Abstract
CB-1 cannabinoid receptors are strongly expressed in the molecular layer of the cerebellar cortex. We have analysed, in patch-clamped Purkinje cells (PCs) in rat cerebellar slices, the effect of the selective CB-1 agonists WIN55,212-2 and CP55,940 and of the selective CB-1 antagonist SR141716-A on excitatory synaptic transmission and synaptic plasticity.
Bath application of both agonists markedly depressed parallel fibre (PF) EPSCs. This effect was reversed by SR141716-A. In contrast, responses of PCs to ionophoretic application of glutamate were not affected by WIN55,212-2.
The coefficient of variation and the paired-pulse facilitation of these PF-mediated EPSCs increased in the presence of WIN55,212-2.
WIN55,212-2 decreased the frequency of miniature EPSCs and of asynchronous synaptic events evoked in the presence of strontium in the bath, but did not affect their amplitude.
WIN55,212-2 did not change the excitability of PFs.
WIN55,212-2 impaired long-term depression induced by pairing protocols in PCs. This effect was antagonized by SR141716-A. The same impairment of LTD was produced by 2-chloroadenosine, a compound that decreases the probability of release of glutamate at PF-PC synapses.
The present study demonstrates that cannabinoids inhibit synaptic transmission at PF-PC synapses by decreasing the probability of release of glutamate, and thereby impair LTD. These two effects might represent a plausible cellular mechanism underlying cerebellar dysfunction caused by cannabinoids.
Marijuana, besides its well-known psychoactive properties, also has a large variety of effects of significant therapeutic potential including analgesic, antiemetic and appetite stimulant actions of particular interest for cancer and AIDS patients. However, Δ-9-tetrahydrocannabinol, the major psychoactive component of marijuana, also impairs a variety of cognitive and performance tasks including memory and motor tasks (Abood & Martin, 1992). Cannabinoid receptors are of two types, Type 1 (CB-1) and Type 2 (CB-2). The latter is mainly localized in the peripheral nervous system and several other tissues, whereas the former is widely expressed in the brain, especially in the neocortex, basal ganglia, hippocampus and cerebellum (see references in Bidaut-Russell, Devane & Howlett, 1990 and in Matsuda, Bonner & Lolait, 1993). In expression systems, these receptors, known to be negatively coupled to adenylate cyclase, inhibit N- and Q-type voltage-gated calcium channels and enhance an inwardly rectifying potassium channel (see references in Mackie, Lai, Wenstenbroek & Mitchell, 1995; Childers & Deadwyler, 1996). Furthermore, in cultured hippocampal neurones, cannabinoids also enhance an A-type K+ current (Deadwyler et al. 1993). Cannabinoids also acutely depress glutamatergic synaptic transmission through a presynaptic mechanism in the hippocampus (Shen, Piser, Seybold & Thayer, 1996) and block induction of long-term potentiation (LTP) in this structure (Nowicky, Teyler & Vardaris, 1987; Terranova, Michaud, Le Fur & Soubrié, 1995; Stella, Schweitzer & Piomelli, 1997).
Despite the fact that CB-1 receptors are particularly well expressed in the cerebellar cortex (Herkenham, Lynn, Jonhson, Melvin, de Costa & Rice, 1991; Matsuda et al. 1993) and that cannabinoids impair motor co-ordination (Stark & Dews, 1980), nothing is presently known about their effects on synaptic transmission and synaptic plasticity in this structure. In particular, this is the case at the level of the synapses between parallel fibres (PFs) and Purkinje cells (PCs) where the characteristics of synaptic transmission are fairly well established (Llano, Marty, Armstrong & Konnerth, 1991), as are some of the mechanisms underlying a form of synaptic plasticity they exhibit: long-term depression (LTD) (see references in Crépel, Hemart, Jaillard & Daniel, 1996). The fact that CB-1 mRNAs are not expressed in PCs, but are abundant in cerebellar granule cells, and that CB-1 receptor binding is found in the molecular layer, suggests that CB-1 receptors are expressed by PFs (Herkenham et al. 1991; Matsuda et al. 1993). Therefore, cannabinoids are good candidates to interact with synaptic transmission and/or synaptic plasticity at these synapses.
These observations make the synapses between PFs and PCs an attractive model to study the effects of cannabinoids on synaptic transmission and synaptic plasticity in the cerebellum and more generally to gain some insights into the cellular mechanisms underlying their behavioural effects.
In the present paper, therefore, we have studied the effects of the selective cannabinoid receptor agonists WIN55,212-2 and CP55,940 (Devane, Dysarz, Johnson, Melvin & Howlett, 1988), and of the selective antagonist SR141716-A (N-(piperidin-1-yl)-5-(-4-chlorophenyl)-1-(2,4-dichlorophenyl)- 4-methyl-1H-pyrazol-3-carboxamide hydrochloride) (Rinaldi- Carmona et al. 1994) on synaptic transmission at PF-PC synapses as well as on the induction of LTD by a classical pairing protocol.
METHODS
Sprague-Dawley rats, age 15-21 days, were stunned and decapitated; sagittal cerebellar slices, 200 μm thick, were cut with a vibroslice and incubated at room temperature in saline solution bubbled with 95% O2-5% CO2 for at least 2 h. The recording chamber was perfused at a rate of 2 ml min−1 with oxygenated saline solution containing (mm): NaCl, 124; KCl, 3; NaHCO3, 24; KH2PO4, 1.15; MgSO4, 1.15; CaCl2, 2; glucose, 10; and 10 μm bicuculline methiodide (a GABAA antagonist; Sigma); final pH 7.35 at 28°C. PCs were directly visualized with Nomarski optics through the × 40 water-immersion objective of an upright microscope (Zeiss). For analysis of miniature EPSCs (mEPSCs), the bathing medium also contained 1 μm tetrodotoxin (TTX) and 100 μm picrotoxin (Sigma). Experiments on strontium-induced asynchronous release of quanta from stimulated synapses were performed by substituting Sr2+ for Ca2+ in the bathing medium (see Results).
Drugs were added to the perfusate. WIN55,212-2, CP55,940 and SR141716-A were generous gifts from Sanofi Recherche (Montpellier, France). Stock solutions of WIN55,212-2, CP55,940 or SR141716-A (10−2 M in dimethylsulphoxide, DMSO) were dissolved in oxygenated Krebs solution at the right concentration (see Results); a sonicator facilitated the dissolving of these DMSO solutions. In pilot experiments (n = 10), we ascertained that the final concentration of DMSO in the bath (0.01-0.02%, depending on experiments) had no detectable effect on bioelectrical or synaptic activities of PCs.
