Abstract
Using an in vitro slice preparation of the rat dorsal lateral geniculate nucleus (dLGN), the properties of retinogeniculate and corticothalamic inputs to thalamocortical (TC) neurones were examined in the absence of GABAergic inhibition.
The retinogeniculate EPSP evoked at low frequency (≤ 0.1 Hz) consisted of one or two fast-rising (0.8 ± 0.1 ms), large-amplitude (10.3 ± 1.6 mV) unitary events, while the corticothalamic EPSP had a graded relationship with stimulus intensity, owing to its slower-rising (2.9 ± 0.4 ms), smaller-amplitude (1.3 ± 0.3 mV) estimated unitary components.
The retinogeniculate EPSP exhibited a paired-pulse depression of 60.3 ± 5.6 % at 10 Hz, while the corticothalamic EPSP exhibited a paired-pulse facilitation of > 150 %. This frequency-dependent depression of the retinogeniculate EPSP was maximal after the second stimulus, while the frequency-dependent facilitation of the corticothalamic EPSP was maximal after the fourth or fifth stimulus, at interstimulus frequencies of 1-10 Hz.
There was a short-term enhancement of the ≤ 0.1 Hz corticothalamic EPSP (64.6 ± 9.2 %), but not the retinogeniculate EPSP, following trains of stimuli at 50 Hz.
The ≤ 0.1 Hz corticothalamic EPSP was markedly depressed by the non-NMDA antagonist 1-(4-amino-phenyl)-4-methyl-7,8-methylene-dioxy-5H-2,3-benzodiazepine (GYKI 52466), but only modestly by the NMDA antagonist 3-((RS)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid ((RS)-CPP), and completely blocked by the co-application of GYKI 52466, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), (RS)-CPP and (5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine (MK-801). Likewise, the corticothalamic responses to trains of stimuli (1-500 Hz) were greatly reduced by this combination of ionotropic glutamate receptor antagonists.
In the presence of GYKI 52466, CNQX, (RS)-CPP and MK-801, residual corticothalamic responses and slow EPSPs, with a time to peak of 2-10 s, could be generated following trains of five to fifty stimuli. Neither of these responses were occluded by 1S,3R-1-aminocyclopentane-1,3-dicarboxylic acid (1S,3R-ACPD), suggesting they are not mediated via group I and II metabotropic glutamate receptors.
The thalamocortical (TC) neurones of the sensory thalamic nuclei receive three excitatory inputs: two are the ascending input from the sensory pathway and the descending input from the cortex, and the third is the modulatory input from the brainstem activating system (Jones, 1985). The sensory and cortical inputs form synapses at different locations on the dendritic arbors of TC neurones, with the sensory input more proximal to the soma and the cortical input more distal. The sensory input to auditory, somatosensory and visual thalamic nuclei has been well characterized as being mediated by both non-NMDA and NMDA ionotropic glutamate receptors both in vitro (Scharfman, Lu, Guido, Adams & Sherman, 1990; Turner, Leresche, Guyon, Soltesz & Crunelli, 1994; Hu, Senatorov & Mooney, 1994; Kao & Coulter, 1997) and in vivo (Salt, 1986; Sillito, Murphy & Salt, 1990a; Sillito, Murphy, Salt & Moody, 1990b; Salt & Eaton, 1991). However, while the contribution of these ionotropic glutamate receptors to corticothalamic synaptic transmission has been established both in vitro and in vivo (Deschênes & Hu, 1990; Scharfman et al. 1990; Eaton & Salt, 1996; Kao & Coulter, 1997), there may be an additional contribution from metabotropic glutamate receptors (mGluRs). Certainly, anatomical studies indicate that the group I mGluRs are opposed to this input on distal TC neurone dendrites in sensory thalamic nuclei (Martin, Blackstone, Huganir & Price, 1992; Liu, Mansour & Jones, 1996; Godwin, Van Horn, Erisir, Sesma, Romano & Sherman, 1996; Vidnyánszky et al. 1996). Indeed, it has been shown that the repetitive stimulation of the corticothalamic but not the retinogeniculate input to the dorsal lateral geniculate nucleus (dLGN) of the guinea-pig evoked a slow synaptic potential, occluded by the presence of the group I and II mGluR agonist 1S,3R-1-aminocyclopentane-1,3-dicarboxylic acid (1S,3R-ACPD) in vitro (McCormick & von Krosigk, 1992). However, while a similar synaptic potential has been observed in the rat ventrobasal (VB) thalamus in vivo (Eaton & Salt, 1996), this slow EPSP was not observed following repetitive stimulation using an in vitro VB thalamic slice preparation (Kao & Coulter, 1997).
The aim of the present study was to make a direct comparison of the retinogeniculate and corticothalamic inputs to the same TC neurone, in order to distinguish these inputs at the cellular level, and to determine the contribution of ionotropic and metabotropic glutamate receptors to the corticothalamic input in the rat dLGN.
METHODS
Slicing procedures
Young adult male pigmented hooded Lister rats (150-200 g) were anaesthetized with halothane (May & Baker) and decapitated using licensed procedures approved by the Home Office. Their brains were then rapidly removed and placed in ice-cold (1-3°C) continuously oxygenated (95% O2-5 % CO2) Krebs medium containing (mm): sucrose, 250; KCl, 3; KH2PO4, 1.25; MgSO4, 5; CaCl2, 1; NaHCO3, 26; and glucose, 10 (Aghajanian & Rasmussen, 1989). In order to maintain the integrity of the sensory and cortical inputs (see Paxinos & Watson, 1986; Bourassa & Deschênes, 1995), two sections were performed at 3-5 deg to the sagittal plane either side of the mid-line, and angled outwards by 10-25 deg in the medio-lateral plane (Fig. 1A and B). The medial aspect of each brain half was then glued to the cutting stage of a Vibroslice (Campden Instruments) with cyanoacrylate adhesive and submerged in the continuously oxygenated Krebs medium, cooled to 2-4°C. Pseudo-sagittal sections (400-500 μm) were made and slices containing the dorsal lateral geniculate nucleus (dLGN) were prepared by excising the main thalamic field plus the adjacent nucleus reticularis thalami, internal capsule and striatum. These slices were placed in a storage chamber, where they were maintained in a continuously oxygenated Krebs medium containing (mm): NaCl, 124; KCl, 3; KH2PO4, 1.25; MgSO4, 5; CaCl2, 1; NaHCO3, 26; and glucose, 10, at room temperature (20-22°C). Slices were left for at least 1 h before being transferred to an interface-type recording chamber, where they were perfused with a similar continuously oxygenated Krebs medium containing (mm): NaCl, 124; KCl, 2; KH2PO4, 1.25; MgSO4, 1; CaCl2, 2; NaHCO3, 26; and glucose, 10. This medium also contained the inhibitory amino acid antagonists bicuculline methiodide (30 μm) and 3-aminopropyl-(diethoxymethyl)-phosphonic acid (CGP 35348, 500-1000 μm), so that synaptic transmission mediated by GABAA and GABAB receptors was blocked and would not influence the properties of recorded neurones. In a few experiments, the antagonists of muscarinic cholinergic, H1 histaminergic, α1 adrenergic and 5HT1/5HT2 serotoninergic receptors (scopolamine, pyrilamine, prazosin and methysergide, respectively) were also included in the Krebs medium to block the neuromodulatory influence of brainstem and hypothalamic fibre input. Slices were maintained at a temperature of 34 ± 1°C and left for a further hour before recording commenced.
