Abstract
Synaptic responses to electrical stimulation of the contralateral pyramidal tract were measured in intracellular recordings from 206 upper limb motoneurones in ten chloralose-anaesthetized macaque monkeys. The objective was to search for evidence of a disynaptic excitatory pathway via C3-C4 propriospinal interneurones similar to that in the cat.
In monkeys with intact spinal cords, only a small proportion of motoneurones (19 %) responded with late EPSPs to repetitive stimulation of the pyramid; only 3 % had segmental latencies that were appropriate for a disynaptic pathway.
From previous studies in the cat, it was expected that a lesion to the dorsolateral funiculus (DLF) at C5 would interrupt the corticospinal input to the spinal segments supplying upper limb muscles, whilst leaving intact excitation transmitted via a C3-C4 propriospinal system, the descending axons of which travel in the ventral part of the funiculus. In five of the monkeys a lesion was made to the DLF at C5 which spared the ventrolateral columns. It severely reduced the monosynaptic EPSPs and disynaptic IPSPs evoked from the pyramidal tract that were present in the intact monkey spinal cord, and which might have masked the presence of disynaptic EPSPs. However, even after the lesion the proportion of motoneurones with such late EPSPs was still low (18 %); 14 % of motoneurones had EPSPs within the disynaptic range.
In addition, some EPSPs with relatively long segmental latencies (> 1·1 ms) were recorded before and after the C5 lesions, but since these effects could be evoked by single stimuli, had stable latencies and did not facilitate with repetitive shocks, it is likely that they represent monosynaptic EPSPs evoked by slowly conducting corticospinal fibres which survived the lesions.
In seven of the monkeys motoneurone responses to stimulation of the ipsilateral lateral reticular nucleus (LRN) were also tested. Most motoneurones showed EPSPs with short latencies (1·2-2·5 ms) and other properties characteristic of monosynaptic activation. This is consistent with the presence of collaterals of C3-C4 propriospinal neurones to the LRN, as demonstrated in the cat.
These short-latency EPSPs evoked from the LRN were just as common before (77 %) as after (75 %) the C5 lesion. They had small amplitudes both before (mean ± s.d. 1·1 ± 0·59 mV) and after (1·2 ± 0·72 mV) the lesion. Unlike the situation in the cat, only a small proportion (16 %) of motoneurones activated from the LRN showed late EPSPs after repetitive stimulation of the pyramid.
The results provide little evidence for significant corticospinal excitation of motoneurones via a system of C3-C4 propriospinal neurones in the monkey. The general absence of responses mediated by such a system in the macaque, under experimental conditions similar to those in which they are seen in the cat, show that extrapolation of results from the cat to the primate should be made with considerable caution.
Direct, cortico-motoneuronal (CM) connections are generally well developed in primates, especially in the Old World monkeys, great apes and man (Phillips, 1971; Heffner & Masterton, 1983; Lemon, 1993). In recent times, much emphasis has been placed on the importance of this direct CM system for cortical control of movement, and there has been relatively little attention given to other indirect pathways through which the motor areas of the cortex might address the α-motoneurone (Jankowska, 1992; Lundberg, 1992; Porter & Lemon, 1993). Anatomical studies demonstrate that localized regions of motor cortex, for example, give widespread projections to many segments of the spinal cord, and that the heaviest projections terminate in the intermediate zone, rather than amongst the motor nuclei (Kuypers, 1981; Armand, 1982; Dum & Strick, 1996; Armand et al. 1997) emphasizing the importance of indirect control.
One of the best-investigated examples of such an indirect control system from the motor cortex is that of the C3-C4 propriospinal system in the cat (Alstermark & Lundberg, 1992). This system (Fig. 1) consists of a population of short propriospinal neurones located in the upper cervical segments which project monosynaptically to the motoneurones in the forelimb segments of the cervical enlargement. These propriospinal neurones receive convergent inputs from a number of descending pathways, including the cortico-, rubro-, reticulo- and tectospinal pathways, as well as afferent inputs from forelimb nerves. In the cat this system represents a major functional linkage between the cortex and the motoneurones, and provides an important integrative system for motor commands originating in different parts of the CNS.
Figure 1. Transmission of corticospinal inputs to cat forelimb motoneurones.
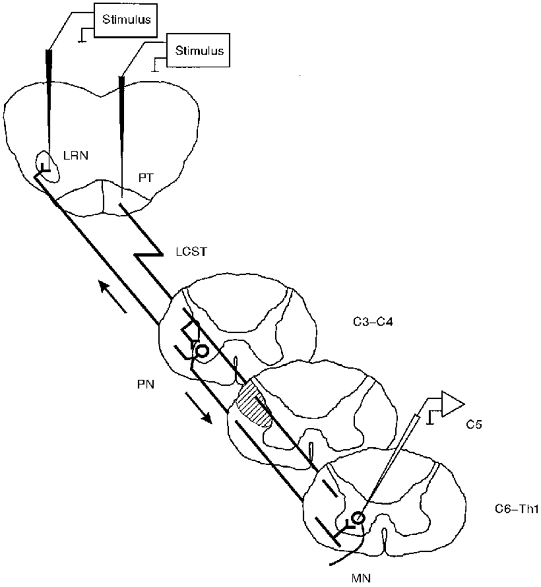
Stimulation of corticospinal fibres in the pyramidal tract (PT) evokes disynaptic excitation in forelimb motoneurones (MN; cervical segments C6-Th1) via propriospinal neurones (PN) which are activated monosynaptically by corticospinal fibres. These neurones are located in upper cervical (C3-C4) segments and they project monosynaptically onto MNs. Since the axons of the PNs are located in the ventrolateral funiculus, a lesion (hatched area) of the lateral corticospinal tract (LCST) at C5 abolishes any corticospinal effects on motoneurones exerted at the segmental level, but leaves intact the disynaptic excitation from C3-C4 PNs. Stimulation of the lateral reticular nucleus (LRN) evokes monosynaptic excitation in motoneurones via ascending axons of the C3-C4 PNs which project to the LRN. The same approach has been taken for the experiments in the monkey.
Although a direct comparison between cat and primate in the organization of corticospinal control is difficult because of the lack of direct CM connections in the cat (Baldissera et al. 1981; Kuypers, 1981), it has been claimed, on the basis of indirect evidence, that a similar system of C3-C4 propriospinal neurones may exist in man (for review, see Pierrot-Deseilligny, 1996). This claim stems from the observation that transmission in pathways mediating non-monosynaptic excitation of upper limb motoneurones from the periphery is modulated during voluntary movement and by transcranial magnetic stimulation (TMS) of the motor cortex (Gracies et al. 1994)
The objective of the experiments described here was to search for direct evidence in the primate for a C3-C4 propriospinal system, similar to that described in the cat, which could transmit disynaptic corticospinal excitation to upper limb motoneurones. The experiments were carried out in the macaque monkey, which provides the best available experimental model of the human motor system. If such a C3-C4 system exists in the primate, then it should be possible to reveal it using exactly the same approach employed by Illert et al. (1977) in the cat. As shown in Fig. 1, this approach involved the demonstration of disynaptic excitation of forelimb motoneurones by repetitive stimulation of the pyramidal tract (PT) in cats with a lesion in the dorsolateral funiculus at the C5 level to interrupt the corticospinal inflow to the forelimb segments. The disappearance of this disynaptic excitation after a C2 corticospinal tract lesion demonstrated that it was mediated by C3-C4 propriospinal neurones, whose axons ran more ventrally in the lateral funiculus (Illert et al. 1978; Alstermark & Lundberg, 1992).
Recordings of responses in monkey and baboon motoneurones to stimulation of the motor cortex or PT are dominated by the fast monosynaptic EPSPs generated by the CM projection, and lack any obvious signs of later, oligosynaptic excitation that might result from significant propriospinal action (Landgren et al. 1962; Shapovalov, 1975; Fritz et al. 1985). However, later EPSPs may have been masked by the disynaptic IPSPs that are seen frequently in response to corticospinal stimulation and which can be strongly facilitated by the repetitive PT stimulation needed to test for propriospinal transmission. Since some of this disynaptic inhibition is probably mediated by segmental interneurones (Jankowska et al. 1976), a C5 lesion of the corticospinal tract in the monkey should abolish both the monosynaptic CM EPSP and some of this disynaptic inhibition. Thus the C5 lesion should reveal any later corticospinal excitation transmitted by a C3-C4 propriospinal system, as originally demonstrated in the cat (Illert et al. 1977).
An important feature of most C3-C4 propriospinal neurones in the cat is that they have an ascending axon collateral to the lateral reticular nucleus (LRN) in the caudal medulla (Illert & Lundberg, 1978; see Fig. 1). Stimulation within the LRN antidromically activates C3-C4 propriospinal neurones and thereby evokes monosynaptic EPSPs in forelimb motoneurones (Alstermark et al. 1981a; Alstermark & Lundberg, 1992). In the cat, it was a consistent finding that a monosynaptic EPSP from the LRN and a disynaptic EPSP from the PT could be evoked from the same motoneurone. In our attempt to identify a similar C3-C4 system in the monkey we therefore also looked for responses of motoneurones to stimulation of the LRN, and compared these effects with those from the PT.
Preliminary accounts of this work have been published previously (Maier et al. 1996a,b)
METHODS
This study was performed in one adult cat (female, 2.6 kg) and in ten adult Macaca fascicularis monkeys (age 2-7 years; weight 2.5-5.5 kg; 5 males, 5 females). All animals were captive bred for research purposes. Animal care and use was in accordance with the UK Animals (Scientific Procedures) Act 1986. Some of the monkeys were used in parallel neuroanatomical studies.
The cat was sedated with 15 mg kg−1 ketamine and 2 mg kg−1 chlorpromazine i.m. Monkeys were sedated with ketamine (10 mg kg−1, i.m.). General anaesthesia was administered using 2 % halothane (cat) or 1.5-2.5 % isoflurane (monkeys) in a 1: 1 O2: N2O mixture. A tracheal cannula was inserted, and one femoral artery and both femoral veins cannulated. Nerve branches to the following muscles of the left arm were dissected free: triceps lateral, long and medial heads, biceps, brachialis and anconeus. Electrodes were also mounted on the following nerves: median and ulnar at the axilla, median and ulnar at the wrist (cuff electrodes), radial nerve at the axilla, deep radial (cuff electrodes) and superficial radial. In six experiments, cuff electrodes only were used on the radial, median and ulnar nerves at the axilla, on the median and ulnar nerves at the wrist, and on the deep radial nerve just above the elbow. An extensive laminectomy to expose the spinal segments from C3 to Th1 was carried out and an occipital craniotomy gave access to the medulla. When surgery was complete, the animal was given α-chloralose and isoflurane was discontinued. In most experiments, the dose of chloralose was increased up to a maximum of 80 mg kg−1i.v. (range 50-80 mg kg−1i.v.). The animal was mounted in a spinal frame and headholder, with clamps on the vertebral column at Th3 and in the lumbar region. The animal was paralysed with pancuronium bromide (Pavulon, Oregon-Technika, Cambridge, UK) at a dose of 0.3 mg kg−1 h−1i.v. and artificially ventilated at a rate of 45 cycles min−1. In the animals with a full dissection of the nerves, a pool was made over the left arm, and nerves mounted on stimulating electrodes and covered in warm mineral oil. A bilateral pneumothorax was carried out. The dura was opened and the spinal cord covered with warm mineral oil. The adequacy of the anaesthesia was continuously assessed by reference to the blood pressure, heart rate and pupillary reflexes. Small doses of pentobarbitone (Sagatal, Rhone Merieux, Harlow, UK) were administered when necessary. The total dose administered was usually in the order of 2-4 mg kg−1i.v. and it was given over the 24-30 h period for which the experiment continued. Body temperature was carefully maintained between 37 and 39°C. Fluid balance was maintained using a slow infusion of lactated Ringer solution into the femoral vein. Routine analysis of blood gases was carried out, supplementary bicarbonate solution added to the Ringer solution as required, and each animal remained in good physiological condition throughout the recording. Mean blood pressure was maintained above 80 mmHg.
