two- to threefold increases occur (Johnson, Robinson & Mann, 1986). This indicates that systemic levels of Ang II, within the physiological range, could affect chemoreceptor function.
A small decrease in receptor binding density was observed following carotid sinus nerve lesion. An attempt was made to produce selective afferent lesions without affecting the efferent parasympathetic innervation. However, given the difficulty of this surgery, such selectivity may not be relied upon. Thus it is possible that a small percentage of the Ang II receptors occur on the terminals of either afferent sensory neurons, or parasympathetic neurons. The presence of Ang II receptors on different elements within the carotid body, the glomus cell and the afferent terminal, might offer an explanation for the biphasic response to Ang II observed in vitro. Indeed this is postulated to be the case for dopamine acting on D2 receptors on glomus cells (inhibitory) and afferent neuron terminals (excitatory) (Gonzalez, Almaraz, Obeso & Rigual, 1994). An alternative explanation might be that the lesion resulted in the loss of a factor, for example a trophic factor, that in turn induced a change in the receptor density. The present study does not allow resolution of this.
It is perhaps surprising that superior cervical ganglionectomy had no detectable effect on AT1 receptor binding in the carotid body. As reported previously, most neurons within the superior cervical ganglion contain a low to moderate density of Ang II receptor binding sites (Castren et al. 1987). Nerve bundles arising from the superior cervical ganglion contain a low density of AT1 receptors, suggesting that the receptor may be transported to a presynaptic site of action. Numerous studies have demonstrated that Ang II, acting presynaptically, potentiates noradrenaline release from sympathetic postganglionic neurons (Story & Ziogas, 1987). Thus it is likely that AT1 receptors are present on the terminals of sympathetic postganglionic neurons in the carotid body. It is probable, however, that the distribution of presynaptic receptors on sympathetic nerve terminals in the carotid body is too diffuse to allow detection by receptor binding.
The interactions between Ang II and catecholamine neurons are widespread with Ang II receptors occurring in many brain regions rich in catecholaminergic neurons (Allen, Chai, Clevers, McKinley, Paxinos & Mendelsohn, 1988; Allen, Paxinos, McKinley, Chai & Mendelsohn, 1991; Song et al. 1992). This association includes dopaminergic neurons in the brain, with Ang II acting presynaptically in the striatum to potentiate dopamine release (Mendelsohn, Jenkins & Berkovic, 1993). Whilst the role of dopamine in the carotid body remains controversial, it has been proposed that dopamine release from glomus cells acts on chemoreceptor afferent terminals to increase activity (Gonzalez, Lopez-Lopez, Obeso, Perez-Garcia & Rocher, 1995; Donnelly, 1995). Given the precedent of Ang II modulating dopamine release in the central nervous system, it is possible that potentiation of the release of dopamine from glomus cells might mediate the excitatory effect of Ang II. Further studies will attempt to address this question directly.
Carotid body chemoreceptor activation results in reflex alteration of respiratory and cardiovascular function (Marshall, 1994). Importantly for this discussion, alterations in fluid and electrolyte homeostasis are also involved through renal and central nervous system effects (Honig, 1989; Marshall & Metcalfe, 1990; Louwerse & Marshall, 1992). Indeed there is evidence that in addition to oxygen and carbon dioxide, the carotid body is responsive to changes in osmolality and temperature (Gallego et al. 1979). Thus it is not surprising that hormones, such as Ang II and atrial natriuretic peptide, which are traditionally implicated in the regulation of the cardiovascular system and fluid and electrolyte homeostasis, might interact with the carotid body to influence chemoreceptor function. Whilst the precise physiological role of this interaction remains to be determined, it is clear that effects of Ang II on the carotid body probably represent one of a number of actions of this peptide in response to alterations in blood gas composition, with direct effects on renal function already outlined (Marshall & Metcalfe, 1990; Louwerse & Marshall, 1992; Neylon et al. 1996). One possibility is that Ang II-induced excitation of carotid chemoreceptors would serve to enhance the renal sympatho-excitation in response to hypoxia and thus promote sodium and water reabsorption. As discussed by Honig (1989) one of the initial responses to hypoxia is diuresis and natriuresis accompanied by decreases in fluid and electrolyte ingestion. Yet, hypoxia increases renal sympathetic nerve activity (Marshall, 1994), which would act to increase salt and water retention. It is tempting to speculate that in hypoxic situations where the pressure to preserve fluid balance is increased, such as following haemorrhage or sodium depletion, elevated systemic Ang II would act at numerous sites to promote fluid and electrolyte conservation, over-riding the normal natriuretic and diuretic responses to hypoxia. This concerted effect would include actions in the brain, to increase renal sympathetic nerve activity and fluid ingestion, the kidney and, as suggested by the observations contained in this manuscript, the carotid body. This would serve to maintain extracellular fluid volume and allow adequate perfusion.
