Abstract
The effects of acute pulmonary venous congestion on the activity of rapidly adapting receptors (RARs) were determined in intact (control and sham-operated) rabbits and in rabbits 6 and 12 weeks after surgical destruction of the mitral valve.
Destruction of the mitral valve increased the mean left atrial pressure (LAP) by approximately 2·6 and 3·8 mmHg, 6 and 12 weeks after surgery, respectively. These changes were accompanied by significant increases in left ventricular weight. The effect of acute increments in LAP on RAR activity was examined against this background of chronic pulmonary venous congestion.
In intact control and sham-operated animals RAR activity increased from 48·8 ± 0·9 to 83·5 ± 3·6 and 121·1 ± 4·7 action potentials min−1 when the LAP was raised by 5 and 10 mmHg, respectively, above control values. Six weeks after surgery only 40 % of RARs were activated in this way.
In animals maintained for 12 weeks after surgery, RAR activity at LAPs of 6·6 ± 1·2 (control), 11·6 ± 1·2 and 16·6 ± 1·2 (mmHg) were 35·5 ± 2·3, 33·8 ± 14·4 and 34·0 ± 3·4 action potentials min−1, respectively. These changes were statistically not significant.
Slowly adapting receptors (SARs) in the lung showed a small but statistically significant increase in activity when the left atrial pressure was acutely elevated in both intact and mitral valve damaged animals.
It is concluded that chronic pulmonary venous congestion resulting from destruction of the mitral valve attenuates the ability of RARs to respond to acute moderate elevations of LAP.
Left ventricular failure is a condition that is characterized by chronic pulmonary venous congestion interspersed by episodes of acute pulmonary alveolar oedema. Previous investigations from this laboratory have demonstrated that the activity of rapidly adapting receptors (RARs) are influenced by fluid fluxes from microvasculature of the airways (Hargreaves et al. 1991). RARs have been shown to respond to acute pulmonary venous congestion with a sustained increase in activity. In those experiments, pulmonary venous congestion was caused by partial obstruction of the mitral valve to raise the left atrial pressure (LAP) by 5-10 mmHg.
Since left ventricular failure usually presents as acute exacerbation of a chronic condition, it is of interest to determine whether the functions of the RARs are modified by persistent mild pulmonary venous congestion. The experiments reported in this paper were undertaken to test the hypothesis that the increase in RAR activity resulting from small acute increments of LAP (5 and 10 mmHg) was attenuated in animals maintained in a state of chronic pulmonary venous congestion. Chronic pulmonary venous congestion was produced surgically by partial destruction of the mitral valve. The experiments were undertaken in anaesthetized New Zealand White rabbits. The data from these animals were compared with intact control animals and sham-operated animals.
METHODS
Experiments were performed on forty-five male New Zealand White rabbits weighing 3.28 ± 0.05 kg (range 2.7-3.8 kg). The protocol was approved by the Animal Use and Care Committee of the University of California, Davis. Two groups of rabbits were studied: intact (control) rabbits and those with surgically induced mitral regurgitation. They were pre-medicated with ketamine HCl (50 mg kg−1, Mallinckrodt Veterinary Inc., Mundelein, IL, USA) and xylazine (5 mg kg−1, Bayer, Shawnee Mission, KA, USA) i.m., and then anaesthetized with pentobarbitone sodium (5 mg kg−1, Veterinary Laboratories Inc., Lenexa, KA, USA) given through an ear vein. Anaesthesia was maintained with pentobarbitone sodium (4 mg kg−1, Veterinary Laboratories Inc.) i.v. every half an hour. After induction of anaesthesia an uncuffed endotracheal tube (i.d. 3 mm and length 5 cm) was introduced through a tracheostomy and was tied in place. The rabbits were artificially ventilated (Model 55-0798; Harvard Apparatus, South Natick, MA, USA) at a rate of 16-20 breaths min−1 and a tidal volume of approximately 8 ml kg−1. The inspired air was supplemented with oxygen and arterial PO2 was maintained above 100 mmHg. The intra-tracheal pressure was measured via a tube (i.d. 1.14 mm, Intramedic polyethylene tubing, Becton Dickinson Co., Sparks, MD, USA) inserted through a side arm into the tracheostomy tube. After connecting to the ventilator, the animals were paralysed with gallamine triethiodide (1.5 mg kg−1, Sigma) i.v. every half an hour. Adequacy of anaesthesia in paralysed animals was monitored by carefully observing them for spontaneous fluctuations in blood pressure and heart rate. They were allowed to recover from gallamine at approximately hourly intervals and tested for absence of a withdrawal reflex and increases in arterial blood pressure and heart rate in response to paw pinch.
Femoral artery and vein were cannulated (i.d. 1.14 mm and 0.86 mm, respectively, using Intramedic polyethylene tubing). The larger was used to measure aortic blood pressure and to withdraw blood samples for blood gas analysis. The smaller was used to introduce anaesthetics, other drugs and i.v. fluids. The arterial PO2, PCO2 and pH were maintained within physiological range by adjusting the respiratory rate and tidal volume and by infusing sodium bicarbonate (8.4 % w/v, Abbott Laboratories, North Chicago, IL, USA) as needed. The body temperature of the animal was maintained at 37 ± 1°C by using a heat pad (Baxter, Model K-20, Valencia, CA, USA). The chest was opened by a mid line sternal incision and the left atrium was exposed by incising the pericardium. The expiratory tube of the ventilator was placed under 1-2 cmH2O to prevent collapse of the lungs after opening the chest. A cannula (i.d. 0.86 mm, Intramedic polyethylene tubing) with a balloon attached to its tip was introduced to the left atrium through its appendage. Inflation of this balloon was used to partially obstruct the mitral valve and thereby to increase the LAP. A second cannula (i.d. 1.14 mm, Intramedic polyethylene tubing) was also introduced into the left atrium and was used to measure the left atrial pressure. The atrial pressures reported in this paper were obtained after adjusting for the position of the tip of the catheter to air post mortem. At the end of each experiment animals were killed with an overdose of pentobarbitone.
