Abstract
The effects of changes in extra- and intracellular pH (pHo and pHi, respectively) on depolarization-evoked rises in intracellular free Ca2+ concentration ([Ca2+]i) and the activity of a Ca2+-dependent K+ channel were investigated in cultured fetal rat hippocampal neurones.
In neurones loaded with 2′,7′-bis-(2-carboxyethyl)-5-(and -6)-carboxyfluorescein (BCECF), changes in pHo evoked changes in pHi. At room temperature, the ratio ΔpHi : ΔpHo (the slope of the regression line relating pHi to pHo) was 0·37 under HCO3−/CO2-buffered conditions and 0·45 under Hepes-buffered conditions; corresponding values at 37 °C were 0·71 and 0·79, respectively. The measurements of changes in pHi evoked by changes in pHo were employed in subsequent experiments to correct for the effects of changes in pHi on the Kd of fura-2 for Ca2+.
In fura-2-loaded neurones, rises in [Ca2+]i evoked by transient exposure to 50 mM K+ were reduced and enhanced during perfusion with acidic and alkaline media, respectively, compared with control responses at pHo 7·3. Fifty percent inhibition of high-[K+]o-evoked rises in [Ca2+]i corresponded to pHo 7·23. In the presence of 10 μM nifedipine, 50 % inhibition of high-[K+]o-evoked responses corresponded to pHo 7·20, compared with a pHo of 7·31 for 50 % inhibition of [Ca2+]i transients evoked by N-methyl-D-aspartate.
Changes in pHi at a constant pHo were evoked by exposing neurones to weak acids or bases and quantified in BCECF-loaded cells. Following pH-dependent corrections for the Kd of fura-2 for Ca2+, rises in [Ca2+]i evoked by high-[K+]o in fura-2-loaded cells were found to be affected only marginally by changes in pHi. When changes in pHi similar to those observed during the application of weak acids or bases were elicited by changing pHo, reductions in pH inhibited rises in [Ca2+]i evoked by 50 mM K+ whereas increases in pH enhanced them.
The effects of changes in pH on the kinetic properties of a BK-type Ca2+-dependent K+ channel were investigated. In inside-out patches excised from neurones in sister cultures to those used in the microspectrofluorimetric studies, with internal [Ca2+] at 20 μM, channel openings at an internal pH of 6·7 were generally absent whereas at pH 7·3 (or 7·8) the open probability was high. In contrast, channel activity in outside-out patches was not affected by reducing the pH of the bath (external) solution from 7·3 to 6·7. In inside-out patches with internal [Ca2+] at 0·7 μM, a separate protocol was applied to generate transient activation of the channel at a potential of 0 mV following a step from a holding level of -80 mV. In this case open probabilities were 0·81 (at pH 7·8), 0·57 (pH 7·3), 0·19 (pH 7·0) and 0·04 (pH 6·7). Channel conductance was not affected by changes in internal pH.
The results indicate that, in fetal rat hippocampal neurones, depolarization-evoked rises in [Ca2+]i mediated by the influx of Ca2+ ions through dihydropyridine-sensitive and -resistant voltage-activated Ca2+ channels are modulated by changes in pHo. The effects of pHo cannot be accounted for by changes in pHi consequent upon changes in pHo. However, changes in pHi affect the unitary properties of a Ca2+-dependent K+ channel. The results support the notion that pHo and/or pHi transients may serve a modulatory role in neuronal function.
The maintenance of acid-base balance within narrow limits is essential for normal cerebral function. It has often been assumed that the acid-base status of brain tissue remains stable under a wide variety of conditions and that, as a result, changes in pH are of little regulatory significance. Contemporary studies, however, have revealed that marked extra- and intracellular pH (pHo and pHi, respectively) shifts occur not only during pathophysiological events such as seizure activity and cerebral ischaemia but also during normal synaptic transmission (reviewed by Chesler & Kaila, 1992; Kaila, 1994). Furthermore, activity-evoked shifts in both pHo and pHi might act to modulate the events which initially caused them. For example, the extracellular alkaline transients evoked in hippocampal slices by the activation of glutamate or γ-aminobutyric acidA (GABAA) receptors can modulate synaptic transmission and augment N-methyl-D-aspartate (NMDA) receptor-mediated responses (Voipio et al. 1995). Conversely mild extracellular acidosis, which may occur during cerebral ischaemia and convulsive activity, has been suggested to be a factor in limiting the extent of neuronal death following ischaemia and in the self-termination of seizures (see Tombaugh & Sapolsky, 1993 and references therein).
The sensitivities of certain ligand-gated ion channels present in mammalian central neurones, notably the NMDA and GABAA receptor-operated channels, to changes in both pHo and pHi have been established and, indeed, the effects of changes in pH on epileptiform and neurodegenerative phenomena have been interpreted largely in terms of modulation of the activities of these ligand-gated receptor-channel complexes (see Kaila, 1994; McBain & Mayer, 1994). However, modulation of voltage-activated conductances by changes in pHo and/or pHi may also play a role. Thus, in rat CA1 hippocampal pyramidal neurones, changes in pHo affect the magnitude of both Ca2+-dependent depolarizing potentials and hyperpolarizing potentials mediated by Ca2+-activated K+ conductances (gK(Ca)) (Church & McLennan, 1989; Church, 1992) and modulate the activity of voltage-activated Ca2+ channels (Tombaugh & Somjen, 1996). However, in many cell types, changes in pHo evoke changes in pHi and it remains unclear whether changes in pHi consequent upon changes in pHo contribute to the known effects of changes in pHo on neuronal excitability. For example, although reduced Ca2+ influx under low pHo conditions could account for the inhibition of potentials mediated by gK(Ca) (Church & McLennan, 1989), a role for changes in pHi either on voltage-activated Ca2+ channels themselves (Church, 1992; Tombaugh & Somjen, 1997) and/or on Ca2+-activated K+ channels cannot be ruled out. In the present study we have examined the effects of changes in pHo and pHi on high-[K+]o-evoked rises in [Ca2+]i and on the activity of a Ca2+-dependent K+ channel in cultured fetal rat hippocampal neurones, an experimental preparation in which changes in pHi can be reliably evoked and quantified.
METHODS
Cell preparation
Primary cultures of fetal rat hippocampal neurones were prepared as described (Baxter & Church, 1996). In brief, 18-day pregnant Wistar rats were anaesthetized with pentobarbitone (50 mg kg−1i.p.) and, following removal of the fetuses, were killed with an intracardiac injection of pentobarbitone. Fetuses were decapitated, hippocampi were removed and, following enzymatic and mechanical dissociation, cells were plated onto glass coverslips coated with poly-D-lysine at a density of 1 × 105 cells cm−2. Cultures were treated with 5-fluorodeoxyuridine to arrest glial cell proliferation and were maintained in a 5 % CO2 atmosphere at 36°C in serum-free, N2-supplemented Dulbecco's modified Eagle's medium (Life Technologies, Burlington, ON, Canada) containing 22 mM NaHCO3. Neurones were used 8-17 days after plating.
Solutions and chemicals
The standard HCO3−/CO2-free perfusion medium contained (mM): NaCl, 136.5; KCl, 3; CaCl2, 2; NaH2PO4, 1.5; MgSO4, 1.5; D-glucose, 10; and Hepes, 10. It was saturated with 100 % air and titrated to the appropriate temperature-corrected pH with 10 M NaOH. In standard HCO3−/CO2-containing media, Hepes was isosmotically replaced by NaCl and solutions contained either 26 mM (room temperature, 20-22°C) or 17.5 mM (37°C) NaHCO3, by equimolar substitution for NaCl, together with the constituents listed above; pH was 7.3 after equilibration with 95 % air-5 % CO2. Low pH HCO3−/CO2-buffered solutions contained either 7 or 10.5 mM NaHCO3 (pH 6.9 after equilibration with 5 % CO2 at 37°C and at room temperature, respectively); high pH solutions contained either 42.5 or 62.5 mM NaHCO3 (pH 7.7 at 37°C and at room temperature, respectively). Additional HCO3−/CO2-buffered media for intermediate pH values were formulated according to the empirically derived equations pH = 6.02 + (1.04 × log[HCO3−]) (37°C) and pH = 5.83 + (1.04 × log[HCO3−]) (room temperature); in all cases, changes in [NaHCO3] were balanced by equimolar changes in [NaCl]. During perfusion with HCO3−-containing media, the atmosphere in the recording chamber contained 95 % air with 5 % CO2. The pH of each solution was re-checked at the appropriate temperature following every experiment.
