Abstract
The aim of this study was to investigate the mechanism of control of Na+,K+-ATPase activity by the cAMP-protein kinase A (PKA) pathway in rat proximal convoluted tubules. For this purpose, we studied the in vitro action of exogenous cAMP (10−3 M dibutyryl-cAMP (db-cAMP) or 8-bromo-cAMP) and endogenous cAMP (direct activation of adenylyl cyclases by 10−5 M forskolin) on Na+,K+-ATPase activity and membrane trafficking.
PKA activation stimulated both the cation transport and hydrolytic activity of Na+,K+-ATPase by about 40 %. Transport activity stimulation was specific to the PKA signalling pathway since (1) db-cAMP stimulated the ouabain-sensitive 86Rb+ uptake in a time- and dose-dependent fashion; (2) this effect was abolished by addition of H-89 or Rp-cAMPS, two structurally different PKA inhibitors; and (3) this stimulation was not affected by inhibition of protein kinase C (PKC) by GF109203X. The stimulatory effect of db-cAMP on the hydrolytic activity of Na+,K+-ATPase was accounted for by an increased maximal ATPase rate (Vmax) without alteration of the efficiency of the pump, suggesting that cAMP-PKA pathway was implicated in membrane redistribution control.
To test this hypothesis, we used two different approaches: (1) cell surface protein biotinylation and (2) subcellular fractionation. Both approaches confirmed that the cAMP-PKA pathway was implicated in membrane trafficking regulation. The stimulation of Na+,K+-ATPase activity by db-cAMP was associated with an increase (+40 %) in Na+,K+-ATPase units expressed at the cell surface which was assessed by Western blotting after streptavidin precipitation of biotinylated cell surface proteins. Subcellular fractionation confirmed the increased expression in pump units at the cell surface which was accompanied by a decrease (-30 %) in pump units located in the subcellular fraction corresponding to early endosomes.
In conclusion, PKA stimulates Na+,K+-ATPase activity, at least in part, by increasing the number of Na+-K+ pumps in the plasma membrane in proximal convoluted tubule cells.
In the kidney proximal convoluted tubule (PCT), the fluid and solute reabsorption rate is controlled by hormones and neurotransmitters which possess receptors coupled to adenylate cyclase and cAMP-dependent protein kinase (PKA) (for review see Morel & Doucet, 1986). The effects of cAMP on the tubular reabsorption processes are mediated by regulation of the activities of ion transporters expressed in both apical and basolateral membrane domains. Sodium apical entry occurs mainly through the amiloride-sensitive Na+-H+ exchanger (Preisig & Rector, 1988) and accessorily through Na+-coupled solute cotransporters (Lapointe et al. 1986). In addition, the recent discovery of an apical Na+ channel (Willmann et al. 1997) raises the possibility of a second amiloride-sensitive Na+ entry pathway. Basolaterally, Na+ exits the cell primarily through Na+,K+-ATPase and subsidiarily through the Na+-HCO3− cotransporter (Ullrich et al. 1977; Yoshitomi et al. 1985). By extruding sodium ions from the renal cells, Na+,K+-ATPase provides the driving force for the vectorial reabsorption processes (Ullrich et al. 1977).
In PCT, short-term stimulation of Na+,K+-ATPase by conditions that activate PKA has been reported by several groups (Giesen et al. 1984; Bertorello & Aperia, 1988; Breton et al. 1994; Beck et al. 1995; Carranza et al. 1996) but the mechanisms have yet to be determined. Besides the possibility of an increase in Na+,K+-ATPase activity due to the kinetic effect of a rise in intracellular Na+ concentration (Skou, 1965), a change in the intrinsic properties of Na+,K+-ATPase (Féraille et al. 1995) or an increased number of active Na+-K+ pump units expressed in plasma membranes (Hundal et al. 1992) may explain the effect of PKA activation.
In this study, we have investigated how the cAMP-PKA signalling pathway controls Na+,K+-ATPase activity in rat PCT. We found that PKA activation stimulated Na+,K+-ATPase activity, at least in part, by increasing the number of Na+-K+ pumps in the plasma membrane.
METHODS
Isolation of single pieces of nephron
Male Wistar rats (130-150 g; Physiologie Institute, Bern, Switzerland) were anaesthetized with pentobarbitone (5 mg (100 g body weight)−1i.p.) and the left kidney was quickly perfused through the abdominal aorta with 4 ml of incubation solution (see composition below) containing 0.18 % (w/v) collagenase (Serva, CLSII, 0.87 U mg−1). In order to reduce renal anoxia, the aorta was ligated above the left renal artery junction just before starting the perfusion. After perfusion, the left kidney was immediately removed and the rat killed by exsanguination. The kidney was sliced into small pyramids which were incubated at 30°C for 20 min in aerated incubation solution containing 0.05 % (w/v) collagenase. The pyramids were then thoroughly rinsed in ice-cold incubation solution and stored in ice until use. The tubules were microdissected under stereomicroscopic control in oxygenated incubation solution (Féraille et al. 1995) containing 120 mm NaCl, 5 mm RbCl, 4 mm NaHCO3, 1 mm CaCl2, 1 mm MgSO4, 0.2 mm NaH2PO4, 0.15 mm Na2HPO4, 5 mm glucose, 10 mm lactate, 1 mm pyruvate, 4 mm glutamine, 4 mm essential and non-essential amino acids (Gibco), 0.03 mm vitamins (Gibco), 0.1 % (w/v) bovine serum albumin (BSA) and 20 mm Hepes, pH 7.45.
86Rb+ uptake
The transport activity of Na+,K+-ATPase was measured in intact isolated tubules by ouabain-sensitive 86Rb+ uptake, as previously described (Cheval & Doucet, 1990). Prior to the 86Rb+ uptake assay, tubules were pre-incubated at 37°C for various times in incubation solution either alone or containing PKA activators and/or inhibitors and/or 5 mm ouabain.
Ouabain-sensitive 86Rb+ uptake was calculated as the difference between the mean values measured in samples incubated in the presence and absence of ouabain. 86Rb+ uptake values are expressed as picomoles of 86Rb+ per millimetre of tubule per minute.
Na+,K+-ATPase activity
The hydrolytic activity of Na+,K+-ATPase was determined, as previously described (Doucet et al. 1979), by a radiochemical assay based on the measurement of 32Pi (inorganic phosphate) released from [γ-32P]ATP after permeabilization of cell membranes. Prior to the ATPase assay, the samples were pre-incubated at 37°C for 15 min in ATPase assay solution either alone or containing 10−3 M db-cAMP.
The total ATPase activity assay solution contained 100 mm NaCl, 50 mm KCl, 10 mm MgCl2, 1 mm EDTA, 100 mm Tris-HCl, 10 mm MgATP, and trace amounts (5 nCi μl−1) of [γ-32P]ATP (Dupont de Nemours, Boston, 2-10 Ci mmol−1). The pH of the solution was adjusted to 7.4. For Na+-independent ATPase activity measurements, NaCl was replaced by 150 mm KCl.