Recording action potentials
Afferent impulses from the pulmonary stretch receptors (SARs) and RARs were recorded from slips of right cervical vagus nerve by using bipolar platinum electrodes. Nerve fibres were split into thin filaments in order to be able to identify fibres with single active units. The raw signals were amplified (Grass Instruments Co., Model CP 511B, W. Warwick, RI, USA) and recorded on a light sensitive paper (Gould Instrument systems, Model TA 11, Valley View, OH, USA). The impulses were counted electronically by using an amplitude discriminator. Conduction velocity of the fibres was measured by stimulating the vagus nerve electrically (strength, 1-6 V; duration, 0.01-0.06 ms) 1.5-2.5 cm caudal to the recording site (Grass Instruments Co., Model SMD 9J).
Identification of nerve fibres from RARs and SARs
The fibres were identified as arising from RARs or SARs on the basis of their response to a sustained hyperinflation of the lungs at three tidal volumes. RARs showed a burst of activity at the peak of the third breath, followed by a rapid adaptation within 1 s (adaptation index > 70 %). SARs showed an increasing and sustained activity with little adaptation to hyperinflation (adaptation index < 20 %). They also have a characteristic rhythmic pattern of discharge with each respiratory cycle. The conduction velocities of the fibres were used to distinguish RARs and SARs from C fibre afferents.
Location of the receptors
At the end of each experiment, the location of the receptor was identified by carefully probing the bronchial tree with a wet cotton tipped applicator with a diameter of 3 mm.
Surgical induction of mitral regurgitation
Rabbits were pre-medicated with ketamine HCl (50 mg kg−1, Mallinckrodt Veterinary Inc.) and xylazine (5 mg kg−1, Bayer) i.m. and were anaesthetized with pentobarbitone sodium (5 mg kg−1, Veterinary Laboratories Inc.) injected through an ear vein. They were allowed to breathe spontaneously. The inspired air was enriched with oxygen. The surgery was performed under aseptic conditions and was completed within half an hour. Rabbits were given peri-operative antibiotics (enrofloxacin, 6 mg kg−1i.m.; BaytrilR, Bayer, KA, USA) as a prophylactic measure to prevent infections. The same dose of enrofloxacin i.m. was continued daily for 5 days postoperatively.
The chest was opened at the left 4th intercostal space and the left lung was allowed to collapse. The left atrial appendage was exposed by opening the pericardium. Then a purse-string suture (6/0 black silk, Ethicon, Somerville, NJ, USA) was inserted into the lateral wall of the left atrial appendage and a pair of microscissors (Straight 14.5 cm, item 15030-14, Fine Science Tools Inc., CA, USA) were introduced into the left atrium through it. The microscissors were then advanced towards the left ventricle until they made contact with the mitral valve. Several cuts (3-4) were made in the anteroseptal cusp of the mitral valve with the microscissors. The scissors were then carefully withdrawn and the purse-string suture was tightened to prevent any bleeding from the left atrium and to prevent any air from entering. The muscles were sutured in layers with chromic gut (3/0, Ethicon) and the pneumothorax was reduced by applying suction to the pleural cavity as the final suture was placed. The skin was sutured using black silk (4/0, Ethicon). Rabbits were then allowed to recover from anaesthesia. Their respiratory rate and heart rate were monitored during this period. These rabbits were then allowed to develop pulmonary venous congestion for either 6 weeks (n= 8) or 12 weeks (n= 16) and at the end of this period SAR and RAR activity was studied. The mitral valve damage was confirmed by post-mortem examination of the mitral valve apparatus.
Some animals were sham operated (n= 5). They underwent the same surgical procedure with the exception that the microscissors were introduced into the left atrium and were withdrawn without damaging the mitral valve. They were maintained for 12 weeks before action potentials were recorded from the vagus nerve. These animals were examined to determine the potential effects of ageing and non-specific changes related to the thoracotomy per se. The left ventricular weight was measured at the end of each experiment after trimming the right ventricle, atrial tissue and ascending aorta away from it.
Experimental protocol
Once an RAR or SAR unit was recognized, the unit was allowed to stabilize for 10 min. Then the number of action potentials were counted each minute for ten consecutive minutes (initial control period). Next the balloon in the left atrium was inflated and the mean LAP was elevated by 5 and 10 mmHg, each for a period of 5 min. At the end of these experimental periods, the left atrial balloon was deflated and the action potentials were recorded for a final control period of 10 min.
Statistical analysis
All the group data were expressed as means ± standard error of mean. The means of group data were compared using an analysis of variance and the difference between means detected by a least significant difference test. A P value < 0.05 was accepted as indicative of significance.