When external Cl− was reduced at a constant pHo, the corresponding methylsulphate salts were substituted. Solutions containing the weak acids sodium propionate (20 or 40 mM, as indicated in the text) or sodium butyrate (20 mM), or the weak base trimethylamine chloride (TMA, 10 mM), were prepared by equimolar substitution for NaCl. For Ca2+-free media, Ca2+ was omitted, [Mg2+] was increased to 4 mM and 100 μM EGTA was added. High-[K+] media contained 50 mM KCl (by substitution for NaCl). In experiments employing NMDA, Mg2+ was omitted and 2 μM glycine added. Nifedipine-containing solutions were handled in the manner previously described (Church et al. 1994). Perfusion lines were replaced following each experiment.
Compounds were obtained from Sigma Chemical Co. with the exceptions of 2-amino-5-phosphonopentanoic acid (AP5) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), which were from Research Biochemicals International, and fura-2 AM and 2′,7′-bis-(2-carboxyethyl)-5-(and -6)-carboxyfluorescein acetoxymethyl ester (BCECF AM), which were from Molecular Probes, Inc.
Recording techniques
Microspectrofluorimetric studies
Fura-2 and BCECF were employed to estimate [Ca2+]i and pHi, respectively. In studies where information was required on the effects of an experimental manoeuvre on both [Ca2+]i and pHi, measurements of [Ca2+]i and pHi were performed separately in parallel experiments conducted on sister cultures. Loading medium contained the same elements as the standard pH 7.3 Hepes-buffered solution (see above) with the isosmotic addition of 3 mM NaHCO3 in place of NaCl (see Baxter & Church, 1996). Coverslips plated with neurones were placed in loading medium containing either 5 μM fura-2 AM for 60 min at 35°C or 2 μM BCECF AM for 30 min at room temperature and then mounted in a temperature-controlled perfusion chamber so as to form the base of the chamber. Neurones were then superfused at a rate of 2 ml min−1 for 15 min with the initial experimental solution at the appropriate temperature prior to the start of an experiment. Both NMDA- (20 μM) and high-[K+]-containing solutions were administered in 1 ml aliquots to the inflow of the perfusion chamber and allowed to remain in contact with neurones for 20 s before washout. Except where indicated, experiments were performed at room temperature in the presence of tetrodotoxin (TTX) at 0.5-1 μM.
[Ca2+]i and pHi were measured using the dual-excitation ratio method, employing a digital fluorescence microscopy system (Atto Instruments, Inc., Rockville, MD, USA; Carl Zeiss Canada Ltd, Don Mills, ON, Canada). Details of the methods employed have been presented previously (Church et al. 1994; Baxter & Church, 1996). In brief, fluorescence emissions from neurones loaded with either fura-2 or BCECF were obtained from multiple neurone somata simultaneously and raw intensity data at each excitation wavelength (334 and 380 nm for fura-2; 488 and 452 nm for BCECF) were corrected for background fluorescence prior to calculation of the ratio. The one-point high-[K+]/nigericin technique was employed to convert background-corrected emission intensity ratios (I488/I452) into pHi values as described (Baxter & Church, 1996). Analysis was restricted to those neurones able to retain BCECF (as judged by raw emission intensity values) throughout the course of an experiment (see Bevensee et al. 1996). Although BCECF is not sensitive to changes in [Ca2+] (Graber et al. 1986), the affinity of fura-2 for Ca2+ is sensitive to changes in pH such that the Kd increases as pH falls (Martínez-Zaguilán et al. 1996). In many experiments employing fura-2, pHi was often changing and the figures depicting changes in [Ca2+]i are (with the exceptions of Figs 4B and 5B) therefore presented as background-corrected I334/I380 ratio values to which pH-dependent corrections for the Kd of fura-2 for Ca2+ were not applied. However, such corrections were applied prior to the quantitative analysis of data, including the estimation of the -log of the dissociation constant (pK) values for H+ inhibition of peak [Ca2+]i transients evoked by high-[K+]o or NMDA (Figs 2B and 3B and D). In the latter cases, pHi values at the peak of evoked [Ca2+]i transients were estimated from experiments in which changes in pHi in response to changes in pHo were measured (see Results) and these pHi values were employed to correct the Kd of fura-2 for Ca2+ according to Martínez-Zaguilán et al. (1996).
Figure 4. Modulation of high-[K+]o-evoked increases in [Ca2+]i by changes in pHi.
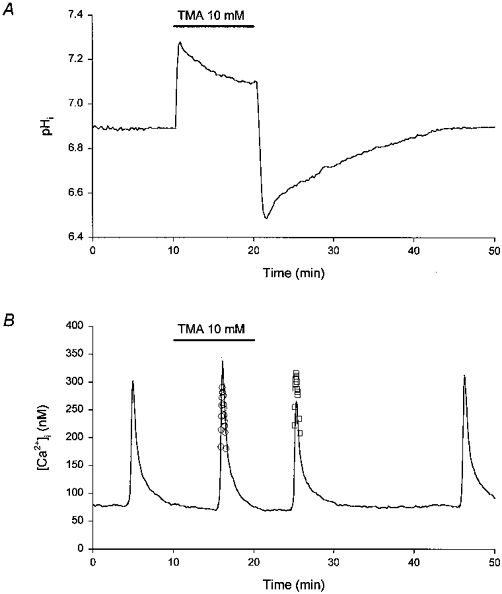
A, a 10 min application of the weak base trimethylamine (TMA, 10 mM) evoked a rise in pHi. Following the withdrawal of TMA, pHi fell to values below the initial resting level and then recovered gradually. The experiment was performed at room temperature in HCO3−/CO2-buffered media (pH 7.3). The record is a mean of data obtained from 17 neurones simultaneously. B, under control conditions (pHo 7.3) an application of 50 mM [K+]o evoked a rise in [Ca2+]i (first response). Subsequent responses to 50 mM K+ were obtained at 6 min after the start of a 10 min period of perfusion with a pH 7.3 medium containing 10 mM TMA (second response), and at 5 and 25 min following the withdrawal of TMA (third and fourth responses, respectively). A single Kd value for fura-2 (169.13, corresponding to a resting pHi of 6.93, measured in 5 experiments of the type illustrated in A) was employed to generate the continuous line. The open circles (○) represent the peak of the rise in [Ca2+]i computed using a Kd 14 % lower than that employed for the continuous line, to reflect the rise in pHi observed at 6 min after the start of application of TMA (see Results). The open squares (□) represent the peak of the rise in [Ca2+]i computed using a Kd 19 % higher than that employed for the continuous line, to reflect the fall in pHi observed at 5 min following the withdrawal of TMA (see Results). The record is a mean of data obtained from 14 neurones simultaneously. The experiment was performed in a sister culture to that employed in the experiment shown in A, under identical conditions.
Figure 5. Comparison of the effects of changes in pHi and pHo on high-[K+]o-evoked increases in [Ca2+]i.
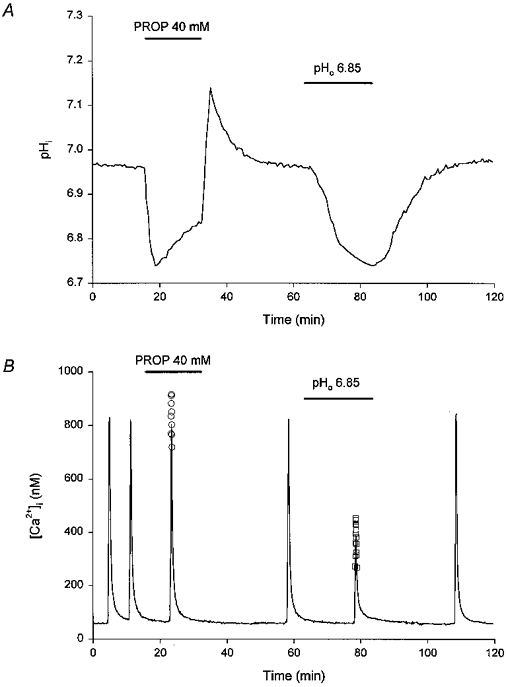
A, a 16 min application of the weak acid propionate (PROP, 40 mM) evoked a fall in pHi. Following the recovery of pHi to the initial resting level, the pH of the perfusate was reduced from 7.3 to 6.85 for 20 min. pHi fell gradually and recovered to the initial resting level upon re-perfusion with pH 7.3 medium. The experiment was performed at room temperature in HCO3−/CO2-buffered media. The record is a mean of data obtained from 7 neurones simultaneously. B, under control conditions (pHo 7.3) two consecutive applications of 50 mM [K+]o evoked stable rises in [Ca2+]i (first and second responses). The third response was obtained 8 min after the start of a 16 min period of perfusion with a pH 7.3 medium containing 40 mM propionate. The final three responses were obtained immediately prior to perfusion with pH 6.85 medium, at 16 min following the start of a 20 min exposure to pH 6.85 medium, and during a period of reperfusion with pH 7.3 medium. A single Kd value for fura-2 (corresponding to the resting pHi measured in 3 experiments of the type illustrated in A) was employed to generate the continuous line. The open circles (○) represent the peak of the rise in [Ca2+]i computed using a Kd 14 % higher than that employed for the continuous line, to reflect the fall in pHi observed at 8 min after the start of application of propionate (see Results). The open squares (□) represent the peak of the rise in [Ca2+]i computed using a Kd 16 % higher than that employed for the continuous line, to reflect the fall in pHi observed at 16 min following the start of exposure to pH 6.85 medium (see Results). The record is a mean of data obtained from 6 neurones simultaneously. The experiment was performed in a sister culture to that employed in the experiment shown in A, under identical conditions.
