Abstract
Electrical stimulation (10 s) of the ethmoidal nerve (EN5) evokes the nasotrigeminal reflex responses, including apnoea, bradycardia and rise in arterial blood pressure. In the present study, we examined the involvement of N-methyl-D-aspartate (NMDA), AMPA/kainate, (γ-aminobutyric acidA (GABAA) and glycine receptors in the Kölliker-Fuse (KF) nucleus in the mediation of the nasotrigeminal reflex responses.
Unilateral injections (n = 6) of 50-100 nl of the NMDA receptor antagonist AP5 into the KF area led to a significant blockade of the EN5-evoked respiratory depression and bradycardia. Injections placed into the midlevel of the KF area were most effective (80-90% blockade). The rise in arterial blood pressure remained unaffected.
Unilateral injections (n = 6) of the AMPA/kainate receptor antagonist CNQX into the KF area failed to block EN5-evoked autonomic responses significantly.
Unilateral injections (n = 5) of the GABAA receptor antagonist bicuculline enhanced the EN5-evoked respiratory depression and bradycardia. The effect persisted for up to 30 s after stimulation. Bicuculline injections into the midlevel of the KF area were most effective. The increase in arterial blood pressure remained unaffected.
Unilateral injections (n = 5) of the glycine receptor antagonist strychnine into the KF area did not produce any significant effects on EN5-evoked autonomic responses.
Our results suggest that the KF area represents a mandatory relay for the nasotrigeminally induced apnoea and bradycardia which are predominantly mediated by NMDA receptors in the KF. Furthermore, it appears that KF neurons are under a potent GABAergic inhibitory control. The EN5-evoked rise in arterial blood pressure was not altered by any of the drugs and, therefore, appears not to be mediated via the KF.
In mammals, the respiratory rhythm is generated by a brainstem neuronal network which produces a continuous pattern of burst discharges that drive the motoneurons of respiratory muscles (Bianchi et al. 1995). This vital rhythm is strongly modulated by sensory afferents arising from the upper and lower airways and also, underlies behavioural and homeostatic changes. A brain area that profoundly modulates the respiratory rhythm is the pontine Kölliker-Fuse (KF) nucleus, characterized as the pontine pneumotaxic centre (Dick et al. 1994; Fung et al. 1994). This refers to the potent influence of the KF on the duration and termination of respiratory phases involving pulmonary afferents. The KF participates also in processing respiratory reflexes, such as the Hering-Breuer reflex (Feldman et al. 1976; Shaw et al. 1989) the chemoreceptor reflex (Koshiya & Guyenet, 1994), the sneeze reflex (Wallois et al. 1995) and the nasotrigeminal reflex (Dutschmann & Herbert, 1996, 1997). Anatomical findings verified the connections of the KF revealing prominent inputs from distinct regions of the nucleus of the solitary tract (NTS) and the ventrolateral medulla (e.g. Herbert et al. 1990) and from spinal and trigeminal neurons which are the primary relays for sensory afferent information from the upper airways and the face (Panneton et al. 1994; Feil & Herbert, 1995).
In the present study, we focus on the role of the KF in mediating the nasotrigeminal reflex. The reflex responses can be evoked by noxious stimulation of the nasal mucosa and comprise apnoea in the expiratory state, activation of laryngeal adductor muscles, bradycardia, and peripheral vasoconstriction, leading to rise in arterial blood pressure (Kratschmer, 1870). Consequently, this vital reflex prevents invasion of noxious substances into the upper airways and, moreover, leads to a reduction in oxygen consumption thereby preventing a rapid progression of asphyxia. The nasotrigeminal reflex plays a key role in the diving response of aquatic mammals which is induced by face immersion (Daly, 1984; Elsner & Daly, 1988). The reflex is mediated by the ethmoidal nerve (EN5), a branch of the ophthalmic division of the trigeminal nerve (Sant'Ambrogio et al. 1995) whose fibres terminate in the pars caudalis of the spinal trigeminal nucleus (Sp5C; Anton & Peppel, 1991; Panneton, 1991). Thus, neurons in the Sp5C represent the first central relay for this reflex circuit (Panneton & Yavari, 1995). Recently, we have provided the first experimental evidence that the KF is also a crucial relay site in the nasotrigeminal reflex circuit, in particular for the trigeminally induced apnoea (Dutschmann & Herbert, 1996). We proposed that the KF might represent the sensory-autonomic interface that relays the trigeminal input from the nasal mucosa to cardiorespiratory neurons in the medulla or spinal cord.
Histological studies from our laboratory and from others demonstrated in the KF immunoreactivities for various GABAA, glycine and NMDA receptor subunits (Guthmann et al. 1996; Herbert et al. 1996; Guthmann et al. 1997) and for AMPA receptor subunits (Chamberlin & Saper, 1995). Therefore, we analysed the roles of NMDA, AMPA/kainate, GABAA and glycine receptors in the KF for the mediation of the nasotrigeminal reflex responses. We performed microinjections of receptor-specific antagonists into the KF and compared the autonomic responses with electrical EN5 stimulation before and after drug injection.
METHODS
Animals and anaesthesia
Twenty-six male Wistar rats (300-400 g) were anaesthetized with a mixture of α-chloralose (150 mg kg−1) and urethane (60 mg kg−1) injected i.p. under light ether pre-anaesthesia. Anaesthesia was maintained by i.v. supplements of α-chloralose as indicated by responses to nociceptive test stimuli. During the experimental sessions the animals were breathing oxygen-enriched air and received i.v. infusions of 0.5 ml h−1 saline containing 10% glucose. The body temperature was maintained at 37°C by a thermostatically controlled heating pad. The experiments were performed in accordance with ethical guidelines for the care and use of animals for experiments and were approved by the local council of animal care.
Surgical preparation and data recording
The femoral artery was cannulated with polyethylene tubing for blood pressure and heart rate recordings, using a Viggo-Spectramed transducer (Spectramed Inc., Oxnard, CA, USA) and a Gould pressure processor (Gould Inc., Valley View, OH, USA). The femoral vein was cannulated for drug and fluid injections. After tracheotomy, a tracheal tube was inserted to record respiratory parameters via a Bell & Howell pressure transducer (Bell & Howell Electronics, Pasadena, CA, USA) calibrated to 1 ml expiratory volume. Thereafter, we isolated the ethmoidal nerve (EN5), which is a sensory branch of the trigeminal nerve innervating the nasal mucosa. The EN5 was identified as a ramification of the ophthalmic nerve, which runs through the foramen ethmoidale on the bottom of the orbital cavity. For electrical stimulation, the EN5 was placed on the exposed tips of Teflon-insulated silver wires and covered with paraffin oil. Thereafter, the animals’ heads were fixed in a stereotaxic frame and a craniotomy was performed. After the surgery, the animals were allowed to stabilize for one hour. All data, such as arterial blood pressure, heart rate, respiratory airflow and respiratory rate were recorded, stored and analysed on a MacLab/8e system.
