Abstract
A clonal cell line, L-S1, has been identified from transfection of human genomic DNA into cultured mouse L-M fibroblasts. Because this transfectant cell line stably expresses a high-affinity serotonin (5-HT) transport mechanism with kinetic and pharmacological properties comparable to those of other serotonin uptake systems, it was used to investigate the mechanistic involvement of Na+ and Cl− ions in the ligand binding and kinetic uptake processes of this system.
Intact transfectant cells, when incubated at low temperature (4 °C), enabled quantitative assessment of imipramine-displaceable 5-[3H]HT binding to the 5-HT transport system. This binding activity is insensitive to the presence of various ligands specific for 5-HT receptor subtypes.
Imipramine-displaceable 5-[3H]HT binding to intact L-S1 cells was shown to be a Cl−-dependent but Na+-independent process. Chloride ions lack binding co-operativity in facilitating ligand binding. Changes in external Cl− concentration altered the Kd but not the Bmax of binding.
The overall transport activity was observed to be highly dependent on both external Na+ and Cl− concentrations, characterized by a 5-HT:Na+:Cl− coupling ratio of 1:1:1 per transport cycle. Alterations in the external concentrations of both Na+ and Cl− ions altered only the Km and not the Vmax of transport.
Both binding and kinetic results are consistent with kinetic modelling predictions of the Cl− ion in facilitating 5-HT binding to the transport system, and of the Na+ ion in enabling translocation of bound 5-HT across the plasma membrane. Thus, Na+ and Cl− ions facilitate mechanistically distinct and discernible functions in the transport cycle.
Inactivation of neurotransmitters following their stimulated release is a key regulatory process of chemical neurotransmission, and for most neurotransmitters this is accomplished via transmitter-specific, high-affinity transport mechanisms residing in the presynaptic neuron terminals and/or glial elements surrounding the synaptic clefts. Additionally, such transport systems are also believed to be critical in mediating pharmaceutical therapy for certain motor and affective disorders. While a great deal of physiological and pharmacological properties are known for amino acid and biogenic amine transmitter uptake systems (reviewed in Iversen, 1975; Kanner, 1983; and Kanner & Schuldiner, 1987), and the structural sequences of several transporters have been recently elucidated (for reviews see Schloss et al. 1992 and Uhl & Hartig, 1992; Amara & Kuhar, 1993), the mechanistic features of these systems remain to be defined in detail.
Previously, a gene transfer strategy was described for the introduction of transmitter-specific, high-affinity transport systems into cultured mouse L-M fibroblast cells (Chang et al. 1989). Two clonal transfectants (L-S1 and L-S2) generated by this strategy exhibit high-affinity 5-HT uptake systems with functional characteristics highly comparable to those previously discerned from the CNS and blood platelets, as well as from mammalian cells heterologously expressing the cloned 5-HT transporter gene (Chang et al. 1989, 1996; Frnka et al. 1991). In particular, these transfectant transport systems have been shown to be dependent on the extracellular presence of both Na+ and Cl− ions, akin to other 5-HT transport systems, but the molecular means whereby these ions facilitate the overall transport cycle awaits identification. In this report, L-S1 cells were used to examine the mechanistic involvement of Na+ and Cl− ions in both the ligand binding process and the overall transport kinetics of the 5-HT uptake system.
METHODS
Culture and transport assay of L-S1 cells
The transfectant cell line L-S1 was maintained in continuous culture with Dulbecco's modified Eagle's medium (Gibco) supplemented with 10 % defined calf serum (Hyclone) and 1 % penicillin-streptomycin (Gibco). The cells were mobilized by trypsinization and seeded into 96-well culture plates such that each well reached confluence by 2-3 days. Confluent cell monolayers were rinsed twice with oxygenated Krebs-Ringer buffer (mm: 128 NaCl, 5.2 KCl, 2.1 CaCl2, 2.9 MgSO4, 5 glucose and 10 Hepes, pH 7.4; supplemented with 0.5 mm pargyline and 0.5 mm ascorbate) and then incubated at 37°C with 5-[3H]HT (New England Nuclear; 15-30 Ci mmol−1). At the end of the incubation, excess radioligands were removed and the cell monolayers washed three times with excess Ringer buffer at room temperature. Incubated cells were then lysed with 1 % sodium dodecyl sulphate (SDS) and transferred into counting vials for liquid scintillation spectrometry. Additional wells were processed in parallel and used for protein determination by the BCA method (Smith et al. 1985) in order to normalize the observed uptake activities. Kinetic measurements of uptake activity were quantified by using various 5-[3H]HT concentrations ranging from 50 nm to 10 μm, and at both 37°C and 4°C (on ice) for each 5-[3H]HT concentration. It was previously determined that L-S1′s 5-HT uptake velocity was linear within 10 min; all transport kinetic measurements therefore utilized this duration of incubation. The uptake velocity measured at 4°C (representing non-specific uptake) was then subtracted from the uptake velocity measured at 37°C (representing total uptake) to obtain the specific uptake velocity for each 5-[3H]HT concentration.
Radioligand binding to transfectant cells
Binding of [3H]ligands to intact L-S1 cells was quantified by using the same 96-well plate format as for transport studies. Plated cells were rinsed with Ringer buffer and then incubated with 50 μl of Ringer buffer containing 5-[3H]HT. All plates were kept on ice (in a refrigerated room maintained at 4°C) for 2 h in order for both 5-[3H]HT and [3H]imipramine (New England Nuclear; 40-70 Ci mmol−1) binding to reach equilibrium, and unbound ligands were subsequently removed with three consecutive washes of ice-cold Ringer buffer. The adherent cells in each well were lysed with 1 % SDS and then subjected to liquid scintillation spectrometry. Preliminary studies indicated that this binding protocal did not significantly affect the viability of the plated cells (as judged by Trypan Blue dye exclusion) and presumably maintained the cellular permeability barrier. Total binding levels were assessed with 5-[3H]HT in concentrations ranging from 0.1 to 0.5 μm (the latter concentration level being saturating); non-specific binding levels were assessed with 5-[3H]HT in the presence of 100 μm imipramine. Specific binding level was defined as the difference between corresponding total and non-specific binding levels. Levels of specific [3H]imipramine binding were similarly defined using concentrations ranging from 10 to 250 nm, and inclusion of 100 μm 5-HT served to assess non-specific binding.