The PF volley was recorded as extracellular field potential with a saline filled electrode placed in the molecular layer 500-800 μm away from the stimulus site. PF stimulation produced a well-characterized extracellular potential (Eccles, Ito & Szentagothai, 1967). To prevent contamination by postsynaptic signals, PF volley experiments were performed in the presence of 20 μm 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; Tocris Cookson) and 10 μm D(-)-2-amino-5-phosphonopentanoic acid (D-AP5, Tocris Cookson).
Recordings using the patch-clamp technique were performed at a somatic level, using an Axopatch 200 amplifier (Axon Instruments). Patch pipettes (2-4 MΩ) were filled with a solution containing (mm): NaCl, 8; potassium gluconate, 70; KCl, 70, or CsCl, 70 (for coefficient of variation experiments); Hepes, 10; ATP-Mg, 2; EGTA, 0.75; pH 7.35 with KOH or CsOH; 300 mosmol l−1. For recordings of quantal events, potassium gluconate and KCl were replaced by 140 mm CsCl and the EGTA concentration was raised to 10 mm. For ionophoresis experiments, 10 mm glutamate was dissolved in standard saline solution (pH 7.5) and delivered in the dendritic field of the recorded PCs through a patch pipette (3-4 MΩ), using negative current pulses.
In the cells retained for the present analysis, access resistance (usually 4-8 MΩ) was partially compensated (50-70%), according to the procedure described by Llano et al. (1991). Cells were held at a membrane potential of -70 mV. As previously reported (Crépel & Jaillard, 1991), parallel fibres were stimulated at 0.33 Hz except during pairing periods (see below), and PF-mediated EPSCs were elicited on 10 mV hyperpolarizing voltage steps that allowed monitoring of the passive electrical properties of the recorded cell throughout the experiment (Llano et al. 1991).
In pairing experiments, after a control period of at least 5 min, two successive pairing protocols separated by 5 min were performed, in an attempt to saturate better LTD. For each of these pairings, the recording mode was changed to current clamp for 1 min, and PF-mediated EPSPs were evoked at 1 Hz during this period, in conjunction with Ca2+ spikes elicited in PCs by both a steady depolarizing current passed through the recording electrode and depolarizing steps timed to coincide with PF-mediated EPSPs. The voltage-clamp mode and the initial frequency of stimulation were resumed just after the end of the pairing protocol.
For paired-pulse facilitation (PPF) experiments (McNaughton, 1982), two successive PF stimulations of the same intensity were applied with an interstimulus interval of 30 ms, and the ratio of the amplitude of the second PF-mediated EPSC over the first one was calculated on-line. As in a previous paper (Blond, Daniel, Jaillard & Crépel, 1997), the contribution of presynaptic factors in the variation of synaptic responses was also established by using the coefficient of variation (CV) method (see references in Kullmann, 1994). CV is given by:
where s is the standard deviation of the amplitude distribution of EPSCs corrected for the background noise, and M is the mean amplitude of EPSCs during the same period. The CV was calculated on sets of fifty EPSCs when the responses were stable over this period. In pilot experiments (n = 6) we ascertained that, as expected (Malinow & Tsien, 1990) and as in a previous study in PCs (Blond et al. 1997), CV as well as PPF increased when PF-mediated EPSCs were inhibited via a presynaptic site of action by 1 μm chloroadenosine (Sigma), but remained unchanged when postsynaptic AMPA receptors where blocked by 0.5 μm CNQX.
For analysis, the synaptic currents in PCs were usually filtered at 5 kHz and digitized on-line at 20 kHz. PF-mediated EPSCs were analysed on-line and off-line by using the Acquis1 computer program (Biologic). For analysis of mEPSCs and of synaptic events evoked in the presence of Sr2+ (see Results), synaptic currents were further filtered at 2 kHz and analysed off-line with Detectivent, Labview-based software developed by N. Ankri (Unité INSERM U 261 and Institut Pasteur, Paris). Briefly, synaptic events were detected on the basis of the slope of their rising phase, and of windows for allowed minimum/maximum times to peak and decay time constants (Ankri, Legendre, Faber & Korn, 1994). These parameters were adjusted so that most visible synaptic events were detected, and, on the contrary, no detection occurred in current traces recorded from the same cells in the presence of 20 μm CNQX added in the bath at the end of the recording session. For each cell, analysis was performed on a few hundred of such selected spontaneous or evoked synaptic events. Although the method of detection did not take into account absolute detection thresholds for mEPSC amplitudes, it is clear from raw and cumulative amplitude distributions of mEPSCs and evoked quantal responses (see Results) that events smaller that 12 pA were probably generally not detected, most of them therefore being likely to be buried in the background noise. Finally, there was no detectable change in the background noise fluctuations of instrumental and cellular origin in the presence of the cannabinoid WIN55,212-2 when compared with the control.
RESULTS
The results of the present study were derived from 129 PCs, retained for analysis on the basis of the stability of their input resistance, holding current (i.e. less than 200 pA at -70 mV) and synaptic responses in control conditions. Stimulation of PFs in the lower third of the molecular layer evoked a fast EPSC (PF-mediated EPSC) (Fig. 1), the characteristics of which have been described previously (Llano et al. 1991). However, decay time constants of synaptic currents for the cells retained for the present study (range, 7.3-10.5 ms) were slightly longer than those obtained by Llano et al. (1991) probably because the PCs in their study were less mature and thus had less extensive dendritic trees.
Figure 1. Effect of WIN55,212-2 on PF-mediated EPSCs.
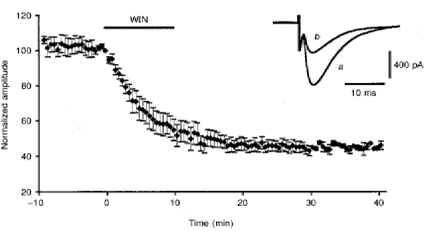
Plot of normalized amplitudes (means ±s.e.m.) of PF-mediated EPSCs against time before, during and after bath application of 1 μm WIN55,212-2 (horizontal filled bar) for 10 min. Insert, superimposed PF-mediated EPSCs recorded in one of these cells before (a) and after (b) the effect of WIN55,212-2.