RESULTS
A total of thirty-five RARs and twenty-one SARs were studied in forty-five rabbits. The arterial blood PO2, PCO2 and pH values were 387.1 ± 13.0 mmHg, 33.0 ± 0.8 mmHg and 7.4 ± 0.01, respectively. The mean arterial pressure, mean LAP, heart rate and peak intra-tracheal pressure were 65.7 ± 2.0 mmHg, 4.7 ± 0.4 mmHg, 178.5 ± 4.7 beats min−1 and 5.7 ± 0.2 mmHg, respectively. The values in the individual groups of animals examined are given in Table 1.
Table 1.
The mean arterial blood pressure (MABP), heart rate (HR), mean left atrial pressure (MLAP) and peak intra-tracheal pressure (PITP) at the commencement of recording action potentials in all the units investigated
| Group | (n) | MABP (mmHg) | HR (beats min−1) | MLAP (mmHg) | PITP (mmHg) |
|---|---|---|---|---|---|
| Intact control | (22) | 68.0 ± 2.5 | 187.7 ± 5.8 | 2.9 ± 0.6 | 4.7 ± 0.1 |
| Sham operated | (4) | 63.3 ± 10.6 | 163.5 ± 9.0 | 4.25 ± 0.4 | 4.8 ± 0.9 |
| PVC 6 weeks | (10) | 55.4 ± 5.5 * | 164.5 ± 16.5 | 5.6 ± 0.5 § | 7.7 ± 0.2 † |
| PVC 12 weeks | (20) | 68.7 ± 3.3 | 178.4 ± 7.5 | 6.2 ± 0.8 § | 6.0 ± 0.5 ‡ |
n,number of experimental runs. PVC, pulmonary venous congestion after surgery.
Significantly different from the values in control rabbits and those with pulmonary venous congestion for 12 weeks.
Significantly different from control rabbits.
Significantly different from all the other values in the same column.
Significantly different from the values in control rabbits and those with pulmonary venous congestion for 6 weeks.
Evidence of mitral valve damage
The mitral valve was examined post mortem in all the animals. In twenty-four animals which underwent surgery on the mitral valve, eighteen were found to have perforations in the anteroseptal leaflet of the valve and the remainder had perforations in both valve leaflets. There was no evidence of healing (Fig. 1). Mitral valve apparatus from control and sham-operated rabbits were normal to visual inspection. The left atria in rabbits with damaged mitral valves were larger than those in control and sham-operated rabbits. The mean LAP in control and sham-operated animals immediately after insertion of the left atrial cannula was 2.5 ± 0.3 mmHg. The corresponding values in the animals studied 6 and 12 weeks after surgery were 5.1 ± 0.7 and 6.2 ± 1.0 mmHg. There is no statistically significant difference between mean LAP from control and sham-operated rabbits. Mean LAP in rabbits 6 and 12 weeks after mitral valve damage was significantly higher than that in control and sham-operated rabbits (P < 0.001). The left ventricular weight had increased from 3.58 ± 0.06 g in intact control rabbits to 5.35 ± 0.22 and 4.86 ± 0.12 g in rabbits with chronic pulmonary venous congestion for 6 and 12 weeks, respectively. Rabbits with chronic pulmonary venous congestion for 6 and 12 weeks showed an increase in left ventricular weight in excess of the gain in body weight. Although sham-operated animals also showed a small but statistically significant increase in left ventricular weight (3.92 ± 0.09 g), their left ventricular weight to body weight ratio was less than that of intact control rabbits. These data are presented in Table 2.
Figure 1. Photographs of mitral valve apparatus as seen from the left atrium.
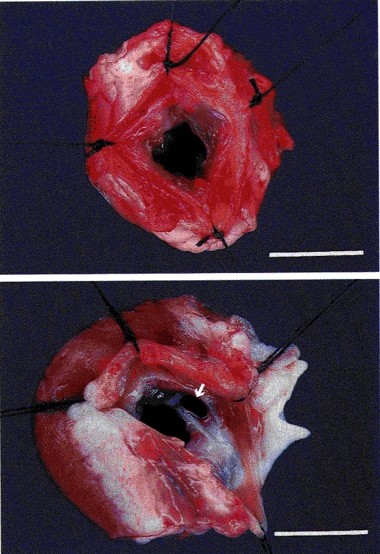
Top, intact control rabbit. Bottom, rabbit with mitral valve damage after 12 weeks. Note that the anteroseptal cusp of the mitral valve is perforated (arrow) and the left ventricle is hypertrophied. Bar indicates 1 cm.
Table 2.
The left ventricular weight (LV wt), body weight and left ventricular weight : body weight ratio (LV wt : body wt) from control and sham-operated rabbits and rabbits with pulmonary venous congestion (PVC)
| LV wt (g) | Body wt (kg) | LV wt : body wt (g kg−1) | |
|---|---|---|---|
| Control | 3.58 ± 0.06 * | 2.94 ± 0.07 * | 1.22 ± 0.03 § |
| Sham operated | 3.92 ± 0.09 * | 3.41 ± 0.05 † | 1.15 ± 0.04 § |
| PVC for 6 weeks | 5.35 ± 0.22 * | 3.33 ± 0.03 † | 1.61 ± 0.06 * |
| PVC for 12 weeks | 4.86 ± 0.12 * | 3.57 ± 0.04 * | 1.36 ± 0.04 * |
Significantly different from all the other values in the same column.
Significantly different from the corresponding values of rabbits with pulmonary venous congestion for 6 and 12 weeks.
