Abstract
In outside-out patches excised from human embryonic kidney (HEK) 293 cells expressing Ca2+-permeable α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate receptor (AMPAR) channels, currents activated by 1 ms glutamate pulses at negative membrane potentials facilitated during and following a repetitive (2 to 100 Hz) agonist application. The degree of facilitation depended on subunit type, membrane potential and stimulation frequency being antagonized by a slow recovery from desensitization.
Activity-dependent current facilitation occurred in Ca2+-permeable but not in Ca2+-impermeable AMPAR channels. Current facilitation, however, does not depend on Ca2+ flux. Rather it reflects a relief from the block of Ca2+-permeable AMPARs by intracellular polyamines since facilitation occurred only in the presence of polyamines and since facilitated currents had a nearly linear current-voltage relation (I-V).
Relief from polyamine block was use dependent and occurred mainly in open channels. The relief mechanism was determined primarily by membrane potential rather than by current flow.
In closed channels the degree of polyamine block was independent of membrane potential. The voltage dependence of the rate of relief from the block in open channels rather than the voltage dependence of the block underlies the inwardly rectifying shape of the I-V at negative potentials.
Currents through native Ca2+-permeable AMPAR channels in outside-out or nucleated patches from either hippocampal basket cells or a subtype of neocortical layer II nonpyramidal cells also showed facilitation.
It is concluded that a use-dependent relief from polyamine block during consecutive AMPAR channel openings underlies current facilitation. This polyamine-AMPAR interaction may represent a new activity-dependent postsynaptic mechanism for control of synaptic signalling.
At glutamatergic synapses in the mammalian central nervous system (CNS), postsynaptic currents mediated by AMPAR channels may be rapidly facilitated or depressed during high frequency or paired-pulse stimulation. Short-term synaptic enhancement occurring on the time scale of tens of milliseconds to several seconds is thought to be due to a short-lasting increase in transmitter release (Zucker, 1994; Fisher et al. 1997). Postsynaptic mechanisms regulating short-term synaptic signalling are less well defined. At certain synapses, a slow recovery from desensitization may contribute to current depression (Trussell et al. 1993). However, no postsynaptic mechanism is known which can lead to a rapid, short-term enhancement of AMPAR-mediated currents.
A number of neurons in the CNS express AMPAR channels which are highly Ca2+-permeable and are blocked by endogenous intracellular polyamines. It is generally assumed that polyamine block of AMPARs is voltage dependent, getting stronger with positive potentials and being relieved at very positive (> +40 mV) potentials producing a characteristic doubly rectifying I-V (Bowie & Mayer, 1995; Koh et al. 1995a; Kamboj et al. 1995; Barnes-Davies & Forsythe, 1996). Heterologous expression of cloned AMPAR subunits has shown that homomeric channels assembled from GluR-A, -C, -D or unedited GluR-B(Q) subunits, which contain glutamine (Q) at the functionally critical Q/R site in the M2 segment, are Ca2+-permeable and are blocked by intracellular polyamines. On the other hand, AMPAR channels containing the edited GluR-B(R) subunit, which have arginine (R) at the Q/R site, are Ca2+-impermeable and insensitive to polyamines. Indeed, in native and heteromeric recombinant channels, Ca2+ permeability and sensitivity to block by intracellular polyamines are functionally determined by the expression level of the GluR-B(R) subunit (Jonas & Burnashev, 1995).
Mechanisms controlling the short-term regulation of AMPAR-mediated currents are of interest because they relate to how the postsynaptic cell encodes presynaptic activity. Nevertheless, molecular mechanisms controlling synaptic signalling are often difficult to identify at the synapse. To study AMPAR channel facilitation, independently of any presynaptic contribution, we mimicked synaptic transmission by applying brief pulses of glutamate to outside-out patches excised from HEK 293 cells expressing different AMPAR subunits or to outside-out and nucleated patches from identified hippocampal and neocortical neurons in brain slices. We find that currents through Ca2+-permeable AMPARs facilitate during and following repetitive stimulation (> 1 Hz). The facilitation does not depend on Ca2+ influx but arises by a use-dependent relief of the block by intracellular polyamines. This polyamine-dependent facilitation may represent a mechanism of enhancing AMPAR-mediated currents which would be entirely postsynaptic in origin.
Some of the results have been reported in abstract form (Rozov et al. 1997).
