Abstract
To investigate the molecular basis of the sublevels induced in the outward current during block by intracellular Mg2+, single-channel currents through inwardly rectifying K+ (IRK1) channels were studied.
cDNA encoding a functional murine IRK1 channel was transfected into COS-1 cells (a Green Monkey kidney cell line) using the liposome method, and voltage clamp experiments were done after 48-72 h.
Intracellular Mg2+ at micromolar concentrations induced sublevels in the outward current at one-third and two-thirds of the unitary amplitude seen in wild-type channels. Replacing Asp 172 with Asn (D172N) and Gln (D172Q) abolished these sublevels, i.e. the channel showed only the fully open and fully blocked states.
Both mutations reduced the Mg2+ sensitivity of the channel at 2 μm Mg2+. However, the Mg2+ sensitivity did not differ significantly at higher concentrations (10 μm) and voltages (+70 mV).
Channels expressed from D172E showed the sublevels, indicating that a negative charge is indispensable to the substate behaviour.
Channels from tandem tetramers of IRK1 with one and two D172N mutant subunits mainly showed sublevels with two-thirds amplitude, while those from tetramers with three D172N mutant subunits showed no sublevels.
These findings suggest that differences in Mg2+ binding patterns lead to different conductive states in a single-barrelled channel.
The phenomenon of inward rectification, whereby the K+ conductance increases under hyperpolarization and decreases under repolarization, has been demonstrated in a variety of cell types (Katz, 1949; Hall et al. 1963; Kandel & Tauc, 1966; Hagiwara & Takahashi, 1974). This behaviour plays an important role in permitting long depolarizing responses. Studies on native cardiac channels and cloned inwardly rectifying K+ (IRK1) channels (Kubo et al. 1993) indicate that inward rectification mainly results from a voltage-dependent block of the channel pore by intracellular Mg2+ (Matsuda et al. 1987; Vandenberg, 1987; Matsuda, 1988) and polyamines (Ficker et al. 1994; Lopatin et al. 1994; Fakler et al. 1995).
The key feature of a Mg2+ block of the cardiac inwardly rectifying K+ channel is that in the presence of intracellular Mg2+ at micromolar concentrations the outward single-channel current fluctuates between four equally spaced conductance levels, including zero current (Matsuda, 1988, 1991a). Three distinct blocked states are also seen during blockade by intracellular Ca2+ (Matsuda & Cruz, 1993) and extracellular Cs+ or Rb+ (Matsuda et al. 1989). The distribution of the current levels agreed reasonably well with the binomial theorem at different probabilities for the blocked state. The substate behaviour can be most simply explained by assuming that the inwardly rectifying K+ channel consists of three identical conducting units that usually function co-operatively to form a single channel and that blocking ions enter and plug each conducting unit independently. Another mechanism that could induce sublevels involves different binding patterns of the blocking ions to sites on the channel protein, leading to different conductive states in a single-barrelled channel.
We confirmed that intracellular Mg2+ induces sublevels in channels expressed by transfecting COS-1 cells with the IRK1 gene, as in native channels (Omori et al. 1997). In the present study, we report that a negatively charged residue at position 172 in the second hydrophobic segment of the IRK1 channel is essential for the substate behaviour, favouring the latter mechanism proposed above. A model to explain the substate behaviour in a single-barrelled channel is proposed.
METHODS
Molecular biology
The IRK1 gene (Kubo et al. 1993) was digested with Hind III and Stu I. The Hind III-Stu I fragment (∼1.8 kb) which contains 5′ untranslated, coding and part of the 3′ untranslated sequences was subcloned into a pTZ19R vector (Pharmacia, Uppsala, Sweden) for mutagenesis or the construction of tandem multimers. Site-directed mutagenesis of the cDNA was performed using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). The mutation was confirmed by sequencing. The DNA fragment containing the mutation was excised by restriction enzymes and used to replace the corresponding fragment in the wild-type cDNA.