Significantly different from the corresponding values of control rabbits and those with pulmonary venous congestion for 12 weeks.
The effect of graded increase in the LAP on the activity of RARs
Action potentials from RARs and SARs from intact control, sham-operated and mitral valve-damaged animals were studied. The characteristic responses of receptors to a sustained hyperinflation of the lung was established in each instance. There were no apparent differences in the responses of the receptors in the three groups of animals. An example of an RAR and an SAR from animals with chronic pulmonary venous congestion for 12 weeks is shown in Fig. 2.
Figure 2. Example of an RAR (A) and an SAR (B) from rabbits with chronic pulmonary venous congestion for 12 weeks.
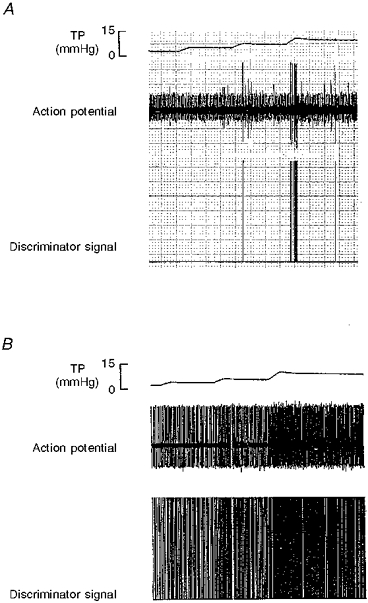
Note that only the RAR shows rapid adaptation to sustained hyperinflation of the lung. (Paper speed of the recordings was 5 mm s−1). TP, tracheal pressure; Action potential, RAR activity recorded in the cervical vagus nerve; Discriminator signal, processed signal from the amplitude discriminator/electronic counter.
Control and sham-operated rabbits
Fifteen RARs were examined; eleven in seven intact control rabbits and four in four sham-operated rabbits. No RAR could be identified from one control rabbit and one sham-operated rabbit. As RARs from the sham-operated animals responded to elevation of LAP in the same manner as those from control rabbits (> 50 % increase in RAR activity at LAP + 5 mmHg and > 100 % increase at LAP + 10 mmHg), they were grouped together when the action potential data were analysed. Of the fifteen units examined, ten were located in the bronchi within 1 cm from the hilum of the lung, four were located in lobar bronchi and one was located in the carina. All the RARs increased their activity when the LAP was increased and returned to the base line activity with the release of pulmonary venous congestion during the final control period. Mean conduction velocity of the fibres was 27.3 ± 4.3 ms−1. Examples of the responses of RARs to acute elevation of LAP is shown in Fig. 3.
Figure 3. Recordings of RAR activity in an intact control rabbit (A) and a rabbit with chronic pulmonary venous congestion for 12 weeks (B) showing their response to acute elevation of left atrial pressure (LAP).
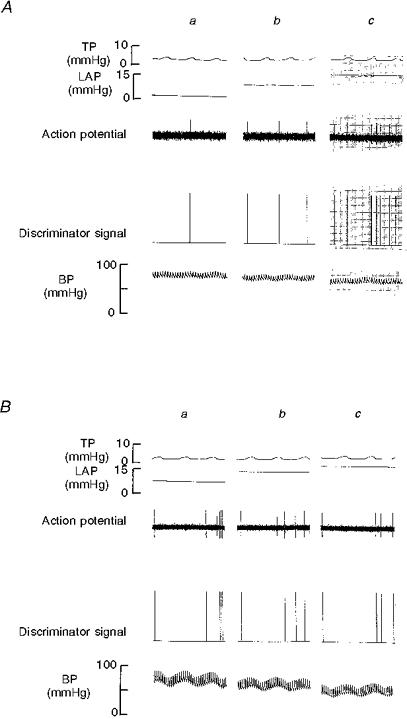
a, initial control period; b, LAP + 5 mmHg; c, LAP + 10 mmHg. Note that in intact control animal A, RAR shows an increase in its activity with acute elevation of LAP whereas in B it does not. TP, tracheal pressure; Action potential, RAR activity recorded in the cervical vagus nerve; Discriminator signal, processed signal from the amplitude discriminator/electronic counter; BP, blood pressure.
When the mean LAP was elevated from the initial control value of 2.9 ± 0.8 to 8.0 ± 0.8 and 12.3 ± 0.8 mmHg, the mean activity of the RARs increased from 48.8 ± 0.9 to 83.5 ± 3.6 and 121.1 ± 4.7 action potentials min−1, respectively (F= 183.72, P < 0.000, ANOVA). During the final control period the activity was 48.8 ± 1.8 action potentials min−1. The mean LAP was 2.1 ± 0.7 mmHg during this period. An example is shown in Fig. 3A and the data are summarized in Fig. 4A.
Figure 4. Average RAR activity (action potentials min−1) in response to increases in left atrial pressure (LAP).