For stimulation of the PT, a monopolar electrode (varnish-insulated tungsten, tip impedance 20-30 kΩ at 1 kHz) was positioned just rostral to obex and 0.5-1.5 mm to the right of the midline. Corticospinal volleys excited from the PT were recorded from the surface of the dorsolateral funiculus (DLF) at a rostral site (usually C4) and at a caudal site close to the region from which motoneurone recordings were made. This caudal electrode was also used for monitoring the volleys evoked by stimulation of the peripheral nerves. The PT electrode was advanced until a large corticospinal volley was evoked. In the monkeys it typically had a latency of 0.6 ms at the C4 electrode and conducted at 70-80 m s−1 (see Edgley et al. 1990; Olivier et al. 1997). Two criteria were used to establish the final stimulation site: low threshold (5-15 μA) and a volley that was close to maximal with a stimulus intensity of 200 μA (i.e. no significant increase in volley size with a 500 μA shock). The volley diminished and then disappeared when the PT electrode was raised by 1-2 mm dorsal to the stimulation site, and its threshold increased considerably. Test stimuli of 10-200 μA (0.1 ms) were delivered to the PT at 3 Hz. Responses to either single or up to four repetitive stimuli (at approximately 333 Hz) were tested.
For stimulation of the LRN (which was carried out in seven of the monkeys) cathodal pulses were delivered through a monopolar tungsten electrode (tip impedance 20-30 kΩ at 1 kHz) introduced from the dorsal surface of the medulla. The electrode was positioned so as to produce a large field potential when recording from within C6-C7 motor nuclei on the ipsilateral side (see Fig. 10). A number of tracks were made in the region 2-4.5 mm lateral from obex and from 2 mm rostral to 4 mm caudal to obex. The electrode was fixed at the position which yielded the field potential at the lowest threshold (typically < 50 μA; Fig. 10A).
Figure 10. Field potential and motoneurone responses to LRN stimulation.
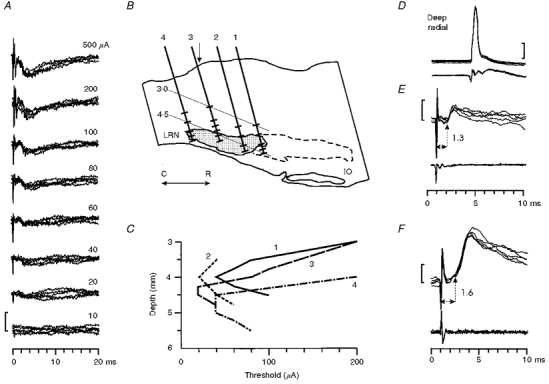
A, field potential responses to stimulation of the lateral reticular nucleus (LRN) with different stimulation intensities (500-10 μA). Note the characteristic slow negative potential (negativity down); an early sharp negativity of unknown origin was seen with the higher intensities (200 and 500 μA). Stimulation site is indicated on the section shown in B: track 2, depth: 4 mm. Five sweeps superimposed for each intensity. Threshold of the slow negativity was 20 μA. Calibration bar = 300 μV. B, reconstruction of 4 different stimulation tracks (1-4) in a saggital section through the medulla 3.75 mm lateral to the midline. Shaded area represents the most clearly defined caudal part of the LRN. Dashed line represents the probable limits of the more dispersed rostral extension of the nucleus (Walberg, 1952). IO = inferior olive, C = caudal, R = rostral. The short bars drawn across each track indicate the depths at which stimulation was tested (mostly between 3 and 5 mm below the surface of the medulla). C, depth-threshold curves for eliciting slow negative field potentials from the tracks and stimulation sites shown in B. The lowest threshold was usually reached within or just dorsal to the LRN. Vertical arrow in B indicates the rostro-caudal position of obex. Recordings made in the deep radial motor nucleus with a low impedance glass microelectrode. E and F, short-latency EPSPs evoked from the LRN (200 μA shock) in two different deep radial motoneurones recorded in monkeys with an intact spinal cord (identification of one motoneurone shown in D). Calibration bars: 20 mV in D and 1 mV in E and F.
Intracellular recordings were made from motoneurones with glass microelectrodes filled with 3 M potassium acetate and having a DC resistance of 4-10 MΩ. A small pressure foot was used in most experiments to reduce movement of the spinal cord. All motoneurones were identified antidromically from the forelimb nerves. Intracellular and surface recordings were digitized directly at 10 kHz using a 1401plus interface (CED, Cambridge, UK). Membrane potential was monitored throughout the recording, and only data from stable periods of recording used for analysis (membrane potential < -50 mV). All latency and amplitude measurements were derived from sets of measurements made from a number of single traces. Measurements are given as means ±s.d. Possible post synaptic potentials smaller than 200 μV were not measured or counted.
In the cat and in five monkeys lesions of the left DLF in the C5 segment were made with fine watchmakers forceps; while the lesion was being made the corticospinal volleys recorded both above and below the lesion site were monitored.
At the end of the experiment, small electrolytic lesions were placed at the PT and LRN stimulation sites and the animal was killed with an intravenous overdose of barbiturate, and perfused through the heart with formol saline. DLF lesions were reconstructed postmortem from paraffin embedded blocks of spinal cord, and the sites of stimulating electrodes confirmed histologically.
RESULTS
Database
Stable recordings were made from a total of 206 identified motoneurones in ten monkeys. Of these, 110 were recorded in monkeys with intact cords: fifty-six were recorded from five monkeys in which no C5 lesion was made, and a further fifty-four before the lesion was made in the other five monkeys. A total of ninety-six were recorded after the lesion. Motoneurones were recorded in the C6-Th1 segments (see Jenny & Inukai, 1983); their identity is listed in Table 1. The effects of LRN stimulation were tested in 145 of the motoneurones (56 in monkeys with intact cords and 89 after the C5 lesion had been made). The single experiment in a cat was carried out to confirm earlier findings (Illert et al. 1977) using the present experimental set-up and anaesthetic procedures (see Discussion).
Table 1.
Number and identity of monkey cervical motoneurones tested from the PT or from both PT and LRN
| Nerve | Triceps* | Biceps** | Radiala | Mediana | Medianw | Ulnara | Ulnarw | Deep radial | Total |
|---|---|---|---|---|---|---|---|---|---|
| PT | 18 | 23 | 11 | 41 | 2 | 37 | 13 | 61 | 206 |
| PT & LRN | 18 | 19 | 9 | 25 | 2 | 16 | 4 | 52 | 145 |
Includes triceps lateral, long and medial heads, anconeus or either of triceps or anconeus.
Includes biceps, brachialis or either of biceps or brachialis. Radiala, Mediana, Ulnara: identified from cuffs on radial, median and ulnar nerves at the axilla. Medianw, Ulnarw: identified from cuffs on median or ulnar nerves at the wrist.
Motoneurone responses to pyramidal tract stimulation before a C5 lesion
EPSPs
A single PT stimulus evoked EPSPs in most motoneurones; an example from a brachialis motoneurone is shown in Fig. 2B -E. The segmental latency of the EPSP was derived from the interval between the positive peak of the descending corticospinal volley and the foot of the EPSP (0.9 ms in Fig. 2E). Figure 2F plots the segmental latency of all EPSPs evoked by a single PT stimulus in the monkeys with intact cords.
Figure 2. Monosynaptic excitation of motoneurones from the PT in monkeys with an intact spinal cord.
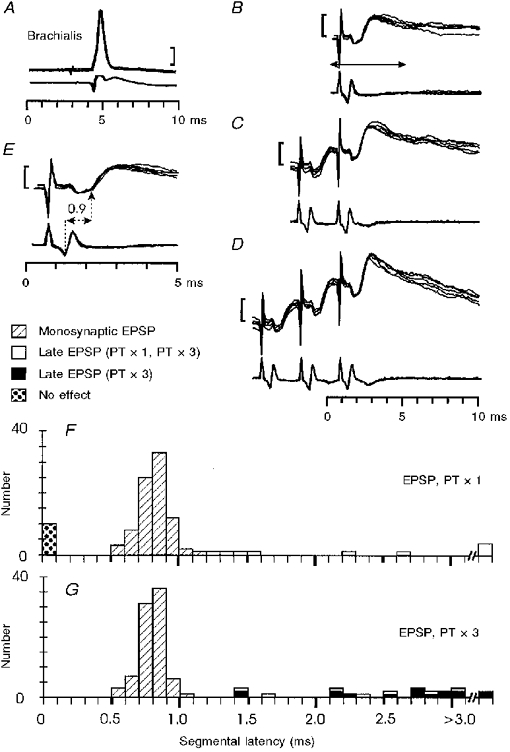
Monosynaptic EPSP in a brachialis motoneurone evoked by PT stimulation. In the specimen records A-E the upper traces display intracellular records of the motoneurone (5 sweeps superimposed), the lower traces records from the surface of the spinal cord at the level of the motoneurone. In this and subsequent figures the calibration bars apply to the intracellular records: 20 mV in A and 1 mV in B-E.A, antidromic identification. B-E, responses to single (B), double (C) and triple (D) PT stimulation at 200 μA. Arrow in B indicates the period of the recording shown at an expanded time scale in E. Segmental latency (onset of EPSP after arrival of corticospinal volley in the surface recording) is 0.9 ms. F and G, distribution of segmental latencies of EPSPs recorded in the sampled motoneurones in response to single (F) and triple (G) PT stimulation at 200 μA. Three categories of response are indicated: monosynaptic EPSPs (hatched columns, n= 84 in both F and G), late EPSPs evoked by single and repetitive PT shocks (open columns, n= 9 and 7 in F and G, respectively), and late EPSPs evoked only by repetitive shocks (black columns n= 16). Unresponsive motoneurones indicated by stippled columns to left of histogram.
Early EPSPs
Most motoneurones had an early EPSP (73 %; 84/110 motoneurones; see Table 2) with a segmental latency of 0.6-1.1 ms (absolute latency 1.3-1.7 ms from the PT stimulus). In the great majority of motoneurones this early EPSP occurred either alone (20/110) or together with a later IPSP (60/110; see below). The corticospinal origin of these excitatory and inhibitory effects was confirmed in every experiment by showing that they disappeared when the PT electrode was raised by 1.5-2 mm.
Table 2.
Effects elicited by pyramidal tract stimulation in cervical motoneurones
| Before C5 lesion | After C5 lesion | |||
|---|---|---|---|---|
| Single (PT × 1) | Repetitive (PT × 3) | Single (PT × 1) | Repetitive (PT × 3) | |
| Monosynaptic EPSP | 20 | 8 | 18 | 8 |
| Monosynaptic EPSP/IPSP | 60 | 55 | 15 | 24 |
| IPSP | 11 | 0 | 8 | 6 |
| Monosynaptic EPSP/late EPSP * | 4 | 21 | 2 | 8 |
| Late EPSP * | 5 | 2 | 8 | 18 |
| No effect | 10 | 0 | 45 | 24 |
| Not tested | 0 | 24 † | 0 | 8 ‡ |
| Total | 110 | 110 | 96 | 96 |
With or without an IPSP.
Two motoneurones responded with spikes.
Three motoneurones responded with spikes.