In all cells tested (n = 14), bath application of 1 μm WIN55,212-2 for 10 min induced a clear decrease in the amplitude of PF-mediated EPSCs that averaged 55.6 ± 13.5% (Fig. 1), and lasted thereafter for the whole duration of the recording session, i.e. usually up to 1 h. Very similar results were obtained with bath application of 400 nm CP55,940, since this compound also induced an irreversible decrease in the amplitude of PF-mediated EPSCs averaging 55.8 ± 19.5% (n = 4). The depressant effect of WIN55,212-2 on PF-mediated EPSCs was induced in only three of eight cells when the concentration of this compound was decreased to as low as 100 nm. Moreover, in these three cells, the decrease in amplitude of PF-mediated EPSCs was about two times smaller than that observed with 1 μm. Therefore, in all the other experiments, WIN55,212-2 was used at a final concentration of 1 or 2 μm in the perfusion medium. Higher concentrations could not be used, because DMSO had deleterious effects on PCs in our experimental conditions at concentrations above 0.02% (corresponding to 2 μm WIN55,212-2). The depressant effect of cannabinoids on PF-mediated EPSCs was never accompanied by any detectable change in passive bioelectrical properties of the cells or of their holding current. Similarly, the kinetics of the PF-mediated EPSCs (Fig. 1) and the presynaptic volley (when the latter was visible in whole-cell recordings) were unchanged. In separate experiments (n = 5), the parallel fibre volley was also recorded as a field potential (see Methods). As shown in Fig. 2, the PF volley was again unaffected by bath application of 2 μm WIN55,212-2 for 20 min.
Figure 2. Lack of effect of WIN55,212-2 on PF volleys recorded as field potentials in the molecular layer.
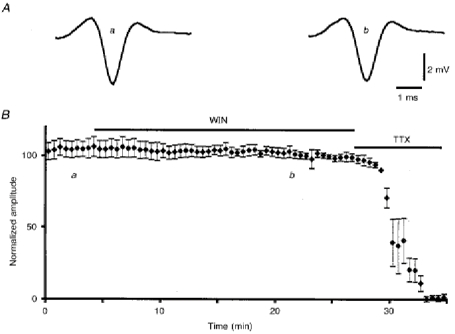
A, examples of 25 averaged PF volleys at the times indicated in B. B, plot of normalized amplitudes (means ±s.e.m.) of PF volleys against time before (a) and during (b) bath application of 2 μm WIN55,212-2, followed by bath application of 1 μm TTX.
Before studying the action of the CB-1 antagonist SR141716-A on the effects of WIN55,212-2 on PF-mediated EPSCs, we ascertained first that bath application of 1 μm SR141716-A alone had no detectable effect on the amplitude and kinetics of PF-mediated EPSCs (n = 9; not illustrated). In a second series of experiments (n = 10), preincubation of the slice for 20 min with the same concentration of SR141716-A or even with a concentration as low as 100 nm (n = 4) totally prevented the depressant effect of bath application of 1 μm WIN55,212-2 (Fig. 3). Furthermore, when WIN55,212-2 (1 μm) was applied for 4-5 min prior to its co-application with 1 μm SR141716-A for the remaining recording period, the latter was able to partially prevent the depressant effect of WIN55,212-2 on the amplitude of PF-mediated EPSCs (n = 9). Indeed, under these conditions, the reduction in amplitude of PF-mediated EPSCs was only 40.3 ± 4.2% against 55.6 ± 3.5% when WIN55,212-2 (1 μm) was applied alone (see before), this difference being significant (Student's t test; P < 0.01) (not illustrated). Finally, bath application of 1 μm SR141716-A did fully reverse the depressant effect of 1 μm WIN55,212-2 when the latter was washed out at the same time as SR141716-A was applied (n = 8; Fig. 4). Therefore, the difficulty of reversing the acute depressant effects of WIN55,212-2 on PF-mediated EPSCs during the recording session (see above) is likely to be due to both the lipophilic nature of this compound and its high affinity for CB-1 receptors. Indeed, both of these properties are likely to render its wash-out very slow, unless dissociation of WIN55,212-2 from CB-1 receptors is speeded up by application of the high affinity antagonist SR141716-A.
Figure 3. Prevention of WIN55,212-2-induced depression of PF-mediated EPSCs by prior application of SR141716-A.
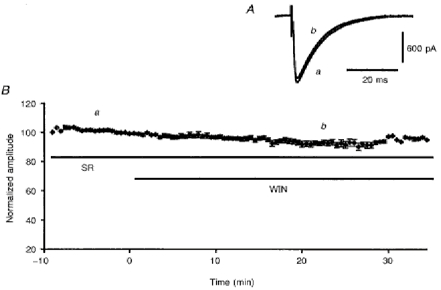
A, superimposed PF-mediated EPSCs recorded in a PC during bath application of 1 μm SR141716-A (a), and 15 min after co-application of 1 μm SR141716-A and 1 μm WIN55,212-2 (b). B, plot of normalized amplitudes (means ±s.e.m.) of PF-mediated EPSCs against time during bath application of 1 μm SR141716-A, followed by bath application of 1 μm SR141716-A and 1 μm WIN55,212-2 (horizontal continuous bars).
Figure 4. Reversal of WIN55,212-2-induced depression of PF-mediated EPSCs by subsequent application of 1 μm SR141716-A.
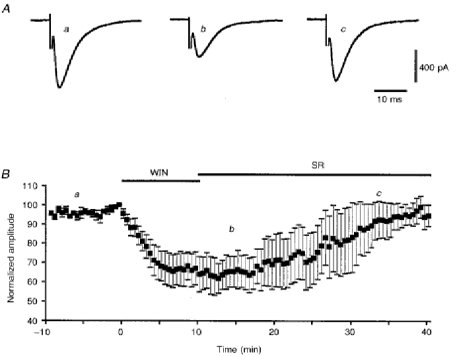
A, averaged PF-mediated EPSCs recorded in one cell at times indicated in B. B, plot of normalized amplitudes (means ±s.e.m.) of PF-mediated EPSCs against time, before (a) and during (b) bath application of 1 μm WIN55,212-2 for 10 min, followed by wash-out of this compound and bath application of 1 μm SR141716-A (c), as indicated by corresponding filled bars.
In order to establish the pre- and/or postsynaptic sites of action of cannabinoids on PF-mediated EPSCs, we compared in three cells the effects of bath application of 1 μm WIN55,212-2 on PF-mediated EPSCs and on inward currents induced in these cells by ionophoretic application of glutamate in their dendritic field. As illustrated in Fig. 5A, PF-mediated EPSCs were again markedly depressed by WIN55,212-2 in these three cells, whereas their responses to glutamate were left unaffected. The latter were abolished by bath application of 5 μm CNQX (not illustrated). This suggests that WIN55,212-2 depressed PF-mediated EPSCs by a presynaptic effect. However, exogenously applied glutamate may act on extrasynaptic receptors as well as on synaptic ones, which weakens the interpretation of such data. We therefore studied this question by additional experiments on PF-mediated EPSCs. In all tested cells (n = 6), PPF of the PF-mediated EPSCs (Fig. 5B) remained stable during the control period (see Methods), and averaged 165.7 ± 13.2% with an interstimulus interval of 30 ms. In these six cells, this PPF markedly increased after bath application of 1 μm WIN55,212-2 for 10 min, to reach a mean value of 220.2 ± 18.2% (Fig. 5). In keeping with these results, which suggest a presynaptic site of action of WIN55,212-2, individual values of CV (see Methods) of the amplitude of PF-mediated EPSCs also markedly increased during the depressant effect of WIN55,212-2 on PF-mediated EPSCs in these six cells as well as in ten other tested cells (Fig. 5). Accordingly, when such individual CV values were averaged for each cell in the control condition and in the presence of WIN55,212-2, and when these averages were then used to deduce the corresponding mean CV values for the sixteen cells tested, the increase in mean CV during WIN55,212-2 application was 94.7 ± 13.4%, i.e. highly significant (Student's t test; P < 0.01). Taken together, these results are consistent with a presynaptic site of action of WIN55,212-2 on PF-mediated EPSCs, although they do not completely exclude an additional postsynaptic effect of this compound (Faber & Korn, 1991).
