Abstract
The medullary raphe, within the ventromedial medulla (VMM), contains putative central respiratory chemoreceptors. To study the mechanisms of chemosensitivity in the raphe, rat VMM neurones were maintained in primary dissociated tissue culture, and studied using perforated patch-clamp recordings. Baseline electrophysiological properties were similar to raphe neurones in brain slices and in vivo.
Neurones were exposed to changes in CO2 from 5% to 3 or 9% while maintaining a constant [NaHCO3]. Fifty-one per cent of neurones (n = 210) did not change their firing rate by more than 20% in response to hypercapnic acidosis. However, 22% of neurones responded to 9% CO2 with an increase in firing rate (‘stimulated’), and 27% of neurones responded with a decrease in firing rate (‘inhibited’).
Chemosensitivity has often been considered an all-or-none property. Instead, a method was developed to quantify the degree of chemosensitivity. Stimulated neurones had a mean increase in firing rate to 298 ± 215% of control when pH decreased from 7.40 to 7.19. Inhibited neurones had a mean increase in firing rate to 232 ± 265% of control when pH increased from 7.38 to 7.57.
Neurones were also exposed to isocapnic acidosis. All CO2-stimulated neurones tested (n = 15) were also stimulated by isocapnic acidosis, and all CO2-inhibited neurones tested (n = 19) were inhibited by isocapnic acidosis. Neurones with no response to hypercapnic acidosis also had no response to isocapnic acidosis (n = 12). Thus, the effects of CO2 on these neurones were mediated in part via changes in pH.
In stimulated neurones, acidosis induced a small increase in the after-hyperpolarization level of 1.38 ± 1.15 mV per −0.2 pH units, which was dependent on the level of tonic depolarizing current injection. In voltage clamp mode at a holding potential near resting potential, there were small and inconsistent changes in whole-cell conductance and holding current in both stimulated and inhibited neurones. These results suggest that pH modulates a conductance in stimulated neurones that is activated during repetitive firing, with a reversal potential close to resting potential.
The two subtypes of chemosensitive VMM neurones could be distinguished by characteristics other than their response to acidosis. Stimulated neurones had a large multipolar soma, whereas inhibited neurones had a small fusiform soma. Stimulated neurones were more likely than inhibited neurones to fire with the highly regular pattern typical of serotonergic raphe neurones in vivo.
Within the medullary raphe, chemosensitivity is a specialization of two distinct neuronal phenotypes. The response of these neurones to physiologically relevant changes in pH is of the magnitude that suggests that this chemosensitivity plays a functional role. Elucidating their mechanisms in vitro may help to define the cellular mechanisms of central chemoreception in vivo.
The respiratory control system is one of the most responsive neuronal systems to acid/base changes. The system-level response is presumed to be due to a high degree of chemosensitivity at the cellular level of specialized brainstem neurones. Early work had suggested that these central respiratory chemoreceptors are located exclusively within the ventrolateral medulla (VLM) (Mitchell et al. 1963; Schlaefke et al. 1970); however, in vitro experiments have identified neurones that increase their firing rate when exposed to acidosis within many brainstem nuclei linked to cardiorespiratory control, including the VLM (Fukuda et al. 1980; Jarolimek et al. 1990; Neubauer et al. 1991; Richerson, 1995), nucleus tractus solitarius (NTS) (Dean et al. 1990), hypothalamus (Dillon & Waldrop, 1992), locus coeruleus (Pineda & Aghajanian, 1997), medullary raphe (Richerson, 1995) and nucleus ambiguus (NA) (Rigatto et al. 1992). This wide distribution of chemosensitive neurones has been interpreted as suggesting that central respiratory chemoreception is a distributed property of the respiratory network. This possibility is also supported by data acquired using in vivo approaches (Sato et al. 1992; Coates et al. 1993; Bernard et al. 1996).
In most cases, the sensitivity of chemosensitive neurones studied in vitro within respiratory nuclei has been less than anticipated based on the degree of sensitivity of the respiratory system as a whole. In some cases, CO2 changes needed to elicit a response have been outside the range expected to occur in vivo. In other cases, changes in firing rate in response to respiratory acidosis have been relatively small. It is possible that chemosensitive neurones with a low degree of sensitivity are central chemoreceptors. However, another alternative is that neurones within many brainstem respiratory regions respond to large acid/base changes, but neurones in only a limited number of regions have large responses to the small changes in CO2 that would typically occur under physiological conditions. If this is the case, all ‘chemoreceptors’ may not play an equal role in respiratory chemoreception.
In most previous in vitro studies identifying neurones responsive to acid/base changes, chemosensitivity has been considered as an all-or-none neuronal property. The magnitude of the stimuli, and the criteria used to define neurones as chemosensitive have been variable. Thus, it remains unclear whether there are differences in the degree of chemosensitivity in different putative chemoreceptor regions. Without more quantitative information about the response of putative chemoreceptor neurones from different respiratory regions to acid/base changes within the normal physiological range, it will be difficult to determine what possible role chemosensitive neurones recorded in vitro play in respiratory chemoreception in vivo.
In this report, the degree of chemosensitivity to physiologically relevant acid/base changes was quantified for neurones from the medullary raphe, a putative chemoreceptor region. To accomplish this goal, a primary dissociated monolayer cell culture preparation was developed. It was found that raphe neurones in tissue culture had baseline electrophysiological properties similar to those seen in brain slices and in vivo. This approach allowed quantification of the response to acid/base disturbances and further examination of the mechanisms of chemosensitivity in raphe neurones. A preliminary report of this work has previously been published (Richerson & Wang, 1997).
METHODS
Sources of chemicals and reagents
Picrotoxin (PTX), tetrodotoxin (TTX), EGTA, EDTA, Hepes, cytosine β-D-arabino-furanoside hydrochloride (Ara-C), papain, cysteine, trypsin inhibitor, bovine serum albumin (BSA), poly-L-ornithine, laminin, streptomycin, penicillin, fibroblast growth factor-5 (FGF-5), and all salts and chemicals not otherwise listed were purchased from Sigma Chemical Co. (±)-2-Amino-5-phosphonopentanoic acid (AP-5), and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), were purchased from Research Biochemicals International. Fetal bovine serum (FBS), basic fibroblast growth factor (bFGF), Neurobasal medium and B27 supplement were purchased from Gibco BRL. Modified Eagle's medium (MEM; no. 56419) and F12 supplement were purchased from JRH Biosciences (Lenexa, KS, USA).
Cell culture
Cultures were prepared from neonatal (P1-P3) Sprague-Dawley rats using aseptic technique. Rats were decapitated, and the medulla was rapidly removed. Transverse cuts were made through the pia mater on the ventral surface of the brainstem at the ponto-medullary border, and immediately caudal to the confluence of the vertebral arteries. Longitudinal cuts were also made down the middle of both pyramids (Fig. 1A). A scalpel blade was used to remove a wedge of tissue with its base at the ventral surface and borders along the previously made cuts, and its apex in the mid-line approximately half way towards the dorsal surface of the medulla (Fig. 1B). The microdissected region contained the raphe pallidus, raphe magnus, a portion of the raphe obscurus and tissue immediately adjacent to these nuclei.
Figure 1. Microdissection of the medullary raphe and surrounding ventromedial medulla (VMM) for preparation of cell culture.
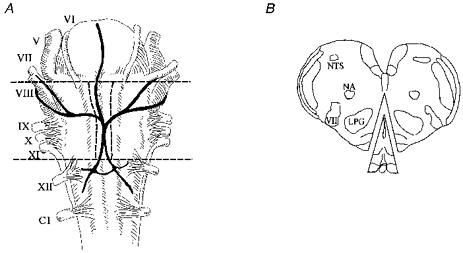
A, ventral view of the cat medulla showing borders of cuts made on the VMM. The locations of the major landmarks of the neonatal rat are similar. V-XII, cranial nerves. C1, 1st cervical root. Adapted with permission from Feldman (1986). B, transverse section of the rat medulla showing wedge of tissue removed for cell culture. NA, nucleus ambiguus. NTS, nucleus tractus solitarius. LPG, nucleus lateralis paragigantocellularis. VII, 7th nerve nucleus.
Dissected tissue was placed in oxygenated Hepes-buffered Ringer solution (mM: NaCl, 130; KCl, 4; MgCl2, 1; CaCl2, 1.5; Hepes, 10; dextrose, 10; NaOH, 3; pH 7.3), then digested for 30 min with papain solution (Hepes-buffered Ringer solution with 1.5 mM CaCl2 (3 mM total), 0.5 mM EDTA, 9 U ml−1 papain, and 0.2 mg ml−1 cysteine, activated for 30 min prior to use). Digested tissue was washed 3 times in trituration solution (10% FBS in MEM with 100 u ml−1 penicillin and 100 μg ml−1 streptomycin; 350 μl trituration solution per animal), then triturated for seven to ten passes, and plated on poly-L-ornithine- and laminin-coated 12 mm round coverslips at a density of 0.5-1 × 105 cells ml−1. Cells were allowed to attach for 40 min, then glial-conditioned medium was added (10% FBS in MEM, or 10% FBS in 60% MEM + 40% Neurobasal Medium with B27 supplement; with penicillin- streptomycin; conditioned for 1 day by glial cultures obtained from the ventromedial medulla, VMM). In some cases, bFGF (0.1-1 ng ml−1) and/or FGF-5 (1–10 ng ml−1) were added to the culture medium to enhance survival (Lindholm et al. 1994). Cells were first fed on days 4–7 with a half-change of Neurobasal-B27 medium to which Ara-C (3–10 μM) was added to inhibit glial growth, and then fed approximately once per week. Recordings were made after cells were grown in culture for 2–94 days (mean, 16.7).