Figure 2. Changes in pHo modulate NMDA-evoked increases in I334/I380 ratio values.
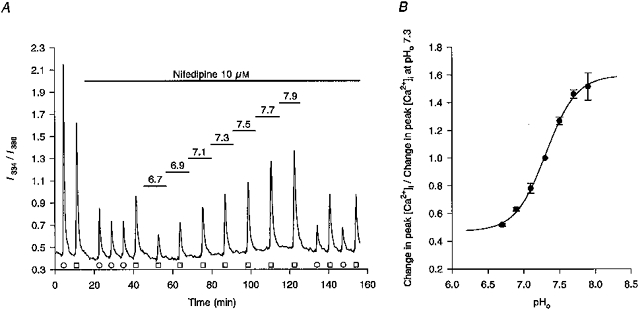
A, under control conditions (pHo 7.3), transient exposures to 50 mM [K+]o (○) or 20 μM NMDA (□) evoked rises in the I334/I380 ratio value. Subsequent addition of 10 μM nifedipine to the Hepes-buffered perfusion medium reduced high-[K+]o-evoked increases in the ratio value by 81 % (third, fourth and fifth responses) and NMDA-evoked increases in the ratio value by 54 % (sixth response). The pH of the perfusion medium was then changed sequentially from 6.7 to 7.9 in 0.2 pH unit increments, for the periods indicated by the bars above the trace, resulting in a gradual increase in NMDA-evoked rises in the I334/I380 ratio values. The final four responses shown are K+- and NMDA-evoked responses upon return to pH 7.3 medium. The record is a mean of data obtained from 10 neurones simultaneously, the experiment being performed at room temperature. B, pH-dependent corrections for the Kd of fura-2 for Ca2+ (see Methods) were applied to the data shown in A and other, similar experiments and a plot was made of pHoversus changes in peak [Ca2+]i responses evoked by NMDA in the presence of 10 μM nifedipine, normalized to the peak of the [Ca2+]i response obtained at pHo 7.3. Each point represents data obtained from a minimum of 3 neuronal populations. The 4-parameter logistic plot (r2= 0.99) had a pK of 7.31. The extrapolated maximum and minimum values were 160 and 47 %, respectively.
Figure 3. Changes in pHo modulate high-[K+]o-evoked increases in I334/I380 ratio values.
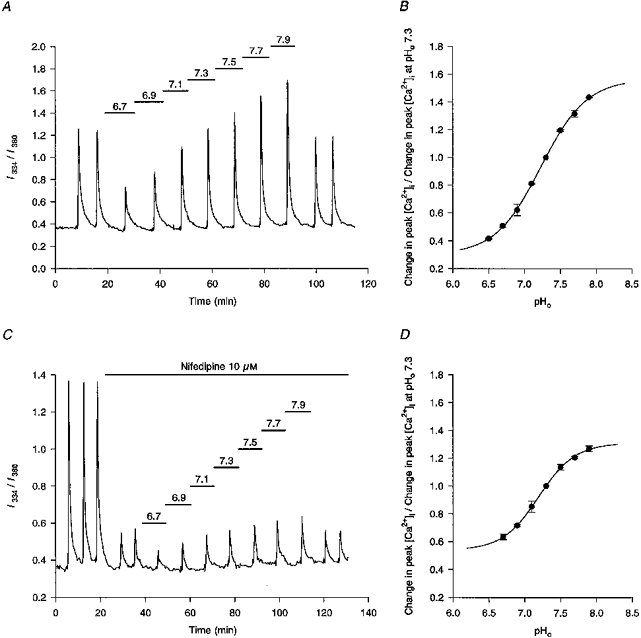
A, under control conditions (pHo 7.3) two consecutive applications of 50 mM [K+]o evoked stable rises in the I334/I380 ratio. Subsequent responses to 50 mM K+ were obtained at the pHo values indicated by the bars above the trace. The final 2 responses were obtained upon reperfusion with pH 7.3 medium. The record is a mean of data obtained from 16 neurones simultaneously. The experiment was performed at room temperature in HCO3−/CO2-buffered media. B, pH-dependent corrections for the Kd of fura-2 for Ca2+ (see Methods) were applied to the data shown in A and other, similar experiments and a plot was made of pHoversus changes in peak high-[K+]o-evoked [Ca2+]i responses, normalized to the peak of the [Ca2+]i response obtained at pHo 7.3. Each point represents data obtained from a minimum of 4 neuronal populations. The 4-parameter logistic plot (r2= 0.99) had a pK of 7.23 and extrapolated maximum and minimum values of 158 and 31 %, respectively. C, under control conditions (pHo 7.3) three consecutive applications of 50 mM [K+]o evoked stable rises in the I334/I380 ratio. Addition of 10 μM nifedipine reduced the high-[K+]o-evoked rises in the I334/I380 ratio by ≈80 % (fourth and fifth responses). Subsequent responses to 50 mM K+ were obtained at the pHo values indicated by the bars above the trace. The final two responses were obtained upon reperfusion with pH 7.3 medium. The record is a mean of data obtained from 7 neurones simultaneously. The experiment was performed at room temperature in Hepes-buffered media containing 40 μM AP5 and 20 μM CNQX throughout. D, pH-dependent corrections for the Kd of fura-2 for Ca2+ were applied to the data shown in C and other, similar experiments and a plot was made of pHoversus changes in peak high-[K+]o-evoked [Ca2+]i responses, normalized to the peak of the [Ca2+]i response obtained at pHo 7.3. Each point represents data obtained from a minimum of 4 neuronal populations. The 4-parameter logistic plot (r2= 0.99) had a pK of 7.20 and extrapolated maximum and minimum values of 131 and 54 %, respectively.
Single channel recordings
The procedures for recording large conductance Ca2+-dependent K+ currents (of the BK type and labelled IBK(Ca)) have been described (McLarnon & Wang, 1991; McLarnon & Sawyer, 1993; McLarnon, 1995). In brief, patch pipettes were fabricated (Corning 7052 glass) and fire polished prior to the excision of inside-out patches from neurones in sister cultures to those utilized in the microspectrofluorimetric studies. For inside-out patches, the bath (internal) solution was (mM): KCl, 140; NaCl, 5; CaCl2, 0.1; and Hepes, 10 (pH 7.3 with 10 M NaOH). The internal [Ca2+] was then reduced either to 0.7 or 20 μM (as indicated in the text) by the addition of EGTA; free [Ca2+] was maintained at a constant value during changes in pH using correction programs described by Fabiato (1988). The patch pipette solution contained (mM): NaCl, 140; KCl, 5; CaCl2, 1; MgCl2, 1; and Hepes, 10 (pH 7.3 with 10 M NaOH), and the electrodes had final resistances of 6-8 MΩ. IBK(Ca) values were recorded with the patch potential (V) set at either 0 or +20 mV. In some experiments in inside-out patches with 0.7 μM internal [Ca2+], a specific protocol was applied consisting of a 10 s prepulse to V= -80 mV prior to V being set at 0 mV. This procedure has previously been shown to lead to activation (at pH 7.3) of IBK(Ca) in inside-out patches excised from cultured fetal rat hippocampal neurones (McLarnon, 1995). Outside-out patches were utilized in some experiments; in these cases, the pipette and bath solutions were reversed.
The amplifier used to record unitary outward IBK(Ca) was an Axopatch-1B (Axon Instruments, Inc.) with a sampling rate of 5 kHz and low-pass filter set at 1 or 2 kHz. Data were stored on computer for subsequent analysis using pCLAMP v. 5.5.1 (Axon Instruments, Inc.). Distributions of amplitudes and open and closed times were collated from the analysis of unitary openings and, except where noted in the text, contained a minimum of 200 events. Channel open probability (Po) was determined by summing individual open times during the recording period and dividing this sum by the total time at a given patch potential. Experiments were performed at room temperature.