Early EPSPs with segmental latencies of < 1.2 ms were classified as monosynaptic (Phillips & Porter, 1964). The mean segmental latency of these early EPSPs (hatched columns in Fig. 2F) was 0.76 ± 0.11 ms (n= 84). Of course, this criterion only identifies EPSPs generated by the fastest corticospinal fibres which dominate the surface recorded volley; it is possible that later EPSPs were also monosynaptic in origin and were mediated by more slowly conducting fibres (see below). The early, monosynaptic EPSPs had a mean rise time of 0.93 ± 0.18 ms (n= 61), they were weakly facilitated by repetitive PT stimulation (usually 3 shocks at 333 Hz; see Fig. 2D), and there was little or no decrease in the segmental latency of the monosynaptic EPSP with repetitive stimulation.
Late EPSPs
Single PT stimuli rarely evoked EPSPs with segmental latencies beyond the monosynaptic range (1.2 ms or longer; see Table 2 and Fig. 2F, open columns). Even in those motoneurones with rather small monosynaptic EPSPs, late responses were rarely present. A total of eighty-six motoneurones were tested with both single and repetitive PT stimuli; late EPSPs were seen after a single stimulus in seven cases (8 %) and in sixteen cases (19 %) for repetitive but not single stimuli. These latter cases are denoted by black columns in Fig. 2G; two examples are shown in Fig. 3. In neither motoneurone was a late EPSP visible with a single PT stimulus (Fig. 3B and F), but a late EPSP was observed after the third shock. In the deep radial motoneurone, it was superimposed on an earlier monosynaptic EPSP (Fig. 3C); in the radial motoneurone, only the late EPSP was visible (Fig. 3G). These late EPSPs had segmental delays (measured from the volley produced by the third shock) of 2.3 and 1.4 ms, respectively (Fig. 3D and H). The mean amplitude, where measurable, of the late EPSPs was 1.4 ± 0.53 mV (n= 15).
Figure 3. Possible disynaptic excitation from the PT in a monkey with an intact spinal cord.
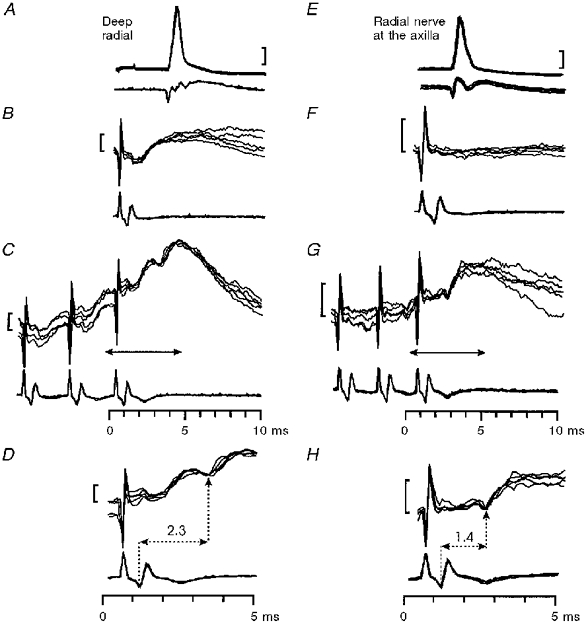
Late, non-monosynaptic EPSP in a deep radial motoneurone (A-D) and a radial motoneurone (E-H) evoked by PT stimulation. A and E, antidromic identification. Responses to single (B, F) and triple (C, G) PT stimulation at 200 μA. Arrows in C and G indicate the periods of the recording shown at an expanded time scale in D and H. The segmental latencies of the late effects were 2.3 ms in D and 1.4 ms in H. Calibration bars: 20 mV in A and E, 1 mV in B-H.
IPSPs
After a single PT shock, some motoneurones responded with an IPSP and no monosynaptic EPSP was present (11/110; Table 2 and Fig. 4A-E). Pure IPSPs were present in all species of motoneurone investigated (particularly triceps), with the exception of intrinsic hand muscle motoneurones (n= 15). In many motoneurones (60/110; Table 2) an IPSP followed the early monosynaptic EPSP; an example is shown in Fig. 4F -J. The IPSP was often larger than the EPSP and was facilitated by repetitive stimulation (see Fig. 4D and I). IPSPs evoked by single PT stimuli had segmental latencies of 1.3-1.9 ms (Fig. 4E,J and K), and were classified as disynaptic in origin (cf. Eccles et al. 1956; Jankowska et al. 1976); they had short rise times (mean 0.96 ± 0.15 ms, n= 12).
Figure 4. Disynaptic inhibition from the PT in monkeys with an intact spinal cord.
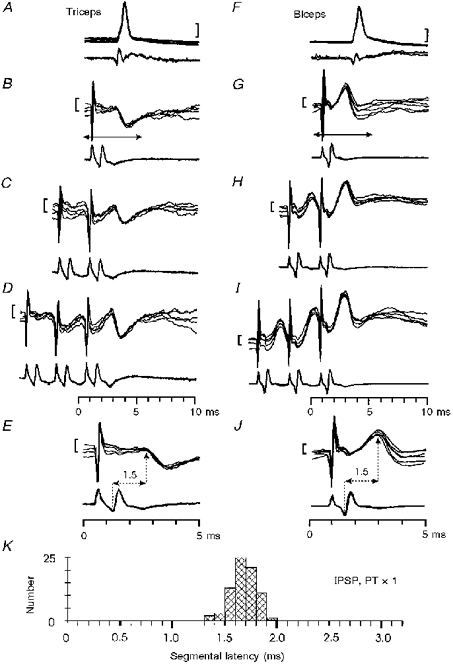
Responses in a triceps (A-E) and a biceps (F-J) motoneurone evoked by PT stimulation. A and F, antidromic identification. Responses to single (B, G), double (C, H) and triple (D, I) PT stimulation at 200 μA. Arrows in B and G indicate the periods of the recording shown at an expanded time scale in E and J. The segmental latency of the IPSP was 1.5 ms in both motoneurones. Calibration bars: 20 mV in A and F, 1 mV in B-E and G-J. K, distribution of segmental latencies of IPSPs recorded in the sampled motoneurones (n= 76) in response to single PT stimulation at 200 μA.
Lesions of the dorsolateral funiculus at C5
The objective was to interrupt most of the lateral corticospinal tract (LCST) fibres. The lesion was intended to include the DLF and a narrow crescent of tissue lying slightly more ventral and lateral, in which many of the corticospinal fibres projecting directly to lamina IX are located (Armand et al. 1997). We did not attempt a complete LCST lesion, since this would have entailed greater damage to the lateral funiculus. The lesion was designed to spare more ventral fibres, including any belonging to a C3-C4 propriospinal system, assuming it to be organized along similar lines to that in the cat (Alstermark & Lundberg, 1992; see Fig. 1).
The lesions were made after several hours of recording from the intact spinal cord and were extended until most, but not necessarily all, of the negative phase of the corticospinal volley in the recording caudal to the lesion was abolished. Volley recordings and lesion reconstructions from all five monkeys in which the lesion was made are shown in Fig. 5 (negativity upwards). The negativity in the volley surviving the lesion was generally smaller in the experiments with the larger lesions (compare PN7 with PN5 in Fig. 5). There was some recovery of the volley in the period, usually lasting up to 10 h, between making the lesion and the end of the experiment.
Figure 5. Schematic representation of the C5 lesions made in five monkeys.
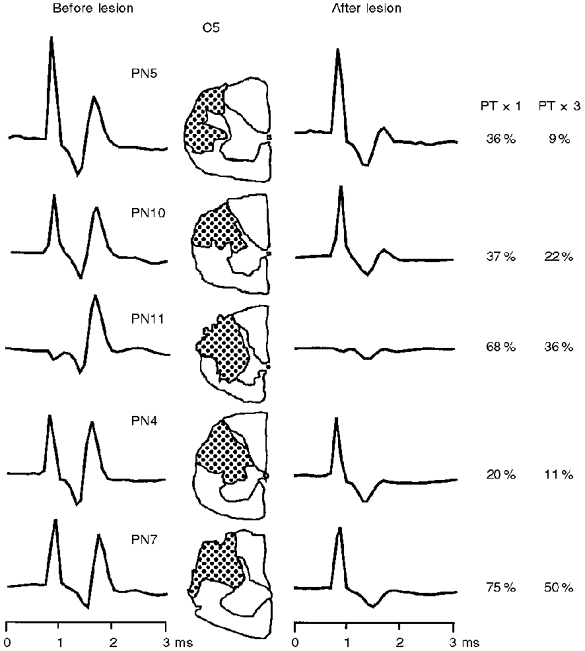
Corticospinal volley in the surface recording evoked by a single PT stimulus of 200 μA is shown before and immediately after the lesion; recordings taken from a site caudal to the lesion. Note the marked reduction of the negativity (upwards) of the corticospinal volley after lesion. There was a general correspondence between the extent of the lesion and the reduction in the volley. In addition the proportion of motoneurones, sampled in the segments caudal to the lesion, which were completely unresponsive to PT stimulation was generally greater in the cases with larger lesions. Percentages of unresponsive motoneurones in each case are given in the right panel, and correspond to data obtained for single (× 1) and repetitive (× 3) PT shocks.
There was an important difference between the cat and monkey in the effect of the DLF lesion on the volleys evoked by repetitive PT stimulation. Figure 6A shows that in the cat the corticospinal volley was almost completely abolished by a C5 lesion (compare recordings rostral (Vr) and caudal (Vc) to the lesion). Repetitive stimulation evoked a late negative potential (arrow in Vr and Vc recordings) which was not seen in responses to single stimuli, and which survived the lesion. Illert et al. (1977) assumed that this volley reflects the activation of C3-C4 propriospinal neurones. In keeping with this suggestion, intracellular recording from motoneurones in this same cat revealed disynaptic EPSPs to repetitive PT stimulation.
Figure 6. Comparison of cord dorsum recordings in the cat and monkey.
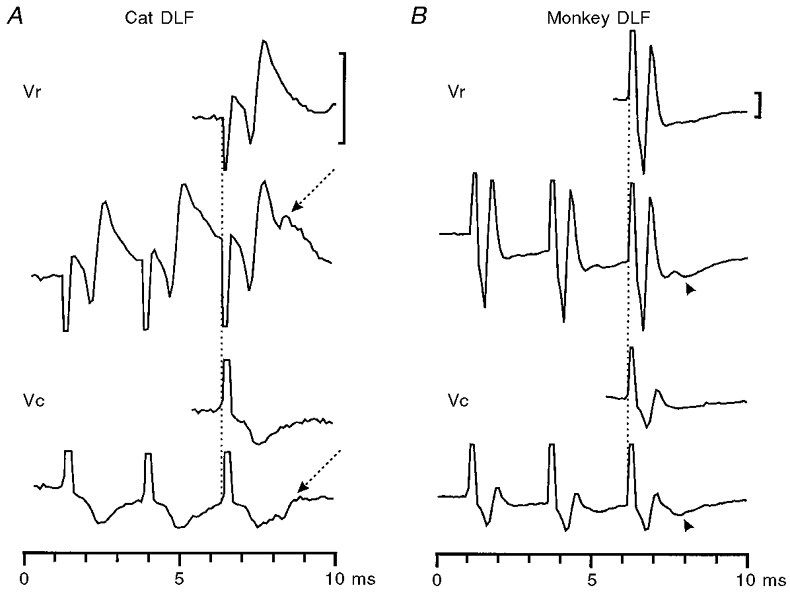
A, cat. Recordings of the corticospinal volley rostral (Vr) and caudal (Vc) to a lesion at C5 (average of 10 sweeps). A single shock (top trace) evoked a corticospinal axonal volley which was abolished by the lesion (see caudal recording, Vc). Repetitive shocks evoked an additional synaptic discharge (arrowed) of presumed propriospinal origin which survived the lesion. In the monkey (B), this synaptic volley was not seen; instead, a small positivity was recorded (arrowhead). The C5 lesion did reduce greatly the corticospinal axonal volley. Calibration bars: 100 μV.