Crude membrane preparations
Cells grown in T-150 culture flasks (Corning) were rinsed twice with Tris-buffered saline (10 mm Tris, 154 mm NaCl, pH 7.8), scraped off the growth surface with a rubber policeman and collected by centrifugation. The cell pellet was then resuspended into ten volumes of homogenization buffer (27 % sucrose, 1 mm EDTA, 20 mm Hepes, pH 7.8) and disrupted with a Polytron (several 5-10 s bursts at intermediate setting). The resulting homogenate was centrifuged at 100g (using the SS-34 rotor in a Sorvall RC-5 centrifuge) for 10 min (at 4°C) to remove particulates, and the supernatant was recentrifuged at 27000g for 15 min (at 4°C). The final pellet was resuspended in homogenization buffer, aliquotted and frozen in liquid N2. The protein content of the membrane preparation was assessed by the BCA method.
Kinetic modelling
Four kinetic models were proposed to account independently for the observed Na+ and Cl− requirements in transport. By assuming that both ions and 5-HT need to bind to the transport system prior to membrane translocation, each model predicts a unique sequence of molecular events for the generalized case of transport binding by an ion and 5-HT. The respective Michaelis-Menten derivations for these models are analogous to those used previously for analysing Na+ dependence in GABA transport (Wheeler & Hollingsworth, 1979; Nelson & Blaustein, 1982). These models and their corresponding predictions are as follows.
Model 1
5-HT (S) binds to the transport system (T) first, followed by ion (I) binding. After reaching equilibrium, the complex of T, S and I then undergoes conformational change(s) and translocates S (and possibly I) inward across the plasma membrane. This scheme can be represented by the reaction:
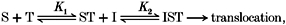
where ST and IST represent complex-intermediates, and K1 and K2 represent intermediate equilibrium constants. Michaelis-Menten derivations based upon the assumptions of this model predict that the observed Km and Vmax (respectively denoted as Km,obs and Vmax,obs) of 5-HT transport are both non-linearly dependent upon [I] (as shown in Table 1).
Table 1.
Kinetic modelling-based predictions of the experimentally observed Michaelis-Menten parameters (Km,obs and Vmax,obs) as functions of the extracellular ion concentration, [I]
| Model | Km,obs | 1/Km,obs | Vmax,obs | |||
|---|---|---|---|---|---|---|
| 1 | ||||||
| 2 | Vmax | |||||
| 3 | Vmax | |||||
| 4 |
Four distinct models were proposed, each incorporating distinct mechanisms whereby a given extracellular ion might support transmembrane transport. Because model 3 describes a higher order reaction than the other models, K1 for model 3 is of a higher order dimension than equilibrium constants of the other models. Note that several kinetic constants of model 4 have been grouped together in the Km,obs and Vmax,obs predictions: K′ = K1K2K4; K″ = K2K3K4; K‴ = K1K2+K3K4.
Model 2
Extracellular ion (I) binds to the complex (T) before S binds. The ternary complex then undergoes the necessary conformational change(s) to enable ligand translocation. This is schematized as:
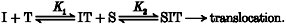
According to this model, Km,obs is predicted to be a non-linear function of [I], while Vmax,obs is expected to be independent of [I] (Table 1).
Model 3
No relative order exists for the binding of S and I to T, but both are necessary in order for translocation to proceed. This is schematized as:
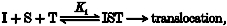
where K1 represents the equilibrium constant (with higher order dimension than equilibrium constants invoked in other models, to reflect a higher order reaction in this model). This model predicts that Km,obs is a non-linear function of [I] whereas Vmax,obs is independent of [I] (Table 1).
Model 4
Both S and I bind to T sequentially, but the relative order is random. This can be schematized as a combination of the following reactions:
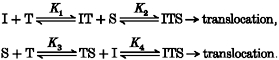
This model predicts that both Michaelis-Menten parameters, as observed experimentally, are non-linear functions of [I] (shown in Table 1).
RESULTS
5-[3H]HT binding to L-S1 cells
Previous analyses of 5-[3H]HT transport by L-S1 cells revealed that the uptake activity was temperature dependent and less active at 4°C than at 37°C (Chang et al. 1989), suggesting that, at the lower temperature, 5-[3H]HT was probably bound to the surface of L-S1 cells and not internalized by membrane translocation. Thus, low temperature incubation was further examined as a feasible means of quantifying 5-[3H]HT binding to the transport complex. Our findings indicated that total 5-[3H]HT binding was diminished with increasing concentrations of imipramine (ranging from 10 nm to 250 μm), reaching saturation by 10 μm imipramine; approximately half-maximal inhibition was attained with 100 nm imipramine. Specific 5-[3H]HT binding to L-S1 cells (using inclusion of 100 μm imipramine in parallel reactions to define non-specific binding) was characterized by a linear Scatchard plot (Fig. 1) with the following parameters: Kd = 109 ± 9 nm, Bmax = 1.11 ± 0.12 pmol mg−1 and Hill coefficient nH = 0.98 ± 0.07. The presence of 100 nm imipramine (the half-maximal inhibitory concentration) in the binding assays altered the Kd but not the Bmax value in the Scatchard profile, suggesting that imipramine is a competitive inhibitor of specific 5-[3H]HT binding to the transport system in the present protocol and thereby competes with other selective inhibitors of 5-HT uptake for binding to common and/or overlapping sites on the transporter (Chang et al. 1993). The Ki of imipramine in inhibiting specific 5-[3H]HT binding was estimated to be 15.1 ± 1.3 nm. No binding activity as such was detectable in untransfected L-M cells. Crude plasma membrane was prepared from L-S1 cells following osmotic shock and used to measure imipramine-displaceable specific 5-[3H]HT binding. The scatchard profile of 5-[3H]HT binding to this membrane preparation was linear and revealed binding Kd (120 ± 11 nm), Bmax (0.69 ± 0.14 pmol mg−1) and nH (1.01 ± 0.08) values strikingly comparable to those of intact cells. Therefore, the binding protocol employed herein is independent of the membrane permeability barrier's integrity and suffices for quantifying 5-[3H]HT interaction with the membrane-integral transport complex of L-S1 cells, presumably without cytoplasmic translocation of the bound ligand.
Figure 1. Scatchard plots of transport-specific 5-[3H]HT binding to intact L-S1 cells.