For each condition, ATPase activity was determined in five to seven replicates. Na+,K+-ATPase activity was taken as the difference between the mean activities of total ATPase and Na+-independent ATPase. Na+,K+-ATPase activity was taken as the Na+-dependent ATPase activity in order to avoid measurement of the ouabain-sensitive, K+-dependent and Na+-independent K+-ATPase activity recently described in rat PCT (Younes-Ibrahim et al. 1995). Results are expressed as picomoles of ATP per millimetre of tubule per minute.
Biotinylation of cell surface proteins
Glomeruli-free cortical tubule suspensions (containing ∼90 % proximal tubules) were prepared from collagenase-treated kidneys, as previously described (Carranza et al. 1996). Isolated tubules were incubated at 37°C for 15 min in BSA-free incubation solution either alone or containing 10−3 M db-cAMP. Incubation was stopped on ice and tubules were pelleted by centrifugation. The supernatant was discarded and tubules were resuspended in biotinylation buffer solution (10 mm Tris-HCl (pH 7.5), 2 mm CaCl2, 50 mm NaCl) containing 1.5 mg ml−1 biotin (EZ-Link sulphosuccinimidobiotin (sulfo-NHS-biotin), Pierce). The biotinylation reaction was performed at 4°C for 1 h with gentle end-over-end motion to ensure mixing. Tubules were then rinsed once with phosphate-buffered saline (PBS)-calcium-magnesium (CM) (composition (mm): 137 NaCl, 2.7 KCl, 8 Na2PO4, 1.5 KH2PO4, 0.1 CaCl2 and 1 MgCl2, pH 7.4) supplied with 100 mm glycine and then washed in this buffer solution for 20 min at 4°C to quench all of the free biotin. After one further rinse with PBS-CM, the tubules were lysed in 200 μl of 1 % (w/v) sodium deoxycholate. After protein content determination of each sample, equal amounts of protein (30 μg) were added to 100 μl of streptavidin-agarose beads (Immunopure immobilized streptavidin, Pierce), diluted in an antiprotease-containing buffer solution (composition: 50 mm Tris-HCl (pH 7.4), 1 M NaCl, 5 mm EDTA, 10 μg ml−1 aprotinin, 50 μg ml−1 leupeptin), and 0.5 % (w/v) digitonin. To ensure equal conditions, volume was adjusted with 1 % (w/v) sodium deoxycholate. Samples were incubated overnight at 4°C with end-over-end motion. The beads were then washed once with rinsing solution A (composition: 150 mm NaCl, 50 mm Tris-HCl (pH 7.4), 5 mm EDTA, 0.5 % digitonin), twice with rinsing solution B (composition: 500 mm NaCl, 50 mm Tris-HCl (pH 7.4), 5 mm EDTA, 0.05 % digitonin), three times with rinsing solution C (composition: 500 mm NaCl, 20 mm Tris-HCl (pH 7.4), 0.2 % BSA, 0.25 % digitonin) and once with 10 mm Tris-HCl (pH 7.4). After this last wash, the samples were resuspended in 100 μl of Laemmli's sample buffer solution (Laemmli, 1970) and submitted to SDS-PAGE. After electrotransfer of proteins from polyacrylamide gels, polyvinylidine difluoride (PVDF) membranes (Immobilon-P, Millipore) were incubated overnight at 4°C with McK1 (Felsenfeld & Sweadner, 1988), a monoclonal anti-Na+,K+-ATPase α1-subunit antibody (courtesy of Dr K. J. Sweadner, Massachusetts General Hospital, Boston, MA, USA) diluted at 1 : 200 (v/v) or with monoclonal anti-Hsp27 antibody (StressGen) diluted 1 : 100 in Tris-buffered saline (TBS)- Tween (composition: 150 mm NaCl, 50 mm Tris, 0.2 % Tween 20, pH 7.5) containing 1 % BSA. The immunoreactivity was detected by the chemiluminescence method (ECL), according to the manufacturer's instructions (Amersham).
Each Western blot had its own internal calibration curve. This calibration curve consisted of loading serial dilutions of total proteins on each gel and thus allowed us to check the accuracy of the signal obtained with experimental samples.
Subcellular fractionation
Cortical tubules (containing ∼90 % PCT cells) from rat kidneys were prepared as previously described (Bertorello, 1992). After removal of the kidneys, the cortices were isolated and the tissue was minced on ice to a paste-like consistency. The preparation was incubated with 7.5 mg (100 ml collagenase)−1 (type I, Sigma) for 60 min at 37°C in 50 ml oxygenated incubation solution (see ‘Isolation of single pieces of nephron’). Incubation was stopped by placing the tissue on ice and pouring it through graded sieves (180, 75, 53 and 38 μm in pore size) to obtain a cell suspension. The cells were collected by centrifugation at 100 g for 4 min and washed three times in ice-cold incubation solution and resuspended. Cell suspensions were then incubated in the presence or absence of 10−3 M db-cAMP for 15 min at 37°C and then incubation was stopped by placing the cell suspension on ice.
Endosomes were fractionated on a flotation gradient as recently described (Chibalin et al. 1997). Briefly, after centrifugation and removal of the incubation solution, proximal tubule cells were suspended in ice-cold homogenization buffer solution containing 3 mm imidazole (pH 7.4), 250 mm sucrose, 2 mm EGTA, 10 mm NaF, 30 mm Na4O7P2, 1 mm Na3VO4, 1 mm PMSF as well as 10 μg ml−1 of leupeptin and 10 μg ml−1 of aprotinin. Cells were gently homogenized by fifteen to twenty strokes in a Dounce homogenizer. The homogenate was centrifuged at 3000 g for 5 min at 4°C. The post-nuclear supernatant was adjusted to 40.6 % sucrose and loaded (1.5 ml) at the bottom of a 5 ml centrifuge tube, to which were added sequentially 16 % sucrose (1.5 ml) in 3 mm imidazole and 0.5 ml EDTA; 10 % sucrose in the same buffer (1 ml); and finally homogenization buffer (1 ml). The samples were centrifuged at 110 000 g for 1 h at 4°C. Early endosomes were collected at the homogenization buffer-10 % sucrose interface, and the late endosomes at the 10 %-16 % sucrose interface. The identity of early and late endosomes was confirmed by the presence of Rab5, a small GTPase that is localized on early endosomes and which controls early endosome fusion, and mannose-6-phosphate receptor, respectively. Basolateral plasma membranes were prepared from the fraction collected at the 16 %-40.6 % sucrose interface using a Percoll gradient. The collected material was diluted in homogenization buffer (see above) and centrifuged at 20 000 g for 20 min at 4°C. The yellow layer was resuspended in the supernatant and centrifuged at 48 000 g for 30 min at 4°C. The supernatant was discarded and the pellet resuspended in 1 ml buffer containing 300 mm mannitol and 12 mm Hepes (pH 7.6). To form a Percoll gradient, 0.19 g undiluted Percoll (Pharmacia Biotech) was added to the 1 ml suspension. The suspension was gently mixed and centrifuged at 48 000 g for 30 min and the ring of basolateral membranes was collected.