Drugs and drug injections
For drug injections into the KF area, we used home-made triple-barrelled micropipettes with a total tip diameter of 50-80 μm. The individual barrels were filled with the excitatory L-glutamic acid (5 mm; Sigma), with saline and either, 2-amino-5-phosphonopentanoic acid, (AP5, 1 mm; RBI), a selective N-methyl-D-aspartic acid (NMDA) receptor antagonist, with 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 1 mm; RBI), a selective α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA)/kainate receptor antagonist, with (-)-bicuculline methbromide (5 mm; Sigma), a selective α-aminobutyric acidA (GABAA) receptor antagonist or with strychnine hydrochloride (5 mm; Sigma) an antagonist for the inhibitory glycine receptor. Each pipette was connected with tubing to a multi-channel Picospritzer (General Valve Corp., Fairfield, NJ, USA). Pressure and opening times were set to allow single puffs of 5-10 nl as measured from the meniscus level in the pipette. Glutamate injections were generally less than 20 nl to avoid depolarization blockade. The injection volumes for the receptor antagonists generally ranged between 50 and 70 nl and occasionally reached 100 nl.
Experimental protocol
Depending on the receptor antagonist injected, two different stimulation protocols were performed. In those cases where antagonists to glutamate receptors were injected, the EN5 was electrically stimulated with a train of pulses (30-40 Hz, 100 μs pulse duration) for 10 s with a maximum intensity of 0.5-1 mA, corresponding to × 5 threshold current. As long as the nerve was intact, this stimulus reliably induced apnoea or suppression of respiration, bradycardia and rise in arterial blood pressure. After lowering the triple-barrelled micropipette into the ipsilateral KF, microinjections of glutamate were made to search for respiratory responsive sites. The pipette was left in those sites where glutamate elicited an apnoea or respiratory suppression. To control for pressure artifacts, injections of similar volumes of saline were made at the same site. Thereafter, two to four EN5 stimulations were performed before drug injection. Thirty to sixty seconds after the drug injection into the KF, EN5 stimulations were performed again, every 2 min at the beginning of the session and every 5-10 min later on until we observed a recovery from the drug effects.
In those experiments where strychnine or bicuculline were injected, we used stimulus intensities close to threshold (20-200 μA). By this means, the augmentation of EN5-evoked autonomic responses, which were to be expected after blockade of inhibitory amino acid receptors, could be evaluated better. The first EN5 stimulation was performed 3-10 min after the bicuculline injection.
At the end of each experiment, the rats received an overdose of anaesthetic (60 mg kg−1 urethane) and were transcardially perfused with saline followed by 500 ml of 4% formalin. Coronal sections through the pons were cut at 50 μm on a freezing microtome, mounted on slides and stained with thionine. Sections through the the dorsal pons, including the parabrachial nucleus (PB) and KF were analysed with a light microscope to localize and reconstruct the pipette track with the aid of a camera lucida. The track produced by the triple-barrelled pipettes as well as the lesions produced by the multiple pressure injections were generally clearly visible in our material. Furthermore, the employed stereotaxic co-ordinates together with an atlas of the rat brain (Paxinos & Watson, 1997) were used to confirm the identified sites. Afterwards, the centres of the injection sites of each individual experiment were pooled into three representative sections through the PB-KF area, as defined previously (Dutschmann & Herbert, 1997).
Data analysis
A total of fourteen rats received injections of either AP5 (n = 7) or CNQX (n = 7) into the dorsal pons. Of these injections, twelve were placed into the KF area (AP5, n = 6; CNQX, n = 6) while two injections were centred into the medial PB (AP5, n = 1; CNQX, n = 1) and considered as controls since they did not show any drug effects. For the statistical evaluation of AP5 and CNQX experiments, we analysed only those cases where the drug injections were centred in the KF area (n = 6 each, compare Fig. 3) and, furthermore, where the EN5 stimulations before drug injections induced a respiratory depression of > 50% (occurring in n = 6 experiments for each drug) and also induced increases in arterial blood pressure of > 5% (n = 6 each). Also, EN5 stimulations evoked decreases in heart rate of > 5% which occurred only in four experiments for each drug.
Figure 3. Semi-schematic line drawings of coronal sections through the PB-KF complex illustrating drug injection sites.
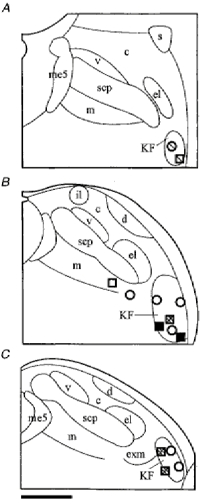
The line drawings of three representative sections through the PB-KF complex (left to right, from rostral to caudal) illustrate the locations of AP5 injections (squares, n = 7) and CNQX injections (circles, n = 7). Different shadings indicate the potency of blocking the EN5-evoked suppression of respiration. Open symbols: blockade < 10%; light shading > 10-40% blockade; dark shading > 40-70% blockade; filled symbols: > 70% blockade. Note that CNQX injections centred in the midlevel KF did not cause considerable blockade. Abbreviations: c, central lateral PB; d, dorsal lateral PB; el, external lateral PB; exm, external medial PB; il, internal lateral PB; KF, Kölliker-Fuse nucleus; m, medial PB; me5, mesencephalic trigeminal tract; s, superior lateral PB; scp, superior cerebellar peduncle and v, ventral lateral PB. Scale bar, 500 μm.
A total of twelve rats received injections of either bicuculline (n = 6) or strychnine (n = 6) into the dorsal pons. Of these injections ten were placed into the KF area (n = 5 for each drug) while two were centred caudally in the medial PB (bicuculline, n = 1) and in the external medial PB (strychnine, n = 1) and considered as controls (compare Fig. 7) since they did not show any drug effects. In those cases where bicuculline was injected into the KF area, we observed that respiratory suppression and bradycardia outlasted stimulation. Here, we determined the total respiratory volume and the maximal drop in heart rate also during three discrete periods after the end of stimulation, i.e. 0-10 s, > 10-20 s, and > 20-30 s post-stimulus.