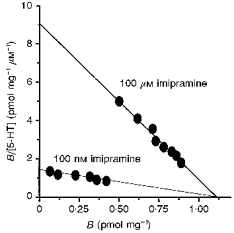
The concentration of 5-[3H]HT ranged from 0.1 to 0.5 μm. Specific binding levels (‘B’) were measured as displacement of total binding by either 100 nm (near the IC50 value) or 100 μm imipramine (fully inhibitory concentration). Each point is the mean of six measurements.
5-HT receptor ligands
Both total and non-specific levels of 5-[3H]HT binding (the latter measured by inclusion of 100 μm imipramine) were quantified in the presence of various 5-HT receptor agonists and antagonists. The repertoire of receptor-specific agents includes 1-(1-naphthyl)-piperazine (a 5HT2 antagonist; Glennon, 1987); 3-tropanylindole-3-carboxylate (a 5HT3 antagonist; Richardson et al. 1985); mianserin (a 5HT2 antagonist; Peroutka, 1988); spiroxatrine (a 5HT1a antagonist; Nelson & Taylor, 1986); (±)-2-dipropylamino-8-hydroxy-1,2,3,4-tetrahydronaphthalene (a 5HT1 agonist; Richardson & Engel, 1986); (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (a 5HT2 agonist; Glennon et al. 1988); and 1-(3-chlorophenyl)piperazine (a 5HT1b agonist; Glennon, 1987). At 100 μm, none of these compounds diminished the specific binding of 5-[3H]HT (from 0.1 to 0.5 μm) to intact L-S1 cells (data not shown). This suggested that the discerned binding activity corresponded to the presence of the high-affinity transport system in L-S1 cells.
[3H]Imipramine binding
The binding protocol described above was reversed to quantify the level of specific [3H]imipramine binding to intact L-S1 cells, using unlabelled 5-HT to assess non-specific binding levels. Total [3H]imipramine binding was saturably inhibited by increasing 5-HT concentrations ranging from 10 nm to 250 μm, reaching saturation by 100 μm; the IC50 was approximately 1 μm. Scatchard analyses of specific [3H]imipramine binding, quantified in the presence of either 1 or 100 μm 5-HT, gave rise to linear plots which shared a common Bmax but possessed differing Kd values (Fig. 2). This indicated that 5-HT is a competitive inhibitor of [3H]imipramine binding. In the presence of 100 μm 5-HT, Scatchard analysis yielded the following imipramine binding parameters: Kd = 6.0 ± 0.4 nm and Bmax = 1.3 ± 0.2 pmol mg−1. The Ki of 5-HT in inhibiting [3H]imipramine binding was estimated to be 0.8 ± 0.1 μm. For comparison, all relevant affinity parameters of 5-[3H]HT uptake, 5-[3H]HT binding and [3H]imipramine binding measured in L-S1 cells are summarized in Table 2.
Figure 2. Scatchard plots of [3H]imipramine binding to L-S1 cells.
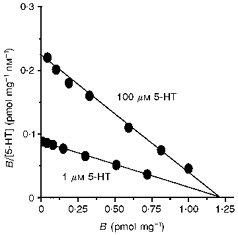
Specific binding levels were defined as displacement of total [3H]imipramine binding by 1 μm (IC50) or 100 μm 5-HT (fully inhibitory concentration); concentration of [3H]imipramine ranged from 0.5 to 100 nm. Each point is the mean of six measurements.
Table 2.
Tabulation of affinity parameters for 5-[3H]HT uptake, 5-[3H]HT binding and [3H]imipramine binding measured in intact L-S1 cells
| 5-[3H]HT uptake | |
| 5-HT Km | 390 ± 45 nm |
| Imipramine Ki | 22.7 ± 3.4 nm |
| 5[3H]HT binding | |
| 5-HT Kd | 109 ± 9 nm |
| Imipramine Ki | 15.1 ± 1.3 nm |
| [3H]Imipramine binding | |
| 5-HT Ki | 800 ± 110 nm |
| Imipramine Kd | 6.0 ± 0.4 nm |
The 5-HT Km and the imipramine Ki values were reported previously (Frnka et al. 1991).
Ion dependences of 5-[3H]HT binding
Specific binding of 5-[3H]HT to L-S1 cells was examined for possible dependence on extracellular Na+ and Cl− concentrations. The findings indicated that specific binding of 5-[3H]HT was essentially invariant with differing external Na+ concentrations between 0 and 128 mm and in the presence of constant external Cl− concentration (137.4 mm; Fig. 3A). Such Na+ independence was established by isotonically substituting extracellular Na+ with Li+; preliminary studies indicated that equivalent substitutions with choline or NMDG during the binding analyses led to comparable findings. Thus, 5-[3H]HT binding to the transport system, in the presence of external Cl− ions, seemed to be Na+ independent. Alterations in the external Cl− concentration ([Cl−]o), however, resulted in reduced specific binding activities with diminishing [Cl−]o but constant [Na+]o (128 mm; Fig. 3B). The apparent dependence of binding on [Cl−]o was further analysed by using varying [Cl−]o in conjunction with a range of 5-[3H]HT concentrations. Scatchard analyses of the binding levels measured under these conditions indicated that binding Bmax remained invariant (within experimental errors), while binding Kd increased with decreasing [Cl−]o (Fig. 4). Thus, presence of external Cl− ions seems strictly to enhance the affinity of 5-HT binding to the transport system.
Figure 3. Dependence of 5-[3H]HT binding on Na+ and Cl−.
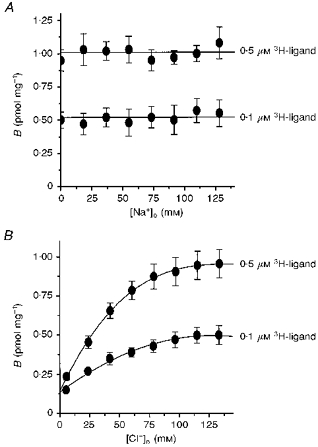
A, sodium dependence of 5-[3H]HT binding. Levels of specific binding (‘B’) were measured at both 0.1 and 0.5 μm of 3H-ligand, and with varying [Na+]o (by isotonic substitution with Li+) and constant [Cl−]o of 137.4 mm. Analogous substitutions with K+, Cs+, Rb+ or choline all led to comparable findings to substitutions with Li+. B, chloride dependence of 5-[3H]HT binding. Specific binding levels (‘B’) were measured at both 0.1 and 0.5 μm 5-[3H]HT with varying [Cl−]o (by isotonic substitution with isethionate ion) and constant [Na+]o of 128 mm. Analogous substitutions by F−, Br− or I− also led to comparable findings. Non-specific binding levels were assessed by inclusion of 100 μm imipramine. Each point is the mean ±s.d. of six values.