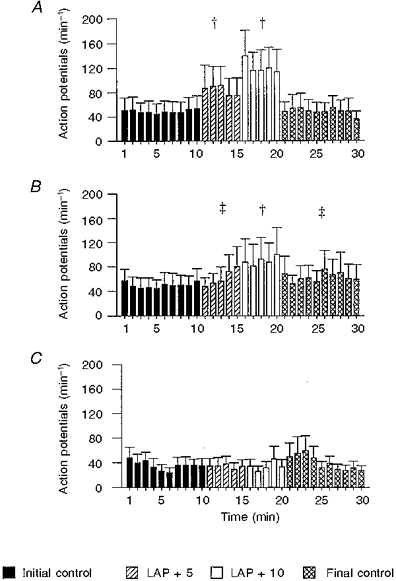
1-10 min, initial control; 11-15 min: +5 mmHg LAP; 16-20 min, +10 mmHg LAP; and 21-30 min, final control. A, intact control + sham-operated rabbits (n= 15); B, pulmonary venous congestion for 6 weeks (n= 10); C, pulmonary venous congestion for 12 weeks (n= 10). Note that RAR from C did not show significant increase in activity with graded increase in LAP. Activity during initial and final control periods in C are significantly lower compared with the corresponding periods in control + sham-operated group. † Significantly different from the initial and final control periods and the other experimental period. ‡ Significantly different from the initial control period.
Rabbits examined 6 weeks after mitral valve surgery
Ten RARs were investigated in six rabbits. In two other animals no RARs could be identified. Out of the ten RARs, seven were located in the bronchi within 1 cm from the hilum of the lung and three were located in one of the lobar bronchi. Four RARs responded in a manner similar to those in control rabbits when the LAP was increased. These four units showed approximately 40 % increase in their activity at LAP +5 and > +100 % increase at LAP +10. There was a small but statistically significant increase in their baseline activity when compared with that of intact control rabbits (56.23 ± 1.6 vs. 48.82 ± 0.9 action potentials min−1). Two RARs increased their activity when the LAP was raised and continued to increase further after the LAP was lowered to control levels. Three RARs showed no significant increase in their activity and one showed a reduction of its activity during the experimental period. The conduction velocity of the fibres was 20.6 ± 3.1 ms−1.
Mean LAP during the initial control period, two experimental periods and the final control period were 5.6 ± 0.5, 10.4 ± 0.7, 16.0 ± 0.7 and 5.7 ± 0.7 mmHg, respectively. Mean RAR activity during the corresponding periods were 49.7 ± 1.4, 61.9 ± 6.1, 89.6 ± 3.2 and 62.9 ± 2.3 action potentials min−1, respectively (F= 28.5, P < 0.0001, ANOVA). These data are shown in Fig. 4B.
Rabbits examined 12 weeks after mitral valve surgery
Ten RAR units were studied in nine rabbits. In four other animals no RARs were identified. Nine receptors were located, three in a lobar bronchus and six in a bronchus within 1 cm from the hilum of the lung. No attempt was made to locate the other receptor. None of ten RARs increased their activity when the LAP was elevated. The conduction velocity of the fibres was 23.8 ± 2.7 ms−1.
Mean LAP during the initial control period, two experimental periods and the final control period were 6.6 ± 1.2, 11.6 ± 1.1, 16.6 ± 1.2 and 6.6 ± 1.2 mmHg, respectively. Mean RAR activity during the corresponding periods were 35.5 ± 2.3, 33.8 ± 14.4, 34.0 ± 3.4 and 39.6 ± 3.9 action potentials min−1, respectively (F= 0.71, P < 0.554, ANOVA). Activity during initial and final control periods are significantly lower compared with the corresponding periods in control + sham-operated group. An example is shown in Fig. 3B and the data are summarized in Fig. 4C. The data have been re-plotted after standardization to a control value of 100 % to highlight these differences (Fig. 5). The mean arterial blood pressure (MABP), heart rate (HR) and peak intra-tracheal pressure (PITP) during various phases of the experiments are given in Table 3.
Figure 5. Change in RAR activity (percentage) activity with graded increases in left atrial pressure (LAP) from control + sham-operated rabbits (n= 15), rabbits with pulmonary venous congestion for 6 weeks (n= 10) and 12 weeks (n= 10).
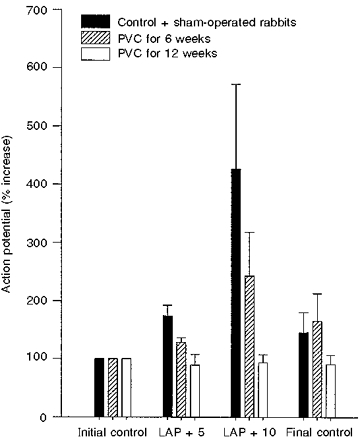
In all three groups of animals, counts for the initial control period were normalized to 100. Note that in rabbits with pulmonary venous congestion (PVC) for 12 weeks, RARs did not show an increase in activity with an increase in LAP.
Table 3.
