Abstract
The interaction of ion channels activated by nicotinic receptor agonists with ion channels gated by extracellular ATP (i.e. P2X receptors) was studied on sympathetic neurons acutely dissociated from coeliac ganglia of the guinea-pig. Patch clamp methods were used to measure the inward current generated through these non-selective cationic channels under voltage clamp.
At the whole cell level, the specific nicotinic receptor agonists nicotine (5-100 μm) or cytisine (50-75 μm) and the P2X receptor agonists ATP (0.1-7 μm) or α,β-methylene ATP (6 μm) were examined separately and in the presence of the other receptor activator. When a nicotinic and P2X receptor agonist were applied together, mutually occlusive effects were generally observed. This occurred even with concentrations of agonists that in themselves generated little to no inward current.
The occlusive effects of nicotinic agonists on ATP-gated currents were blocked by the nicotinic receptor/ion channel blocker hexamethonium (150 μm). The occlusive effects of ATP analogues on inward currents generated by nicotinic agonists were blocked by the P2X receptor antagonist suramin (100 μm).
Mutual occlusion of the effects of nicotinic agonists and ATP analogues were also observed when currents through single channels were studied in excised (outside-out) patches.
The results suggest that nicotinic receptors and P2X ATP receptors do not act independently in these sympathetic neurons.
Ligand-gated channels are receptors that transduce the binding of agonist into the flow of ionic current; these events occur within a fraction of a millisecond and with enormous amplification of the initiating chemical signal (Hille, 1992). In the vertebrate nervous system, the transmitter acetylcholine (ACh) stimulates nicotinic receptors that incorporate ion channels as part of the receptor protein, thus exciting the skeletal neuromuscular junction, autonomic ganglia and the central nervous system (for reviews see Sargent, 1993; DeVillers-Thiery, Galzi, Eisele, Bertrand, Bertrand & Changeux, 1993; Lindstom, 1996; Albuquerque et al. 1997). Similarly, neurally released ATP, acting on P2X ATP receptors (ATP receptors which incorporate an ion channel), mediates excitation of mammalian smooth muscle and neurons in the autonomic and central nervous systems (Sneddon & Westfall, 1984; Sneddon & Burnstock, 1985; Illes & Norenberg, 1993; Brake & Julius, 1996; Silinsky, Von Kugelgen, Smith & Westfall, 1997). With regard to fast synaptic transmission between mammalian neurons, ATP is released from nerve endings and acts on P2X receptors to mediate fast excitatory synaptic transmission in the medial habenula (Edwards, Gibb & Colquhoun, 1992), the enteric nervous system (Galligan & Bertrand, 1994), the dorsal horn of the spinal cord (Bardoni, Goldstein, Lee, Gu & MacDermott, 1997) and between neurons cultured from sympathetic ganglia (Gerzanich, Matsumoto, North & Silinsky, 1991; Silinsky, Gerzanich & Vanner, 1992; Silinsky & Gerzanich, 1993; see also Evans, Derkach & Surprenant, 1992).
At nicotinic synapses ATP is released with acetylcholine (Silinsky, 1975; Redman & Silinsky, 1994) and within milliseconds of a nerve impulse (Silinsky & Redman, 1996). Whilst the recent molecular biological approaches suggest that P2X ATP receptors and nicotinic receptors have different transmembrane dispositions (for reviews see DeVillers-Thiery et al. 1993; Brake & Julius, 1996; North & Barnard, 1997), some evidence suggests that extracellular ATP and nicotinic receptors may interact. Indeed nicotinic receptors possess an external binding site for ATP (Carlson & Raftery, 1993; Schrattenholz et al. 1994; Zimmerman, 1994) and both nicotinic receptors and ion channels gated by extracellular ATP appear together in both developing and adult neurons (Nakazawa, Fujimori, Takanaka & Inoue, 1991; Silinsky et al. 1992; Silinsky & Gerzanich, 1993). Of interest in this regard is the report that, in rat phaeochromocytoma cells, the combined effects of ATP and nicotine were less than additive; additive effects would be predicted to occur if the currents arose from two independent populations of ion channels (Nakazawa et al. 1991). It has subsequently been found that even very low ATP concentrations inhibit the action of nicotine both on rat phaeochromocytoma cells and in ganglionic-like receptors expressed in Xenopus oocytes when studied on macroscopic ionic currents (Nakazawa, Ito, Koizumi, Ohno & Inoue, 1995). However, the interactions between ATP and nicotine have not been studied at the single channel level.