Figure 4. Scatchard plots of specific 5-[3H]HT binding with varying [Cl−]o.
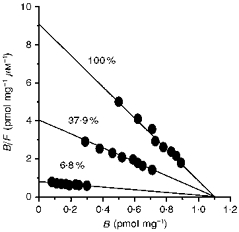
Using isethionate substitution, [Cl−]o at 6.8 % (9.4 mm), 37.9 % (52.1 mm) and 100 % (137.4 mm) were used for quantifying specific binding at 5-[3H]HT concentrations ranging from 0.1 to 0.5 μm. Non-specific binding levels were assessed using 100 μm imipramine. Each point is the mean of six measurements.
Ion dependences of 5-[3H]HT uptake
It was previously shown that L-S1 cells exhibit a saturable velocity profile in transporting 5-[3H]HT, characterized by Michaelis-Menten parameters (Km = 0.39 ± 0.10 μm, Vmax = 2.14 ± 0.55 pmol mg−1 min−1, nH = 0.95 ± 0.09) which are highly comparable to those of other serotonin transport systems (Chang et al. 1989, 1996). The apparent lack of ligand co-operativity during transport agrees with the observed lack of ligand co-operativity during binding to the transport complex, and further suggests that each cycle of 5-HT transport translocates one 5-HT molecule. When [Na+]o was isotonically altered to varying extents, the initial velocity of 5-[3H]HT uptake increased to a saturable level with increasing [Na+]o (Fig. 5A). But a Na+-independent component was observable even without added [Na+]o and may reflect low-level contamination of Na+ in Li+-substituted buffers. When this Na+-independent component was subtracted out of the overall velocity profile, the resulting Na+-dependent profile yielded a linear Hill plot with unitary slope (nH = 0.98 ± 0.11). This suggested that Na+ lacks co-operativity in facilitating 5-HT transport and that one Na+ ion is likely to be involved in each cycle of 5-HT transport. Kinetic analyses of 5-[3H]HT uptake were conducted with varying ligand concentrations (50 nm to 10 μm), and for each ligand concentration with Ringer buffers containing 100 % (128 mm), 33.3 % (42.7 mm) or 0 % of the normal [Na+]o level. Lineweaver-Burk analyses of the resulting velocity profiles revealed all linear profiles which shared a common ordinate intercept. This indicated that changing [Na+]o had no apparent effect on the observed transport Vmax, but elevated the observed transport Km with decreasing [Na+]o. Inverses of the experimentally derived Km (‘Km,obs‘) values were plotted as a function of the corresponding [Na+]o and gave rise to a linear relationship which intersected the axes close to the origin (Fig. 5B).
Figure 5. Sodium dependence of 5-HT uptake.
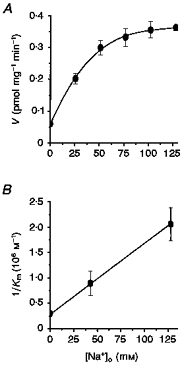
A, initial velocity profile of 5-[3H]HT (0.1 μm) uptake as a function of [Na+]o. External [Na+] was changed by isotonic replacement with Li+. A Na+-independent component of transport was evident at 0 mm [Na+]o. Removing this Na+-independent component gives a velocity profile characterized with unitary Hill coefficient. B, relationship between 1/Km,obs and [Na+]o. The linearity of this relationship suggests that model 3 is most consistent with experimental findings. The Km,obs values were attained from kinetic analyses (using 50 nm to 10 mm 5-[3H]HT) conducted with 100, 33.3 and 0 % of the physiological [Na+]o level. Lineweaver-Burk analyses of the results of such kinetic analyses revealed invariant Vmax,obs but changing Km,obs as a function of [Na+]o. Each point is the mean ±s.d. of six measurements.
Initial velocities of 5-[3H]HT uptake also exhibited significant dependence upon [Cl−]o, and approached saturation with increasing [Cl−]o (Fig. 6A). By extrapolating the velocity profile to the level of zero [Cl−]o, however, no Cl−-independent component was discernible. This contrasted with the binding results whereby specific 5-[3H]HT binding was discernible even at zero [Cl−]o. The Cl−-dependent velocity profile yielded a Hill plot characterized by an unitary slope (nH = 0.95 ± 0.08). Thus, analogous to Na+ dependence, Cl− participation in 5-HT transport seems to lack co-operativity and each cycle of 5-HT transport is likely to involve a 5-HT:Na+:Cl− coupling ratio of 1:1:1. Kinetic analyses with Ringer buffers containing 100 % (137.4 mm), 37.9 % (52.1 mm) or 6.8 % (9.4 mm) [Cl−]o yielded velocity profiles which, when analysed by Lineweaver-Burk plots, were linear and all shared a common ordinate intercept, a strong indication that Cl− facilitates 5-HT transport by increasing the overall affinity of the transport system for 5-HT (by lowering transport Km) and not affecting the overall transport capacity (invariant Vmax). Plotting the inverse of each Km,obs value against the corresponding [Cl−]o yielded a non-linear, saturating relationship which intersected the origin (Fig. 6B), in contrast to that of the Na+ dependence findings. Because the ion dependences of 5-HT uptake discerned herein are indistinguishable from those previously observed by heterologous expression of the cloned serotonin transporter cDNA (Chang et al. 1996), it is reasonable to assume that the present findings of [Na+]o and [Cl−]o effects on Km,obs are truly reflective of such ion effects on native 5-HT uptake systems.
Figure 6. Chloride dependence of 5-HT uptake.