Changes in mean left atrial pressure (MLAP), mean arterial blood pressure (MABP), heart rate (HR), and peak intra-tracheal pressure (PITP) during graded increase in left atrial pressure (LAP) when RARs were investigated
| LAP (mmHg) | Initial control | +5 mmHg | +10 mmHg | Final control |
|---|---|---|---|---|
| Intact control + sham (n= 15) | ||||
| MLAP (mmHg) | 2.9 ± 0.8 | 8 ± 0.8 | 12.3 ± 0.8 | 2.1 ± 0.7 |
| MABP (mmHg) | 65.7 ± 3.7 | 53.9 ± 4.0 * | 45.2 ± 5.8 § | 62.1 ± 3.8 |
| HR (beats min−1) | 179.6 ± 6.9 | 180.3 ± 7.6 | 175.9 ± 12 | 173.5 ± 8.2 |
| PITP (mmHg) | 4.7 ± 0.2 | 5.3 ± 0.3 | 5.5 ± 0.3 * | 5.1 ± 0.3 |
| PVC 6 weeks (n= 10) | ||||
| MLAP (mmHg) | 5.6 ± 0.5 | 10.4 ± 0.7 | 16 ± 0.7 | 5.7 ± 0.7 |
| MABP (mmHg) | 55.4 ± 5.5 | 50.1 ± 5.5 | 39.2 ± 6.1 | 54.9 ± 5.9 |
| HR (beats min−1) | 164.5 ± 16.5 | 176.5 ± 14.1 | 176.3 ± 18.8 | 144.4 ± 18.8 |
| PITP (mmHg) | 7.7 ± 0.2 | 8.3 ± 0.2 | 8.8 ± 0.5 | 8.6 ± 0.4 |
| PVC 12 weeks (n= 10) | ||||
| MLAP (mmHg) | 6.6 ± 1.2 | 11.6 ± 1.1 | 16.6 ± 1.2 | 6.6 ± 1.2 |
| MABP (mmHg) | 64.6 ± 6.2 | 49.9 ± 7.1 | 43.8 ± 5.5 § | 59.7 ± 4.6 |
| HR (beats min−1) | 165.3 ± 11.8 | 170.5 ± 14.9 | 171.1 ± 18.5 | 155.9 ± 11.8 |
| PITP (mmHg) | 5.4 ± 0.7 | 5.7 ± 0.7 | 6.0 ± 0.7 | 5.5 ± 0.7 |
n,number of experimental runs.
Significantly different from initial control.
Significantly different from initial and final controls.
The effect of graded increase in the LAP on the activity of SARs
Intact control rabbits
Nine rabbits were used to study the activity of SARs. Eleven SARs were recorded from seven rabbits. Stable single units could not be recorded from two rabbits. Six of the SARs were located in lobar bronchi and four were in bronchi within 1 cm from the hilum of the lung. We did not attempt to locate the other SAR. The conduction velocity of the fibres was 37.6 ± 5.2 ms−1. Mean LAP during the initial control period, two experimental periods and the final control period were 3.4 ± 0.7, 8.8 ± 0.7, 13.4 ± 0.7 and 3.2 ± 0.4 mmHg, respectively. Mean SAR activity during the corresponding periods was 1061 ± 5.1, 1222.4 ± 8.8, 1262.8 ± 12.1 and 1064.7 ± 15.4 action potentials min−1, respectively. (F= 67.6, P < 0.0001, ANOVA) These data are shown in Fig. 6A.
Figure 6. Average SAR activity (action potentials min−1) in response to increases in left atrial pressure (LAP).
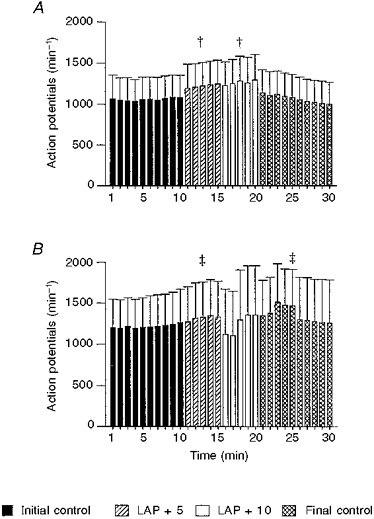
1-10 min, initial control; 11-15 min, +5 mmHg LAP; 16-20 min, +10 mmHg LAP; and 21-30 min, final control. A, intact control (n= 11); B, pulmonary venous congestion for 12 weeks (n= 10). † Significantly different from the initial and final control periods and the other experimental period. ‡ Significantly different from the initial control period.
Rabbits examined 12 weeks after mitral valve surgery
Ten SARs were identified from five rabbits. Stable single units could not be recorded in two rabbits. Seven units were located in a bronchus within 1 cm from the hilum of the lung. One unit was located in right lower lobe bronchus. No attempts were made to locate the other two SARs. Mean conduction velocity of the fibres was 25.3 ± 3.7 ms−1.
Mean LAP during the initial control period, two experimental periods and the final control period were 5.9 ± 1.2, 10.9 ± 1.2, 17.1 ± 1.4 and 5.8 ± 1.2 mmHg, respectively. Mean SAR activity during the corresponding periods were 1219.1 ± 6.9, 1319.2 ± 13.0, 1247.2 ± 55.0 and 1157.3 ± 30.3 action potentials min−1, respectively (F= 6.23, P < 0.002, ANOVA). These data are presented in Fig. 6B. The MABP, HR and PITP during the experiments are given in Table 4.
Table 4.