Figure 7. Semi-schematic line drawings of coronal sections through the PB-KF complex illustrating drug injection sites.
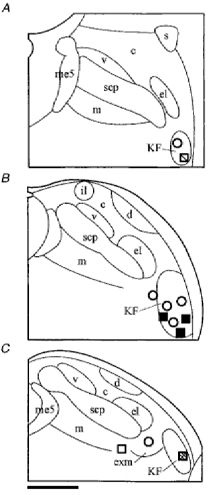
Line drawings of three representative sections through the PB-KF complex (left to right, from rostral to caudal) demonstrating the locations of bicuculline injections (squares, n = 6) and strychnine injections (circles, n = 6). Different shadings indicate the potency of the respective drug in extending the EN5-evoked respiratory suppression 0-10 s post-stimulus. Open symbols < 10% respiratory suppression 0-10 s post-stimulus; light shading > 10-40% respiratory suppression 0-10 s post-stimulus; dark shading > 40-80% respiratory suppression 0-10 s post-stimulus; filled symbols > 80-100% respiratory suppression 0-10 s post-stimulus. Abbreviations: c, central lateral PB; d, dorsal lateral PB; el, external lateral PB; exm, external medial PB; il, internal lateral PB; KF, Kölliker-Fuse nucleus; m, medial PB; me5, mesencephalic trigeminal tract; s, superior lateral PB; scp, superior cerebellar peduncle and v, ventral lateral PB. Scale bar, 500 μm.
EN5 stimulations resulted in apnoea or respiratory suppression, bradycardia and rise in arterial blood pressure. In order to quantify the effects to EN5 stimulations before and after drug injections, we calculated: (i) the total respiratory volume by adding up the expiratory volumes of single breaths occurring during the 10 s of EN5 stimulation, (ii) the mean arterial blood pressure during the 10 s of stimulation, and (iii) the maximal drop in heart rate occurring in response to EN5 stimulation. These values were then compared with control values which were taken 10 s before every individual stimulation performed. The effects of the EN5 stimulations (before and after drug injections) were expressed as percentage change of the control values. The values achieved following EN5 stimulations before drug injection were compared with those following EN5 stimulations after drug injection and those after recovery.
The respiratory response to EN5 stimulation was the most sensitive and consistent parameter modulated by drug injections. For statistical analysis of the drug effects, we used those stimulations that caused maximal changes in respiratory response to EN5 stimulation within the first 10-20 min after drug injection. The same stimulation served also for evaluation of the heart rate and blood pressure responses. Those experiments that did not show any clear changes in EN5-evoked autonomic responses 30 min after drug injection were considered to be ineffective.
The sites of drug injections are depicted in three standardized sections through the PB-KF from rostral to caudal, as defined previously (Dutschmann & Herbert, 1997). Blocking efficiency (AP5 and CNQX) was calculated according to Dutschmann & Herbert (1996). To indicate the efficiency of enhancing respiratory suppression (bicuculline and strychnine), we determined the value of respiratory suppression occurring within the first 10 s after EN5 stimulation.
For the analysis of numerical data, we used the analysis of variances (ANOVA) followed by a Fisher Least Squares Differences post hoc test. The tests were a priori restricted to the differences between stimulations before and after drug injection and the differences between stimulations after drug injection and at recovery. Probability values at P < 0.05 were considered to be significant. All values were expressed as means ±s.e.m.
RESULTS
Effect of NMDA and AMPA/kainate receptor blockade in the KF area on the nasotrigeminal reflex responses
Neither AP5 nor CNQX injections alone led to significant changes in the control values of respiration, heart rate or arterial blood pressure. Rats received unilateral injections of 50-100 nl of AP5 (1 mm) into the KF area (individual experiment presented in Fig. 1) which resulted in a significant blockade of the EN5-evoked respiratory depression (n = 6; Fig. 2). EN5 stimulation before AP5 injection resulted in a mean respiratory depression of 74.5 ± 8.2% (P < 0.001) whereas EN5 stimulation after AP5 blockade of NMDA receptors in the KF area resulted in a respiratory depression of only 33 ± 8.9% (P < 0.01). After 1-1.5 h the respiratory depression had recovered to 67.5 ± 8.6% (P < 0.01). EN5 stimulations before AP5 injections induced, in four of the six rats, bradycardia with a mean value of 17 ± 3.5% decrease in heart rate (P < 0.01, Fig. 2). The remaining two rats showed no bradycardia in response to EN5 stimulation and, therefore, were not included in the analysis. After AP5 injections, the EN5-evoked bradycardia was significantly reduced to a mean value of only 4 ± 0.6% decrease in heart rate (P < 0.05). The AP5 effect on the EN5-evoked bradycardia recovered partly to a mean heart rate decrease of 10 ± 4%. Interestingly, we observed that in those rats where AP5 strongly blocked the EN5-evoked respiratory depression, the bradycardiac response was also strongly blocked. The six rats investigated also showed a significant EN5-evoked rise in blood pressure with a mean increase of 15 ± 1.7% before AP5 injections (P < 0.001). However, the blood pressure response was not significantly changed after blockade of NMDA receptors in the KF area (Fig. 2).
Figure 1. Autonomic responses to a glutamate injection into the KF and to electrical stimulation of the ethmoidal nerve before and after NMDA receptor blockade in the KF.
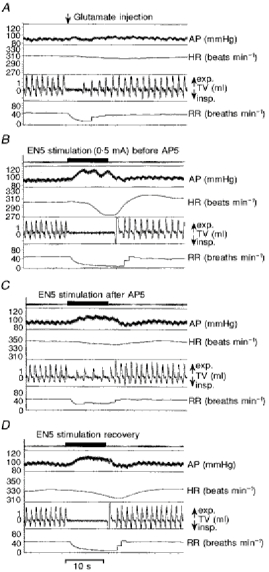
Recordings of arterial blood pressure (AP), heart rate (HR), tidal volume (TV), and respiratory rate (RR) in a single experiment (A-D) analysing the role of NMDA receptors in the KF for mediating the nasotrigeminal reflex responses. A, autonomic responses to a discrete glutamate injection (∼20 nl, 1 mm) into the KF. This injection characterizes the respective site as a locus where apnoea can be induced. B, autonomic responses to an EN5 stimulation (0.5 mA; horizontal bar) before drug injection. C, responses to EN5 stimulation after an AP5 injection (∼70 nl, 1 mm). D, responses to EN5 stimulation 70 min after AP5 injection documenting the recovery of the autonomic response.