For construction of tetrameric cDNA, two Pst I-sites were separately introduced by site-directed mutagenesis in the 5′ untranslated sequences adjoining the initiation codon and in the 3′ untranslated sequences, including the third position of the termination codon. The two mutant cDNAs were ligated at the Pst I-site to make dimeric cDNA. The amino acid sequence of the linking region between two subunits was Ile(428)-Trp-Leu-Gln-Pro-Met(1). To form tetrameric cDNA, we made a cDNA with Pst I-sites in both the 5′ and 3′ untranslated sequences and used it for insertion into the dimeric cDNA. Although most of the transformed bacterial colonies made trimeric or self-ligated dimeric cDNA, ∼2-3% of the transformed colonies produced tetrameric cDNA inserted as the concatemer with correct direction. All the constructed tandem tetramers could be digested completely by Pst I. The direction of the inserted cDNAs of the constructs were confirmed by restriction enzyme digestion (Bgl II).
For expression in COS-1 cells (Riken Gene Bank, Tsukuba, Japan), cDNA was subcloned into pSVL expression vector (Pharmacia). COS-1 cells were transiently transfected with the expression plasmid (1 μg per 35 mm dish) using Lipofectamine (Gibco BRL, Gaithersburg, MD, USA) according to the manufacturer's protocol. Currents were recorded from cells 48-72 h after transfection.
Electrophysiology
The coverslips (3 mm × 18 mm) on which COS-1 cells were grown were transferred to a recording chamber. Whole-cell and single-channel currents were recorded using a heat-polished patch electrode (Hamill et al. 1981) with an EPC-7 patch clamp amplifier (List Electronic, Darmstadt, Germany). Pipettes were made from capillaries of hard borosilicate glass (Pyrex), and for single-channel recording were coated near the tips with silicone to reduce electrical capacitance. Single-channel current records were obtained from inside-out patches in high-K+ solution. The electrode resistance ranged between 5 and 15 MΩ when filled with a pipette solution containing (mm): KCl, 150; CaCl2, 1; Hepes, 5 (pH 7.4). Whole-cell currents were recorded in Tyrode solution using electrodes filled with high-K+, Mg2+-free solution (electrode resistance, 2-5 MΩ). Tyrode solution contained (mm): NaCl, 140; NaH2PO4, 0.33; KCl, 5.4; CaCl2, 1.8; MgCl2, 0.5; Hepes, 5; glucose, 5.5; pH was adjusted to 7.4 with NaOH. High-K+ solution contained (mm): potassium aspartate, 60; KCl, 65; KH2PO4, 1; MgCl2, 0-3.62; EDTA, 2-5; K2ATP, 3; Hepes, 5; the pH was adjusted to 7.4 with KOH. The free Mg2+ concentration was calculated by means of a computer program (Fabiato & Fabiato, 1979; Tsien & Rink, 1980) using the dissociation constants (Martell & Smith, 1974) corrected for temperature and ionic strength (Harrison & Bers, 1989). Experiments were carried out at 23-26°C.
Data were collected on digital audiotape using a PCM data recorder (RD-120TE, TEAC, Tokyo, Japan) and stored for subsequent computer analysis (PC-98XL, NEC, Tokyo, Japan). The unitary currents were filtered using a four-pole low-pass Bessel filter (FV-665, NF, Kanagawa, Japan) with a -3 dB corner frequency of 1.2 kHz and sampled at 5 kHz, unless otherwise indicated. Mean open-channel currents were calculated as follows. A threshold was set just above the zero current level and data points below the threshold were averaged. The difference between each datum point lying above the threshold and the averaged baseline was calculated, and the differences were integrated. The resulting integrated value was then divided by the duration of the channel opening. Membrane potentials were corrected for the liquid junction potential at the tip of the patch pipette in Tyrode solution and for that at the tip of the indifferent reference electrode filled with Tyrode solution and placed in the bathing solution (Matsuda, 1991a).
Averaged results throughout this paper are given as means ±s.d. Student's unpaired t test was performed, and P values of less than 0.05 were regarded as significant.
