Abstract
The effect of rapid shortening on rate of force regeneration (dF/dtR) was examined in single, intact frog (Rana temporaria) skeletal muscle fibres (3·0 °C). Step releases leading to unloaded shortening were applied after 500 ms of stimulation, during the plateau of an isometric tetanus. Initial mean sarcomere length ranged from 2·05 to 2·35 μm; force regeneration after shortening was at 2·00 μm.
Values for dF/dtR following a 25 nm half-sarcomere−1 release were 3·17 ± 0·17 (mean ± s.e.m., n= 8) times greater than the initial rate of rise of force before release (dF/dtI). As release size was increased from 25 to 175 nm half-sarcomere−1, the relationship between release size and dF/dtR decreased sharply before attaining a plateau value that was 1·34 ± 0·09 times greater than dF/dtI. Despite wide variations in dF/dtR, the velocity of unloaded shortening remained constant (2·92 ± 0·08 μm half-sarcomere−1 s−1; n= 8) for the different release amplitudes used in this study.
To investigate its role in the attenuation of dF/dtR with increased shortening, the effects of rapid ramp (constant velocity) shortening on intracellular free Ca2+ concentration ([Ca2+]i) were monitored using the Ca2+-sensitive fluorescent dye furaptra. Compared with an isometric contraction, rapid fibre shortening was associated with a transient increase in [Ca2+]i while force regeneration after shortening was associated with a transient reduction in [Ca2+]i. The greatest reductions in [Ca2+]i were associated with the largest amplitude ramps.
Cross-bridge-mediated modifications of the Ca2+ affinity of troponin C (TnC) may explain the fluctuations in [Ca2+]i observed during and after ramps. Associated fluctuations in TnC Ca2+ occupancy could play a role in the reduction of dF/dtR with increasing release size.
Force generation is regulated by Ca2+ binding to troponin C (TnC) (reviewed by Ashley et al. 1991). Conformational changes in the regulatory complex of the thin filament induced by Ca2+ binding to TnC lead to the formation of the strongly bound, force-generating myosin-actin complex. In turn, co-operative interactions between attached cross-bridges and TnC subunits may reversibly increase the Ca2+ affinity of TnC of vertebrate skeletal muscle (Bremel & Weber, 1972; Güth & Potter, 1987). Hence the number of attached cross-bridges may modulate the Ca2+ occupancy of TnC, and thereby the activation level of the thin filament.
Edman (1980) has demonstrated that the rate of force regeneration (dF/dtR) following high velocity, unloaded shortening of intact frog skeletal muscle fibres decreases as the extent of shortening is increased. Stiffness measurements made on intact frog skeletal muscle fibres indicate that, compared with an isometric contraction, the number of attached cross-bridges is reduced during high velocity shortening (Julian & Sollins, 1975). Thus if myosin binding increases the Ca2+ affinity of TnC, high velocity shortening could reduce the Ca2+ affinity of TnC, raising the free myoplasmic Ca2+ concentration ([Ca2+]i) of intact, skeletal muscle fibres. Because the kinetics of force generation of skinned rabbit skeletal muscle fibres are Ca2+ sensitive (Brenner, 1988), shortening-induced reductions in the Ca2+ affinity of TnC could reduce the dF/dtR of intact skeletal muscle fibres.
Indirect evidence for shortening-induced reduction in the Ca2+ affinity of TnC has been obtained from isolated, intact cardiac muscle as well as from invertebrate muscle fibres. Allen & Kurihara (1982), using the Ca2+-sensitive, bioluminescent protein aequorin, showed elevations in [Ca2+]i during rapid shortening of rat and cat papillary muscles. Similarly, Gordon & Ridgway (1987) and Ridgway & Gordon (1984) used aequorin to show that shortening during relaxation from a twitch increases [Ca2+]i in giant barnacle fibres. However, Wang & Fuchs (1995) have directed attention to the fact that increased [Ca2+]i during shortening has never been reported for vertebrate skeletal muscle fibres (but see Edman, 1996). Indeed, studies performed on skinned rabbit skeletal muscle fibres by Fuchs and colleagues do not support the idea that myosin binding increases the Ca2+ affinity of TnC (Fuchs & Wang, 1991; Wang & Fuchs, 1995).
The working hypothesis for the experiments described here was that reductions in the number of force-generating cross-bridges attached to the thin filament during rapid shortening reduce the Ca2+ affinity of TnC, thereby reducing the Ca2+ occupancy of TnC. It follows that re-attachment of force-generating cross-bridges during force regeneration after shortening would co-operatively increase the Ca2+ affinity of TnC. This working hypothesis leads to the testable hypothesis that, compared with an isometric contraction, the [Ca2+]i of intact skeletal muscle fibres will be increased during rapid shortening and decreased during force regeneration following shortening. We report here that results obtained from tetanized, intact, skeletal muscle fibres from frogs are in agreement with this prediction. Biphasic fluctuations in [Ca2+]i associated with shortening and force regeneration support the view that cross-bridge attachment modulates Ca2+ occupancy of TnC. Associated changes in thin filament activation may, in turn, modulate dF/dtR following shortening. Preliminary accounts of this work have appeared elsewhere (Vandenboom et al. 1997a, b).
METHODS
Methods were approved by the Harvard Medical Area Standing Committee on Animals to comply with Federal, State and Harvard regulations governing the humane care and use of laboratory animals. Frogs (Rana temporaria) were stored at 4°C and killed by decapitation followed by double-pithing immediately after removal from cold storage. Single intact fibres were dissected from tibialis anterior muscles which had been excised and transferred to a dissecting dish. The microdissection methods and the experimental chamber have been described by Morgan et al. (1997). For these experiments, the chamber was mounted on an inverted microscope (Nikon Diaphot 300) fitted with a PTI (Photon Technology International, South Brunswick, NJ, USA) photometer system and a PTI Deltascan 4000 illumination system. All experiments were carried out at 3.0 ± 0.1°C. The standard Ringer solution contained (mm): 115 NaCl, 2.5 KCl, 1.8 CaCl2, 2.15 Na2HPO4, 0.85 NaH2PO4, pH 7.2.
Individual fibres were mounted by attaching the tendon at one end to a force transducer and the tendon at the other end to a servomotor. Care was taken in the mounting procedure to minimize end-compliance. Mean sarcomere length (SL) was determined from photographs taken at three different areas along the fibre at rest. This information was then used to calibrate fibre length to SL. A micrometer drive was used to adjust fibre length during experiments.