P2X ATP-gated channels in celiac ganglia of the guinea-pig possess markedly different properties from nicotinic channels. Specifically, the conductance of the purine receptor in these sympathetic neurons near the normal cell resting potential is approximately half that of the nicotinic receptors and the ATP-gated channel is prone to long apparent openings with a high degree of flickering (see Silinsky & Gerzanich, 1993; Silinsky & Redman, 1996). As preliminary experiments suggest that the actions of ATP and nicotinic agonists are not independent in these neurons (see p. 209 in Silinsky & Gerzanich, 1993), it is therefore of interest to determine if P2X receptors and nicotinic receptors interact at coeliac ganglia, where the single channel events have a distinct signature of conductance and gating patterns. This paper describes such an electrophysiological study using the patch clamp method in both whole cell and excised (outside-out) configurations. The results, which focus on the occlusive effects of receptor agonists, suggest that nicotinic agonists and P2X agonists do not act independently on guinea-pig sympathetic neurons.
METHODS
Preparation of acutely dissociated neurons containing ATP-gated channels and nicotinic receptors
Procedures followed previously were used for acute dissociation of sympathetic neurons (Matsumoto, Gruener & Kreulen, 1993; Silinsky & Gerzanich, 1993; Silinsky & Redman, 1996). Briefly, young adult guinea-pigs (150-250 g) were killed by inhalation of CO2 and then exsanguinated. Coeliac ganglia were then dissected from young adult guinea-pigs and treated sequentially in papain and collagenase (1 mg ml−1) + dispase (4 mg ml−1) dissolved in Ca2+/Mg2+-free Hanks’ balanced salt solution. The cells were then triturated, washed, centrifuged and plated onto collagen (Collaborative Biomedical, Bedford, MA, USA) or poly-D-lysine- (Sigma) coated coverslips in feeding medium (supplemented by Eagle's essential medium or Liebovitz's L15 medium). Cells were used 3-48 h after dissociation.
Electrophysiology
Patch electrodes (5-25 MΩ) were fabricated using a four-stage pull on a horizontal microelectrode puller (Flaming-Brown P87), coated with Sylgard resin, fire polished, and filled with internal solution. Currents were recorded using an Axopatch-1D or Axopatch 200A amplifier (Axon Instruments) and recorded on videotape, or computer (Digidata 1200 A/D Converter, Axon Instruments). Cells were held at a potential of -110 mV to allow unambiguous comparisons between whole cell and single channel records, but simlar results at the whole cell level were observed near the normal resting potential of the cell (-50 to -60 mV). Currents were either analysed directly from computer data files or were played back and refiltered for analysis at 1-5 kHz (-3 dB, 4 pole Bessel), sampled at 4-8 kHz, and analysed. Hard copy of the data was obtained by importing the ASCII files into SigmaPlot 4 (Windows version) and then exporting the data to Microsoft PowerPoint. In some experiments, the data traces were scanned using an Ultra Scan III Scanner, edited with Adobe Photoshop software, and then exported to PowerPoint.
Solutions and methods of application
The internal solution used to fill the patch electrodes contained (mm): Hepes, 10; EGTA, 10; and caesium gluconate, 140 (to block K+ channels). The holding potentials were corrected for liquid junction potentials (-10 mV). External solutions (pH 7.4) contained (mm): NaCl, 140; glucose, 10; Hepes, 10; CaCl2, 2.5; and KCl, 3; gravity fed into the bath. As P2X receptor activation is highly dependent upon pH (see e.g. Wildman, King & Burnstock, 1997), we tested the effects of the various agonists on pH at concentrations 100-fold over what we employed in these patch clamp experiments, and found no significant pH change using these solutions. Agonists were applied by a ‘fast-flow’ delivery system consisting of a series of six capillary tubes (300 μm inner diameter, Garner glass) fed by gravity from reservoirs. Bars on figures indicate the duration of application of nicotinic or purine receptor agonists.