Changes in mean left atrial pressure (MLAP), mean arterial blood pressure (MABP), heart rate (HR) and peak intra-tracheal pressure (PITP) during graded increase in left atrial pressure (LAP) when SARs were investigated
| LAP (mmHg) | Initial control | +5 mmHg | +10 mmHg | Final control |
|---|---|---|---|---|
| Intact control (n= 11) | ||||
| MLAP (mmHg) | 3.4 ± 0.7 | 8.8 ± 0.7 | 13.4 ± 0.7 | 3.2 ± 0.4 |
| MABP (mmHg) | 69.5 ± 3.6 | 59.7 ± 2.6 * | 51.8 ± 4.3 § | 70.4 ± 4.9 |
| HR (beats min−1) | 189.9 ± 8.3 | 186.5 ± 7.5 | 180 ± 9.8 | 179.55 ± 6.4 |
| PITP (mmHg) | 4.7 ± 0.2 | 5.3 ± 0.3 | 5.6 ± 0.3 | 4.7 ± 0.2 |
| PVC 12 weeks (n =10) | ||||
| MLAP (mmHg) | 5.9 ± 1.2 | 10.9 ± 1.2 | 17.1 ± 1.4 | 5.8 ± 1.2 |
| MABP (mmHg) | 72.8 ± 2.0 | 64.7 ± 4.3 | 47 ± 5.6 † | 67.1 ± 3.5 |
| HR (beats min−1) | 191.4 ± 7.6 | 192.2 ± 10.0 | 216.3 ± 12.6 | 185.9 ± 12.4 |
| PITP (mmHg) | 6.6 ± 0.7 | 6.9 ± 0.6 | 8.0 ± 0.6 | 6.9 ± 0.6 |
n, number of experimental runs.
Significantly different from initial control.
Significantly different from initial and final controls.
Significantly different from the other values in the same row.
DISCUSSION
In anaesthetized intact animals acute pulmonary venous congestion caused by increasing the mean LAP to levels insufficient to cause overt pulmonary (alveolar) oedema (+5 to +10 mmHg), stimulates RARs in a graded and sustained manner. It has been suggested that this increase in activity was due to the ability of the RAR to sense the minute fluid fluxes across the pulmonary capillary wall (Hargreaves et al. 1991). It has also been shown previously that stimulation of RARs leads to reflex increases in respiratory rate (Kappagoda et al. 1989), tracheal tone (Kappagoda et al. 1988) and mucus secretion (Yu et al. 1989). Patients with chronic left ventricular dysfunction develop wheezing, dyspnoea and tachypnoea specially when their LAP is increased transiently as during exercise. It has been suggested that some of these symptoms may be due to activation of reflexes originating from sensory receptors in the lung (Braunwald, 1992). The physiological properties of RARs indicate that they may be involved in a reflex mechanism by which the patients with chronic left ventricular dysfunction develop these symptoms.
However, the experimental studies in support of such a claim are based on studies undertaken in intact anaesthetized animals. The present study was undertaken to investigate the response of the RARs to acute, graded increases in LAP in rabbits with surgically induced damage to the mitral valve. Thus the left atrial pressure was elevated acutely against a background of chronic pulmonary venous congestion. There are no studies reported where the activity of the RARs have been examined in such a situation. The most important finding in the present study was that the RARs did not respond to graded increases in LAP after 12 weeks of chronic pulmonary venous congestion.
Experimental models of chronic pulmonary venous congestion
There are several forms of experimental heart failure and in many of them pulmonary venous congestion has been induced as a concurrent unavoidable event. These methods have included the creation of arteriovenous shunts (Zucker et al. 1977), ventricular pacing (Armstrong et al. 1986) and damage to the mitral valve (Freeman & LeWinter, 1984; Kleaveland et al. 1988). The forms which are most similar to the one used here are those reported by Freeman & LeWinter (1984) and by Kleveland et al. (1988). In both these examples, mitral regurgitation was induced in dogs by damaging the mitral valves and chordae. In the former, the mitral valve was approached through the left ventricular wall while in the latter the valve was approached through the aortic valve. Freeman & LeWinter (1984) demonstrated an increase in mean LAP of approximately 12 mmHg in dogs with mitral valve damage compared with the control state. In the present study, the increase in LAP was approximately 3.5 mmHg above that in control animals. This difference could be explained in part by the extent of damage to the mitral valve and in part by the time interval which elapsed between the damage to the valve and the measurement of LAP. In the present study LAP was measured 6-12 weeks after surgery while Freeman & LeWinter (1984) reported measurements made immediately after. It is possible that there was a greater degree of damage to the left ventricle immediately after such surgery in dogs.
In the present study, the left ventricular mass was also increased by 49.4 % (6 weeks of mitral regurgitation) and 35.8 % (12 weeks of mitral regurgitation). In addition the left ventricular weight : body weight ratio showed that the left ventricle hypertrophied in excess of the gain in body weight. These increases are greater than those reported by Kleaveland et al. (1988) and Spinale et al. (1993).
Effect of chronic pulmonary venous congestion on the activity of RARs
RARs are known to respond to acute changes in Starling forces in the pulmonary vasculature, such as an increase in pulmonary venous pressure, obstruction of the pulmonary lymphatic drainage and a reduction in the plasma colloid osmotic pressure (Hargreaves et al. 1991). The responses to acute increases in LAP were attenuated 12 weeks after damage to the mitral valve. Only 40 % of the RARs responded after 6 weeks and none after 12 weeks. In contrast, the units from sham-operated and intact control animals responded to small acute increases in LAP. These findings suggest that this attenuation of the response of RARs to acute pulmonary venous congestion is a process that occurs over a period of several weeks.
The SARs showed an increase in activity when the LAP was acutely increased. This response was probably associated with reduction in lung compliance secondary to pulmonary venous congestion (see peak intra-tracheal pressure in Table 4). Twelve weeks after surgery, the SARs continued to show a qualitatively similar response to an acute increase in LAP.