Vu and dF/dtR
Maximum unloaded shortening velocity (Vu) was determined using the slack test technique introduced by Edman (1979). The dF/dtR values were obtained from force traces recorded during the slack tests. Fibres were stimulated at 20-30 pulses s−1 to produce fused tetani of 1.0 s duration. Consecutive tetani were separated by 120 s rest periods. During the plateau of isometric force generation a step release (termed ‘release’ hereafter) was applied by a servomotor to one end of the fibre to allow shortening under unloaded conditions. Releases varied in amplitude from 25 to 175 nm half-sarcomere−1, in 25 nm half-sarcomere−1 increments. Initial fibre SL was adjusted to between 2.05 and 2.35 μm so that the final SL at which force was regenerated was always 2.00 μm. Slack time, the elapsed time between the introduction of the release and the onset of force regeneration, was determined using techniques described by Julian et al. (1986a). Release size plotted against slack time yields a straight line with slope equal to Vu (Edman, 1979).
The effect of shortening on dF/dtR, defined as the slope of the force record as it passes through a fixed load of 0.20 Fo (where Fo is the maximal isometric force at a SL of 2.00 μm), was determined for each release size. The initial rate of force generation before release (dF/dtI), defined as the slope of the force record as it passes through a fixed load of 0.20 Fo at a SL of 2.05 μm, was also determined. To calculate dF/dtR and dF/dtI, force records from slack tests were differentiated and then digitally low-pass filtered (corner frequency, 100 Hz). Similar procedures were used to determine dF/dtR and dF/dtI in furaptra-loaded fibres used during fluorescence experiments.
In control experiments, the effect of stimulus duration on dF/dtR was probed by applying 25 nm half-sarcomere−1 releases at different times during the course of an isometric tetanus. Fibres were stimulated (20-25 pulses s−1 for 1.0 s) once every 120 s and one release was applied per tetanus. Releases were applied at 25 ms intervals, up to 250 ms, as well as 500 and 750 ms after the start of stimulation. Other control experiments were conducted to determine the rapidity with which the increased rate of force rise observed during stimulation dissipated following cessation of stimulation. To do this, fibres were stimulated to produce two closely spaced isometric tetani; stimulus duration was 1 s for the first and 0.5 s for the second contraction. The elapsed time between the end of one stimulation period and the beginning of the next was 1 s, a period of time long enough for the onset of force generation from the second contraction to occur almost immediately (approximately 100 ms) following the completion of force relaxation from the first contraction. Tetanus pairs were separated by 180 s and SL was 2.15 μm.
Monitoring [Ca2+]i
[Ca2+]i was monitored using the ratiometric Ca2+-sensitive fluorescent dye furaptra (mag-fura-2; Molecular Probes, Eugene, OR, USA). Furaptra was introduced by soaking fibres for 30 min in a solution containing 10 μM dye at 20°C. Furaptra was present as the acetoxymethyl ester (AM) form and the dispersing agent Pluronic F-127 (0.01 % w/v) was used. In one experiment, the potassium salt form of furaptra was injected into a fibre by impaling the fibre with a micropipette with 1 μl of 10 mm furaptra in the tip, backfilled with 150 mm KCl. Dye was injected iontophoretically by passing -15 nA of current for 10 min.
For the experiments in which [Ca2+]i was monitored, fibres were stimulated at 15-25 pulses s−1 for 1-2 s duration to produce fused tetani. During these experiments, pairs of isometric (control) contractions were alternated with pairs of contractions incorporating ramp releases (test). All contractions were separated by 120 s of rest. The SL for isometric contractions was 2.00 μm. Shortening during test contractions was initiated after 500 ms of stimulation during the plateau of isometric force generation. Ramp releases (termed ‘ramps’ hereafter) were used to avoid introducing slack, with attendant motion artifacts, to fibres during shortening. A 5 nm half-sarcomere−1 step preceding the ramp was used to discharge the elastic strain residing in attached cross-bridges or in series with attached cross-bridges, to drop force to near zero. The rate of the ramp was carefully adjusted such that the velocity of shortening approached, but did not exceed, Vu. The rate of the ramp used for different size releases was constant for any given fibre. Thus during each ramp, developed force was reduced to near zero, but fibres did not go slack. The initial SL for test contractions was 2.05, 2.15, 2.35 or 2.70 μm, with force regeneration after ramps at a fixed SL of 2.00 μm. The ramp sizes corresponding to these initial SLs were 25, 75, 175 and 350 nm half-sarcomere−1, respectively.
Within each pair of control and test contractions, the fibre was illuminated during the first contraction with excitation wavelengths centred on 380 nm and during the second contraction centred on 344 nm. The illumination wavelength was set by a diffraction grating monochromator with entrance and exit slit widths set at 10 nm. The resulting fluorescence was monitored in a 40 nm band centred at 510 nm, set by an interference filter (Omega Optical, Brattleboro, VT, USA). Analog signals proportional to light intensity were recorded from a photomultiplier tube using a digital oscilloscope (Nicolet, Model Pro 20, Madison, WI, USA). Raw 344 nm and 380 nm light signals were treated in the following way. The relatively small background and autofluorescence signals, produced by illuminating the chamber and fibre before addition of dye, were subtracted at each excitation wavelength. Each record was then digitally low-pass filtered (corner frequency, 200 Hz) using digital signal processing software (VuPoint3, Maxwell Labs, La Jolla, CA, USA). The ratio, R, reporting [Ca2+]i, was formed by dividing the 344 nm fluorescence response by the 380 nm fluorescence responses from paired tetani.
The effect of shortening on [Ca2+]i was determined by subtracting the R signal generated from paired isometric contractions from the R signal generated from paired contractions incorporating ramps. The resulting waveform was termed furaptra ΔR. The magnitude of the decline in furaptra ΔR during force regeneration following shortening was determined by smoothing records using boxcar averaging (equal weighting of all points) with boxcar width equal to one stimulation period. The amplitude of the decline in furaptra ΔR (in absolute ratio units) was then calculated by subtracting the maximum decline evident in the furaptra ΔR record following shortening from the steady-state furaptra ΔR level achieved after the force regeneration phase at a SL of 2.00 μm.
