Abstract
The effect of externally applied ATP on cytosolic free Ca2+ concentration ([Ca2+]i) was tested in single isolated rat neurohypophysial nerve terminals by fura-2 imaging. The release of vasopressin (AVP) and oxytocin (OT) upon ATP stimulation was also studied from a population of terminals using specific radioimmunoassays.
ATP evoked a sustained [Ca2+]i increase, which was dose dependent in the 1-100 μM range (EC50= 4·8 μM). This effect was observed in only ≈40 % of the terminals.
Interestingly, ATP, in the same range (EC50= 8·6 μM), evoked AVP, but no significant OT, release from these terminals.
Both the [Ca2+]i increase and AVP release induced by ATP were highly and reversibly inhibited by suramin, suggesting the involvement of a P2 purinergic receptor in the ATP-induced responses. Pyridoxal-5-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), another P2 purinergic receptor antagonist, strongly reduced the ATP-induced [Ca2+]i response.
To further characterize the receptor, different agonists were tested, with the following efficacy: ATP = 2-methylthio-ATP > ATP-γ-S > α,β-methylene-ATP > ADP. The compounds adenosine, AMP, β,γ-methylene-ATP and UTP were ineffective.
The ATP-dependent [Ca2+]i increase was dependent on extracellular Ca2+ concentration ([Ca2+]o). Fluorescence-quenching experiments with Mn2+ showed that externally applied ATP triggered a Mn2+ influx. The ATP-induced [Ca2+]i increase and AVP release were independent of and additive to a K+-induced response, in addition to being insensitive to Cd2+. The ATP-induced [Ca2+]i increase was strongly reduced in the presence of Gd3+. These results suggest that the observed [Ca2+]i increases were elicited by Ca2+ entry through a P2X channel receptor rather than via a voltage-dependent Ca2+ channel.
We propose that ATP, co-released with neuropeptides, could act as a paracrine-autocrine messenger, stimulating, via Ca2+ entry through a P2X2 receptor, the secretion of AVP, in particular, from neurohypophysial nerve terminals.
ATP has been shown to act as a neurotransmitter in both the peripheral and central nervous systems (Inoue & Nakazawa, 1992; Edwards & Gibb, 1993), yet little is known about central ATP-mediated transmission (Séguéla et al. 1996). Co-storage of ATP with acetylcholine and catecholamines has been demonstrated for a variety of peripheral and central synaptic vesicles (for review see Zimmermann, 1994). ATP co-released with these neurotransmitters can act as a fast excitatory agent on both cholinergic (Edwards et al. 1992; Evans et al. 1992; Silinsky et al. 1992) and adrenergic (Vizi et al. 1992; Kurz et al. 1993; Buller et al. 1996) neurones. Furthermore, in endocrine cells, ATP can act as an agonist of hormone release. For example, ATP stimulates the release of catecholamines from PC12 cells (Inoue et al. 1992) and of luteinizing hormone from gonadotrophs (Tomic et al. 1996). On the other hand, ATP has been shown to inhibit calcium currents in chromaffin cells (Currie & Fox, 1996). Finally, although ATP has been shown to activate a ligand-gated ion channel in cholinergic terminals (Sun & Stanley, 1996), it has never been shown to increase intraterminal calcium levels directly or to stimulate release from CNS terminals.
The neurohypophysis is the original model CNS neurosecretory system (Douglas & Poisner, 1964), releasing neuropeptide hormones synthesized by the magnocellular neurones of the hypothalamus. The release of the neuropeptides is triggered by specific firing patterns of the hypothalamic neurones (Cazalis et al. 1985). This system has been used to demonstrate the direct relationship between [Ca2+]i increase and neurohormone secretion (Brethes et al. 1987). Isolated neurohypophysial terminals, which consist almost exclusively of oxytocin- (OT) and vasopressin- (AVP) releasing nerve terminals, provide an excellent model system for studying the regulation of secretion at the terminal level, free from the complex array of synaptic effects present throughout the rest of the CNS.
In order to study any possible ATP feedback regulation of CNS terminals directly, we used isolated neurohypophysial terminals to measure the effect of extracellular ATP on both intraterminal Ca2+ levels (by fura-2 imaging) and AVP/OT release (by specific radioimmunoassays). A preliminary account of this work has appeared in abstract form (Troadec et al. 1997).
METHODS
All standard chemicals were purchased from Sigma-Aldrich (France) with the exception of fura-2 AM, which was obtained from Molecular Probes (USA).
Preparation of isolated neurohypophysial nerve terminals
Isolated nerve terminals were prepared from male Wistar rats (200-300 g) as previously described by Cazalis et al. (1987a). Animals were killed by decapitation with a guillotine following the guidelines laid down by the French/European ethical committee, and the pituitary isolated. After removal of the anterior lobe and the pars intermedia, the neural lobe was homogenized at 37°C in 100 μl of a solution containing (mM): sucrose, 270; EGTA, 0.1 or 2; Hepes, 20; and adjusted to pH 7.2 with Tris. For release experiments the solution containing the dissociated neurohypophysis was centrifuged at 100 g for 1 min with the supernatant, then centrifuged at 2400 g for 4 min. The resulting pellet was then resuspended in physiological saline at 37°C that contained (mM): NaCl, 140; KHCO3, 5; MgCl2, 1; CaCl2, 2.2; glucose, 10; Hepes, 10, buffered to pH 7.2 with Tris; and bovine serum albumin at 0.01 % (w/v). For [Ca2+]i monitoring experiments, the centrifugations were omitted, and the homogenate of one neurohypophysis was directly placed onto a glass coverslip forming the bottom of a 35 mm Petri dish, and put in an open recording chamber. After 10 min, the nerve terminals were bathed with 200 μl of normal Locke buffer at 37°C (mM: NaCl, 140; KCl, 5; Hepes, 20; glucose, 10; MgCl2, 1; and CaCl2, 2.2, pH adjusted to 7.4 with NaOH) for 20 min. The osmolarity of the solutions used in this study ranged between 290 and 300 mosmol l−1. In both cases a highly purified suspension of isolated nerve terminals was obtained (Nordmann et al. 1987). The preparation contained the two types of previously described terminals, endings and swellings (Toescu & Morris, 1990), both known to be able to release neurohormones upon depolarization (Nordmann & Dayanithi, 1988).
[Ca2+]i measurements
For loading of the Ca2+-sensitive fluorescent indicator fura-2, isolated nerve terminals were superfused (1 ml min−1) with normal Locke buffer containing 1 μM fura-2 acetoxymethyl ester (AM) in DMSO (0.01 % final concentration) for 30 min at 37°C. The nerve terminals were then rinsed with normal Locke buffer for 20 min to permit maximum hydrolysis of the acetoxymethyl ester. The flow rate of the bathing solution was increased to 2 ml min−1 in order to remove from the recording chamber all free-floating nerve terminals, red blood cells and incompletely dissociated tissue resulting from the homogenization process. The 35 mm Petri dish was secured to the stage of an inverted microscope (Nikon Diaphot TDM) in a temperature-controlled device (Medical Systems, NY, USA). Peptidergic nerve terminals were identified using phase-contrast optics (e.g. Fig. 1D). A rapid change in the superfusion solution was accomplished with the use of a 10-port distribution valve connected to a series of solution reservoirs that were attached to a micromanipulator-controlled outlet on top of the chamber.
Figure 1. Calcium imaging of isolated nerve terminals.