METHODS
Transverse slices of 200–300 μm thickness were prepared from the brains of 12- to 14-day-old Wistar rats killed by decapitation. Cells were identified visually using infrared differential contrast video microscopy (Stuart et al. 1993) and according to their firing pattern following current injection (Koh et al. 1995b). All recordings from recombinant AMPARs were made from HEK 293 cells transiently (GluR-D, GluR-A/B(R), GluR-B(N)) or stably (GluR-A, GluR-B(Q)) expressing AMPAR subunits. Recordings from transiently expressing cells were made 2 days after transfection (Burnashev et al. 1992). Experiments with stably expressing cells were made 1–2 days after plating. All subunits tested were in the ‘flip’ form (Sommer et al. 1990).
Glutamate (1–3 mM) was applied using a piezo-controlled (P 245.70, Physik Instrumente, Waldbronn, Germany) fast application system with a double-barrel application pipette (Colquhoun et al. 1992). Unless otherwise noted, durations of the glutamate pulses were 1 ms for outside-out patches (Hamill et al. 1981) and 2 ms for nucleated patches (Sather et al. 1992). Recordings of the I-V using voltage ramps were made as in Burnashev et al. 1992. Currents were recorded using an EPC-7 amplifier with PULSE software (HEKA Elektronik, Lambrecht, Germany), filtered at 3–5 kHz bandwidth (−3 dB) with a 8 pole low pass Bessel filter and digitized at 10–20 kHz. All analysis was done off-line using IGOR PRO (WaveMetrics, Inc., Lake Oswego, OR). Mean data are given as means ±s.e.m. unless otherwise noted. All recordings were made at room temperature (22–24°C).
The standard extracellular solution was (mM): 135 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 10 Hepes (pH 7.2 with NaOH). In some experiments, the same solution without divalents was used to which 10 mM CaCl2 or 10 mM MgCl2 was added. In experiments with variable Na+ concentrations, the reference extracellular solution contained (mM): 135 NaCl, 1.8 CaCl2, 5 Hepes (pH 7.2 with NaOH). In solutions with a reduced Na+ concentration, the 135 NaCl was replaced by either 30 mM NaCl and 105 mM N-methyl-D-glucamine (NMDG) or 10 mM NaCl and 125 mM NMDG. Patches isolated from brain slices were recorded in the presence of 50 μM D-2-amino-5-phosphonopentanoic acid (D-AP5).
Two intracellular solutions were used (mM): (a) 135 NaCl, 0.5 EGTA, 4 Mg-ATP, 5 Hepes (pH 7.2 with NaOH); or (b) 110 KCl, 0.5 EGTA, 20 Na2-ATP, 5 Hepes (pH 7.2 with NaOH). The ‘b’ solution was used to achieve polyamine-free conditions; spermine was added to this solution in concentrations of 7, 25, 50 or 100 μM yielding approximate free concentrations of 0.5, 2, 3.9 or 7.7 μM, respectively (Watanabe et al. 1991). Mg2+ and polyamines competitively bind to ATP but because Mg2+ is bound preferentially (Frausto da Silva & Williams, 1993), solution ‘a’ chelates polyamines much less efficiently than solution ‘b’.
RESULTS
Subunit-specific and frequency-dependent facilitation of AMPAR-mediated currents
In outside-out patches excised from HEK 293 cells expressing Ca2+-permeable AMPAR subunits, 1 ms applications of glutamate induced inward currents which rapidly deactivated. The amplitudes of these currents remained unchanged during trains of low frequency activation (< 1 Hz) (data not shown). However, with application frequencies of 2 Hz or greater, current amplitudes increased depending on subunit type and stimulation frequency (Fig. 1). Figure 1A (left and middle) illustrates trains of repetitive activation of Ca2+-permeable GluR-D channels at −60 mV. At both 14 and 33 Hz, the amplitudes of the currents gradually enhanced or facilitated relative to the initial amplitude in the train (dashed line) reaching a steady-state level of facilitation near the end of the train. After a 5 s delay without activation, currents returned to their initial amplitude. The degree of steady-state facilitation depended on stimulation frequency being, on average, 32 ± 1.3 % (n = 4) at 14 Hz and 10 ± 1.4 % (n = 6) at 33 Hz. Currents through Ca2+-permeable unedited GluR-B(Q) channels also facilitated (Fig. 1B) at 14 Hz (38 ± 1.4 %, n = 7) and at 33 Hz (29 ± 3.3 %, n = 9).