Figure 5. Effect of WIN55,212-2 on paired-pulse facilitation (PPF) and coefficient of variation (CV) of PF-mediated EPSCs.
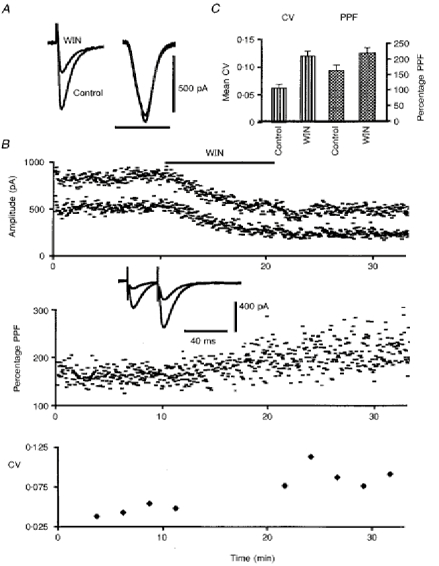
A, superimposed sweeps of PF-mediated EPSCs (left traces) and glutamate-induced inward currents (right traces) elicited in one PC in control conditions and in the presence of 1 μm WIN55,212-2 (WIN) in the bath. The ionophoretic current pulses were 25 nA in amplitude and 400 ms in duration. Note the absence of any effect of WIN55,212-2 on the responses to glutamate application. Horizontal calibration bar, 50 ms for PF-mediated EPSCs and 1 s for glutamate-induced responses. B, plot of the amplitude, PPF and CV of PF- mediated EPSCs in one PC (upper, middle and lower graphs, respectively) against time, before and during bath application of 1 μm WIN55,212-2 for 10 min as indicated by the corresponding filled bar. In the upper graph, amplitudes of the first and second responses have been superimposed. In the middle and lower graphs, PPF and the CV were calculated as indicated in Methods. Insert, superimposed sweeps of PF-mediated EPSCs elicited in this PC by 2 successive PF stimulations with an interstimulus interval of 30 ms in control condition, and in the presence of 1 μm WIN55,212-2 in the bath. C, histogram of mean CV and PPF (means +s.e.m.) in control condition and in the presence of 1 μm WIN55,212-2 in the bath (WIN) for all tested cells (see Results).
Another approach to localize the site of action of WIN55,212-2 consists of examining the evolution of the size and frequency of spontaneous miniature EPSCs (mEPSCs) under the action of this compound compared with these parameters in drug-free medium. As previously described (Barbour, 1993), all PCs recorded in the presence of TTX, bicuculline and picrotoxin in the bath (n = 6; see Methods) exhibited clear-cut mEPSCs (Fig. 6A-C). Their mean cumulative distribution of amplitudes (Fig. 6E) also showed that mEPSCs larger than 40 pA were rare, as already mentioned by Barbour (1993). The glutamatergic nature of these spontaneous events was confirmed by their disappearance when 20 μm CNQX was added to the bathing medium (see Methods). However, the mean detection rate of mEPSCs in the present study (0.26 ± 0.1 Hz) was clearly lower than that previously reported (Barbour, 1993). A larger dendritic filtering of mEPSCs in our recordings than that occurring in the more immature PCs used in Barbour's study, together with the use of different methods for detection of mEPSCs, might well explain part of this discrepancy. In particular, and although our method of detection did not take into account absolute detection thresholds for mEPSC amplitudes (see Methods), it is clear from raw and cumulative amplitude distributions of mEPSCs (see Fig. 6D and E) that events smaller that 12 pA were generally not detected, and were therefore likely to be buried in the background noise. Additionally, differences in the strains of rats and/or in their developmental stage might also contribute to the differences between our results and those of Barbour's study. As illustrated in Fig. 6, bath application of 1 μm WIN55,212-2 did not induced any sizeable change in the time course and in the raw amplitude distribution of mEPSCs in each of these six cells (Fig. 6C and D), and accordingly, their mean cumulative distribution of amplitudes (Fig. 6E) was also left unaffected (n = 6; Kolmogorov-Smirnov test, P < 0.05). In contrast, such an application significantly decreased (n = 6; Student's paired t test; P < 0.05) the mean detection rate of mEPSCs to 0.20 ± 0.1 Hz (Fig. 6B).
Figure 6. Effects of WIN55,212-2 on mEPSCs.
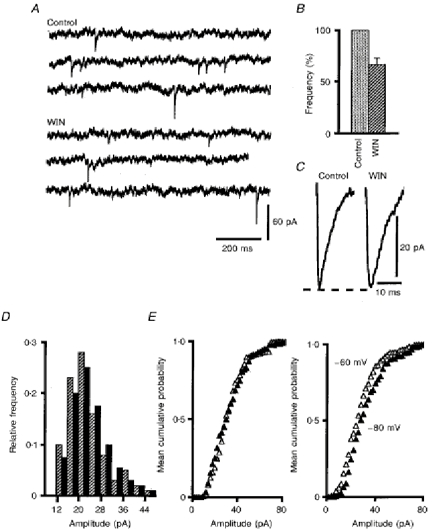
A, example of mEPSCs recorded in one PC under control conditions and in the presence of 1 μm WIN55,212-2 (WIN) in the bath. B, histograms of mean detection rates of mEPSCs (+s.e.m.) in these two solutions; histograms are normalized relative to the mean detection rate in control solution. C, averaged representative mEPSCs (n = 35) from the same cell as in A in standard solution (Control) and in the same medium containing 1 μm of WIN55,212-2 (WIN). D, superimposed histograms of the amplitude distributions of mEPSCs for the cell illustrated in A and C, under control conditions (black bars) and in the presence of 1 μm WIN55,212-2 (hatched bars) in the bath. E, left graph, cumulative mean amplitude distributions of mEPSCs in control solution (▵) and in the presence of 1 μm WIN55,212-2 (▴). E, right graph, cumulative mean amplitude distributions of mEPSCs recorded in control solution at -60 mV (▵) and at -80 mV (▴).