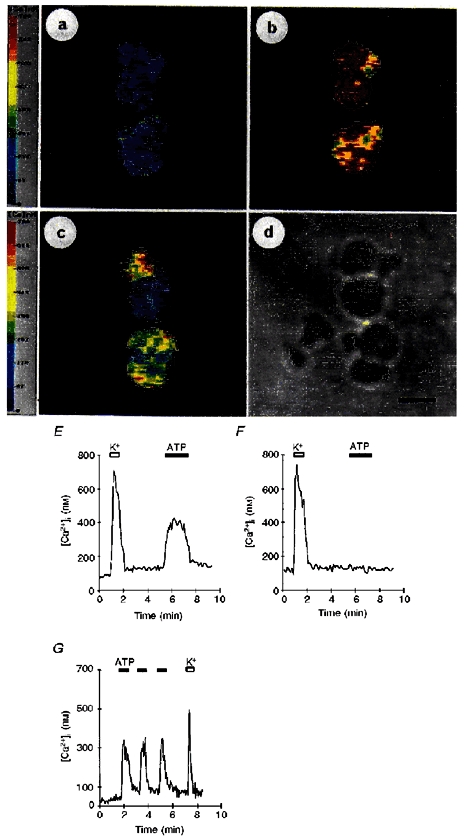
A, images of a number of neurohypophysial terminals obtained under resting conditions. B, images observed after perfusion with 50 mM K+. Note that all terminals displayed a rise in [Ca2+]i. C, after washing, the same terminals were exposed to 100 μM ATP. Images show that not all terminals have responded to ATP. Pseudocolour bars on the left represent levels of [Ca2+]i, as indicated. D, same group of nerve terminals observed with phase-contrast microscopy; scale bar, 4 μm. E, traces represent the [Ca2+]i response recorded from an ATP-sensitive terminal selected from the above images. F, trace represents the [Ca2+]i response recorded from an ATP-insensitive terminal. G, successive, short (1 min) 100 μM ATP challenges all evoked similar responses. The subsequent exposure to 50 mM K+ challenge (indicated by open bar) was routinely performed as a control stimulus to compare the amplitude of the response with the previous ATP challenges. Filled bars, ATP applications.
The inverted microscope was fitted with a CCD camera (Darkstar 800-Photonic Science, 10−6 lx). Regions of isolated nerve terminals were selected using a × 100 oil-immersion epifluorescence objective (Nikon Fluor, NA 1.30). The set-up consisted of a 100 W xenon lamp and a digitally controlled chopper wheel (lambda 10, Sutter Instruments), which alternated between 350 ± 10 and 380 ± 10 nm excitation filters (or 360 ± 10 nm for Mn2+ quenching experiments). This wheel was equipped with a shutter in order to control the exposure time. The emitted light was filtered through a 400 nm dichroic mirror and a 510 nm barrier filter. Four consecutive images acquired by the camera were averaged to reduce the signal-to-noise ratio, and images were digitized to 255 grey levels. For each experimental run, a field of 132 μm × 132 μm was recorded, containing between ten and forty nerve terminals. Each experiment was repeated at least twice, on two different animals.
[Ca2+]i values were calculated from the ratio between recordings at 350 and 380 nm, in accordance with the equation given by Grynkiewicz et al. (1985). Values of Rmin, Rmax and Fo/Fs (i.e. ratio of emitted fluorescence intensity at 380 nm excitation under Ca2+-limiting and Ca2+-saturating conditions) were determined using solutions of defined Ca2+ concentration and were 0.3, 3.4 and 7.5, respectively. The Ca2+Kd value of 224 for fura-2 was taken from Grynkiewicz et al. (1985). Photobleaching was minimized by decreasing illumination intensity with neutral filters and by spacing the frame acquisition rate.
The technique of Mn2+ quenching of fura-2 (Hallam & Rink, 1985) was also used on the isolated neurohypophysial nerve terminals. MnCl2 (1 mM) was included in the external medium containing 2.2 mM CaCl2, and fluorescence was monitored at 360 nm, the isosbestic point for fura-2 AM. Quenching of fura-2 fluorescence by Mn2+ was evaluated by calculating quenching slopes (absolute values) before ATP application and during the 10 s following the ATP challenge.
Measurement of AVP and OT release
For each experimental run, the isolated terminals from two neurohypophyses were equally distributed and loaded onto four filters, i.e. each filter received about 25 % of the total terminals. The release of AVP or OT from each filter was considered as an independent experiment (n= 1). Experiments were repeated at least two to three times to gather sufficient sample size. The experiments were performed to see the effect of more than one substance at one time. One out of the four filters was used for control test (either ATP or K+ depending on the experimental design), the second and third filters were used for testing substance I (e.g. CdCl2 or suramin etc.) and the fourth filter was used to test a different substance, substance II.
Following loading of isolated nerve terminals onto filters (0.45 μm Acrodisc, LCP-VDF, Gelman Sciences, USA), they were perfused (Minipulse Peristaltic Pump, Gilson, USA) for 45 min with normal Locke solution at a flow rate of 50 μl min−1 followed by 45 min of a low Na+ (40 mM) saline that contained (mM): NaCl, 40; N-methyl-D-glucamine chloride (NMG-Cl), 100; KHCO3, 5; MgCl2, 1; CaCl2, 2.2; glucose, 10; Hepes, 10, buffered to pH 7.2 with Tris; and bovine serum albumin at 0.01 % (w/v). The flow rate was slowly increased during this period to 100 μl min−1. Collection of the perfusate over 4 min periods started 60 min after loading the nerve terminals onto the filter, as has previously been described (Cazalis et al. 1987b; Wang et al. 1997). AVP and OT content in each fraction were then determined by radioimmunoassay as already described (Cazalis et al. 1985, 1987a) with necessary modifications (Wang et al. 1997). The antisera were kindly given by Dr John Bicknell, Babraham, UK.
In the AVP and OT release experiments reported in this paper, the results represent the average AVP or OT content of standard aliquots taken from the collected fractions arising from at least three separate groups of isolated nerve terminals. Internal control groups arising from the same pool of isolated nerve terminals were run for comparison with test groups. Differences in the total amount of AVP and OT released between experiments resulted from differences in the amount of each preparation loaded onto the filters and from heterogeneity of secretory responsiveness between preparations. In a number of experiments we have used the S2/S1 protocol for analysis described by Dayanithi et al. (1992). In these experiments the nerve terminals received two stimulation periods, control and test, defined as S1 and S2, respectively, separated by a recovery period. The amount of hormone release during each period was calculated by subtracting the amount of hormone released under basal conditions from that observed during, and directly after, the stimulus.
Statistical analysis
The [Ca2+]i reported throughout the text (means ±s.e.m.) were calculated solely for the ATP responsive terminals. The experimental results were analysed by Mann-Whitney non-parametric test using Instat 2.01 for Macintosh (GraphPad Software, USA). Results are expressed as means ±s.e.m., and P values < 0.05 were considered significant. EC50 values were calculated by fitting a Langmuir isotherm to the data using Sigma plot (Jandel Scientific, USA).
RESULTS
ATP evokes an [Ca2+]i increase in CNS nerve terminals
In normal Locke buffer, the resting [Ca2+]i was 73 ± 4 nM (n= 197) as shown in Fig. 1A,E and F. The control stimulus used in this study was a normal Locke buffer containing high (50 mM) K+ which evoked a rise (Fig. 1B,E, and F) in [Ca2+]i of 587 ± 12 nM above resting or basal levels in 90 ± 1 % of the terminals tested (n= 43). Exposure to 100 μM extracellular ATP induced a single, rapid increase (345 ± 32 nM, n= 43) in [Ca2+]i but in only 40 ± 2 % of the terminals tested (see e.g. Fig. 1C). The response was maintained during the entire duration of the ATP application (Fig. 1E), and then [Ca2+]i returned to near-basal levels once ATP was washed out (Fig. 1E). Successive ATP stimulations separated by short (30-60 s) periods resulted in identical increases in [Ca2+]i (first challenge, 284 ± 31 nM; second challenge, 299 ± 39 nM; third challenge, 310 ± 36 nM; n= 8; Fig. 1G).