Data analysis
Results are reported as means ±s.e.m. with the accompanying n value referring either to the number of cell populations (i.e. number of coverslips) analysed (microspectrofluorimetric studies) or to the number of patches (single channel studies). The number of neurones from which data were obtained in the former experiments was 1853. Statistical comparisons were performed using Student's two-tailed t test with a 95 % confidence limit.
RESULTS
Microspectrofluorimetric studies
In initial experiments we determined the maximum extent to which pHi changed in response to exposure to HCO3−/CO2- and Hepes-buffered media at a variety of different pH values (Fig. 1). At room temperature, the ratio ΔpHi : ΔpHo (the slope of the regression line relating pHi to pHo) was 0.37 under HCO3−/CO2-buffered conditions (n= 7) and 0.45 under Hepes-buffered conditions (n= 5). The ΔpHi : ΔpHo ratio values obtained at 37°C were greater than those observed at room temperature, being 0.71 under HCO3−/CO2-buffered conditions (n= 6) and 0.79 under Hepes-buffered conditions (n= 4). The equations relating pHi to pHo (see Fig. 1, legend) were employed to estimate changes in pHi consequent upon changes in pHo, in order to apply pH-dependent corrections for the Kd of fura-2 for Ca2+ in subsequent experiments in which [Ca2+]i was measured (see Methods).
Figure 1. Dependence of pHi on pHo.
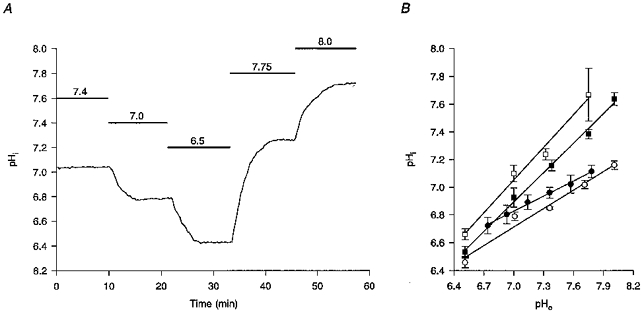
A, in an experiment conducted at 37 °C under HCO3−/CO2-buffered conditions, decreasing or increasing pHo for the periods indicated by the bars above the trace and to the values shown above the bars, decreased and increased pHi, respectively. The record is a mean of data obtained from 9 neurones simultaneously. B, linear regression analysis of the dependence of pHi on pHo at both room temperature (•) and at 37 °C (▪) under HCO3−/CO2-buffered conditions and under Hepes-buffered conditions (○ and □, respectively). Each point represents data obtained from a minimum of 4 neuronal populations; error bars are s.e.m. The equations relating pHi to pHo at room temperature and at 37 °C under HCO3−/CO2-buffered conditions were, respectively, pHi= 4.27 + (0.37 × pHo) (r2= 0.99) and pHi= 1.92 + (0.71 × pHo) (r2= 0.99). The equations relating pHi to pHo at room temperature and at 37 °C under Hepes-buffered conditions were, respectively, pHi= 3.60 + (0.45 × pHo) (r2= 0.98) and pHi= 1.52 + (0.79 × pHo) (r2= 0.99).
The effects of changes in pHo (n= 4 in both HCO3−/CO2- and Hepes-buffered media) on rises in I334/I380 ratio values evoked by 20 μM NMDA were then examined (Fig. 2A). Nifedipine (10 μM) was present to limit the contribution of Ca2+ influx via L-type high voltage-activated (HVA) Ca2+ channels to the response (see below). No differences were observed in the effects of changes in pHo on rises in I334/I380 ratio values under the different buffering conditions and the results were therefore pooled after appropriate (i.e. buffering condition-specific) corrections for the pH dependence of the Kd of fura-2 for Ca2+ had been applied. Nifedipine (10 μM) reduced NMDA-evoked rises in [Ca2+]i by 48 ± 2 % under control conditions at pHo 7.3. Compared with responses evoked at pHo 7.3, NMDA-evoked rises in [Ca2+]i were reduced by 37 ± 1 % at pHo 6.9 and increased by 46 ± 3 % at pHo 7.7. Figure 2B summarizes the pHo dependence of NMDA-evoked rises in [Ca2+]i; 50 % inhibition corresponded to a pHo of 7.31.
In the neurones employed, rises in [Ca2+]i evoked by transient application of 50 mM K+ are mediated primarily by dihydropyridine-sensitive (L-type) HVA Ca2+ channels, with smaller contributions from ω-conotoxin GVIA-sensitive (N-type) HVA Ca2+ channels and Ca2+ channels insensitive to dihydropyridines and ω-conotoxin GVIA but sensitive to crude funnel-web spider venom (Church et al. 1994). High [K+]o-evoked rises in I334/I380 ratio values were reduced by 96 ± 1 % (n= 4) during perfusion with Ca2+-free medium, indicating that the release of Ca2+ from internal stores is not involved to an appreciable extent in the high-[K+]o response in our experimental preparation. The addition of 40 μM AP5 and 20 μM CNQX to Ca2+-containing perfusion medium reduced K+-evoked rises in I334/I380 ratio values by 6 ± 4 % (n= 6), indicating that endogenously released glutamate contributes little to the response (see Church et al. 1994).
Under both HCO3−/CO2-buffered (n= 6) and Hepes-buffered (n= 6) conditions, lowering pHo attenuated rises in I334/I380 ratio values evoked by 50 mM K+ whereas high-[K+]o-evoked rises in ratio values were enhanced during exposure to high pH media (Fig. 3A). After corrections for the pH dependence of the Kd of fura-2 for Ca2+ had been applied, perfusion with pH 6.9 and pH 7.7 media were found to reduce and increase, respectively, high-[K+]o-evoked rises in [Ca2+]i by 38 ± 4 % and 32 ± 2 %, compared with control responses evoked at pHo 7.3. Since the rise in [Ca2+]i evoked by 50 mM K+ is dominated by Ca2+ influx through L-type Ca2+ channels, the results suggest that changes in pHo modulate the activity of this subtype of HVA Ca2+ channel. A 50 % inhibition of the high-[K+]o-evoked rise in [Ca2+]i corresponded to a pHo of 7.23 (Fig. 3B). Perfusion with methylsulphate-substituted media containing 91 mM Cl− (the same as in the high-[HCO3−], pH 7.7 medium) at pH 7.3 did not affect the magnitude of high-[K+]o-evoked rises in I334/I380 ratio values (n= 6; not shown).
To examine the effects of changes in pHo on rises in [Ca2+]i mediated by Ca2+ influx through dihydropyridine-resistant HVA Ca2+ channels, 10 μM nifedipine (a maximally effective concentration in the neurones employed; see Church et al. 1994) was added to the perfusate. Addition of nifedipine (10 μM) to pH 7.3 medium reduced high-[K+]o-evoked rises in I334/I380 ratio values by 79 ± 2 % (n= 7) and the residual K+-evoked [Ca2+]i transients were attenuated during perfusion with low pH media and enhanced during perfusion with high pH media (Fig. 3C). After correcting for the pH dependence of the Kd of fura-2 for Ca2+, a 50 % inhibition of high-[K+]o-evoked rises in [Ca2+]i recorded in the presence of 10 μM nifedipine was found to correspond to pHo 7.20 (Fig. 3D).
In order to determine whether the effects of changes in pHo on K+-evoked rises in [Ca2+]i reflected changes in pHoper se or were secondary to changes in pHi consequent upon changes in pHo, weak acids and bases were employed to change pHi at a constant pHo (Fig. 4). In five experiments of the type illustrated in Fig. 4A, 10 mM TMA evoked a rise in pHi of 0.26 ± 0.02 pH units measured at 6 min following its introduction, whereas a reduction in pHi of 0.21 ± 0.03 pH units was observed at 5 min following its washout. In paired experiments performed on sister cultures, high-[K+]o was applied such that the peaks of the ensuing rises in [Ca2+]i occurred at 6 min following the introduction of the weak base and at 5 min following its withdrawal. As shown in Fig. 4B (continuous line), the [Ca2+]i transient appeared to be enhanced when pHi was raised above resting levels (a 17 ± 6 % increase) whereas the response appeared to be reduced when pHi was below resting levels (a 22 ± 6 % decrease), suggesting that changes in pHi at a constant pHo modulate K+-evoked rises in [Ca2+]i. However, when corrections for the effects of pH on the Kd of fura-2 for Ca2+ were applied (a 14 % decrease in the Kd at the time when pHi was increased by 0.26 pH units and a 19 % increase when pHi was decreased by 0.21 pH units), it became apparent that changing pHi at a constant pHo had only a minor effect on the magnitude of high-[K+]o-evoked rises in [Ca2+]i. Thus, following the application of pH-dependent corrections for the Kd of fura-2 for Ca2+, K+-evoked [Ca2+]i transients increased by 1 ± 6 % during exposure to 10 mM TMA and decreased by 2 ± 6 % following its washout (Fig. 4B).