Proteins from basolateral membrane, early and late endosome fractions were analysed by SDS-PAGE (7.5-15 %) using Laemmli's buffer system, and identification of the α-subunit of Na+,K+-ATPase was performed using a monoclonal antibody raised against the α1-subunit (Pietrini et al. 1992) (courtesy of Dr M. J. Caplan, Yale University, New Haven, CT, USA).
Statistics
The data are given as means ±s.e.m. from n independent experiments. Each independent experiment was performed with one kidney from one rat. Statistical comparisons between two groups were performed by Student's t test for unpaired data. P values less than 0.05 were considered significant.
RESULTS
Effect of PKA activation on ouabain-sensitive 86Rb+ uptake and Na+,K+-ATPase activity
To examine the role of the cAMP signalling pathway in the regulation of Na+,K+-ATPase in rat proximal convoluted tubules, we increased the intracellular cAMP content by addition of dibutyryl-cAMP (db-cAMP) or 8-bromo-cAMP (8-Br-cAMP), two cell-permeant analogues of cAMP, or by direct activation of adenylyl cyclases by forskolin.
An increase in the cellular levels of cAMP stimulated the cation transport activity of Na+,K+-ATPase measured in intact rat PCTs in the presence of a rate-limiting intracellular Na+ concentration (Cheval & Doucet, 1990). As shown in Fig. 1, a 15 min preincubation at 37°C of PCTs in the presence of either 10−3 M db-cAMP or 10−3 M 8-Br-cAMP stimulated ouabain-sensitive 86Rb+ uptake by approximately 40 % without acting on ouabain-insensitive 86Rb+ uptake (data not shown). Under the same conditions, 10−5 M forskolin also stimulated ouabain-sensitive 86Rb+ uptake by about 32 % and did not alter ouabain-insensitive 86Rb+ uptake (data not shown). Since all PKA activators produced similar effects, db-cAMP was used in subsequent experiments.
Figure 1. Activation of the cAMP-dependent protein kinase pathway stimulates ouabain-sensitive 86Rb+ uptake in rat PCT.
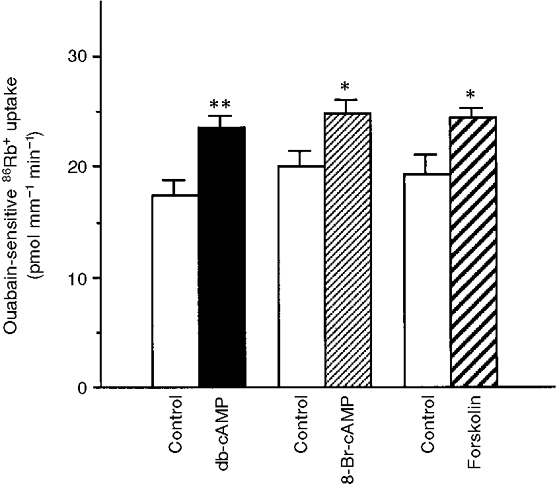
Ouabain-sensitive 86Rb+ uptake was measured under initial rate conditions in PCTs preincubated for 15 min at 37 °C in the absence (Control) or presence of one of the following PKA activators: 10−3 M db-cAMP, 10−3 M 8-Br-cAMP, or 10−5 M forskolin. Results are means +s.e.m. from 4-7 independent experiments. *P < 0.05, **P < 0.01.
To assess whether this stimulatory effect resulted from the activation of PKA, PCTs were incubated in the presence of two structurally different PKA inhibitors, N-(2-((p-bromocinnamyl)amino)ethyl)-5-isoquinolinesulphonamide HCl (H-89) and Rp-cyclic 3′,5′-hydrogen phosphorothiolate adenosine triethylammonium salt (Rp-cAMPS). As shown in Fig. 2A, preincubation of samples for 30 min at 37°C in the presence of PKA inhibitors (5 × 10−5 M H-89 or 10−3 M Rp-cAMPS) did not significantly alter basal ouabain-sensitive 86Rb+ uptake but prevented the stimulatory effect of db-cAMP on ouabain-sensitive 86Rb+ uptake. By contrast, preincubation of the tubules in the presence of 5 × 10−7 M GF109203X (GFX), a specific PKC inhibitor, did not alter basal ouabain-sensitive 86Rb+ uptake but appeared to increase the stimulatory effect of db-cAMP on ouabain-sensitive 86Rb+ uptake (Fig. 2B). However, the difference between the db-cAMP-treated group and the GFX plus db-cAMP group was not significant (P= 0.17). H-89, Rp-cAMPS and GF109203X had no effect on ouabain-insensitive 86Rb+ uptake (data not shown).
Figure 2. The effects of db-cAMP on ouabain-sensitive 86Rb+ uptake are prevented when PKA but not PKC is inhibited.
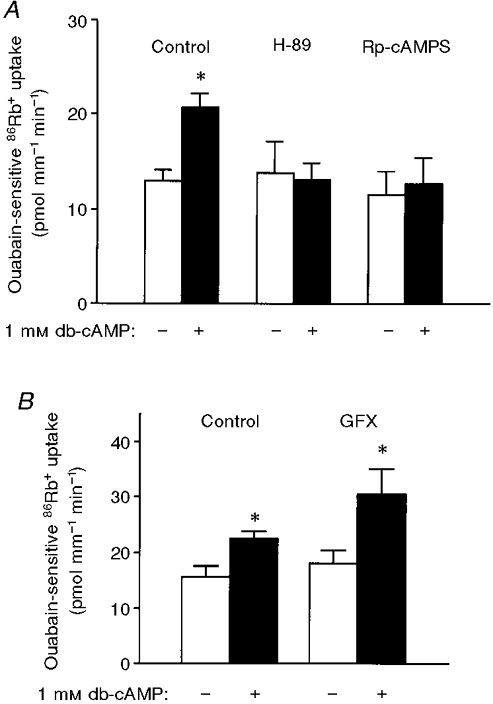
Ouabain-sensitive 86Rb+ uptake was measured as described in Fig. 1 *P < 0.05. Results are means +s.e.m. from 4-5 independent experiments. A, PCTs were preincubated for 30 min at 37 °C in the absence (Control) or presence of either PKA inhibitor (5 × 10−5 M H-89 or 10−3 M Rp-cAMPS), and in the presence (+) or absence (-) of 10−3 M db-cAMP. B, PCTs were preincubated for 30 min at 37 °C in the absence (Control) or presence of a PKC inhibitor (5 × 10−7 M GF109203X, GFX), and in the presence (+) or absence (-) of 10−3 M db-cAMP.