Data presentation and statistics
Drugs applications were repeated two to four times to assure reproduceability of results. The general statistical procedures that have been used in this laboratory in the past were followed (e.g. Silinsky & Gerzanich, 1993). Thus, after testing for normality, an analysis of variance was performed. This was followed, where appropriate, by a multiple comparisons procedure of the normally distributed data (i.e. Student's t test). Specifically, the Bonferroni t test was employed to test for differences between groups. This procedure is the most conservative of the multiple comparison procedures and is appropriate when no greater than four groups are being compared (Glantz, 1992). As will be evident from the figures, the reported effects were so large that statistically significant differences were generally observed at P < 0.01. Hence, for the data in Fig. 1, the s.e.m. was less than or equal to 2% of the mean for the effect of each concentration of each drug. Using these procedures, in the overwhelming majority of dissociations (26 out of 29 preparations of acutely dissociated neurons), non-additive interactions between nicotinic and purinergic agonists were observed. In the remaining three dissociations, the effects of the agonists were simply additive.
Figure 1. Inhibition by nicotine (5 μm) of currents generated by ATP (6 μm).
In this experiment, after a rough concentration-response curve was constructed for nicotine (A), a concentration of 5 μm nicotine was chosen to examine the interaction with 6 μm ATP (B). Note the complete occlusion of the inward current produced by 6 μm ATP in the presence of nicotine. The effect was fully reversible (C). Traces A, B and C were recorded continuously from the same cell. In all figures, drugs were applied by rapid fast-flow superfusion during the period represented by the bar. The cell was held at -110 mV in the whole cell recording mode.
Detailed statistical analyses of channel openings and closings in excised patches were not performed in this study for the following reasons. (i) In most instances, multiple ATP-gated channels were present in the patch. (ii) Nicotinic receptors in outside-out patches tend to run down (see e.g. Sivilotti, McNeil, Lewis, Nassar, Schoepfer & Colquhoun, 1997). (iii) The relevant details of the difference between nicotinic channels and ATP-gated channels in coeliac neurons have been reported previously (Silinsky & Gerzanich, 1993). Specifically, the mean amplitude of the ATP-gated current at -90 mV is -2.4 ± 0.15 pA (n = 5), with a conductance of this P2X receptor at -50 mV of 22 pS. The nicotinic channel conductance was approximately twice that value with a kinetic pattern of bursts and gaps similar to that which had been reported previously for autonomic ganglia in cell-attached patches and very different from the prolonged flickering produced by full agonists at these P2X ATP receptors (see e.g. Figs 5 and 6). (iv) In most experiments, one agonist fully occluded the single channel currents generated by the other receptor activator. In the experiments in which it was possible to approximate the opening of a single ATP-gated channel (e.g. Fig. 6), the mean open probability (po, as determined from the proportion of time the channel is open during drug application), is reported.
Figure 5. The mutually occlusive effects of ATP (6 μm) and cytisine (60 μm) at the single channel level.
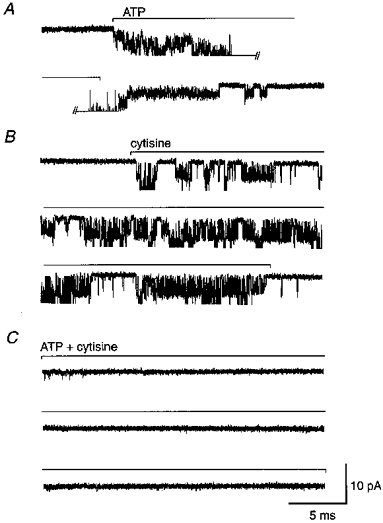
Note that shortly after application of ATP, multiple ATP-gated channels are opened and saturation of the amplifier is observed. After the cessation of superfusion with ATP, discrete openings characteristic of ATP are observed as the extracellular concentration of ATP declines. In B, application of cytisine opens nicotinic receptor-gated ion channels in the same patch. As above, saturation of the patch clamp amplifier occurred with the opening of two or more nicotinic receptors. In C, the combination of ATP (6 μm) + cytisine (60 μm) reveals mutual occlusion of the discrete channel openings. The effect was reversible (data not shown). The excised patch was made from the same cell as in Fig. 5 using the same concentrations of agonists and the same holding potential (-110 mV). For further details, see text.