Figure 1. The preparation of rat mid-brain slices containing the dLGN and maintaining the continuity of retinogeniculate and corticothalamic fibre inputs.
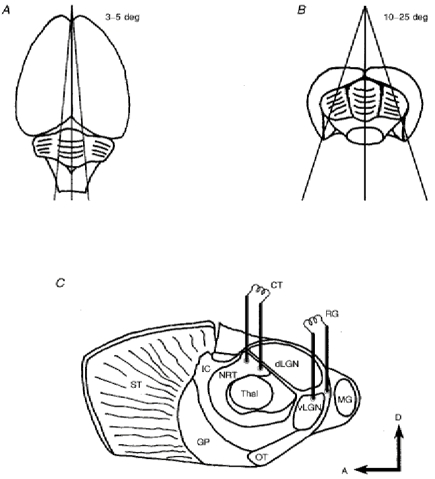
A and B, the dorsal and caudal views of the rat brain, respectively, showing the position and angle of the two initial cuts used to produce the blocks of brain tissue for subsequent slice preparation. C, the locations of the stimulating electrodes required to activate retinogeniculate and corticothalamic fibre inputs and evoke EPSPs in the dLGN TC neurones of rat mid-brain slices cut at the angles illustrated in A and B. Abbreviations: A, anterior; CT, corticothalamic; D, dorsal; dLGN, dorsal lateral geniculate nucleus; GP, globus pallidus; IC, internal capsule; MG, medial geniculate nucleus; NRT, nucleus reticularis thalami; OT, optic tract; RG, retinogeniculate; ST, striatum; Thal, main field of the thalamus; vLGN, ventral lateral geniculate nucleus.
Recording procedures
Using the current-clamp technique, intracellular recordings were made with sharp standard-walled glass microelectrodes (TF120, Clark Electromedical), filled with 1 M potassium acetate (final tip resistance, 80-120 MΩ). To generate EPSPs of retinogeniculate fibre origin, a bipolar stimulating electrode was placed in the ventral lateral geniculate nucleus (vLGN) close to its caudal border with the optic tract (Fig. 1C, RG). To generate EPSPs of corticothalamic fibre origin, a second bipolar stimulating electrode was placed in the nucleus reticularis thalami (NRT) region of the thalamic field adjacent to the dLGN (Fig. 1C, CT). The two electrodes were placed approximately equidistant from the recording site, and EPSPs evoked using 100 μs square wave pulses of current (constant current, 30-500 μA or voltage, 3-50 V) in a variety of single- or multiple-stimulus protocols. Following amplification with an Axoprobe 1A (Axon Instruments), voltage and current records were stored on an analog tape recorder (Racal Recorders Ltd) and later analysed with pCLAMP software (Axon Instruments), having been digitized at 1-20 kHz and acquired via the DigiData 1200A interface (Axon Instruments). The criteria for the distinction of TC cells and local circuit interneurones were similar to those described previously for the rat dLGN (Crunelli, Kelly, Leresche & Pirchio, 1987a; Williams, Turner, Anderson & Crunelli, 1996).
Data analysis
Quantitative results were tested for the normality of their distribution using the Kolmogorov-Smirnov test, and expressed in the text and figures as means ±s.e.m. Statistical significance was tested using Student's t test, either unpaired or paired depending on the experimental design.
Material sources
6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 3-((RS)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid ((RS)-CPP) were purchased from Tocris Cookson; bicuculline methiodide, prazosin, pyrilamine, scopolamine and tetrodotoxin (TTX) from Sigma; and (5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d]cyclohepten-5,10-imine (MK-801) and methysergide from RBI. CGP 35348 was a gift from Dr W. Fröstl (Ciba-Geigy, Basel), and 1-(4-amino-phenyl)-4-methyl-7,8-methylene-dioxy-5H-2,3-benzodiazepine (GYKI 52466) was a gift from Dr I. Tarnawa (Institute for Drug Research, Budapest). All drugs were applied in the perfusion medium.
RESULTS
Characterization of the retinogeniculate and corticothalamic input to the dLGN evoked by a single stimulus at low frequency
Low-frequency stimulation (≤ 0.1 Hz) of either the retinogeniculate or the corticothalamic fibres could generate an EPSP, which in turn could evoke a low-threshold Ca2+ potential (LTCP) at resting membrane potential (RMP), in the presence of the GABAA and GABAB receptor antagonists bicuculline and CGP 35348, respectively (Fig. 2). These EPSPs could not be evoked in the presence of TTX, illustrating their dependence on the propagation of action potentials along their respective input fibre pathways (Fig. 2B). In addition, these events could be distinguished by a number of their response characteristics. Firstly, upon membrane potential depolarization, stimulation of the region through which the corticothalamic fibres passed could lead to antidromic activation of the TC neurone axon (Fig. 2A). This observation illustrates that the integrity of both thalamocortical and corticothalamic fibres can be maintained in this slice preparation. Thus, all subsequent analysis was performed at membrane potentials, usually RMP, where no antidromic spikes were observed for single-stimulus responses.
Figure 2. The EPSPs recorded from a dLGN TC neurone when stimulating the retinogeniculate and corticothalamic fibres in the same slice, and their dependence on action potential propagation.
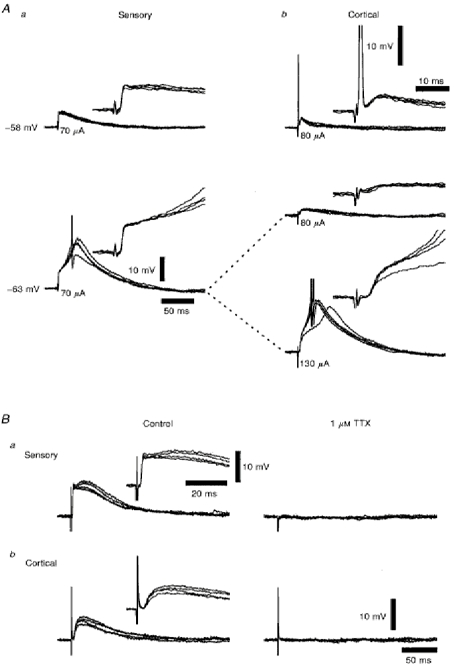
Aa and b, two series of four superimposed voltage records showing that both retinogeniculate (a) and corticothalamic (b) EPSPs were able to evoke LTCPs at resting membrane potential (-63 mV), and that the stimulation of corticothalamic fibres (80 μA) could evoke an antidromic spike at a more depolarized membrane potential (-58 mV), indicating the continuity of thalamocortical fibres to the stimulation site. The insets illustrate the onset of the EPSPs and the antidromic spikes over a shorter time course. Spike amplitudes have been truncated for clarity. Stimulus intensities are denoted below each set of traces. Firing threshold was -45 mV. Ba and b, two series of four superimposed voltage records of retinogeniculate (a) and corticothalamic (b) EPSPs in the absence and presence of 1 μm TTX, illustrating their dependence on the Na+ current which underlies the action potential. The insets illustrate the onset and rising phase of the EPSPs over a shorter time course. Membrane potential was -64 mV.