Nerve terminals were stimulated with increasing concentrations, from 1 μM to 2 mM, of ATP (Fig. 2). The amplitude of the [Ca2+]i response increased as a function of [ATP] (Fig. 2A). In order to obtain a clear dose-response relationship, the peak amplitudes of the [Ca2+]i responses were calculated (peak amplitude minus basal) and pooled from individual terminals which were exposed to only one ATP concentration. A small response was evoked by extracellularly applied 1 μM ATP (56 ± 19 nM, n= 7; Fig. 2B). [Ca2+]i increases were 85 ± 7, 183 ± 22 and 262 ± 21 nM for 2.5, 5 and 10 μM ATP, respectively (n= 22; Fig. 2B). The response was dose dependent (EC50= 4.8 μM) up to 100 μM, the concentration which evoked a maximal effect (Fig. 2B). Higher ATP concentrations had no significant (P= 0.62) further effect on the [Ca2+]i increase, e.g. 344 ± 15 nM for 2 mM ATP (n= 25).
Figure 2. Dose-dependent effects of ATP.
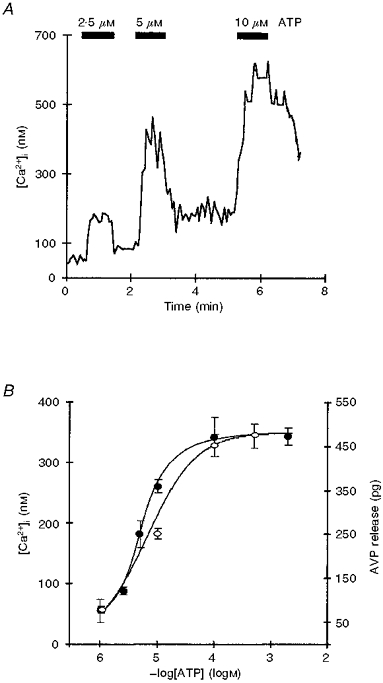
A, effect of increasing ATP concentrations (2.5, 5 and 10 μM, indicated by filled bars) on [Ca2+]i of a single neurohypophysial nerve terminal. B, dose-response curve of the log of extracellular ATP concentration vs. resulting changes in [Ca2+]i (•) and AVP release (○). Groups of nerve terminals were exposed to different concentrations of ATP, and evoked [Ca2+]i (nM) and AVP (pg) increases were calculated by subtracting the basal Ca2+ and AVP levels. The observed EC50 for [Ca2+]i is 4.8 μM and for AVP release it is 8.6 μM ATP. Symbols represent means and error bars represent s.e.m.
ATP induces AVP release from isolated nerve terminals
To test the possibility that the ATP-induced [Ca2+]i increase was able to trigger subsequent neurohormone secretion, the effect of ATP on the release of AVP from isolated neurohypophysial nerve terminals was tested. As was the case for the ATP-dependent [Ca2+]i response, a small ATP-dependent increase in AVP release was recorded beginning with 1 μM ATP (AVP 80 ± 7 pg above basal, n= 3; Fig. 2B). Perfusion of the nerve terminals with 10 μM extracellular ATP (Fig. 2B) evoked a larger AVP response (252 ± 12 pg above basal, n= 4). The maximal effect was obtained with 100 μM ATP (452 ± 25 pg, n= 4; see Fig. 3A), and the application of higher ATP concentrations (e.g. 500 μM) evoked no significantly (P= 0.44) larger increases in AVP release (475 ± 28 pg above basal, n= 4). The EC50 value for AVP release was 8.6 μM ATP (see Fig. 2B).
Figure 3. Effects of ATP on AVP and OT release.
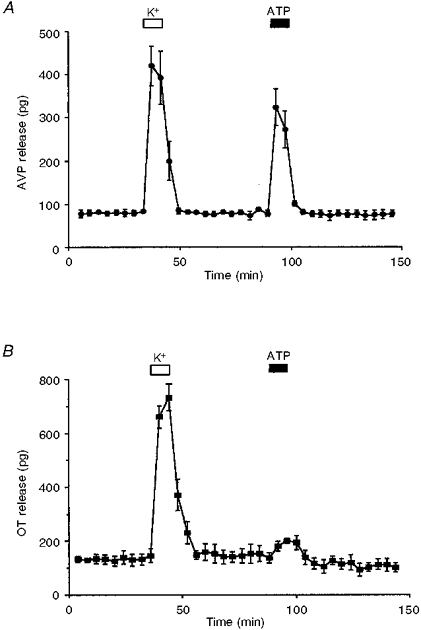
A, time course of AVP release from isolated nerve terminals first exposed to a depolarizing stimulus (50 mM K+, open bar), and then to ATP (100 μM, filled bar). The results are expressed as amount (in pg) of AVP released per 4 min fraction. B, time course of OT release. Nerve terminals were first stimulated with 50 mM K+ and then with 100 μM ATP. The results are expressed as amount (in pg) of OT released per 4 min fraction. Note that ATP, even at 100 μM, only induced a very small increase in OT release.
The time course of release was also analysed. Secretion was first evoked by high K+ depolarizations lasting 8 min, which gave an increase in AVP release of 782 ± 40 pg above basal hormone levels (n= 5). After a resting period of 40 min, perfusion with 100 μM ATP (Fig. 3A) resulted in another increase in hormone release (452 ± 25 pg above basal AVP, n= 4).
ATP induces only a weak OT release
In order to study the effect of ATP on OT release, we measured OT release simultaneously from the same perfusate samples as AVP (Fig. 3B). Basal content was 95 ± 12 pg OT (n= 14). Stimulation with 50 mM K+ triggered an increase in OT release of 1472 ± 48 pg OT (n= 4). Subsequently, a small increase in OT release was observed with 100 μM ATP (133 ± 18 pg above basal, n= 3), but the observed increase did not reach statistical significance (P= 0.14). This response was the maximum observed, since 500 μM ATP induced a similar response (to 148 ± 9 pg OT above basal; n= 7). Furthermore, OT release was not affected by 10 μM ATP (40 ± 19 pg, n= 4; data not shown).
Interestingly, similar results for OT release were observed when the 100 μM ATP stimulation was performed before the stimulation with 50 mM K+, thus excluding the possibility of depletion of releasable OT due to pre-stimulation with 50 mM K+ (see e.g. Fig. 3B).
Effect of purinergic receptor antagonists
The effect of suramin, a P2 purinergic receptor antagonist, was tested on the ATP-induced [Ca2+]i increase (Fig. 4A). After 3 min of preincubation in normal Locke buffer containing 300 μM suramin, nerve terminals were re-stimulated with 100 μM ATP in the presence of suramin. The effect of ATP on the [Ca2+]i response was abolished (ATP control, 259 ± 22 nM; suramin plus ATP, 24 ± 8 nM; inhibition, 91 ± 3 %; n= 37; Fig. 4A). This inhibition by the P2 antagonist was reversible. Suramin did not affect the high K+-induced [Ca2+]i increase (high K+ control, 548 ± 37 nM; high K+ plus suramin, 541 ± 34 nM; n= 10, P= 0.88; data not shown). When suramin was used at 100 μM, the response to ATP challenge showed a partial inhibition (45 ± 5 %; ATP control, 237 ± 22 nM; suramin plus ATP, 127 ± 17 nM; n= 19; data not shown). Most importantly, the release of AVP induced by ATP was strongly abolished by 300 μM suramin (evoked release for K+, 625 ± 49 pg; ATP, 517 ± 37 pg; suramin plus ATP, 115 ± 11 pg; n= 4; Fig. 4B). Similarly, when suramin was used at only 100 μM, the ATP-evoked AVP release was partially inhibited (59 ± 2 %; n= 3, data not shown).
Figure 4. Effect of suramin on ATP responses.