RESULTS
Effects of mutations of D172 on gating
As reported previously (Stanfield et al. 1994; Wible et al. 1994), mutations which neutralize a negatively charged residue at position 172 (D172N and D172Q) affect onset of the inward currents on hyperpolarization (Fig. 1). Both cells transfected with wild-type IRK1 and those transfected with mutants showed inward rectification under whole-cell recording. In the former, voltage steps to levels more negative than the equilibrium potential for K+ (EK) induced inward currents which increased with time during the first several milliseconds. In contrast, onset of inward currents through channels formed from D172N and D172Q mutants was virtually instantaneous.
Figure 1. Instantaneous onset of inward currents in mutant channels.
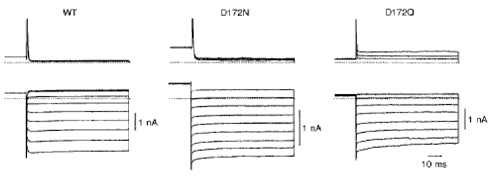
Whole-cell currents recorded from COS-1 cells transfected with wild-type (WT) and mutant (D172N and D172Q) forms of IRK1. Superimposed current records were elicited by 300 ms depolarizations from -19 to +40 mV in 30 mV increments (upper panel) and by hyperpolarizations from -57 to -136 mV in 10 mV increments (lower panel). Holding potential, -48 mV. Here and in subsequent figures unless otherwise stated the dotted line indicates the zero current level.
Time-dependent increase in the inward current through the inwardly rectifying K+ channel is ascribed to activation of the channel and/or unblock of intracellular polyamines upon hyperpolarization (Ficker et al. 1994; Lopatin et al. 1994). The instantaneous onset of inward currents in mutant channels indicates that the open probability of mutant channels at positive potentials is higher than that of wild-type channels. Indeed, outward currents through mutant channels could be recorded in steady-state conditions at potentials more positive than +50 mV (Fig. 2), while those through wild-type channels could not be recorded (Omori et al. 1997). Figure 2A shows single-channel currents recorded in steady-state conditions in the inside-out configuration without intracellular divalent cations and polyamines. EK, predicted from 150 mm extracellular and intracellular K+ concentration, was 0 mV. The unitary current-voltage (i-V) relationships were almost linear (Fig. 2B) and the channel conductance averaged 32.0 ± 2.1 pS (n = 21 experiments) in D172N and 29.7 ± 1.5 pS (n = 11) in D172Q. The conductance of D172N was not significantly different from that of wild-type channels (34.1 ± 2.0 pS, n = 13; Omori et al. 1997), but the conductance of D172Q was smaller than those of wild-type and D172N channels.
Figure 2. Single-channel currents recorded from mutant channels (D172N) expressed in COS-1 cells in the inside-out configuration.
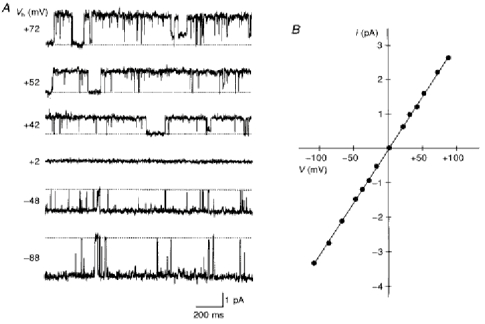
A, steady-state currents filtered and digitized at 1 kHz. Numbers to the left of each current trace refer to the holding potential (Vh). Mg2+-free intracellular solution. B, single-channel i-V relationship obtained from the same patch. The slope conductance of the unitary current was 31.1 pS.
Effects of mutations of D172 on the block by intracellular Mg2+
Inward rectification depends, in part, on voltage-dependent block of channels by intracellular Mg2+ (Matsuda et al. 1987; Vandenberg, 1987). Conductance sublevels at one-third and two-thirds of the unitary amplitude of the outward currents were induced by low intracellular Mg2+ in the cardiac inwardly rectifying K+ channel (Matsuda, 1988, 1991a) and channels expressed by transfection with IRK1 (Omori et al. 1997). Such sublevels were not observed in mutant channels. Instead, the outward currents fluctuated between only the fully open and fully blocked (zero current) levels during the low intracellular Mg2+ blockade (Fig. 3). With larger depolarizations transition became faster and blocked times were prolonged.