Figure 2. Bar diagrams summarizing the effects of NMDA and AMPA receptor blockade in the KF area on the EN5-evoked autonomic responses.
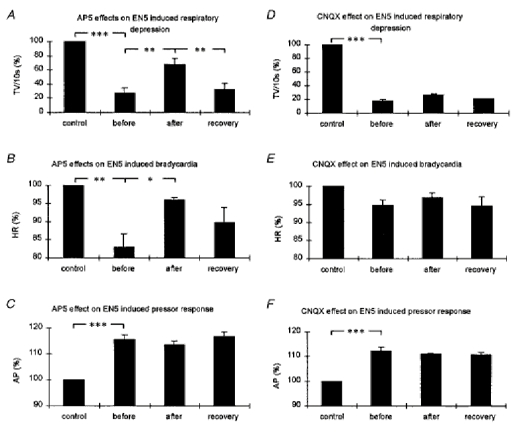
The bar diagrams illustrate the tidal volumes (A and D, TV (10 s)−1; n = 6), the maximal drop in heart rate (B and E, HR; n = 4), and the mean arterial blood pressure (C and F, AP; n = 6) in response to EN5 stimulation before and after pharmacological manipulations (A, B and C, AP5 experiments; D, E and F, CNQX experiments). Control, 10 s before every EN5 stimulation; before, EN5 stimulation before AP5 or CNQX injection; after, EN5 stimulation after AP5 or CNQX injection; recovery, EN5 stimulation at recovery. All values are expressed as a percentage ±s.e.m.*P < 0.05, **P < 0.01, ***P < 0.001.
Injections of the AMPA/kainate receptor antagonist CNQX into the KF area of six rats caused only minor, insignificant alterations of EN5-evoked autonomic responses (Fig. 2). The EN5-evoked respiratory depression changed from 82 ± 7.3% before, to 73 ± 7.6% after, the CNQX injection and recovered to 79 ± 6.8% after 1-1.5 h. Four of the six rats showed changes in bradycardia from 5 ± 0.6% decrease in heart rate before, to 3 ± 1.3% after CNQX injection with a recovery back to 5 ± 2.1%. In six rats, the increase in arterial blood pressure changed from 12 ± 1.6% before, to 10 ± 0.5% after CNQX injection.
Verification of the injection sites revealed a topography with respect to the efficiency of blocking the EN5-evoked respiratory depression and bradycardia (Fig. 3). AP5 injections placed ventrally into the midlevel KF area resulted in the most effective blockade of EN5-evoked respiratory depression (84.1 and 94.2% blockade, Fig. 3B). The remaining injection sites in the midlevel and caudal aspects of the KF area resulted in a blockade of 61% and 46-48%, respectively. An injection into the rostral KF area caused blockade of only 31%. An ineffective AP5 injection was found to be centred in the medial PB. In contrast, CNQX injections in the caudal and midlevel KF area resulted in no blockade at all or in minor blockade of up to 16%. Only one rostral CNQX injection led to a 30% blockade of EN5-evoked respiratory suppression.
Effect of GABAA and glycine receptor blockade in the KF area on the nasotrigeminal reflex responses
Injections of 50-100 nl strychnine (5 mm) into the KF area did not elicit any changes in the control values of respiration, heart rate or arterial blood pressure. In contrast, injections of 50-100 nl bicuculline (5 mm) into the KF area resulted in perturbations of the respiratory rhythm (Fig. 4, upper panel). Overall, the respiratory minute volume was slightly reduced (from 99 ± 4.8 to 84 ± 7.2 ml min−1). Analysis of five experiments with unilateral injections of the GABAA receptor antagonist bicuculline into the KF area (individual experiment shown in Fig. 4) revealed a highly significant enhancement of the EN5-evoked respiratory depression (Fig. 5A). EN5 stimulation close to threshold resulted in a 43.7 ± 7.4% (P < 0.001) depression of respiration before drug injections. These weak stimulations, performed a few minutes after bicuculline injections, led in all animals to a complete cessation of breathing (100%) during the 10 s of EN5 stimulation (P < 0.001). Moreover, the apnoeas and later on respiratory suppression persisted for up to 30 s after the end of stimulation (Fig. 6). Statistical analysis of the post-stimulus respiratory activity revealed a significant suppression of breathing within the first 20 s after stimulation (0-10 s post-stimulus, P < 0.01; 10-20 s post-stimulus, P < 0.05). Approximately 1 h after the bicuculline injections the EN5-evoked suppression of breathing had significantly recovered (Figs 6 and 7). EN5 stimulations close to threshold resulted in only minor bradycardiac responses, i.e. 1.2 ± 0.6% decrease in heart rate. Yet, after bicuculline injections we observed a significant enhancement of the bradycardiac response to 22.2 ± 8.3% (P < 0.01) decrease in heart rate during the 10 s of stimulation (Figs 6 and 7). Comparable with the respiratory response, the bradycardia persisted also for up to 30 s after stimulation. Statistical analysis of the post-stimulus heart rate revealed a significant decrease within the first 20 s after end of stimulus (0-10 s post-stimulus, P < 0.01; 10-20 s post-stimulus, P < 0.05). Approximately 1 h after the bicuculline injections the EN5-evoked fall in heart rate had significantly recovered (Figs 6 and 7). EN5 stimulations close to threshold resulted in a slight increase of arterial blood pressure of 8.8 ± 1.9% (P < 0.05). In contrast to the EN5-evoked cardiorespiratory responses, the rise in arterial blood pressure was not significantly enhanced by bicuculline injections into the KF. After drug injection, the mean pressor response was even slightly reduced to a 6.5 ± 0.8% increase; however, this response was not significant (Fig. 6).
Figure 4. Autonomic responses to electrical stimulation of the ethmoidal nerve before and after GABAA receptor blockade in the KF.