Motion correction
Ratiometric dyes allow correction of the signal for motion artifacts, i.e. changes in light intensity caused by variations in the amount of dye contributing to the signal (Grynkiewicz et al. 1985), either through translation or rotation of the fibre, or through movement of the fibre relative to the focal plane of the microscope. In our system the furaptra fluorescence response to illumination at 344 nm is Ca2+ insensitive (i.e. 344 nm is the ‘isosbestic’ wavelength of furaptra). Thus, any light fluctuations observed during excitation at 344 nm are considered to be related to fibre motion. Fluorescence responses obtained during excitation at 380 nm have both Ca2+-sensitive and Ca2+-insensitive components. Thus, dividing light signals obtained at 344 nm by those obtained at 380 nm from consecutive contractions of the fibre eliminates the Ca2+-insensitive component and the resultant ratio R is free of motion artifact. The effectiveness of the ratio technique in cancellation of motion signals is illustrated in Fig. 1 of Morgan et al. (1997). During shortening, the fibre segment undergoing the least translational motion was the end opposite the servomotor. Thus, the mask from which fibre fluorescence was monitored was positioned as close as possible to that end.
Statistical analysis
Data are reported as means ±s.e.m. Mechanical and fluorescence data were analysed using a one-way repeated measures analysis of variance (ANOVA). The Student-Newman-Kuels test was used for determining significance among different release sizes (SigmaStat, Jandel Corporation, CA, USA). The value of P used throughout the study was 0.05.
RESULTS
Vu and dF/dtR
Length and force records from a typical slack test experiment are shown in Fig. 1A. Variations in isometric force development before releases were due to differences in initial SL. Maximal force regeneration after shortening (at SL = 2.00 μm) was similar for all releases. Values for dF/dtR were always increased with respect to values for dF/dtI, with the magnitude of this increase being diminished with increasing release size. This effect can be seen in Fig. 1B, where the force records shown in Fig. 1A are replotted on an expanded time scale. The relationship between dF/dtR and release size during slack tests is presented in Fig. 2 (•). After a 25 nm half-sarcomere−1 release, dF/dtR was 3.17 ± 0.17 times greater than dF/dtI. Values for dF/dtR decreased sharply as release size increased from 25 to 175 nm half-sarcomere−1. For release sizes in excess of approximately 175 nm half-sarcomere−1, a curve fitted to the dF/dtR data showed an asymptotic return towards dF/dtI.
Figure 1. Effects of step releases on force and the rate of force regeneration (dF/dtR) after shortening of intact frog skeletal muscle fibres.
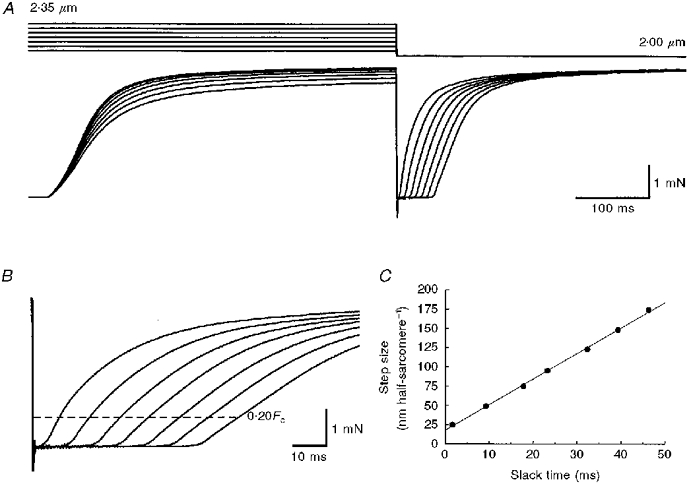
A, representative length (top) and force (bottom) records from one experiment. Step length releases were used to drop force to zero and to allow the fibre to shorten at maximum (unloaded) shortening velocity (Vu). Releases were introduced from the plateau of an isometric tetanus after 500 ms of stimulation. Starting mean sarcomere length (SL) varied from 2.05 to 2.35 μm but force regeneration was always at a fixed SL of 2.00 μm. B, force records shown in A on an expanded time scale, showing slowing of rate of rise of force with increasing release size. The dashed line represents 20 % of peak isometric tetanic force (Fo) after releases, the force at which dF/dtR was calculated for each release. C, plot of release size versus slack time for force records shown in A and B. The line is a least-squares regression fitted to the data (Vu= 3.33 μm half-sarcomere−1 s−1). Fibre 960929.
Figure 2. Effect of release size on dF/dtR in intact frog skeletal muscle fibres.
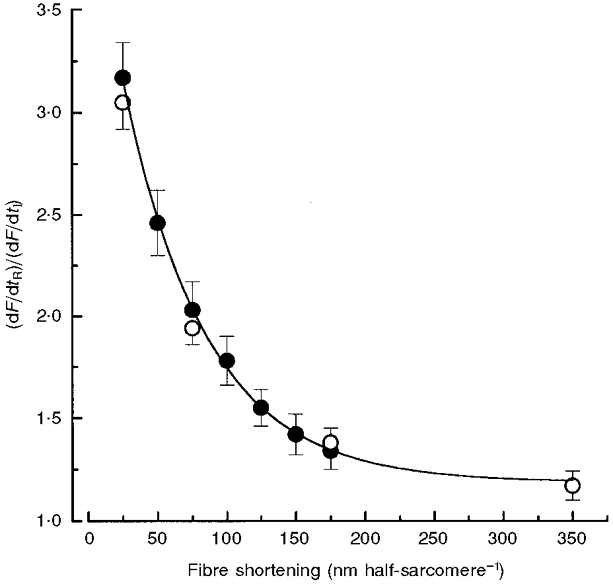
Filled symbols show the effect of step releases on dF/dtR (means ±s.e.m., n= 8). The curve is a first-order exponential decay fit to step release data, extrapolated to 350 nm half-sarcomere−1. Open symbols show the effect of ramps on dF/dtR (n= 7) in a separate set of fibres from which fluorescence data were collected. The effect of shortening on dF/dtR was similar whether step releases or ramps were used. Note that the value for the 350 nm half-sarcomere−1 ramp fell onto the curve fitted to step release data. All dF/dtR data were obtained during force regeneration at a SL of 2.00 μm, and normalized to values for dF/dtI. Absolute values for dF/dtI prior to step release and ramps were 53.8 ± 3.5 (n= 8) and 56.1 ± 10.7 mN s−1 (n= 7), respectively. All release and ramp dF/dtR values were significantly greater than corresponding dF/dtI values (P < 0.05).
Compared with releases applied 500 ms after the start of stimulation, dF/dtR was reduced when releases were applied less than 150 ms after the start of stimulation. Release times and respective reductions in dF/dtR were: 25 ms, 70 ± 3 %; 50 ms, 55 ± 4 %; 75 ms, 35 ± 3 %; 100 ms, 13 ± 1 %; and 125 ms, 9 ± 4 %. In contrast, releases applied between 150 and 250 ms and at 750 ms after the start of stimulation produced values for dF/dtR that were not significantly different from those due to releases applied at 500 ms. Release times with their corresponding reductions in dF/dtR were: 150 ms, 3 ± 4 %; 175 ms, 2 ± 1 %; 200 ms 4 ± 2 %; 225 ms, 2 ± 2 %; 250 ms, 2 ± 6 %; and 750 ms, 1 ± 3 %. The early release experiments (≤ 250 ms) were performed on one fibre (n= two releases per time point) and the later release experiments were performed using a second fibre (n= four to eight releases per time point). In all cases release size was 25 nm half-sarcomere−1.