In the monkey experiments there was almost no sign of the late negative potential: in some cases there was a small positivity after the second and third stimulus (arrowhead in Fig. 6B). Because of the polarity of this potential and because extracellular recordings in the ventral horn revealed an interneuronal field at the appropriate latency after PT stimulation, it is probable that, rather than being a tract volley, this small potential represents the activation of segmental interneurones or motoneurones by the remaining corticospinal fibres.
Motoneurone responses to pyramidal tract stimulation after a C5 lesion
Motoneurones with no response to PT stimulation
A much smaller proportion of motoneurones responded to PT stimulation than before the lesion. In the intact cord, single PT stimuli produced responses, excitatory or inhibitory, in 91 % of the sampled motoneurones (100/110; Table 2). After the lesion only 51/96 (53 %) motoneurones showed a response. With repetitive stimuli before the lesion, all motoneurones responded, whereas only 74 % (67/91) did so after it. It was generally the case that a higher proportion of unresponsive motoneurones were found in the monkeys with larger lesions, which had little if any negativity in the surviving corticospinal volley (see data in panel to right of Fig. 5).
EPSPs evoked by PT stimulation after DLF lesions at C5
Early EPSPs
Figure 7A-E shows responses from a deep radial motoneurone with a small monosynaptic EPSP after the lesion. Single PT stimuli produced early EPSPs (segmental latency < 1.2 ms) in only 36 % of the recorded motoneurones (35/96), compared with 76 % (84/110) before the lesion (Table 2). The segmental latencies of all EPSPs recorded after the lesion are plotted in Fig. 7K (PT × 1); these latencies were determined with reference to the small corticospinal volley which survived the lesion or, if none were present, to the latency of the pre-lesion volley. Early EPSPs in the monosynaptic range had a distribution similar to that for EPSPs recorded in the monkeys with intact cords (see Fig. 2F); the mean latency of these effects was 0.82 ± 0.1 ms (n= 35). The rise times of these early EPSPs were also unchanged. Comparison of Fig. 9AandC shows that there was a significant reduction in the amplitude of monosynaptic EPSPs as a result of the C5 lesion; the mean amplitudes were 1.87 ± 1.0 mV (n= 62) before and 1.03 ± 0.59 mV (n= 33) after the lesion (Student's t test, P < 0.001).
Figure 7. Monosynaptic excitation of motoneurones from the PT in monkeys with a C5 lesion.
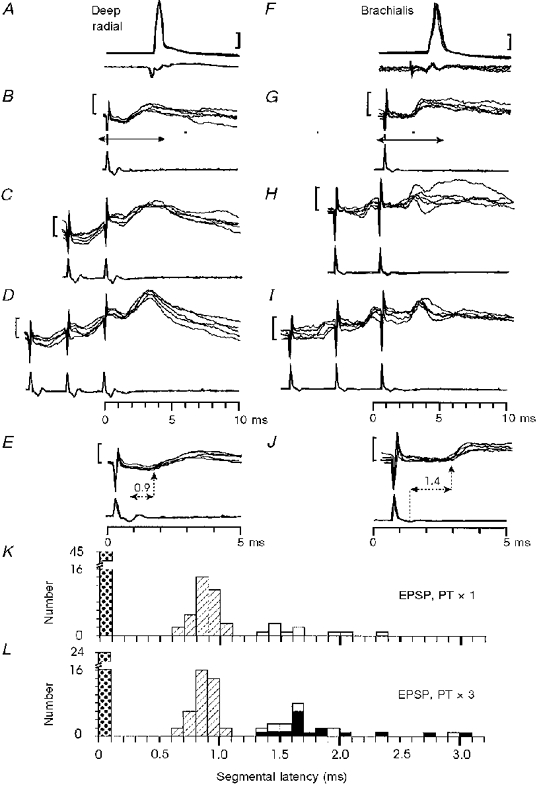
Monosynaptic EPSP in a deep radial motoneurone (A-E) and late EPSP in brachialis motoneurone (F-J) evoked by PT stimulation of 200 μA. A and F, antidromic identification. Responses to single (B, G), double (C, H) and triple (D, I) PT stimulation. Arrows in B and G indicate the periods of the recording shown at an expanded time scale in E and J. The segmental latencies of the effects were 0.9 ms in E and 1.4 ms in J. Calibration bars: 20 mV in A and F, 1 mV in B-E and G-J.K and L, distribution of segmental latencies of EPSPs recorded in the sampled motoneurones in response to single (K, n= 90) and triple (L, n= 90) PT stimulation at 200 μA. Key as in Fig. 2
Figure 9. Amplitude distribution of EPSPs evoked from the PT before and after the C5 lesion.
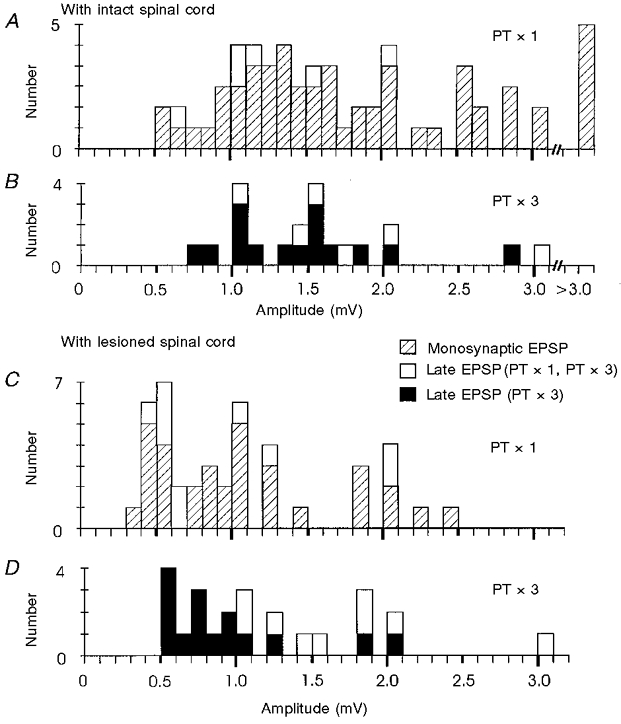
Responses to 200 μA PT stimulation before (A, B) and after (C, D) a C5 lesion. A and C, amplitudes of monosynaptic and late EPSPs evoked by single PT stimulation before (A) and after (C) the lesion. B and D, amplitudes of late EPSPs that were evoked by repetitive PT stimulation before (B) and after (D) lesion.
Late EPSPs
In comparing the results before and after the C5 lesion, it can be seen that there was a small increase in the number of late EPSPs after the lesion (Figs 2F and 7K (open columns) and Table 2). For single PT shocks, the proportions were 8 % (9/110 motoneurones) before the lesion and 10 % (10/96) after it. These late EPSPs had segmental latencies of 1.3-2.3 ms (Fig. 7K). After the lesion single PT stimuli evoked small late EPSPs, with no accompanying early EPSP, in eight motoneurones. An example is shown in Fig. 7F -J. With a single shock, this motoneurone responded with an EPSP having a segmental latency of 1.4 ms (Fig. 7G and J); with repetitive stimulation (Fig. 7H and I) there was no reduction in the latency of the response, nor was there any facilitation of the EPSP. These characteristics (response to a single shock, constant latency and weak facilitation) applied to 7/8 of these motoneurones and clearly distinguished these late EPSPs from other late effects recorded after the lesion, which were evoked only by repetitive stimuli (see below). Late EPSPs evoked by single shocks resembled monosynaptic EPSPs recorded before the lesion (compare with Fig. 7A-E and Fig. 2). It is possible that these late EPSPs resulted from monosynaptic activation of motoneurones by slow corticospinal fibres that had survived the lesion. They were generally rather small in amplitude (see Fig. 9C, open columns).
The second type of late EPSP recorded after the lesion was elicited by repetitive PT stimuli only; the proportion of motoneurones responding with this type of late EPSP was much the same before (19 %; 16/86) and after the lesion (18 %; 16/88, respectively). These effects could have resulted from corticospinal activation of an interneuronal system, such as a C3-C4 propriospinal relay. Two examples, from deep radial and biceps motoneurones, are shown in Fig. 8; there was no response to a single PT shock in either motoneurone, but a late EPSP appeared after the third shock (segmental latencies 1.8 and 1.6 ms, Fig. 8E and J). The segmental latencies of all late EPSPs evoked by repetitive stimulation are plotted in Fig. 7L (black columns); they ranged from 1.3 to 3.0 ms. Those with latencies of 1.3 to 1.9 ms (12/88 motoneurones, 14 %) were considered to be within the disynaptic range; some appeared to cluster at around 1.6 ms (Fig. 7L).
Figure 8. Possible disynaptic excitation from the PT in monkeys with a C5 lesion.
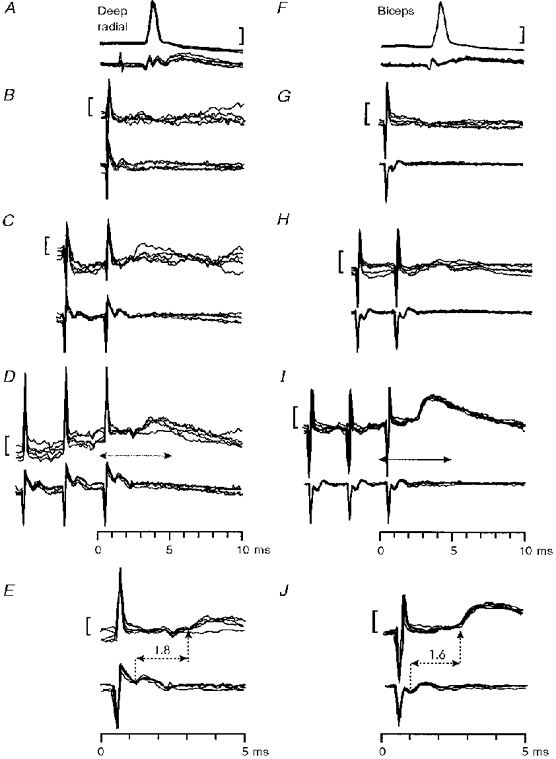
Late non-monosynaptic EPSP in a deep radial motoneurone (A-E) and in a biceps motoneurone (F-J) evoked by PT stimulation of 200 μA. A and F, antidromic identification. Responses to single (B, C), double (C, H) and triple (D, I) PT stimulation at 200 μA. Arrows in D and I indicate the periods of the recording shown at an expanded time scale in E and J. The segmental latencies of the late effects were 1.8 ms in E and 1.6 ms in J. Calibration bars: 20 mV in A and F, 1 mV in B-E and G-J.
The example shown in Fig. 8F-J shows a rather robust EPSP, with an amplitude of about 1.0 mV. In most motoneurones these late effects were both labile and small (Fig. 9D, black columns; mean amplitude 0.93 ± 0.47 mV, n= 16). A more representative example is shown in Fig. 8A-E; in this case, the probability of recording a late EPSP was rather low with two shocks (Fig. 8C), and was still inconsistent with three shocks (Fig. 8D), suggesting a rather weak linkage. These late EPSPs were recorded in motoneurones supplying a variety of muscles, i.e. there was no preferential distribution for any particular species of motoneurone. Note that no late EPSPs were observed in twenty-four motoneurones which lacked any early EPSP or IPSP, which, in the intact cord, might have masked any late effects.
IPSPs evoked by PT stimulation after DLF lesions at C5
Small IPSPs with segmental latencies of 1.4 to 2.0 ms were seen after the lesion. The proportion of motoneurones responding with IPSPs (with or without other excitatory effects) was smaller after the lesion (23/96 motoneurones; 24 %) than before (71/110; 65 %, see Table 2 and Fig. 13). A similar reduction was seen when the proportions responding to repetitive PT stimulation were compared before and after the lesion (64 % (55/86) and 34 % (30/88), respectively).