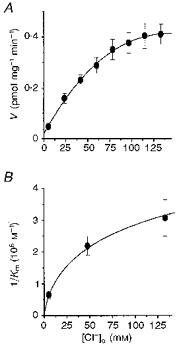
A, initial velocity profile of 5-[3H]HT (0.1 μm) uptake as a function of [Cl−]o. External [Cl−] was changed by isotonic replacement with isethionate. No Cl−-independent component of transport was evident, and the velocity profile was characterized with a unitary Hill coefficient. B, relationship between 1/Km,obs and [Cl−]o. The hyperbolic nature of this relationship and its intersection with the origin suggests that model 2 is most consistent with experimental findings. The Km,obs values were attained from kinetic analyses (using 50 nm to 10 mm 5-[3H]HT) conducted with 100, 37.9 and 6.8 % of the physiological [Cl−]o level. Lineweaver-Burk analyses of the results of such kinetic analyses revealed invariant Vmax,obs but changing Km,obs as a function of [Cl−]o. Each point is the mean ±s.d. of six analyses.
DISCUSSION
Transfectant cell models of high-affinity 5-HT uptake
One of the essential processes of synaptic neurotransmission is the inactivation of the released transmitter in the synaptic domain, which is mainly accomplished by transmitter-specific uptake (transport) mechanisms residing in the pre-synaptic membranes and/or in apposing glial cells. Novel models of such transport systems have been generated by transfecting human genomic DNA into mouse fibroblast L-M cells, resulting in clonal cell lines each of which stably manifests a high-affinity neurotransmitter uptake system (Chang et al. 1989). This approach has yielded two clonal cell lines, L-S1 and L-S2, which possess physiological and pharmacological characteristics of 5-[3H]HT transport that are comparable to other systems of 5-HT transport (Frnka et al. 1991; Chang et al. 1993). Both of these transfectant cell lines were used for the mechanistic analyses of ion requirements in the ligand binding process of 5-HT transport. Because the results for both cell lines are essentially identical for all of the described studies, only those of L-S1 are presented as representative of both cell lines. While the transgenic cell lines used for these studies may represent heterologous expression of the human serotonin transporter gene within the mouse fibroblastic cell host, it is nevertheless conceivable that the observed transport activities correspond to trans-activation of the endogenous mouse gene resulting from fortuitous integration of regulatory human element(s). It is noteworthy, however, that heterologous expression of both the human and the mouse 5-HT transporter genes have revealed kinetic and ion-dependent uptake properties which are indistinguishable from each other (Ramamoorthy et al. 1993; Chang et al. 1996). Thus, the findings herein should be mechanistically pertinent to both human and mouse 5-HT transporters, and likely to be generalizable to other mammalian 5-HT transport mechanisms.
5-[3H]HT binding protocol
Previous studies have illustrated the feasibility of uncoupling the ligand binding process apart from the translocation process of a neurotransmitter transport cycle (Wood & Wyllie, 1982). Observations that low incubating temperature rendered intact L-S1 cells apparently incapable of transporting exogenous 5-[3H]HT prompted the use of a low incubating temperature to uncouple the ligand binding process from the rest of the transport-related mechanisms. Since imipramine was shown to be a competitive inhibitor of 5-[3H]HT transport in the transfectant cells (Frnka et al. 1991), it was considered a suitable candidate as competitor for quantification of specific 5-[3H]HT binding to intact transfectant cells. By this protocol, the presence of a single and saturable binding process in intact L-S1 cells was observed and shown to be competitively inhibited by imipramine (consistent with competitive inhibition of 5-HT transport by imipramine). The lack of ligand co-operativity discerned for this binding process also agreed with the lack of ligand co-operativity during transport. The observed binding Kd was also comparable with the observed transport Km previously reported (Frnka et al. 1991; Chang et al. 1996), and supports the notion that ligand binding is rate limiting in this transport process. Analyses of crude membrane preparations derived from L-S1 cells provided additional indications that the observed binding activities were independent of the integrity of the permeability barrier, and therefore did not incorporate ligand translocation activities of the system.
When the binding protocol was reversed and [3H]imipramine binding level was quantified by using unlabelled 5-HT as competitor, a saturable binding process was again observed and shown to be competitively inhibited by 5-HT. The observed Kd of [3H]imipramine binding to intact L-S1 cells compared favourably with Kd values discerned from CNS-derived preparations as well as blood platelets (D'Amato et al. 1987; Langer & Galzin, 1988; Rovescalli et al. 1989), and with imipramine's Ki in inhibiting 5-[3H]HT transport by L-S1 cells (Frnka et al. 1991). Additionally, binding of both [3H]imipramine and 5-[3H]HT to these cells manifested the same Bmax values. Furthermore, [3H]imipramine's Kd was also equivalent to imipramine's Ki in inhibiting both 5-[3H]HT binding and transport. All of these congruences between the binding parameters and the overall agreements with the transport properties (summarized in Table 2) strongly indicate that 5-HT and imipramine indeed bind to, and mutually compete for, common/overlapping sites on the 5-HT transport system of L-S1 cells. Since 5-HT and selective serotonin reuptake inhibitors (SSRIs) as well as cocaine congeners all share a common pharmacophore structure that is important for transporter interaction (Chang et al. 1993 and the authors’ unpublished observations), it is highly conceivable that imipramine and other tricyclic antidepressants also compete with other serotonin uptake inhibitors at common and/or overlapping sites on the transport complex.
The apparent discrepancy between 5-HT's Ki in inhibiting [3H]imipramine binding and 5-[3H]HT's Kd in binding suggested a mechanistic distinction in the manner whereby 5-HT and imipramine interact with the 5-HT transport system. It is noteworthy that this phenomenon has been observed previously for blood platelets (Talvenheimo et al. 1983) and, while presently inexplicable, does not invalidate the current binding protocol. Rather, this phenomenon may point to a unique feature of imipramine interaction with the transport system.
It is also noteworthy that direct comparisons of both 5-[3H]HT transport Vmax with its binding Bmax provide an estimate of transport ‘turnover’ rate (2-3 min−1) which is notably slower than in other models of transmembrane 5-HT uptake. Several possibilities may be envisioned to account for this observation: over-estimation of whole cell binding density; kinetic slowing of the transporter in the context of fibroblastic expression; aberrant assembly of transporter complex on the fibroblast cell surface (such that specific binding activity is enabled without corresponding transport capacity). This observation is unlikely to be due to excessive cell lysis during the binding and uptake manipulations that rendered the reduced Vmax/Bmax ratio since cell viabilities were monitored during such analyses and deemed to be within an acceptable range.