The effect on dF/dtI of terminating and restarting stimulation was also examined. When stimulation was stopped and then restarted within 100-200 ms of the completion of relaxation, the dF/dtI of the second tetanus was 88 ± 3 % (n= 2) of the first.
Increasing amplitudes of release had no detectable effect on Vu. The evidence for this is the linear relationship between release size and slack time, an example of which is shown in Fig. 1C. Thus our results show that, under the conditions of these experiments, Vu was constant over the range 25-175 nm half-sarcomere−1 of shortening, despite large variations in dF/dtR. Mean Vu for all fibres was 2.92 ± 0.08 μm half-sarcomere−1 s−1 (n= 8).
Furaptra fluorescence changes during and after ramps
Figure 3 shows results from a typical ramp experiment from which fluorescence measurements were made on a furaptra-loaded fibre. Fig. 3A and B shows length and force records corresponding to isometric (b) and ramped (a) contractions. The corresponding furaptra ratios for the isometric and ramped contractions are shown superimposed in Fig. 3C. The ratio records have been offset slightly to facilitate comparison of the difference in the records after the ramp, when SL for both contractions was 2.00 μm. The difference between the two ratio signals is furaptra ΔR, shown in Fig. 3D, where upward and downward deflections in furaptra ΔR indicate that [Ca2+]i is elevated and reduced during the ramped compared with the isometric contraction, respectively.
Figure 3. Effect of rapid ramps on force and furaptra fluorescence in a tetanized frog skeletal muscle fibre.
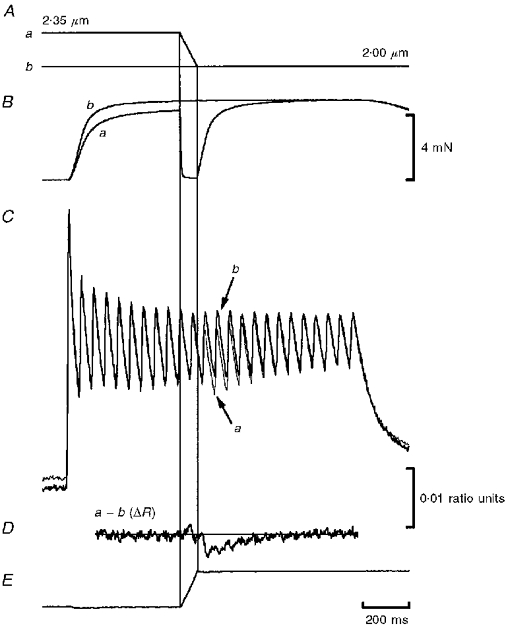
Length, force and furaptra records from a typical experiment. The fibre was stimulated at 20 pulses s−1 for 1.5 s. A, during stimulation, fibre SL was either fixed at 2.00 μm (isometric control) (b) or changed from 2.35 to 2.00 μm by applying a ramp release to one end of the fibre (a). B, the ramp was of sufficient speed to reduce force to near zero and to allow fibre shortening to occur at near Vu. The isometric (thick line) and ramped (thin line) ratios shown in C were obtained by dividing the 344 nm by the 380 nm fluorescence signals obtained from paired tetani. The furaptra ΔR, obtained by subtracting the isometric from the ramped ratio (a - b) is shown in D. E, 344 nm signal from the ramped contraction, showing that the fluctuations in furaptra ΔR we interpreted as changes in [Ca2+]i were not artifacts due to fibre motion. Fibre 970205.
In our apparatus, 344 nm excitation is isosbestic for furaptra, i.e. the fluorescence response during excitation at 344 nm is Ca2+ insensitive. Thus changes in the 344 nm signal are due to fibre motion. This can be seen in Fig. 3E, which shows that the isosbestic response mirrors the applied length change. That is, the ramp decrease in fibre length results in a ramp increase in fibre volume within the mask, which, in turn, gives rise to a ramp increase in fluorescence. It is important to note that the movement record in Fig. 3E bears no resemblance to the furaptra ΔR record shown in Fig. 3D, indicating that the furaptra ΔR response is not an artifact due to fibre motion.
The effects of shortening and force regeneration on furaptra ΔR are shown in Fig. 4 for four different ramp amplitudes. Mean furaptra ΔR records for fibres ramped from initial SLs of 2.05 (n= 8), 2.15 (n= 8), 2.35 (n= 10) and 2.70 μm (n= 6) to a fixed SL of 2.00 μm are shown. In each panel, the ΔR response rose soon after fibre shortening began (with a delay of 10-20 ms). Note that the furaptra ΔR records shown in Fig. 4C and D were elevated only during fibre shortening.
Figure 4. Effect of ramp amplitude on furaptra ΔR of tetanized frog skeletal muscle fibres.
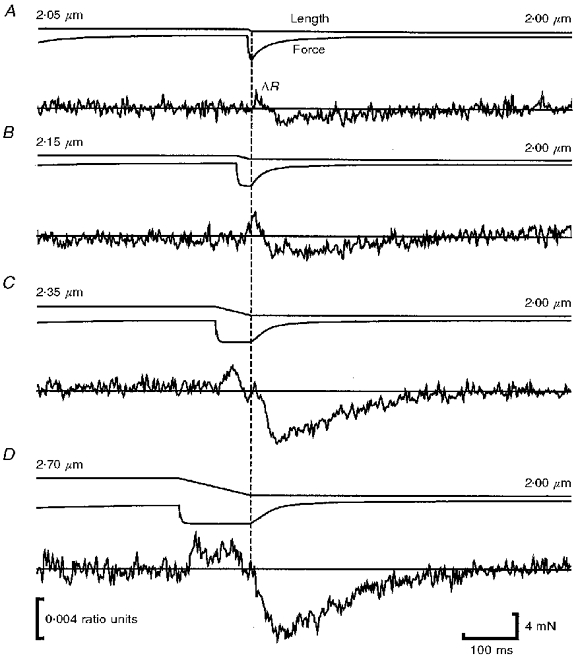
Representative length and force records are shown with corresponding mean furaptra ΔR records for each amplitude of ramp used. Fibres were ramped from a SL of 2.05 to 2.00 μm (n= 8; A), 2.15 to 2.00 μm (n= 8; B), 2.35 to 2.00 μm (n= 10; C) and 2.70 to 2.00 μm (n= 6; D). Before averaging, all ΔR records were time-shifted to be synchronized with respect to the end of the ramp. This was necessary due to slight variations in ramp duration among fibres due, in turn, to variations in Vu among fibres. A continuous line is drawn through the zero point of the mean furaptra ΔR record for each release to facilitate comparison of the effect of ramp amplitude. Although changes in fibre ΔR were also evident during or shortly after shortening, we have attempted to quantify changes in fibre ΔR only during the force regeneration phase of each procedure, when SLs for test and control contractions were the same. The records in each panel have been aligned so that force regeneration at the end of shortening occurred at the same time point in each panel (shown by dashed vertical line).