Figure 13. Proportion of responses to PT and LRN stimulation in motoneurones recorded before and after a C5 lesion.
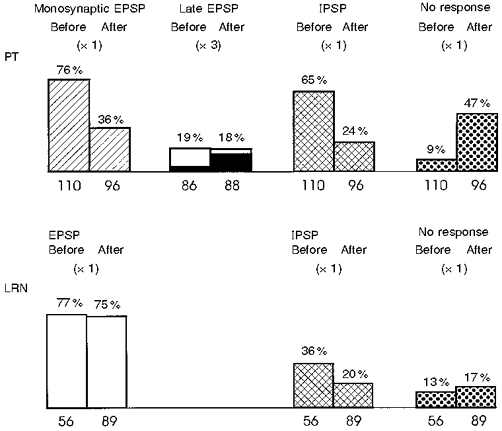
Response categories for PT data: monosynaptic EPSP, with a segmental latency < 1.2 ms; late EPSP, segmental latency of (>= 1.2 ms and evoked by PT × 3 but not by PT × 1 (black columns are late EPSPs in the disynaptic range, 1.3-1.9 ms). IPSP, recorded in motoneurones with or without preceding EPSP. Total number of motoneurones tested for each condition given at base of each column. The C5 lesion caused a large reduction in the proportion of EPSPs and IPSPs evoked from the PT, but there was little effect on either the late EPSPs from the PT, or on effects from the LRN.
Stimulation of the LRN
Figure 10A shows examples of the motor nucleus field potentials evoked from the LRN. A glass microelectrode was advanced into the C6 segment and positioned at a site which gave large antidromic field potentials from stimulation of the deep radial and biceps nerves. While recording was carried out from the motor nucleus, several tracks were made with a tungsten electrode through the ipsilateral medulla, using a search stimulus of 500 μA. The field potentials evoked from the LRN had a characteristic slow negativity which began at around 1.8 ms and peaked at 3.0 ms, declining to baseline after 10-15 ms; its threshold from the site illustrated was 20 μA. A reconstruction of some of the tracks on a parasagittal section of the medulla is shown in Fig. 10B. The current-distance plots (Fig. 10C) for the slow negative potential showed that sites at which it was elicited at low threshold (< 60 μA) were concentrated within the LRN or just dorsal to it, particularly in or near the caudal part of the nucleus, as indicated by the subsequent histology (shaded area in Fig. 10B).
Motoneurone responses to LRN stimulation before and after a C5 lesion
EPSPs
Most motoneurones showed EPSPs from single stimuli to the LRN, 43/56 (77 %) before the C5 lesion and in 67/89 (75 %) after it. Examples from before and after the lesion are shown in Fig. 10E and F and Fig. 12A and E, respectively. These EPSPs had short, stable absolute latencies of 1.3-1.6 ms which did not show shortening or facilitation with repetitive stimulation (Fig. 12B and F). In some cases a later EPSP was evoked by repetitive stimulation (arrow in Fig. 12F). It was not possible to estimate the segmental latency of the postsynaptic responses evoked by LRN stimulation, because no clear volley responsible for the effects could be discerned in the surface recording; therefore only the absolute latency, measured from the time of the stimulus, is given for the LRN data.
Figure 12. Comparison of motoneurone responses to stimulation of the PT and LRN after the C5 lesion.
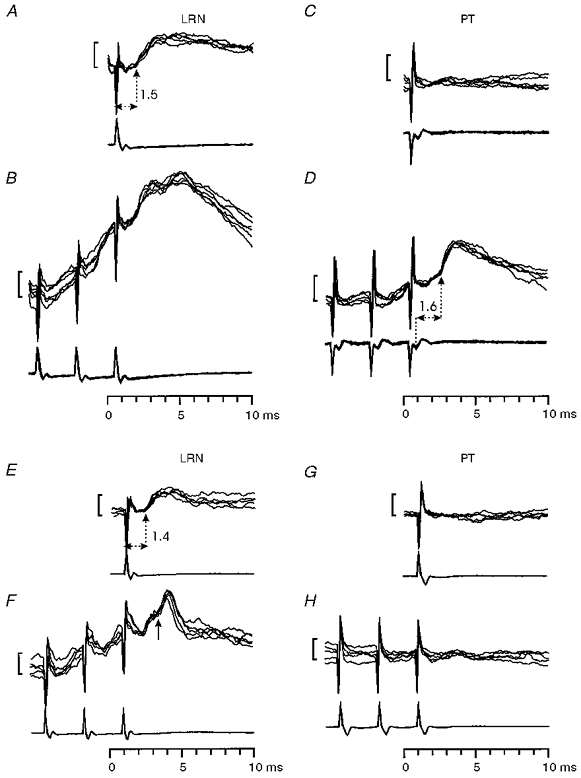
A-D, biceps motoneurone with an EPSP from the LRN and a late EPSP at a disynaptic latency from the PT. The LRN-evoked EPSP had a latency of 1.5 ms (A). Single PT stimuli did not evoke any effects (C), but triple shocks produced a late EPSP, with a segmental latency of 1.6 ms (D). All stimulation intensities were 200 μA. Calibration bars in all panels: 1 mV. E-H, responses in a median motoneurone to single (E and G) and triple (F and H) stimuli delivered to the LRN (E and F) and PT (G and H) with 200 μA shocks. The EPSP from the LRN had a latency of 1.4 ms. A later EPSP (arrowed) was evoked by repetitive stimulation (F). Neither single nor repetitive PT stimulation evoked any response in this motoneurone.
The properties of EPSPs evoked by single LRN stimuli before and after the C5 lesion are illustrated by the histograms in Fig. 11. The absolute latency ranged from 1.2 to 4.2 ms, with most < 2.0 ms and was similar before (mean ±s.d. 1.74 ± 0.56 ms) and after (1.81 ± 0.55 ms) the lesion. The distribution of rise times of the LRN evoked EPSPs is shown in Fig. 11B; only a few of these were sharply rising (ca 1.0 ms), and many had long rise times exceeding 1.5 ms (e.g. Fig. 12A). The mean rise time was 1.49 ± 0.53 ms before the lesion and 1.47 ± 0.49 ms after it. The distribution of EPSP amplitudes evoked from the LRN by a single 200 μA stimulus is plotted in Fig. 11C; most were rather small, nearly all being less than 2 mV. The mean amplitude was 1.1 ± 0.59 mV before the lesion and 1.2 ± 0.74 mV after it.
Figure 11. Properties of EPSPs evoked from the LRN before and after the C5 lesion.
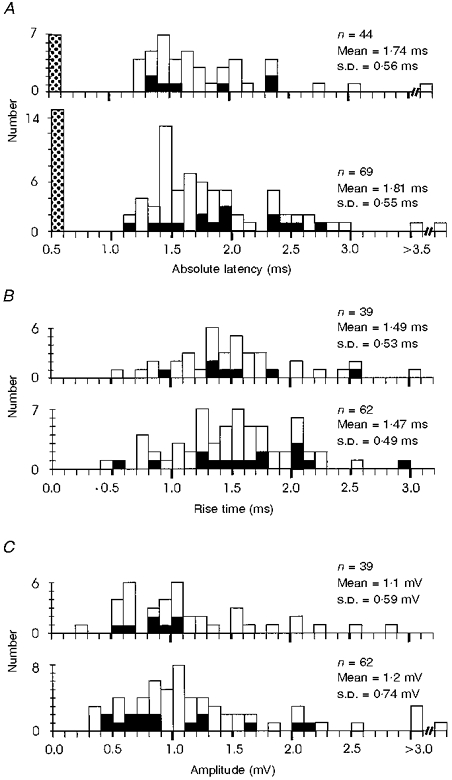
A, distribution of absolute latencies of EPSPs recorded in monkeys before (upper histogram) and after a C5 DLF lesion from 43 and 67 motoneurones, respectively, in response to single LRN stimuli of 200 μA. One motoneurone yielded both an early and a late EPSP. Stippled columns at extreme left: motoneurones unresponsive to LRN (no EPSP). The black columns represent those motoneurones with late EPSPs evoked by repetitive (3 ×) but not single PT stimuli. B, distribution of rise times of EPSPs evoked by stimulation of the LRN at 200 μA in 39 motoneurones before the lesion (upper histogram) and in 62 motoneurones after it. EPSPs were not contaminated by ensuing IPSPs. C, distribution of EPSP amplitudes in these same motoneurones.
In some experiments it was possible to record from the same motoneurone while making a number of different penetrations with the stimulating electrode in the vicinity of the LRN. EPSPs were reliably evoked from loci within and just dorsal to the LRN, but not from more medial tracks that did not pass close to the nucleus.
IPSPs
LRN stimulation evoked IPSPs with absolute latencies ranging from 1.8 to 3.3 ms. In most cases the IPSP was observed immediately after the short-latency EPSP. A slightly higher proportion of motoneurones showed IPSPs before the lesion than after it (20/56; 36 % compared with 18/89; 20 %, respectively).
Comparison of motoneurone responses to stimulation of the PT and LRN
It was of considerable interest to compare the occurrence of responses to PT and LRN stimulation in individual motoneurones recorded after the C5 lesion: if the short-latency EPSPs evoked from the LRN were mediated by a C3-C4 propriospinal system similar to that in the cat, motoneurones with such EPSPs should also show disynaptic EPSPs to repetitive stimulation of the PT. Figure 12A-D shows an example of a biceps motoneurone with responses consistent with this expectation: it responded to LRN stimulation with an EPSP (Fig. 12A) which had a short latency (1.5 ms) and which did not facilitate with repetitive stimulation (Fig. 12B). This motoneurone did not respond to a single PT shock (Fig. 12C), but yielded an EPSP in the disynaptic range (segmental latency 1.6 ms) with repetitive stimuli (Fig. 12D). Of the sixty-seven motoneurones giving EPSPs to LRN stimulation after the lesion, fifteen (22 %) showed such responses to repetitive PT stimulation (black columns in Fig. 11); eleven (16 %) of these were at disynaptic latencies. However, just as many of the motoneurones showing EPSPs from the LRN gave no response at all to PT stimulation (n= 15) and rather more gave only monosynaptic effects (22 at short and 10 at longer latencies). The remainder (5) showed IPSPs.
In general, then, the effect of the C5 lesion on responses to LRN stimulation was much less marked than on those evoked from the PT. This is shown in Fig. 13, which summarizes the proportion of EPSPs and IPSPs in motoneurones recorded before and after the C5 lesion. EPSPs from the LRN were almost as common after (75 %) as before the lesion (77 %). There was a small reduction in the number of IPSPs recorded (36 % before vs. 20 % after). For the PT effects, in contrast, the lesion produced a striking reduction in the occurrence of both monosynaptic EPSPs (from 76 to 36 %) and IPSPs (from 65 to 24 %) evoked by single stimuli (PT × 1). The amplitudes of the EPSPs were also reduced by the lesion (see Fig. 9), whereas there was little effect on EPSPs evoked from the LRN (see Fig. 11C). Many motoneurones (47 %) were unresponsive to PT stimulation after the lesion.
As reported above, late EPSPs evoked by triple stimuli (PT × 3) were seen infrequently, and their occurrence was similar before and after the lesion (19 and 18 %, respectively). EPSPs within the disynaptic range (black columns in Fig. 13) were slightly more frequent after the lesion (14 %) than before it (3 %). These results suggest that whereas the C5 lesion interrupted a significant proportion of the corticospinal tract projecting to the lower cervical segments, there was only a minor effect on the fibre systems mediating responses evoked by stimulation in the vicinity of the LRN.