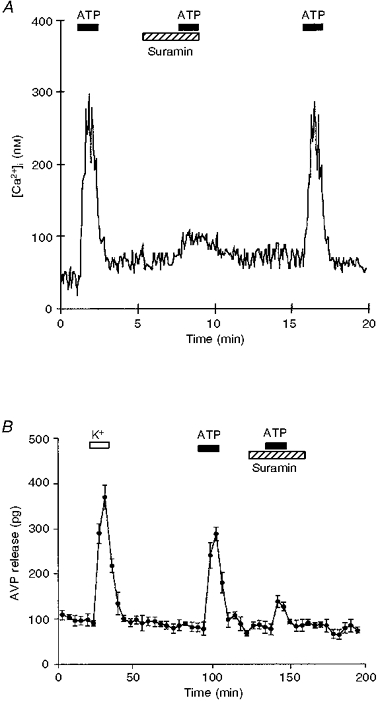
A, in fura-2-loaded neurohypophysial nerve terminals, after a control ATP (100 μM) exposure, another 100 μM ATP stimulation failed to elicit a response when nerve terminals were preincubated with 300 μM suramin (indicated by hatched bar). After washing away the purinergic receptor inhibitor, the ATP-dependent [Ca2+]i increase was restored. B, isolated nerve terminals were first challenged with high (50 mM) K+ followed by 100 μM ATP. The terminals were then preincubated with 300 μM suramin 10 min before the second ATP exposure. Note that the ATP-evoked AVP release is strongly inhibited by suramin.
Another P2 purinergic receptor antagonist, pyridoxal-5-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), was tested on the ATP-induced [Ca2+]i increase. After 1 min of preincubation in normal Locke buffer containing 10 μM PPADS, the nerve terminals were stimulated with 100 μM ATP in the presence of PPADS. Figure 5A shows that the [Ca2+]i increase induced by ATP was strongly inhibited by PPADS (ATP control, 455 ± 75 nM; PPADS plus ATP, 48 ± 28 nM; inhibition, 93 ± 4 %; n= 7). The recovery of the ATP response after PPADS was incomplete, however.
Figure 5. Effect of purinergic receptor agonists and antagonists on ATP responses.
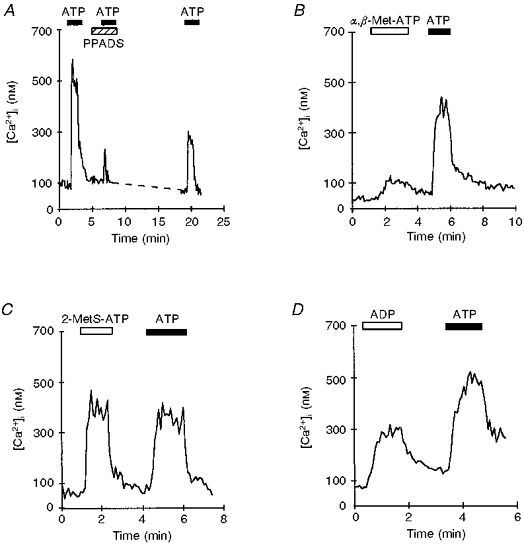
A, trace represents the effect of PPADS on the ATP-induced [Ca2+]i increase in an individual nerve terminal. After a control ATP response, preincubation with 10 μM PPADS (indicated by hatched bar), strongly inhibited the [Ca2+]i response to 100 μM ATP. After washing away the inhibitor, the ATP-dependent [Ca2+]i increase was partially restored. B, 100 μM α,β-methylene-ATP (α,β-Met-ATP, open bar) elicited a much weaker response than a subsequent 100 μM ATP challenge. C, application of 100 μM 2-methylthio-ATP (2-MetS-ATP, open bar) evoked an [Ca2+]i response very similar in amplitude and duration to that observed during a 100 μM ATP challenge. D, representative traces showing the effects of both 1 mM ADP and 100 μM ATP on [Ca2+]i in a single nerve terminal. ADP at lower concentrations had little or no effect (not shown). Filled bars indicate time of ATP application.
Effect of purinergic receptor agonists
Adenosine as well as AMP (up to 1 mM, 3 experimental runs) was ineffective in increasing [Ca2+]i in fura-2-loaded nerve terminals. Adenosine and AMP are known to be more effective than ATP in stimulating P1-type purinergic receptors, suggesting that P1 receptors were not involved in mediating the ATP-evoked [Ca2+]i increase of nerve terminals. In another series of experiments, fura-2-loaded isolated nerve terminals were challenged with extracellular ADP, in the range of 1-100 μM, and no response was observed (3 experimental runs). However, at a higher concentration (1 mM), the increase in [Ca2+]i was observed in 40 ± 3 % of the terminals (191 ± 20 nM, n= 36). These terminals were still capable of responding to 100 μM ATP (325 ± 47 nM, n= 36; Fig. 5D). The perfusion of terminals with 100 μM ATP-γ-S, a non-hydrolysable analogue of ATP, induced a rise in [Ca2+]i (128 ± 6 nM, n= 29) in 34 ± 3 % of the terminals.
Uridine triphosphate (UTP), used in concentrations up to 1 mM, had no effect on [Ca2+]i (5 experimental runs). Exposure to the agonist α,β-methylene-ATP (100 μM) induced a weak increase in [Ca2+]i (32 ± 3 nM in 17 ± 5 % of the terminals; ATP control, 158 ± 14 nM; n= 18; Fig. 5B), while no response was recorded when nerve terminals were perfused with 100 μM β,γ-methylene-ATP (3 experimental runs). We also tested 2-methylthio-ATP (100 μM), which evoked a clear increase in [Ca2+]i (240 ± 13 nM in 38 ± 10 % of the terminals; ATP control, 216 ± 18 nM; n= 50; Fig. 5C). 2-Methylthio-ATP and ATP were equally effective in increasing the [Ca2+]i of neurohypophysial nerve terminals.
Extracellular Ca2+ and the ATP-induced [Ca2+]i increase
In Ca2+-free conditions (5 mM EGTA), externally applied ATP did not affect the [Ca2+]i of neurohypophysial nerve terminals (basal [Ca2+]i, 80 ± 5 nM; ATP challenge in the absence of external Ca2+, 79 ± 5 nM; n= 56; Fig. 6A). The effect of this Ca2+ depletion was reversible, since 100 μM ATP induced a normal [Ca2+]i response following the reintroduction of 2.2 mM Ca2+ into the normal Locke buffer (223 ± 14 nM, n= 56; Fig. 6A). During a maintained 100 μM ATP perfusion, a decrease of extracellular Ca2+ concentration from 2.2 mM (normal Locke buffer) to 100 μM triggered a marked decrease in [Ca2+]i (365 ± 46 nM to 147 ± 15 nM, n= 23; Fig. 6B). The effect of this external Ca2+ decrease was reversible: [Ca2+]i rose to normal levels (n= 13) following reintroduction of 2.2 mM Ca2+ into the perfusion medium (346 ± 48 nM; Fig. 6B).
Figure 6. Effect of [Ca2+]o concentration on ATP-induced [Ca2+]i increase.
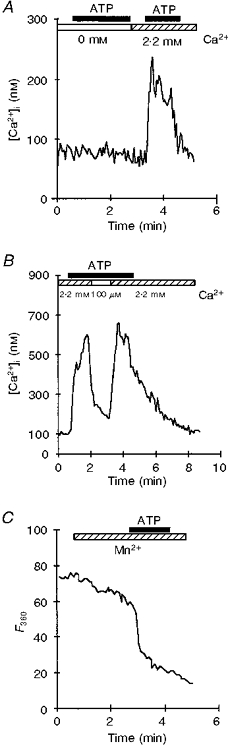
A, in Ca2+-free Locke buffer (indicated by open bar), exposure to 100 μM ATP had no effect on the [Ca2+]i of isolated neurohypophysial nerve terminals. After returning to normal [Ca2+]o (hatched bar), the expected increase in [Ca2+]i was observed in response to ATP. B, during a 100 μM ATP-evoked [Ca2+]i increase, the normal (2.2 mM) [Ca2+]o was decreased to 100 μM (indicated by open bar), with a corresponding decrease in [Ca2+]i; [Ca2+]i returned to maximum upon switching back to normal [Ca2+]o. C, trace illustrates the effect of ATP on fura-2 fluorescence quenching by Mn2+. The entry of Mn2+ into the cytoplasm was monitored by fluorescence-quenching measurements at 360 nm in fura-2-loaded neurohypophysial nerve terminals. The perfusion of 1 mM MnCl2-containing normal Locke buffer (hatched bar), resulted in a slow decrease in fura-2 fluorescence, followed by a more rapid drop at the onset of 100 μM ATP perfusion. Fluorescence intensity is expressed in terms of grey levels. Filled bars indicate duration of ATP application.