The records in Fig. 4 show a decline in furaptra ΔR following fibre shortening. Although evident for all ramps, this decline was most easily observed following ramps of 175 and 350 nm half-sarcomere−1. Note that the furaptra ΔR records corresponding to these ramps increased during shortening but did not decline below baseline until after the fibre had stopped shortening. Thus the decline in furaptra ΔR appeared to be coupled to force regeneration; moreover, furaptra ΔR continued to decline until force regeneration was very nearly complete. Thereafter the decline in furaptra ΔR was reversed, with furaptra ΔR returning to zero after several hundred milliseconds.
The coupling between the decline in furaptra ΔR and force regeneration was less evident for small ramps. For example, the decline in furaptra ΔR for the 25 and 75 nm half-sarcomere−1 ramps, shown in Fig. 4A and B, occurred relatively late after the start of force regeneration. This effect may be explained by the observation that furaptra ΔR was rising when force regeneration was initiated. Thus, the processes responsible for release of Ca2+ into and removal of Ca2+ from the myoplasm may have overlapped. In contrast, sufficient time elapsed between the start and end of the 175 and 350 nm half-sarcomere−1 ramps that the rise and fall in furaptra ΔR appeared as separate events.
The amplitude of the decline in furaptra ΔR for different ramps shown in Fig. 4 is plotted against ramp size in Fig. 5. The decline in ΔR for the 25, 75, 175 and 350 nm half-sarcomere−1 ramps was equivalent to 3.3 ± 1.1 (n= 8), 5.4 ± 1.0 (n= 8), 10.0 ± 1.1 (n= 10) and 13.8 ± 1.6 % (n= 6) of the mean tetanic ratio from control contractions, respectively.
Figure 5. Relation between amplitude of fall in furaptra ΔR record and ramp amplitude in tetanized frog skeletal muscle fibres.
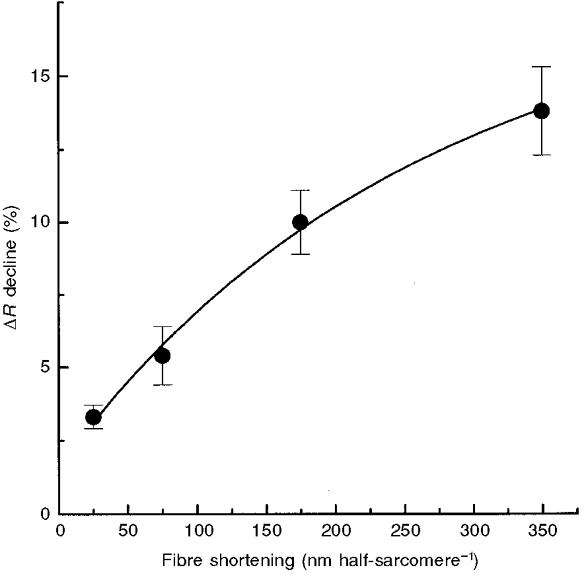
Plot of the decline in mean furaptra ΔR during the force regeneration phase of each release versus ramp amplitude for all fibres used in these experiments (means ±s.e.m., n= 6-10). The decline in mean furaptra ΔR is expressed as a percentage of mean tetanic fluorescence levels of control isometric contractions. All ΔR declines were significantly greater than zero (P < 0.05) and, with the exception of the two smallest ramps, significantly different from each other (P < 0.05).
Elimination of possible dye artifacts
There is a possibility that the fluctuations in ΔR we observed and that we attribute to alterations in [Ca2+]i were artifacts associated with the use of furaptra or to AM loading of the dye. In addition to Ca2+, furaptra binds Mg2+, albeit with a much lower affinity (Raju et al. 1989). To alleviate concern that our observations were the result of Mg2+ rather than Ca2+ fluctuations, we performed control experiments identical to those shown in Fig. 3 with fibres loaded with the AM form of the fluorescent Ca2+ indicator fura-2. Compared with furaptra, fura-2 is relatively insensitive to Mg2+ (Konishi et al. 1991, Konishi 1993). Fura-2-loaded fibres indicated a decline in [Ca2+]i during the force regeneration phase that was qualitatively similar to those shown in Fig. 4 for furaptra-loaded fibres. Thus changes in the furaptra fluorescence ratio associated with force regeneration were not due exclusively to fluctuations in [Mg2+].
The AM form of the dye used in these experiments diffuses freely into subcellular fractions such as mitochondria and the sarcoplasmic reticulum (SR), raising the possibility that changes in fluorescence that we interpreted as changes in [Ca2+]i could have been biased by changes in fluorescence within cellular compartments excluding the myoplasm. To test this we performed control experiments on a fibre injected with a membrane-impermeant form of furaptra. The fluorescence responses from those experiments were similar to those shown in Fig. 4, indicating that our results were not contaminated by changes in fluorescence originating from non-myoplasmic Ca2+.
DISCUSSION
The goal of this study was to monitor tetanic [Ca2+]i during and after rapid ramps leading to unloaded shortening and force regeneration in intact frog skeletal muscle fibres. This was done in an attempt to determine whether changes in [Ca2+]i could explain the slowing of force recovery with increasing release size. Compared with an isometric control contraction, ramps produced transient, biphasic fluctuations in [Ca2+]i. Shortening was associated with an increased [Ca2+]i while force regeneration after shortening was associated with a reduced [Ca2+]i, an effect that increased with ramp duration and size. Cross-bridge-mediated modification of the Ca2+ affinity of TnC during shortening and force regeneration could explain the observed fluctuations in [Ca2+]i, as well as the reduced rate of rise of force observed with increased shortening.