Experiments analogous to those with TMA were performed with the weak acid propionate (n= 5). At 6 min after the start of exposure to 20 mM propionate pHi decreased by 0.10 ± 0.02 pH units, whereas pHi increased by 0.10 ± 0.02 pH units at 6 min following its washout. After pH-dependent corrections for the Kd of fura-2 for Ca2+ were applied, peak [Ca2+]i responses were found to be increased by 3 ± 5 % at the time at which pHi was decreased by 0.10 pH units and changed by 0 ± 2 % at the time at which pHi was increased by 0.10 pH units. Similar results were obtained when 20 mM butyrate was employed as the weak acid (n= 3; not shown).
In the final series of experiments, high-[K+]o-induced rises in [Ca2+]i were evoked initially during perfusion with pH 7.3 medium containing propionate at 40 mM (under which conditions pHi falls at a constant pHo) and subsequently during exposure to low pH medium (under which conditions both pHo and pHi fall); corresponding pHi changes were examined in parallel experiments in sister cultures. In three experiments of the type shown in Fig. 5A, exposure to 40 mM propionate (pHo= 7.3) evoked a fall in pHi of 0.15 ± 0.01 pH units measured at 8 min after the start of exposure to the weak acid. Following the washout of propionate and the return of pHi to normal resting levels, exposure to a pH 6.85 medium evoked a fall in pHi of 0.17 ± 0.04 pH units measured at 16 min following the start of perfusion. Figure 5B illustrates one of three corresponding experiments conducted in fura-2-loaded neurones. After correcting for the pH dependence of the Kd of fura-2 for Ca2+, peak [Ca2+]i responses to 50 mM K+ were found to be increased by 10 ± 5 % at 8 min following the start of perfusion with 40 mM propionate; in contrast, corrected peak [Ca2+]i responses to 50 mM K+ were reduced by 45 ± 4 % at 16 min following the start of perfusion with pH 6.85 medium. Since pHi values were similar under both conditions, the results indicate that the attenuation of K+-evoked rises in [Ca2+]i during exposure to low pH media reflects reductions in pHo rather than pHi. Similar paired experiments (n= 3) were performed in which cells were exposed initially to pH 7.3 medium containing 10 mM TMA and subsequently to pH 7.85 medium. Measured at 5 min following the start of perfusion with 10 mM TMA, pHi increased by 0.29 ± 0.04 pH units, at which time K+-evoked rises in [Ca2+]i (corrected for the pH dependence of the Kd of fura-2 for Ca2+) were found to be increased by 12 ± 7 %. The pHi increase observed at 15 min following the start of exposure of the neurones to pH 7.85 medium was 0.23 ± 0.02 pH units, at which time corrected high-[K+]o-evoked [Ca2+]i transients were increased by 38 ± 3 %.
Single channel recordings
The findings detailed above indicate that changes in pHi at a constant pHo exert only a limited effect on the magnitude of rises in [Ca2+]i mediated by Ca2+ influx through HVA Ca2+ channels. However, previous studies in hippocampal neurones have indicated that reductions in pHi at a constant pHo attenuate both the fast after-hyperpolarization (AHP) observed following a single depolarizing current-evoked action potential and the slow AHP observed after a train of action potentials (Church, 1992). Because these hyperpolarizing potentials are mediated by gK(Ca) (see Storm, 1990), the possibility exists that changes in the magnitudes of potentials mediated by gK(Ca) previously observed in hippocampal neurones during changes in pHo (Church & McLennan, 1989) may reflect not only changes in Ca2+ influx (consequent upon changes in pHo) but also direct effects of changes in pHi on Ca2+-activated K+ channels. We therefore examined the effects of changes in internal and external pH on the unitary properties of a Ca2+-dependent K+ channel in, respectively, inside-out and outside-out patches excised from cultured hippocampal neurones. We have characterized previously some of the properties of this BK-type channel in hippocampal neurones, which include a Hill coefficient for activation of ∼2, a Mg2+-dependent modulation of kinetic behaviour, block by low concentrations of external TEA and open-channel blockade by class III antiarrhythmic agents (McLarnon & Wang, 1991; McLarnon & Sawyer, 1993; McLarnon, 1995).
Representative unitary recordings of IBK(Ca) from an inside-out patch are shown in Fig. 6A (upper trace), where internal pH is 7.3, internal [Ca2+] is 20 μM and V is +20 mV; the internal and external [K+] were 140 and 5 mM, respectively. An estimate for the unitary (slope) conductance could be derived from measurements of the amplitudes of currents recorded for V over the range 0 to +60 mV (data not shown) and yielded a value of 102 pS, similar to values obtained for BK-type channels in other cell types with physiological-like K+ across inside-out patches (see Barrett et al. 1982). A reduction of internal [Ca2+] to 0.7 μM (lower trace of Fig. 6A) led to cessation of channel activity in six out of six inside-out patches examined (also see McLarnon & Sawyer, 1993). The effects on unitary IBK(Ca) of reducing internal pH at a constant internal [Ca2+] (20 μM) are shown in Fig. 6B, where the upper trace shows currents recorded at pH 7.3 (V=+20 mV). Reducing pH to 6.7 caused a marked decrease in the number of channel openings with activity restored upon reperfusion with pH 7.3 medium. The amplitudes of the currents were unchanged and were not altered by reductions in internal pH at other patch potentials (not shown); hence, unitary conductance was independent of internal pH. In four additional experiments, channel activity evident at pH 7.3 was effectively abolished when internal pH was reduced to pH 6.7 (also see Kume et al. 1990). Distributions of open times at pH 7.3 and pH 6.7 (V=+20 mV) are presented in Figs 6C and D, respectively. The distributions were fitted with single exponential functions, as previously employed in studies of BK-type channels (e.g. Christensen & Zeuthen, 1987), and the mean open times were 19.8 ± 0.4 ms (pH 7.3) and 5.4 ± 1.7 ms (pH 6.7). Thus, in inside-out patches, reducing internal pH from 7.3 to 6.7 caused a marked decrease in the number of open events and mean open time (n= 1) or a cessation of channel activity (n= 4). For the single patch, there were insufficient events at pH 6.7 to permit fitting of a closed time distribution; however, an estimate of a mean closed time was inferred from the occasional events and yielded a value near 300 ms; the corresponding value at pH 7.3 was 4.8 ± 1.8 ms (collation of 760 events). It should be noted that closed time distributions for the BK-type channel in hippocampal neurones require two-component fits (e.g. McLarnon & Sawyer, 1993) and the use of mean values to compare closed times serves only as an approximation for the effects of changes in pH on this quantity.
Figure 6. Unitary properties of a Ca2+-dependent K+ channel.
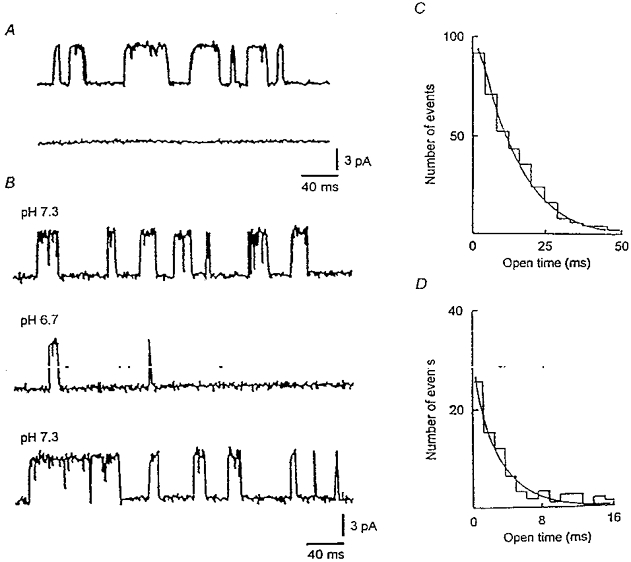
A, single channel current recordings from an inside-out patch with internal (bath) [Ca2+] at 20 μM (upper trace) and lack of activity when internal [Ca2+] was decreased to 0.7 μM (lower trace). In both records, the internal pH was 7.3 and the patch potential was +20 mV. Channel activity was restored upon reperfusion with medium containing 20 μM Ca2+ (not shown). B, openings from a different inside-out patch with internal [Ca2+] at 20 μM and internal pH values of 7.3 (upper trace), 6.7 (middle trace) and following return to pH 7.3 (lower trace). The patch potential was +20 mV for all records. C, distribution of open times (at V=+20 mV) at pH 7.3 (collation of 462 events); the mean open time was 19.8 ms. D, distribution of open times (at V=+20 mV) at pH 6.7 (collation of 107 events); the mean open time was 5.4 ms.