The second and most distinctive difference between these inputs was that while the corticothalamic EPSP was graded with increasing stimulus intensity, indicating that it was composed of a large number of small unitary events (Fig. 3Ab and Bb), the retinogeniculate EPSP was all-or-none in nature or had two distinct amplitude levels with increasing stimulus intensity, indicating that it was composed of one or two large-amplitude unitary events (Fig. 3Aa and Ba). These sensory EPSPs also varied in amplitude from 2 to 35 mV (estimated following membrane potential hyperpolarization) so that some sensory EPSPs were suprathreshold at RMP. This type of response to retinogeniculate input in the presence of bicuculline has previously been observed using a coronal slice preparation from the guinea-pig brain (Paulsen & Heggelund, 1994; estimated EPSP amplitude at -60 to -70 mV using a 50-100 MΩ input resistance for TC neurones and assuming there is no electrotonic decay, 2.5-30 mV), suggesting that these observations are not dependent on the orientation of the slice preparation. Finally, there were faster latencies to onset and rise times (Table 1) for the retinogeniculate EPSP (Fig. 3Aa, insets) than for the corticothalamic EPSP (Fig. 3Ab, insets).
Figure 3. Characterization of the retinogeniculate and corticothalamic EPSPs recorded from the same TC neurone.
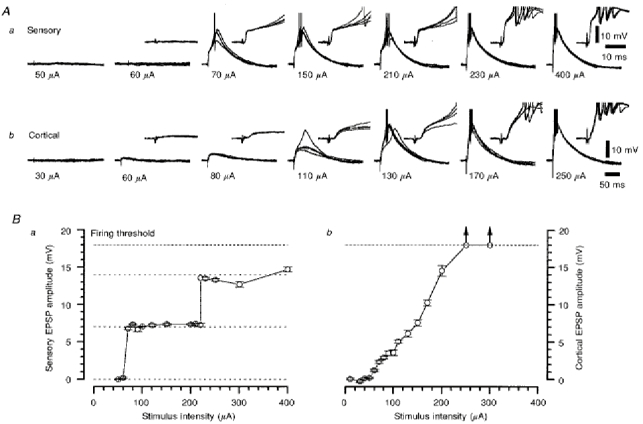
Aa and b, two series of four superimposed voltage records of retinogeniculate (a) and corticothalamic (b) EPSPs evoked at the stimulus intensities denoted below each set of traces. The insets illustrate the onset and rising phase of the EPSPs over a shorter time course. Action potentials have been truncated for clarity. Membrane potential was -70 mV. Ba and b, the stimulus intensity-EPSP amplitude response relationships for the retinogeniculate (a) and corticothalamic (b) EPSPs, as constructed from the data shown in Aa and Ab, respectively. The arrows indicate that at these stimulus intensities, the corticothalamic EPSP was suprathreshold at the time at which the peak measurement was made.
Table 1.
Retinogeniculate and corticothalamic EPSP parameters
| EPSP | Peak amplitude (mV) | Latency to onset (ms) | 10–90% rise time (ms) |
|---|---|---|---|
| Retinogeniculate | 10.3 ± 1.6 | 1.1 ± 0.3 | 0.8 ± 0.1 |
| Corticothalamic | 1.3 ± 0.3* | 2.3 ± 0.3* | 2.9 ± 0.4* |
Retinogeniculate and corticothalamic EPSP parameters were determined from the first unitary responses of their respective stimulus intensity-response relationships at threshold.
Significantly different from retinogeniculate EPSP (P < 0.001, Student's paired t test).
Paired stimuli characterization of retinogeniculate and corticothalamic inputs
The retinogeniculate and corticothalamic EPSPs were further distinguished by the nature of these responses to paired stimuli of varying interval (100-2000 ms). Since the retinogeniculate input is composed of single or double unitary EPSPs, stimulus intensities were selected that were supramaximal for the first unitary EPSP, and under these constraints the conditioned (second) EPSP response was smaller than the conditioning (first) EPSP response for interstimulus intervals of 100-700 ms (Fig. 4Aa), with a mean depression of 60.3 ± 5.6 % (n = 6) at an interval of 100 ms. In contrast, the stimulus intensity for the corticothalamic EPSP was selected so that the EPSP did not evoke a LTCP, in most cases. Under these circumstances, the conditioned (second) EPSP was greatly facilitated over the conditioning (first) EPSP response (Fig. 4Ab), with a >150 % (n = 6) mean facilitation at 100 ms, with the second EPSP evoking action potential firing, either directly or via a LTCP.
Figure 4. The paired-pulse properties of retinogeniculate and corticothalamic EPSPs recorded from the same TC neurone.
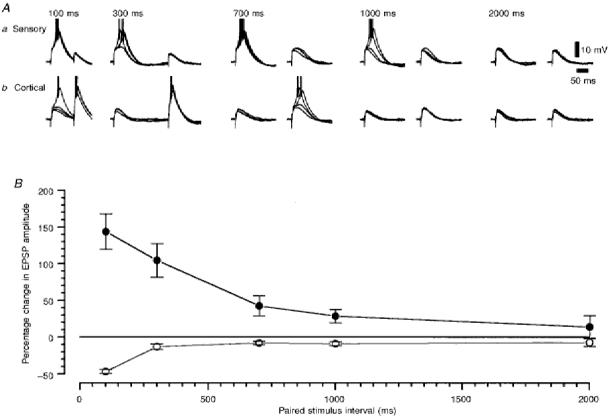
A, two series of four superimposed voltage records of retinogeniculate (a) and corticothalamic (b) EPSPs evoked by pairs of stimuli at the time intervals indicated at the top of the panel. Action potentials have been truncated for clarity. Membrane potential was -77 mV. B, the paired stimulus interval-percentage change in EPSP amplitude relationship for the retinogeniculate and corticothalamic EPSPs, as constructed from the data shown in A. ^, sensory; •, cortical.
Frequency dependence of TC neurone inputs to multiple stimuli
The retinogeniculate and corticothalamic fibres were stimulated with trains of five stimuli in order to examine the frequency-dependent nature of these EPSPs in more detail. For a small-amplitude retinogeniculate EPSP, there was a sustained depression in amplitude from the second EPSP onwards during these trains, and a greater depression with increasing frequency between 1 and 10 Hz (Fig. 5). At frequencies between 33.3 and 100 Hz, there was temporal summation of the individual EPSPs, leading to a prolonged membrane depolarization, and so the TC neurone could generate burst-firing output by evoking a LTCP. At 333 and 500 Hz, the time course of this summation appears to be so rapid that membrane potential is not depolarized long enough for the TC neurones to generate a LTCP in every case. For large-amplitude EPSPs, which evoked a LTCP after the first stimulus, a similar profile was observed, with sustained depression of the subsequent EPSPs between 1 and 10 Hz. At higher frequencies of stimulation, there was also summation of the depressed EPSPs to maintain a depolarized membrane potential, but this rarely reached firing threshold subsequent to the LTCP, unless the initial EPSP was very large. In contrast, the resulting series of EPSPs for the corticothalamic input showed an increasing facilitation in amplitude, which was maximal after the fourth or fifth EPSP, with a greater overall facilitation with increasing frequency between 1 and 10 Hz (Fig. 6). At frequencies between 33.3 and 500 Hz, there was further temporal summation and facilitation, such that at frequencies above 100 Hz, membrane potential could be maintained at firing threshold after the offset of the train, with burst-firing output that outlasted that evoked by the LTCP or the duration of the stimulus train. With increased numbers of stimuli in the train, these responses could lead to a prolonged depolarization of membrane potential sufficient to inactivate the action potentials (Fig. 7B), unlike an initially suprathreshold retinogeniculate EPSP, where frequency-dependent depression was still clear (Fig. 7A).