Speculation on mechanisms
This lack of response of RARs to acute elevations of LAP could be due to their chronic exposure to elevated pulmonary venous pressure resulting from either gradual destruction of nerve endings themselves or a change in the ability of RARs to respond to alterations in extravascular fluid volumes in the vicinity of the receptors. Alterations in the activity of sensory receptors have been demonstrated in other types of myelinated fibres originating from the cardiovascular system. For instance, Zucker & Gilmore (1985) suggested that myelinated afferents from the left atrium are structurally damaged in animals with congestive heart failure. McCubbin et al. (1956) observed resetting of baroreceptors in dogs who sustained hypertension for 6 weeks or more. Thorén et al. (1983) demonstrated resetting in non-myelinated fibres originating from aortic arch baroreceptors in rats and suggested that this effect was probably due to an increase in collagen content in the aortic wall in the later stages of hypertension. Since the RARs are still activated in animals with chronic pulmonary venous congestion by a sustained inflation of the lung, it appears unlikely that there is an abnormality in the ability of the receptor to generate action potentials. The inability of these receptors to respond is probably related to the nature of the change in extracellular fluid volume in the vicinity of the receptor which results from an acute elevation of left atrial pressure. It is likely that the fluid fluxes which occur with acute increases in LAP in animals maintained in a state of chronic pulmonary venous congestion are proportionately smaller than those in intact animals. Under these circumstances, relative change in extracellular fluid volume would be less and may not be recognized by RAR. In summary, the present study has demonstrated for the first time that the ability of the RARs to respond to moderate acute elevations of LAP is attenuated when the rabbits are maintained in a state of chronic pulmonary venous congestion.
Acknowledgments
This work is supported by a Program Project Grant from the National Institutes of Health (HL 52165). The authors wish to thank Ms Malina Karim, MSc, for assistance during surgery. This work was undertaken as partial fulfilment of the requirements for a PhD thesis at the University of Ruhuna, Sri Lanka by S. G.
References
- Armstrong PWA, Stopps TP, Ford SE, De Bold AJ. Rapid ventricular pacing in the dog: pathophysiologic studies of heart failure. Circulation. 1986;74:1075–1084. doi: 10.1161/01.cir.74.5.1075. [DOI] [PubMed] [Google Scholar]
- Braunwald E. Mechanisms of dyspnoea. In: Braunwald E, editor. Heart Disease - A Textbook of Cardiovascular Medicine. Philadelphia: W. B. Saunders Company; 1992. p. 450. chap. 16. [Google Scholar]
- Freeman GL, LeWinter MM. Role of parietal pericardium in acute, severe mitral regurgitation in dogs. American Journal of Cardiology. 1984;54:217–219. doi: 10.1016/0002-9149(84)90332-1. 10.1016/0002-9149(84)90332-1. [DOI] [PubMed] [Google Scholar]
- Hargreaves M, Ravi K, Kappagoda CT. Responses of slowly and rapidly adapting receptors in the airways of rabbits to changes in the Starling forces. The Journal of Physiology. 1991;432:81–97. doi: 10.1113/jphysiol.1991.sp018377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappagoda CT, Man GCW, Ravi K, Teo KK. Reflex tracheal contraction during pulmonary venous congestion in the dog. The Journal of Physiology. 1988;402:335–346. doi: 10.1113/jphysiol.1988.sp017207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappagoda CT, Ravi K, Teo KK. Effect of pulmonary venous congestion on respiratory rate in dogs. The Journal of Physiology. 1989;408:115–128. doi: 10.1113/jphysiol.1989.sp017450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleaveland JP, Kussmau WG, Vinciguerra T, Diters R, Carabello BA. Volume overload hypertrophy in a closed chest model of mitral regurgitation. American Journal of Physiology. 1988;254:H1034–1041. doi: 10.1152/ajpheart.1988.254.6.H1034. [DOI] [PubMed] [Google Scholar]
- McCubbin JW, Green JH, Page IH. Baroreceptor function in chronic renal hypertension. Circulation Research. 1956;4:205–210. doi: 10.1161/01.res.4.2.205. [DOI] [PubMed] [Google Scholar]
- Spinale FG, Ishihra K, Zile M, DeFryte G, Crawford FA, Carabello BA. Structural basis for changes in left ventricular function and geometry because of chronic mitral regurgitation and after correction of volume overload. Journal of Thoracic Cardiovascular Surgery. 1993;106:1147–1157. [PubMed] [Google Scholar]
- Thorén P, Andresen MC, Brown AM. Resetting of aortic baroreceptors with non-myelinated afferent fibres in spontaneously hypertensive rats. Acta Physiologica Scandinavica. 1983;117:91–97. doi: 10.1111/j.1748-1716.1983.tb07182.x. [DOI] [PubMed] [Google Scholar]
- Yu J, Schultz HD, Goodman J, Coleridge JCG, Coleridge HM, Davis B. Pulmonary rapidly adapting receptors reflexly increase airway secretion in dogs. Journal of Applied Physiology. 1989;67:682–687. doi: 10.1152/jappl.1989.67.2.682. [DOI] [PubMed] [Google Scholar]
- Zucker IH, Earle AM, Gilmore JP. The mechanism of adaptation of left atrial stretch receptors in dogs with chronic congestive heart failure. Journal of Clinical Investigation. 1977;60:323–331. doi: 10.1172/JCI108780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker IH, Gilmore JP. Aspects of cardiovascular reflexes in pathologic states. Federation Proceedings. 1985;44:2400–2407. [PubMed] [Google Scholar]