A change in the size of mEPSCs is classically interpreted as a postsynaptic modification, whereas a change in their frequency points towards a presynaptic phenomenon. Before drawing any conclusion from these results, we first ascertained that our recording conditions and analysing methods were sensitive enough to detect moderate changes in amplitude of mEPSCs. For this purpose, mEPSCs were recorded in three PCs successively held at -60 and -80 mV. The significant (Kolmogorov-Smirnov test, P < 0.05) voltage-dependent shift of the mean cumulative distributions of amplitudes of mEPSCs for these three cells clearly validates our procedure (Fig. 6E). We then used another method, based upon substitution of Sr2+ for Ca2+, to confirm the presynaptic site of action of WIN55,212-2 on PF-mediated EPSCs. This was also done because some mEPSCs in the previous experiments may have come from climbing fibre synapses, in addition to those coming from PF-PC synapses. When Sr2+ is substituted for Ca2+ in the bathing medium, the stimulation-evoked synchronous release of transmitter is reduced, but asynchronous release of quanta is markedly and selectively enhanced, allowing detailed analysis of evoked quantal events (Miledi, 1966; see also references in Oliet, Malenka & Nicoll, 1996). In the seven cells tested, this treatment led to a marked decrease in the amplitude of PF-mediated EPSCs (Fig. 7A), and to the appearance of numerous asynchronous quantal events which lasted for a few hundred milliseconds after the stimulus. Figure 7B illustrates such asynchronous events recorded from one of these seven cells. This figure also illustrates the fact that, as in hippocampal neurones (Oliet et al. 1996), the kinetics and the mean cumulative distribution of amplitudes of these events were very similar to those of mEPSCs (Fig. 7C). The mean detection rate of these evoked quanta during the 400 ms following the stimulus was 1 ± 0.25 Hz. As for mEPSCs, bath application of 1 μm WIN55,212-2 did not change their raw amplitude distribution in any of the seven cells tested (Fig. 7D) and, accordingly, their mean cumulative distribution of amplitudes (Fig. 7E) was also left unaffected (n = 7; Kolmogorov-Smirnov test, P < 0.05). In contrast, here again such an application significantly decreased (n = 7; Student's paired t test; P < 0.05) the mean detection rate to 0.46 ± 0.08 Hz (Fig. 7F). These results strongly suggest that the site of action of WIN55,212-2 on PF-mediated EPSCs is entirely presynaptic (see Discussion).
Figure 7. Effects of WIN55,212-2 on Sr2+-induced asynchronous EPSCs.
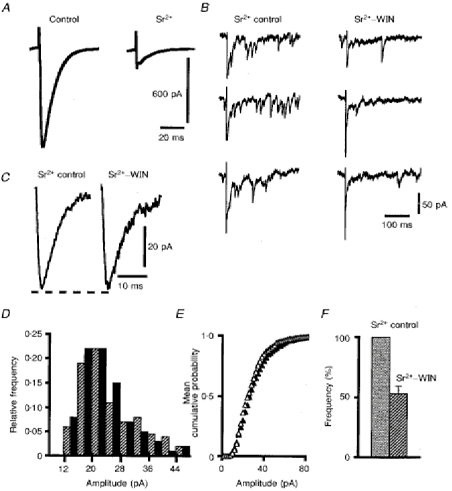
A, averaged PF-mediated EPSCs (n = 50) recorded in one PC in standard solution (control) and after Sr2+ was substituted for Ca2+. B, examples of evoked asynchronous events recorded in this PC in control solution (Sr2+ control) and in the same medium containing 1 μm of WIN55,212-2 (Sr2+-WIN). Note the reduction of the amplitude of the residual stimulation-evoked synchronous EPSC under the action of WIN55,212-2. C, averaged representative Sr2+-induced asynchronous EPSCs in control solution (Sr2+ control) and in the same medium containing 1 μm of WIN55,212-2 (Sr2+-WIN). D, superimposed histograms of the amplitude distributions of Sr2+-induced asynchronous EPSCs for the cell illustrated in A-C, under control conditions (black bars) and in the presence of 1 μm WIN55,212-2 (hatched bars) in the bath. E, cumulative mean amplitude distributions of asynchronous events in control solution (▵) and in the presence of 1 μm WIN55,212-2 (▴). F, histogram of mean detection rates (+s.e.m.) of asynchronous events in these two solutions; the histogram is normalized relative to the mean detection rate in the control solution.
Finally, in control experiments (9 cells), the pairing protocols of PF-mediated EPSCs with Ca2+ spikes (see Methods) induced a clear-cut LTD of PF-mediated EPSCs in all cells tested, with a mean reduction in the amplitude of synaptic responses of 41.4 ± 4.6% (Fig. 8). As previously described (see references in Crépel et al. 1996), no change in the kinetics of PF-mediated EPSCs was observed during expression of LTD (Fig. 8A). When the same pairing protocols were performed in the presence of 1 μm SR141716-A in the bath, a LTD as large as that seen in control conditions was still induced since the mean decrease in amplitude of PF-mediated EPSCs was 41.3 ± 3.9% (n = 9; Fig. 8B). In marked contrast, when WIN55,212-2 (1 μm) was added to the perfusion medium 10-20 min prior to the pairing protocols, LTD was partly impaired, since the mean decrease of PF-mediated EPSCs was only 23.7 ± 4.7% (n = 9) against 41.4 ± 4.6%, this difference being significant (Student's t test; P < 0.05; Fig. 8). This impairment of LTD by WIN55,212-2 was reversed by 1 μm SR141716-A when this drug was co-applied in the bath with WIN55,212-2, since, in this case, the mean decrease in amplitude of PF-mediated EPSCs during LTD (34.2 ± 2.1%; n = 9) was no longer significantly different from the control value (Student's t test; P < 0.05), and was significantly different (Student's t test; P < 0.05) from that obtained with bath application of WIN55,212-2 alone (Fig. 8B).
Figure 8. Effect of WIN55,212-2, SR141716-A and 2-chloroadenosine on LTD of PF-mediated EPSCs.