In order to better characterize the db-cAMP effects on Na+,K+-ATPase, we performed time and dose dependence experiments. The time course of the db-cAMP (10−3 M) effect on ouabain-sensitive 86Rb+ uptake is shown in Fig. 3A. Stimulation of ouabain-sensitive 86Rb+ uptake by db-cAMP was already apparent after 5 min of preincubation at 37°C and reached a plateau after 15 min. The effect of db-cAMP on ouabain-sensitive 86Rb+ uptake was concentration dependent with a threshold effect observed at 10−5 M (Fig. 3B).
Figure 3. The effects of db-cAMP on ouabain-sensitive 86Rb+ are time and dose dependent.
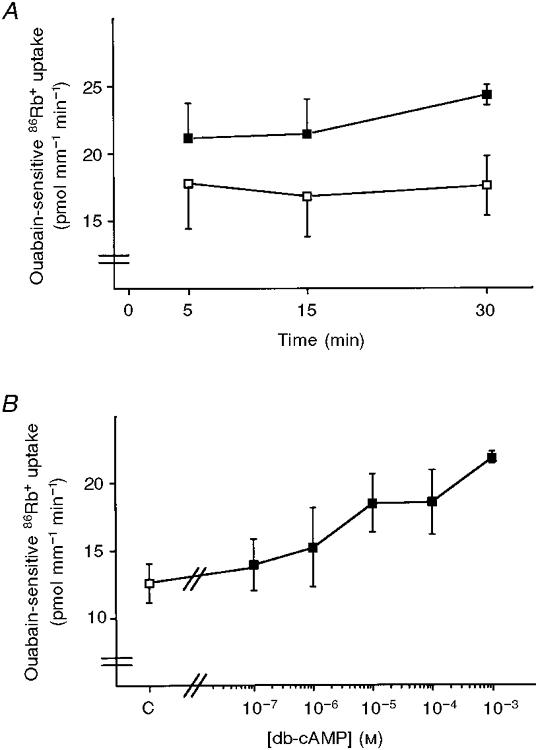
Ouabain-sensitive 86Rb+ uptake was measured as described in Fig. 1A, PCTs were preincubated for 5, 15 or 30 min at 37 °C in the absence (□) or presence (▪) of 10−3 M db-cAMP. Results are means and s.e.m. from 3 independent experiments. B, PCTs were preincubated for 30 min at 37 °C in the absence (□) or presence (▪) of db-cAMP at various concentrations from 10−7 to 10−3 M. Results are means ±s.e.m. from 6 independent experiments.
The stimulatory effects of db-cAMP on ouabain-sensitive 86Rb+ uptake could be mediated either by modulating the intracellular Na+ concentration through an increase in apical entry or by acting directly on the pump. Thus we investigated the effect of db-cAMP on the hydrolytic activity of Na+,K+-ATPase in the absence of a Na+ gradient. In PCTs preincubated for 15 min in the presence of 10−3 M db-cAMP, Na+,K+-ATPase activity measured in the presence of a saturating concentration of sodium (100 mm) was stimulated by about 45 % (in pmol ATP mm−1 min−1: control, 48.07 ± 6.0; db-cAMP, 69.79 ± 5.0, P > 0.05), indicating that db-cAMP increased the Vmax of Na+,K+-ATPase. Na+-independent ATPase activity was not altered by db-cAMP (data not shown). This increase represents either a direct effect on maximal enzyme turnover or an increase in the number of active Na+,K+-ATPase units.
To evaluate the possibility of an additional effect of db-cAMP on the Na+ affinity of Na+,K+-ATPase and/or on intracellular Na+ concentration (secondary to an alteration in its apical entry), we calculated the efficiency of Na+,K+-ATPase for each experimental condition (control or db-cAMP) as the ratio of the mean of corresponding 86Rb+ uptake values and the mean of Na+,K+-ATPase activity values, and assuming a stoichiometry of two Rb+ ions transported for each hydrolysed ATP molecule. In agreement with previous results (Cheval & Doucet, 1990), we found that in intact PCT cells, i.e. under intracellular Na+ rate-limiting conditions, Na+,K+-ATPase functioned at 16 % of its maximal capacity under basal conditions. The respective calculated efficiency for db-cAMP treated groups was similar (15 %), strongly suggesting that db-cAMP did not significantly alter the Na+ affinity of Na+,K+-ATPase and that its effect is independent of Na+ availability.
Effects of db-cAMP on the cell surface expression of Na+,K+-ATPase
To assess whether or not db-cAMP modified the cell surface expression of Na+,K+-ATPase, we used the biotinylation- streptavidin precipitation procedure. To adapt this technique to our system we first determined the accessibility of Na+,K+-ATPase to the biotin derivative. In renal epithelial cells, Na+,K+-ATPase is located in the basolateral plasma membrane to which the tubular basal membrane can prohibit the access of the sulfo-NHS-biotin. Since the Na+,K+-ATPase α-subunit was detected only in collagenase-treated tubules (Fig. 4A), digestion of tubular basal membrane with collagenase was used for all subsequent experiments.
Figure 4. Determination of experimental conditions for the biotinylation assay.
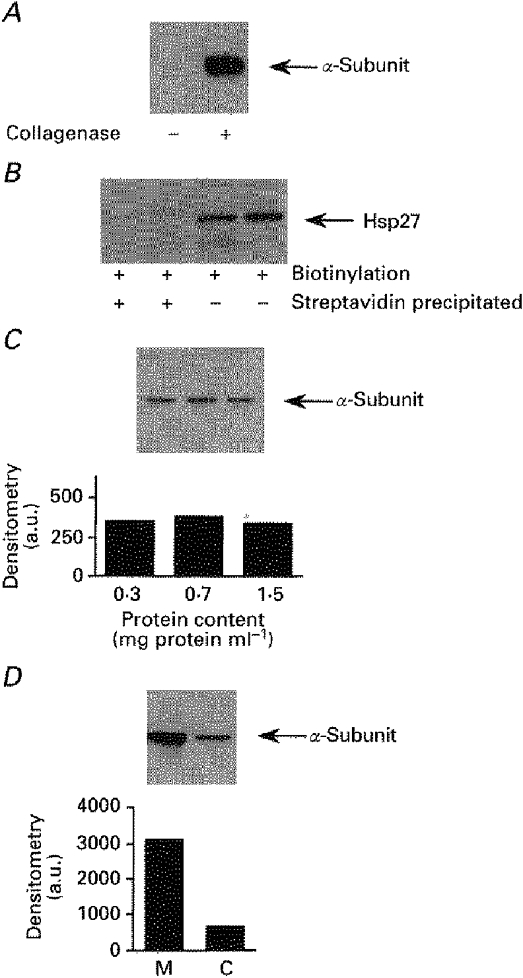
After biotinylation and lysis of cortical tubules, equal amounts of total protein were submitted to streptavidin precipitation. The Na+,K+-ATPase α1-subunit was detected with McK1, a monoclonal antibody raised against Na+,K+-ATPase α1-subunit. A, effect of removal of the basal membrane on Na+,K+-ATPase accessibility. Cortical tubules were obtained from untreated (left lane) or collagenase-treated (right lane) kidneys. B, absence of intracellular protein biotinylation. Hsp27, a cytosolic protein, was detected by immunoblot before (right 2 lanes) and after (left 2 lanes) streptavidin precipitation of biotinylated samples. C, biotinylation of cell surface protein is performed under saturating conditions. Increasing amounts of cortical tubules were incubated in the presence of 1.5 mg ml−1 biotin and equal amounts of total protein extracts were submitted to streptavidin precipitation. The bottom panel represents densitometry results of the upper blot. D, suspensions of tubules obtained from the kidney medulla (M) or cortex (C) were biotinylated and equal amounts of total proteins were submitted to streptavidin precipitation. The bottom panel represents densitometry results of the upper blot.