Figure 5. The frequency-dependent depression for a series of five consecutive retinogeniculate EPSPs evoked at frequencies between 0.1 and 500 Hz.
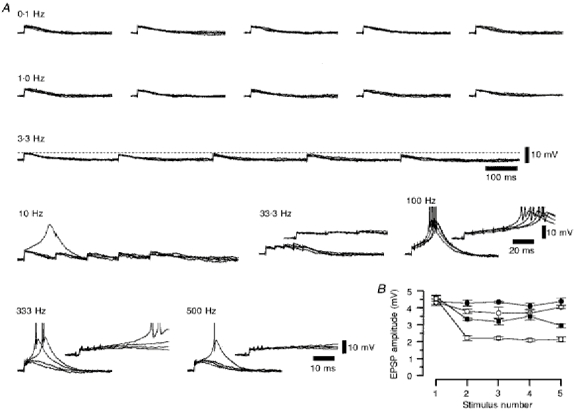
A, a series of four superimposed voltage records of the retinogeniculate EPSPs evoked by five stimuli at the frequency denoted above each set of traces. The insets illustrate the temporal summation of the depressed retinogeniculate EPSP with increasing frequency. Action potentials have been truncated for clarity. Membrane potential was -67 mV. B, a graph showing the change in retinogeniculate EPSP amplitude with increasing stimulus number, constructed from the data shown in A. •, 0.10 Hz; ^, 1.00 Hz; ▪, 3.33 Hz; □, 10.0 Hz.
Figure 6. The frequency-dependent facilitation for a series of five consecutive corticothalamic EPSPs evoked at frequencies between 0.1 and 500 Hz.
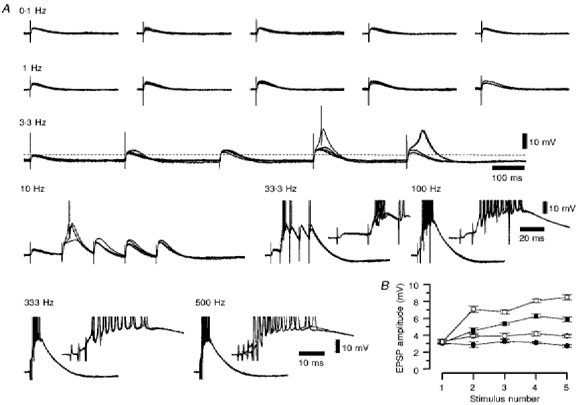
A, a series of four superimposed voltage records of the corticothalamic EPSPs evoked by five stimuli at the frequency denoted above each set of traces. The insets illustrate that there is a very robust wind-up of the corticothalamic EPSP with increasing frequency. Action potentials have been truncated for clarity. Membrane potential was -68 mV. B, a graph showing the change in corticothalamic EPSP amplitude with increasing stimulus number, constructed from the data in shown A. •, 0.10 Hz; ^, 1.00 Hz; ▪, 3.33 Hz; □, 10.0 Hz.
Figure 7. Retinogeniculate and corticothalamic responses to trains of ten stimuli at 100 Hz recorded from the same TC neurone.
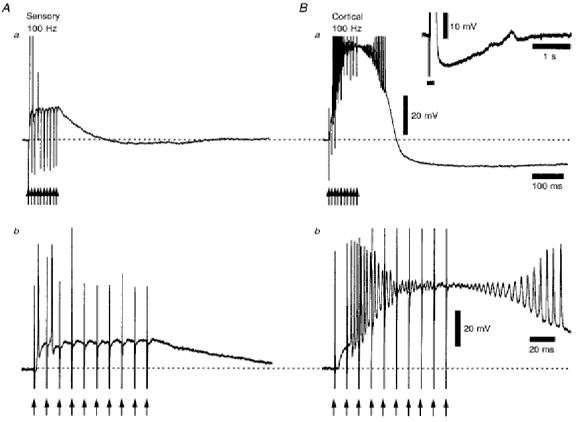
Aa and b, single-voltage records of the retinogeniculate response, which was suprathreshold until the third stimulus (Ab). Following the offset of the stimulus train, there was a small after-hyperpolarization following repolarization of membrane potential (Aa). Ba and b, single-voltage records of the corticothalamic response, which was subthreshold after the first stimulus, but became more depolarized with each successive stimulus (Bb). The action potentials evoked after the second stimulus became diminished in amplitude during this depolarization, recovering their height during the repolarization of membrane potential at the offset of the stimulus train. Following this response, there was a pronounced long-lasting after-hyperpolarization (Ba), the full time course of which is shown in the inset, where the horizontal filled bar is the time period of the stimulus train. The arrows indicate the times of stimulation during the train. Action potentials in Aa and Ba have been truncated for clarity. Membrane potential was -61 mV.
Short-term potentiation
The corticothalamic EPSP evoked by a single stimulus at low frequency was facilitated in amplitude for 2-5 min, before returning to control values, following six to nine 50 Hz trains containing five stimuli (Fig. 8). This process was repeatable in the same neurone and independent of EPSP amplitude (Fig. 8), so that the mean enhancement was 64.6 ± 9.2 % (n = 6) for a range of amplitudes between 1.8 and 9.1 mV. The retinogeniculate EPSP evoked at low frequency showed no change in amplitude following similar stimulus trains (n = 4; data not shown).
Figure 8. The short-term potentiation of the low-frequency corticothalamic EPSP following trains of five stimuli at 50 Hz.
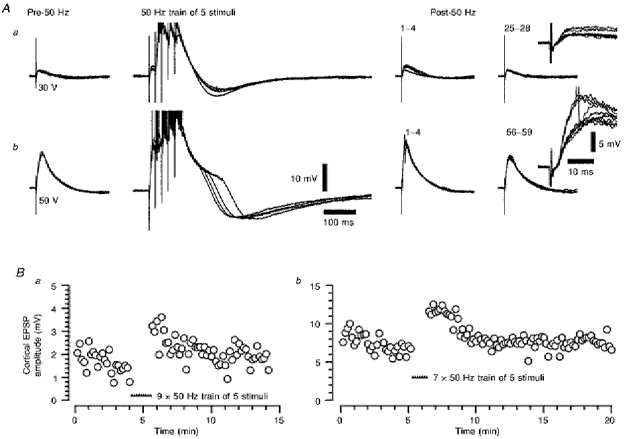
Aa and b, two series of four superimposed voltage responses illustrating the potentiation of a relatively small (Aa) and a relatively large EPSP (Ab) recorded from the same TC neurone, and the probable lack of dependence on whether a LTCP was evoked by the EPSP or the voltage excursion during the 50 Hz stimulation. The numbers above the post-50 Hz records denote the low-frequency stimulus number after the last 50 Hz train. All low-frequency stimulation voltage records have been overlaid in the insets on the right of these series. Note the difference in the repolarizing phase of the responses to the trains of five stimuli between 30 and 50 V stimulation, probably reflecting the differential activation of suprathreshold voltage-dependent conductances by the response to 50 V stimulation. Action potentials have been truncated for clarity. Membrane potential was -64 mV. Ba and b, the time course of the potentiation of the corticothalamic EPSP by 50 Hz stimulation from Aa and Ab, respectively. Filled triangles indicate the times of stimulus trains.