Electrophysiological recordings
Coverslips were transferred to a recording chamber (E. W. Wright, Guilford, CT, USA) mounted on a fixed-stage upright microscope (Axioskop FS, Carl Zeiss, Inc.), and maintained at room temperature throughout the experiments. Neurones were continuously superfused at a rate of 3–4 ml min−1 with oxygenated Ringer solution (mM: NaCl, 124; KCl, 3; MgCl2, 2; dextrose, 10; NaH2PO4, 1.3; NaHCO3, 26; CaCl2, 2; pH 7.4 at 5% CO2−95% O2). After a brief period in Ringer solution, most neurones were also recorded in Ringer solution with PTX (100 μM), AP-5 (50 μM), and CNQX (10 μM) to block ionotropic GABAergic and glutamatergic receptors. Acid/base changes were made using methods described previously (Richerson, 1995). Bath pH was continuously measured with a pH electrode (MI-414; Microelectrodes, Inc., Londonderry, NH, USA) placed in the inflow to the recording chamber. When changes in solution were made, the change in pH in the chamber was well fitted with a single exponential function with a time constant of 1.07 ± 0.47 min (mean ±s.d.; n = 7).
All recordings were made using the amphotericin perforated-patch technique (Rae et al. 1991), since whole-cell recording results in loss of repetitive firing and chemosensitivity in raphe neurones (Richerson, 1995). Electrodes (4–10 MΩ) were made from borosilicate glass (Corning 7052) using a micropipette puller (P-97; Sutter Instrument Co.), and filled with intracellular solution containing (mM): potassium methanesulphonate, 135; KCl, 10; Hepes, 5; and EGTA, 1; pH 7.2; osmolarity, 275 ± 10 mosmol l−1. Input resistance was usually >200 MΩ (seal resistance ≥1 GΩ; access resistance typically 10–40 MΩ). Neurones were considered healthy with resting potential ≤-45 mV, action potential height ≥60 mV, and maintenance of repetitive firing if present early in the recording. Recordings were amplified (Axopatch-1D, Axon Instruments), filtered (10 kHz low-pass), and acquired at ten kilosamples per second with a computerized data acquisition system (AT-MIO-16F-5, National Instruments, Austin, TX, USA) using custom-written software. Data were stored simultaneously in original form on tape (Neurocorder DR-484, Neuro Data Instruments Corp., New York) and after reduction on magnetic storage media.
Recordings were first made in current clamp mode. If a cell did not have spontaneous firing, cells were depolarized to induce firing. If a neurone required continuous hyperpolarization to decrease firing rate or stabilize membrane potential, that neurone was not used to study chemosensitivity. Changes in firing rate were measured in response to changes in CO2/pH in spontaneously firing and depolarized neurones. The response was monitored at the time of the experiment using computerized real-time analysis. The duration of exposure to each stimulus was varied to permit a steady-state response to be reached. The duration of exposure was therefore not constant, since neurones required a variable period before the new firing rate level reached a steady state.
Changes in resting whole-cell conductance were measured in voltage clamp mode at a holding potential of either −50 or −60 mV. Resting whole-cell conductance was calculated from the change in steady-state current with voltage steps of −10 mV (330 ms, 1 Hz). The mean conductance was averaged continuously from 10 pulses every 10 s. The reversal potential of the current modulated by CO2/pH was estimated as the intersection of the slope conductances extrapolated from holding and step potentials at two different CO2 levels.
Anatomy
To correlate neuronal morphology with electrophysiology, neurones were visualized with Nomarski differential interference contrast (DIC) optics, and digital micrographs were made of each neurone prior to recording. Digital images were imported into a graphical drawing program and the outline of each cell was drawn by hand.
During these experiments, initial observations suggested a relationship between morphology and chemosensitivity. The purpose of this study was to examine the mechanisms of chemosensitive neurones, not to define the population properties of VMM neurones in culture. Therefore, neurones were selected based on their morphology, in order to increase the chance that recordings were from chemosensitive neurones. Thus, the values reported here for percentage of chemosensitive cells, ratio of stimulated vs. inhibited neurones, percentage of neurones with spontaneous firing, mean firing rates, etc. are not a random sample of the culture population.
Data analysis
Most CNS neurones typically have spontaneous variations in firing rate during recordings in all types of preparation. If such a spontaneous change in firing rate occurs coincident with a change in CO2/pH, a neurone could be erroneously labelled as chemosensitive. In the present experiments, neurones were observed to have changes in firing rate that sometimes occurred coincidentally with acid/base changes, but did not occur consistently. If a response to a single exposure to an acid/base stimulus was considered sufficient evidence for chemosensitivity, these neurones would have been inaccurately classified as chemosensitive. To avoid this type of ‘false positive’ error, the following criteria were used to define neurones as chemosensitive.
Preliminary classification of neurones was based on changes in firing rate in response to changes in CO2. Spikes were detected with a threshold detection software algorithm, and firing rate plots were generated using a moving average technique (bin size, 1–2 s; 20 bins per point). Neurones were considered candidates for chemosensitivity if they demonstrated a response that was: (1) reproducible and reversible on four or more acid/base changes (at least two exposures to an acid/base disturbance, with washout each time); (2) consistent in time course for each stimulus; and (3) opposite in sign if the neurone was exposed to both acidosis and alkalosis. This approach was more effective in screening out neurones with random changes in firing rate than a response to a single change in CO2.
Final classification of neurones as chemosensitive was based on statistical analysis of changes in firing rate vs. pH. Mean firing rate and pH were calculated for 30 s epochs while cells were exposed to four or more transitions in acid/base status. The time of onset of the change in pH was used to identify the time of transition between different CO2/pH levels (Fig. 3B). These transition times were used to separate each trace into intervals (usually 5–10 min each). The first 30–60 s in each interval was excluded in all subsequent analyses, to eliminate the period of solution exchange. Neurones were defined as chemosensitive if acid/base changes resulted in a statistically significant increase or decrease in mean firing rate of at least 20% per change in pH of 0.2 units (P ≤ 0.05 level; Student's two-tail t test), for at least four separate transitions. The 20% level was chosen because changes less than 20% commonly occur randomly, and because a change in firing rate of 10–20% has been used in previous studies to define neurones as chemosensitive. Further work may reveal that a change in firing rate of 10, 50, or 100% is more closely correlated with a chemoreceptor role in vivo. However, based on the 20% threshold, neurones were categorized as stimulated, inhibited, or unresponsive to hypercapnia and/or acidosis. This approach would have underestimated the sensitivity of neurones that had a fast transient response to slow changes in acidosis as previously seen in neurones of the VLM in brain slices (Jarolimek et al. 1990), but neurones with that type of response were not seen in VMM cultures.
Figure 3. Hypercapnic acidosis stimulated some neurones cultured from the VMM.
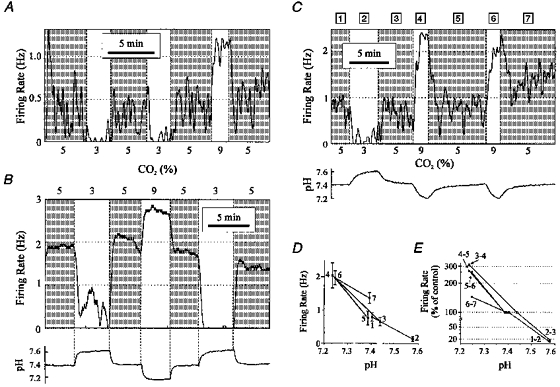
A, example of a ‘stimulated’ chemosensitive neurone. Shown is firing rate vs. time as hypocapnic alkalosis and hypercapnic acidosis were induced by changing CO2 from 5% to 3% and from 5% to 9%. B, example of a second stimulated neurone. Shown are firing rate (top) and pH (bottom). Acid/base changes were induced as in A. The times of transition between CO2 levels are shown (vertical dashed lines). C, example of a third stimulated neurone. Shown are firing rate (top) and pH (bottom). The numbers above the firing rate plot correspond to the intervals for calculations of mean firing rate and pH in D and E. D, steady state firing rate vs. pH for the neurone shown in C. Numbers correspond to the intervals at different CO2 levels. Error bars are ±s.d.E, firing rate (percentage of control) vs. pH for each transition between CO2 levels (same neurone as in C and D). The firing rates at the test CO2 levels were normalized to the firing rate for the adjacent intervals at 5% CO2. For this neurone, a change in pH from a mean of 7.39 to a mean of 7.23 resulted in a mean increase in firing rate to 285% of control. Note the logarithmic vertical scale, discontinuous at 85%.
Rather than relying solely on an arbitrary threshold value, it is important that the degree of chemosensitivity is quantified. The most accurate estimate of the degree of chemosensitivity is possible in neurones exposed to a large number of stimuli. Therefore, a subgroup of neurones was selected for detailed analysis that included only stimulated (n = 25) and inhibited (n = 19) neurones exposed to at least six transitions in acid/base status. For these neurones, the responses to acidosis were analysed separately from those to alkalosis. Firing rate and pH were measured prior to and after each transition in acid/base status. For each transition, the response to the test CO2 level (3 or 9%) was then calculated as a percentage of firing rate during the adjacent control interval at 5% CO2. At 9% CO2 the pH usually decreased by 0.2 units, and at 3% CO2 pH usually increased by 0.2 units, but there was some variability. Therefore, the response to acidosis and to alkalosis of each neurone was normalized to a change in pH of 0.2 pH units, using the equation:
Firing rate (% of control) per 0.2 pH units =
where FRT is mean firing rate at the test CO2 level as a percentage of control, pHT is mean pH at the test CO2 level, and pHC is mean pH at the control CO2 level (near 7.4). If the normalized response for any transition was less than 1% of control, it was assigned the value of 1%.
A second analysis was also performed on 134 neurones defined as stimulated, inhibited and unresponsive to respiratory acidosis exposed to four or more CO2 transitions. The mean slope of the relationship between firing rate and pH was determined for these neurones over the entire pH range tested. Since the relationship between these two parameters was unknown in advance, the analysis was performed in two ways. In one, it was assumed that there was a linear relationship between firing rate and pH, and in the other it was assumed there was a linear relationship between log10(firing rate) and pH. There was variability, but the relationship between firing rate and pH more closely resembled a log relationship (see Figs 3E, 4A and 6A). For example, in a neurone in which alkalosis of 0.2 pH units induced a decrease in firing rate to 50% of control, acidosis of 0.2 pH units would typically induce an increase in firing rate to around 200% of control. Both analyses gave qualitatively similar results, but for illustrative purposes we have chosen to represent the summary data for all classified neurones using a log relationship. A value, termed the chemosensitivity index (CI), was calculated from the slope of the log firing rate vs. pH relationship. The CI represented the firing rate of a neurone, as a percentage of control, in response to a decrease in pH of 0.2 units:
Figure 4. Summary of chemosensitivity of neurones stimulated by hypercapnic acidosis.