Figure 3. Neutralization of D172 abolishes the substate behaviour in Mg2+ block.
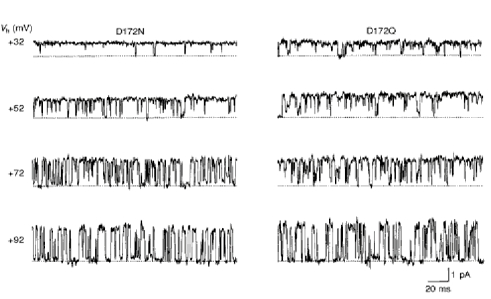
Outward currents recorded in steady-state conditions in the presence of 2 μm intracellular Mg2+. Mutant channels (D172N, left-hand panel; D172Q, right-hand panel) fluctuate between the fully open and the fully blocked levels.
To compare the extent of Mg2+ block in wild-type and mutant channels, we calculated the mean open-channel current and normalized it to the unitary current with no Mg2+ blockade (Fig. 4). Normalized currents were larger in the mutant channel (D172N) than in the wild-type channel. However, there was no significant difference when the extent of the block increased at +70 mV and +90 mV with 10 μm Mg2+. This finding suggests that a binding site other than D172 is critical in Mg2+ blockade.
Figure 4. Comparison of Mg2+ block between wild-type and mutant (D172N) channels.
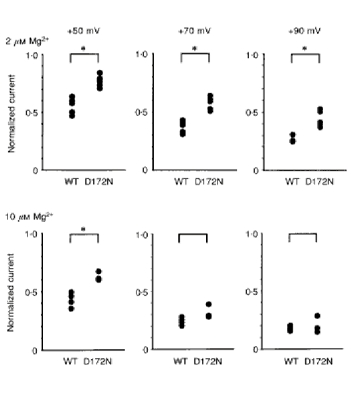
Outward currents were evoked by stepping the membrane potential for 130-140 ms every 1 s in the wild-type channels, while outward currents through the mutant channels were recorded in steady-state conditions. Outward mean open channel currents in the presence of intracellular Mg2+ were calculated and normalized to the unitary amplitude without Mg2+. Numbers at the top of each column refer to the potential level during the depolarizing steps from -48 mV (WT) or the holding potential (D172N). *P < 0.05.
Sublevels were preserved in D172E
To test whether a negative charge at position 172 or the Asp residue per se is crucial for the substate behaviour, we replaced Asp at position 172 with Glu (D172E). Figure 5 shows whole-cell and single-channel currents through channels expressed from D172E. Both a time-dependent increase of the inward current on hyperpolarization and the sublevels with low intracellular Mg2+ were preserved in this mutant. This result indicates that it is not the aspartate residue in particular, but rather a negative charge, which is required for the substate behaviour.
Figure 5. Time-dependent onset of inward currents and sublevels were conserved in D172E.
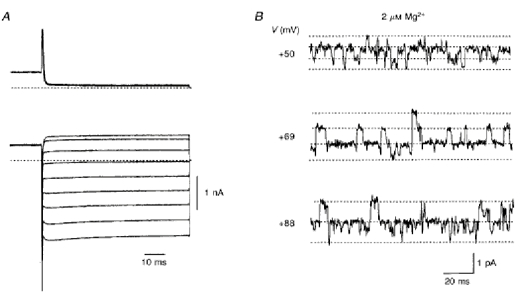
A, whole-cell currents recorded from a cell transfected with D172E. Voltage protocol was the same as in Fig. 1. B, outward unitary currents in D172E in response to the voltage steps indicated from -48 mV show the same sublevels as for wild-type IRK1 with 2 μm Mg2+. The dotted lines indicate, from bottom to top, zero, one-third, two-thirds and fully open current levels. At +88 mV, fully open current levels were not observed.