A second important prerequisite for this procedure was the impermeability of the plasma membrane to sulfo-NHS-biotin. As shown in Fig. 4B, Hsp27, an abundant cytosolic protein, was detected by Western blotting (monoclonal antibody from StressGen) in total cellular extracts but not in the streptavidin precipitated samples, indicating the impermeability of the plasma membrane to sulfo-NHS-biotin.
We further checked whether the biotinylation procedure was performed under saturating conditions. Increasing amounts of cortical tubules (from 0.3 to 1.5 mg total protein ml−1) were incubated in the presence of the same amount of sulfo-NHS-biotin. After cell lysis, equal amounts of total protein extracts (30 μg) were incubated with an excess of streptavidin-coupled agarose beads. As shown in Fig. 4C, similar amounts of Na+,K+-ATPase α-subunits were recovered under each condition, indicating that maximal labelling of the plasma membrane Na+-K+ pumps was achieved.
Finally, the intensity of the Na+,K+-ATPase α-subunit signal detected by immunoblot after streptavidin precipitation of biotinylated proteins should be proportional to the number of pump units expressed at the cell surface. This was confirmed by a threefold greater cell surface expression of Na+,K+-ATPase in the inner stripe of the outer medulla than in the cortex (Fig. 4D), in agreement with the known differences in expression of the enzyme between these two parts of the kidney tissue (McDonough et al. 1994).
Based on these results, we assumed that the biotinylation- streptavidin precipitation procedure was appropriate for the purpose of the experiment. Thus, after incubation in the presence or absence of 10−3 M db-cAMP for 15 min at 37°C, cortical tubules were subjected to the biotinylation- streptavidin precipitation procedure. Western blotting analysis showed a 40 % increase in Na+,K+-ATPase α-subunit immunoreactivity in db-cAMP-treated samples compared with control samples (Fig. 5), indicating that db-cAMP increased the cell surface expression of Na+,K+-ATPase.
Figure 5. The number of Na+,K+-ATPase units is increased at the cell surface after db-cAMP treatment in rat cortical tubules.
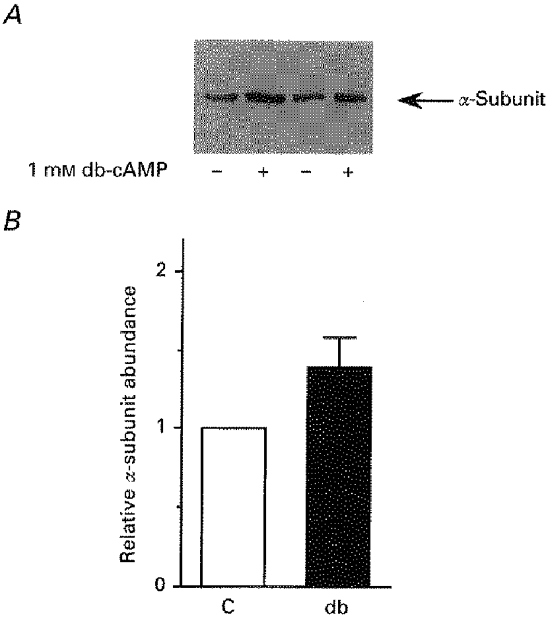
Cortical tubules (≈90 % PCTs) isolated from collagenase-treated kidneys were preincubated for 15 min at 37 °C in the absence (C, control) or presence of 10−3 M db-cAMP (db) before biotinylation for 1 h with 1.5 mg ml−1 sulfo-NHS-biotin at 4 °C. Tubules were then lysed with 1 % sodium deoxycholate and equal amounts of total proteins (30 μg) were precipitated on streptavidin beads. At least 2 samples were precipitated for each group of lysates. Proteins were separated by SDS-PAGE and transferred to PVDF membranes before Western blotting with the McK1 antibody. A, representative immunoblot from 9 independent experiments. B, scanning results from 9 independent experiments. Results are given as means +s.e.m. of the relative intensities; db-cAMP increased the cell surface expression of Na+,K+-ATPase by 41 ± 19 % (P < 0.05).
Effects of db-cAMP on Na+,K+-ATPase distribution in cellular membrane compartments
In order to confirm that db-cAMP increased the number of Na+,K+-ATPase units present in the plasma membrane, we performed subcellular fractionation experiments. After incubation of cortical tubules in the presence or absence of 10−3 M db-cAMP, the Na+,K+-ATPase α-subunit was detected by Western blotting in plasma membrane, early or late endosome fractions. As shown in Fig. 6, db-cAMP induced a net increase in α-subunit abundance in the plasma membrane fraction that was proportional to the reciprocal net loss observed in the early endosome fraction, while no changes were found in the late endosome fraction. These results suggest that db-cAMP induces a redistribution of Na+,K+-ATPase between an intracellular compartment and the plasma membrane.
Figure 6. Increase in Na+,K+-ATPase units in the plasma membrane after db-cAMP treatment is associated with a decrease in pump units in early endosomes in rat cortical tubule cells.