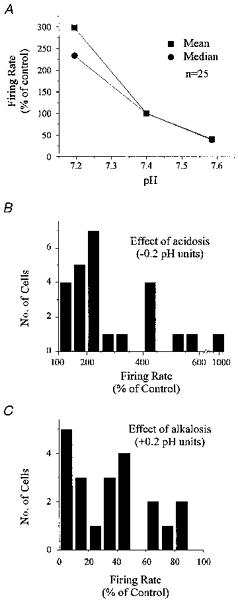
A, mean and median firing rate (percentage of control) are plotted against pH for 25 stimulated neurones exposed to at least six transitions in CO2. B, histogram of responses to hypercapnic acidosis of the same stimulated neurones as in A. Responses were normalized to a change in pH of −0.2 units. Most neurones increased their firing rate to more than 200% of control when CO2 was increased from 5% to 9%. C, histogram of responses to hypocapnic alkalosis of the same stimulated neurones as in A. Responses were normalized to a change in pH of +0.2 units. Most neurones decreased their firing rate to below 50% of control when CO2 was decreased from 5% to 3%.
Figure 6. Summary of chemosensitivity of neurones inhibited by hypercapnic acidosis.
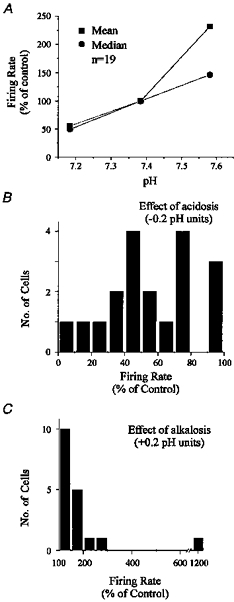
A, mean and median firing rate (percentage of control) are plotted against pH for 19 inhibited neurones exposed to at least six transitions in CO2. B, histogram of responses to hypercapnic acidosis of the same inhibited neurones as in A. Responses were normalized to a change in pH of −0.2 units. Most neurones decreased their firing rate to less than 60% of control when CO2 was increased from 5% to 9%. C, histogram of responses to hypocapnic alkalosis of the same inhibited neurones as in A. Responses were normalized to a change in pH of +0.2 units. Most neurones had a smaller response to alkalosis than to acidosis. In most cases firing rate did not increase above 200% of control when CO2 was decreased from 5% to 3%.
Chemosensitivity index (CI) =
where FR9 is mean firing rate (percentage of control) at 9% CO2, FR3 is mean firing rate (percentage of control) at 3% CO2, pH9 is mean pH at 9% CO2, and pH3 is mean pH at 3% CO2. In those neurones only exposed to either 3 or 9% CO2, the values at 5% CO2 were used in the above equation in place of the missing values.
Thus, the CI was the mean slope of the entire log firing rate vs. pH relationship. The CI would be 200% for a stimulated neurone whose firing rate doubled in response to a decrease in pH of 0.2, and would also be 200% for a stimulated neurone whose firing rate decreased to 50% in response to an increase in pH of 0.2. It would be 25% for an inhibited neurone whose firing rate decreased to 50% in response to a decrease in pH of 0.1 units.
As a measure of regularity of neuronal firing, the coefficient of variation (CV) of the interspike interval (ISI) was calculated for the entire period of recording from each neurone using 30 s epochs (Mason, 1997). ISI was measured for each spike pair, and was used to calculate the mean, standard deviation (s.d.) and CV of ISI for each epoch. To calculate the mean, s.d., and CV of ISI for the entire recording, any epoch with only three spikes or fewer (firing rate, ≤0.1 Hz), and the 10% of epochs with the largest CV of ISI, were not included in the calculation, to obtain a more representative estimate of firing regularity.
The CV of the absolute ISI would not accurately reflect the beat-to-beat regularity of a neurone whose firing rate was slowly ramping up in frequency, despite a highly regular firing pattern from one spike to the next. Thus, a second approach was also used to measure variability of ISI. In this case, the relative ISI was calculated for each spike as the ratio of ISI after a spike (ISIA) to ISI before a spike (ISIB): ISIA/ISIB. The relative ISI was calculated for ≤10000 spikes from each neurone, and plotted as a histogram. The mean relative ISI and standard deviation of the relative ISI were calculated after elimination of all relative ISI values greater than 10.0, to eliminate the contribution from long pauses in firing. This second approach may have resulted in a more representative estimate of regularity of firing in these neurones in which changes in firing frequency were induced by acid/base changes; however, both methods gave qualitatively similar results.
After-hyperpolarization (AHP) level was measured as the minimum membrane potential immediately following each spike. Spike threshold was measured as the time of the positive inflection of the 1st derivative of membrane potential at the onset of each spike. Spike duration was calculated as the width of each spike at spike threshold. Each of these values was obtained as the mean of four consecutive spikes at the steady state firing rate for each CO2 level.
Data presented in the form x±y are means ± standard deviation. Statistical significance was tested using the Mann-Whitney- Wilcoxon test unless otherwise noted.
RESULTS
Baseline electrophysiology of cultured ventromedial medulla (VMM) neurones
Of the first 100 neurones recorded in Ringer solution with PTX, AP-5 and CNQX, twenty-four spontaneously fired repetitively at a highly regular rate, with each action potential followed by a prominent AHP, and a ramp depolarization to the next spike (Fig. 2A). Another twenty-six neurones spontaneously fired action potentials repetitively, but without such a high degree of regularity, and usually with a less prominent AHP (Fig. 2B). An additional twenty-three neurones fired with one of these two patterns of activity when cells were depolarized. Thus, a total of 73 out of 100 neurones (73%) displayed continuous or intermittent repetitive firing with an AHP. The remaining neurones (27%) fired spontaneously or after current injection with intermittent, non-sustained firing with no AHP or ramp depolarization (n = 18), or with bursting (n = 9). As described in Methods, these values do not necessarily reflect the distribution of firing patterns of all neurones in culture, since neurones were selected based on morphology to increase the chance of recording chemosensitive neurones.
Figure 2. Baseline electrophysiological properties of cultured VMM neurones.
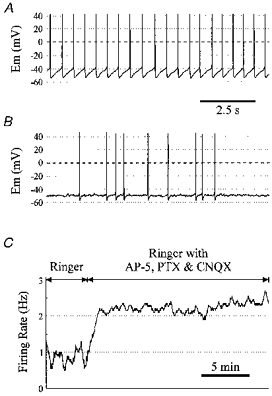
A, membrane potential of a neurone that fired spontaneously with a highly regular firing pattern. For this neurone, the s.d. of the relative ISI was 0.16, the CV of ISI was 0.14, and the mean firing rate was 1.65 Hz. B, membrane potential of a different neurone that fired more irregularly, but with a prominent AHP. For short periods of time, this neurone could fire more regularly, but without the same high degree of regularity as the neurone in A. For this neurone, the s.d. of the relative ISI was 1.90, the CV of ISI was 1.89, and the mean firing rate was 0.24 Hz. C, firing rate vs. time for a cultured VMM neurone. Blockade of ionotropic glutamate and GABA receptors led to an increase in firing rate.
Of the first ninety-eight neurones in which data were obtained in both Ringer solution and Ringer solution with PTX, AP-5 and CNQX (an overlapping, but different subset from that above), spontaneous firing occurred in 47% in Ringer solution. After blockade of glutamatergic and GABAergic neurotransmission, the percentage of cultured VMM neurones with spontaneous firing increased to 64%, approximately equal to that seen previously in neurones recorded from the rostral ventral medulla in brain slices (76%) (Richerson, 1995). Of forty-six neurones that fired spontaneously in Ringer solution, the firing rate increased in thirty-eight in Ringer solution with PTX, AP-5, and CNQX (Fig. 2C). The firing rate of neurones that fired spontaneously in the latter solution was 0.91 ± 1.48 Hz (0–8.5 Hz) in Ringer solution, and increased to 1.69 ± 1.89 Hz (0.1-9.5 Hz) after blockade of GABAergic and glutamatergic transmission (P < 0.01; Wilcoxon signed rank test). Thus, firing was suppressed in culture by GABAergic inhibition.
Chemosensitivity of cultured VMM neurones
From a total of 210 recordings stable enough to meet the criteria for analysis of chemosensitivity, 22% (n = 46) of neurones were stimulated, 27% (n = 56) inhibited, and 51% (n = 108) unaffected by hypercapnic acidosis. Three examples of neurones stimulated by hypercapnic acidosis are shown in Fig. 3. In the first neurone (Fig. 3A), hypocapnic alkalosis induced by a decrease in CO2 from 5% to 3% resulted in a reversible and reproducible decrease in firing rate. The mean firing rate during the first exposure to 3% CO2 (0.034 Hz) was 10% of the mean firing rate during the first interval at 5% CO2 (0.352 Hz), and 11% of the firing rate during the subsequent interval at 5% CO2 (0.326 Hz). Hypercapnic acidosis induced a response opposite to that caused by hypocapnic alkalosis.
The second example of a stimulated neurone (Fig. 3B) includes the pH trace, and illustrates the method used to determine transitions in acid/base status. These transitions were visually determined as the time of the initial change in pH. Note that this neurone had a higher baseline firing rate, but still had a robust response to both hypercapnic acidosis and hypocapnic alkalosis.
The third example of a stimulated neurone (Fig. 3C) includes the pH trace, and also illustrates the method used to quantify the relationship between mean firing rate and pH. For each epoch (30 s), mean firing rate and pH were calculated. The values for firing rate were plotted against pH for each interval at a given CO2 level (Fig. 3D). The percentage change in firing rate for each transition was then calculated (Fig. 3E; note log scale). In this neurone, an increase in CO2 to 9% resulted in a mean decrease in pH from 7.41 to 7.24, and a mean increase in firing rate to 246% of control. A decrease in CO2 to 3% resulted in a mean increase in pH from 7.41 to 7.58, and a mean decrease in firing rate to 18% of control. Note that in this neurone a rapid response was still seen, despite relatively slow changes in bath pH.