Tandem tetramer with wild-type IRK1 and D172N
The findings presented so far suggest that the different ways in which Mg2+ binds are responsible for the sublevels in a single-barrelled channel (see Discussion). Therefore, we examined the effect of the number of negative charges at D172 on the sublevels by constructing tandem multimers with wild-type IRK1 and D172N. It has been reported that IRK1 channels (Yang et al. 1995b) and other strongly rectifying K+ channels (BIR10; Glowatzki et al. 1995) are tetrameric, like voltage-gated K+ channels (MacKinnon, 1991; Tytgat & Hess, 1992). Although our previous study on the subunit stoichiometry did not support the notion that IRK1 channels consist of four subunits (Omori et al. 1997), we constructed tandem tetramers containing wild-type IRK1 and D172N (Fig. 6). Channels expressed from tetrameric cDNAs containing D172N linked as the fourth unit to a wild-type trimeric IRK1 (WT3-D172N) showed sublevels during the block by intracellular Mg2+. However, the substate behaviour was different from that in wild-type channels: the channel fluctuated between fully open, two-thirds open and zero current levels. In one of twelve patches, the channel did not show sublevels. A high open probability at +90 mV suggests that this channel contained more than three D172N subunits (see below). Channels expressed from tetramers containing D172N linked as the second and third units (WT-(D172N)2-WT) also showed sublevels of two-thirds amplitude. In two of seven patches, sublevels of one-half of the unit amplitude were also observed. However, they were so infrequent that the amplitude histogram showed the peak corresponding to the two-thirds level but not the peak corresponding to the one-half level. Channels expressed from tetramers containing three mutant subunits (WT-(D172N)3) did not show sublevels. In two of fourteen patches, sublevels of two-thirds were observed, presumably owing to one or two wild-type subunit(s) replacing mutant subunit(s).
Figure 6. Outward currents of channels expressed from tetrameric cDNAs containing wild-type IRK1 and D172N.
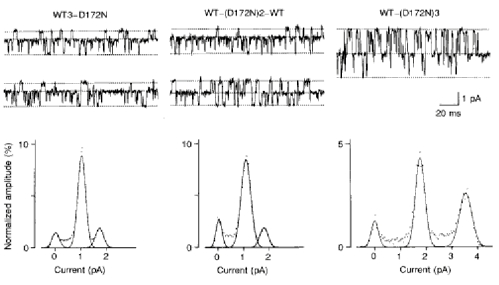
Steady-state currents were recorded at +52 mV in the presence of 2 μm intracellular Mg2+ (upper panel). The dotted lines indicate, from bottom to top, current levels for zero, two-thirds (or one-half) and fully open in WT3-D172N (left panel) and WT-(D172N)2-WT (middle panel), and for zero, one channel open and two channels open in WT-(D172N)3 (right panel). Lower panel shows histograms of current amplitude from the same patch as in the upper panel. The curve is the sum of three Gaussian distributions fitted by a least-squares method. The parameters (area, mean, s.d.) were as follows. In WT3-D172N: 0.104, 0.11, 0.14; 0.686, 1.10, 0.15; and 0.149, 1.78, 0.16. In WT-(D172N)2-WT: 0.131, 0.05, 0.11; 0.676, 1.08, 0.18; and 0.128, 1.76, 0.16. In WT-(D172N)3: 0.09, 0.03, 0.14; 0.399, 1.76, 0.18; and 0.318, 3.49, 0.24.
Effects of D172N mutation on steady-state open probability
As mentioned above, outward unitary currents were recorded in steady-state conditions at more positive potentials in channels expressed by transfection with D172N or D172Q monomers. To determine the effect of the number of D172N subunits on steady-state activation, we constructed the amplitude histogram of the steady-state outward currents in the absence of intracellular Mg2+ and polyamines and calculated the open probability (Fig. 7). The data were fitted to Boltzmann distributions to estimate the slope factor and the voltage of half-activation. As the number of D172N mutant subunits in tetrameric cDNAs increased, the steady-state open probability-membrane potential relation shifted in the positive direction. The slope factor yielded the gating charge of 3.4 (WT), 2.6 (WT3-D172N), 2.8 (WT-(D172N)2- WT), 2.6 (WT-(D172N)3) and 1.3 (D172N monomer).
Figure 7. Steady-state open probability in channels expressed from WT, WT3-D172N, WT-(D172N)2-WT, WT-(D172N)3 and D172N.