Figure 1. Activity-dependent facilitation of current through Ca2+-permeable AMPARs.
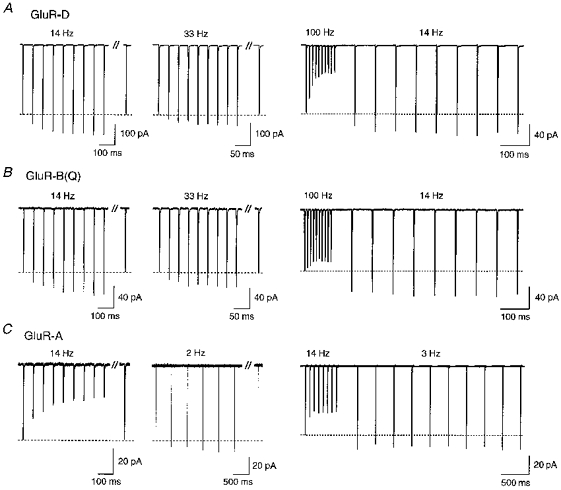
Glutamate-activated currents (1 ms application of 3 mM glutamate) in outside-out patches isolated from HEK 293 cells expressing Ca2+-permeable GluR-D (A), GluR-B(Q) (B) or GluR-A (C) channels. All measurements were made within 5 min after patch formation using pipette solution ‘a’. A, GluR-D channels. Current amplitudes elicited by glutamate applications facilitated relative to that of the first current in the train (dashed line) by 28 and 13 % at 14 and 33 Hz, respectively. Delayed currents separated by the double slash were recorded approximately 5 s after the end of the train. Right trace, glutamate was initially applied at 100 Hz, then, following a delay of 70 ms, at 14 Hz. With the decrease in application frequency, the steady-state facilitation was 29 %. B, GluR-B(Q) channels. At 14 and 33 Hz stimulation, steady-state facilitation was 35 and 24 %, respectively. Right trace, sustained facilitation was 38 %. C, GluR-A channels. At 14 Hz stimulation currents decayed (left trace), but facilitated at 2 Hz stimulation (17 %). Right trace, glutamate was initially applied at 14 Hz, then at 3 Hz where currents were facilitated by 17 %. In all experiments shown, the membrane potential was −60 mV. Each trace is the average of 5–10 current sweeps.
For both GluR-D and GluR-B(Q) channels, current amplitudes depressed at 100 Hz stimulation (right panels in Fig. 1A, B) reflecting that desensitization dominates current amplitudes at this higher frequency. However, following a delay of 70 ms, which is sufficient time for most of the channels to recover from desensitization (see below), current amplitudes were facilitated, and remained facilitated at a lower (14 Hz) stimulation rate, being increased by 22 ± 5.6 % (n = 3) and 38 ± 3.8 % (n = 21) in GluR-D and GluR-B(Q) channels, respectively. The influence of desensitization on current amplitudes is seen more strongly in Ca2+-permeable GluR-A channels which show the slowest recovery from desensitization of all ‘flip’ form subunits (A. Rozov & N. Burnashev, unpublished data). Consistent with this, currents in GluR-A channels declined at 14 Hz but facilitated at lower application frequencies (2 Hz, Fig. 1C) or at 33 Hz in the presence of cyclothiazide (see Fig. 12). Further, as in GluR-D and GluR-B(Q) channels, a high frequency train of glutamate pulses induced a sustained current facilitation at a lower stimulation rate in GluR-A channels (21 ± 2.4 %, n = 9; Fig. 1C, right panel) but less frequent applications were necessary in order to get comparable effects. In contrast to Ca2+-permeable AMPAR channels, currents in Ca2+-impermeable heteromeric GluR-A/B(R) channels (excess of cDNA for GluR-B(R) subunit) did not facilitate (n = 9) at any stimulation frequency (0.5-100 Hz; data not shown).
Figure 12. Polyamine-dependent current facilitation counteracts channel desensitization.