In conclusion, the rat carotid body contains a high density of Ang II AT1 receptors located on the primary chemoreceptor element, the glomus cell. Activation of this receptor by physiological concentrations of Ang II induces a predominantly excitatory effect on afferent chemoreceptor activity. This may represent another mechanism by which circulatory and volume status could influence the response to cardiorespiratory perturbations.
Acknowledgments
The expert technical assistance of Ms E. Clune is gratefully acknowledged. This work was supported by a grant from the Australian National Health and Medical Research Council. The author is the recipient of an R. D. Wright fellowship from the Australian National Health and Medical Research Council.
References
- Allen AM, Chai SY, Clevers J, McKinley MJ, Paxinos G, Mendelsohn FAO. Localization and characterization of angiotensin II receptor binding and angiotensin converting enzyme in the human medulla oblongata. Journal of Comparative Neurology. 1988;269:249–264. doi: 10.1002/cne.902690209. [DOI] [PubMed] [Google Scholar]
- Allen AM, Paxinos G, McKinley MJ, Chai SY, Mendelsohn FAO. Localization and characterization of angiotensin II receptor binding sites in the human basal ganglia, thalamus, midbrain, pons and cerebellum. Journal of Comparative Neurology. 1991;312:291–298. doi: 10.1002/cne.903120211. [DOI] [PubMed] [Google Scholar]
- Assmussen E, Nielsen M. Studies on the initial increase in O2-capacity of the blood at low oxygen pressure. Acta Physiologica Scandinavica. 1945;9:75–87. [Google Scholar]
- Castren E, Kurihara M, Gutkind JS, Saavedra JM. Specific angiotensin binding sites in the rat stellate and superior cervical ganglia. Brain Research. 1987;422:347–351. doi: 10.1016/0006-8993(87)90942-5. [DOI] [PubMed] [Google Scholar]
- Donnelly DF. Does catecholamine secretion mediate the hypoxia-induced increase in nerve activity? Biological Signals. 1995;4:304–309. doi: 10.1159/000109457. [DOI] [PubMed] [Google Scholar]
- Gallego R, Eyzaguirre C, Monti-Bloch L. Thermal and osmotic responses of arterial receptors. Journal of Neurophysiology. 1979;42:665–680. doi: 10.1152/jn.1979.42.3.665. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors. From natural stimuli to sensory discharges. Physiological Reviews. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Lopez-Lopez JR, Obeso A, Perez-Garcia MT, Rocher A. Cellular mechanisms of oxygen chemoreception in the carotid body. Respiration Physiology. 1995;102:137–147. doi: 10.1016/0034-5687(95)00069-0. 10.1016/0034-5687(95)00069-0. [DOI] [PubMed] [Google Scholar]
- Honig A. Peripheral arterial chemoreceptors and reflex control of sodium and water homeostasis. American Journal of Physiology. 1989;257:R1282–1302. doi: 10.1152/ajpregu.1989.257.6.R1282. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Robinson MM, Mann JFE. The role of the renal renin-angiotensin system in thirst. In: De Caro G, Epstein AN, Massi M, editors. The Physiology of Thirst and Sodium Appetite. New York: Plenum Press; 1986. pp. 161–180. [Google Scholar]
- Kholwadwala D, Donnelly DF. Maturation of carotid chemoreceptor sensitivity to hypoxia: in vitro studies in the new-born rat. The Journal of Physiology. 1992;453:461–473. doi: 10.1113/jphysiol.1992.sp019239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louwerse AM, Marshall JM. The role of the renin-angiotensin system in the cardiovascular response to systemic hypoxia in the anaesthetized rat with Pa,CO2 maintained. The Journal of Physiology. 1992;452:319P. [Google Scholar]