Figure 6. The occlusion of ATP-gated channel openings (6 μm ATP, A) by nicotine (5 μm, B).
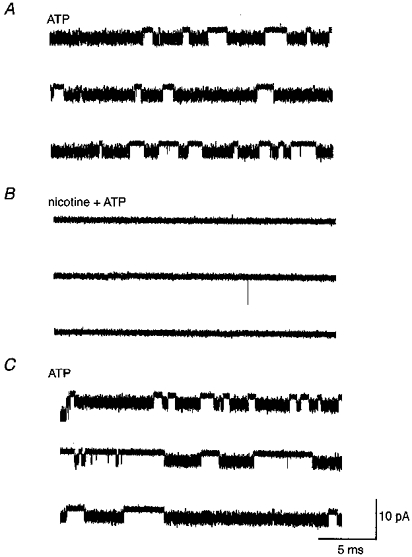
The effect is reversible (C). An excised (outside-out) patch was held at -110 mV.
Drugs
Cytisine was obtained from Research Biochemicals International. The remaining compounds were obtained from Sigma Chemical Company.
RESULTS
Mutually occlusive effects of nicotine and purine receptor agonists on the macroscopic currents recorded in the whole cell configuration
Figure 1 illustrates the typical effect of nicotine and the interaction of nicotine with ATP at the whole cell level. After applying 5, 50 and 100 μm nicotine (Fig. 1A), ATP (6 μm) was applied first in the absence and then in the presence of 5 μm nicotine (Fig. 1B). Note the complete occlusion of ATP receptor activation by the concomitant application of nicotine (5 μm, Fig. 1B), even though this concentration of nicotine produced only a small inward current in this neuron (Fig. 1A). This effect was reversible (Fig. 1C). Occlusion also occurred when nicotine produced larger inward currents. For example, when high concentrations (100 μm) of nicotine were applied together with ATP in the experiment of Fig. 1, the response to 100 μm nicotine + 6 μm ATP was only 2% greater than the response to 100 μm nicotine alone (data not shown). In a total of twenty-six different dissociations, occlusive effects of nicotine were observed, with the combined presence of ATP (6 μm) and nicotine (5-100 μm) being far less than additive. In twenty-six experiments, nicotine (5 μm) co-applied with ATP (6 μm) reduced the response to 31.3 ± 4.6% compared with responses to ATP alone (mean ± 1 s.e.m.; range, 1-82%). In two other experiments using these concentrations of agonists, the co-application of agonists produced responses greater than either agonist alone but the combined response was significantly smaller than the sum of the two independent effects.
If nicotinic and P2X receptors are heterogeneous receptors that have entered into multimeric complexes (see e.g. Bean, 1992; Nakazawa et al. 1991, 1995), then it might be expected that P2X receptor activation could occlude the gating of nicotinic receptors. The experiment of Fig. 2, in which the non-hydrolysable P2X receptor agonist α,β-methylene ATP was employed, shows this to be the case. Note that α,β-methylene ATP (Fig. 2, α,β-mATP) failed to produce a significant inward current at a concentration of 6 μm in this experiment; when applied together with nicotine, however, this concentration of α,β-methylene ATP reduced the current generated by nicotine by greater than 50% in this experiment. In all nine experiments using these concentrations of agonists, α,β-methylene ATP when co-applied with nicotine, reduced the response to nicotine to 44 ± 6% of its initial value (mean ± 1 s.e.m., range 10-63%).
Figure 2. The P2X ATP receptor agonist α,β-methylene ATP (6μm) inhibits nicotinic receptor activation.
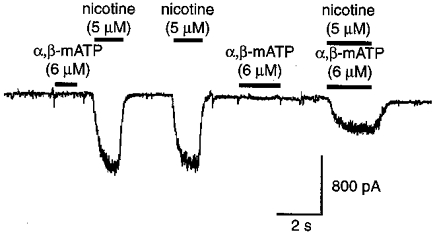
Note that a concentration of α,β-methylene ATP that produces no significant inward current in this experiment, reduced the inward current produced by 5 μm nicotine by greater than 50%. The cell was held at -110 mV in the whole cell recording mode.