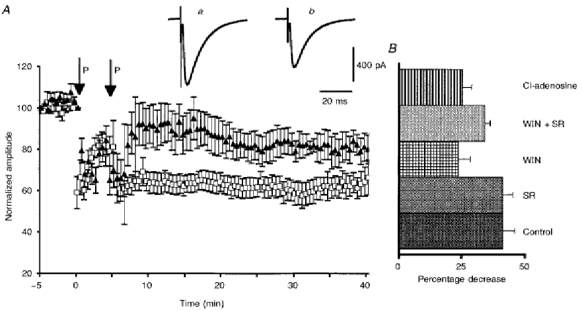
A, plots of normalized amplitudes (means ±s.e.m.) of PF-mediated ESPCs against time before and after 2 successive pairing protocols (P; see Methods), under control conditions (□), and in the presence of 1 μm WIN55,212-2 in the bath (▴). Inserts show EPSCs recorded in a PC under control conditions (a) and during expression of LTD (b). B, histogram of the mean decrease in amplitude of PF-mediated EPSCs during expression of LTD induced under control conditions, or in the presence of WIN55,212-2 (WIN), SR141716-A (SR), Win + SR, or 2-chloroadenosine (Cl-adenosine).
The present data suggest that, via a presynaptic effect on PF terminals, cannabinoids are able to impair LTD, the induction and expression of which are generally considered as postsynaptic (see Discussion). We further tested this hypothesis in another set of experiments by replacing WIN55,212-2 by 2-chloroadenosine, another compound that depresses synaptic transmission at PF-PC synapses (Kocsis, Eng & Bhisitkul, 1984) by decreasing the probability of release of glutamate (Blond et al. 1997). When 1 μm 2-chloroadenosine was added to the perfusion medium 10 min prior to the pairing protocols, i.e. a concentration which reduces the amplitude of PF-mediated EPSCs by about the same as 1 μm WIN55,212-2, LTD was again impaired, since the mean decrease of PF-mediated EPSCs was only 25.3 ± 3.7% (n = 6) against 41.4 ± 4.6% in control, this difference being significant at P < 0.05 (Student's t test; Fig. 8B). Therefore, these data show that 2-chloroadenosine is as potent as WIN55,212-2 in reducing cerebellar LTD.
DISCUSSION
The results of the present study establish for the first time that the cannabinoid receptor agonist WIN55,212-2 inhibits glutamatergic transmission at PF-PC synapses via a presynaptic action, and impairs LTD induction in PCs. The fact that CP55,940, another specific CB-1 receptor agonist (Devane et al. 1988) fully reproduces the depressant effect of WIN55,212-2 on PF-mediated EPSCs, and that the specific cannabinoid receptor antagonist SR141716-A antagonizes the effects of WIN55,212-2 on PF-mediated EPSCs and on LTD, confirms that these effects are mediated through the activation of CB-1 receptors. Before discussing further the present results, it is worth mentioning that we used synthetic CB-1 receptor agonists rather than the putative endogenous agonist(s) as its (their) identification requires clarification (Stella et al. 1997).
Concerning the acute effects of cannabinoid agonists on excitatory synaptic responses, our results agree well with recently published data on the effects of cannabinoids on glutamatergic transmission in hippocampal cultures (Shen et al. 1996), with the exception, however, of the higher concentrations of WIN55,212-2 required to obtain consistent effects of this compound on PF-mediated EPSCs in the present study. The lipophilic nature of WIN55,212-2 and SR141716-A might well be responsible for this difference, as it renders their access to synapses more difficult in acute slices than in cultures, and thus probably decreases the actual concentration of these compounds in the synaptic cleft. This is certainly even more true for PF-PC synapses, which are isolated from outer extracellular spaces by a diffusion barrier (Barbour, Keller, Llano & Marty, 1994). Otherwise, our results are entirely consistent with those of Shen et al. (1996). In particular, the absence of effect of WIN55,212-2 on responses of PCs to ionophoretic application of glutamate, together with the increase in the CV and PPF during the depressant effect of WIN55,212-2 on PF-mediated EPSCs, suggests a presynaptic location of CB-1 receptors on PFs. This fits well with the studies that show the presence of messenger RNA coding for CB-1 receptors in the cerebellar granular layer (see references in Matsuda et al. 1993) and with the presence of the CB-1 receptor in the molecular layer as revealed by receptor binding autoradiography (Herkenham et al. 1991). However, interpretation of the CV is not unambiguous (Faber et al. 1991; see also Blond et al. 1997), and interpretation of PPF experiments is complicated by the existence of the so-called supranormal period of PFs (Gardner-Medwin, 1972) which, by itself, can contribute to the increase of second PF-mediated EPSCs in PPF experiments.
The notion of a presynaptic site for mediating the effects of cannabinoid agonists on PF-mediated EPSCs is also supported by the observations that WIN55,212-2 markedly decreases the mean detection rate of mEPSCs and of asynchronous synaptic events without affecting their amplitude. Interestingly, the fact that the mean frequency of mEPSCs is less affected by WIN55,212-2 than the mean frequency of Sr2+-evoked EPSCs (see Results) suggests that cannabinoids might have a more potent inhibitory effect on the presynaptic voltage-gated calcium channels involved in release of evoked synaptic events than on the mechanisms involved in mEPSC generation. This latter interpretation is supported by recent observations showing that, at synapses of the spinal dorsal horn, factors modifying calcium channel function differentially affect evoked and spontaneous EPSCs (Bao, Li & Perl, 1997). It also fits well with results obtained in expression systems, which demonstrate an inhibition of voltage-gated calcium channels by cannabinoid agonists (see references in Mackie et al. 1995; Childers et al. 1996).
If WIN55,212-2 primarily acts on presynaptic calcium channels involved in glutamate release at PF-PC synapses, the probability of release at PF terminals should be reduced, whereas the number of release sites should be unaffected. On the other hand, if WIN55,212-2 decreases the excitability of PFs, the number of release sites should be reduced without affecting their probability of release. The fact that WIN55,212-2 does not change the PF volley strongly argues against the second possibility. In contrast, this absence of effect does not contradict the postulated inhibition of presynaptic calcium channels by WIN55,212-2, since blockade of these channels does not significantly affect PF excitability (Mintz, Sabatini & Regehr, 1995).
Recently, Stella et al. (1997) described the properties of sn-2-arachidonylglycerol (2-AG), a putative endogenous cannabinoid. Surprisingly enough, this compound strongly inhibits LTP in the hippocampus, but has no detectable acute effect on excitatory synaptic transmission, in marked contrast with all other cannabinoids tested so far. One possible explanation might rely on the lower affinity of 2-AG for CB-1 as compared with WIN55,212-2 and CP55,940, if the threshold concentration for effects of cannabinoids on synaptic plasticity happens to be lower than that for acute effects on synaptic transmission.
To summarize, the most likely interpretation of the present results is that cannabinoids depress synaptic transmission at PF-PC synapses by reducing the probability of release of glutamate. Moreover, this effect is likely to result from an inhibition of presynaptic calcium channels.