The increased furaptra ΔR observed during ramps suggests that shortening mobilizes Ca2+ into the myoplasm while the decline in furaptra ΔR after shortening suggests that force regeneration removes Ca2+ from the myoplasm. Because the Ca2+-binding capacity of the thin filament is large at 0.1- 0.3 mm (Ashley et al. 1991), while tetanic [Ca2+]i of frog skeletal muscle fibres is only 5-20 μM (Konishi et al. 1991), it seems feasible that mechanically induced alterations in the Ca2+ affinity of TnC are observable as changes in [Ca2+]i. It is difficult to conceive of a mechanism by which the other major Ca2+-buffering systems, parvalbumin (PV) and the SR, could be affected by shortening. Indeed, Ca2+ release in response to shortening has been observed in chemically skinned skeletal muscle fibres (Stephenson & Wendt, 1984), a preparation that has no PV or functional SR, as well as in skinned cardiac fibres (Allen & Kentish, 1988).
An advantage of using furaptra is that it is a relatively low affinity reporter of [Ca2+] (KD, 44 μM; Raju et al. 1989). Consequently, compared with other commonly used dyes such as fura-2 or indo-1, changes in furaptra R are approximately proportional to changes in [Ca2+]i, with no saturation of the fluorescence signal over the range of concentrations thought to be present in the myoplasm of tetanized frog skeletal muscle fibres. No attempt was made in this study to calibrate furaptra so that R could be transformed into [Ca2+]i. Because of its low affinity for Ca2+, furaptra is also better able to track rapid changes in [Ca2+]i with high fidelity compared with higher affinity dyes such as fura-2 or fluo-3 (Konishi et al. 1991).
Interpretation of changes in furaptra fluorescence
Our results may be interpreted in terms of a model for activation in which formation of the force-generating acto-myosin complex increases the Ca2+-binding affinity of TnC (Bremel & Weber, 1972; Güth & Potter, 1987; Zot & Potter, 1989). Rapid ramps leading to high velocity shortening, with the attendant reduction in the number of attached cross-bridges, could reduce the Ca2+ occupancy of TnC compared with the isometric condition. The Ca2+ released during shortening would appear in the myoplasm before being bound by PV or resequestered by the SR, thus accounting for the transient increase in [Ca2+]i observed. Reattachment of cross-bridges during force regeneration could remove Ca2+ from the myoplasm by increasing the Ca2+ affinity of TnC, thus accounting for the decrease in [Ca2+]i we observed.
The measured decline in furaptra ΔR increased as the amplitude of the ramp used to induce rapid shortening was increased. During these experiments, initial SL was increased and thus myofilament overlap before shortening was reduced as release size was increased. If Ca2+ release from TnC during shortening rapidly followed cross-bridge detachment from the thin filament, the increase in furaptra ΔR during shortening should be inversely related to ramp amplitude. Instead, the increases in furaptra ΔR upon shortening as ramp amplitude increased suggests that mobilization of Ca2+ into the myoplasm during shortening is dominated by other factors. One possible factor is that release of Ca2+ from TnC during rapid shortening lags cross-bridge detachment and, consequently, increases as the duration of unloaded shortening increases. That is, for a given redistribution of cross-bridges, large amplitude ramps leading to relatively long periods of shortening would mobilize more Ca2+ from TnC than relatively brief, small amplitude ramps. The magnitude of the subsequent resequestration of Ca2+ by TnC as force is regenerated, and thus the fall in the furaptra ΔR record, would increase with prolonged shortening duration. Another possible contributor to the increased decline in furaptra ΔR associated with larger ramp amplitudes may have been increases in the difference between the amount of myofilament overlap before the release and the amount of overlap during force regeneration, after release (i.e. Δoverlap) as ramp size was increased from 25 to 350 nm half-sarcomere−1. Based on myofilament overlap alone, the potential for the formation of force-generating cross-bridge attachments at a SL of 2.00 μm is equal following all ramp amplitudes. However, initial SL was increased and the number of initial force-generating cross-bridge attachments was decreased as ramp amplitudes were increased from 25 to 350 nm half-sarcomere−1. Hence, the number of newly recruited myosin binding sites on the thin filament beyond the region of initial myofilament overlap is increased as ramp amplitude increases. Thus, attachment of newly recruited cross-bridges may have contributed to the greater decline in furaptra ΔR observed for increased ramp sizes due to increases in the Ca2+ affinity of TnC induced by myosin binding (Swartz et al. 1996).
For all ramp amplitudes, the decline in furaptra ΔR observed during force regeneration was greater in amplitude than the increase in furaptra ΔR observed during shortening. These results suggest that the decline in [Ca2+]i during force regeneration exceeds the increase in [Ca2+]i during shortening. One possible explanation for this discrepancy is that the composite on-rate of the myoplasmic Ca2+ buffers is relatively greater than their composite off-rate. Under such conditions, a rapid increase in [Ca2+]i would be expected to be buffered more effectively than a rapid decline. This discrepancy could also be explained on the basis of differences in Δoverlap. Because myofilament overlap is greater during force regeneration than during shortening, the potential for removal of Ca2+ due to newly recruited cross-bridges exceeds the potential for release of Ca2+ due to cross-bridge detachment. A related possibility is that, because the region of overlap increases during shortening, there is a concomitant increase in the number of potential myosin-actin interactions during shortening. It may be that, in opposition to Ca2+ mobilization from the region of myofilament overlap present at the initiation of the ramp, there is increased binding of Ca2+ to TnC in the region of newly formed myosin-actin complexes, a region that is increasing during shortening. That is, the release of Ca2+ from TnC during shortening may be partially offset by uptake of Ca2+ from new regions of myofilament overlap where actin-myosin cross-bridge complexes of the type and number appropriate for unloaded shortening are being formed.
Relation to previous work
Several studies document different effects of rapid releases on [Ca2+]i in intact frog skeletal muscle fibres. Caputo et al. (1994) used the Ca2+-sensitive fluorescent indicator fluo-3 to show that shortening transiently reversed the decline of [Ca2+]i during relaxation from a tetanus. Edman (1996), using fluo-3, showed that [Ca2+]i was also transiently increased during a shortening step applied at the beginning of a tetanus. In contrast, Taylor et al. (1981) found that tetanic [Ca2+]i of aequorin-loaded fibres was reduced by shortening, similar to the original report of reduced [Ca2+]i in aequorin-loaded single mouse skeletal fibres by Allen (1978). Cecchi et al. (1984) used aequorin to show that quick releases exceeding 4-5 % fibre length resulted in a ‘momentary fall’ in tetanic [Ca2+]i. No increase in tetanic [Ca2+]i during shortening was observed by Cecchi et al. (1984). Thus the present work is unique in that we show that, compared with isometric controls, fibre shortening and force regeneration after shortening result in transient increases and decreases in tetanic [Ca2+]i, respectively. Thus our results provide indirect evidence that cross-bridge interaction with the thin filament reversibly alters the affinity of TnC for Ca2+ in intact frog skeletal muscle fibres.