Mutually occlusive effects of nicotine and purine receptor agonists are likely to be receptor mediated
It would appear of interest to examine if the mutually occlusive effects of nicotinic agonists and P2X agonists are blocked by antagonists at nicotinic receptors and P2X receptors. The results of Fig. 3 show this to be the case. Specifically, Fig. 3A shows that the occlusive effects of nicotine (6 μm) on P2X receptor activation by ATP is blocked by the nicotinic receptor/channel blocker hexamethonium (150 μm, n = 8), suggesting that the occlusion is receptor mediated. The occlusive effects of P2X agonists on the responses to nicotinic agonists appeared to be receptor mediated as well. Figure 3B shows that a 100 nm concentration of ATP (a concentration that in itself produced no inward current), occludes the action of nicotine and this occlusion is blocked by the P2X antagonist suramin (100 μm, n = 4).
Figure 3. Prevention of occlusion by antagonists of nicotinic receptors (A) and ATP receptors (B).

In A, note that the nicotinic receptor/channel blocker hexamethonium (150 μm) prevents the occlusive effect of nicotine on ATP-gated inward currents. In B, the ATP-receptor antagonist suramin (100 μm) prevents the occlusive effect of ATP on nicotinic receptors. Cells were held at -110 mV in the whole cell recording mode.
Mutually occlusive effects of the nicotinic receptor agonist cytisine and ATP analogues on macroscopic currents
It was of interest to examine if cytisine, a nicotinic agonist that interacts with a circumscribed set of residues on the extracellular binding domains of the ganglionic nicotinic receptor (see Figl, Cohen, Quick, Davidson & Lester, 1992), also interacts with P2X ATP receptors. Figure 4 depicts the result of such an experiment. Note that cytisine (60 μm), which produces an inward current about half that of 6 μm ATP in this experiment, reversibly eliminated any additional response that would be expected if the effects of the two agents were additive. Similar lack of additivity was observed in eight other experiments using cytisine and ATP analogues (for quantitative details, see legend to Fig. 4).
Figure 4. Lack of additivity of ATP (6 μm) and the nicotinic receptor agonist cytisine (60 μm).
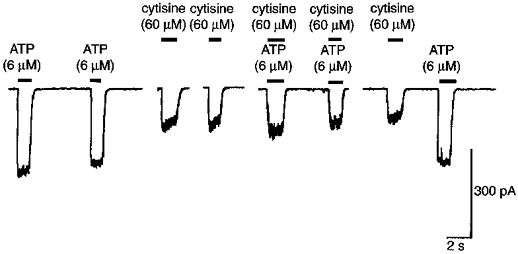
The cell was held at -110 mV in the whole cell recording mode (from the same recording as Fig. 4). In 4 of 5 experiments using these concentrations of cytisine and ATP, co-application of cytisine and ATP reduced the response of ATP to 59 ± 11% of control (mean ± 1 s.e.m., range 41-90%). In the remaining experiment, the combined effects were less than that of sum of the two independent effects. In 2 of 3 experiments using α,β-methylene ATP (6 μm) and cytisine (60 μm), cytisine reduced the response of α,β-methylene ATP (6 μm) to 75% and 74% of control. In the remaining experiment, the effects of α,β-methylene ATP (6 μm) and cytisine were less than the sum of the two independent effects.
The results described thus far could be interpreted as occlusive interactions between nicotinic and purine receptors that are dependent upon the synthesis of soluble second messengers by ATP receptor activation and not via a membrane-delimited linkage to an ion channel. To eliminate this possibility, we performed the remaining experiments on excised (outside-out) patches of neuronal membrane.
Mutually occlusive effects of nicotinic and agonists and ATP receptor agonists on excised patches
The remaining figures show that the mutually occlusive relationship observed using the whole cell recording configuration is maintained at the level of discrete ion channels studied in outside-out patches. Figure 5 is from a fortuitous experiment in which it was possible to study the interaction on a single neuron at both the macroscopic whole cell level (see Fig. 4) and the level of discrete ion channels (Fig. 5) using the same concentrations of agonists. Note that ATP (6 μm, Fig. 5A) applied to this patch opened several channels which soon become off scale at this recording gain, and produced its characteristic flickery burst of activity seen most clearly on the single channel openings observed shortly after the cessation of ATP superfusion. When the same patch was superfused with cytisine (60 μm, Fig. 5B), nicotinic channel openings similar to those described previously for this agent in sympathetic neurons were observed (Sivilotti et al. 1997, Fig. 1). In contrast, when both cytisine (60 μm) and ATP (6 μm) were applied together, no significant degree of channel activity was observed (Fig. 5C). Re-application of cytisine alone again revealed characteristic nicotinic openings albeit of reduced frequency (data not shown).