Excitatory amino acid receptor pharmacology of the corticothalamic input to the dLGN evoked by a single stimulus at low frequency
The amplitude of the corticothalamic EPSP was only modestly depressed by the competitive NMDA receptor antagonist (RS)-CPP (10-30 μm), with the predominant effect being a reduced ability of the EPSP to evoke a LTCP and so generate burst firing. In contrast, this EPSP was markedly reduced by the non-competitive non-NMDA antagonist GYKI 52466 (100 μm), with the remaining EPSP in the presence of GYKI 52466 having a slower time course (Fig. 9B and C). In addition, this EPSP was completely suppressed in the presence of both these antagonists (Fig. 9C), or both these antagonists plus the mixed competitive non-NMDA and NMDA glycine-site antagonist CNQX and the non-competitive NMDA antagonist MK-801 (Fig. 10A). Thus, the corticothalamic EPSP evoked at low frequency was solely mediated by ionotropic glutamate receptors and had a similar profile to that exhibited by the retinogeniculate EPSP, which was also predominantly mediated by non-NMDA excitatory amino acid receptors, with the NMDA component able to modulate EPSP-evoked burst firing (Scharfman et al. 1990; Turner et al. 1994; Paulsen & Heggelund, 1994).
Figure 9. The ionotropic glutamate receptor pharmacology of the corticothalamic EPSP.
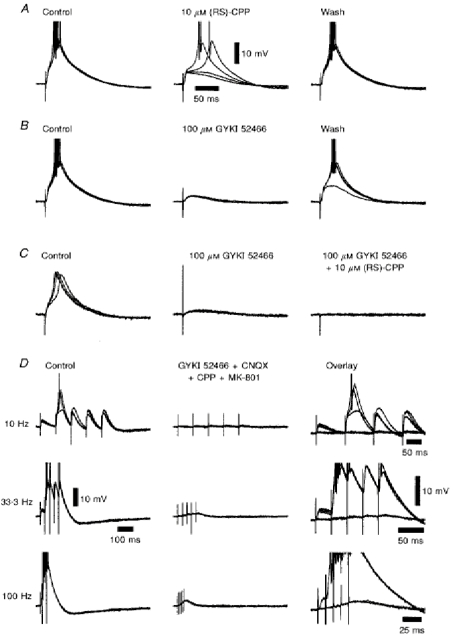
A, a series of four superimposed voltage records illustrating the small depression of the corticothalamic EPSP caused by the NMDA antagonist (RS)-CPP. B, a further series of four superimposed voltage records, recorded from the same TC neurone, illustrating the marked depression of the corticothalamic EPSP caused by the non-NMDA antagonist GYKI 52466. C, a series of four superimposed voltage records from a different TC neurone illustrating the sequential depression of the corticothalamic EPSP by GYKI 52466, and the remaining slower EPSP (obtained in the presence of GYKI 52466) by (RS)-CPP, resulting in a complete suppression of the corticothalamic response. The calibration bars in A also apply to B and C.D, three series of four superimposed voltage records in the absence and presence of 20 μm CNQX, 100 μm GYKI 52466, 10 μm (RS)-CPP and 3 μm MK-801, plus an overlay of all records at each frequency, illustrating the depression of the facilitated corticothalamic response to trains of five stimuli between 10 and 100 Hz by a combination of ionotropic glutamate receptor antagonists. Action potentials have been truncated for clarity. Membrane potential was -69 mV in both A and B, -67 mV in C, and -68 mV in D.
Figure 10. The residual response and slow EPSP evoked by stimulation of corticothalamic fibres, using a series of trains at 50 Hz, in the presence of a combination of ionotropic glutamate receptor antagonists.
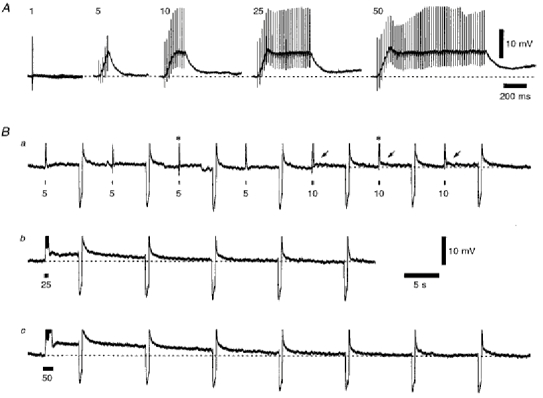
A, on the left are four superimposed voltage records to a single stimulus, followed by a series of individual voltage records evoked by an increasing number of stimuli at 50 Hz, illustrating the development of the residual wind-up of the corticothalamic response in the presence of 20 μm CNQX, 100 μm GYKI 52466, 10 μm (RS)-CPP and 3 μm MK-801. The vertical deflections are the stimulus artefacts. Ba-c, a series of individual voltage records from the same TC neurone, but on a longer time scale, illustrating the development of the slow EPSP after the residual responses illustrated in A. The horizontal filled bars indicate the time period of each stimulus train, and the asterisks in Ba denote the residual responses to five and ten stimuli illustrated in A. The arrows in Ba highlight the small slow EPSPs evoked by ten stimuli. Membrane potential was -64 mV.
Residual corticothalamic responses and slow EPSPs
In the presence of CNQX, GYKI 52466, CPP and MK801, and with the single-stimulus corticothalamic EPSP completely blocked, trains of five stimuli were still able to evoke depolarizing responses of increasing amplitude with increasing frequency, but very much diminished in amplitude compared with control (Fig. 9D), indicating that they were predominantly mediated by ionotropic glutamate receptors. These residual responses could be maintained during trains of up to fifty stimuli at 50 Hz (Fig. 10A), and for trains of twenty or more stimuli were followed by slow EPSPs with times to peak of between 2 and 10 s (Fig. 10Bb and c), depending on stimulus intensity (data not shown). These slow EPSPs are temporally very similar to the slow ‘metabotropic’ EPSP described previously (McCormick & von Krosigk, 1992). However, neither these slow EPSPs nor the residual responses (Fig. 11) were occluded by the presence of the group I and II mGluR agonist 1S,3R-ACPD (100 μm, n = 3), even though this concentration of the agonist was sufficient to maximally depolarize membrane potential via these mGluRs. In addition, these responses were resistant to the inclusion of the antagonists of muscarinic, H1, α1 and 5HT1/5HT2 receptors scopolamine, pyrilamine, prazosin and methysergide, respectively, in the Krebs medium (all 3 μm; n = 3, data not shown), indicating that they are unlikely to result from the stimulation of fibres of brainstem or hypothalamic origin. Therefore, the response of TC neurones to multiple stimuli applied to corticothalamic fibres was predominantly mediated by ionotropic glutamate receptors and not mGluRs or muscarinic, H1, α1 and 5HT1/5HT2 receptors, and so the nature of the residual responses and slow EPSP remain to be elucidated.