Ion dependences of 5-[3H]HT binding
Previous studies using blood platelets reported that Cl− independence in [3H]imipramine binding was presumably reflective of Cl− independence in 5-[3H]HT binding to the high-affinity transport system (Lingjaerde, 1971; Talvenheimo et al. 1979). In like manner, Na+ dependence in 5-[3H]HT binding to platelet membrane vesicles was presumed to be indicative of the same ion dependence in 5-[3H]HT binding to the 5-HT transporters (Talvenheimo et al. 1979). By applying the binding protocol developed herein for the transfectant cells, we have observed that 5-[3H]HT binding to the transfectant 5-HT transport system was Na+ independent and Cl− dependent. Moreover, the Cl− dependence seemed to prominently influence the Kd, but not the Bmax, of ligand binding, thereby suggesting that the Cl− ion participates in the overall transport cycle by facilitating the 5-HT binding process; this postulation was subsequently substantiated by kinetic modelling of Cl− dependence in transport kinetics. The apparent Na+ independence of steady-state 5-[3H]HT binding is a departure from prior observations of Na+ dependence for both 5-[3H]HT and [3H]imipramine binding to the platelet 5-HT transport system (Talvenheimo & Rudnick, 1980; Talvenheimo et al. 1983); this discrepancy cannot be readily reconciled, although kinetic modelling of Na+ dependence in transport kinetics indicates that the ligand binding process may indeed be Na+ independent.
Ion dependence of 5-[3H]HT transport
The ion dependences of 5-[3H]HT transport kinetics were examined for direct comparisons with the ion dependences observed for 5-[3H]HT binding. Kinetic results strongly indicated that this transport system is both Na+ dependent and Cl− dependent. The [Na+]o dependence of 5-[3H]HT uptake observed for L-S1 cells satisfies an important requirement of neurotransmitter uptake systems in general, and serotonin transport in particular (Sneddon, 1969). Similarly, the dependence on [Cl−]o is another verification of the authenticity of this uptake system, since studies with blood platelets and brain-derived membrane vesicles revealed the existence of this ion dependence for 5-HT transport (Lingjaerde, 1971; Nelson & Rudnick, 1982; Reith et al. 1989). Kinetic analyses of these ion dependences further revealed the lack of co-operativity for both Na+ and Cl− in facilitating 5-[3H]HT transport by L-S1 cells. The deduced coupling ratio between 5-HT, Na+ and Cl− is likely to be 1:1:1, which agrees with previous studies in other 5-HT transport systems as well as in heterologous expression of the mouse serotonin transporter cDNA (Sneddon, 1969; Nelson & Rudnick, 1982; Talvenheimo et al. 1983; Wood, 1987; Ganapathy et al. 1989; Chang et al. 1996). Assuming that Na+ and Cl− are cotransported with 5-HT, these findings would suggest that 5-HT uptake is therefore an electrogenic process during ligand influx and with a net charge of +1, since at physiological pH 5-HT is predominantly protonated at the alkylamino group. Whether or not the overall transport cycle is also electrogenic depends upon the nature and relative number of outward ion flow accompanying each cycle of inward 5-HT translocation.
Alterations in the extracellular concentration of either ion led to changes in apparent transport Km but not apparent transport Vmax. By comparison, other studies have reported changes in [Na+]o which led strictly to changes in either kinetic parameter (Sneddon, 1969; Briley & Langer, 1981; Talvenheimo et al. 1983). Also, changes in [Cl−]o have been shown to either alter Vmax alone (Lingjaerde, 1971), or both Km and Vmax (Nelson & Rudnick, 1982). Based upon the binding results presented herein, it seemed consistent that the observed Cl− dependence would be observed in transport kinetics. These results collectively indicated that 5-[3H]HT binding is critically dependent upon the extracellular presence of Cl− when in the presence of extracellular Na+. In this particular regard, it is highly noteworthy that a recent study of platelet 5-HT transporters also revealed the Cl− ion's ability to increase 5-HT binding to the transporter in the concomitant presence of extracellular Na+ (Humphreys et al. 1994). The kinetic dependence on Na+, while not observed in the ligand binding process, could conceivably reflect the generally accepted notion that the transmembrane Na+ gradient provides at least part of the bioenergetics for this transport system (Sneddon, 1971; Rudnick, 1977). The transmembrane K+ concentration gradient has been shown to be contributory to 5-HT transport kinetics but not an absolute requirement for the overall uptake process (Nelson & Rudnick, 1979; Cool et al. 1990). It has been postulated that K+ efflux stimulates the restoration of the transporter ligand-binding site to an externally accessible conformation, following transporter-mediated influx of the bound 5-HT. While such conformational restoration is unlikely to be the rate-limiting step in the overall transport cycle, it nevertheless warrants further study in order to describe more comprehensively the K+ ion's participation in this transport mechanism. More significantly, it will be of interest to delineate better the K+ dependence of this system in relation to the dependence on extracellular Cl− ions as described herein.
Kinetic models of ion dependence
To rationalize the observed kinetic effects of [Na+]o and [Cl−]o alterations, four generalized kinetic models were proposed wherein each described a distinct relative order of 5-HT/ion binding to the transport system prior to ligand translocation. Based upon the specific premise of each model, Michaelis-Menten derivations led to predictions of both Km,obs and Vmax,obs as distinct functions of the given extracellular ion concentration (summarized in Table 1). These predictions were then compared with the attained Km,obs and Vmax,obs values.
In the case of Na+ dependence, variations in [Na+]o resulted in no change of Vmax,obs. Since models 1 and 4 both predicted Vmax,obs as a function of [Na+]o, neither model is compatible with the present observations. Models 2 and 3, however, both predicted that Vmax,obs would be independent of [Na+]o, which is consistent with experimental findings. Since model 2 predicted 1/Km,obs as a non-linear, saturating function of [Na+]o, whereas model 3 predicts 1/Km,obs as a linear function of [Na+]o, model 3 is more appropriate for accounting for the observed Na+ dependence in transport kinetics. In terms of Cl− dependence, variations in [Cl−]o also resulted in no apparent change in transport Vmax. Again, models 1 and 4 are not appropriate. The 1/Km,obs values, when plotted against the corresponding [Cl−]o levels, yielded a hyperbolic relationship, suggesting that model 2 is most compatible with the observed Cl− dependence in transport kinetics.