- McQueen DS. Effects of some polypeptides on carotid chemoreceptor activity. In: Belmonte C, Pallot DJ, Acker H, Fidone S, editors. Arterial Chemoreceptors. Leicester, UK: Leicester University Press; 1981. pp. 299–308. [Google Scholar]
- McQueen DS, Dashwood MR, Cobb VJ, Bond SM, Marr CG, Spyer KM. Endothelins and rat carotid body: autoradiographic and functional pharmacological studies. Journal of the Autonomic Nervous System. 1995;53:115–125. doi: 10.1016/0165-1838(94)00179-n. 10.1016/0165-1838(94)00179-N. [DOI] [PubMed] [Google Scholar]
- Marshall JM. Peripheral chemoreceptors and cardiovascular regulation. Physiological Reviews. 1994;74:543–594. doi: 10.1152/physrev.1994.74.3.543. [DOI] [PubMed] [Google Scholar]
- Marshall JM, Metcalfe JD. The cardiovascular response to systemic hypoxia in the cat: the role of the renin-angiotensin system. In: Acker H, Trzebski A, O'Regan RG, editors. Chemoreceptors and Chemoreceptor Reflexes. New York: Plenum Press; 1990. pp. 147–154. [Google Scholar]
- Mendelsohn FAO, Jenkins TA, Berkovic SF. Effects of angiotensin II on dopamine and serotonin turnover in the striatum of conscious rats. Brain Research. 1993;613:221–229. doi: 10.1016/0006-8993(93)90902-y. 10.1016/0006-8993(93)90902-Y. [DOI] [PubMed] [Google Scholar]
- Neylon M, Marshall JM, Johns EJ. The role of the renin-angiotensin system in the renal response to moderate hypoxia in the rat. The Journal of Physiology. 1996;491:479–488. doi: 10.1113/jphysiol.1996.sp021232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake PJ, Jennings DB. Angiotensin II stimulates respiration in awake dogs and antagonizes baroreceptor inhibition. Respiration Physiology. 1993;91:335–351. doi: 10.1016/0034-5687(93)90110-v. [DOI] [PubMed] [Google Scholar]
- Potter EK, McCloskey DI. Respiratory stimulation by angiotensin II. Respiration Physiology. 1979;36:367–373. doi: 10.1016/0034-5687(79)90048-3. 10.1016/0034-5687(79)90048-3. [DOI] [PubMed] [Google Scholar]
- Rose CE, Kimmel DP, Godine RL, Kaiser DL, Carey RM. Synergistic effects of acute hypoxemia and hypercapnic acidosis in conscious dogs. Circulation Research. 1983;53:202–213. doi: 10.1161/01.res.53.2.202. [DOI] [PubMed] [Google Scholar]
- Song K, Allen AM, Paxinos G, Mendelsohn FAO. Mapping of angiotensin II receptor subtype heterogeneity in rat brain. Journal of Comparative Neurology. 1992;316:467–484. doi: 10.1002/cne.903160407. [DOI] [PubMed] [Google Scholar]
- Story DF, Ziogas J. Interaction of angiotensin with noradrenergic neuroeffector transmission. Trends in Pharmacological Science. 1987;8:269–271. 10.1016/0165-6147(87)90201-X. [Google Scholar]
- Timmermans PBMWM, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JAM, Smith RD. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacological Reviews. 1993;45:205–251. [PubMed] [Google Scholar]
- Wang Z-Z, He L, Stensaas LJ, Dinger BG, Fidone S. Localization and in vitro action of atrial natriuretic peptide in the cat carotid body. American Journal of Physiology. 1991;70:942–946. doi: 10.1152/jappl.1991.70.2.942. [DOI] [PubMed] [Google Scholar]