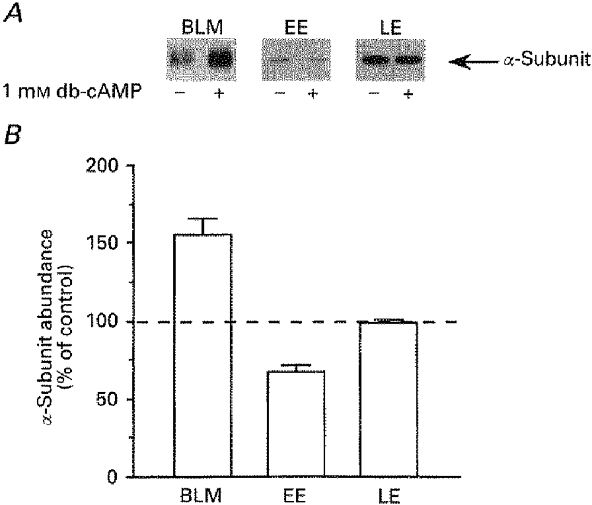
Cortical tubule cell suspensions (≈90 % PCT cells) were preincubated for 15 min at 37 °C in the absence or presence of 10−3 M db-cAMP before subcellular fractionation. Equal amounts of protein from both control and db-treated samples were loaded onto SDS-PAGE. The loaded amounts for each fraction were 5 μg of protein from basolateral membrane (BLM), and 1 μg from early (EE) and late endosomes (LE). A, representative immunoblots of the α-subunit of Na+,K+-ATPase with an anti-α1-subunit monoclonal antibody. B, scanning results from 3 independent experiments. Data are expressed as a percentage of control values and are means +s.e.m.; db-cAMP increased the amount of α-subunit by 55 ± 10 % (P < 0.05) in BLM, decreased the amount of α-subunit by 33 ± 4 % (P < 0.05) in EE and did not alter the α-subunit content of LE (-2 ± 3 %).
DISCUSSION
The results of this study indicate that the stimulatory effects of the cAMP-PKA pathway on Na+,K+-ATPase activity are mainly due to an increase in the number of Na+,K+-ATPase units present in the plasma membrane of the proximal tubule. The effect of db-cAMP on the 86Rb+ uptake and the change in the number of pump units expressed at the cell surface were of the same order of magnitude (∼30-40 %) under conditions of rate limiting Na+ concentration.
Our results are in agreement with studies demonstrating a stimulatory effect of cAMP on proximal tubule Na+,K+-ATPase activity measured in intact cells by (a) ouabain-sensitive oxygen consumption (Beck et al. 1995), (b) Na+,K+-ATPase-dependent basolateral membrane potential variation (Breton et al. 1994), (c) ouabain-sensitive 86Rb+ uptake (Carranza et al. 1996), or in permeabilized isolated PCTs (Bertorello & Aperia, 1988, 1989) and kidney cortex homogenate (Giesen et al. 1984) by the hydrolytic activity of the enzyme. However, one study has reported inhibition of Na+,K+-ATPase by db-cAMP (Ribeiro & Mandel, 1992) while another one did not find any effect of either exogenous or endogenous cAMP on Na+,K+-ATPase activity (Satoh et al. 1993). These discrepancies are neither explained by species differences nor by specific experimental conditions, such as temperature, oxygenation status, and bicarbonate or calcium concentrations in the incubation solutions.
In the kidney proximal convoluted tubule, solute and water reabsorptions are under the control of hormones and neurotransmitters possessing receptors coupled to adenylate cyclase and cAMP-dependent protein kinase (PKA) which can exert contradictory effects according to the ion transport system considered. For example in microperfused proximal tubule, cAMP decreases bicarbonate (McKinney & Myers, 1980) and increases chloride (Wang et al. 1995) reabsorption. These contradictory effects of cAMP depend on the regulation of ion transporters expressed in both apical and basolateral membrane domains. The inhibitory action of cAMP on bicarbonate reabsorption may result from a co-ordinated inhibition of the apical Na+-H+ exchanger (Weinman, Shenolikar & Kahn, 1987) and of the basolateral Na+-HCO3− cotransporter (Ruiz & Arruda, 1992). Therefore, the stimulatory effect of cAMP on chloride reabsorption requires both apical and basolateral Cl− transport that is independent of acid-base transport; this is most likely to be via cAMP-regulated apical (Darvish et al. 1994) and basolateral Cl− channels (Seki et al. 1995). The basolateral Cl− extrusion is favoured by the Na+,K+-ATPase-dependent hyperpolarization that follows exposure of cells to cAMP (Breton et al. 1994). In addition, the stimulation of Na+,K+-ATPase can also increase the driving force for paracellular Cl− reabsorption. Furthermore, the sustained stimulation of Na+,K+-ATPase transport activity by cAMP implies an adequate Na+ entry independent of the Na+-H+ exchanger. The organic solutes-Na+-coupled cotransporters and the described apical amiloride-sensitive Na+ conductance (Willmann et al. 1997) are potential candidates. In the PCT this Na+ conductance could be created by epithelial Na+ (ENa) channels similar to those described in the collecting duct (Willmann et al. 1997). These channels might be activated when Na+ entry through the apical Na+-H+ exchanger is decreased. Indeed, ENa channel activity is closely related to Na+ availability and is upregulated by a decreased rate of Na+ entry (Frindt et al. 1993; Willmann et al. 1997). Conversely, ENa channel activity is downregulated when the rate of the Na+ entry exceeds the rate of the Na+ exit driven by the Na+,K+-ATPase activity (Silver et al. 1993). Furthermore, cAMP increases the amiloride-sensitive Na+ conductance of the apical membrane (Schafer & Troutman, 1990) through direct stimulation of ENa channels in the collecting duct (Frindt & Palmer, 1996). Thus, one may postulate that in PCT, ENa channel activity regulation is similar to that observed in the collecting duct. Taken together, it is fair to postulate that cAMP promotes a shift from sodium bicarbonate towards sodium chloride reabsorption in PCT. In this context, the stimulation of Na+,K+-ATPase by cAMP could play a key role in the enhanced NaCl reabsorption by maintaining or increasing the electrochemical gradients necessary to the sodium and chloride reabsorptions independently of the apical Na+-H+ exchanger and basolateral Na+-HCO3− cotransporter activities.
Our results show that activation of PKA increases the Vmax of Na+,K+-ATPase as well as the number of plasma membrane pump units in rat PCTs. Thus an increase in enzyme turnover or activation of latent pump units already present in plasma membranes does not contribute to the increase in Vmax. In addition, the rapid time course of the db-cAMP effect on the pump activity makes the synthesis of new pump units unlikely. Therefore, the observed proportional redistribution of Na+-K+ pumps between plasma membrane and early endosomes can fully explain the cAMP-induced increase in Na+,K+-ATPase activity. This result suggests that latent intracellular Na+-K+ pumps located in an early endosomal compartment can be translocated in response to PKA activation in PCT cells. However, a PKA-induced decrease in the endocytosis rate of Na+,K+-ATPase cannot be excluded.