Combining the kinetic models most consistent with the experimental findings provide the notion that Cl− binding to the transport system is a requisite for 5-HT binding, whereas Na+ binding is unimportant for 5-HT binding but necessary for translocation of bound 5-HT. This is entirely consistent with the observed Cl− dependence and Na+ independence in 5-HT binding to intact L-S1 cells, and also consistent with the generally accepted notion that Na+-dependent transport processes derive at least part of their driving force from the transmembrane Na+ electrochemical gradient. The notion that Cl− facilitates 5-HT binding is not only in accord with the binding and transport studies described herein, but also in agreement with findings in the dopamine uptake system suggests that Cl− enhances ligand binding to the transporter (Amejdki-Chab et al. 1992). Moreover, the presently proposed roles of Na+ and Cl− in facilitating 5-HT transport is still consistent with previous studies indicating that this transport system is energetically driven, at least in part, by several transmembrane ion gradients (Sneddon, 1971; Rudnick, 1977; Keyes & Rudnick, 1982; Nelson & Rudnick, 1982; Talvenheimo et al. 1983; Cool et al. 1990).
The high degree of agreement between the observed ion dependences in transport/binding studies and the theoretical predictions of kinetic modelling lends significant validity to the following mechanistic assumptions which are inherent to the modelling approach. First, Na+ and Cl− bindings to the transport system necessarily precede translocation of the bound 5-HT. Second, the two ion dependences are functionally independent of each other. Third, once both ions and 5-HT are appropriately bound to the transport system, all of the subsequent events in the transport cycle will proceed to translocate 5-HT across the plasma membrane. Fourth, 5-HT and ion bindings are rapidly equilibratable steps relative to the translocation process. Fifth, the observed transport kinetics can be adequately modelled by Michaelis-Menten analyses. Further studies should lend additional support to these mechanistic assumptions and the proposed roles of Na+ and Cl− ions arising from the present studies in other models of high-affinity 5-HT transport, as well as other Na+- and Cl−-dependent neurotransmitter transport mechanisms.
Acknowledgments
The authors gratefully acknowledge Dr Jerome Frnka and Dr Gary Rudnick for valuable discussions during these studies. This work was supported by grants from the National Institute on Drug Abuse to A. S. C. (DA06743) and the National Eye Institute to D. M.-K. L. (EY02423).
References
- Amara SG, Kuhar MJ. Neurotransmitter transporters: Recent progress. Annual Review of Neuroscience. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Amejdki-Chab N, Costentin J, Bonnet J-J. Kinetic analysis of the chloride dependence of the neuronal uptake of dopamine and effect of anions on the ability of substrates to compete with the binding of the dopamine uptake inhibitor GBR 12783. Journal of Neurochemistry. 1992;58:793–800. doi: 10.1111/j.1471-4159.1992.tb09327.x. [DOI] [PubMed] [Google Scholar]
- Briley M, Langer SZ. Sodium-dependency of [3H]imipramine binding to rat cerebral cortex. European Journal of Pharmacology. 1981;72:377–380. doi: 10.1016/0014-2999(81)90580-x. [DOI] [PubMed] [Google Scholar]
- Chang AS, Chang SM, Starnes DM. Structure-activity relationships of serotonin transport: Relevance to nontricyclic antidepressant interactions. European Journal of Pharmacology. 1993;247:239–248. doi: 10.1016/0922-4106(93)90191-b. [DOI] [PubMed] [Google Scholar]
- Chang AS, Chang SM, Starnes DM, Schroeter S, Bauman AL, Blakely RD. Cloning and expression of the mouse serotonin transporter. Molecular Brain Research. 1996;43:185–192. doi: 10.1016/s0169-328x(96)00172-6. [DOI] [PubMed] [Google Scholar]
- Chang AS, Frnka JV, Chen D, Lam DM-K. Characterization of a genetically reconstituted high-affinity system for serotonin transport. Proceedings of the National Academy of Sciences of the USA. 1989;86:9611–9615. doi: 10.1073/pnas.86.23.9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool DR, Leibach FH, Ganapathy V. Modulation of serotonin uptake kinetics by ions and ion gradients in human placental brush-border membrane vesicles. Biochemistry. 1990;29:1818–1822. doi: 10.1021/bi00459a022. [DOI] [PubMed] [Google Scholar]
- D'Amato RJ, Largent BL, Snowman AM, Snyder SH. Selective labelling of serotonin uptake sites in rat brain by [3H]citalopram contrasted to labelling of multiple sites by [3H]imipramine. Journal of Pharmacology and Experimental Therapeutics. 1987;242:364–371. [PubMed] [Google Scholar]
- Frnka JV, Chang AS, Lam DM-K. Pharmacological characteristics of high-affinity serotonin uptake systems established through gene transfer. Journal of Pharmacology and Experimental Therapeutics. 1991;256:734–740. [PubMed] [Google Scholar]
- Ganapathy V, Kulanthaivel P, Tiruppathi C, Mahesh VB, Leibach FH. Inactivation of the human placental serotonin transporter by tyrosyl group-specific reagents. Journal of Pharmacology and Experimental Therapeutics. 1989;251:9–15. [PubMed] [Google Scholar]
- Glennon RA. Central serotonin receptors as targets for drug research. Journal of Medicinal Chemistry. 1987;30:1–12. doi: 10.1021/jm00384a001. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Seggel MR, Soine WH, Herrick-Davis K, Lyon RA, Titeler M. [125I]-1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane: An iodinated radioligand that specifically labels the agonist high affinity state of 5HT2 serotonin receptors. Journal of Medicinal Chemistry. 1988;31:5–7. doi: 10.1021/jm00396a003. [DOI] [PubMed] [Google Scholar]
- Humphreys CJ, Wall SC, Rudnick G. Ligand binding to the serotonin transporter: Equilibria, kinetics, and ion dependence. Biochemistry. 1994;33:9118–9125. doi: 10.1021/bi00197a014. [DOI] [PubMed] [Google Scholar]