The impairment of LTD by WIN55,212-2 is in keeping with inhibition by cannabinoid agonists of another type of synaptic plasticity, LTP in the hippocampus (Nowicky et al. 1987; Terranova et al. 1995; Stella et al. 1997). One interpretation might be that, as in the recent experiments by Stella et al. (1997), WIN55,212-2 impairs LTD by a mechanism independent of those responsible for the acute effects of this compound on synaptic transmission. However, another possibility deserves to be mentioned, which explains our results without any need for new and still unknown mechanisms. In the cerebellum, all available evidence points towards a postsynaptic site of induction and expression of LTD (Ito, 1993; Linden, 1994; Hémart, Daniel, Jaillard & Crépel, 1995), whereas the results of the present study indicate that WIN55,212-2 acts at a presynaptic level. One interpretation might be that, by decreasing the release of glutamate at presynaptic PF terminals, WIN55,212-2 leads to a lower activation of AMPA and metabotropic postsynaptic receptors at individual PF-PC synapses. This, in turn, is likely to inhibit LTD induction since this change in synaptic strength is known to require activation of these receptors (Linden, Dickinson, Smeyne & Connor, 1991; Aiba et al. 1994; Conquet et al. 1994; Shigemoto, Abe, Nomura, Nakanishi & Hirano, 1994; Hémart et al. 1995). If this were the case, any other compound reducing the probability of glutamate release at PF-PC synapses should have the same inhibiting effect on LTD. Indeed, 2-chloroadenosine was also potent in impairing LTD, thus reinforcing the above interpretation.
The partial inhibition of LTD by WIN55,212-2 might also rely on the involvement of NO in LTD (Crépel & Jaillard, 1990; Ito & Karachot, 1990; Shibuki & Okada, 1991; Crépel et al. 1994) since, as suggested by recent work in our laboratory (Crépel et al. 1994), NO is likely to be produced by depolarization of presynaptic elements during LTD induction. Therefore, activation of presynaptic CB-1 might decrease this depolarization-induced NO production by reducing Ca2+ entry through presynaptic voltage-gated Ca2+ channels, and thus lead to an attenuated LTD. This hypothesis is supported by Hillard, Peltier & Muthian (1995) who report that the activation of cannabinoid receptors in cerebellar granule cells decreases KCl-stimulated NOS activity.
Finally, because synthesis of the two known potential endogenous cannabinoids, anandamide and 2-AG, is calcium dependent, it could occur in PCs during the induction of LTD. Moreover, in their recent paper, Stella et al. (1997) show that synthesis of the potential endogenous cannabinoid 2-AG occurs through the hydrolysis of diacylglycerol (DAG) and also depends upon phospholipase C activity, an enzyme activated during LTD induction (Crépel et al. 1996). Thus, endogenous cannabinoids might be produced by PCs during induction of LTD and then modulate this plasticity by a presynaptic effect. However, the absence of an effect of SR141716-A on induction of LTD in the present study suggests that, in our experimental conditions, the increase in cytosolic calcium and DAG concentration in PCs during the pairing protocol leading to LTD does not promote production and release of endogenous cannabinoids from these neurones. Whatever the way and the conditions of production of cannabinoids at the PF-PC synapses, the present study establishes for the first time that cannabinoids inhibit glutamatergic transmission at PF-PC synapses by a presynaptic action, and thereby impair LTD induction in PCs. These two effects represent a plausible cellular mechanism underlying cerebellar dysfunction caused by cannabinoids.
Acknowledgments
This work was supported by Sanofi Recherche. We thank Dr Pascal Legendre for helpful discussion during experiments on quantal analysis and Dr Boris Barbour for helpful comments on the manuscript. We also thank Gérard Sadoc for the Acquis1 software and Norbert Ankri for the Detectivent software used in this study.
References
- Abood ME, Martin BR. Neurobiology of marijuana abuse. Trends in Pharmacological Sciences. 1992;13:201–206. doi: 10.1016/0165-6147(92)90064-d. 10.1016/0165-6147(92)90064-D. [DOI] [PubMed] [Google Scholar]
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- Ankri N, Legendre P, Faber D, Korn H. Automatic detection of spontaneous synaptic responses in central neurons. Journal of Neuroscience Methods. 1994;52:87–100. doi: 10.1016/0165-0270(94)90060-4. 10.1016/0165-0270(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Bao J, Li J, Perl E. Different presynaptic calcium channels influence evoked EPSCs and spontaneous, miniature EPSCs in rodent spinal lamina I and II neurons. The Journal of Physiology. 1997;504.P:175P. [Google Scholar]
- Barbour B. Synaptic currents evoked in Purkinje cells by stimulating individual granule cells. Neuron. 1993;11:759–769. doi: 10.1016/0896-6273(93)90085-6. [DOI] [PubMed] [Google Scholar]
- Barbour B, Keller BU, Llano I, Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994;12:1331–1343. doi: 10.1016/0896-6273(94)90448-0. [DOI] [PubMed] [Google Scholar]
- Bidaut-Russell M, Devane W, Howlett A. Cannabinoid receptors and modulation of cyclic AMP accumulation in the rat brain. Journal of Neurochemistry. 1990;55:21–26. doi: 10.1111/j.1471-4159.1990.tb08815.x. [DOI] [PubMed] [Google Scholar]
- Blond O, Daniel H, Jaillard D, Crépel F. Pre and postsynaptic effects of nitric oxide at synapses between parallel fibers and Purkinje cells; involvement in cerebellar long-term depression. Neuroscience. 1997;77:945–954. doi: 10.1016/s0306-4522(96)00524-6. [DOI] [PubMed] [Google Scholar]
- Childers S, Deadwyler S. Role of cyclic AMP in the actions of cannabinoid receptors. Biochemical Pharmacology. 1996;52:819–827. doi: 10.1016/0006-2952(96)00419-4. [DOI] [PubMed] [Google Scholar]
- Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Condé F, Collingridge GL, Crépel F. Motor deficit and impairment of synaptic plastivity in mice lacking mGluR1. Nature. 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- Crépel F, Audinat E, Daniel H, Hemart N, Jaillard D, Rossier J, Lambolez B. Cellular locus of the nitric oxide-synthase involved in cerebellar long-term depression induced by high external potassium concentration. Neuropharmacology. 1994;33:1399–1405. doi: 10.1016/0028-3908(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Crépel F, Hemart N, Jaillard D, Daniel H. Cellular mechanisms of long-term depression in the cerebellum. Behavioral and Brain Sciences. 1996;19:347–353. [Google Scholar]
- Crépel F, Jaillard D. Protein kinases, nitric oxide and long-term depression of synapses in the cerebellum. NeuroReport. 1990;1:133–136. doi: 10.1097/00001756-199010000-00013. [DOI] [PubMed] [Google Scholar]