A total of twenty-five stimulated neurones were exposed to at least six transitions in CO2. This subset of neurones was analysed in the same way as the neurone shown in Fig. 3C-E. The mean and median firing rate (percentage of control) were plotted for these twenty-five stimulated neurones (Fig. 4A). The sensitivity of these neurones was high, with firing rate increasing to 298 ± 215% of control when pH decreased from 7.40 ± 0.02 to 7.19 ± 0.04 due to an increase in CO2 from 5% to 9%. When CO2 was decreased from 5% to 3%, firing rate decreased to 41 ± 34% of control as pH increased from 7.40 ± 0.02 to 7.59 ± 0.04. The median change in firing rate was to 233 and 39% of control in response to 9% and 3% CO2, respectively. Baseline firing rate at 5% CO2 was 1.05 ± 0.85 Hz.
The responses of these twenty-five stimulated neurones to 9% CO2 were normalized to a decrease in pH of 0.2 units, and plotted as a histogram (Fig. 4B). The responses of neurones also exposed to 3% CO2 (n = 21) were normalized to an increase in pH of 0.2 units and plotted as a histogram (Fig. 4C). In most stimulated neurones, firing rate increased to more than 200% of control in response to a decrease in pH of 0.2 units, and also decreased to less than 50% in response to an increase in pH of 0.2 units.
Many neurones inhibited by hypercapnic acidosis also had a high degree of sensitivity. Two examples of inhibited neurones are shown in Fig. 5. In both examples, a decrease in pH resulted in a decrease in firing rate, but in the first example (Fig. 5A) cessation of firing required an increase in CO2 to 9%, while in the other example (Fig. 5B), firing did not begin until CO2 decreased below 5%.
Figure 5. Hypercapnic acidosis inhibited some neurones cultured from the VMM.
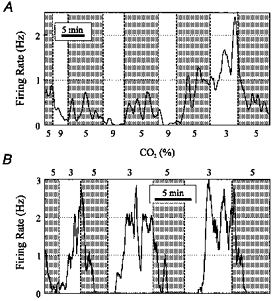
A, example of an ‘inhibited’ neurone. Shown is firing rate vs. time as acid/base changes were induced by changing CO2 from 5% to 9% and from 5% to 3%. B, example of a second inhibited neurone. Note that this neurone did not fire until CO2 dropped below the baseline of 5%. This was not the case for most neurones, since tonic current injection was used in most neurones that were not spontaneously active to induce firing near 1 Hz at 5% CO2.
A total of nineteen inhibited neurones were exposed to at least six transitions in CO2. This subset of neurones was analysed in the same way as the neurone shown in Fig. 3C-E. The mean and median firing rate (percentage of control) were plotted for these inhibited neurones (Fig. 6A). In response to a decrease in CO2 from 5% to 3%, firing rate increased to 232 ± 265% of control when pH increased from a mean of 7.38 to 7.57. When CO2 was increased from 5% to 9%, firing rate decreased to 56 ± 26% of control as pH decreased from a mean of 7.38 to 7.18. The median change in firing rate was to 146 and 50% of control in response to 3 and 9% CO2, respectively. Baseline firing rate at 5% CO2 for these nineteen neurones was 1.12 ± 1.02 Hz. Histograms of normalized responses were plotted in the same way as for stimulated neurones (Fig. 6B and C). Compared with the responses to both acidosis and alkalosis of stimulated neurones, the responses of inhibited neurones were smaller. However, the degree of sensitivity of inhibited neurones was still large, especially to acidosis.
Other neurones meeting the criteria for definition of chemosensitivity had a similar degree of chemosensitivity as the subset of stimulated and inhibited neurones with six or more transitions that were selected for in-depth analysis. A histogram of the CI for 134 neurones with four or more transitions (including stimulated, inhibited, and no response) is shown (Fig. 7). These data indicate that a large subset of neurones cultured from the VMM increased or decreased their firing rate by more than 50% in response to a decrease in pH of 0.2 units.
Figure 7. Summary of chemosensitivity in VMM neurones.
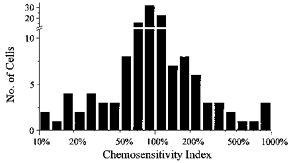
Plotted is a histogram of the chemosensitivity index (CI) for 134 cultured VMM neurones defined as stimulated, inhibited, or unresponsive to respiratory acidosis. The CI is a calculated value that normalizes the response of a neurone to hypercapnic acidosis of −0.2 pH units. A large percentage of these neurones increased or decreased their firing rate by ±50% of control in response to hypercapnic acidosis. Note log scale.
Response to isocapnic acidosis of chemosensitive neurones
It remains unclear whether central respiratory chemoreception in vivo results from a direct effect of CO2, H+, OH−, HCO3−, or more than one of these. To determine whether chemosensitivity of raphe neurones is due to changes in CO2, or whether changes in pH alone are sufficient to induce a response, neurones were exposed to isocapnic acid/base changes.
All neurones stimulated by hypercapnic acidosis that were tested were also stimulated by isocapnic acidosis (n = 15; Fig. 8A), and all neurones inhibited by hypercapnic acidosis that were tested were inhibited by isocapnic acidosis (n = 18; Fig. 8B). Neurones that were unresponsive to hypercapnic acidosis did not respond to isocapnic acidosis (n = 12). Thus, the response to ‘metabolic’ acidosis was qualitatively the same as ‘respiratory’ acidosis. Further work is needed to determine whether both forms of acidosis are quantitatively equal in their effect, and whether changes in CO2 have an effect independent of pH. Rather than referring to neurones as CO2-stimulated and CO2-inhibited, we will use the terms acidosis-stimulated and acidosis-inhibited, or shorten this to simply ‘stimulated neurones’ and ‘inhibited neurones.‘
Figure 8. Chemosensitive neurones also responded to isocapnic acid/base changes.
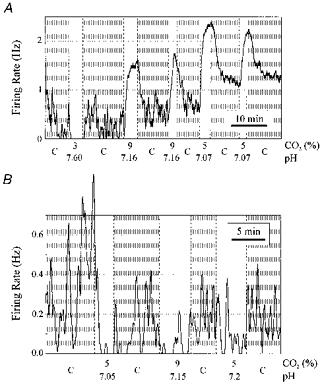
A, stimulated neurone. Changes in firing rate were induced by a decrease in CO2 from 5% to 3% and by an increase in CO2 from 5% to 9%. When this neurone was subsequently exposed to isocapnic acidosis, there was also an increase in firing rate. Letter C on the abscissa is the control period at 5% CO2 and pH 7.4. B, inhibited neurone. This neurone was inhibited by both hypercapnic acidosis and by isocapnic acidosis.
Firing pattern of chemosensitive neurones
The two subtypes of chemosensitive neurone had different firing patterns. Stimulated neurones were more likely than inhibited neurones to display regular, repetitive firing with a low CV of ISI, and a low s.d. of the relative ISI. Examples of membrane potential of the two subtypes of chemosensitive neurone are shown (Fig. 9). The stimulated neurone (Fig. 9A and B) fired action potentials at a highly regular rate, with each spike followed by an AHP and a ramp depolarization to the next spike. During normocapnia (Fig. 9A), membrane potential reached a plateau prior to reaching spike threshold, and firing rate had some minor irregularity. At times, firing would stop for prolonged periods, but when the neurone began to fire again the regular pattern resumed. Hypercapnic acidosis (9% CO2, Fig. 9B) caused an increase in slope of the interspike depolarization, which became nearly linear, and firing became even more regular. Despite the decrease in regularity at low CO2 levels, the s.d. of relative ISI was 0.33 (mean firing rate, 1.42 Hz), indicating that on average firing rate was highly regular.
Figure 9. Effect of acid/base changes on membrane potential of stimulated and inhibited neurones.
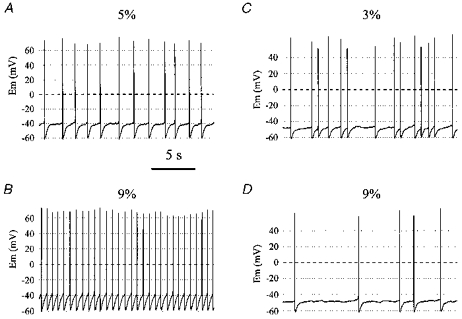
A, membrane potential of a stimulated neurone during exposure to 5% CO2. Note the regular firing pattern (s.d. of relative ISI was 0.33 for entire recording) and the prominent AHP. B, membrane potential of the same neurone as in A during exposure to 9% CO2. The firing rate increased and the firing pattern became even more regular. C, membrane potential of an inhibited neurone at 3% CO2. Note that the firing pattern is more irregular (s.d. of relative ISI was 1.38) than the neurone in A and B. D, membrane potential of the same neurone as in C after CO2 was increased to 9%. Note that firing rate decreased.
In contrast to the regular firing pattern of many stimulated neurones, most inhibited neurones fired more irregularly. An example of membrane potential of an inhibited neurone is shown (Fig. 9C and D). In this neurone, hypocapnic alkalosis (Fig. 9C) resulted in an increase in firing rate, and hypercapnic acidosis (Fig. 9D) led to a decrease in firing rate. Each spike was followed by an AHP, and firing was relatively irregular. At higher rates, firing would become slightly more regular. However, on average firing was much more irregular than most stimulated neurones. The s.d. of relative ISI was 1.38 (mean firing rate, 1.46 Hz).