Caputo et al. (1994) reported that releasing frog skeletal muscle fibres to shorten from 2.20 to 2.08 μm sarcomere−1 did not alter tetanic [Ca2+]i. However, the rate of shortening used by Caputo et al. (1994) was only a small fraction of Vu. Consequently, the reduction in the number of force-generating cross-bridges attached to the thin filament during shortening was probably much smaller than in the present study. To verify that this discrepancy is due to differences in shortening rate, we applied slow length ramps to change SL from 2.35 to 2.00 μm during the plateau of isometric force generation in tetanically stimulated fibres. In accord with the results of Caputo et al. (1994), this manoeuvre did not measurably alter [Ca2+]i (data not shown).
dF/dtRversus dF/dtI
Our results confirm that the rate of force regeneration from a release applied during the plateau of an isometric tetanus is greater than the initial rate of force generation (Jewell & Wilkie, 1958). We observed that, after the first 250 ms of a tetanic contraction, dF/dtR does not depend upon the duration of stimulation. This rules out the possibility that the observed decrease in dF/dtR with increasing release size is due to the concomitant increase in the duration of the tetanus before the measurement is made. The fact that the increase in dF/dtR with respect to dF/dtI was abolished when the fibre was allowed to relax briefly between two bouts of tetanic stimulation suggests that this increase was not due to metabolic factors. Instead, the increase in dF/dtR with respect to dF/dtI may be due to increased activation of the contractile apparatus by increased Ca2+ binding and/or cross-bridge attachment to the thin filament.
Effect of release size on dF/dtR
The reductions in dF/dtR we observed in these experiments with increasing release size cannot be explained on the basis of slowed cross-bridge cycling. That is, since unloaded shortening velocity was not influenced, the effect of increasing release size on the attenuation of dF/dtR most probably involved some impairment in the activation of the contractile element. Because size-dependent reductions in dF/dtR have also been reported for skinned skeletal muscle fibres (Ekelund & Edman, 1982), it is unlikely that shortening effects on dF/dtR observed in intact fibres involves impaired Ca2+ release from the SR.
Edman & Kiessling (1971) were the first to propose that co-operative interactions between cross-bridge attachment to actin and the Ca2+-binding affinity of TnC could produce alterations in the mechanical properties of skeletal muscle fibres. Our results are in general agreement with this hypothesis. For example, the increased fluctuations in [Ca2+]i observed with increased release size may have been related to shortening-induced decreases in the Ca2+ affinity of TnC. Because the rate constants of force regeneration of skinned skeletal muscle fibres are known to be Ca2+ sensitive (Brenner, 1988), it seems feasible that the relative decline in dF/dtR with increased shortening was a consequence of decreased Ca2+ activation of the thin filament.
The declining effect of release size on dF/dtR may be explained by noting that, in skinned skeletal muscle fibres, the relation between [Ca2+] and ktr, the rate constant for force redevelopment following release and restretch, is very steep when the [Ca2+] in the bathing medium yields force > 50 %Fo, and is relatively unaffected by changes in [Ca2+] when the [Ca2+] in the bathing medium yields forces < 50 %Fo (Regnier et al. 1996). In our experiments, fibres were released to shorten during force production well in excess of 50 %Fo. Thus even minor alterations in the Ca2+ occupancy of TnC, evident as increases in the furaptra ΔR associated with shortening, may have been causally related to the slowed recovery of force associated with longer release sizes.
Julian et al. (1997b) have shown that when bathing [Ca2+] is decreased to levels that decrease force development extensively (e.g. to 30 % of Fo), the Vu of skinned frog skeletal muscle fibres is also decreased. The absence of an effect of release size on Vu in the present work suggests that, even for the largest releases used, any resulting decrease in thin filament activation did not approach that achieved by Julian et al. (1986b) in skinned fibres by lowering bathing [Ca2+]. Our results showing large variations in dF/dtR with no change in Vu following different releases (c.f. Edman, 1980) can be explained if, at high [Ca2+]i, Vu of intact frog skeletal muscle fibres is less sensitive to variations in the Ca2+ affinity of TnC than is dF/dtR.
Conclusions
Rapid shortening increases, while force regeneration after shortening decreases, tetanic [Ca2+]i of intact, frog skeletal muscle fibres. These results are consistent with the idea that cross-bridge interaction with the thin filament alters the Ca2+ affinity and thus the Ca2+ occupancy of TnC. In turn, shortening-induced variations in the Ca2+ occupancy of TnC may modulate the kinetics of force recovery.
Acknowledgments
This work was supported by National Institutes of Health grant HL 35032 (to F. J. J.).