DISCUSSION
The main objective of this study was to look for transmission of corticospinal commands to primate cervical motoneurones by a group of C3-C4 propriospinal neurones similar to those in the cat described by Lundberg, Illert, Alstermark and others (Illert et al. 1977; Baldissera et al. 1981; Alstermark & Lundberg, 1992). The results obtained give no support for a significant transmission of corticospinal commands by such a route in the macaque. Late EPSPs from the pyramidal tract with latencies in the di- or oligosynaptic range were small and were observed in only a small proportion of motoneurones. The experiments demonstrate clear differences in the organization of corticospinal control between the macaque monkey and cat which may be of considerable significance for understanding the mediation of this control in man.
Lack of evidence for C3-C4 propriospinal transmission in the macaque
We followed the experimental strategy used in previous experiments in the cat (Illert et al. 1977) using repetitive stimulation of the PT to search for disynaptic excitation of upper limb motoneurones, and to prove with lesions of the LCST at C5 that they were mediated by a relay above this segmental level (see Fig. 1). If such a propriospinal system exists in the primate with characteristics similar to that in the cat, we would have expected to find a considerable proportion of motoneurones with disynaptic excitation from the pyramid.
Motoneurone responses to PT stimulation in monkeys with intact spinal cords
The motoneurone recordings demonstrate the dominance of monosynaptic EPSPs from the pyramid (segmental latency < 1.2 ms, Fig. 2F); these were often followed by disynaptic inhibition (segmental latency 1.2-1.9 ms, Fig. 4K). This confirms the obvious lack of oligosynaptic excitatory effects in such preparations: Fritz et al. (1985) found only 5/76 hand and forearm motoneurones with such late EPSPs in response to PT stimulation (see Porter & Lemon, 1993, p. 137). Late EPSPs are also absent in the responses of cervical motoneurones to stimulation of the motor cortex and pyramidal tract (Landgren et al. 1962; Shapovalov, 1975), although this may have been due to the effects of the anaesthetics used (see below). These findings also agree with the EMG and motor unit responses evoked in wrist and hand muscles of the awake monkey from the primary motor cortex or pyramidal tract (Cheney & Fetz, 1985; Cheney et al. 1985; Mantel & Lemon, 1987; Lemon et al. 1987; Lemon & Mantel, 1989; see Porter & Lemon, 1993). These responses are characterized by single excitatory peaks of brief duration, often followed by a period of suppression. Although one cannot exclude the contribution of some non-monosynaptic effects to the single excitatory peaks seen in these studies, it is nevertheless noteworthy that no distinctive secondary peaks, which might correspond to a later phase of excitation, have been reported.
It is expected that synaptic effects mediated by interneuronal descending pathways to motoneurones will require facilitation by repetitive stimulation in order to transmit; in the cat disynaptic EPSPs evoked by repetitive PT stimulation increased in amplitude and shortened in latency with increasing numbers of stimuli (Baldissera et al. 1981). However, the overall proportion of motoneurones here which responded with late EPSPs after repetitive stimulation of the PT was small. If only cells with late EPSPs to repetitive, but not to single, stimuli are considered, then only 19 % (16/86) of tested motoneurones in monkeys with intact cords showed responses consistent with transmission by a C3-C4 propriospinal relay. Only three motoneurones (3 %) had late EPSPs within the disynaptic range.
Lesions of the LCST in C5
It is possible that disynaptic pyramidal IPSPs, which were facilitated by repetitive stimulation (see Fig. 4) obscured any late EPSPs, thereby leading to an underestimation of the number of motoneurones with di- or oligosynaptic excitation. In the lumbar segments of the monkey, Ia inhibitory interneurones contribute to this disynaptic inhibition (Jankowska et al. 1976), and it is likely that a similar mechanism operates in the cervical cord. We performed a C5 lesion of the DLF to reduce the amount of this inhibition and, at the same time, to prove that any disynaptic excitation which might be unmasked in this way is relayed via more rostrally located interneurones.
The C5 lesions were intended to interrupt most of the LCST fibres located in the upper parts of the DLF, including those that enter lamina IX directly, and presumably giving rise to CM contacts (Armand et al. 1997). Recordings from the DLF were used to judge the size of the LCST lesion. However, this method will preferentially detect the activity of the fast-conducting tract fibres, although the tract is made up mainly of small, slower fibres (Haggqvist, 1937; Humphrey & Corrie, 1978). After the lesion, between 70 and 95 % of the negative phase of the corticospinal volleys was abolished in the different experiments. This indicates a considerable destruction of the LCST, at least of the fast fibres, and this was confirmed by histological reconstruction of the lesions. The remaining negativity of the corticospinal volley does not necessarily mean that a considerable proportion of the LCST survived the lesion. In contrast to the situation in the cat (Illert et al. 1974), the remaining volley will have included activity conducted by anterior corticospinal tract fibres in the ventral funiculus, as indicated by recordings from dissected spinal quadrants in the monkey (M. A. Maier, M. Illert, P. A. Kirkwood, J. Nielsen & R. N. Lemon, unpublished observations). The extent of the lesions was further confirmed by the great reduction in the occurrence and amplitude of the monosynaptic CM EPSPs after the lesion (Figs 5, 9 and 13).
Responses to PT stimulation after C5 lesions
Forty-five of ninety-six motoneurones recorded after the lesion showed no early response at all (either EPSP or IPSP) to PT stimulation; many of these (26 %; 24/91) were also unresponsive to repetitive stimuli. These twenty-four motoneurones should have been the ideal candidates for displaying late EPSPs in case of a suprasegmental relay, since any masking of excitatory effects by inhibition should have been removed by the lesion. However, no such effects were seen.
Early EPSPs and IPSPs
Some motoneurones showed the same pattern as that seen before the lesion: an early EPSP with or without an IPSP. Single PT stimuli evoked a monosynaptic EPSP in 36 % (35/96) motoneurones after the C5 lesion, compared with 76 % (84/110) before it. These EPSPs had identical segmental latencies and rise times to those recorded in monkeys with intact cords (Figs 2F and 7K), and were probably produced by CM synapses supplied by fast-conducting corticospinal fibres that had survived the lesion; as expected, they were somewhat smaller than before the lesion (Fig. 9A and C).
There was as large a reduction in the occurrence of disynaptic inhibition (segmental latencies 1.4-1.9 ms; Table 2 and Fig. 13) as in monosynaptic excitation. With a single stimulus, 65 % of the sampled motoneurones showed early IPSPs before the lesion, which included motoneurones with a preceding monosynaptic EPSP. After the lesion, the proportion was only 24 %. For repetitive PT stimuli the proportions were 75 and 36 %, respectively. The parallel reduction in both monosynaptic excitation and inhibition is best explained by the interruption of a significant portion of CM input to motoneurones and of corticospinal input to segmental inhibitory interneurones in the cervical enlargement.
Late EPSPs
A clear distinction is drawn between late EPSPs elicited by single and by repetitive PT stimulation. We suggest that the former represent monosynaptic action by slow corticospinal fibres that have survived the C5 lesion, while the latter represent activation of the motoneurone via di- or oligosynaptic pathways. Late EPSPs evoked by single PT stimuli were recorded in 10/96 motoneurones (10 %) and had segmental latencies of 1.2 ms or more. These late EPSPs were only weakly facilitated in amplitude by repetitive stimuli and did not show any reduction in latency. Although the segmental latency is outside the monosynaptic range defined for the fastest fibres contributing to the early corticospinal volley, these features are nevertheless all characteristic of a monosynaptic linkage. The late EPSPs observed had absolute latencies of 1.9 ms or more, and could have been produced by corticospinal fibres conducting in the range of 20-30 m s−1. Since slow fibres by far outnumber the fast ones, it is possible that a considerable number of them survived the C5 lesion. Spike-triggered averages of EMG in the awake monkey also suggest that slowly conducting pyramidal tract neurones can exert direct effects on hand muscle motoneurones (Fetz & Cheney, 1980; Lemon et al. 1992). Late EPSPs may have been difficult to record before the C5 lesion because they were masked by the large IPSPs following fast fibre input.
The second group consists of 16/88 motoneurones (18 %) in which late EPSPs were evoked by two or three PT stimuli, while a single stimulus was without effect. This category of late EPSP (see Fig. 8) closely resembles those seen in the cat after a comparable C5 lesion of the LCST. The segmental latencies ranged from 1.3 to 3.0 ms; twelve cells showed effects in the range 1.3-1.9 ms (see small peak in Fig. 7L), values which are typical of a disynaptic linkage (Eccles et al. 1956). Thus these twelve EPSPs (14 % of the tested sample) best approach the criteria for transmission through a C3-C4 propriospinal system. Most of these EPSPs were small (< 1.0 mV; Fig. 9D). Because of the rarity of these effects and their small amplitude it was impractical to investigate them further, such as by transection of the corticospinal tract at C2 to determine directly whether these EPSPs involved a spinal or a supraspinal relay (e.g. via the brainstem, Illert et al. 1981). Moreover, since the lesions of the corticospinal tract at C5 may have been incomplete we cannot rule out the possibility that some or all of these late EPSPs were generated by segmental interneurones. Even if all of these EPSPs were generated by transmission through a C3-C4 propriospinal system, the low incidence and the small amplitudes are surprising in view of the common occurrence and large amplitude of such effects in cats with a C5 lesion of the LCST (Illert et al. 1977). This leads us to the conclusion that, compared with the situation in the cat, transmission through a C3-C4 propriospinal system is far less effective or even absent in the macaque.
Factors that might contribute to the lack of propriospinal transmission of corticospinal input
Anaesthesia
It might be argued that the lack of evidence for C3-C4 propriospinal transmission may be due to the susceptibility of such a system to anaesthesia. Four points can be made to counter this argument. First, the anaesthetic (α-chloralose) used in this study was identical to that used in the cat studies (Illert et al. 1977), in which EPSPs mediated by the C3-C4 system were common. We have confirmed this observation in one cat in this laboratory. In four of the five monkeys in which a C5 lesion was made the maximum dose of chloralose was 70 mg kg−1i.v. In two of these animals no additional barbiturate was necessary, and in the other two the total doses of barbiturate given were only 4 and 2.2 mg kg−1i.v., respectively. Thus it is unlikely that the level of anaesthesia depressed propriospinal transmission in these experiments. There was in any case no correlation between the dose of anaesthetics used and the proportion of late EPSPs observed in the different animals. Second, many of the sampled motoneurones showed oligosynaptic responses to stimulation of cutaneous nerves, high-threshold muscle afferents and the LRN (see Fig. 12B and F) confirming that the preparations were not overly depressed by anaesthesia. Third, the results obtained here, which emphasize the importance of the direct, monosynaptic projection, are consistent with observations made in the awake, unanaesthetized monkey (see above). Finally, the same anaesthetic regime has been used for experiments in the New World squirrel monkey (Saimiri sciureus) in which late EPSPs, possibly of propriospinal origin, were encountered more frequently (Maier et al. 1997).
Sample of motoneurones
The present experiments sampled over 100 motoneurones in ten monkeys with intact cords and a further ninety-six after C5 lesions in five of them. These motoneurones supplied a wide variety of muscles acting at the elbow, wrist and fingers, identical with the motor nuclei known in the cat to receive prominent C3-C4 propriospinal excitation (Illert et al. 1977). Thus it is unlikely that any significant propriospinal effects were missed in the current study because of an inadequate or unrepresentative sample.
Size of lesion
It should be considered whether the C5 lesions were so extensive that they also interrupted the axons of the putative C3-C4 propriospinal neurones. In the cat, the descending axons of these neurones run in the ventral half of the lateral funiculus, and are therefore spared by a dorsolateral funiculus lesion (Illert et al. 1977, Illert & Lundberg, 1978; Alstermark et al. 1981b). Were the location of such axons in the monkey similar to that in the cat, they should have been spared by the DLF lesion, at least in monkeys PN4, PN7 and PN10. However, there was no prevalence of motoneurones with late EPSPs evoked by repetitive PT stimuli in these cases. Furthermore, the occurrence of EPSPs evoked from the LRN was unaffected by the lesions (see below).