Figure 6 shows a representative experiment in which a predominantly single channel event produced by ATP (Fig. 6A) is reversibly occluded by the addition of nicotine (Fig. 6B). Note that the control probability of ATP-gated channel opening (po = 0.75, Fig. 6A) is reduced to 0 by the addition 5 μm nicotine (Fig. 6B). Re-application of ATP alone restored po to 0.66 (Fig. 6C). Similar occlusive effects of nicotine and ATP were observed in a total of twenty experiments at the single channel level.
DISCUSSION
The results demonstrate that ATP receptor agonists and nicotinic receptor agonists do not act independently in sympathetic neurons of the guinea-pig. Specifically, the aggregate inward current produced by concomitant application of these agonists is much less than the current predicted from the effects of the agonists applied separately. Indeed, in most experiments, concentrations of agonist that produced little to no inward current, produced dramatic occlusion of the inward current generated through the other ligand-gated ionic channel (see Figs 1–3 and 6). These results are not dependent on the holding potential of the cells as similar occlusion occurs when cells are held at closer to the normal resting potential of coeliac neurons (data not shown). In addition, these occlusions are not due to blockade of one receptor type by a mechanism involving intracellular Ca2+ entering via the other conductance pathway as these interactions persist in Ca2+-free solutions (Nakazawa, 1994; E. M. Silinsky, unpublished observations).
These occlusive interactions between nicotinic agonists and ATP analogues are likely to be receptor mediated as (i) they are blocked by the nicotinic receptor/channel antagonist hexamethonium or by the ATP antagonist, suramin (Fig. 3; Silinsky & Gerzanich, 1993; see also Nakazawa, 1994), and, (ii) a saturation of the inhibitory effect occurs as the concentration of occluding agonist is increased (data not shown). These results are thus consistent with the suggestion that subunits from P2X receptors and nicotinic receptors can form a heteromeric non-selective cation channel (Nakazawa et al. 1991; Bean, 1992). These putative channel complexes would possess binding sites in which either transmitter can activate the receptor via a specific recognition site and reduce the effect of the other agent. Alternatively, P2X receptors could possess a nicotinic inhibitory site and nicotinic receptors could contain an inhibitory ATP binding site. Of note in this regard is that in the sequence of the cloned ATP channels, neither a conventional ATP binding site nor a nicotinic recognition site has been described (Buell, Collo & Rassendren, 1996). In contrast, nicotinic receptor sequences indeed have a predictable Walker ATP binding motif (Carlson & Raftery, 1993; Zimmermann, 1994). It is also possible that the co-expression of nicotinic receptors and P2X receptors is necessary for the mutually occlusive effects to be observed, as no such interaction has been found in one type of cloned P2X receptors expressed in frog oocytes (see e.g. Wildman et al. 1997).
In the results described herein, the data demonstrate an occlusive interaction between nicotinic agonists and purine receptor activators. In contrast, in developing skeletal muscle, ATP activates channels of small conductance that depolarize the developing skeletal muscle (Hume & Thomas, 1988; Thomas, Zawisa, Lin & Hume, 1991), and potentiates the activation of nicotinic receptors by ACh in dissociated rat skeletal muscle (Lu & Smith, 1991). In preliminary experiments, we too have found that ACh and some other nicotinic ligands can potentiate the actions of ATP on celiac neurons and produce intriguing, time-dependent effects at the level of single ionic channels. These results will be the topic of a subsequent investigation.
Acknowledgments
This work was supported by a research grant from the NIH (NS12782). We are grateful to Ms Shirley Foster for her invaluable assistance with the dissociations of sympathetic neurons.