Figure 11. The slow EPSP and residual response to corticothalamic fibre stimulation in a combination of ionotropic glutamate antagonists, and the effect of the group I and II mGluR agonist 1S,3R-ACPD.
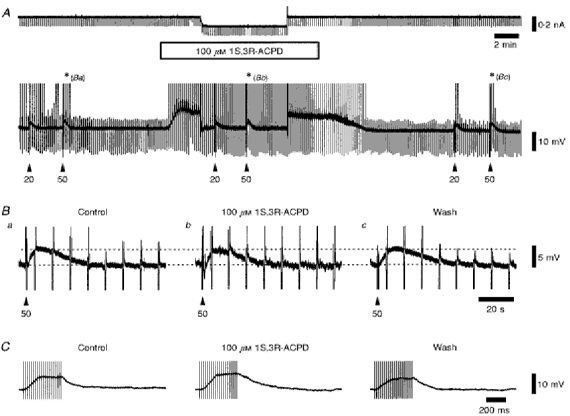
A, continuous current and voltage records in the absence and presence of 100 μm 1S,3R-ACPD, while in the constant presence of 20 μm CNQX, 100 μm GYKI 52466, 10 μm (RS)-CPP and 3 μm MK-801. The upward deflections on the voltage record are the LTCPs generated at the offset of the current pulse, some of which evoke action potentials. These have been truncated for clarity. The voltage artefacts of the stimulus trains are marked by the arrowheads. Ba-c, a series of individual voltage records illustrating the amplitude and time course of the slow corticothalamic EPSP before, during and after the application of 1S,3R-ACPD, as illustrated by the asterisks in A, showing the lack of occlusion by the mGluR agonist. The downward deflections are the voltage responses to injected current, as shown in A, and the upward deflections are as described for A, except for the stimulus artefact as indicated by the arrowheads. Membrane potential was -67 mV. C, a series of individual voltage records illustrating the residual corticothalamic response to twenty stimuli at 50 Hz, and the lack of occlusion of this response by 100 μm 1S,3R-ACPD in the presence of 20 μm CNQX, 100 μm GYKI 52466, 10 μm (RS)-CPP and 3 μm MK-801. The vertical deflections are the stimulus artefacts. Membrane potential was -61 mV.
DISCUSSION
The main conclusions of this study are as follows. (1) The stimulation at low frequency of corticothalamic fibres passing through the NRT evoked an EPSP in rat dLGN TC neurones with similar pharmacology to that evoked by stimulation of retinogeniculate fibres (Turner et al. 1994). (2) These retinogeniculate and corticothalamic EPSPs differed in terms of their latency to onset, rising phase, stimulus intensity-response relationship, and response to paired stimuli, consistent with the differences in synapse location, fibre input and the previously noted frequency-dependent synaptic mechanisms observed with the corticothalamic input (Jones, 1985; Deschênes & Hu, 1990). (3) High-frequency stimulation of the corticothalamic but not the retinogeniculate input could result in the robust depolarization of membrane potential sufficient to maintain or inactivate action potential firing and lead to a short-term potentiation of the response to this input. (4) This high-frequency corticothalamic response was predominantly mediated by ionotropic glutamate receptors, and appears not to be mediated by mGluRs activated by 1S,3R-ACPD.
The retinogeniculate and corticothalamic EPSPs of TC neurones evoked by low-frequency stimulation
The differences in the latency to onset and rise time of the retinogeniculate and corticothalamic EPSPs probably reflects differences in the underlying synaptic currents and the different dendritic locations of their respective inputs on TC neurones (Jones, 1985). Since only one or two unitary events appear to make up the retinogeniculate EPSP in this slice preparation, the present study suggests that this EPSP is a large-amplitude event arising from discrete single- or double-fibre input. This is consistent with the direct anatomical evidence in the cat, where one or two retinogeniculate axon fibres probably make up the total sensory synaptic input onto X cells (Hamos, Van Horn, Raczkowski & Sherman, 1987), and indirect estimates of between one and four retinogeniculate axons per TC neurone for the rat dLGN based on neuronal numbers (Sefton & Dreher, 1994).
The graded nature of the corticothalamic EPSP, shown both in this study and in vivo (Deschênes & Hu, 1990), suggests that this EPSP consists of numerous small-amplitude unitary events, generated by multifibre input. Certainly, in the cat dLGN, each corticothalamic axon provides only a small percentage of the total number of axon terminals (Robson, 1983). The anatomical evidence in the rat dLGN is consistent with this, since numerous en passant corticothalamic terminals arise from a single axon over a length of several hundred micrometres (Bourassa & Deschênes, 1995), supporting the idea of the convergence of the cortical input onto individual TC neurones (Sherman & Koch, 1986).
Glutamate receptor pharmacology of the corticothalamic EPSP in TC neurones evoked by low-frequency stimulation
It is clear from this study that the corticothalamic EPSP of rat dLGN TC neurones, like the retinogeniculate EPSP evoked by low-frequency stimulation, is predominantly mediated by non-NMDA receptors, has a relatively small NMDA receptor-mediated component that can modulate burst firing, and is solely mediated by these ionotropic glutamate receptors (Turner et al. 1994). Indeed, the suppression by CPP of the burst firing associated with the corticothalamic EPSP generated via the LTCP is consistent with the block of burst discharge by AP5 and CPP seen in the cat and rat, respectively, in vivo (Deschênes & Hu, 1990; Eaton & Salt, 1996). In contrast, the majority of the corticothalamic EPSCs were almost completely blocked by AP5 in the rat VB thalamus in vitro from animals at postnatal day 16-28 (Kao & Coulter, 1997), suggesting that there is a developmental change in the relative contribution of ionotropic glutamate receptors to the corticothalamic EPSP of TC neurones.
Frequency dependence of the response of TC neurones to retinogeniculate and corticothalamic fibre stimulation
Perhaps the most interesting difference between the retinogeniculate and corticothalamic responses of TC neurones in the rat dLGN is their frequency-dependent depression or facilitation, respectively, to trains of five stimuli. The lack of failures for the unitary retinogeniculate EPSP observed in this study suggests that there is a high probability of release for this input, so that for any subsequent stimuli there would be a reduced number of quanta available for release. In contrast, although the facilitation of the corticothalamic response was initially suggested to be dependent on NMDA receptor activation (Deschênes & Hu, 1990), there is evidence that this facilitation is likely to result from an increased probability of release for each successive stimulus in rat VB thalamus in vitro (Castro-Alamancos, Landisman & Connors, 1997).