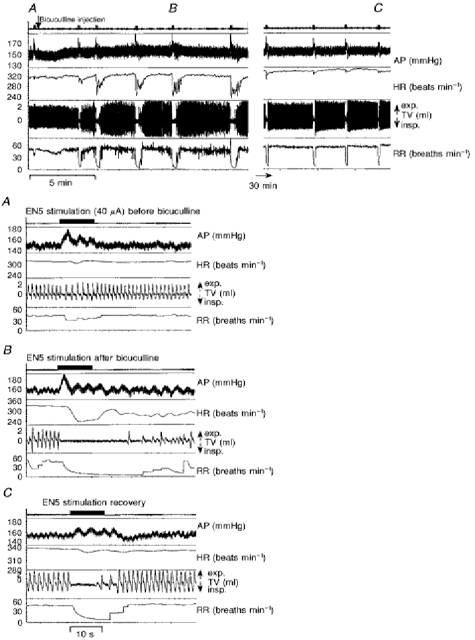
Recordings of arterial blood pressure (AP), heart rate (HR), tidal volume (TV), and respiratory rate (RR) of a single experiment analysing the role of GABAA receptors in the KF for the mediation of the nasotrigeminal reflex responses. The upper panel illustrates the autonomic responses to EN5 stimulations before and after an injection of bicuculline (∼70 nl, 5 mm) into the KF and the long-term development of these responses. Squares on the upper trace indicate individual EN5 stimulations. Those labelled A-C are shown as blown ups in the lower panels. A, responses to EN5 stimulation close to threshold (40 μA) before the bicuculline injection. B, responses to EN5 stimulation after the bicuculline injection. C, responses to EN5 stimulation 1 h after the drug injection (recovery).
Figure 5. Bar diagrams summarizing the effects of GABAA and glycine receptor blockade in the KF on the EN5-evoked autonomic responses.
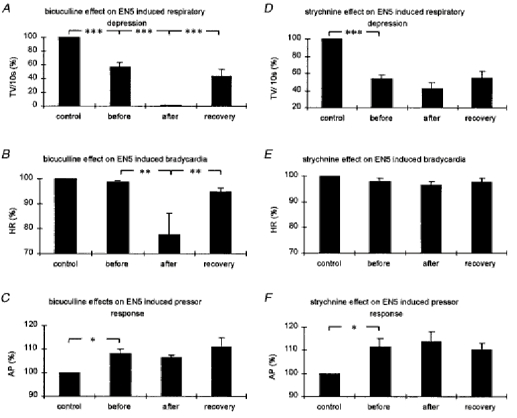
The bar diagrams illustrate the tidal volumes (A and D, TV (10 s)−1; n = 5), the maximal drop in heart rate (B and E, HR; n = 5), and the mean arterial blood pressure (C and F, AP; n = 5) in response to EN5 stimulation before and after pharmacological manipulations (A, B and C, bicuculline experiments; D, E and F, strychnine experiments). Control, 10 s before every EN5 stimulation; before, EN5 stimulation before bicuculline or strychnine injection; after, EN5 stimulation after bicuculline or strychnine injection; recovery, EN5 stimulation at recovery. All values are expressed as percentage ±s.e.m.*P < 0.05, **P < 0.01, ***P < 0.001.
Figure 6. Bar diagrams summarizing the effects of GABAA receptor blockade in the KF on the EN5-evoked respiratory and cardiac response.
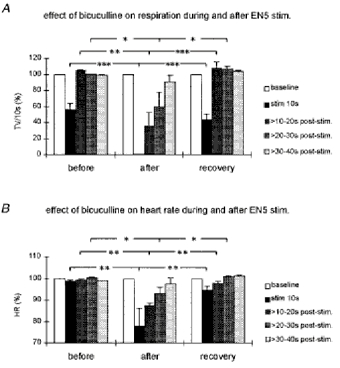
The bar diagrams illustrate the tidal volume (TV (10 s)−1; A) and the maximal drop in heart rate (HR; B) during and at three time windows after EN5 stimulations (close to threshold). Before, EN5 stimulation before bicuculline injection into the KF; after, EN5 stimulation after bicuculline injection; recovery, EN5 stimulation at recovery. All values are expressed as percentage ±s.e.m. *P < 0.05, **P < 0.01 and ***P < 0.001.
Strychnine injections into the KF area did not result in significant changes of EN5-evoked autonomic responses following electrical stimulations close to threshold. In five experiments (Fig. 5D, E and F), we found after strychnine injections a slight enhancement of respiratory depression (from 46.2 ± 4.9 to 58 ± 7.5%), a bradycardiac response (from 2 ± 1 to 3.2 ± 1.5%) and an increase in arterial blood pressure (from 11.3 ± 3.4 to 13 ± 4.2%).
Verification of injection sites in the KF area revealed a topography with respect to the efficiency of drug effects on the EN5-evoked respiratory depression and bradycardia. Three of the midlevel bicuculline injections resulted in a prominent suppression of breathing during the 10 s of stimulation and for the following 10 s after end of stimulation (83 -100%). Another two injections rostrally and caudally in the KF area were considerably less efficient (27.5 and 40%, respectively). One injection placed caudally in the medial PB was ineffective (Fig. 7). In contrast, strychnine injections into the KF area never led to a comparable enhancement of the EN5-evoked responses, even if they were centred in the midlevel KF (Fig. 7).
DISCUSSION
In the present study, we determined the role of excitatory and inhibitory amino acid receptors in the KF area involved in the mediation of nasotrigeminal reflex responses. Our data revealed that antagonism of NMDA receptors profoundly blocked the EN5-evoked apnoea and bradycardia. In contrast, antagonism of AMPA/kainate receptors only resulted in weak effects. Blockade of GABAA receptors led to a pronounced enhancement of the EN5-evoked apnoea and bradycardia which persisted for several seconds after end of stimulation. Antagonism of glycine receptors in the KF showed only weak effects on the nasotrigeminal cardiorespiratory reflex response. Histological reconstructions revealed that both the most effective AP5 and bicuculline injection sites, were located ventrally in the midlevel KF area. Furthermore, our experiments showed that the blood pressure response remained largely unaffected by any of the pharmacological manipulations in the KF area. Recent data indicate that it is the medial nucleus of the solitary tract (NTS) which mediates the EN5-evoked pressor response (Dutschmann & Herbert, 1998).
Technical considerations
Block of synaptic transmission in the KF area was performed by unilateral injections of receptor-specific antagonists. Since we attempted to achieve a substantial blockade of neurotransmission in the KF area, the amounts of drugs injected ranged from 50 nl to occasionally 100 nl. In the latter cases, drugs might have diffused in effective concentrations into adjacent pontine regions also implicated in respiratory control, such as the ventrolateral pons with the A5 noradrenergic cell group (Jodkowski et al. 1997) which is located at least 1 mm ventral and caudal to the KF area. However, lines of evidence support our assumption that it is basically neurons in the KF area that account for the described drug effects. The locations of the drug injection sites in the PB-KF area revealed a topography with respect to their potency of blocking or enhancing the EN5-evoked cardiorespiratory responses. Drug injections placed approximately 500 μm apart from the most effective sites in the midlevel KF area were already considerably less effective or not effective at all. Furthermore, the location of the proposed relay site in the KF area as revealed with our drug injections is in good accordance with a recently published c-fos study from our laboratory (Dutschmann & Herbert, 1997).