References
- Albuquerque EX, Alkondon M, Pereira E, Castro NG, Schrattenholz A, Barbosa TF, Bonfante-Cabarcas R, Aracava Y, Eisenberg HM, Maelicke A. Properties of neuronal nicotinic acetylcholine receptors: Pharmacological characterization and modulation of synaptic function. Journal of Pharmacology and Experimental Therapeutics. 1997;280:1117–1136. [PubMed] [Google Scholar]
- Bardoni R, Goldstein PA, Lee J, Gu JG, MacDermott AB. ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. Journal of Neuroscience. 1997;17:5297–5304. doi: 10.1523/JNEUROSCI.17-14-05297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. Pharmacology and electrophysiology of ATP-activated ion channels. Trends in Pharmacological Sciences. 1992;13:87–90. doi: 10.1016/0165-6147(92)90032-2. [DOI] [PubMed] [Google Scholar]
- Brake AJ, Julius D. Signalling by extracellular nucleotides. Annual Review of Cell and Developmental Biology. 1996;12:19–41. doi: 10.1146/annurev.cellbio.12.1.519. [DOI] [PubMed] [Google Scholar]
- Buell G, Collo G, Rassendren F. P2X receptors: an emerging channel family. European Journal of Neuroscience. 1996;8:2221–2228. doi: 10.1111/j.1460-9568.1996.tb00745.x. [DOI] [PubMed] [Google Scholar]
- Carlson BJ, Raftery MA. Specific binding of ATP to an extracellular site on Torpedo acetylcholine receptor. Biochemistry. 1993;32:7329–7333. doi: 10.1021/bi00080a002. [DOI] [PubMed] [Google Scholar]
- Devillers-Thiery A, Galzi JL, Eisele JL, Bertrand S, Bertrand JP, Changeux JP. Functional architecture of the nicotinic acetylcholine receptor: A prototype of ligand-gated channels. Journal of Membrane Biology. 1993;136:97–112. doi: 10.1007/BF02505755. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Derkach V, Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Figl A, Cohen BN, Quick MW, Davidson N, Lester HA. Regions of beta 4/beta 2 subunit chimeras that contribute to the agonist selectivity of neuronal nicotinic receptors. FEBS Letters. 1992;308:245–248. doi: 10.1016/0014-5793(92)81284-s. 10.1016/0014-5793(92)81284-S. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, Bertrand PP. ATP mediates fast synaptic potentials in enteric neurons. Journal of Neuroscience. 1994;14:7563–7571. doi: 10.1523/JNEUROSCI.14-12-07563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzanich V, Matsumoto S, North RA, Silinsky EM. ATP receptors in guinea-pig celiac neurons. Society for Neuroscience Abstracts. 1991;21:410. [Google Scholar]
- Glantz SA. Primer of Biostatistics. New York: McGraw Hill Inc.; 1992. [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer Associates Inc.; 1992. [Google Scholar]
- Hume RI, Thomas SA. Multiple action of adenosine 5′-triphosphate on chick skeletal muscle. Journal of Physiology. 1988;406:503–524. doi: 10.1113/jphysiol.1988.sp017393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes P, Norenberg W. Neuronal ATP receptors and their mechanism of action. Trends in Pharmacological Sciences. 1993;14:50–54. doi: 10.1016/0165-6147(93)90030-n. 10.1016/0165-6147(93)90030-N. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. Neuronal nicotinic receptors. In: Nararahashi T, editor. Ion Channels. Vol. 4. New York: Plenum Press; 1996. pp. 377–450. [DOI] [PubMed] [Google Scholar]
- Lu Z, Smith DO. Adenosine 5′-triphosphate increases acetylcholine channel opening frequency in rat skeletal muscle. Journal of Physiology. 1991;436:45–56. doi: 10.1113/jphysiol.1991.sp018538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto SG, Gruener RP, Kreulen DL. Neurotransmitter properties of guinea-pig sympathetic neurons grown in dissociated cell culture-I. Adult neurons. Neuroscience. 1993;57:1135–1145. doi: 10.1016/0306-4522(93)90055-k. 10.1016/0306-4522(93)90055-K. [DOI] [PubMed] [Google Scholar]