Since there is also facilitation of corticothalamic, but not cerebellothalamic, EPSPs in the ventrolateral (VL) thalamus in vivo (Deschênes & Hu, 1990; Timofeev & Steriade, 1997), it would appear that this facilitation is a feature common to the corticothalamic input located on the distal dendrites of TC neurones in the thalamus. Indeed, the facilitation of the corticothalamic EPSP at frequencies between 3.3 and 33.3 Hz observed both in this study and in vivo (Deschênes & Hu, 1990) is reminiscent of the augmenting responses observed during thalamic radiation or intrathalamic stimulation in vivo (Mishima & Ohta, 1992; Steriade & Timofeev, 1997). This would suggest that this facilitation is one of the thalamic mechanisms by which the augmenting responses can be either generated or reinforced. However, it is clear that the augmenting responses to intrathalamic stimulation involve not only the facilitation of EPSPs but also the facilitation and depression of IPSPs recorded from TC neurones, and so reflect the interplay of a number of short-term plastic mechanisms at the excitatory and inhibitory synapses of the thalamus (Steriade & Timofeev, 1997).
Glutamate receptor pharmacology of the response to high-frequency stimulation of corticothalamic fibres in TC neurones
The fact that the response to trains of stimuli applied to the corticothalamic input of TC neurones is predominantly mediated by ionotropic glutamate receptors is consistent with the partial depression of this response by the NMDA antagonist ketamine (Deschênes & Hu, 1990), and the depression of the augmenting response in the VB thalamus by the non-specific ionotropic glutamate antagonist kynurenic acid (Mishima & Ohta, 1992). Although there is a wealth of anatomical evidence indicating the presence of mGluRs in the thalamus opposed to corticothalamic terminals (Martin et al. 1992; Liu et al. 1996; Godwin et al. 1996; Vidnyánszky et al. 1996), the lack of a synaptic mGluR-mediated response observed in this study is consistent with the lack of a similar response in the rat VB thalamus in vitro (Kao & Coulter, 1997). In fact, although a mGluR-mediated EPSP has been reported in the rat VB thalamus in vivo (Eaton & Salt, 1996), this response only occurred after an EPSP-IPSP complex, which generated burst firing by evoking a LTCP. This suggests that this mGluR-mediated response was dependent upon the interplay of thalamus and cortex in the intact preparation and that these conditions may need to be mimicked in order to observe it in vitro.
Physiological role of the properties of retinogeniculate and corticothalamic EPSPs in thalamocortical function
Under physiological conditions, both the retinogeniculate and corticothalamic responses of TC neurones have prominent inhibitory components (Mushiake, Shosaku & Kayama, 1984; Steriade, Deschênes, Domich & Mulle, 1985; Bloomfield & Sherman, 1988). As a result, there is a significant problem in interpreting the role of the properties of these EPSPs, as observed in this study, without an understanding of the contribution of inhibition to these responses. For example, a graded relationship between retinogeniculate EPSP amplitude and stimulus intensity was observed in the rat dLGN, in the presence of a GABAA but not a GABAB receptor antagonist (Crunelli, Kelly, Leresche & Pirchio, 1987b). It has subsequently been shown that this EPSP is controlled by the tonic activation of presynaptic GABAB receptors, so that large-amplitude EPSPs were only evoked in the complete absence of inhibition (Emri, Turner & Crunelli, 1996), consistent with those seen in this study. Likewise, the response of a TC neurone to sustained stimulation of the retinogeniculate fibres displayed varying degrees of depression, from little to very pronounced, when inhibition was present (Turner et al. 1994). Thus, the level of inhibition appears to be critical in determining the amplitude of the retinogeniculate EPSP and the response to sustained input in the rat dLGN.
A similar scenario could be expected for the corticothalamic response at low frequencies of stimulation. However, the extent to which the corticothalamic response to high-frequency stimulation was able to depolarize membrane potential (in comparison with the retinogeniculate response, see Fig. 7), together with the electrotonic compactness of rat dLGN TC neurones (Crunelli et al. 1987a), suggests that this response could potentially exert a powerful frequency-dependent influence over TC neurone excitability, even when inhibition is present. Indeed, this robust response, with action potential inactivation, observed both in this study and in vivo (Deschênes & Hu, 1990), probably underlies the large depolarizing events that are initiated by augmenting responses or high-frequency stimulation in the cortex (Steriade, Oakson & Diallo, 1976; Roy, Clercq, Steriade & Deschênes, 1984). In addition, augmenting responses are believed to be important in establishing the reverberations in the thalamocortical loop that underlie the spindle oscillation in the thalamus. If the facilitation of the corticothalamic EPSP by repetitive stimuli does in part contribute to these responses, this facilitation could then represent one of the cellular mechanisms by which the corticothalamic input is able to control the spatiotemporal coherence of the spindle oscillation in the thalamus (Contreras, Destexhe, Sejnowski & Steriade, 1996).
Acknowledgments
Supported by The Wellcome Trust. We wish to thank Ciba-Geigy for the CGP 35348, and Dr I. Tarnawa (Institute for Drug Research, Budapest) for the GYKI 52466.
References
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–338. doi: 10.1002/syn.890030406. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Sherman SM. Postsynaptic potentials recorded in neurons of the cat's lateral geniculate nucleus following electrical stimulation of the optic chiasm. Journal of Neurophysiology. 1988;60:1924–1945. doi: 10.1152/jn.1988.60.6.1924. [DOI] [PubMed] [Google Scholar]
- Bourassa J, Deschênes M. Corticothalamic projections from the primary visual cortex in rats: a single fiber study using biocytin as an antegrade tracer. Neuroscience. 1995;66:253–263. doi: 10.1016/0306-4522(95)00009-8. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Landisman CE, Connors BW. Mechanisms of facilitation at corticothalamic synapses in the ventrobasal thalamus. Society for Neuroscience Abstracts. 1997;23:230.5. [Google Scholar]
- Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science. 1996;274:771–774. doi: 10.1126/science.274.5288.771. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Kelly JS, Leresche N, Pirchio M. The ventral and dorsal lateral geniculate nucleus of the rat: intracellular recordings in vitro. Journal of Physiology. 1987a;384:587–601. doi: 10.1113/jphysiol.1987.sp016471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Kelly JS, Leresche N, Pirchio M. On the excitatory post-synaptic potential evoked by stimulation of the optic tract in the rat lateral geniculate nucleus. The Journal of Physiology. 1987b;384:603–618. doi: 10.1113/jphysiol.1987.sp016472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschênes M, Hu B. Electrophysiology and pharmacology of the corticothalamic input to lateral thalamic nuclei: an intracellular study in the cat. European Journal of Neuroscience. 1990;2:140–152. doi: 10.1111/j.1460-9568.1990.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Eaton SA, Salt TE. Role of N-methyl-D-aspartate and metabotropic glutamate receptors in corticothalamic excitatory postsynaptic potentials in vivo. Neuroscience. 1996;73:1–5. doi: 10.1016/0306-4522(96)00123-6. 10.1016/0306-4522(96)00123-6. [DOI] [PubMed] [Google Scholar]