Previous studies revealed that evocation and expression of the diving bradycardia depends on co-operative sensory inputs arising from the trigeminal system, the lungs, and from chemo- and baroreceptors (Elsner & Daly, 1988). Activation of the EN5 leads to apnoea in the expiratory state (silent pulmonary stretch receptors) which is mandatory for a bradycardia to occur (Daly, 1984). The strength of bradycardia is then reinforced by increasing chemoreceptor drive (Elsner & Daly, 1988). Since in our experiments rats were generally well oxygenated, the chemoreceptor drive most likely did not contribute to a prominent drop in heart rate. Additionally, EN5 stimulation causes pressor response and, thus, baroreceptor activation. However, there is ample evidence that the baroreflex is not the major cause for the bradycardia seen in our study. First, from our traces it appears that the EN5-evoked changes in blood pressure do not correlate well with the changes in heart rate. Second, disinhibition in the KF area lead to a more rapid onset and a stronger expression of the bradycardiac response while the pressor response remained unaffected. Third, after disinhibition in the KF area both apnoea and bradycardia were enhanced and outlasted the end of stimulation while the blood pressure response never showed such a performance. In general, our data strongly suggest that heart rate and respiration are more closely associated with each other than heart rate and blood pressure (see also discussion below) and, therefore, the contribution of the baroreflex to the bradycardia might be minor.
We found that selective antagonism of AMPA/kainate receptors failed to cause a significant blockade of EN5-evoked responses. We conclude that AMPA/kainate receptors in the KF area play only a minor role for mediating the reflex responses. This finding does not fit with the general idea that NMDA-mediated synaptic events depend on a preceding depolarization evoked by AMPA/kainate receptors. Yet, it has been reported that NMDA receptors do mediate synaptic transmission in vivo without a preceding non-NMDA receptor depolarization (Headley & Grillner, 1991; Salt & Eaton, 1991). This is in line with an in vitro study showing that NMDA receptors can be blocked by antagonists at negative membrane potentials (Burgard & Hablitz, 1993). Furthermore, from our in situ hybridization studies we know that KF neurons express almost exclusively mRNAs for the NR1 and the NR2D subunits (Guthmann et al. 1997) which have been shown to form heteromeric NMDA receptors with a weak Mg2+ block (Kuner & Schopfer, 1996).
Role of excitatory amino acid receptors in the KF
Our present data show that in the KF, NMDA receptors and not AMPA/kainate receptors are essential for mediating the EN5-evoked apnoea and bradycardia. Likewise, AMPA/kainate receptors are also not involved in pontine respiratory termination (Pierrefiche et al. 1994). These findings agree with several previous studies demonstrating that NMDA receptors are crucial for the expression of pontine pneumotaxic mechanisms. Systemic or local blockade of NMDA receptors after vagotomy led to an apneustic breathing pattern known to originate predominantly in the pontine pneumotaxic centre (Pierrefiche et al. 1994; Ling et al. 1994; Fung et al. 1994; Cassus-Soulanis et al. 1995). The physiological findings are supported by anatomical data on the distribution of glutamate receptors in the KF. A major role for NMDA receptors in the KF is indicated by a ligand binding study demonstrating that in the PB-KF area a higher level of L-[3H]glutamate binding sites was found, compared with the remaining pons (Monaghan & Cotman, 1985). Recent work in our laboratory revealed that KF neurons are strongly immunoreactive for the NR1 subunit of the NMDA receptor (Guthmann et al. 1997). In contrast, immunoreactivities for the AMPA receptor subunits GluR1, GluR2/3 and GluR4 are fairly weak in the KF while they are prominent in lateral PB nuclei (Chamberlin & Saper, 1995).
Role of inhibitory amino acid receptors in the KF
We show that KF neurons which mediate the EN5-evoked apnoea and bradycardia are under strong GABAergic inhibition. Furthermore, disinhibition of KF neurons by GABAA receptor blockade led to a long-lasting disturbance of respiratory activity. These physiological findings are in good agreement with our anatomical data demonstrating that the KF is heavily innervated by GABAergic fibres and also expresses several GABAA receptor subunits (Guthmann et al. 1996). In contrast, we found that glycine in the KF is apparently not relevant for the nasotrigeminal reflex response although both glycine-ergic fibres and glycine receptors are present in all parts of the KF (Herbert et al. 1996). The source of GABAergic projections to the KF is unknown. Potential inhibitory afferents may arise from various brainstem nuclei, especially respiratory nuclei in the ventrolateral medulla and the NTS. This is suggested by electrophysiological recordings showing that the KF receives inhibitory input from medullary respiratory neurons (Dick et al. 1994) which might in part be driven by vagal pulmonary afferents (Feldman et al. 1976; Shaw et al. 1989) relaying the Hering-Breuer reflex via the NTS to the KF. This inhibitory input from the pulmonary stretch receptors is of particular interest. It was shown that trigeminal stimulation was most effective in eliciting cardiorespiratory responses at those times where pulmonary stretch receptors were minimally active, i.e. during apnoea in the expiratory state. This is regarded to be essential for the maintenance of diving bradycardia and apnoea (Daly, 1984; Elsner & Daly, 1988). Thus, lack of pulmonary inhibitory (GABAergic?) drive to the KF leads to an enhancement of trigeminally induced cardiorespiratory responses. This observation is reminiscent of our data demonstrating a dramatic enhancement of EN5-evoked cardiorespiratory responses after reducing inhibition in the KF by means of GABAA receptor blockade. Thus, we speculate that the effects observed in our experiments might in fact represent blockade of the inhibitory input from the pulmonary stretch receptors in the KF. The disinhibition of KF neurons finally leads to an extremely sensitive system that can be perturbed by otherwise ineffective stimuli, such as weak EN5 stimuli.