Facilitation needed from other sources
In the cat, the propriospinal neurones receive widespread convergence from several descending pathways (including rubro-, tecto- and reticulospinal fibres) and from peripheral inputs (Illert et al. 1978). Recordings from motoneurones have shown that these inputs facilitate each other strongly, with a dominant action from the PT (Illert et al. 1977). It is possible that in the monkey more late EPSPs would have been obtained if some of these additional inputs had been activated, providing spatial facilitation of the presumed propriospinal neurones. This may be the case, but it is worth pointing out that such a requirement for effective propriospinal transmission would suggest a corticospinal contribution which on its own is rather weak, in line with the other findings of our experiments. In the cat, PT stimulation can produce propriospinal activation of many motoneurones without the need for spatial facilitation from other inputs (Illert et al. 1977). One further possibility is that, in the primate, feed-forward inhibition of the C3-C4 system (Alstermark et al. 1984) is particularly strong. In the cat such inhibition has been suggested to explain the small disynaptic EPSPs in some categories of motoneurones (Alstermark & Sasaki, 1986a).
Lack of evidence for a ‘propriospinal volley’ in the macaque
In the cat, recordings were made by Illert et al. (1974) from a dissected spinal half at the C7 level; these recordings provide the most sensitive method of detecting descending volleys. In response to PT stimulation these recordings revealed an early corticospinal tract volley at around 1 ms. Repetitive stimuli elicited a later volley at around 2 ms. This volley survived a lesion to the corticospinal tract at C5, and it was considered to reflect the activity in the axons of C3-C4 propriospinal neurones discharged by corticospinal input and running in the ventrolateral funiculus. In recordings from the surface of the spinal cord this synaptic volley is seen as a discharge following the corticospinal tract volley. This finding was confirmed in the cat experiment (Fig. 6A) where recordings were made from the DLF caudal to the C5 lesion and an additional later volley was evident after the third PT stimulus (open arrow in Fig. 6A, Vc). No such volley was detected in the DLF recordings from the lesioned monkeys (see Vc in Fig. 6B). Interestingly, a late volley has been reported by Maier et al. (1997) in their study of the squirrel monkey, in which late EPSPs in response to repetitive PT activation were relatively common.
Comparison of motoneurone responses evoked from the LRN and PT
Our objective in stimulating the lateral reticular nucleus (LRN) was again based on the organization of the C3-C4 propriospinal system in the cat, in which it has been demonstrated that most C3-C4 propriospinal neurones have an ascending collateral to the LRN (see Alstermark & Lundberg, 1992; Tantisira et al. 1996). Stimulation of the LRN evokes monosynaptic EPSPs in cat forelimb motoneurones, and both anatomical and electrophysiological evidence indicates that this is largely due to the excitation of the ascending collaterals of C3-C4 propriospinal neurones which also have descending connections to the motoneurones (Alstermark et al. 1981a, 1990; Alstermark & Sasaki, 1986a,b; Alstermark & Lundberg, 1992).
Our experiments compared the responses of motoneurones to LRN and PT stimulation. Although many motoneurones responded with EPSPs to stimulation of the LRN (77 % before and 75 % after the C5 lesion), only a small proportion (16 %) responded to repetitive PT stimulation with EPSPs in the disynaptic range. This result is in strong contrast to the cat, where all motoneurones with monosynaptic EPSPs from the LRN also show disynaptic EPSPs to repetitive stimulation of the PT (Alstermark et al. 1981a; Alstermark & Sasaki, 1986a,b).
The interpretation of this result depends on the origin of the EPSPs evoked from the LRN. It is likely that many of these were monosynaptic in origin since they were evoked by single shocks, and had short, stable latencies which did not shorten with repetitive stimulation; nor did their amplitude show much facilitation (e.g. Fig. 12B and F). These effects were qualitatively very much like those in the cat. Both the field potentials and the EPSPs were evoked from within the LRN or just dorsomedial to it, where the ascending axons of C3-C4 propriospinal neurones have been shown to pass (Illert & Lundberg, 1978; Alstermark et al. 1981a; Alstermark & Sasaki, 1986b). In contrast to the effects evoked from the PT, the EPSPs from the LRN were not affected by the C5 lesion (Fig. 13). This would be consistent with the involvement of axons running in the ventral half of the lateral funiculus, where the C3-C4 propriospinal axons lie in the cat (Illert et al. 1978; Lundberg, 1992; Alstermark et al. 1990; Tantisira et al. 1996).
However, in the monkey, in contrast to the cat, there is as yet no anatomical or electrophysiological evidence for a population of C3-C4 neurones with an ascending collateral to the LRN (see Alstermark et al. 1981a, 1990; Tantisira et al. 1996). Further, it is possible that the monosynaptic EPSPs evoked from the LRN result from activation of ascending or descending systems passing in the vicinity of the LRN (see Alstermark et al. 1981a). Whatever the origin of these EPSPs in the monkey, it is interesting that they were much smaller than in the cat: most EPSPs were less than 2 mV (mean 1.2 mV; Fig. 11). In the cat, LRN stimulation evoked EPSPs almost all of which had amplitudes greater than 2 mV, including those recorded in large, fast motoneurones; mean amplitudes were 1.8-6.6 mV according to the muscle innervated (Alstermark et al. 1981a; Alstermark & Sasaki, 1986a,b). Such quantitative comparisons between different studies in different species can be problematic. Nevertheless, the absence of large EPSPs from the LRN suggests that any input relayed by this pathway will have comparatively small effects on motoneurones, and is thus entirely consistent with the general lack of disynaptic EPSPs from the pyramid.
Thus although we cannot attribute with any certainty the EPSPs evoked from the LRN to activation of a C3-C4 propriospinal system, we can conclude that if such system does exist in the macaque monkey, then, under the conditions of these experiments, it is not responsible for transmission of any significant corticospinal excitation to upper limb motoneurones.
Implications for observations made in man
Pierrot-Deseilligny and his colleagues (see Pierrot-Desilligny, 1996 for a review) have demonstrated in man non-monosynaptic inputs from peripheral afferents onto motor nuclei supplying a wide variety of upper limb muscles. It has been suggested that these inputs may be mediated by a class of premotoneurones in the upper cervical segments (Gracies et al. 1991). The interpretation of these results has been strongly influenced by the findings in the cat, and it has been proposed that a C3-C4 system in man is organized along the same lines as that found in the cat (Pierrot-Deseilligny, 1996). Particular emphasis has been placed on the excitatory nature of such a relay and on the dominant effect from the corticospinal tract, demonstrated by using transcranial magnetic stimulation (TMS) to facilitate non-monosynaptic excitation from the periphery (Gracies et al. 1994).
Our results are in conflict with this view. Given that both macaque and man are Old World primates, one would predict that if a C3-C4 propriospinal transmission of corticospinal inputs is well-developed in man, then it should also be prevalent in the monkey. Although we found some signs of C3-C4 propriospinal transmission, they were weak and few in number and by no means comparable with the situation in the cat. A critical issue in the interpretation of the results obtained in human subjects is the presumed location of the premotoneurones transmitting corticospinal inputs in the upper cervical region, similar to the C3-C4 system in the cat. Our data have stressed the importance of segmental inputs to upper limb motoneurones: i.e. those that are mediated by corticospinal projections to the same segments in which the target motoneurones are located. In the cat both C3-C4 and segmental interneurones contribute to the disynaptic EPSPs from the pyramid (Alstermark & Sasaki, 1985). In the monkey, there are heavy projections to the intermediate zone of these segments from all motor areas of the frontal lobe (Kuypers, 1981; Armand, 1982; Dum & Strick, 1996; Armand et al. 1997). Many of these projections may represent connections to segmental inhibitory interneurones (cf. Jankowska et al. 1976), which are responsible for the widespread IPSPs recorded in this study. However, connections to segmental excitatory interneurones may be present (cf. Fetz et al. 1996), as in the cat, and some non-monosynaptic effects described in man may be mediated in this way.
Evolutionary significance of cortico-motoneuronal versus propriospinal systems
There are many similarities between the organization of the corticospinal system in man and macaque monkey: its areas of origin, its distribution of fibre sizes and pattern of termination in the spinal cord (Kuypers, 1981; Porter & Lemon, 1993). There are extensive projections in both primates to lamina IX, and a well-defined proximal to distal gradient in the strength of CM input (Clough et al. 1968; Palmer & Ashby, 1992; Porter & Lemon, 1993). In the New World squirrel monkey, in which the CM projection to hand and arm motoneurones is rather weak, non-monosynaptic excitation of these same motoneurones from the corticospinal tract is more common than in the macaque (Maier et al. 1997). In the cat, CM connections are completely absent (Kuypers, 1981; Baldissera et al. 1981). In the Old World primate, our results stress the relative importance of direct CM versus indirect propriospinal connections for transmission of corticospinal commands to motoneurones. This may be related to the profound differences between primates and subprimates in the use of the upper limb as an organ of reach, exploration and grasp, with far less emphasis on body support and locomotion (Sherrington, 1906).
General conclusions
These experiments did not replicate in the primate those in the cat which demonstrated a disynaptic excitatory pathway from pyramidal tract neurones to upper limb motoneurones via C3-C4 propriospinal interneurones. The exceptions to this conclusion were minor, and the production of possible disynaptic EPSPs at the segmental level of the motoneurones or via brainstem relays cannot be ruled out. Whether or not C3-C4 propriospinal interneurones exist in the primate remains to be revealed by further work. However, the present study indicates that it is unlikely that a significant proportion of the corticospinal excitation is relayed by such a system. Therefore deductions about its existence in man and other primates, based on data from the cat, should now be regarded with great caution.
Acknowledgments
We should like to thank Nora Philbin, Chris Seers, Natalia Ognjenovic and Kully Sunner for excellent technical support. This work was funded by The Wellcome Trust.