To test for the possibility of ATP-induced Ca2+ entry through the plasma membrane, we quenched the fura-2 fluorescence with extracellular Mn2+. Fluorescence was monitored at 360 nm, the isosbestic point for fura-2. At this wavelength, the fluorescence of fura-2 is not dependent on Ca2+ concentration, and the fluorescence signal could only be altered by Mn2+ influx. When Locke buffer containing 1 mM MnCl2 and 2.2 mM CaCl2 was perfused, a slow decrease of fluorescence was observed (quenching slope, 0.22 ± 0.03 grey levels s−1). ATP (100 μM) dramatically accelerated the quenching (quenching slope, 0.45 ± 0.04 grey levels s−1, n= 12), suggesting that ATP opened a cation channel permeable to Mn2+ in the plasma membrane (n= 21; Fig. 6C).
To test the possible influence of voltage-dependent Ca2+ channels on the ATP-evoked [Ca2+]i increase and AVP release, it was of interest to test K+-stimulated terminals for any additional effect of extracellular ATP on [Ca2+]i and then correlate this with the effect on AVP release. After a control high K+ depolarization, nerve terminals were perfused with a solution containing high K+ in combination with 100 μM ATP. This mixture evoked an [Ca2+]i increase of 392 ± 11 nM above that with only a control depolarization (n= 15; Fig. 7A).
Figure 7. High K+- and ATP-induced [Ca2+]i increase and AVP release were additive.
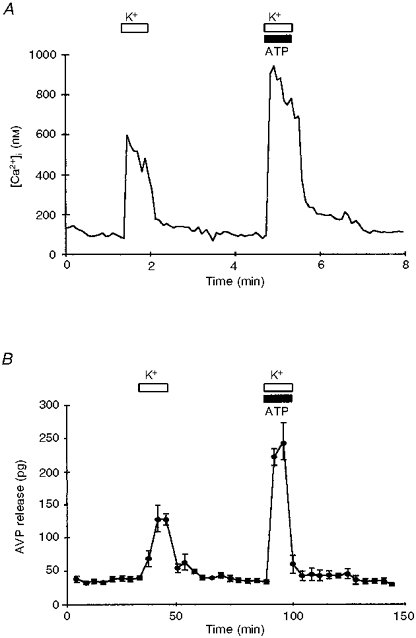
A, trace shows [Ca2+]i increase observed after application of 50 mM K+ alone (open bar) or in combination with 100 μM ATP (open and filled bars). B, similarly, AVP release in nerve terminals stimulated with 50 mM K+ alone and then with simultaneous K+ and ATP. Note that in both cases an additive response was observed when ATP and K+ were given together.
Similarly, 100 μM ATP and high K+ when given in combination evoked release of 433 ± 15 pg AVP (n= 4). In these experiments 50 mM K+ stimulation alone evoked release of 229 ± 12 pg AVP above basal levels (Fig. 7B).
Furthermore, the [Ca2+]i response induced by 100 μM ATP was not significantly affected by 100 μM Cd2+ (control ATP, 380 ± 38 nM; ATP plus Cd2+, 361 ± 15 nM; n= 30; Fig. 8A), a blocker of neurohypophysial high voltage-activated Ca2+ channels (Wang et al. 1992). In contrast, the [Ca2+]i reached with 50 mM K+ was strongly reduced in the presence of 100 μM Cd2+ (50 mM K+, 446 ± 46 nM; 50 mM K+ plus Cd2+, 36 ± 10 nM; n= 6; Fig. 8B). In parallel, we analysed the effect of Cd2+ on both K+- and ATP-induced AVP release from the nerve terminals. Figure 8C clearly illustrates that the K+-induced release of AVP was strongly blocked by the non-specific Ca2+ channel blocker, Cd2+ (K+-induced AVP release, 488 ± 36 pg; K+-induced AVP release in the presence of Cd2+, 92 ± 8 pg; n= 3), whereas the release of AVP induced by ATP was unaffected (ATP-evoked AVP release, 324 ± 29 pg; ATP-evoked AVP release in the presence of Cd2+, 317 ± 26 pg; n= 3).
Figure 8. Effect of Cd2+ and Gd3+ on the ATP- and high K+-induced [Ca2+]i increase and AVP release.
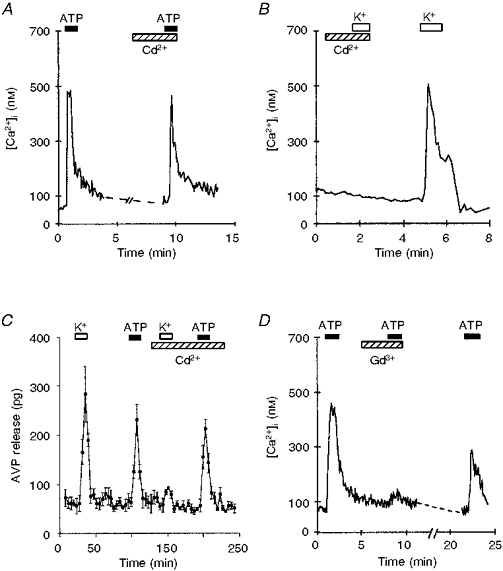
A, after ATP challenge, the nerve terminals were perfused for 5 min with buffer containing 50 μM CdCl2 (hatched bar). The presence of Cd2+ did not affect the ATP-induced [Ca2+]i response. B, the nerve terminal was perfused for 5 min in a 50 μM CdCl2-containing buffer (hatched bar), then depolarized with 50 mM K+. Application of K+ was repeated after washing off the Cd2+. The presence of Cd2+ completely blocked the depolarization-induced [Ca2+]i increase. C, AVP release from isolated nerve terminals was first measured in response to high K+ followed by 100 μM ATP. K+- but not ATP-induced AVP release was strongly inhibited by 50 μM Cd2+. D, traces illustrate fura-2-loaded nerve terminal responses to 100 μM ATP, in the presence and absence of 10 μM Gd3+ (indicated by hatched bar). The inhibitory effect was partially reversed by subsequent washout.
La3+, also commonly used to inhibit Ca2+ channels, was effective in blocking the K+-induced [Ca2+]i response (50 mM K+, 336 ± 40 nM; 50 mM K+ plus La3+, 47 ± 27 nM; n= 4), while ineffective on the ATP-induced [Ca2+]i response (control ATP, 296 ± 69 nM; ATP plus La3+, 310 ± 35 nM; n= 15; data not shown). However, exposure to a 100 μM ATP challenge in the presence of 10 μM gadolinium (Gd3+) showed in a strong, partially reversible inhibition of the calcium response (control ATP, 307 ± 25 nM; ATP plus Gd3+, 10 ± 7 nM; inhibition, 97 ± 1 %; n= 23; Fig. 8D).
DISCUSSION
ATP induces an increase in [Ca2+]i and in AVP release in nerve terminals
The present results clearly show that [Ca2+]i increases in isolated neurohypophysial nerve terminals in response to applications of extracellular ATP. This [Ca2+]i increase was observed for low concentrations of ATP, and was dose dependent in the 1-100 μM range. In addition, AVP secretion increased after applications of extracellular ATP, with a similar dose dependence (EC50= 8.6 μM compared with 4.8 μM for [Ca2+]i increases). This strongly suggests that ATP triggered the AVP release via an [Ca2+]i increase. The [Ca2+]i increase and subsequent AVP release from nerve terminals resulting from K+ depolarization vs. ATP stimulation are distinct as shown in Fig. 7. In both cases, the ATP response was additive with that of high K+.