Potential interactions of KF neurons with the respiratory rhythm generator and with cardiac vagal motoneurons
A most likely mechanism for centrally induced apnoeas is a tonic activation of postinspiratory neurons, some of which exert an inhibitory influence on the inspiratory network (Remmers et al. 1986). Interestingly, Dick et al. (1994) showed that in the KF many neurons are found that exhibit a peak activity during postinspiration, indicating that the EN5-evoked apnoea might be due to activation of postinspiratory neurons in the medullary respiratory network via the KF. Batsel & Lines (1979) provided evidence that EN5-evoked apnoeas are indeed brought about by tonic drive of medullary postinspiratory neurons, thus, supporting the outlined hypothesis. In our experiments, the EN5-evoked apnoea was accompanied by bradycardia and both responses could be blocked or enhanced by either AP5 or bicuculline injections into the KF, respectively. This parallel modulation of the cardiorespiratory responses suggests a co-operative processing of both responses. It has long been known that postinspiratory neurons, besides shutting off inspiration, also disinhibit cardiac vagal motoneurons which cause bradycardia (Richter & Spyer, 1990). We propose that tonic activation of postinspiratory neurons by the KF is a suitable means to account for the EN5-evoked apnoea and bradycardia. Furthermore, there are direct projections from the KF to cardiac vagal motoneurons as revealed by transneuronal virus tracing (Ter Horst et al. 1996). These direct KF projections could also participate in mediating the EN5-evoked bradycardia.
Clinical implications of the nasotrigeminal reflex response
The nasotrigeminal reflex is generally considered a life-protecting, oxygen-conserving reflex that firstly, induces an apnoea to prevent invasion of noxious substances into the airways and secondly, adjusts the cardiovascular system such that progression of asphyxia is considerably slowed down. However, this reflex response is also discussed as a primary cause of pathological, life-threatening phenomena, in particular with respect to the sudden infant death syndrome (SIDS; for review, see Lobban, 1991). Several lines of evidence indicate that in terrestrial mammals and humans the diving reflex matures during the first month of postnatal life. Facially induced autonomic responses were found to be much stronger in neonates compared with adults (Tchobroutsky et al. 1969; Smith et al. 1976). Similar observations have been made for pre-term infants compared with full-term infants. Nasal stimulations with airpuffs revealed that in pre-term infants cardiorespiratory responses occurred more often and were generally more pronounced than those elicited in full-term infants (Ramet et al. 1990). We speculate that the hyperactive diving reflex in young postnatal mammals might represent one means leading to apnoea and/or cardiac arrest as assumed to occur in SIDS victims.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (He1842/6-1) and by the Graduiertenkolleg Neurobiologie Tübingen. The authors thank Helga Zillus, Dr Peter Pilz and Dr Jo Ostwald for their help during the course of the study and Professor Dr D. W. Richter for valuable suggestions on the manuscript.
References
- Anton F, Peppel P. Central projections of trigeminal primary afferents innervating the nasal mucosa: a horseradish peroxidase study in the rat. Neuroscience. 1991;41:629–641. doi: 10.1016/0306-4522(91)90354-q. 10.1016/0306-4522(91)90355-R. [DOI] [PubMed] [Google Scholar]
- Batsel HL, Lines AJ. Discharge of respiratory neurons in sneezes resulting from ethmoidal nerve stimulation. Experimental Neurology. 1979;58:410–424. doi: 10.1016/0014-4886(78)90097-3. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubié M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiological Reviews. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Burgard EC, Hablitz JJ. Developmental changes in NMDA and non-NMDA receptor-mediated synaptic potentials in the neocortex. Journal of Neurophysiology. 1993;69:725–745. doi: 10.1152/jn.1993.69.1.230. [DOI] [PubMed] [Google Scholar]
- Cassus-Soulanis S, Foutz AS, Denavit-Saubié M. Involvement of NMDA receptors in inspiratory termination in rodents: effects of wakefulness. Brain Research. 1995;679:35–33. doi: 10.1016/0006-8993(95)00205-5. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. Differential distribution of AMPA-selective glutamate receptor subunits in the parabrachial nucleus of the rat. Neuroscience. 1995;68:435–443. doi: 10.1016/0306-4522(95)00129-7. [DOI] [PubMed] [Google Scholar]
- de Burgh Daly M. Breath-hold diving: mechanisms of cardiovascular adjustments in the mammal. Recent Advances in Physiology. 1984;10:201–245. [Google Scholar]
- Dick TE, Bellingham MC, Richter DW. Pontine respiratory neurons in anesthetized cats. Brain Research. 1994;636:259–269. doi: 10.1016/0006-8993(94)91025-1. 10.1016/0006-8993(94)91025-1. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H. The Kölliker-Fuse nucleus mediates the trigeminally induced apnea in the rat. NeuroReport. 1996;7:1432–1436. doi: 10.1097/00001756-199605310-00022. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H. Fos expression in the rat parabrachial and Kölliker-Fuse nuclei after electrical stimulation of the trigeminal ethmoidal nerve and water stimulation of the nasal mucosa. Experimental Brain Research. 1997;117:97–110. doi: 10.1007/s002210050203. 10.1007/s002210050203. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H. The medial nucleus of the solitary tract mediates the trigeminally evoked pressor response. NeuroReport. 1998;9:1053–1057. [PubMed] [Google Scholar]
- Elsner R, de Burgh Daly M. Coping with asphyxia: lessons from seals. News in Physiological Sciences. 1988;3:65–69. [Google Scholar]
- Feil K, Herbert H. Topographic organization of spinal and trigeminal somatosensory pathways to rat parabrachial and Kölliker-Fuse nuclei. Journal of Comparative Neurology. 1995;353:506–528. doi: 10.1002/cne.903530404. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Cohen MI, Wlotsky P. Powerful inhibition of pontine respiratory neurons by pulmonary afferent activity. Brain Research. 1976;104:341–346. doi: 10.1016/0006-8993(76)90629-6. 10.1016/0006-8993(76)90629-6. [DOI] [PubMed] [Google Scholar]