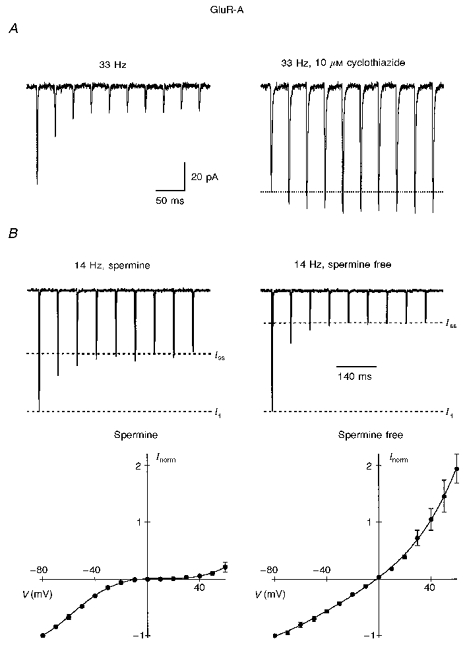
A, currents in GluR-A channels were recorded within 5 min after formation of outside-out patch using intracellular solution ‘a’. At 33 Hz stimulation, current amplitudes strongly decayed (left trace). After partial removal of desensitization by 10 μM cyclothiazide, currents in the same patch facilitated by 21 % at the same stimulation frequency. Membrane potential was −60 mV. B, spermine attenuates current depression at the end of a train. Currents were recorded using intracellular solution ‘b’ with either 7 μM spermine (left) or no spermine (right) added. Steady-state current amplitude at the end of a train (Iss) with spermine was 71 % larger than without spermine. For each cell, 5–10 current traces at −60 mV were averaged and then normalized to the amplitude of the first current in the train (I1; left, 5 cells; right, 3 cells). Buffering of spermine was verified by the shape of the I-V (lower panel) which was inwardly rectifying in the presence of spermine (left) but outwardly rectifying in the spermine-free solution (right). (It should be noted that GluR-A channels are the most sensitive AMPAR subunits to polyamines that we have tested; see also Bowie and Mayer, 1995.)
In summary, currents in Ca2+-permeable but not Ca2+-impermeable AMPARs show an activity-dependent facilitation. Depending on desensitization properties, the same stimulation frequency may induce either current facilitation or depression. Since facilitation occurs in the absence of any presynaptic contribution as well as any other ion channels, it presumably is a property of Ca2+-permeable AMPARs. To identify its mechanism, we primarily studied GluR-B(Q) channels stably expressed in HEK 293 cells. Currents through these channels, as in other Ca2+-permeable AMPAR subunits, facilitate but show a rapid recovery from desensitization (τ < 5 ms) allowing for extensive manipulations without the use of cyclothiazide.
Current facilitation is not triggered by Ca2+ or Mg2+ ions
Facilitation was observed only in Ca2+-permeable AMPAR channels. Ca2+ influx, however, is not required for facilitation. Figure 2 shows repetitive activation (14 Hz) of GluR-B(Q) channels in the same patch bathed either in the standard extracellular solution with 10 mM Ca2+ or the same solution without added divalents. Currents facilitated in both instances and to the same degree, showing a steady-state facilitation of 23.3 ± 11 % and 22.5 ± 6.6 % (n = 4), respectively. Similar results were obtained in 10 mM Mg2+ (16.4 ± 5.1 %) and nominally Mg2+-free solutions (17.4 ± 2 %) measured within the same cells (n = 6). In addition, facilitation still occurred (15 ± 4.3 %, n = 3, 33 Hz) even in divalent-free extracellular (2.5 mM EGTA and 2.5 mM EDTA) and intracellular (10 mM EGTA and 10 mM EDTA) solutions. Additional evidence supporting the idea that Ca2+ influx is not the factor inducing facilitation is the observation that, in the presence of 1.8 mM extracellular Ca2+, currents facilitated more strongly at −40 mV than at −80 mV (44 ± 5.7 %vs. 16 ± 3.7 %, n = 8 for each, 33 Hz) in direct contrast to what would be expected if Ca2+ influx was the trigger.
Figure 2. Calcium influx is not required to induce facilitation.
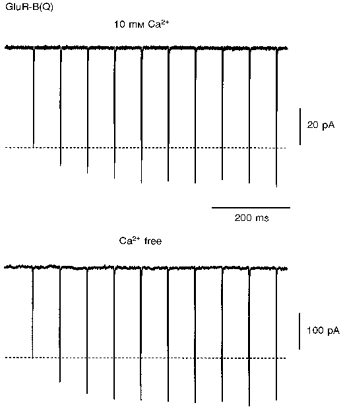
Glutamate-activated currents in an outside-out patch containing GluR-B(Q) channels recorded within 5 min after patch formation using pipette solution ‘a’. The patch was bathed either in the standard extracellular solution with added 10 mM Ca2+ (upper trace) or the same solution with no added divalents (lower trace). Currents facilitated to a similar extent (steady-state facilitation was 38 and 45 %, respectively; 14 Hz stimulation). Recordings were made first in the 10 mM Ca2+ solution. Holding potential was −60 mV. Note the smaller current size in 10 mM Ca2+.
