AMPA-preferring glutamate receptors lacking the GluR2 subunit are highly permeable to Ca2+ ions. Ca2+ permeability is controlled by a single amino acid in the second membrane-associated domain, the so-called ‘Q/R-site’. In addition to Ca2+ permeability, the Q/R site influences the sensitivity of the receptor complex to block by polyamine spider toxins and internal polyamines. The voltage-dependent block by internal polyamines gives rise to inward rectification of AMPA receptors lacking the GluR2 subunit (Bowie & Mayer 1995; Koh et al. 1995; Kamboj et al. 1995).
Most principal neurones of the mammalian CNS express high levels of GluR2 suggesting that AMPA receptor Ca2+ permeability is low in these cells. In contrast, some hippocampal and neocortical local circuit GABAergic inhibitory interneurones possess glutamate receptors with inwardly rectifying current-voltage (I-V) relationships and appreciable Ca2+ permeability. The presence of these receptors is correlated with a low abundance of GluR2 mRNA expression (Jonas & Burnashev, 1995). Despite knowledge of the presence of these receptors in interneurones, no clear physiological function (other than Ca2+ permeability) has been attributed to them to distinguish them from ‘conventional’ AMPA receptors. An article by Rozov et al. (1998) in this issue of The Journal of Physiology identifies a unique physiological role for these receptors, moving internal block by polyamines from the biophysical to the physiological arena. They demonstrate that a use-dependent unblock by internal polyamines potentially could confer a novel mechanism of short-term synaptic plasticity at these receptors.
Using brief, rapid applications of glutamate to mimic synaptic transmission Rozov et al. (1998) demonstrate that currents through recombinant Ca2+-permeable AMPA receptors are facilitated upon repetitive stimulation (Fig. 1). This facilitation is frequency dependent and the number of glutamate pulses required for maximal facilitation differs depending on the receptor subunit studied. Surprisingly, this current facilitation appears not to depend on Ca2+ permeation (or ion flux for that matter) through these receptors but arises from a voltage- and use-dependent relief of block by internal polyamines. Relief of block by polyamines requires that the channels open and, as would be expected from our previous knowledge of polyamine block, the rate of unblock is more rapid at more negative potentials. Removal of internal polyamines by washout or by omitting them from the internal solution results in a loss of the facilitatory mechanism. These data suggest that receptors are ‘tonically’ blocked by polyamines and that upon repetitive activation block is relieved. During current facilitation, the I-V relationship is temporarily transformed from inwardly rectifying to linear, consistent with a relief of block of the channel by polyamines. Facilitation of currents lasts only for a limited time before reblock of the channel occurs. The reblocking mechanism proceeds without the requirement for the channel to open, suggesting that polyamines do not only act as classical open channel blockers as was previously thought. The actual mechanism whereby polyamines ‘reblock’ the channel, however, remains to be determined.
Figure 1. Frequency-dependent facilitation of currents through Ca2+-permeable AMPA receptors.
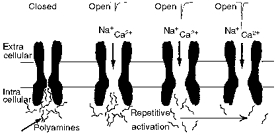
In the closed state the inner pores of glutamate receptors are tonically blocked by polyamines (left). Following receptor activation only a modest amount of current flows due to the occlusion of the pore by polyamines. On repetitive activation significant relief of channel block occurs allowing greater current flow through the channel. Consequently, at a given voltage, current amplitudes are maximal only once the significant polyamine block is removed (right).
Polyamine block of Ca2+-permeable AMPA receptors confers a strong voltage dependence to the rise time of currents. Since the channel is blocked by polyamines in the closed state, significant unblock of the channel must occur before the steady-state current amplitude is reached. This has the effect of slowing the time course of current activation at more positive voltages: a result not seen in the absence of internal polyamines. This would suggest that the rise times of synaptic currents through native Ca2+-permeable AMPA receptors would also be voltage dependent and slowed compared with Ca2+-impermeable receptors. Whether this is indeed the case remains to be determined; however, such a mechanism will have major consequences for the temporal integration of synaptic transmission in a cell possessing these receptors. Of particular interest, the voltage-dependent rate of channel unblock confers a hysteresis to the I-V relationship during rapid voltage ramps. One can envision that EPSP amplitudes in cells containing these receptors will not only be strongly influenced by the frequency of synaptic transmission but also by the membrane potential ‘history’ of the postsynaptic cell resulting in a complex pattern of modulation.
Given the recent observations that conventional mechanisms of long term potentiation (LTP) are absent from most hippocampal interneurone types (McBain & Maccaferri, 1997), these data provide an alternative mechanism for plasticity for excitatory synapses onto these cells. Indeed, the authors demonstrate that current facilitation exists at native Ca2+-permeable AMPA receptors in patches excised from both dentate gyrus basket cells and neocortical layer II interneurones, suggesting that such facilitation is not a product of recombinant receptor systems. This facilitation is distinct from conventional forms of LTP seen in principal neurones. Furthermore the relative brevity of the facilitation (∼5 s) provides only a limited time window in which plasticity can occur. Since polyamines are an essential component of the intracellular milieu, it is likely that the free polyamine concentration and the metabolic state of the cell will be permissive for the occurrence of such a mechanism of plasticity. Whether such facilitation exists at synapses containing Ca2+-permeable receptors remains to be determined. The results by Rozov et al., however, demonstrate a novel mechanism that will undoubtedly provide a rapid but transient facilitation in Ca2+ entry during periods of intense neuronal activity.
References
- Bowie D, Mayer ML. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Jonas P, Burnashev N. Neuron. 1995;15:987–990. doi: 10.1016/0896-6273(95)90087-x. 10.1016/0896-6273(95)90087-X. [DOI] [PubMed] [Google Scholar]
- Kamboj SK, Swanson GT, Cull-Candy SG. The Journal of Physiology. 1995;486:297–303. doi: 10.1113/jphysiol.1995.sp020812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh DS, Burnashev N, Jonas P. The Journal of Physiology. 1995;486:305–312. doi: 10.1113/jphysiol.1995.sp020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Maccaferri G. Canadian Journal of Physiology and Pharmacology. 1997;75:488–494. 10.1139/cjpp-75-5-488. [PubMed] [Google Scholar]
- Rozov A, Zilberter Y, Wollmuth LP, Burnashev N. The Journal of Physiology. 1998;511:361–377. doi: 10.1111/j.1469-7793.1998.361bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