Only a subpopulation of nerve terminals was sensitive to ATP
A subpopulation of fura-2-loaded nerve terminals was insensitive to the application of any concentration of ATP. This heterogeneity was consistently observed and is unlikely to result from nerve terminal damage, since most of the nerve terminals (90 %), including those that did not respond to ATP, exhibited an increase in [Ca2+]i in response to depolarization.
Considering only 40 % of the tested nerve terminals responded to ATP and that AVP release, in particular, was strongly affected by this nucleotide, the simplest hypothesis is that ATP-sensitive nerve terminals preferentially contain AVP rather than OT. Functional differences have previously been reported between these two types of neuropeptidergic terminals. The electrical activities invading the two types are different, with a single burst of activity for OTergic neurones and a phasic pattern of activity for AVPergic neurones (Cazalis et al. 1985; Dayanithi et al. 1996). Furthermore, it has recently been demonstrated that Q-type Ca2+ channels are present on a subset of the neurohypophysial terminals where, in combination with N-and L-type channels, they control AVP but not OT peptide secretion (Wang et al. 1997).
Our results show that extracellular ATP triggers significant AVP but weak OT secretion. The nerve terminals that exhibit an ATP-induced [Ca2+]i rise could thus belong to the AVPergic population. The small OT secretion observed after ATP stimulation could originate from a subpopulation of terminals that contain both AVP and OT. Indeed, in hypothalamic supraoptic neurones, in situ hybridization has revealed coexpression of the messenger RNA for OT and AVP in a small population of cells (Kiyama & Emson, 1990). In addition, in supraoptic and paraventricular nuclei of lactating rats, some neurones display both OT- and AVP-related electrical activity, suggesting the possibility of coexistence of the two peptides (Moos & Ingram, 1995). Most recently, it was found that a small percentage of supraoptic nucleus neurones responded to both AVP and OT with increases in [Ca2+]i (Sabatier et al. 1997). Immunoblots of individual terminals also show the existence of terminals containing both neurohormones (D. Moorman, H. Curran & J. R. Lemos, unpublished observations). It is noteworthy that the percentage of the cell population displaying this mixed phenotype was about 10 % in in situ hybridization, electrophysiological and immunoblot studies. These data are all consistent with the hypothesis that ATP-sensitive terminals are AVPergic.
The ATP-dependent [Ca2+]i increase and AVP release involves a P2X purinergic receptor
Suramin has been shown to act as an antagonist to several types of P2 purinergic receptors, inhibiting various responses to extracellular ATP (Dunn & Blakely, 1988; Den Hertog et al. 1989a,b; Hoyle et al. 1990; Hourani et al. 1992; Bailey & Hourani, 1995). In the present study, suramin reversibly inhibited both ATP-induced [Ca2+]i increase and AVP release (Fig. 4). These results strongly suggest that a P2 purinergic receptor is involved in mediating the ATP-evoked Ca2+ concentration increase and subsequent AVP release observed in nerve terminals.
ATP had no effect when applied in the absence of external Ca2+. A temporary decrease in [Ca2+]o leads to a corresponding decrease in the ATP-dependent [Ca2+]i. Experiments performed in the presence of extracellular Mn2+ showed an acceleration of fura-2 quenching upon ATP stimulation, indicative of Mn2+ entry (Hallam & Rink, 1985). In earlier studies, Mn2+ has been shown to share common pathways with Ca2+ influx across the plasma membrane (Hallam & Rink, 1989; Jacob, 1990), and more recently it has been shown that the P2X purinoceptor is permeable to Mn2+ (Capiod, 1998).
The inorganic cation Cd2+, known to block voltage-sensitive Ca2+ channels involved in the high K+-induced [Ca2+]i increase in neurohypophysial nerve terminals (Bourne & Trifaro, 1982; Biagi & Enyeart, 1990; Brown et al. 1990; Boland et al. 1991; Wang et al. 1992; Stuenkel, 1994), did not affect the ATP-induced [Ca2+]i response or AVP release (Fig. 8A and C). It seems, therefore, unlikely that voltage-sensitive Ca2+ channels are involved in the observed response to ATP. The lanthanide Gd3+ has been reported to block several types of Ca2+ channels (Lansman, 1990) as well as non-specific cationic channels (Yang & Sachs, 1989). The ATP-dependent [Ca2+]i response in neurohypophysial nerve terminals was blocked by extracellular Gd3+. These results, taken together, suggest that exposure to ATP opens a channel permeable to Ca2+ in the plasma membrane of these neurosecretory nerve terminals, again consistent with ATP acting through a P2X receptor (Evans et al. 1996).
The ATP-induced [Ca2+]i increase was not elicited by adenosine. Furthermore, ATP-γ-S, a non-hydrolysable analogue of ATP, could also induce an increase in [Ca2+]i, confirming that ATP itself, rather than its degradation products, was eliciting the response. In our study, 2-methylthio-ATP was equipotent to ATP and more potent than α,β-methylene-ATP. It is now well established that 2-methylthio-ATP and ATP are more potent than α,β-methylene-ATP as agonists of specific P2X purinoceptors, such as P2X2 (Kennedy & Leff, 1995). Perhaps the strongest evidence for the involvement of a P2X receptor in the observed ATP-dependent [Ca2+]i increase is its dependence on external calcium. Furthermore, of these purinoceptors only the P2X2 subtype is blocked by suramin and PPADS (see Figs 4 and 5) and has the same relative agonist potency ratios. Therefore, we conclude that the ATP response in the nerve terminals is via a P2X2 receptor.
ATP could act as a positive feedback on neuropeptide secretion
Our study presents evidence that extracellular ATP, by triggering an [Ca2+]i increase, induces a dose-dependent increase in resting AVP secretion. ATP evoked an [Ca2+]i increase and AVP release even in the presence of Cd2+, showing that ATP-induced [Ca2+]i increase and AVP release from the nerve terminals do not depend on voltage-dependent Ca2+ channels. Thus, ATP is a stimulator of AVP secretion, via an increase in [Ca2+]i, acting directly at the level of the neurohypophysial nerve terminals, and independent of hypothalamic central signals (see Fig. 9).
Figure 9. Model of proposed ATP effects on neurohypophysial terminals.
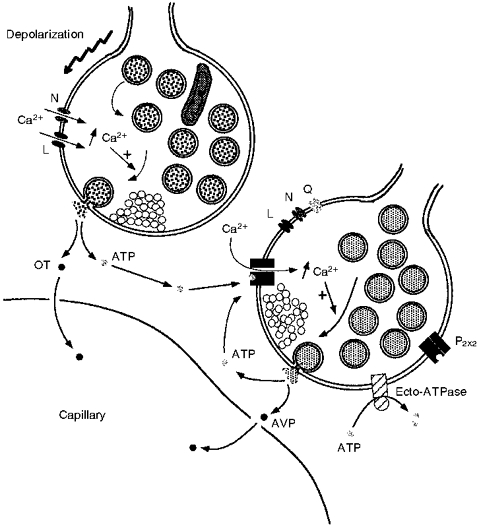
Depolarization of neurohypophysial nerve terminals opens voltage-gated Ca2+ channels (L- and N-types), triggering neurohormone secretion via an [Ca2+]i increase. In this particular example, this results in the exocytosis of neurosecretory granules containing OT and co-stored ATP into the extracellular space. The co-released ATP could then trigger, via a P2X2 purinergic receptor and not via L-, N- or Q-type channels, an [Ca2+]i increase and subsequent AVP (as well as more ATP) release from neighbouring vasopressinergic terminals. This positive feedback on AVP release by ATP would be terminated by ecto-ATPases known to be localized on the terminal membrane. Thus, ATP could act as an autocrine and paracrine regulator of secretion from CNS nerve terminals.