References
- Alstermark B, Kümmel H, Pinter MJ, Tantisira B. Integration in descending motor pathways controlling the forelimb in the cat. 17. Axonal projection and termination of C3-C4 propriospinal neurones in the C6-Th1 segments. Experimental Brain Research. 1990;81:447–461. doi: 10.1007/BF02423494. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Lindström S, Lundberg A, Sybirska E. Integration in descending motor pathways controlling the forelimb of the cat. 8. Ascending projection to the lateral reticular nucleus from C3-C4 propriospinal neurones also projecting to forelimb motoneurones. Experimental Brain Research. 1981a;42:282–298. doi: 10.1007/BF00237495. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Lundberg A. The C3-C4 propriospinal system: target reaching and food-taking. In: Jami L, Pierrot-Deseilligny E, Zytnicki D, editors. Muscle Afferents and Spinal Control of Movement. London: Pergamon Press; 1992. pp. 327–354. [Google Scholar]
- Alstermark B, Lundberg A, Norrsell U, Sybirska E. Integration in descending motor pathways controlling the forelimb in the cat. 9. Differential behavioural defects after spinal cord lesions interrupting defined pathways from higher centres to motoneurones. Experimental Brain Research. 1981b;42:299–318. doi: 10.1007/BF00237496. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Lundberg A, Sasaki S. Integration in descending motor pathways controlling the forelimb in the cat. 12. Interneurones which may mediate descending feed-forward inhibition and feed-back inhibition from the forelimb to C3-C4 propriospinal neurones. Experimental Brain Research. 1984;56:308–322. doi: 10.1007/BF00236286. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Sasaki S. Integration in descending motor pathways controlling the forelimb in the cat. 13. Corticospinal effects in shoulder, elbow, wrist, and digit motoneurones. Experimental Brain Research. 1985;59:353–364. doi: 10.1007/BF00230915. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Sasaki S. Integration in descending motor pathways controlling the forelimb in the cat. 14. Differential projection to fast and slow motoneurones from excitatory C3-C4 propriospinal neurones. Experimental Brain Research. 1986a;63:530–542. doi: 10.1007/BF00237476. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Sasaki S. Integration in descending motor pathways controlling the forelimb in the cat. 15. Comparison of the projection from excitatory C3-C4 propriospinal neurones to different species of forelimb motoneurones. Experimental Brain Research. 1986b;63:543–556. doi: 10.1007/BF00237477. [DOI] [PubMed] [Google Scholar]
- Armand J. The origin, course and terminations of corticospinal fibers in various mammals. Progress in Brain Research. 1982;57:330–360. doi: 10.1016/S0079-6123(08)64136-9. [DOI] [PubMed] [Google Scholar]
- Armand J, Olivier E, Edgley SA, Lemon RN. The postnatal development of corticospinal projections from motor cortex to the cervical enlargement in the macaque monkey. Journal of Neuroscience. 1997;17:251–266. doi: 10.1523/JNEUROSCI.17-01-00251.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In: Brookhart JB, Mountcastle VB, editors. Handbook of Physiology, The Nervous System. II. Bethesda: American Physiological Society; 1981. pp. 509–595. [Google Scholar]
- Cheney PD, Fetz EE. Comparable patterns of muscle facilitation evoked by individual corticomotoneuronal (CM) cells and by single intracortical microstimuli in primates. Evidence for functional groups of CM cells. Journal of Neurophysiology. 1985;53:786–804. doi: 10.1152/jn.1985.53.3.786. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE, Palmer SS. Patterns of facilitation and suppression of antagonist forelimb muscles from motor cortex sites in the awake monkey. Journal of Neurophysiology. 1985;53:805–820. doi: 10.1152/jn.1985.53.3.805. [DOI] [PubMed] [Google Scholar]
- Clough JFM, Kernell D, Phillips CG. The distribution of monosynaptic excitation from the pyramidal tract and from primary spindle afferents to motoneurons of the baboon's hand and forearm. The Journal of Physiology. 1968;198:145–166. doi: 10.1113/jphysiol.1968.sp008598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. Journal of Neuroscience. 1996;16:6513–6525. doi: 10.1523/JNEUROSCI.16-20-06513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Fatt P, Landgren S. Central pathway for direct inhibitory action of impulses in largest affeent nerve fibres to muscle. Journal of Neurophysiology. 1956;19:75–98. doi: 10.1152/jn.1956.19.1.75. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Eyre JA, Lemon RN, Miller S. Excitation of the corticospinal tract by electromagnetic and electrical stimulation of the scalp in the macaque monkey. The Journal of Physiology. 1990;425:301–320. doi: 10.1113/jphysiol.1990.sp018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE, Cheney PD. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. Journal of Neurophysiology. 1980;44:751–772. doi: 10.1152/jn.1980.44.4.751. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Perlmutter SI, Maier MA, Flament D, Fortier PA. Response patterns and postspike effects of premotor neurons in cervical spinal cord of behaving monkeys. Canadian Journal of Physiology and Pharmacology. 1996;74:531–546. 10.1139/cjpp-74-4-531. [PubMed] [Google Scholar]
- Fritz N, Illert M, Kolb FP, Lemon RN, Muir RB, Van Der Burg J, Wiedemann E, Yamaguchi T. The cortico-motoneuronal input to hand and forearm motoneurones in the anaesthetized monkey. The Journal of Physiology. 1985;366:20P. [Google Scholar]
- Gracies JM, Meunier S, Pierrot-Deseilligny E. Evidence for corticospinal excitation of presumed propriospinal neurons in man. The Journal of Physiology. 1994;475:509–518. doi: 10.1113/jphysiol.1994.sp020089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracies JM, Meunier S, Pierrot-Deseilligny E, Simonetta M. Pattern of propriospinal-like excitation to different species of human upper-limb motoneurones. The Journal of Physiology. 1991;434:151–167. doi: 10.1113/jphysiol.1991.sp018463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggqvist G. Faseranalytische studien uber die pyramidenbahn. Acta Psychiatrica et Neurologica Scandinavica. 1937;12:457–466. [Google Scholar]
- Heffner RS, Masterton RB. The role of the corticospinal tract in the evolution of human digital dexterity. Brain, Behavior and Evolution. 1983;23:165–183. doi: 10.1159/000121494. [DOI] [PubMed] [Google Scholar]
- Humphrey DR, Corrie WS. Properties of pyramidal tract neuron system within a functionally defined subregion of primate motor cortex. Journal of Neurophysiology. 1978;41:216–243. doi: 10.1152/jn.1978.41.1.216. [DOI] [PubMed] [Google Scholar]
- Illert M, Jankowska E, Lundberg A, Odutola A. Integration in descending motor pathways controlling the forelimb in the cat. 7. Effects from the reticular formation on C3-C4 propriospinal neurones. Experimental Brain Research. 1981;42:269–281. doi: 10.1007/BF00237494. [DOI] [PubMed] [Google Scholar]
- Illert M, Lundberg A. Collateral connections to the lateral reticular nucleus from cervical propriospinal neurones projecting to forelimb motoneurones in the cat. Neuroscience Letters. 1978;7:167–172. doi: 10.1016/0304-3940(78)90162-3. 10.1016/0304-3940(78)90162-3. [DOI] [PubMed] [Google Scholar]
- Illert M, Lundberg A, Padel Y, Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 5. Properties of and monosynaptic connections on C3-C4 propriospinal neurones. Experimental Brain Research. 1978;33:101–130. doi: 10.1007/BF00238798. [DOI] [PubMed] [Google Scholar]
- Illert M, Lundberg A, Tanaka R. Disynaptic corticospinal effects in forelimb motoneurones in the cat. Brain Research. 1974;75:312–315. doi: 10.1016/0006-8993(74)90752-5. 10.1016/0006-8993(74)90752-5. [DOI] [PubMed] [Google Scholar]
- Illert M, Lundberg A, Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 3. Convergence on propriospinal neurons transmitting disynaptic excitation from the corticospinal tract and other descending tracts. Experimental Brain Research. 1977;29:323–346. doi: 10.1007/BF00236174. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Progress in Neurobiology. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Padel Y, Tanaka R. Disynaptic inhibition of spinal motoneurones from the motor cortex in the monkey. Journal of Physiology. 1976;258:467–487. doi: 10.1113/jphysiol.1976.sp011431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny AB, Inukai J. Principles of motor organization of the monkey cervical spinal cord. Journal of Neuroscience. 1983;3:567–575. doi: 10.1523/JNEUROSCI.03-03-00567.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers HGJM. Anatomy of the descending pathways. In: Brookhart JM, Mountcastle VB, editors. Handbook of Physiology, The Nervous System. II. Bethesda: American Physiological Society; 1981. pp. 597–666. [Google Scholar]
- Landgren S, Phillips CG, Porter R. Minimal synaptic actions of pyramidal impulses on some alpha motoneurones of the baboon's hand and forearm. The Journal of Physiology. 1962;161:91–111. doi: 10.1113/jphysiol.1962.sp006875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN. Cortical control of the primate hand. The 1992 G. L. Brown Prize Lecture. Experimental Physiology. 1993;78:263–301. doi: 10.1113/expphysiol.1993.sp003686. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Mantel GWH. The influence of changes in the discharge frequency of corticospinal neurones on hand muscles in the monkey. The Journal of Physiology. 1989;413:351–378. doi: 10.1113/jphysiol.1989.sp017658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN, Muir RB, Mantel GWH. The effects upon the activity of hand and forearm muscles of intracortical stimulation in the vicinity of corticomotor neurones in the conscious monkey. Experimental Brain Research. 1987;66:621–637. doi: 10.1007/BF00270695. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Werner W, Bennett KMB, Flament DA. The proportion of slow and fast pyramidal tract neurones producing post-spike facilitation of hand muscles in the conscious monkey. The Journal of Physiology. 1992;459:166P. [Google Scholar]
- Lundberg A. To what extent are brain commands for movements mediated by spinal interneurones. Behavioral and Brain Sciences. 1992;15:775–776. [Google Scholar]
- Maier M, Illert M, Kirkwood PA, Nielsen J, Lemon RN. Investigation into a possible C3-C4 propriospinal transmission of corticospinal excitation to forearm motoneurons in the macaque monkey. Society for Neuroscience. 1996a;22:430. [Google Scholar]
- Maier MA, Illert M, Kirkwood PA, Nielsen J, Lemon RN. Lack of evidence for C3-C4 propriospinal transmission of corticospinal excitation to forearm motoneurones in the anaesthetized macaque monkey. The Journal of Physiology. 1996b;494.P:63P. doi: 10.1111/j.1469-7793.1998.191bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier MA, Olivier E, Baker SN, Kirkwood PA, Morris T, Lemon RN. Direct and indirect corticospinal control of arm and hand motoneurons in the squirrel monkey (Saimiri sciureus) Journal of Neurophysiology. 1997;78:721–733. doi: 10.1152/jn.1997.78.2.721. [DOI] [PubMed] [Google Scholar]
- Mantel GWH, Lemon RN. Cross-correlation reveals facilitation of single motor units in thenar muscles by single corticospinal neurones in the conscious monkey. Neuroscience Letters. 1987;77:113–118. doi: 10.1016/0304-3940(87)90617-3. 10.1016/0304-3940(87)90617-3. [DOI] [PubMed] [Google Scholar]
- Olivier E, Edgley SA, Armand J, Lemon RN. An electrophysiological study of the postnatal development of the corticospinal system in the macaque monkey. Journal of Neuroscience. 1997;17:267–276. doi: 10.1523/JNEUROSCI.17-01-00267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer E, Ashby P. Corticospinal projections to upper limb motoneurones in humans. The Journal of Physiology. 1992;448:397–412. doi: 10.1113/jphysiol.1992.sp019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips CG. Proceedings of 3rd International Congress of the Primatology Society. Basel: Karger; 1971. Evolution of the corticospinal tract in primates with special reference to the hand; pp. 2–23. [Google Scholar]
- Phillips CG, Porter R. The pyramidal projection to motoneurones of some muscle groups of the baboon's forelimb. Progress in Brain Research. 1964;12:222–245. doi: 10.1016/s0079-6123(08)60625-1. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Transmission of the cortical command for human voluntary movement through cervical propriospinal premotoneurons. Progress in Neurobiology. 1996;48:489–517. doi: 10.1016/0301-0082(96)00002-0. 10.1016/0301-0082(96)00002-0. [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon RN. Corticospinal Function and Voluntary Movement. Oxford: Oxford University Press; 1993. p. 428. [Google Scholar]
- Shapovalov AI. Neuronal organization and synaptic mechanisms of supraspinal motor control in vertebrates. Reviews of Physiology Biochemistry and Pharmacology. 1975;72:1–54. doi: 10.1007/BFb0031545. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. The Integrative Action of the Nervous System. New Haven: Yale University Press; 1906. [Google Scholar]
- Tantisira B, Alstermark B, Isa T, Kummel H, Pinter M. Motoneuronal projection pattern of single C3-C4 propriospinal neurones. Canadian Journal of Physiology and Pharmacology. 1996;74:518–530. 10.1139/cjpp-74-4-518. [PubMed] [Google Scholar]
- Walberg F. The lateral reticular nucleus of the medulla oblongata in mammals. A comparative anatomical study. Journal of Comparative Neurology. 1952;96:283–344. doi: 10.1002/cne.900960205. [DOI] [PubMed] [Google Scholar]