- Fung M-L, Wang W, St John WM. Involvement of pontile NMDA receptors in inspiratory termination in rat. Respiration Physiology. 1994;96:177–188. doi: 10.1016/0034-5687(94)90125-2. 10.1016/0034-5687(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Guthmann A, Dutschmann M, Wagner T, Herbert H. NMDA-receptor subunit immunoreactivity in the rat autonomic brainstem and colocalization with Fos induced by nasal stimulation. Society for Neuroscience Abstracts. 1997;23:725–285. 7. [Google Scholar]
- Guthmann A, Fritschy J-M, Ottersen OP, Torp R, Herbert H. GABA, GAD and GABAA-receptors in the rat parabrachial complex. Society for Neuroscience Abstracts. 1996;22:1040–414. 2. [Google Scholar]
- Headley PM, Grillner S. Excitatory amino acids and synaptic transmission: the evidence for a physiological function. In: Lodge D, Collingridge GL, editors. The Pharmacology of Excitatory Amino Acids, Trends in Pharmacological Science, A Special Report. Cambridge, UK: Elsevier Trends Journals; 1991. pp. 30–36. [DOI] [PubMed] [Google Scholar]
- Herbert H, Guthmann A, Ottersen OP, Zafra F. Glycine, glycine-receptors and glycine-transporters in the rat parabrachial complex. Society for Neuroscience Abstracts. 1996;22:1041–414. 8. [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. Journal of Comparative Neurology. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Jodkowski JS, Coles SK, Dick TE. Prolongation in expiration evoked from ventrolateral pons of adult rats. Journal of Applied Physiology. 1997;82:377–381. doi: 10.1152/jappl.1997.82.2.377. [DOI] [PubMed] [Google Scholar]
- Koshiya N, Guyenet PG. Role of the pons in the carotid sympathetic chemoreflex. American Journal of Physiology. 1994;267:R508–518. doi: 10.1152/ajpregu.1994.267.2.R508. [DOI] [PubMed] [Google Scholar]
- Kratschmer F. Über Reflexe von der Nasenschleimhaut auf Athmung und Kreislauf. Sitzungsberichte der Mathematisch-Naturwissenschafftlichen Classe, Akademie der Wissenschaften. 1870;XVI:147–170. [Google Scholar]
- Kuner T, Schopfer R. Multiple structural elements determine subunit specificity of Mg2+ block in NMDA receptor channels. Journal of Neuroscience. 1996;16:3549–3558. doi: 10.1523/JNEUROSCI.16-11-03549.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Karius DR, Speck DF. Role of N-methyl-D-aspartate receptors in the pontine pneumotaxic mechanism in the cat. Journal of Applied Physiology. 1994;76:1138–1143. doi: 10.1152/jappl.1994.76.3.1138. [DOI] [PubMed] [Google Scholar]
- Lobban CR. The human dive reflex as primary cause of SIDS. A review of the literature. The Medical Journal of Australia. 1991;155:561–563. [PubMed] [Google Scholar]
- Monaghan DT, Cotman CW. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in the rat brain. Journal of Neuroscience. 1985;5:2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneton MW. Primary afferent projections from the upper respiratory tract in the muskrat, Ondatra zibethicus. Journal of Comparative Neurology. 1991;308:51–65. doi: 10.1002/cne.903080106. [DOI] [PubMed] [Google Scholar]
- Panneton WM, Johnson SN, Christensen ND. Trigeminal projections to the peribrachial region in the muskrat. Neuroscience. 1994;58:605–625. doi: 10.1016/0306-4522(94)90085-x. 10.1016/0306-4522(94)90085-X. [DOI] [PubMed] [Google Scholar]
- Panneton MW, Yavari P. A medullary dorsal horn relay for the cardiorespiratory responses evoked by stimulation of the nasal mucosa in the muskrat, Ondatra zibethicus. Neuroscience. 1995;58:605–625. doi: 10.1016/0006-8993(95)00597-j. 10.1016/0306-4522(94)90085-X. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Pierrefiche O, Foutz AS, Champagnat J, Denavit-Saubié M. NMDA and non-NMDA receptors may play distinct roles in timing mechanisms and transmission in the feline respiratory network. The Journal of Physiology. 1994;474:509–523. doi: 10.1113/jphysiol.1994.sp020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramet J, Praud JP, D'Allest A-M, Dehan M, Gaultier C. Trigeminal airstream stimulation. Maturation-related cardiac and respiratory responses during REM sleep in human infants. Chest. 1990;98:92–96. doi: 10.1378/chest.98.1.92. [DOI] [PubMed] [Google Scholar]
- Remmers JE, Richter DW, Ballantyne D, Bainton CR, Klein JP. Reflex prolongation of stage I of expiration. Pflügers Archiv. 1986;407:190–198. doi: 10.1007/BF00580675. [DOI] [PubMed] [Google Scholar]
- Richter DW, Spyer KM. Cardiorespiratory control. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. New York, Oxford: Oxford University Press; 1990. pp. 189–207. chap. 11. [Google Scholar]
- Salt TE, Eaton SA. Sensory excitatory postsynaptic potentials mediated by NMDA and non-NMDA receptors in the thalamus in vivo. European Journal of Neuroscience. 1991;3:296–300. doi: 10.1111/j.1460-9568.1991.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Sant'Ambrogio G, Tusbone H, Sant'Amgrogio FB. Sensory information from the upper airway: role in the control of breathing. Respiration Physiology. 1995;102:1–16. doi: 10.1016/0034-5687(95)00048-i. [DOI] [PubMed] [Google Scholar]
- Shaw C-F, Cohen MI, Brandhardt R. Inspiratory-modulated neurons of the rostrolateral pons: effects of pulmonary afferent input. Brain Research. 1989;485:179–184. doi: 10.1016/0006-8993(89)90681-1. [DOI] [PubMed] [Google Scholar]
- Smith JB, McCubbin JA, McLean WT, Jr, Toole JF. The diving reflex in infants: a preliminary report. Transactions of the American Neurological Association. 1976;101:294–295. [PubMed] [Google Scholar]
- Tchobroutsky C, Merlet C, Rey P. The diving reflex in rabbit, sheep and newborn lamb and its afferent pathways. Respiration Physiology. 1969;8:108–117. doi: 10.1016/0034-5687(69)90048-6. [DOI] [PubMed] [Google Scholar]
- Ter Horst GJ, Hautvast RWM, De Jongste MJL, Korf J. Neuroanatomy of cardiac activity-regulating circuitry: a transneuronal retrograde viral labelling study in the rat. European Journal of Neuroscience. 1996;8:2029–2041. doi: 10.1111/j.1460-9568.1996.tb00723.x. [DOI] [PubMed] [Google Scholar]
- Wallois F, Gros F, Masmoudi K, Larnicol N. c-FOS-like immunoreactivity in cat brainstem evoked by sneeze-inducing air puff stimulation of the nasal mucosa. Brain Research. 1995;687:143–154. doi: 10.1016/0006-8993(95)00487-b. [DOI] [PubMed] [Google Scholar]