The channel Po is the product of mean open time and frequency of channel opening. Overall, for inside-out patches with internal pH of 7.3 and internal [Ca2+] of 20 μM, the channel Po at V=+20 mV was significantly (P < 0.05; n= 5) reduced from 0.81 ± 0.06 to 0.06 ± 0.03 when internal pH was lowered from 7.3 to 6.7. The reduction in Po reflected a decrease in both the frequency of channel opening and the mean duration of the open state, with the effect of acidosis to lower the Po being greater compared to its effect in reducing mean open time. In essence, reducing internal pH from 7.3 to 6.7 had the most significant effect in increasing closed time. A similar result has been documented for a Ca2+-activated maxi K+ channel in epithelium where, over the pH range 7.4 to 6.4, acidosis increased closed time by a factor of 10 whereas open time was diminished 2-fold (Christensen & Zeuthen, 1987).
To determine whether a change in external pH might also affect channel activity, we examined the effects of reducing external pH on unitary IBK(Ca) in outside-out patches (n= 4). In this configuration the unitary conductance, derived by recording currents at patch potentials of 0 mV and +20 mV, was estimated at 115 pS, a value close to that established for channel conductance using inside-out patches (see above). Reducing the pH of the bath (external) solution from 7.3 to 6.7 had no effect on channel activity in outside-out patches; channel Po remained unchanged near 0.9 and amplitudes of the unitary currents were unaffected (data not shown). Therefore, over the pH range 7.3 to 6.7, changes in extracellular pH had no effects in altering the unitary properties of IBK(Ca) in hippocampal neurones.
In inside-out patches, increasing internal pH from 7.3 to 7.8 (n= 2) had little effect on channel mean open times (18.7 ± 1.6 ms and 20.3 ± 1.3 ms at pH 7.3 and pH 7.8, respectively) or Po (0.76 ± 0.05 and 0.81 ± 0.06). As previously discussed by Christensen & Zeuthen (1987) and Kume et al. (1990), the lack of dependence of Po on internal pH when pH was > 7.3 could indicate that binding sites for activation of IBK(Ca) were saturated at the internal [Ca2+] of 20 μM. However, when internal [Ca2+] was reduced from 20 to 0.7 μM at an internal pH of 7.3, no activation of IBK(Ca) was evident at maintained potentials of 0 or +20 mV (see Fig. 6A; also see Fig. 1B in McLarnon & Sawyer (1993) for a plot of Po for the channel versus internal [Ca2+] in cultured fetal rat hippocampal neurones). Therefore, in order to examine possible differences in channel activity between pH 7.3 and pH 7.8, we employed a protocol (McLarnon, 1995) which, at an internal [Ca2+] of 0.7 μM and physiological K+ across an inside-out patch, leads to transient activation of IBK(Ca). The procedure was to initially hold the excised patch at V= -80 mV for 10 s and then step V to 0 mV for 1 s to activate outward unitary IBK(Ca). In Fig. 7, records of IBK(Ca) are shown commencing 4 ms after V had returned to 0 mV from the holding level of -80 mV (to eliminate capacitative artefacts), at four different internal pH values. The protocol was repeated in three additional patches and the overall Po values were determined by collation of events (for 10 steps at each pH value) over the first 70 ms following the step to 0 mV. Estimated in this manner, the Po values with internal pH at 6.7, 7.0, 7.3 and 7.8 were 0.04 ± 0.03, 0.19 ± 0.06, 0.57 ± 0.11 and 0.81 ± 0.13, respectively.
Figure 7. Effects of changes in internal pH on transient activation of IBK(Ca).
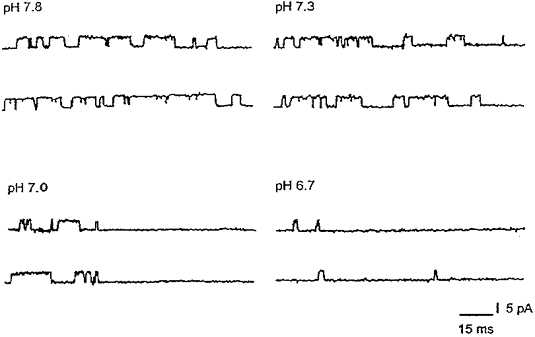
The protocol was to initially hold the potential of the inside-out patch at -80 mV for 10 s and then to step V to 0 mV. The records shown commence 4 ms following the steps to 0 mV. No further events were evident following the final closures shown in each of the traces, despite a maintained potential of 0 mV (see McLarnon, 1995). Po values were determined from the analysis of 10 steps, for times of 70 ms following each step, for each of the pH values tested; internal [Ca2+] was 0.7 μM throughout.
DISCUSSION
Effects of changes in pHo
The sensitivities of voltage-activated Ca2+ channels to changes in pHo have been documented in a variety of cell types (e.g. Klöckner & Isenberg, 1994b; Ou-Yang et al. 1994). In all cases, falls in pHo reduce Ca2+ currents whereas rises in pHo increase them. Recently, Tombaugh & Somjen (1996) reported the effects of changes in pHo on HVA Ca2+ currents in acutely dissociated adult rat hippocampal CA1 neurones under voltage clamp; the pK for the effect of pHo on whole-cell Ca2+ current amplitude (7.1) was close to the value (7.2) found in the present study for inhibition of K+-evoked rises in [Ca2+]i which, in the fetal neurones employed, are dominated by Ca2+ influx through L-type HVA Ca2+ channels. We also find that [Ca2+]i transients dependent upon Ca2+ flux through dihydropyridine-resistant HVA Ca2+ channels are modulated by changes in pHo (pK= 7.2), a finding which indicates that changes in neurotransmitter release, in addition to changes in postsynaptic excitability, probably participate in the known effects of changes in pHo on synaptic transmission in hippocampal slices (Balestrino & Somjen, 1988; Drapeau & Nachshen, 1988; Church & McLennan, 1989).
The pK established in the present study for inhibition of NMDA-evoked rises in [Ca2+]i (7.3) compares well not only to values found for pHo modulation of NMDA receptor-mediated currents in neurones and in wild-type NR1A/NR2B receptors expressed in Xenopus oocytes (McBain & Mayer, 1994; Kashiwagi et al. 1996) but also to the pHo sensitivities of HVA Ca2+ channels. Because the application of NMDA (or glutamate) under non-voltage-clamped conditions evokes rises in [Ca2+]i mediated, at least in part, by voltage-activated Ca2+ channels, the present results suggest that the anticonvulsant and neuroprotective effects of mildly reduced pHo may reflect decreases in Ca2+ flux through voltage- as well as ligand-operated Ca2+ channels.
Effects of changes in pHi
Initial studies in neurones indicated that the change in pHi occasioned by a change in pHo was relatively small (e.g. Moody, 1984; Tolkovsky & Richards, 1987) but it now appears that the dependence of neuronal pHi on pHo is more direct. In the present study, the ratio ΔpHi : ΔpHo was 0.71 under HCO3−/CO2-buffered conditions at 37°C, similar to values obtained in rat cortical neurones (0.78; Ou-Yang et al. 1993) and synaptosomes (0.68; Sánchez-Armass et al. 1994). The basis for the steep dependence of pHi on pHo in neurones is unknown although, by analogy with other cell types (see Wakabayashi et al. 1997), it may involve the modulation of the activity of pHi regulating mechanism(s) by changes in pHo. The dependence of pHi on pHo has a number of potential implications, including the possibility that changes in pHo occurring during pathophysiological events such as cerebral ischaemia may modulate not only ionic conductances and transport mechanisms which are sensitive to changes in pHo but also those which are sensitive to changes in pHi (e.g. Na+-Ca2+ exchange; see below).