The examples of stimulated and inhibited neurones shown (Fig. 9) represent the most common firing pattern for the two types of chemosensitive neurone, with stimulated neurones more likely to have a regular firing pattern. When histograms of relative ISI for the two types of chemosensitive neurones were constructed, most stimulated neurones had a narrow distribution of relative ISI around a central value of 1.0 (Fig. 10A), while inhibited neurones were more likely to have a broad distribution (Fig. 10B). A plot of the standard deviation of relative ISI vs. firing rate for stimulated and inhibited neurones (Fig. 10C) illustrates the tendency for stimulated neurones to have a lower variability of ISI. To ensure that this population was made up of two homogeneous groups, seventy-nine neurones were selected from the 102 total stimulated and inhibited neurones (in a manner blinded to the firing pattern) as those with the largest responses. Some stimulated neurones fired irregularly, and some inhibited neurones fired more regularly, as in brain slices (Richerson, 1995). However, in general stimulated neurones fired with much more regularity than inhibited neurones. The s.d. of the relative ISI, and the CV of ISI were both significantly different for the two groups (P < 0.0004).
Figure 10. Stimulated neurones were more likely to fire with a high degree of regularity than inhibited neurones.
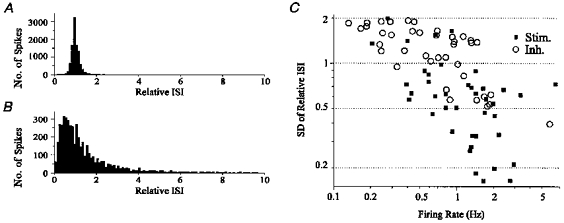
A, histogram of relative ISI for a stimulated neurone. The distribution of relative ISI was narrow, with a peak at 1.0, indicating a high degree of regularity. The CV of ISI for this neurone was 0.09, and the s.d. of relative ISI was 0.33. B, histogram of relative ISI for an inhibited neurone. The distribution of relative ISI was broad, indicating a large degree of irregularity. The CV of ISI for this neurone was 0.78, and the s.d. of the relative ISI was 1.50. C, s.d. of relative ISI vs. firing rate for 79 stimulated (▪) and inhibited (○) neurones. Stimulated neurones were more likely to have a low s.d. of relative ISI than inhibited neurones (P < 0.0004). Note double log scale. When the CV of ISI was plotted against firing rate, the distribution was similar, and the difference between the two groups was also statistically significant (P < 0.0004).
In any individual neurone, there was a tendency for variability of the ISI to decrease as firing rate increased. Therefore, some of the difference in regularity of firing could be attributed to differences in firing rate, which was slightly higher in stimulated neurones (1.4 ± 1.1 Hz; n = 40) than in inhibited neurones (0.9 ± 0.9 Hz; n = 39). However, differences in firing rate could not account entirely for the difference in variability of the ISI, since stimulated neurones were still more likely to have a low variability of the ISI than inhibited neurones with similar firing rates. These differences in regularity of firing, as well as the shape of the membrane potential trajectory, were most evident when visually comparing membrane potential recordings of stimulated and inhibited neurones at similar firing rates (e.g. Fig. 9A vs. C).
There were no apparent differences between the two types of chemosensitive neurones in AHP level (stimulated: −56.3 ± 8.7 mV, −40.7 to −70.4 mV, n = 18; inhibited: −61.0 ± 6.6 mV, −53.3 to −71.9 mV, n = 9; both groups included neurones with tonic depolarizing current) or spike duration (stimulated: 5.1 ± 2.0 ms, 3.1-10.9 ms, n = 18; inhibited: 4.7 ± 1.7 ms, 3.1-8.5 ms, n = 9). Neurones that were not chemosensitive were heterogeneous in their firing pattern and variability of ISI, with some unresponsive neurones having a highly regular discharge pattern, and others firing more irregularly.
Effect of acid/base changes on membrane potential and spike duration in stimulated neurones
Changes in AHP level, spike threshold and spike duration were measured in response to CO2-induced acid/base disturbances in eighteen stimulated neurones. These variables were measured at CO2 levels of 3, 5 and 9%, and normalized to a change in pH of −0.2 units. There was a depolarization of AHP level of 1.38 ± 1.15 mV (0.07-3.73 mV) per −0.2 pH units. There was also an increase in spike threshold of 0.76 ± 0.7 mV per −0.2 pH units. Spike duration was prolonged from a baseline of 5.1 ± 2.0 ms, by 0.22 ± 0.23 mV per −0.2 pH units. Each of these differences was statistically significant (P < 0.05; Wilcoxon signed rank test).
In brain slices, AHP decreases slightly in stimulated medullary raphe neurones in response to acidosis (Richerson, 1995), the opposite of what occurred in VMM neurones in tissue culture. We initially thought this might represent a difference in the properties of neurones in culture compared with brain slices. In the current experiments tonic depolarizing current injection was used in many neurones to increase firing rate to near 1 Hz at 5% CO2, which is close to the baseline firing rate for these neurones in vivo (Jacobs & Azmitia, 1992; Veasey et al. 1995). In contrast, all recordings from brain slices had been made without any current injection. We hypothesized that the small difference between our findings in culture, and the previous findings in brain slices, might be due to the use of tonic depolarizing current injection. This possibility was supported by the observation that the effect on AHP level with acidosis increased with larger levels of current injection. Thus, the increase in AHP in response to acidosis was 0.69 ± 0.54 mV per −0.2 pH units for neurones with current injection of ≤40 pA (n = 7), and 1.82 ± 1.30 mV per −0.2 pH units for those with current injection of >40 pA (n = 10), which was significantly different (P < 0.05; Student's two-tail t test).
Effect of acid/base changes on resting whole-cell conductance
In peripheral chemoreceptor neurones voltage clamped near resting potential, acidosis causes an inward shift in holding current (Buckler & Vaughan-Jones, 1994). As a first step in characterizing ionic mechanisms of chemosensitivity in VMM neurones, similar experiments were performed to measure changes in resting whole-cell conductance induced by hypercapnic acidosis. In some stimulated neurones, an increase in CO2 led to a small decrease in net outward current, and a decrease in whole-cell conductance.
Figure 11A and B shows the result of a voltage clamp experiment in a stimulated neurone with a CI of 212%. Whole-cell conductance was measured in voltage clamp at a holding potential of −50 mV (zero current level, −57 mV), using −10 mV steps. In response to hypercapnic acidosis, there was a decrease in outward holding current, and a decrease in resting conductance. However, most stimulated neurones had only small and inconsistent changes in resting conductance in response to hypercapnic acidosis, despite large changes in firing rate. The resting conductance in response to acid/base changes (as a percentage of control) was calculated in seven stimulated neurones, using the same analysis as for firing rate. In all stimulated neurones exposed to alkalosis (n = 5), there was an increase in conductance, although it only increased by 5.5 ± 2.0% of control per 0.2 pH units. In two of six neurones exposed to acidosis, there was a statistically significant decrease in conductance to 92% of control, but in the other four neurones the conductance during hypercapnia was not significantly different from the conductance during normocapnia. The change in conductance for those neurones exposed to acidosis (n = 6) was −3.8 ± 3.2% of control. Thus, changes in resting conductance in these neurones were small and inconsistent, as opposed to the large changes in firing rate seen in current clamp.
Figure 11. Acid/base disturbances induced small and inconsistent changes in resting whole-cell conductance.
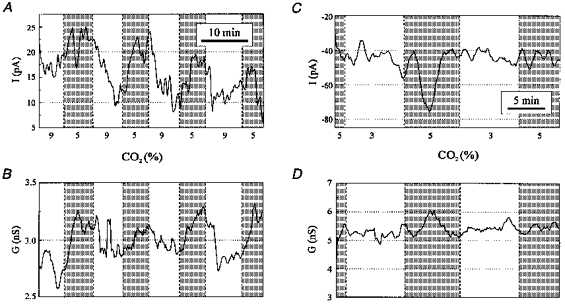
A, stimulated neurone with a CI of 212%. Acidosis induced a decrease in the outward holding current. Recording was made in voltage clamp at a holding potential of −50 mV (resting potential, −57 mV). There was a consistent decrease in outward holding current with hypercapnic acidosis, although the maximum change was only 10 pA. B, whole-cell conductance of the same neurone as in A, measured from a holding potential of −50 mV, with voltage steps to −60 mV. Recording was simultaneous with that in A. Hypercapnic acidosis induced a small decrease in whole-cell conductance to an average of 92% of control. C, inhibited neurone. Alkalosis had no effect on holding current in this inhibited neurone (same neurone as in Fig. 5B). Holding potential, −60 mV (resting potential, −50 mV). D, resting whole-cell conductance of the same neurone as in C, measured from −60 to −70 mV. Recording was simultaneous with that in C. There was no change in whole-cell conductance with hypocapnic alkalosis.
For the neurone shown in Fig. 11A-B, the mean reversal potential of the conductance modulated by acid/base changes calculated using all seven transitions was −81 ± 31 mV. However, during two transitions the change in conductance was small (3 and 4% of control), possibly leading to an error in estimating the reversal potential. When only the five transitions with a change in conductance >6% were used, the reversal potential was −65 ± 15 mV. When the same analysis was applied to other neurones stimulated by respiratory acidosis, the small changes in conductance resulted in a large variability of the estimated reversal potential for each transition. Thus, the ionic selectivity of the chemosensitive current(s) could not be accurately determined in most neurones due to the small changes in conductance.
In inhibited neurones, decreases in firing rate induced by hypercapnic acidosis were accompanied by little or no change in resting whole-cell conductance or holding current. The whole-cell resting conductance and holding current are shown (Fig. 11C and D) for the same inhibited neurone as in Fig. 5B. Despite the large change in firing rate induced by hypocapnic alkalosis in this neurone, there was no change in whole-cell resting conductance or holding current. This was a consistent observation in inhibited neurones, with 7/7 neurones having no significant change in conductance with acid/base changes, and a large variability in response with multiple exposures to acid/base changes. The change in conductance for those neurones exposed to acidosis (n = 6) was −4.7 ± 4.2% of control. The change in conductance for those neurones exposed to alkalosis (n = 7) was 1.7 ± 6.7% of control.
In neurones with no change in firing rate in response to acid/base disturbances, there was also no change in holding current or conductance when CO2 was changed (n = 4).