References
- Allen DG. Shortening of tetanized skeletal muscle causes a fall of intracellular calcium concentration. The Journal of Physiology. 1978;275:63P. [PubMed] [Google Scholar]
- Allen DG, Kentish JC. Calcium concentration in the myoplasm of skinned ferret ventricular muscle following changes in muscle length. The Journal of Physiology. 1988;407:489–503. doi: 10.1113/jphysiol.1988.sp017427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG, Kurihara S. The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. The Journal of Physiology. 1982;327:79–94. doi: 10.1113/jphysiol.1982.sp014221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley CA, Mulligan IP, Lea TJ. Ca2+ and activation mechanisms in skeletal muscle. Quarterly Reviews of Biophysics. 1991;24:1–73. doi: 10.1017/s0033583500003267. [DOI] [PubMed] [Google Scholar]
- Bremel RD, Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nature New Biology. 1972;238:97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: Implications for regulation of muscle contraction. Proceedings of the National Academy of Sciences of the USA. 1988;85:3265–3269. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C, Edman KAP, Lou F, Sun Y-B. Variation in myoplasmic Ca2+ concentration during contraction and relaxation studied by the indicator fluo-3 in frog skeletal muscle fibres. The Journal of Physiology. 1994;478:137–148. doi: 10.1113/jphysiol.1994.sp020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi G, Griffiths PJ, Taylor S. Sugi H, Pollock GH, editors. Changes in intracellular Ca2+ induced by shortening imposed during tetanic contractions Contractile Mechanisms in Muscle. Advances in Experimental Medicine and Biology. 1984;170:455–472. doi: 10.1007/978-1-4684-4703-3_41. [DOI] [PubMed] [Google Scholar]
- Edman KAP. The velocity of unloaded shortening and its relation to sarcomere length and isometric tension in vertebrate muscle fibres. The Journal of Physiology. 1979;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KAP. Depression of mechanical performance by active shortening during twitch and tetanus of vertebrate muscle fibres. Acta Physiologica Scandinavica. 1980;109:15–26. doi: 10.1111/j.1748-1716.1980.tb06559.x. [DOI] [PubMed] [Google Scholar]
- Edman KAP. Fatigue vs. shortening-induced deactivation in striated muscle. Acta Physiologica Scandinavica. 1996;156:183–192. doi: 10.1046/j.1365-201X.1996.t01-1-198000.x. 10.1046/j.1365-201X.1996.t01-1-198000.x. [DOI] [PubMed] [Google Scholar]
- Edman KAP, Kiessling A. The time course of the active state in relation to sarcomere length and movement studied in single skeletal muscle fibres of the frog. Acta Physiologica Scandinavica. 1971;81:182–196. doi: 10.1111/j.1748-1716.1971.tb04891.x. [DOI] [PubMed] [Google Scholar]
- Ekelund MC, Edman KAP. Shortening induced deactivation of skinned fibres of frog and mouse striated muscle. Acta Physiologica Scandinavica. 1982;116:189–199. doi: 10.1111/j.1748-1716.1982.tb07129.x. [DOI] [PubMed] [Google Scholar]
- Fuchs F, Wang Y-P. Force, length, and Ca2+ troponin C affinity in skeletal muscle. American Journal of Physiology. 1991;261:C787–792. doi: 10.1152/ajpcell.1991.261.5.C787. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Ridgway EB. Extra calcium on shortening in barnacle muscle. Is the decrease in calcium binding related to decreased cross-bridge attachment, force, or length. Journal of General Physiology. 1987;90:321–340. doi: 10.1085/jgp.90.3.321. 10.1085/jgp.90.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Güth K, Potter JD. Effect of rigor and cycling cross-bridges on the structure of troponin C and on the Ca2+ affinity of the Ca2+-specific regulatory sites in skinned rabbit psoas fibers. Journal of Biological Chemistry. 1987;262:13627–13635. [PubMed] [Google Scholar]
- Jewell BR, Wilkie DR. An analysis of the mechanical components in frog's striated muscle. The Journal of Physiology. 1958;143:515–540. doi: 10.1113/jphysiol.1958.sp006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian FJ, Rome LC, Stephenson DG, Striz S. The maximum speed of shortening in living and skinned frog muscle fibres. The Journal of Physiology. 1986a;370:181–190. doi: 10.1113/jphysiol.1986.sp015929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian FJ, Rome LC, Stephenson DG, Striz S. The influence of free calcium on the maximum speed of shortening in skinned frog muscle fibres. The Journal of Physiology. 1986b;380:257–273. doi: 10.1113/jphysiol.1986.sp016284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian FJ, Sollins MR. Variation of muscle stiffness with tension at increasing speeds of shortening. Journal of General Physiology. 1975;66:287–302. doi: 10.1085/jgp.66.3.287. 10.1085/jgp.66.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M, Hollingworth S, Harkins AB, Baylor SM. Myoplasmic calcium transients in intact frog skeletal muscle fibers monitored with the fluorescent indicator furaptra. Journal of General Physiology. 1991;97:271–301. doi: 10.1085/jgp.97.2.271. 10.1085/jgp.97.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M, Suda N, Kurihara S. Fluorescence signals from the Mg2+/Ca2+ indicator furaptra in frog skeletal muscle fibers. Biophysical Journal. 1993;64:223–239. doi: 10.1016/S0006-3495(93)81359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL, Claflin DR, Julian FJ. The relationship between tension and slowly varying intracellular calcium concentration in intact frog skeletal muscle. The Journal of Physiology. 1997;500:177–192. doi: 10.1113/jphysiol.1997.sp022008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju B, Murphy LA, Levy LA, Hall RD, London RE. A fluorescent indicator for measuring cytosolic free magnesium. American Journal of Physiology. 1989;256:C540–548. doi: 10.1152/ajpcell.1989.256.3.C540. [DOI] [PubMed] [Google Scholar]
- Regnier M, Martyn DA, Chase PB. Calmidazolium alters Ca2+ regulation of tension redevelopment rate in skinned skeletal muscle. Biophysical Journal. 1996;71:2786–2794. doi: 10.1016/S0006-3495(96)79471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway EB, Gordon AM. Muscle calcium transient. Effect of post-stimulus length changes in single fibers. Journal of General Physiology. 1984;83:75–103. doi: 10.1085/jgp.83.1.75. 10.1085/jgp.83.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson DG, Wendt IR. Length dependence of changes in sarcoplasmic Ca2+ concentration and myofibrillar Ca2+ sensitivity in striated muscle fibres. Journal of Muscle Research and Cell Motility. 1984;5:243–272. doi: 10.1007/BF00713107. [DOI] [PubMed] [Google Scholar]
- Swartz DR, Moss RL, Greaser ML. Calcium alone does not activate the thin filament for S1 binding to rigor myofibrils. Biophysical Journal. 1996;71:1891–1904. doi: 10.1016/S0006-3495(96)79388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SR, Lopez JR, Griffiths PJ, Cecchi G. Calcium in excitation-contraction coupling of frog skeletal muscle. Canadian Journal of Physiology and Pharmacology. 1981;60:489–502. doi: 10.1139/y82-068. [DOI] [PubMed] [Google Scholar]
- Vandenboom R, Claflin DR, Julian FJ. Plateau in shortening-induced reduction of force development rate in frog skeletal muscle fibers. Biophysical Journal. 1997a;72:381a. [Google Scholar]
- Vandenboom R, Claflin DR, Julian FJ. Effect of rapid releases and force regeneration on free myoplasmic [Ca2+] of intact frog skeletal muscle fibers. Canadian Journal of Applied Physiology. 1997b;22(suppl.):62P. doi: 10.1111/j.1469-7793.1998.171bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-P, Fuchs F. Osmotic compression of skinned cardiac and skeletal muscle bundles: Effects on force regeneration, Ca2+ sensitivity and Ca2+ binding. Journal of Molecular and Cellular Cardiology. 1995;27:1235–1244. doi: 10.1016/s0022-2828(05)82385-5. [DOI] [PubMed] [Google Scholar]
- Zot AS, Potter JD. Reciprocal coupling between troponin C and myosin cross-bridge attachment. Biochemistry. 1989;28:6751–6756. doi: 10.1021/bi00442a031. [DOI] [PubMed] [Google Scholar]