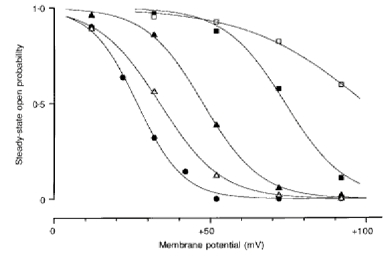
The steady-state open probability was calculated from the amplitude histogram. Currents were filtered with a -3 dB corner frequency of 0.5 kHz and digitized at 1 kHz. The data represent the average of three to five experiments and are fitted with the Boltzmann equation. The slope factor and the voltage of half-activation, respectively, for each channel were: 7.36 mV and +26.6 mV (WT, •), 9.64 mV and +33.8 mV (WT3-D172N, ▵), 8.96 mV and +47.7 mV (WT-(D172N)2-WT, ▴), 9.65 mV and +74.0 mV (WT-(D172N)3, ▪) and 19.0 mV and +99.8 mV (D172N, □).
DISCUSSION
The aspartate residue in the second hydrophobic segment of the IRK1 channel is thought to form part of the site for Mg2+ and polyamine blockade (Stanfield et al. 1994; Wible et al. 1994; Ficker et al. 1994). However, little is known of the block by intracellular Mg2+ of D172 mutant channels at the level of single channels (Wible et al. 1994). We recorded single-channel currents through D172 mutant channels and found that the negative charge at this site affects the substate behaviour in the presence of low intracellular Mg2+.
A triple-barrelled structure of the inwardly rectifying K+ channel has been proposed as the simplest model to explain the three distinct blocked states during blockade by intracellular Mg2+ or Ca2+ and extracellular Rb+ or Cs+ and the binomial distribution of occupancies of each current level (Matsuda, 1988; Matsuda et al. 1989; Matsuda & Cruz, 1993), and has attracted the attention of investigators of ion channels (Hille, 1992; Aldrich, 1993). This model requires a common gate to regulate conducting units so that they act as a single channel in the absence of blockers. Simple transitions between the fully open and zero conductance levels in mutant channels are interpreted, in terms of a triple-barrelled channel, by assuming that removal of the negative charge at D172 makes a common gate close as one of three units is plugged by a Mg2+ ion. It is, however, more likely that removal of the negative charge hinders Mg2+ ions binding to this site. The experiments on tandem tetramers containing D172N mutant subunits support the idea that different Mg2+ binding patterns produce the sublevels in a single-barrelled channel.
The finding that channels expressed from the D172N monomer were blocked without showing sublevels indicates that binding of Mg2+ to the D172 site induces partially blocked states but not the fully blocked state. Evidently, at least one more Mg2+ binding site apart from D172 should exist to produce the zero current level. Glutamate at position 224 in the putative cytoplasmic carboxyl domain is one such candidate (Taglialatela et al. 1994; Yang et al. 1995a). Channels expressed from tetramers with one or two D172N subunits showed sublevels with two-thirds of the unitary amplitude, but not with one-third, and those expressed from tetramers with three D172N subunits showed no sublevels. These findings suggest that one Mg2+ ion binds to a pair of aspartate carboxylates to produce a partial block, and support the following hypothesis for the molecular basis of substate behaviour in a single-barrelled channel.