Morphology of chemosensitive VMM neurones
Neurones cultured from the VMM had a variety of shapes, including fusiform, pyramidal, multipolar and round. For most chemosensitive neurones, the morphology was well visualized, permitting ‘video lucida’ drawings of the soma and proximal neurites. A composite drawing was made of the first twenty-three well-visualized neurones that were stimulated (Fig. 12A) or inhibited (Fig. 12B) by hypercapnic acidosis.
Figure 12. Morphology of stimulated neurones was different from that of inhibited neurones.

A, ‘video lucida’ drawings of the first 10 well-visualized stimulated neurones. Note that all stimulated neurones had medium to large sized somata. Most had irregular cell bodies, often with three major poles. In all cases, there were at least three major processes coming off the soma. Subsequent recordings have shown these features to be highly consistent. B, ‘video lucida’ drawings of the first 13 well-visualized inhibited neurones. Note that all inhibited neurones had small to medium sized somata, and all but one had fusiform cell bodies. In most cases there were two major processes, one coming off each pole of the soma. Subsequent recordings have shown these features to also be highly consistent.
Neurones stimulated by hypercapnic acidosis were all medium to large. These neurones had three or more processes coming directly off an irregularly shaped soma, with a tendency toward a pyramidal shape. The morphology of inhibited neurones was different from stimulated neurones. All but one inhibited neurone had small to medium sized, fusiform somata. In most cases, there were two major processes, one arising from each end. In some cases, there was more than one process at one end, or a minor process coming directly off the soma, but the soma still had a fusiform appearance. One neurone inhibited by hypercapnic acidosis had a medium-sized soma with three relatively large processes coming off the soma giving it a triangular appearance (Fig. 12B, asterisk). There was little overlap in the soma size of the two groups, with the largest inhibited cell (soma width, 22 μm) approximately the same size as the smallest stimulated cell (soma width, 21 μm). Two neurones stimulated by hypercapnic acidosis (Fig. 12A, asterisk and double asterisk) had a slightly fusiform appearance, but both had a medium to large soma, and three larger processes. One of these two neurones (Fig. 12A, double asterisk) responded to hypercapnic acidosis with an increase in firing rate to only 121% of control.
Neurones that were not chemosensitive had a variety of shapes and sizes. Some neurones of the unresponsive group had similar morphology to neurones in the two groups of chemosensitive neurones. Although this made it difficult to use morphology to predict if a cell was chemosensitive, morphology alone could be used to predict whether a neurone would be stimulated or inhibited if it was chemosensitive. During recent experiments it has been possible to use morphology to predict in advance, with a high degree of accuracy, whether a neurone will be stimulated. The differences in morphology seen in the first twenty-three chemosensitive neurones have been highly consistent for all subsequent cells studied.
DISCUSSION
Use of tissue culture for studying chemosensitivity
Most previous work on respiratory control has been done in vivo, in intact brain preparations (Richerson & Getting, 1990; Smith et al. 1991), or in brain slices (Fukuda et al. 1980; Dekin et al. 1985). Tissue culture also offers a number of advantages, and has been widely adopted in neurobiology (Banker & Goslin, 1991). However, it has only seen limited use in the field of respiratory neurophysiology (Neubauer et al. 1991; Rigatto et al. 1992; Wagner & Dekin, 1993; Stea et al. 1995). We began by attempting to use brain slices to obtain quantitative data on chemosensitive neurones, but we found that that approach was not well suited for the purpose of the current experiments. Thus, we have chosen to use a long-term, primary dissociated tissue culture preparation to study central chemosensitivity, for the following reasons: (1) patch clamp seals are more stable, permitting better quantification of responses to pH; (2) bath-applied substances (e.g. CO2, H+, ions and ion channel blockers) can be controlled better at the neuronal membrane in monolayer culture, compared with thick en bloc tissue and brain slices; (3) microdissection completely eliminates synaptic input from neurones outside of the region studied, ensuring that chemosensitivity is intrinsic to that region, which is difficult to prove in more intact preparations even when using solutions designed to block synaptic transmission; (4) neurones have time to recover from the post-traumatic and post-ischaemic changes that are common using brain slices (Richerson & Messer, 1995); and (5) cut dendrites, which could be important for chemosensitivity (Pilowsky et al. 1993), regrow in culture. Finally, development of a tissue culture system for chemosensitive neurones offers the potential to perform a host of future experiments using a variety of modern neurobiological techniques to study the cellular and molecular basis of central chemoreception.
In general, most ‘neurones and glial cells in culture are remarkably similar to neurones and glia in situ’ (Banker & Goslin, 1991). Neuronal properties in culture are more likely to reflect their counterparts in vivo if the neurones are phylogenetically and ontogenetically primitive, which is the case for brainstem respiratory neurones. Intrinsic properties of individual neurones, such as chemosensitivity, are also more likely to be preserved than network properties. However, when using tissue culture there is a concern that the properties of neurones may not be the same as those in vivo. This concern can be alleviated by comparing data from neurones in culture with data from brain slices and in vivo.
There is a large body of existing data on raphe neurones in brain slices and in vivo that can be used to validate our results in tissue culture. Neurones of the medullary raphe have a characteristic firing behaviour in vivo (Jacobs & Azmitia, 1992; Veasey et al. 1995; Mason, 1997) and in brain slices (Richerson, 1995), which is similar to that of dorsal raphe neurones in the mid-brain (Jacobs & Azmitia, 1992). These neurones display repetitive firing with a prominent AHP, and a ramp depolarization between spikes. Firing can occur either spontaneously, or with depolarization or pharmacological treatment (e.g. with adrenergic agonists) (Vandermaelen & Aghajanian, 1983). In a subset of medullary raphe neurones, firing is monotonously regular (Jacobs & Azmitia, 1992). This highly regular firing pattern, with a low CV of ISI, has been correlated with a serotonergic phenotype in vivo (Mason, 1997).
Neurones cultured from the VMM had firing patterns and membrane potential trajectories that were indistinguishable from those seen in the medullary raphe in brain slices and in vivo. Many cultured VMM neurones fired with the highly regular firing pattern typical of serotonergic neurones in vivo (Mason, 1997). Chemosensitivity of cultured VMM neurones was qualitatively similar to neurones in brain slices within the medullary raphe (Richerson, 1995). Thus, these basic properties of medullary raphe neurones appeared to be well preserved in VMM cultures.
We cannot be certain that all electrophysiological properties of cultured raphe neurones were identical to their counterparts in vivo. It is probable that some properties were different from those in brain slices, such as the predominance of GABAergic inhibition in culture, and many aspects of network interactions. The morphology of cultured neurones could not be the same as in vivo, since neurones and glia are grown on a two-dimensional surface and have lost their innervation targets. However, the baseline electrophysiological properties and chemosensitivity were preserved, and these were the properties we were interested in studying. Thus, we conclude that tissue culture is a suitable model system for studying chemosensitivity of raphe neurones. Tissue culture can be used to generate hypotheses, which can then be further tested in more intact systems, including brain slice and in vivo preparations.
Quantification of chemosensitivity of cultured VMM neurones
Respiratory output in vivo increases by 2- to 6-fold in response to a 10 mmHg increase in CO2 (from 5% to ∼6.3%) (St John, 1977; Cunningham et al. 1986). Individual neurones with a similar degree of chemosensitivity have been difficult to identify in vitro. Most in vitro studies have defined a chemosensitive CNS neurone as one that responds to any degree of acidosis with an increase in firing rate to 10–20% above control (i.e. 1.1- to 1.2-fold). That is, chemosensitivity has been considered as an all-or-none neuronal property. In addition, the changes in pH used have sometimes been larger than would occur in vivo, and the criteria used have not always ensured that random changes in firing rate would be excluded. In a previous report a more quantitative approach was used, measuring transient responses of VLM neurones to small changes in pH with extracellular recordings (Jarolimek et al. 1990). Quantification of responses is important, because the degree of chemosensitivity may be relevant to whether individual neurones could play a role in respiratory control in vivo.
We have developed a quantitative approach to studying chemosensitivity, and have used this to characterize the magnitude of steady-state responses of putative central respiratory chemoreceptors to physiologically relevant changes in acid/base status. We found that the VMM contained all cellular elements required for chemosensitivity. In addition, the degree of chemosensitivity to changes in pH between 7.2 and 7.6 approached the sensitivity of the respiratory system as a whole. At a mean firing rate of 1.05 Hz, stimulated neurones were highly sensitive to both increases and decreases in CO2 from a baseline of 5%. Thus, our results are consistent with these neurones having large responses to physiological changes in acid/base status in vivo.
A large response to small acid/base changes is not necessarily required for a neurone to play a role in respiratory chemoreception in vivo. Neurones with small responses to physiological degrees of acidosis could contribute to the output of the respiratory system through summation, or via downstream amplification mechanisms. However, it is unlikely that responses to very large changes in pH are involved in normal chemoreception. Instead, it is possible that some regions function as the primary site of respiratory chemoreception under physiological conditions, while other regions contribute to the systems response under pathological conditions of extreme acidosis. The advantage of quantification of responses, compared with treating chemosensitivity as an all-or-none neuronal property, is that based on the relative degree of chemosensitivity, together with information on the strength of connections with respiratory neurones, inferences can be made about whether a given region is likely to contribute to the normal central chemoreceptor response.
Specificity of chemosensitivity within the VMM
The morphology and baseline firing pattern of stimulated neurones were different from those of inhibited neurones, with large, multipolar neurones with a highly regular firing pattern more likely to be stimulated, and small, bipolar neurones with an irregular firing pattern more likely to be inhibited. This phenotypic specialization suggests that chemosensitivity is programmed into the developmental sequence of specific neurones, resulting in a predictable combination of these properties. This specificity also indicates that chemosensitivity is not a ubiquitous or random property of CNS neurones in culture.
A low interspike variability of medullary raphe neurones in vivo has been found to correlate with immunoreactivity for serotonin (Mason, 1997), suggesting that stimulated neurones contain serotonin. Some neurones without chemosensitivity had similar baseline electrophysiology and morphology to these stimulated neurones. This is consistent with previous observations that only a subset of medullary raphe neurones is responsive to inhaled carbon dioxide in the cat in vivo (Veasey et al. 1995). The medullary raphe has been proposed to play a variety of functional roles, and only a subset of these may involve modulation by pH.