- Iversen LL. Uptake processes for biogenic amines. In: Iversen LL, Iversen SD, Snyder SH, editors. Handbook of Psychopharmacology. Vol. 3. New York: Plenum Press; 1975. pp. 381–442. [Google Scholar]
- Kanner BI. Bioenergetics of neurotransmitter transport. Biochimica et Biophysica Acta. 1983;726:293–316. doi: 10.1016/0304-4173(83)90013-7. [DOI] [PubMed] [Google Scholar]
- Kanner BI, Schuldiner S. Mechanism of transport and storage of neurotransmitters. CRC Critical Reviews in Biochemistry. 1987;22:1–38. doi: 10.3109/10409238709082546. [DOI] [PubMed] [Google Scholar]
- Keyes SR, Rudnick G. Coupling of transmembrane proton gradients to platelet serotonin transport. Journal of Biological Chemistry. 1982;257:1172–1176. [PubMed] [Google Scholar]
- Langer SZ, Galzin AM. Studies on the serotonin transporter in platelets. Experientia. 1988;44:127–130. doi: 10.1007/BF01952194. [DOI] [PubMed] [Google Scholar]
- Lingjaerde O., Jr Uptake of serotonin in blood platelets in vitro. I. The effects of chloride. Acta Phyiologica Scandinavica. 1971;81:75–83. doi: 10.1111/j.1748-1716.1971.tb04878.x. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Blaustein MP. GABA efflux from synaptosomes: Effects of membrane potential, and external GABA and cations. Journal of Membrane Biology. 1982;69:213–223. doi: 10.1007/BF01870400. [DOI] [PubMed] [Google Scholar]
- Nelson PJ, Rudnick G. Coupling between platelet 5-hydroxytryptamine and potassium transport. Journal of Biological Chemistry. 1979;254:10084–10089. [PubMed] [Google Scholar]
- Nelson PJ, Rudnick G. The role of chloride ion in platelet serotonin transport. Journal of Biological Chemistry. 1982;257:6151–6155. [PubMed] [Google Scholar]
- Nelson DL, Taylor EW. Spiroxatrine: A selective serotonin 1A receptor antagonist. European Journal of Pharmacology. 1986;124:207–208. doi: 10.1016/0014-2999(86)90147-0. 10.1016/0014-2999(86)90147-0. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ. 5-Hydroxytryptamine receptor subtypes. Annual Review of Neuroscience. 1988;11:45–60. doi: 10.1146/annurev.ne.11.030188.000401. 10.1146/annurev.ne.11.030188.000401. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, Blakely RD. Antidepressant- and cocaine-sensitive human serotonin transporter: Molecular cloning, expression, and chromosomal localization. Proceedings of the National Academy of Sciences of the USA. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith MEA, Zimanyi I, O'Reilly CA. Role of ions and membrane potential in uptake of serotonin into plasma membrane vesicles from mouse brain. Biochemical Pharmacology. 1989;38:2091–2097. doi: 10.1016/0006-2952(89)90062-2. 10.1016/0006-2952(89)90062-2. [DOI] [PubMed] [Google Scholar]
- Richardson BP, Engel G. The pharmacology and function of 5HT3 receptors. Trends in Neurosciences. 1986;9:424–428. 10.1016/0166-2236(86)90137-2. [Google Scholar]
- Richardson BP, Engel G, Donatsch P, Stadler PA. Identification of serotonin M-receptor subtypes and their specific blockade by a new class of drugs. Nature. 1985;316:126–131. doi: 10.1038/316126a0. [DOI] [PubMed] [Google Scholar]
- Rovescalli AC, Brunello N, Riva M, Galimberti R, Racagni G. Effect of different photoperiod exposure on [3H]imipramine binding and serotonin uptake in the rat brain. Journal of Neurochemistry. 1989;52:507–514. doi: 10.1111/j.1471-4159.1989.tb09149.x. [DOI] [PubMed] [Google Scholar]
- Rudnick G. Active transport of 5-hydroxytryptamine by plasma membrane vesicles isolated from human blood platelets. Journal of Biological Chemistry. 1977;252:2170–2174. [PubMed] [Google Scholar]
- Schloss P, Mayser W, Betz H. Neurotransmitter transporters: A novel family of integral plasma membrane proteins. FEBS Letters. 1992;307:76–80. doi: 10.1016/0014-5793(92)80905-v. 10.1016/0014-5793(92)80905-V. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Analytical Biochemistry. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sneddon JM. Sodium-dependent accumulation of 5-hydroxytryptamine by rat blood platelets. British Journal of Pharmacology. 1969;37:680–688. doi: 10.1111/j.1476-5381.1969.tb08506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon JM. Relationship between internal Na+/K+ and the accumulation of 14C-5-hydroxytryptamine by rat platelets. British Journal of Pharmacology. 1971;43:834–844. doi: 10.1111/j.1476-5381.1971.tb07220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talvenheimo J, Fishkes H, Nelson PJ, Rudnick G. The serotonin transporter-imipramine “receptor”: Different sodium requirements for imipramine binding and serotonin translocation. Journal of Biological Chemistry. 1983;258:5115–6119. [PubMed] [Google Scholar]
- Talvenheimo J, Nelson PJ, Rudnick G. Mechanism of imipramine inhibition of platelet 5-hydroxytryptamine transport. Journal of Biological Chemistry. 1979;254:4631–4635. [PubMed] [Google Scholar]
- Talvenheimo J, Rudnick G. Solubilization of the platelet plasma membrane serotonin transporter in an active form. Journal of Biological Chemistry. 1980;255:8606–8611. [PubMed] [Google Scholar]
- Uhl GR, Hartig PR. Transporter explosion: Update on uptake. Trends in Pharmacological Sciences. 1992;13:421–425. doi: 10.1016/0165-6147(92)90133-q. 10.1016/0165-6147(92)90133-Q. [DOI] [PubMed] [Google Scholar]
- Wheeler DD, Hollingsworth RG. A model of GABA transport by cortical synaptosomes from the Long-Evans rat. Journal of Neuroscience Research. 1979;4:265–289. doi: 10.1002/jnr.490040405. [DOI] [PubMed] [Google Scholar]
- Wood MD. Examination of the relationship between the uptake site for 5-hydroxytryptamine and the high affinity binding site for [3H]imipramine. II. The role of sodium ions. Neuropharmacology. 1987;26:1081–1085. doi: 10.1016/0028-3908(87)90251-6. 10.1016/0028-3908(87)90251-6. [DOI] [PubMed] [Google Scholar]
- Wood MD, Wyllie MG. Resolution of monoamine uptake into binding and translocation components. Neuropharmacology. 1982;21:375–378. doi: 10.1016/0028-3908(82)90104-6. 10.1016/0028-3908(82)90104-6. [DOI] [PubMed] [Google Scholar]