A set of four aspartate carboxylates can interact simultaneously with two Mg2+ ions, inducing the one-third level in the case where another binding site is free of Mg2+. If only one Mg2+ ion binds to the D172 site and another binding site is free, the two-thirds level appears. Thus, transitions between substates during the open state of the channels can be described as:
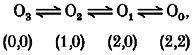 |
where O0, O1, O2 and O3 are the substates of zero, one-third, two-thirds and fully open, respectively. The numbers in parentheses represent the number of Mg2+ ions binding to the D172 site (left) and to another site (right). We assume that the binding of two Mg2+ ions to a site more interior than D172 results in the zero current level. Mutant channels from WT3-D172N and WT-(D172N)2-WT have three or two aspartate carboxylates at position 172 respectively, and cannot interact with two Mg2+ ions simultaneously. Therefore, the O1 state does not appear in these mutants, and transitions during the open state can be described as:
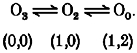 |
In channels from the WT-(D172N)3 tetramer and the D172N monomer where no Mg2+ ion can bind to the D172 site, O2 and O1 states do not occur. Transitions during the open state of the channel can be described as:
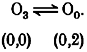 |
In the present experiments, the extent of Mg2+ block was not significantly different in wild-type and mutant (D172N) channels at higher Mg2+ concentrations and voltages. This implies that the D172 site is a subsidiary Mg2+ binding site which produces a partial block. With increasing Mg2+ concentration and depolarizations, the probability that the site other than D172 is occupied by two Mg2+ ions should increase, resulting in a complete block. In this situation, Mg2+ binding to D172 is of little importance.
Inward rectification was ascribed to voltage-dependent Mg2+ block and an intrinsic gating mechanism that closes the channels under depolarization (Matsuda, 1991b). Blockade of the IRK1 channel by intracellular polyamines (Ficker et al. 1994; Lopatin et al. 1994) seems to obviate the necessity for intrinsic gating for inward rectification. We measured the steady-state open probability at least 40 min after isolating patches and perfusing a 0.06 ml chamber with polyamine-free, ATP (3 mm)-containing solution at a rate of 1.5 ml min−1. Polyamines are effectively bound to ATP (Watanabe et al. 1991; Fakler et al. 1995). It is thought that the concentration of polyamines at the time of measurement should have been reduced to subnanomolar levels (Yang et al. 1995a; Yamashita et al. 1996), the lowest possible level attained for a limited time because of run-down of the channel activity. The finding that the steady-state open probability decreases with larger depolarizations even after profound washout of polyamines may suggest that IRK1 channels possess an intrinsic rectification mechanism. The negatively charged residues at 172, which interact with Mg2+ and polyamines under the physiological conditions, might act as voltage sensors in the absence of intracellular blockers. In this context, the reduced ability of the channel to sense voltage may reflect shifts of the voltage of half-activation in the positive direction as the number of negative charges decreased, and a reduction in the gating charge in D172N mutant channels. Further studies using a more rapid and efficient perfusion system will provide evidence for the intrinsic rectification mechanism.
Acknowledgments
We thank L. Y. Jan for IRK1 cDNA. This work was supported by grants from the Ministry of Education, Science and Culture of Japan.
References
- Aldrich R. Advent of a new family. Nature. 1993;362:107–108. doi: 10.1038/362107a0. 10.1038/362107a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. Journal de Physiologie. 1979;75:463–505. [PubMed] [Google Scholar]
- Fakler B, Brandle U, Glowatzki E, Weidemann S, Zenner H-P, Ruppersberg JP. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell. 1995;80:149–154. doi: 10.1016/0092-8674(95)90459-x. [DOI] [PubMed] [Google Scholar]
- Ficker E, Taglialatela M, Wible BA, Henley CM, Brown AM. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994;266:1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fakler G, Brandle U, Rexhausen U, Zenner H-P, Ruppersberg JP, Fakler B. Subunit-dependent assembly of inward-rectifier K+ channels. Proceedings of the Royal Society B. 1995;261:251–261. doi: 10.1098/rspb.1995.0145. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. Journal of Membrane Biology. 1974;18:61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hall AE, Hutter OP, Noble D. Current-voltage relations of Purkinje fibres in sodium-deficient solutions. Journal of Physiology. 1963;166:225–240. doi: 10.1113/jphysiol.1963.sp007102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harrison SM, Bers DM. Correction of proton and Ca association constants of EGTA for temperature and ionic strength. American Journal of Physiology. 1989;256:C1250–1256. doi: 10.1152/ajpcell.1989.256.6.C1250. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland: Sinauer Associates; 1992. Gating mechanisms; pp. 472–503. [Google Scholar]