The specificity of chemosensitivity for only a subset of cultured VMM neurones does not address the broader question of whether a high degree of chemosensitivity is unique to the medullary raphe, or to a restricted subset of respiratory nuclei. To date, the degree of intrinsic chemosensitivity to physiological changes in pH seen here has not been reported in other respiratory nuclei. However, in most cases experiments have been performed in brain slices, and chemosensitivity has not been quantified. We found that the degree of chemosensitivity of raphe neurones was qualitatively similar in culture to that in brain slices (Richerson, 1995), but it is possible that the degree of chemosensitivity has been underestimated in other regions due to buffering of acid/base changes, ischaemic/traumatic injury of neurones, and/or loss of dendrites, when using brain slices. To determine whether other regions contain neurones with the same degree of chemosensitivity as in the medullary raphe, and to quantify the potential contributions of each of the putative chemoreceptor areas, it will be important to compare the degree of intrinsic chemosensitivity using recording techniques comparable to the ones we have used here. However, even if a high degree of chemosensitivity is not unique to the raphe, the magnitude of these response to small acid/base changes and the known involvement of the raphe in respiratory control makes it likely that defining the mechanisms of chemosensitivity in these neurones will contribute to our understanding of central chemoreception in vivo.
Cellular mechanisms of chemosensitivity
Chemosensitive neurones of the medullary raphe were sensitive to both hypercapnic (‘respiratory’) acidosis and isocapnic (‘metabolic’) acidosis. Thus, a change in pH alone was sufficient to induce a response. This indicates that a change in PCO2 is not necessary for a response to occur, although gaseous CO2 could still contribute to the response. A change in [HCO3−] occurs with both hypercapnic acidosis and with isocapnic acidosis, although the changes are opposite in the extracellular solution, making it unlikely that the responses resulted from a change in extracellular [HCO3−]. However, changes in intracellular [HCO3−] cannot be predicted. Therefore, further work is needed to study CO2, H+, and HCO3− independently to determine their contributions to the response.
In brain slices, there is a slight hyperpolarization of the AHP level in response to acidosis in stimulated neurones in the absence of current injection (Richerson, 1995). In contrast, in the present study we found that there was a slight depolarization of the AHP level in response to acidosis in stimulated neurones, which was dependent on the amount of tonic depolarizing current injection. In both cases the changes were small and variable, but these results suggest that acidosis inhibits an ion channel with a reversal potential near resting potential. Inhibition of such an ion channel would increase firing rate with little change in AHP level, but the decrease in conductance would lead to greater depolarization during tonic current injection. The reversal potential of the current modulated by pH was difficult to measure accurately due to the small changes in conductance, but if it was indeed −65 mV as indicated in the stimulated neurone with the greatest conductance change, its modulation would have the effect we observed on AHP level. This mechanism would also explain why there was no effect of acidosis on resting potential in neurones without spontaneous firing.
Our voltage clamp results indicated that there was only a small and inconsistent effect on resting whole-cell conductance in stimulated raphe neurones, which was probably not sufficient to explain the large changes in firing rate. This finding is different from what has been observed in chemosensitive neurones of the carotid body (Buckler & Vaughan-Jones, 1994), where there were large changes in resting whole-cell conductance and holding current. This indicates that the mechanisms of chemoreception are different in the two types of chemoreceptors.
The large changes seen in firing rate must have been due to substantial changes in conductance. However, the lack of an effect on resting conductance suggested that the candidate ion channel for chemotransduction was voltage and time dependent, and was not open when membrane potential was held constant at a potential close to resting potential. We propose that the current inhibited by acidosis was activated by repetitive firing. This is true of a variety of candidate channels found in the medullary raphe and dorsal raphe, including A-current (Aghajanian, 1985), calcium current (Burlhis & Aghajanian, 1987), calcium-activated potassium current (Bayliss et al. 1997), inwardly rectifying potassium current (Williams et al. 1988), and a depolarization-activated cation current (Penington & Kelly, 1993). However, none of these currents has a reversal potential near resting potential. If our estimate of reversal potential is accurate, our results are consistent with chemotransduction being due to either an unidentified current, or a combination of more than one of the above currents. Further definition of the cellular mechanisms of chemosensitivity in these neurones will require isolation of individual currents with specific voltage-clamp protocols and pharmacological agents.
Functional role of chemosensitivity in the medullary raphe
The medullary raphe has been associated with a variety of functions, including respiratory control. Neurones of the medullary raphe project to respiratory nuclei, including the NTS, NA, VLM and phrenic motor nucleus (Holtman et al. 1984; Smith et al. 1989; Jacobs & Azmitia, 1992). Electrical stimulation of the medullary raphe can lead to an increase or a decrease in respiratory output, depending on the specific location of the electrode (Lalley, 1986a). Such an effect could result if there were two pools of neurones in the raphe, one that stimulated and one that inhibited respiratory output. It has previously been proposed that CO2-stimulated and CO2-inhibited neurones in the medullary raphe act in a push-pull manner to modulate respiratory output (Richerson, 1995). Thus, although stimulated neurones are more classically linked to a chemoreceptor function, neurones strongly inhibited by acidosis may also play a chemoreceptor role.
The mean firing rate of stimulated neurones was 1.05 ± 0.85 Hz, which is typical of neurones within the raphe nuclei in vivo and in brain slices (Jacobs & Azmitia, 1992; Richerson, 1995; Veasey et al. 1995; Mason, 1997). Three neurotransmitters are colocalized in a subset of medullary raphe neurones: serotonin (5-HT), thyrotrophin-releasing hormone (TRH), and substance P (Holtman et al. 1984; Jacobs & Azmitia, 1992; Dean et al. 1993), each of which stimulates respiratory output (Dekin et al. 1985; Lalley, 1986b; Moss & Inman, 1989). In spinal cord slices, electrical stimulation of axons from the medullary raphe induces release of 5-HT alone at firing rates <1 Hz, 5-HT and TRH are co-released at firing rates between 1 Hz and 3 Hz, and all three neurotransmitters are released at firing rates >3 Hz (Iverfeldt et al. 1989). Thus, the dynamic range over which acid/base changes modulated the firing rate of stimulated cultured VMM neurones overlaps the firing rate of raphe neurones in vivo, and spans the range which differentially affects release of three neurotransmitters with potent effects on respiration.
Until recently (Richerson, 1995), the medullary raphe had not been proposed to contain central respiratory chemoreceptors. In cats in vivo, inhalation of CO2 results in an increase in firing rate of a subset of medullary raphe neurones (Veasey et al. 1995), and increases c-fos expression in the medullary raphe (Larnicol et al. 1994). Microinjections of acetazolamide into the medullary raphe of rats in vivo increases minute ventilation, presumably due to local acidosis (Bernard et al. 1996). Selective ablation of serotonergic neurones in rats in vivo with 5,7-dihydroxytryptamine treatment results in a decrease in baseline minute ventilation, an increase in arterial PCO2 and a reduction in the ventilatory response to increased PCO2 (Mueller et al. 1984). Abnormalities of the human homologue of the medullary raphe have recently been reported in infants who died of sudden infant death syndrome (SIDS; Kinney et al. 1995), which has been linked to a decrease in cardiorespiratory drive and/or arousal in response to a hypercarbic stimulus during sleep (Richerson, 1997). Thus, there is now accumulating in vivo and in vitro evidence that this region may contain a subset of respiratory chemoreceptors.
It is unknown whether the chemosensitive medullary raphe neurones studied here are respiratory chemoreceptors. However, it is likely that these in vitro responses to small changes in pH have functional consequences in vivo. The raphe and serotonin have been linked to a variety of non-respiratory brain functions which are also affected by blood CO2 levels, such as arousal (Jacobs & Azmitia, 1992), seizure threshold (Kovacs & Zoll, 1974; Tecott et al. 1995), cerebrovascular control (Bonvento et al. 1991) and cardiovascular control (Jacobs & Azmitia, 1992). Defining the cellular mechanisms of chemosensitivity in raphe neurones may help to determine how acid/base changes modulate cardiorespiratory and/or other pH-sensitive CNS functions.
Acknowledgments
This work was supported by NIH grant R01HL52539, and by the Veterans Affairs Medical Center (VAMC).