In the present study, and in agreement with the findings of Tombaugh & Somjen (1996), changes in pHi consequent upon changes in pHo failed to account for the effects of changes in pHo on K+-evoked [Ca2+]i transients (see Fig. 5). Nevertheless, the activities of HVA Ca2+ channels in a variety of cell types are modulated by changes in pHi (e.g. Kaibara & Kameyama, 1988; Mironov & Lux, 1991; Klöckner & Isenberg, 1994a). Furthermore, it has been found that changes in pHi affect HVA Ca2+ currents in acutely dissociated adult rat hippocampal neurones under whole-cell voltage clamp, although changes in Ca2+ currents were evoked by pHi changes of unknown magnitude and pK values could not be estimated (Tombaugh & Somjen, 1997). In the present study, however, changes in pHi at a constant pHo failed to affect markedly the magnitude of K+-evoked rises in [Ca2+]i once corrections for the pH dependence of the Kd of fura-2 for Ca2+ had been applied (Fig. 4). A number of explanations may account for this finding. First, as pointed out by Dixon et al. (1993), in all cell types studied for which data are available, the pHi for 50 % suppression of HVA Ca2+ currents (∼6-6.5) is below normal resting pHi, suggesting in turn that the relatively small perturbations of pHi from rest employed in the present study may not have been sufficient to affect noticeably responses mediated by Ca2+ flux through HVA Ca2+ channels. However, in agreement with findings in other cell types (e.g. Moody, 1980), we have observed that reductions in pHi (at a constant pHo) of greater magnitude than those employed in the present study act to increase K+-evoked [Ca2+]i transients in hippocampal neurones (J. Church & K. A. Baxter, unpublished observations). Second, under our experimental conditions (in which outward K+ currents were not suppressed; cf. Tombaugh & Somjen, 1997), the effects of changes in pHi on gK(Ca) (and, possibly, other K+ conductances; see Moody, 1984) may have acted to offset any direct effect of pHi on Ca2+ flux through HVA Ca2+ channels. For example, an inhibitory effect of a decrease in pHi on Ca2+ influx may have been attenuated by concomitant blockade of gK(Ca), the result being that changes in pHi at a constant pHo have little net effect on the magnitude of depolarization-evoked [Ca2+]i transients. In this regard, it has been shown in CA3 pyramidal neurones that blockade of gK(Ca) is associated with an increased accumulation of internal Ca2+ during repetitive firing (Müller & Connor, 1991; see also Drapeau & Nachshen, 1988). Third, a number of mechanisms which participate in the control of neuronal Ca2+ homeostasis, including ATP-dependent Ca2+ efflux and forward Na+-Ca2+ exchange, are inhibited by low pHi (Dipolo & Beaugé, 1982). Falls in pHi acting on these, and other, mechanisms might also shift the balance between a reduction in Ca2+ entry via voltage-activated Ca2+ channels and the size of depolarization-evoked [Ca2+]i transients.
We conclude that K+-evoked [Ca2+]i transients in fetal rat hippocampal neurones are more sensitive to modulation by changes in pHo than changes in pHi, and that changes in pHi do not mediate the effects of changes in pHo on these responses. The results also indicate that pHi-dependent changes in the activities of HVA Ca2+ channels (Tombaugh & Somjen, 1997) do not alone determine the net effect of a change in pHi on the magnitude of depolarization-evoked [Ca2+]i transients. The latter will reflect the sum of the effects of pHi on the various ionic conductances and transport mechanisms involved in intracellular Ca2+ homeostasis and which are sensitive to changes in pHi.
pH modulation of a Ca2+-dependent K+ channel
Although K+-evoked [Ca2+]i transients are more sensitive to changes in pHo than to changes in pHi, changes in pHi (at a constant pHo) had marked effects on the kinetic properties of a BK-type Ca2+-dependent K+ channel. The IBK(Ca) examined in the present study probably mediates the fast AHP observed in CA1 pyramidal cells because, in six neurones impaled in hippocampal slices, the class III antiarrhythmic agent tedisamil inhibited the fast AHP with no effect on the slow AHP (J. Church, unpublished observations); the effect of tedisamil to inhibit the IBK(Ca) examined here has been reported previously (McLarnon & Wang, 1991). Thus, the present results may reflect previous findings that reductions in pHi at a constant pHo inhibit fast AHPs in CA1 neurones in the face of only minor reductions in the width of Ca2+-mediated depolarizing potentials (Church, 1992). The results also suggest that changes in the magnitude of fast AHPs evoked by changes in pHo (Church, 1992) may reflect not only alterations in Ca2+ influx (mediated by changes in pHo) but also changes in the activity of Ca2+-dependent K+ channels (mediated by changes in pHi consequent upon changes in pHo). They also uncover another mechanism for modulation of large conductance Ca2+-activated K+ channels, in addition to cellular redox potential (DiChiara & Reinhart, 1997; Wang et al. 1997), which occurs downstream from Ca2+ influx and indicate that changes in pHi are able to uncouple the activation of BK-type channels from the internal Ca2+ load.
Our observation that changes in pHi modulate a IBK(Ca) in hippocampal neurones is consistent with reports which indicate that protons acting on the cytoplasmic side of the membrane suppress Ca2+-activated K+ currents in a variety of cell types in an especially sensitive manner (Christensen & Zeuthen, 1987; Kume et al. 1990; Copello et al. 1991; Laurido et al. 1991; Peers & Green, 1991). In all cases in which it has been examined, reductions in internal pH lower Po and, in the present study, this effect was tentatively ascribed to an increase in the closed time of the channel (see Christensen & Zeuthen, 1987). At an internal [Ca2+] of 20 μM there was little effect on IBK(Ca) when internal pH was increased above 7.3 whereas, when internal [Ca2+] was reduced to 0.7 μM, graded increases in Po were observed when internal pH was raised from 6.7 to 7.0 to 7.3 to 7.8. These observations suggest that, as in other cell types (e.g. Christensen & Zeuthen, 1987; Kume et al. 1990), protons affect the IBK(Ca) in hippocampal neurones by modifying channel gating rather than by changing single channel conductance, although more detailed studies of channel kinetics are required to determine whether protons compete with Ca2+ at regulatory binding sites (e.g. Christensen & Zeuthen, 1987; Copello et al. 1991) or whether they exert their actions via an allosteric site on the channel complex (e.g. Laurido et al. 1991).
Given the sensitivity of slow AHPs in hippocampal neurones to changes in pHo and pHi (Church & McLennan, 1989; Church, 1992), and the fact that SK channels are more sensitive to Ca2+ ions than BK channels (Lancaster et al. 1991), it will be interesting to determine the effects of changes in pHi on small conductance (SK-type) Ca2+-activated K+ channel(s) which underlie slow AHPs. In addition, future studies should assess whether the effects of pHi in rat hippocampal neurones are relatively selective for Ca2+-activated K+ currents (as is the case in type I carotid body cells; Peers & Green, 1991) or whether changes in pHi can also affect Ca2+-independent K+ currents. Given the lability of neuronal pHi and the importance of Ca2+-dependent K+ channels for the regulation of Ca2+ influx and neurotransmitter release presynaptically and for the integration of synaptic potentials and neuronal firing behaviour postsynaptically (Storm, 1990), the sensitivity of Ca2+-activated K+ channels to changes in pHi may have important implications for neuronal function.
Acknowledgments
We thank Dr K. Abdel-Hamid for his participation in initial experiments and Ms S. Atmadja and Ms M. Grunert for the preparation and maintenance of the neuronal cultures. Financial support was provided by operating grants to J. C. from the Medical Research Council of Canada and to J. G. McL. from the Natural Sciences and Engineering Research Council of Canada. K. A. B. was the recipient of a Heart and Stroke Foundation of BC and Yukon Summer Studentship.