ATP is known to be co-stored in many secretory vesicles (Zimmermann, 1994), and has even been shown to be present in neurohypophysial secretory vesicles (Gratzl et al. 1980; Zimmermann, 1994). ATP is expected to be co-released from neurohypophysial terminals during stimulation. We have shown that low (1 μM) ATP concentrations triggered an increase in both [Ca2+]i and subsequent AVP release, with maximal effects at 100 μM ATP. Concentrations in this range could easily be reached in the neurohypophysial extracellular space as a result of neurosecretory granule exocytosis. Even if the intragranular ATP concentration in neurohypophysial granules was as low as 2 mM (Gratzl et al. 1980), the exocytosis of neuropeptides probably induces a sufficient local increase (4-40 μM, within the diameter of the majority of terminals; see Chow & Von Rüden, 1995) in extracellular ATP concentration to evoke a substantial rise in [Ca2+]i and in AVP secretion. Furthermore, if the concentration of ATP is higher near the site of release, as in chromaffin granules where it is in the order of 100 mM (Zimmermann, 1994), one would expect a similar effect for concentrations (up to 2 mM) in excess of 100 μM. It is, therefore, likely that the ATP concentrations temporarily and locally achieved by neuropeptide secretion can further stimulate release via positive feedback. In addition to the autocrine effect, ATP release could elicit secretion in nearby terminals that are electrically inactive, thus recruiting them in the response (see Fig. 9).
Finally, the effect of ATP needs to be terminated. This is particularly necessary since maintained perfusion of ATP evoked a sustained [Ca2+]i increase and AVP release (Figs 1E and 3A). Previously, we have provided evidence that an ecto-ATPase is present on the neurohypophysial nerve terminals (Thirion et al. 1996). The breakdown of ATP by ecto-ATPases could terminate the action of ATP, and end the positive feedback loop (Fig. 9).
ATP is a novel stimulator of the neurohypophysis
In conclusion, we have demonstrated that, in neurosecretory nerve terminals, ATP by stimulating a P2X2 receptor induces an increase in [Ca2+]i, subsequently triggering AVP, but not OT, release. This would imply stimulation of the terminals and of neuropeptide release independent of depolarization-evoked secretion. Our results provide strong evidence for a novel role of extracellular ATP as a stimulator of release in the neurohypophysis. Furthermore, since ATP is co-stored in many secretory vesicles (Zimmermann, 1994), autocrine and/or paracrine regulation of secretion by ATP could be a ubiquitous phenomenon found in nerve terminals in the central nervous and other secretory systems.
Acknowledgments
We thank Drs Philippe Pougeol and Hélène Widmer (University of Massachusetts Medical Center, Worcester, USA) for critical comments on this manuscript. The imaging experiments were performed in the ‘Centre Commun de Microscopie Appliquée’ of Nice-Sophia Antipolis University, with the help of J. P. Laugier. AVP and OT antisera were kindy supplied by Dr John Bicknell, AFRC Institute, Babraham, UK. The financial support of the CNRS (UMR 6548 and UPR 9055) and NIH (NS29470) is gratefully acknowledged.
References
- Bailey SJ, Hourani SMO. Effects of suramin on contractions of the guinea-pig vas deferens induced by analogues of adenosine 5′-triphosphate. British Journal of Pharmacology. 1995;114:1125–1132. doi: 10.1111/j.1476-5381.1995.tb13324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi BA, Enyeart JJ. Gadolinium blocks low- and high-threshold calcium currents in pituitary cells. American Journal of Physiology. 1990;259:C515–520. doi: 10.1152/ajpcell.1990.259.3.C515. [DOI] [PubMed] [Google Scholar]
- Boland LM, Brown TA, Dingledine R. Gadolinium block of calcium channels: influence of bicarbonate. Brain Research. 1991;563:142–150. doi: 10.1016/0006-8993(91)91527-8. [DOI] [PubMed] [Google Scholar]
- Bourne GW, Trifaro JM. The gadolinium ion: a potent blocker of calcium channels and catecholamine release from cultured chromaffin cells. Neuroscience. 1982;7:1615–1622. doi: 10.1016/0306-4522(82)90019-7. [DOI] [PubMed] [Google Scholar]
- Brethes D, Dayanithi G, Letellier L, Nordmann JJ. Depolarization-induced Ca2+ increase in isolated neurosecretory nerve terminals measured with Fura-2. Proceedings of the National Academy of Sciences of the USA. 1987;84:1439–1443. doi: 10.1073/pnas.84.5.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Boland LM, Dingledine R. Gadolinium non-selectively blocks both N-type and L-type calcium channels. Society for Neuroscience Abstracts. 1990;16:512. [Google Scholar]
- Buller KM, Khanna S, Sibbald JR, Day TA. Central noradrenergic neurons signal via ATP to elicit vasopressin responses to haemorrhage. Neuroscience. 1996;73:637–642. doi: 10.1016/0306-4522(96)00156-x. [DOI] [PubMed] [Google Scholar]
- Capiod T. ATP-activated cation currents in single guinea-pig hepatocytes. The Journal of Physiology. 1998;507:795–805. doi: 10.1111/j.1469-7793.1998.795bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalis M, Dayanithi G, Nordmann JJ. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. The Journal of Physiology. 1985;369:45–60. doi: 10.1113/jphysiol.1985.sp015887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalis M, Dayanithi G, Nordmann JJ. Hormone release from isolated nerve endings of the rat neurohypophysis. The Journal of Physiology. 1987a;390:55–70. doi: 10.1113/jphysiol.1987.sp016686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalis M, Dayanithi G, Nordmann JJ. Requirements for hormone release from permeabilized nerve endings isolated from the rat neurohypophysis. The Journal of Physiology. 1987b;390:71–91. doi: 10.1113/jphysiol.1987.sp016687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RH, Von Rüden L. Single Channel Recording. USA: Plenum Press; 1995. Electrochemical detection of secretion from single cells; pp. 245–277. [Google Scholar]
- Currie KP, Fox AP. ATP serves as a negative feedback inhibitor of voltage-gated Ca2+ channel currents in cultured bovine adrenal chromaffin cells. Neuron. 1996;16:1027–1036. doi: 10.1016/s0896-6273(00)80126-9. [DOI] [PubMed] [Google Scholar]
- Dayanithi G, Stuenkel EL, Nordmann JJ. Hormone release from nerve terminals in the presence of opioid receptor agonist and antagonists. Experimental Brain Research. 1992;90:539–545. doi: 10.1007/BF00230936. [DOI] [PubMed] [Google Scholar]
- Dayanithi G, Widmer H, Richard P. Vasopressin-induced intracellular Ca2+ increase in isolated rat supraoptic cells. The Journal of Physiology. 1996;490:713–727. doi: 10.1113/jphysiol.1996.sp021180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Hertog A, Nelemans A, Van Der Akker J. The inhibitory action of suramin on the P2-purinoceptor in smooth muscle cells of guinea-pig taenia coli. European Journal of Pharmacology. 1989a;166:531–534. doi: 10.1016/0014-2999(89)90370-1. 10.1016/0014-2999(89)90370-1. [DOI] [PubMed] [Google Scholar]
- Den Hertog A, Van Der Akker J, Nelemans A. Suramin and the inhibitory junction potential in taenia coli of the guinea-pig. European Journal of Pharmacology. 1989b;173:207–209. doi: 10.1016/0014-2999(89)90522-0. 10.1016/0014-2999(89)90522-0. [DOI] [PubMed] [Google Scholar]