Polyamine sensitivity underlies AMPAR facilitation
Ca2+-permeable AMPAR channels are blocked by endogenous polyamines in a voltage-dependent manner producing a characteristic doubly rectifying I-V. High Ca2+ permeability and block by polyamines are dissociated by substituting histidine (H) (Curutchet et al. 1992) or asparagine (N) (Burnashev et al. 1992) at the Q/R site in the M2 region of AMPAR subunits. Mutant GluR-B(N) channels have a high Ca2+ permeability, but a linear I-V, reflecting the fact that they are insensitive to polyamines (Burnashev et al. 1992; Koh et al. 1995a). Currents in these channels do not facilitate (data not shown), suggesting that facilitation may be related to polyamine sensitivity.
In outside-out patches, the doubly rectifying I-V of polyamine-sensitive AMPAR channels linearizes over time due to washout of intracellular polyamines (Kamboj et al. 1995; Koh et al. 1995a; Bowie & Mayer, 1995; Isa et al. 1996). Figure 3A illustrates that the I-V of GluR-B(Q) channels measured within 1 min after patch formation was doubly rectifying (open circles) and currents facilitated upon repetitive glutamate application (upper trace). Fifteen minutes later, the I-V was more linear (filled circles) and currents now no longer facilitated. When the endogenous polyamine spermine, a high affinity channel blocker (Kamboj et al. 1995), was added to the pipette solution (Fig. 3B), the shape of the I-V and facilitation properties remained essentially unaffected during the recording time (up to 40 min). Thus, the presence of intracellular polyamines appears necessary for facilitation to occur.
Figure 3. Facilitation depends on intracellular polyamines.
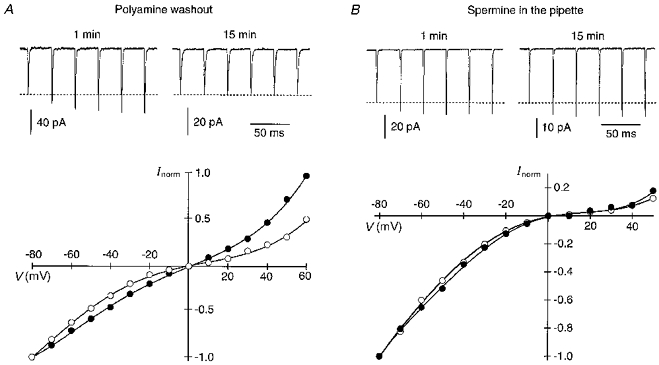
A, washout of intracellular polyamines results in linearization of the I-V relation and disappearance of facilitation. Upper traces, currents in GluR-B(Q) channels, recorded around 1 min after patch formation, using pipette solution ‘b’, showed a 40 % steady-state facilitation (33 Hz) whereas 15 min later in the same patch, facilitation was no longer present. Holding potential was −60 mV. Lower panel, I-V relations, normalized to the current amplitudes at −80 mV, correspond to currents measured around 1 min (○) and 15 min (•) after patch formation (I-V recorded at 0.2 Hz). Qualitatively similar results were obtained in 11 patches. B, facilitation does not washout when spermine is added to the intracellular solution (pipette solution ‘b’ with added spermine (25 μM)). Same experimental protocol as in A. Upper traces, currents showed a steady-state facilitation of 23 and 29 % at 1 and 15 min, respectively, after patch formation. Lower panel, I-V relations correspond to currents measured around 1 min (○) and 15 min (•) after patch formation. Facilitation did not wash out in all 25 patches tested. Note that in both A and B the amplitude of the first current in the trains at 15 min was smaller due to current rundown which occurred during relatively long-lasting experiments regardless of the absence or presence of spermine in the pipette (in both instances, currents are reduced by about 50 %).
Facilitation arises from a relief from polyamine block
Current amplitudes in GluR-B(Q) channels typically depress during a 100 Hz train of glutamate pulses but following a delay are facilitated (Fig. 1B, right panel). During the delay, channels recover from desensitization, a process that apparently occurs more quickly than that mediating recovery from facilitation. We took advantage of this difference in time course to further characterize the mechanism underlying current facilitation in AMPAR channels.