Since the cAMP pathway evokes activation of at least one protein kinase, one may hypothesize that phosphorylation processes are implicated in the redistribution of Na+-K+ pumps. Na+,K+-ATPase α-subunit phosphorylation has been shown to occur in vitro (Bertorello et al. 1991; Chibalin et al. 1992) and in intact cells (Beguin et al. 1994; Fisone et al. 1994; Carranza et al. 1996). We have recently shown that an increase in the phosphorylation status of Na+,K+-ATPase occurs concomitantly with ouabain-sensitive 86Rb+ uptake stimulation by db-cAMP in intact cells of rat kidney cortex (Carranza et al. 1996). However, the definite relationship between this cAMP-stimulated phosphorylation and the effect of the PKA pathway on Na+,K+-ATPase activity remains to be determined. It would be a valuable endeavour to test whether Na+,K+-ATPase α-subunit phosphorylation by PKA regulates the distribution of Na+-K+ pumps between plasma membrane and intracellular compartments.
In conclusion, our data indicate that activation of cellular PKA by cAMP stimulates Na+,K+-ATPase activity in rat proximal tubules. This effect is probably mediated by the insertion of additional Na+,K+-ATPase units in the plasma membrane, implying that the cAMP-PKA pathway contributes in the control of membrane trafficking.
Acknowledgments
The authors wish to express particular thanks to Dr S. D. Dreher for the kind gift of the anti-Hsp27 monoclonal antibody and to Dr P. Y. Martin for critical reading of the manuscript. We also thank Miss A. Bryson for checking the English of the manuscript. This work was supported by grants 31-40-386-94 and 31-50-643.97 from the Swiss National Science Research Foundation to H. Favre and E. Féraille.
References
- Beck JS, Marsolais M, Noël J, Breton S, Laprade R. Dibutyryl cyclic adenosine monophosphate stimulates the sodium pump in rabbit renal cortical tubules. Renal Physiology and Biochemistry. 1995;18:21–26. doi: 10.1159/000173895. [DOI] [PubMed] [Google Scholar]
- Beguin P, Beggah AT, Chibalin AV, Burgener-Kairuz P, Jaisser F, Mathews PM, Rossier BC, Cotecchia S, Geering K. Phosphorylation of the Na,K-ATPase α-subunit by protein kinase A and C in vitro and in intact cells. Identification of a novel motif for PKC-mediated phosphorylation. Journal of Biological Chemistry. 1994;269:24437–24445. [PubMed] [Google Scholar]
- Bertorello A, Aperia A. Pertussis toxin modulates dopamine inhibition of Na,K-ATPase activity in rat proximal convoluted segments. In: Skou J, Norby J, Maunsbach A, Esman M, editors. The Na+,K+-pump, part B, Cellular Aspects. New York: Liss; 1988. pp. 353–356. [Google Scholar]
- Bertorello A, Aperia A. Regulation of Na+-K+-ATPase activity in kidney proximal tubules: involvement of GTP binding proteins. American Journal of Physiology. 1989;256:F57–62. doi: 10.1152/ajprenal.1989.256.1.F57. [DOI] [PubMed] [Google Scholar]
- Bertorello AM. Diacylglycerol activation of protein kinase C results in a dual effect on Na+,K+-ATPase activity from intact proximal tubule cells. Journal of Cell Science. 1992;101:343–347. doi: 10.1242/jcs.101.2.343. [DOI] [PubMed] [Google Scholar]
- Bertorello AM, Aperia A, Walaas SI, Nairn AC, Greengard P. Phosphorylation of the catalytic subunit of Na+,K+-ATPase inhibits the activity of the enzyme. Proceedings of the National Academy of Sciences of the USA. 1991;88:11359–11362. doi: 10.1073/pnas.88.24.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton S, Beck JS, Laprade R. cAMP stimulates proximal convoluted tubule Na+-K+-ATPase activity. American Journal of Physiology. 1994;266:F400–410. doi: 10.1152/ajprenal.1994.266.3.F400. [DOI] [PubMed] [Google Scholar]
- Carranza ML, Féraille E, Kiroytcheva M, Rousselot M, Favre H. Stimulation of ouabain-sensitive 86Rb+ uptake and Na+,K+-ATPase α-subunit phosphorylation by a cAMP-dependent signalling pathway in intact cells from rat kidney cortex. FEBS Letters. 1996;396:309–314. doi: 10.1016/0014-5793(96)01121-0. [DOI] [PubMed] [Google Scholar]
- Cheval L, Doucet A. Measurement of Na-K-ATPase-mediated rubidium influx in single segments of rat nephron. American Journal of Physiology. 1990;259:F111–121. doi: 10.1152/ajprenal.1990.259.1.F111. [DOI] [PubMed] [Google Scholar]
- Chibalin AV, Katz AI, Berggren P-O, Bertorello AM. Receptor-mediated inhibition of renal Na+-K+-ATPase is associated with endocytosis of its α- and β-subunits. American Journal of Physiology. 1997;273:C1458–1465. doi: 10.1152/ajpcell.1997.273.5.C1458. [DOI] [PubMed] [Google Scholar]
- Chibalin AV, Vasilets LA, Hennekes H, Pralong D, Geering K. Phosphorylation of Na,K-ATPase α-subunits in microsomes and in homogenates of Xenopus oocytes resulting from the stimulation of protein kinase A and protein kinase C. Journal of Biological Chemistry. 1992;267:22378–22384. [PubMed] [Google Scholar]
- Darvish N, Winaver J, Dagan D. Diverse modulations of chloride channels in renal proximal tubules. American Journal of Physiology. 1994;267:F716–724. doi: 10.1152/ajprenal.1994.267.5.F716. [DOI] [PubMed] [Google Scholar]
- Doucet A, Katz AI, Morel F. Determination of Na-K-ATPase activity in single segments of the mammalian nephron. American Journal of Physiology. 1979;237:F105–113. doi: 10.1152/ajprenal.1979.237.2.F105. [DOI] [PubMed] [Google Scholar]
- Felsenfeld DP, Sweadner KJ. Fine specificity mapping and topography of an isozyme-specific epitope of the Na,K-ATPase catalytic subunit. Journal of Biological Chemistry. 1988;263:10932–10942. [PubMed] [Google Scholar]
- Féraille E, Carranza ML, Buffin-Meyer B, Rousselot M, Doucet A, Favre H. Protein kinase C-dependent stimulation of Na+-K+-ATPase in rat proximal convoluted tubules. American Journal of Physiology. 1995;268:C1277–1283. doi: 10.1152/ajpcell.1995.268.5.C1277. [DOI] [PubMed] [Google Scholar]