- Douglas WW, Poisner AM. Stimulus-secretion coupling in a neurosecretory organ: the role of calcium in the release of vasopressin from the neurohypophysis. The Journal of Physiology. 1964;172:1–18. doi: 10.1113/jphysiol.1964.sp007399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn PN, Blakely AGH. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. British Journal of Pharmacology. 1988;93:243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Gibb AJ. ATP - a fast neurotransmitter. FEBS Letters. 1993;325:86–89. doi: 10.1016/0014-5793(93)81419-z. 10.1016/0014-5793(93)81419-Z. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Derkach V, Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Lewis C, Virginio C, Lundstrom K, Buell G, Surprenant A, North RA. Ionic permeability of, and divalent cation effect on, two ATP-gated cation channels (P2X receptors) expressed in mammalian cells. The Journal of Physiology. 1996;497:413–422. doi: 10.1113/jphysiol.1996.sp021777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzl M, Torp-Pedersen C, Daertt DA, Treiman M, Thorn NA. Isolation and characterization of secretory granules from bovine neurohypophyses. Hoppe-Seyler's Z. Physiology and Chemistry. 1980;361:1615–1628. doi: 10.1515/bchm2.1980.361.2.1615. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hallam TJ, Rink TJ. Agonists stimulate divalent cation channels in the plasma membrane. FEBS Letters. 1985;186:175–179. doi: 10.1016/0014-5793(85)80703-1. 10.1016/0014-5793(85)80703-1. [DOI] [PubMed] [Google Scholar]
- Hallam TJ, Rink TJ. Receptor-mediated Ca2+ entry: diversity of function and mechanism. Trends in Pharmacological Sciences. 1989;10:8–10. doi: 10.1016/0165-6147(89)90092-8. 10.1016/0165-6147(89)90092-8. [DOI] [PubMed] [Google Scholar]
- Hourani SMO, Hall DA, Nieman CJ. Effect of the P2-purinoceptor antagonist suramin on human platelet aggregation induced by adenosine 5′-diphosphate. British Journal of Pharmacology. 1992;105:453–457. doi: 10.1111/j.1476-5381.1992.tb14274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle CHV, Knight GE, Burnstock G. Suramin antagonizes responses to P2-puriceptor agonist and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. British Journal of Pharmacology. 1990;99:617–621. doi: 10.1111/j.1476-5381.1990.tb12979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Nakazawa K. ATP receptor-operated Ca2+-influx and catecholamine release from neuronal cells. News in Physiological Sciences. 1992;7:56–59. [Google Scholar]
- Jacob R. Agonist-stimulated divalent cation entry into single human umbilical vein endothelial cells. The Journal of Physiology. 1990;421:55–57. doi: 10.1113/jphysiol.1990.sp017933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C, Leff P. How should P2X purinoceptors be classified pharmacologically. Trends in Pharmacological Sciences. 1995;16:168–174. doi: 10.1016/s0165-6147(00)89010-0. 10.1016/S0165-6147(00)89010-0. [DOI] [PubMed] [Google Scholar]
- Kiyama H, Emson PC. Evidence for co-expression of oxytocin and vasopressin messenger ribonucleic acids in magnocellular neurosecretory cells: simultaneous demonstration of two neurohypophysin messenger ribonucleic acids by hybridization histochemistry. Journal of Neuroendocrinology. 1990;2:257–259. doi: 10.1111/j.1365-2826.1990.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Kurz K, Von Kügelgen I, Starke K. Prejunctional modulation of noradrenaline release in mouse and rat vas deferens: contribution of P1- and P2-purinoceptors. British Journal of Pharmacology. 1993;110:1465–1472. doi: 10.1111/j.1476-5381.1993.tb13986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansman JB. Blockade of current through single calcium channels by trivalent lanthanide cations. Effect of ionic radius on the rates of ion entry and exit. Journal of General Physiology. 1990;95:679–696. doi: 10.1085/jgp.95.4.679. 10.1085/jgp.95.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos FC, Ingram CD. Electrical recordings of magnocellular supraoptic and paraventricular neurons displaying both oxytocin- and vasopressin-related activity. Brain Research. 1995;669:309–314. doi: 10.1016/0006-8993(94)01296-t. 10.1016/0006-8993(94)01296-T. [DOI] [PubMed] [Google Scholar]
- Nordmann JJ, Dayanithi G. Release of neuropeptides does not only occur at nerve terminals. Bioscience Reports. 1988;8:471–483. doi: 10.1007/BF01121646. [DOI] [PubMed] [Google Scholar]
- Nordmann JJ, Dayanithi G, Lemos JR. Isolated neurosecretory nerve endings as a tool for studying the mechanism of stimulus-secretion coupling. Bioscience Reports. 1987;7:411–426. doi: 10.1007/BF01362504. [DOI] [PubMed] [Google Scholar]
- Sabatier N, Richard P, Dayanithi G. L-, N- and T- but neither P- nor Q-type Ca2+ channels control vasopressin-induced Ca2+ influx in magnocellular vasopressin neurones isolated from rat supraoptic nucleus. The Journal of Physiology. 1997;503:253–268. doi: 10.1111/j.1469-7793.1997.253bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguéla P, Haghighi A, Soghomonian JJ, Cooper E. A novel neuronal P2X ATP receptor ion channel with widespread distribution in the brain. Journal of Neuroscience. 1996;16:448–455. doi: 10.1523/JNEUROSCI.16-02-00448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky EM, Gerzanich V, Vanner SM. ATP mediates excitatory synaptic transmission in mammalian neurones. British Journal of Pharmacology. 1992;106:762–763. doi: 10.1111/j.1476-5381.1992.tb14408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuenkel EL. Regulation of intracellular calcium and calcium buffering properties of rat isolated neurohypophysial nerve endings. The Journal of Physiology. 1994;481:251–271. doi: 10.1113/jphysiol.1994.sp020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XP, Stanley EF. An ATP-activated, ligand-gated ion channel on a cholinergic presynaptic nerve terminal. Proceedings of the National Academy of Sciences of the USA. 1996;93:1859–1863. doi: 10.1073/pnas.93.5.1859. 10.1073/pnas.93.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirion S, Troadec JD, Nicaise G. Cytochemical localization of ecto-ATPases in rat neurohypophysis. Journal of Histochemistry and Cytochemistry. 1996;44:103–111. doi: 10.1177/44.2.8609366. [DOI] [PubMed] [Google Scholar]
- Toescu EC, Morris JF. Morphometric analysis of nerve endings isolated from bovine and rat neurohypophysis. Journal of Anatomy. 1990;173:1–17. [PMC free article] [PubMed] [Google Scholar]
- Tomic M, Jobin RM, Vergara LA, Stojilkovic SS. Expression of purinergic receptor channels and their role in calcium signaling and hormone release in pituitary gonadotrophs. Journal of Biological Chemistry. 1996;271:21200–21208. doi: 10.1074/jbc.271.35.21200. 10.1074/jbc.271.35.21200. [DOI] [PubMed] [Google Scholar]
- Troadec JD, Thirion S, Nicaise G, Lemos JR, Dayanithi G. The effects of ATP on intracellular Ca2+ and vasopressin release from isolated rat neurohypophysial terminals. The Journal of Physiology. 1997;506.P:66P. doi: 10.1111/j.1469-7793.1998.089bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizi ES, Sperlagh B, Baranyi M. Evidence that ATP released from the postsynaptic site by noradrenaline, is involved in mechanical responses of guinea-pig vas deferens: cascade transmission. Neuroscience. 1992;50:455–465. doi: 10.1016/0306-4522(92)90437-7. 10.1016/0306-4522(92)90437-7. [DOI] [PubMed] [Google Scholar]
- Wang G, Dayanithi G, Kim S, Hom D, Nadasdi L, Kristipati R, Ramachandran J, Stuenkel EL, Nordmann JJ, Newcomb R, Lemos JR. Role of Q-type Ca2+ channels in vasopressin secretion from neurohypophysial terminals of the rat. The Journal of Physiology. 1997;502:351–363. doi: 10.1111/j.1469-7793.1997.351bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XM, Treistman SN, Lemos JR. Two types of high-threshold calcium current inhibited by ω-conotoxin in nerve terminals of rat neurohypophysis. The Journal of Physiology. 1992;445:181–199. doi: 10.1113/jphysiol.1992.sp018919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XC, Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989;243:1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Signalling via ATP in the nervous system. Trends in Neurosciences. 1994;17:420–426. doi: 10.1016/0166-2236(94)90016-7. 10.1016/0166-2236(94)90016-7. [DOI] [PubMed] [Google Scholar]