- Kandel ER, Tauc L. Anomalous rectification in the metacerebral giant cells and its consequences for synaptic transmission. Journal of Physiology. 1966;183:287–304. doi: 10.1113/jphysiol.1966.sp007867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. Les constantes electriques de la membrane du muscle. Archives des Sciences Physiologiques. 1949;3:285–299. [Google Scholar]
- Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993;362:127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991;350:232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- Martel AE, Smith RM. Critical Stability Constants, vol. 1, Amino Acids. New York: Plenum Press; 1974. [Google Scholar]
- Matsuda H. Open-state substructure of inwardly rectifying potassium channels revealed by magnesium block in guinea-pig heart cells. Journal of Physiology. 1988;397:237–258. doi: 10.1113/jphysiol.1988.sp016998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Effects of external and internal K+ ions on magnesium block of inwardly rectifying K+ channels in guinea-pig heart cells. Journal of Physiology. 1991a;435:83–99. doi: 10.1113/jphysiol.1991.sp018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Magnesium gating of the inwardly rectifying K+ channel. Annual Review of Physiology. 1991b;53:289–298. doi: 10.1146/annurev.ph.53.030191.001445. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Cruz JDS. Voltage-dependent block by internal Ca2+ ions of inwardly rectifying K+ channels in guinea-pig ventricular cells. Journal of Physiology. 1993;470:295–311. doi: 10.1113/jphysiol.1993.sp019859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Matsuura H, Noma A. Triple-barrel structure of inwardly rectifying K+ channels revealed by Cs+ and Rb+ block in guinea-pig heart cells. Journal of Physiology. 1989;413:139–157. doi: 10.1113/jphysiol.1989.sp017646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Saigusa A, Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+ Nature. 1987;325:156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Omori K, Oishi K, Matsuda H. Inwardly rectifying potassium channels expressed by gene transfection into the Green Monkey kidney cell line COS-1. Journal of Physiology. 1997;499:369–378. doi: 10.1113/jphysiol.1997.sp021934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield PR, Davies NW, Shelton PA, Sutcliffe MJ, Khan IA, Brammar WJ, Conley EC. A single aspartate residue is involved in both intrinsic gating and blockage by Mg2+ of the inward rectifier, IRK1. Journal of Physiology. 1994;478:1–6. doi: 10.1113/jphysiol.1994.sp020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglialatela M, Wible BA, Caporaso R, Brown AM. Specification of pore properties by the carboxyl terminus of inwardly rectifying K+ channels. Science. 1994;264:844–847. doi: 10.1126/science.8171340. [DOI] [PubMed] [Google Scholar]
- Tsien RY, Rink TJ. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochimica et Biophysica Acta. 1980;599:623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Tytgat J, Hess P. Evidence for cooperative interactions in potassium channel gating. Nature. 1992;359:420–423. doi: 10.1038/359420a0. [DOI] [PubMed] [Google Scholar]
- Vandenberg CA. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proceedings of the National Academy of Sciences of the USA. 1987;84:2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Kusama-Eguchi K, Kobayashi H, Igarashi K. Estimation of polyamine binding to macromolecules and ATP in bovine lymphocytes and rat liver. Journal of Biological Chemistry. 1991;266:20803–20809. [PubMed] [Google Scholar]
- Wible BA, Taglialatela M, Ficker E, Brown AM. Gating of inwardly rectifying K+ channels localized to a single negatively charged residue. Nature. 1994;371:246–249. doi: 10.1038/371246a0. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Horio Y, Yamada M, Takahashi N, Kondo C, Kurachi Y. Competition between Mg2+ and spermine for a cloned IRK2 channel expressed in a human cell line. Journal of Physiology. 1996;493:143–156. doi: 10.1113/jphysiol.1996.sp021370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Jan YN, Jan LY. Control of rectification and permeation by residues in two distinct domains in an inward rectifier K channel. Neuron. 1995a;14:1047–1054. doi: 10.1016/0896-6273(95)90343-7. [DOI] [PubMed] [Google Scholar]
- Yang J, Jan YN, Jan LY. Determination of the subunit stoichiometry of an inwardly rectifying potassium channel. Neuron. 1995b;15:1441–1447. doi: 10.1016/0896-6273(95)90021-7. [DOI] [PubMed] [Google Scholar]