- Fisone G, Cheng S X.-J, Nairn AC, Czernik AJ, Hemmings HC, Jr, Höög J-O, Bertorello AM, Kaiser R, Bergman T, Jörnvall H, Aperia A, Greengard P. Identification of the phosphorylation site for cAMP-dependent protein kinase on Na+,K+-ATPase and effects of site-directed mutagenesis. Journal of Biological Chemistry. 1994;269:9368–9373. [PubMed] [Google Scholar]
- Frindt G, Palmer LG. Regulation of Na channels in the rat cortical collecting tubule: effects of cAMP and methyl donors. American Journal of Physiology. 1996;271:F1086–1092. doi: 10.1152/ajprenal.1996.271.5.F1086. [DOI] [PubMed] [Google Scholar]
- Frindt G, Silver RB, Windhager EE, Palmer LG. Feedback regulation of Na channels in rat CCT. II. Effects of inhibition of Na entry. American Journal of Physiology. 1993;264:F565–574. doi: 10.1152/ajprenal.1993.264.3.F565. [DOI] [PubMed] [Google Scholar]
- Giesen EM, Imbs JL, Grima M, Schmidt M, Schwartz J. Modulation of renal ATPase activities by cyclic AMP. Biochemical and Biophysical Research Communications. 1984;120:619–624. doi: 10.1016/0006-291x(84)91300-7. [DOI] [PubMed] [Google Scholar]
- Hundal HS, Marette A, Mitsumoto Y, Ramlal T, Blostein R, Klip A. Insulin induces translocation of the α2 and β1 subunits of the Na+/K+-ATPase from intracellular compartments to the plasma membrane in mammalian skeletal muscle. Journal of Biological Chemistry. 1992;267:5040–5043. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapointe J-Y, Laprade R, Cardinal J. Characterization of the apical membrane ionic permeability of the rabbit proximal convoluted tubule. American Journal of Physiology. 1986;250:F339–347. doi: 10.1152/ajprenal.1986.250.2.F339. [DOI] [PubMed] [Google Scholar]
- McDonough AA, Magyar CE, Komatsu Y. Expression of Na+-K+-ATPase α- and β-subunits along rat nephron: isoform specificity and response to hypokalemia. American Journal of Physiology. 1994;267:C901–908. doi: 10.1152/ajpcell.1994.267.4.C901. [DOI] [PubMed] [Google Scholar]
- McKinney TD, Myers P. Bicarbonate transport by proximal tubules: effect of parathyroid hormone and dibutyryl cyclic AMP. American Journal of Physiology. 1980;238:F166–174. doi: 10.1152/ajprenal.1980.238.3.F166. [DOI] [PubMed] [Google Scholar]
- Morel F, Doucet A. Hormonal control of kidney functions at the cell level. Physiological Reviews. 1986;66:377–468. doi: 10.1152/physrev.1986.66.2.377. [DOI] [PubMed] [Google Scholar]
- Pietrini G, Matteoli M, Banker G, Caplan MJ. Isoforms of the Na,K-ATPase are present in both axons and dendrites of hippocampal neurons in culture. Proceedings of the National Academy of Sciences of the USA. 1992;89:8414–8418. doi: 10.1073/pnas.89.18.8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisig PA, Rector FC., Jr Role of Na+-H+ antiport in rat proximal tubule NaCl absorption. American Journal of Physiology. 1988;255:F461–465. doi: 10.1152/ajprenal.1988.255.3.F461. [DOI] [PubMed] [Google Scholar]
- Ribeiro CP, Mandel LJ. Parathyroid hormone inhibits proximal tubule Na+-K+-ATPase activity. American Journal of Physiology. 1992;262:F209–216. doi: 10.1152/ajprenal.1992.262.2.F209. [DOI] [PubMed] [Google Scholar]
- Ruiz OS, Arruda JAL. Regulation of the renal Na-HCO3 cotransporter by cAMP and Ca-dependent protein kinases. American Journal of Physiology. 1992;262:F560–565. doi: 10.1152/ajprenal.1992.262.4.F560. [DOI] [PubMed] [Google Scholar]
- Satoh T, Cohen HT, Katz AI. Different mechanisms of renal Na-K-ATPase regulation by protein kinases in proximal and distal nephron. American Journal of Physiology. 1993;265:F399–405. doi: 10.1152/ajprenal.1993.265.3.F399. [DOI] [PubMed] [Google Scholar]
- Schafer JA, Troutman SL. cAMP mediates the increase in apical membrane Na+ conductance produced in rat CCD by vasopressin. American Journal of Physiology. 1990;259:F823–831. doi: 10.1152/ajprenal.1990.259.5.F823. [DOI] [PubMed] [Google Scholar]
- Seki G, Taniguchi S, Uwatoko S, Suzuki K, Kurokawa K. Activation of the basolateral Cl− conductance by cAMP in rabbit renal proximal tubule S3 segments. Pflügers Archiv. 1995;430:88–95. doi: 10.1007/BF00373843. [DOI] [PubMed] [Google Scholar]
- Silver RB, Frindt G, Windhager EE, Palmer LG. Feedback regulation of Na channels in rat CCT. I. Effects of inhibition of Na pump. American Journal of Physiology. 1993;264:F557–564. doi: 10.1152/ajprenal.1993.264.3.F557. [DOI] [PubMed] [Google Scholar]
- Skou JC. Enzymatic basis for active transport of Na+ and K+ across cell membrane. Physiological Reviews. 1965;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Ullrich KJ, Capasso G, Rumrich G, Papavassiliou F, Klöss S. Coupling between proximal tubular transport processes. Studies with ouabain, SITS and HCO3−-free solutions. Pflügers Archiv. 1977;368:245–252. doi: 10.1007/BF00585203. [DOI] [PubMed] [Google Scholar]
- Wang T, Segal AS, Giebisch G, Aronson PS. Stimulation of chloride transport by cAMP in rat proximal tubules. American Journal of Physiology. 1995;268:F204–210. doi: 10.1152/ajprenal.1995.268.2.F204. [DOI] [PubMed] [Google Scholar]
- Weinman EJ, Shenolikar S, Kahn AM. cAMP -associated inhibition of Na+-H+ exchanger in rabbit kidney brush-border membranes. American Journal of Physiology. 1987;252:F19–25. doi: 10.1152/ajprenal.1987.252.1.F19. [DOI] [PubMed] [Google Scholar]
- Willmann JK, Bleich M, Rizzo M, Schmidt-Hieber M, Ullrich KJ, Greger R. Amiloride-inhibitable Na+ conductance in rat proximal tubule. Pflügers Archiv. 1997;434:173–178. doi: 10.1007/s004240050380. [DOI] [PubMed] [Google Scholar]
- Yoshitomi K, Burckhardt BC, Frömter E. Rheogenic sodium-bicarbonate cotransport in the peritubular cell membrane of rat renal proximal tubule. Pflügers Archiv. 1985;405:360–366. doi: 10.1007/BF00595689. [DOI] [PubMed] [Google Scholar]
- Younes-Ibrahim M, Barlet-Bas C, Buffin-Meyer B, Cheval L, Rajerison R, Doucet A. Ouabain-sensitive and -insensitive K-ATPases in rat nephron: effect of K depletion. American Journal of Physiology. 1995;268:F1141–1147. doi: 10.1152/ajprenal.1995.268.6.F1141. [DOI] [PubMed] [Google Scholar]