References
- Aghajanian GK. Modulation of a transient outward current in serotonergic neurons by α1-adrenoceptors. Nature. 1985;315:501–503. doi: 10.1038/315501a0. [DOI] [PubMed] [Google Scholar]
- Banker G, Goslin K. Culturing Nerve Cells. Cambridge, MA, USA: MIT Press; 1991. [Google Scholar]
- Bayliss DA, Li YW, Talley EM. Effects of serotonin on caudal raphe neurons: inhibition of N- and P/Q-type calcium channels and the afterhyperpolarization. Journal of Neurophysiology. 1997;77:1362–1374. doi: 10.1152/jn.1997.77.3.1362. [DOI] [PubMed] [Google Scholar]
- Bernard DG, Li A, Nattie EE. Evidence for central chemoreception in the midline raphe. Journal of Applied Physiology. 1996;80:108–115. doi: 10.1152/jappl.1996.80.1.108. [DOI] [PubMed] [Google Scholar]
- Bonvento G, Lacombe P, Mackenzie ET, Fage D, Benavides J, Rouquier L, Scatton B. Evidence for differing origins of the serotonergic innervation of major cerebral arteries and small pial vessels in the rat. Journal of Neurochemistry. 1991;56:681–689. doi: 10.1111/j.1471-4159.1991.tb08203.x. [DOI] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of hypercapnia on membrane potential and intracellular calcium in rat carotid body type I cells. The Journal of Physiology. 1994;478:157–171. doi: 10.1113/jphysiol.1994.sp020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlhis TM, Aghajanian GK. Pacemaker potentials of serotonergic dorsal raphe neurons: Contribution of a low-threshold Ca2+ conductance. Synapse. 1987;1:582–588. doi: 10.1002/syn.890010611. [DOI] [PubMed] [Google Scholar]
- Coates EL, Li A, Nattie EE. Widespread sites of brain stem ventilatory chemoreceptors. Journal of Applied Physiology. 1993;75:5–14. doi: 10.1152/jappl.1993.75.1.5. [DOI] [PubMed] [Google Scholar]
- Cunningham DJC, Robbins PA, Wolff CB. Integration of respiratory responses to changes in alveolar partial pressures of CO2 and O2 and in arterial pH. In: Cherniack NS, Widdicombe JG, editors. Handbook of Physiology, section 3, The Respiratory System, Control of Breathing. II. Bethesda, MD, USA: American Physiologicl Society; 1986. pp. 475–528. [Google Scholar]
- Dean C, Marson L, Kampine JP. Distribution and co-localization of 5-hydroxytryptamine, thyrotropin-releasing hormone and substance-P in the cat medulla. Neuroscience. 1993;57:811–822. doi: 10.1016/0306-4522(93)90026-c. 10.1016/0306-4522(93)90026-C. [DOI] [PubMed] [Google Scholar]
- Dean JB, Bayliss DA, Erickson JT, Lawing WL, Millhorn DE. Depolarization and stimulation of neurons in nucleus tractus solitarii by carbon dioxide does not require chemical synaptic input. Neuroscience. 1990;36:207–216. doi: 10.1016/0306-4522(90)90363-9. 10.1016/0306-4522(90)90363-9. [DOI] [PubMed] [Google Scholar]
- Dekin MS, Richerson GB, Getting PA. Thyrotropin-releasing hormone induces rhythmic bursting in neurons of the nucleus tractus solitarius. Science. 1985;229:67–69. doi: 10.1126/science.3925552. [DOI] [PubMed] [Google Scholar]
- Dillon GH, Waldrop TG. In vitro responses of caudal hypothalamic neurons to hypoxia and hypercapnia. Neuroscience. 1992;51:941–950. doi: 10.1016/0306-4522(92)90531-6. 10.1016/0306-4522(92)90531-6. [DOI] [PubMed] [Google Scholar]
- Feldman JL. Neurophysiology of breathing in mammals. In: Bloom FE, editor. Handbook of Physiology, section 1, The Nervous System, Intrinsic Regulatory Systems of the Brain. IV. Bethesda, MD, USA: American Physiological Society; 1986. pp. 463–524. [Google Scholar]
- Fukuda Y, See WR, Honda Y. H+-sensitivity and pattern of discharge of neurons in the chemosensitive areas of the ventral medulla oblongata of rats in vitro. Pflügers Archiv. 1980;388:53–61. doi: 10.1007/BF00582628. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr, Norman WP, Skirboll L, Dretchen KL, Cuello C, Visser TJ, Hokfelt T, Gillis RA. Evidence for 5-hydroxytryptamine, substance P, and thyrotropin- releasing hormone in neurons innervating the phrenic motor nucleus. Journal of Neuroscience. 1984;4:1064–1071. doi: 10.1523/JNEUROSCI.04-04-01064.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverfeldt K, Serfozo P, Diaz AL, Bartfai T. Differential release of coexisting neurotransmitters: frequency dependence of the efflux of substance P, thyrotropin releasing hormone and [3H]serotonin from tissue slices of rat ventral spinal cord. Acta Physiologica Scandinavica. 1989;137:63–71. doi: 10.1111/j.1748-1716.1989.tb08721.x. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiological Reviews. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jarolimek W, Misgeld U, Lux HD. Neurons sensitive to pH in slices of the rat ventral medulla oblongata. Pflügers Archiv. 1990;416:247–253. doi: 10.1007/BF00392060. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Filiano JJ, Sleeper LA, Mandell F, Valdes-Dapena M, White WF. Decreased muscarinic receptor binding in the arcuate nucleus in sudden infant death syndrome. Science. 1995;269:1446–1450. doi: 10.1126/science.7660131. [DOI] [PubMed] [Google Scholar]
- Kovacs DA, Zoll JG. Seizure inhibition by median raphe nucleus stimulation in rat. Brain Research. 1974;70:165–169. doi: 10.1016/0006-8993(74)90224-8. [DOI] [PubMed] [Google Scholar]
- Lalley PM. Responses of phrenic motoneurones of the cat to stimulation of medullary raphe nuclei. The Journal of Physiology. 1986a;380:349–371. doi: 10.1113/jphysiol.1986.sp016290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM. Serotoninergic and non-serotoninergic responses of phrenic motoneurones to raphe stimulation in the cat. The Journal of Physiology. 1986b;380:373–385. doi: 10.1113/jphysiol.1986.sp016291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larnicol N, Wallois F, Berquin P, Gros F, Rose D. c-fos-like immunoreactivity in the cat's neuraxis following moderate hypoxia or hypercapnia. Journal de Physiologie. 1994;88:81–88. doi: 10.1016/0928-4257(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Harikka J, da Penha Berzaghi M, Castren E, Tzimagiorgis G, Hughes RA, Thoenen H. Fibroblast growth factor-5 promotes differentiation of cultured rat septal cholinergic and raphe serotonergic neurons - comparison with the effects of neurotrophins. European Journal of Neuroscience. 1994;6:244–252. doi: 10.1111/j.1460-9568.1994.tb00267.x. [DOI] [PubMed] [Google Scholar]
- Mason P. Physiological identification of pontomedullary serotonergic neurons in the rat. Journal of Neurophysiology. 1997;77:1087–1098. doi: 10.1152/jn.1997.77.3.1087. [DOI] [PubMed] [Google Scholar]
- Mitchell RA, Loeschcke HH, Massion WH, Severinghaus JW. Respiratory responses mediated through superficial chemosensitive areas on the medulla. Journal of Applied Physiology. 1963;18:523–533. doi: 10.1152/jappl.1963.18.3.523. [DOI] [PubMed] [Google Scholar]
- Moss IR, Inman JG. Neurochemicals and respiratory control during development. Journal of Applied Physiology. 1989;67:1–13. doi: 10.1152/jappl.1989.67.1.1. [DOI] [PubMed] [Google Scholar]
- Mueller RA, Towle AC, Breese GR. Supersensitivity to the respiratory stimulatory effect of TRH in 5,7-dihydroxytryptamine-treated rats. Brain Research. 1984;298:370–373. doi: 10.1016/0006-8993(84)91440-9. [DOI] [PubMed] [Google Scholar]
- Neubauer JA, Gonsalves SF, Chou W, Geller HM, Edelman NH. Chemosensitivity of medullary neurons in explant tissue cultures. Neuroscience. 1991;45:701–708. doi: 10.1016/0306-4522(91)90282-s. [DOI] [PubMed] [Google Scholar]
- Penington NJ, Kelly JS. Ionic dependence of a slow inward tail current in rat dorsal raphe neurones. The Journal of Physiology. 1993;464:33–48. doi: 10.1113/jphysiol.1993.sp019623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilowsky P, Llewellynsmith IJ, Arnolda L, Lipski J, Minson J, Chalmers J. Are the ventrally projecting dendrites of respiratory neurons a neuroanatomical basis for the chemosensitivity of the ventral medulla oblongata. Sleep. 1993;16:S53–55. [PubMed] [Google Scholar]
- Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience. 1997;77:723–743. doi: 10.1016/s0306-4522(96)00485-x. [DOI] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. Journal of Neuroscience Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Response to CO2 of neurons in the rostral ventral medulla in vitro. Journal of Neurophysiology. 1995;73:933–944. doi: 10.1152/jn.1995.73.3.933. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Sudden infant death syndrome: The role of central chemosensitivity. Neuroscientist. 1997;3:3–7. [Google Scholar]
- Richerson GB, Getting PA. Preservation of integrative function in a perfused guinea pig brain. Brain Research. 1990;517:7–18. doi: 10.1016/0006-8993(90)91001-w. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Messer C. Effect of composition of experimental solutions on neuronal survival during rat brain slicing. Experimental Neurology. 1995;131:133–143. doi: 10.1016/0014-4886(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W. Ventromedial medullary neurons have a high degree of intrinsic chemosensitivity. Society for Neuroscience Abstracts. 1997;23:434. [Google Scholar]
- Rigatto H, Fitzgerald SC, Willis MA, Yu C. In search of the central respiratory neurons: II. Electrophysiologic studies of medullary fetal cells inherently sensitive to CO2 and low pH. Journal of Neuroscience Research. 1992;33:590–597. doi: 10.1002/jnr.490330411. [DOI] [PubMed] [Google Scholar]
- Sato M, Severinghaus JW, Basbaum AI. Medullary CO2 chemoreceptor neuron identification by c-fos immunocytochemistry. Journal of Applied Physiology. 1992;73:96–100. doi: 10.1152/jappl.1992.73.1.96. [DOI] [PubMed] [Google Scholar]
- Schlaefke ME, See WR, Loeschcke HH. Ventilatory response to alterations of H+ ion concentration in small areas of the ventral medullary surface. Respiration Physiology. 1970;10:198–212. doi: 10.1016/0034-5687(70)90083-6. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. Journal of Comparative Neurology. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- St John WM. Integration of peripheral and central chemoreceptor stimuli by pontine and medullary respiratory centers. Federation Proceedings. 1977;36:2421–2427. [PubMed] [Google Scholar]
- Stea A, Jackson A, Macintyre L, Nurse CA. Long-term modulation of inward currents in O2 chemoreceptors by chronic hypoxia and cyclic AMP in vitro. Journal of Neuroscience. 1995;15:2192–2202. doi: 10.1523/JNEUROSCI.15-03-02192.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Research. 1983;289:109–119. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. Journal of Neuroscience. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner PG, Dekin MS. GABAb receptors are coupled to a barium-insensitive outward rectifying potassium conductance in premotor respiratory neurons. Journal of Neurophysiology. 1993;69:286–289. doi: 10.1152/jn.1993.69.1.286. [DOI] [PubMed] [Google Scholar]
- Williams JT, Colmers WF, Pan ZZ. Voltage- and ligand-activated inwardly rectifying currents in dorsal raphe neurons in vitro. Journal of Neuroscience. 1988;8:3499–3506. doi: 10.1523/JNEUROSCI.08-09-03499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