- Nakazawa K. ATP-activated current and its interaction with acetylcholine-activated current in rat sympathetic neurons. Journal of Neuroscience. 1994;14:740–750. doi: 10.1523/JNEUROSCI.14-02-00740.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Fujimori K, Takanaka A, Inoue K. Comparison of adenosine-triphosphate- and nicotine-activated inward currents in rat phaeochromocytoma cells. Journal of Physiology. 1991;434:647–660. doi: 10.1113/jphysiol.1991.sp018491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Ito K, Koizumi S, Ohno Y, Inoue K. Reduction of acetylcholine-activated current by low concentrations of extracellular adenosine 5′-triphosphate. Life Sciences. 1995;57:351–356. doi: 10.1016/0024-3205(95)02188-o. [DOI] [PubMed] [Google Scholar]
- North RA, Barnard EA. Nucleotide receptors. Current Opinion in Neurobiology. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. 10.1016/S0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- Redman RS, Silinsky EM. ATP released together with acetylcholine as the mediator of neuromuscular depression at frog motor nerve endings. Journal of Physiology. 1994;477:117–127. doi: 10.1113/jphysiol.1994.sp020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent PB. The diversity of neuronal acetylcholine receptors. Annual Review of Neuroscience. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Schrattenholz A, Roth U, Achuhen A, Schager HJ, Godovac-Zimmerman J, Albuquerque EX, Maelicke A. Identification of purine binding sites on Torpedo acetylcholine receptor. Journal of Receptor Research. 1994;14:197–208. doi: 10.3109/10799899409066031. [DOI] [PubMed] [Google Scholar]
- Silinsky EM. On the association between transmitter secretion and the release of adenine nucleotides from mammalian motor nerve terminals. Journal of Physiology. 1975;247:145–162. doi: 10.1113/jphysiol.1975.sp010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky EM, Gerzanich V. On the excitatory effects of ATP and its role as a neurotransmitter in coeliac neurons of the guinea-pig. Journal of Physiology. 1993;464:197–212. doi: 10.1113/jphysiol.1993.sp019630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky EM, Gerzanich V, Vanner SM. ATP mediates excitatory synaptic transmission in mammalian neurones. British Journal of Pharmacology. 1992;106:762–763. doi: 10.1111/j.1476-5381.1992.tb14408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky EM, Von Kugelgen I, Smith A, Westfall D. Functions of extracellular nucleotides in peripheral and central neuronal tissues. In: Turner JT, Weisman G, Fedan JS, editors. The P2 Nucleotide Receptors. Totowa, NJ, USA: Humana Press Inc.; 1997. pp. 259–290. Chap. 11. [Google Scholar]
- Silinsky EM, Redman RS. Synchronous release of ATP and neurotransmitter within milliseconds of a motor nerve impulse in the frog. Journal of Physiology. 1996;492:815–822. doi: 10.1113/jphysiol.1996.sp021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivilotti LG, McNeil DK, Lewis TM, Nassar MA, Schoepfer R, Colquhoun D. Recombinant nicotinic receptors, expressed in Xenopus oocytes, do not resemble native rat sympathetic ganglion receptors in single-channel behaviour. Journal of Physiology. 1997;500:123–138. doi: 10.1113/jphysiol.1997.sp022004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon P, Burnstock G. ATP as a cotransmitter in the rat tail artery. European Journal of Pharmacology. 1985;106:149–152. doi: 10.1016/0014-2999(84)90688-5. 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- Sneddon P, Westfall D. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. Journal of Physiology. 1984;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, Zawisa MJ, Lin X, Hume RI. A receptor that is highly specific for extracellular ATP in developing chick skeletal muscle in vitro. British Journal of Pharmacology. 1991;103:1963–1969. doi: 10.1111/j.1476-5381.1991.tb12360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman SS, King BF, Burnstock G. Potentiation of ATP-responses at a recombinant P2X2 receptor by neurotransmitters and related substances. British Journal of Pharmacology. 1997;120:221–224. doi: 10.1038/sj.bjp.0700903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H. Signalling via ATP in the nervous system. Trends in Neurosciences. 1994;17:420–426. doi: 10.1016/0166-2236(94)90016-7. 10.1016/0166-2236(94)90016-7. [DOI] [PubMed] [Google Scholar]