- Crépel F, Jaillard D. Pairing of pre- and postsynaptic activities in cerebellar Purkinje cells induces long-term changes in synaptic efficacy in vitro. The Journal of Physiology. 1991;432:123–141. doi: 10.1113/jphysiol.1991.sp018380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler S, Hampson R, Bennett B, Edwards T, Mu J, Pacheco M, Ward S, Childers S. Cannabinoids modulate potassium current in cultured hippocampal neurons. Receptors Channels. 1993;1:121–134. [PubMed] [Google Scholar]
- Devane W, Dysarz F, Johnson M, Melvin L, Howlett A. Determination and characterisation of a cannabinoid receptor in rat brain. Molecular Pharmacology. 1988;34:605–613. [PubMed] [Google Scholar]
- Eccles JC, Ito M, Szentagothai J. The Cerebellum as a Neuronal Machine. Berlin: Springer-Verlag; 1967. [Google Scholar]
- Faber DS, Korn H. Applicability of coefficient of variation method for analyzing synaptic plasticity. Biophysical Journal. 1991;60:1288–1294. doi: 10.1016/S0006-3495(91)82162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner-Medwin AR. An extreme supernormal period in cerebellar parallel fibres. The Journal of Physiology. 1972;222:357–371. doi: 10.1113/jphysiol.1972.sp009802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hémart N, Daniel H, Jaillard D, Crépel F. Receptors and second messengers involved in long-term depression in rat cerebellar slices in vitro: a reappraisal. European Journal of Pharmacology. 1995;7:45–53. doi: 10.1111/j.1460-9568.1995.tb01019.x. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn A, Jonhson M, Melvin L, de Costa B, Rice K. Characterisation and localisation of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. Journal of Neuroscience. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Peltier B, Muthian S. Activation of the cannabinoid receptor of cerebellar granule cells decreases KCl stimulated nitric oxide synthase activity. FASEB Journal. 1995;9:A403. [Google Scholar]
- Ito M. Synaptic plasticity in the cerebellar cortex and its role in motor learning. Canadian Journal of the Neurological Sciences. 1993;20:S70–74. [PubMed] [Google Scholar]
- Ito M, Karachot L. Messengers mediating long-term desensitization in cerebellar Purkinje cells. NeuroReport. 1990;1:129–132. doi: 10.1097/00001756-199010000-00012. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Eng DL, Bhisitkul RB. Adenosine selectively blocks parallel fiber-mediated synaptic potentials in rat cerebellar cortex. Proceedings of the National Academy of Sciences of the USA. 1984;81:6531–6534. doi: 10.1073/pnas.81.20.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM. Amplitude fluctuations of dual-component EPSCs in hippocampal pyramidal cells: implications for long-term potentiation. Neuron. 1994;12:1111–1120. doi: 10.1016/0896-6273(94)90318-2. 10.1016/0896-6273(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Linden DJ. Input-specific induction of cerebellar long-term depression does not require presynaptic alteration. Learning and Memory. 1994;1:121–128. [PubMed] [Google Scholar]
- Linden DJ, Dickinson MH, Smeyne M, Connor JA. A long-term depression of AMPA currents in cultured cerebellar Purkinje neurons. Neuron. 1991;7:81–89. doi: 10.1016/0896-6273(91)90076-c. 10.1016/0896-6273(91)90076-C. [DOI] [PubMed] [Google Scholar]
- Llano I, Marty A, Armstrong CM, Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. The Journal of Physiology. 1991;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Lai Y, Wenstenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in At20 cells transfected with rat brain cannabinoid receptor. Journal of Neuroscience. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton B. Long-term synaptic enhancement and short-term potentiation in rat fascia dentata act through different mechanisms. The Journal of Physiology. 1982;324:249–262. doi: 10.1113/jphysiol.1982.sp014110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Tsien R. Presynaptic changes revealed by whole cell recordings of long-term potentiation in rat hippocampal slices. Nature. 1990;346:177–180. doi: 10.1038/346177a0. 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- Matsuda L, Bonner T, Lolait S. Localisation of cannabinoid receptor mRNA in rat brain. Journal of Comparative Neurology. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- Miledi R. Strontium as a substitute for calcium in the process of transmitter release at the neuromuscular junction. Nature. 1966;212:1233–1234. doi: 10.1038/2121233a0. [DOI] [PubMed] [Google Scholar]
- Mintz I, Sabatini B, Regehr W. Calcium control of transmitter release at a cerebellar synapse. Neuron. 1995;15:675–688. doi: 10.1016/0896-6273(95)90155-8. 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- Nowicky A, Teyler T, Vardaris R. The modulation of long term potentiation by Δ-9-tetrahydrocannabinol in the rat hippocampus, in vitro. Brain Research Bulletin. 1987;19:663. doi: 10.1016/0361-9230(87)90052-9. 10.1016/0361-9230(87)90052-9. [DOI] [PubMed] [Google Scholar]
- Oliet S, Malenka R, Nicoll R. Bidirectional control of quantal size by synaptic activity in the hippocampus. Science. 1996;271:1294–1297. doi: 10.1126/science.271.5253.1294. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Clandra B, Congy C, Martinez S, Maruani J, Néliat G, Caput D, Ferrara P, Soubrié P, Brelière JC, Le Fur G. SR 141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Letters. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. 10.1016/0014-5793(94)00773-X. [DOI] [PubMed] [Google Scholar]
- Shen M, Piser T, Seybold V, Thayer S. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. Journal of Neuroscience. 1996;16:4322–4334. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuki K, Okada D. Endogenous nitric-oxide release required for long-term synaptic depression in the cerebellum. Nature. 1991;349:326–328. doi: 10.1038/349326a0. 10.1038/349326a0. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Abe T, Nomura S, Nakanishi S, Hirano T. Antibodies inactivating mGluR1 metabotropic glutamate receptor block long-term depression in cultured Purkinje cells. Neuron. 1994;12:1245–1255. doi: 10.1016/0896-6273(94)90441-3. 10.1016/0896-6273(94)90441-3. [DOI] [PubMed] [Google Scholar]
- Stark P, Dews P. Cannabinoids. I. Behavioural effects. Journal of Pharmacology and Experimental Therapeutics. 1980;214:124–130. [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Terranova J, Michaud J, Le Fur G, Soubrié P. Inhibition of long-term potentiation in rat hippocampal slices by anandamide and WIN55212–2: reversal by SR141716 A, a selective antagonist of CB1 cannabinoid receptors. Naunyn-Schmiedeberg's Archives of Pharmacology. 1995;352:576–579. doi: 10.1007/BF00169393. [DOI] [PubMed] [Google Scholar]