References
- Balestrino M, Somjen GG. Concentration of carbon dioxide, interstitial pH and synaptic transmission in hippocampal formation of the rat. The Journal of Physiology. 1988;396:247–266. doi: 10.1113/jphysiol.1988.sp016961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JN, Magleby KL, Pallotta BS. Properties of single calcium-activated potassium channels in cultured rat muscle. The Journal of Physiology. 1982;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter KA, Church J. Characterization of acid extrusion mechanisms in cultured fetal rat hippocampal neurones. The Journal of Physiology. 1996;493:457–470. doi: 10.1113/jphysiol.1996.sp021396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevensee MO, Schwiening CJ, Boron WF. Use of BCECF and propidium iodide to assess membrane integrity of acutely isolated CA1 neurons from rat hippocampus. Journal of Neuroscience Methods. 1996;58:61–75. doi: 10.1016/0165-0270(94)00159-e. [DOI] [PubMed] [Google Scholar]
- Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends in Neurosciences. 1992;15:396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Christensen O, Zeuthen T. Maxi K+ channels in leaky epithelia are regulated by intracellular Ca2+, pH and membrane potential. Pflügers Archiv. 1987;408:249–259. doi: 10.1007/BF02181467. [DOI] [PubMed] [Google Scholar]
- Church J. A change from HCO3−-CO2- to Hepes-buffered medium modifies membrane properties of rat CA1 pyramidal neurones in vitro. The Journal of Physiology. 1992;455:51–71. doi: 10.1113/jphysiol.1992.sp019290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church J, Fletcher EJ, Abdel-Hamid K, MacDonald JF. Loperamide blocks high-voltage-activated calcium channels and N-methyl-D-aspartate-evoked responses in rat and mouse cultured hippocampal pyramidal neurons. Molecular Pharmacology. 1994;45:747–757. [PubMed] [Google Scholar]
- Church J, McLennan H. Electrophysiological properties of rat CA1 pyramidal neurones in vitro modified by changes in extracellular bicarbonate. The Journal of Physiology. 1989;415:85–108. doi: 10.1113/jphysiol.1989.sp017713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copello J, Segal Y, Reuss L. Cytosolic pH regulates maxi K+ channels in Necturus gall-bladder epithelial cells. The Journal of Physiology. 1991;434:577–590. doi: 10.1113/jphysiol.1991.sp018487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiChiara TJ, Reinhart PH. Redox modulation of hslo Ca2+-activated K+ channels. Journal of Neuroscience. 1997;17:4942–4955. doi: 10.1523/JNEUROSCI.17-13-04942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipolo R, Beaugé L. The effect of pH on Ca2+ extrusion mechanisms in dialyzed squid axons. Biochimica et Biophysica Acta. 1982;688:237–245. doi: 10.1016/0005-2736(82)90599-5. [DOI] [PubMed] [Google Scholar]
- Dixon DB, Takahashi K-I, Copenhagen DR. L-Glutamate suppresses HVA calcium current in catfish horizontal cells by raising intracellular proton concentration. Neuron. 1993;11:267–277. doi: 10.1016/0896-6273(93)90183-r. [DOI] [PubMed] [Google Scholar]
- Drapeau P, Nachshen DA. Effects of lowering extracellular and cytosolic pH on calcium fluxes, cytosolic calcium levels, and transmitter release in presynaptic nerve terminals isolated from rat brain. Journal of General Physiology. 1988;91:305–315. doi: 10.1085/jgp.91.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods in Enzymology. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Graber ML, DiLillo DC, Friedman BL, Pastoriza-Munoz E. Characteristics of fluoroprobes for measuring intracellular pH. Analytical Biochemistry. 1986;156:202–212. doi: 10.1016/0003-2697(86)90174-0. [DOI] [PubMed] [Google Scholar]
- Kaibara M, Kameyama M. Inhibition of the calcium channel by intracellular protons in single ventricular myocytes of the guinea-pig. The Journal of Physiology. 1988;403:621–640. doi: 10.1113/jphysiol.1988.sp017268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K. Ionic basis of GABAA receptor channel function in the nervous system. Progress in Neurobiology. 1994;42:489–537. doi: 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Kashiwagi K, Fukuchi J-I, Chao J, Igarashi K, Williams K. An aspartate residue in the extracellular loop of the N-methyl-D-aspartate receptor controls sensitivity to spermine and protons. Molecular Pharmacology. 1996;49:1131–1141. [PubMed] [Google Scholar]
- Klöckner U, Isenberg G. Intracellular pH modulates the availability of vascular L-type Ca2+ channels. Journal of General Physiology. 1994a;103:647–663. doi: 10.1085/jgp.103.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöckner U, Isenberg G. Calcium channel current of vascular smooth muscle cells: Extracellular protons modulate gating and single channel conductance. Journal of General Physiology. 1994b;103:665–678. doi: 10.1085/jgp.103.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume H, Takagi K, Satake T, Tokuno H, Tomita T. Effects of intracellular pH on calcium-activated potassium channels in rabbit tracheal smooth muscle. The Journal of Physiology. 1990;424:445–457. doi: 10.1113/jphysiol.1990.sp018076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Nicoll RA, Perkel DJ. Calcium activates two types of potassium channels in rat hippocampal neurons in culture. Journal of Neuroscience. 1991;11:23–30. doi: 10.1523/JNEUROSCI.11-01-00023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurido C, Candia S, Wolff D, Latorre R. Proton modulation of a Ca2+-activated K+ channel from rat skeletal muscle incorporated into planar bilayers. Journal of General Physiology. 1991;98:1025–1043. doi: 10.1085/jgp.98.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Mayer ML. N-methyl-D-aspartic acid receptor structure and function. Physiological Reviews. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- McLarnon JG. Inactivation of a high conductance calcium dependent potassium current in rat hippocampal neurons. Neuroscience Letters. 1995;193:5–8. doi: 10.1016/0304-3940(95)11651-c. [DOI] [PubMed] [Google Scholar]
- McLarnon JG, Sawyer D. Effects of divalent cations on the activation of a calcium-dependent potassium channel in hippocampal neurons. Pflügers Archiv. 1993;424:1–8. doi: 10.1007/BF00375095. [DOI] [PubMed] [Google Scholar]
- McLarnon JG, Wang X-P. Actions of cardiac drugs on a calcium-dependent potassium channel in hippocampal neurons. Molecular Pharmacology. 1991;39:540–546. [PubMed] [Google Scholar]
- Martínez-Zaguilán R, Parnami G, Lynch RM. Selection of fluorescent ion indicators for simultaneous measurements of pH and Ca2+ Cell Calcium. 1996;19:337–349. doi: 10.1016/s0143-4160(96)90074-3. [DOI] [PubMed] [Google Scholar]
- Mironov SL, Lux HD. Cytoplasmic alkalinization increases high-threshold calcium current in chick dorsal root ganglion neurones. Pflügers Archiv. 1991;419:138–143. doi: 10.1007/BF00372999. [DOI] [PubMed] [Google Scholar]
- Moody W. Appearance of calcium action potentials in crayfish slow muscle fibres under conditions of low intracellular pH. The Journal of Physiology. 1980;302:335–346. doi: 10.1113/jphysiol.1980.sp013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody W. Effects of intracellular H+ on the electrical properties of excitable cells. Annual Review of Neuroscience. 1984;7:257–278. doi: 10.1146/annurev.ne.07.030184.001353. [DOI] [PubMed] [Google Scholar]
- Müller W, Connor JA. Cholinergic input uncouples Ca2+ changes from K+ conductance activation and amplifies intradendritic Ca2+ changes in hippocampal neurons. Neuron. 1991;6:901–905. doi: 10.1016/0896-6273(91)90230-w. [DOI] [PubMed] [Google Scholar]
- Ou-Yang Y, Kristián T, Mellergård P, Siesjö BK. The influence of pH on glutamate- and depolarization-induced increases of intracellular calcium concentration in cortical neurons in primary culture. Brain Research. 1994;646:65–72. doi: 10.1016/0006-8993(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Ou-Yang Y, Mellergård P, Siesjö BK. Regulation of intracellular pH in single rat cortical neurons in vitro: A microspectrofluorometric study. Journal of Cerebral Blood Flow and Metabolism. 1993;13:827–840. doi: 10.1038/jcbfm.1993.105. [DOI] [PubMed] [Google Scholar]
- Peers C, Green FK. Inhibition of Ca2+-activated K+ currents by intracellular acidosis in isolated type I cells of the neonatal rat carotid body. The Journal of Physiology. 1991;437:589–602. doi: 10.1113/jphysiol.1991.sp018613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Armass S, Martínez-Zaguilán R, Martínez GM, Gillies RJ. Regulation of pH in rat brain synaptosomes. I. Role of sodium, bicarbonate, and potassium. Journal of Neurophysiology. 1994;71:2236–2248. doi: 10.1152/jn.1994.71.6.2236. [DOI] [PubMed] [Google Scholar]
- Storm JF. Potassium currents in hippocampal pyramidal cells. Progress in Brain Research. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Sapolsky RM. Evolving concepts about the role of acidosis in ischemic neuropathology. Journal of Neurochemistry. 1993;61:793–803. doi: 10.1111/j.1471-4159.1993.tb03589.x. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Somjen GG. Effects of extracellular pH on voltage-gated Na+, K+ and Ca2+ currents in isolated rat CA1 neurons. The Journal of Physiology. 1996;493:719–732. doi: 10.1113/jphysiol.1996.sp021417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh GC, Somjen GG. Differential sensitivity to intracellular pH among high- and low-threshold Ca2+ currents in isolated rat CA1 neurons. Journal of Neurophysiology. 1997;77:639–653. doi: 10.1152/jn.1997.77.2.639. [DOI] [PubMed] [Google Scholar]
- Voipio J, Paalasmaa P, Taira T, Kaila K. Pharmacological characterization of extracellular pH transients evoked by selective synaptic and exogenous activation of AMPA, NMDA, and GABAA receptors in the rat hippocampal slice. Journal of Neurophysiology. 1995;74:633–642. doi: 10.1152/jn.1995.74.2.633. [DOI] [PubMed] [Google Scholar]
- Wakabayashi S, Shigekawa M, Pouysségur J. Molecular physiology of vertebrate Na+/H+ exchangers. Physiological Reviews. 1997;77:51–74. doi: 10.1152/physrev.1997.77.1.51. [DOI] [PubMed] [Google Scholar]
- Wang Z-W, Nara M, Wang Y-X, Kotlikoff MI. Redox regulation of large conductance Ca2+-activated K+ channels in smooth muscle cells. Journal of General Physiology. 1997;110:35–44. doi: 10.1085/jgp.110.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]