- Emri zs, Turner JP, Crunelli V. Tonic activation of presynaptic GABAB receptors on thalamic sensory afferents. Neuroscience. 1996;72:689–698. doi: 10.1016/0306-4522(95)00590-0. 10.1016/0306-4522(95)00590-0. [DOI] [PubMed] [Google Scholar]
- Godwin DW, Van Horn SC, Erisir A, Sesma M, Romano C, Sherman SM. Ultrastructural localization suggests that retinal and cortical inputs access different metabotropic glutamate receptors in the lateral geniculate nucleus. Journal of Neuroscience. 1996;16:8181–8192. doi: 10.1523/JNEUROSCI.16-24-08181.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamos JE, Van Horn SC, Raczkowski D, Sherman SM. Synaptic circuits involving an individual retinogeniculate axon in the cat. Journal of Comparative Neurology. 1987;259:165–192. doi: 10.1002/cne.902590202. [DOI] [PubMed] [Google Scholar]
- Hu B, Senatorov V, Mooney D. Lemniscal and non-lemniscal synaptic transmission in rat auditory thalamus. The Journal of Physiology. 1994;479:217–231. doi: 10.1113/jphysiol.1994.sp020290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. New York: Plenum Press; 1985. [Google Scholar]
- Kao C-Q, Coulter DA. Physiology and pharmacology of corticothalamic stimulation-evoked responses in rat somatosensory thalamic neurones in vitro. Journal of Neurophysiology. 1997;77:2661–2676. doi: 10.1152/jn.1997.77.5.2661. [DOI] [PubMed] [Google Scholar]
- Liu X-B, Mansour RM, Jones EG. Differential location of two metabotropic glutamate receptor subtypes in the somatosensory thalamus of the rat. Society for Neuroscience Abstracts. 1996;22:414.6. [Google Scholar]
- McCormick DA, von Krosigk M. Corticothalamic activation modulates thalamic firing through glutamate ‘metabotropic’ receptors. Proceedings of the National Academy of Sciences of the USA. 1992;89:2774–2778. doi: 10.1073/pnas.89.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Blackstone CD, Huganir RL, Price DL. Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron. 1992;9:259–270. doi: 10.1016/0896-6273(92)90165-a. 10.1016/0896-6273(92)90165-A. [DOI] [PubMed] [Google Scholar]
- Mishima K, Ohta M. Reappraisal of the corticothalamic and thalamocortical interactions that contribute to the augmenting response in the rat. Japanese The Journal of Physiology. 1992;42:211–221. doi: 10.2170/jjphysiol.42.211. [DOI] [PubMed] [Google Scholar]
- Mushiake S, Shosaku A, Kayama Y. Inhibition of thalamic ventrobasal complex neurons by glutamate infusion into the thalamic reticular nucleus of the rat. Journal of Neuroscience Research. 1984;12:93–100. doi: 10.1002/jnr.490120109. [DOI] [PubMed] [Google Scholar]
- Paulsen O, Heggelund P. The quantal size at retinogeniculate synapses determined from spontaneous and evoked EPSCs in guinea-pig thalamic slices. The Journal of Physiology. 1994;480:505–511. doi: 10.1113/jphysiol.1994.sp020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic Press; 1986. [Google Scholar]
- Robson JA. The morphology of corticofugal axons to the dorsal lateral geniculate nucleus of the cat. Journal of Comparative Neurology. 1983;216:89–103. doi: 10.1002/cne.902160108. [DOI] [PubMed] [Google Scholar]
- Roy JP, Clercq M, Steriade M, Deschênes M. Electrophysiology of neurons of the lateral thalamic nuclei in cat: mechanisms of long-lasting hyperpolarizations. Journal of Neurophysiology. 1984;51:1220–1235. doi: 10.1152/jn.1984.51.6.1220. [DOI] [PubMed] [Google Scholar]
- Salt TE. Mediation of thalamic sensory input by both NMDA receptors and non-NMDA receptors. Nature. 1986;322:263–265. doi: 10.1038/322263a0. [DOI] [PubMed] [Google Scholar]
- Salt TE, Eaton SA. Sensory excitatory postsynaptic potentials mediated by NMDA and non-NMDA receptors in the thalamus in vivo. European Journal of Neuroscience. 1991;3:296–300. doi: 10.1111/j.1460-9568.1991.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Lu S-M, Guido W, Adams P R, Sherman SM. N-Methyl-D-aspartate (NMDA) receptors contribute to EPSPs of cat lateral geniculate neurons recorded in thalamic slices. Proceedings of the National Academy of Sciences of the USA. 1990;87:4548–4552. doi: 10.1073/pnas.87.12.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton AJ, Dreher B. Visual system. In: Paxinos G, editor. The Rat Nervous System. San Diego: Academic Press; 1995. pp. 833–898. [Google Scholar]
- Sherman SM, Koch C. The control of retinogeniculate transmission in the mammalian lateral geniculate nucleus. Experimental Brain Research. 1986;63:1–20. doi: 10.1007/BF00235642. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Murphy PC, Salt TE. The contribution of the non-N-methyl-D-aspartate group of excitatory amino acid receptors to retinogeniculate transmission in the cat. Neuroscience. 1990a;34:273–280. doi: 10.1016/0306-4522(90)90137-s. 10.1016/0306-4522(90)90137-S. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Murphy PC, Salt TE, Moody CI. Dependence of retinogeniculate transmission in cat on NMDA receptors. Journal of Neurophysiology. 1990b;63:347–355. doi: 10.1152/jn.1990.63.2.347. [DOI] [PubMed] [Google Scholar]
- Steriade M, Deschênes M, Domich M, Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. Journal of Neurophysiology. 1985;54:1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- Steriade M, Oakson G, Diallo A. Cortically elicited spike-wave afterdischarges in thalamic neurons. Electroencephalography and Clinical Neurophysiology. 1976;41:641–644. doi: 10.1016/0013-4694(76)90009-2. 10.1016/0013-4694(76)90009-2. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I. Short-term plasticity during intrathalamic augmenting responses in decorticated cats. Journal of Neuroscience. 1997;17:3778–3795. doi: 10.1523/JNEUROSCI.17-10-03778.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Steriade M. Fast (mainly 30–100 Hz) oscillations in the cat cerebellothalamic pathway and their synchronization with cortical potentials. The Journal of Physiology. 1997;504:153–168. doi: 10.1111/j.1469-7793.1997.153bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JP, Leresche N, Guyon A, Soltesz I, Crunelli V. Sensory input and burst firing output of thalamocortical cells: the role of NMDA and non-NMDA receptors. The Journal of Physiology. 1994;480:281–295. doi: 10.1113/jphysiol.1994.sp020359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidnyánszky Z, Görcs TJ, Négyessy L, Borostyánköi Z, Kuhn R, Knöpfel T, Hámori J. Immunocytochemical visualization of the mGluR1a metabotropic glutamate receptor at synapses of corticothalamic terminals originating from area 17 of the rat. European Journal of Neuroscience. 1996;8:1061–1071. doi: 10.1111/j.1460-9568.1996.tb01273.x. [DOI] [PubMed] [Google Scholar]
- Williams SR, Turner JP, Anderson CM, Crunelli V. Electrophysiological and morphological properties of interneurones in the rat dorsal lateral geniculate nucleus in vitro. Journal of Physiology. 1996;490:129–147. doi: 10.1113/jphysiol.1996.sp021131. [DOI] [PMC free article] [PubMed] [Google Scholar]