Figure 4A compares the I-V of facilitated currents with that of unfacilitated or control currents in GluR-B(Q) channels recorded with spermine in the pipette. To obtain the control I-V (open circles), patches were held at −80 mV and 20 ms after the step to the test potential (−80 to +60 mV), a 1 ms pulse of glutamate was applied. As expected for channels blocked by spermine, the control I-V was doubly rectifying. In contrast, the I-V for test currents (filled circles) measured 180 ms after a 100 Hz train of glutamate pulses applied at −80 mV (see inset) was nearly linear. Figure 4B shows the relative increase in the amplitude of facilitated (If) versus control (Ic) currents as a function of the test potential. The relative current enhancement, (If - Ic)/Ic, which is an index of the extent to which channels are unblocked at each potential compared with the control I-V, gets stronger with more positive potentials reaching a maximum at +30 mV, and then is reduced at more positive potentials. This pattern of enhancement closely parallels the presumed affinity of polyamines for the channels (cf. Bowie & Mayer, 1995). Thus, as indexed by the shape of the I-V, facilitated currents reflect a reduced polyamine block. Apparently, the high frequency train of glutamate pulses at negative potentials relieves channels from polyamine block.
Figure 4. High frequency stimulation relieves AMPAR channels from polyamine block.
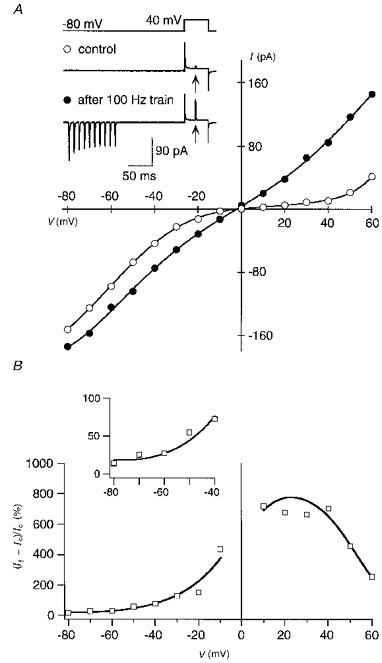
Currents in GluR-B(Q) channels were recorded with 25 μM spermine added to intracellular solution ‘b’. A, I-V relations for control (○) and facilitated currents (•). In control, glutamate was applied 20 ms after stepping the potential from −80 mV to various test potentials (−80 to +60 mV) at 0.2 Hz. An identical voltage protocol was used for the facilitated currents except that a 100 Hz train (10 pulses) of glutamate was applied at −80 mV (conditioning glutamate pulses), 160 ms before the step to the test potential. Inset, example recordings at a test potential of +40 mV; arrows indicate glutamate application during the test step. For the I-V, smooth lines are fitted 6th (control) or 8th order polynomials, and each point represents the mean of 10 sweeps. B, relative increase in current amplitudes for facilitated currents at different test potentials (data from I-V s shown in A). Ic, control currents; If, facilitated currents. Smooth lines are derived from the fitted polynomials, whereas symbols (□) correspond to the experimental points. Data between −10 and +10 mV are omitted. Inset shows data points in the potential range from −80 to −40 mV on an expanded vertical scale. Similar results were obtained in 15 other experiments.
Polyamines block closed AMPAR channels
Facilitated currents return to their initial amplitude within about 5 s after the end of a train (Fig. 1, left and middle traces), suggesting that during this time closed channels are reblocked by polyamines. On the other hand, the results in Fig. 4A suggest that the process of reblock of closed channels must take longer than 180 ms (time between the end of the high frequency train and test application in the inset). To quantify the time course of reblock of closed channels, we stimulated GluR-B(Q) channels with a 14 Hz train of glutamate pulses at −80 mV, and then measured the amplitude of a test current at +40 mV or at −60 mV following a variable time period (Fig. 5). The decline of test current amplitudes with increasing time was approximated by a single exponential with time constants of 118 ± 12 and 375 ± 27 ms, (n = 6) at +40 and −60 mV, respectively. Thus, closed channels are blocked by spermine at both positive and negative membrane potentials with the rate of reblock being strongly voltage dependent. These results also indicate that polyamine block of AMPAR channels does not act as a ‘classical’ open channel blocker (see Hille, 1992).
Figure 5. Rate of polyamine reblock of closed AMPAR channels.
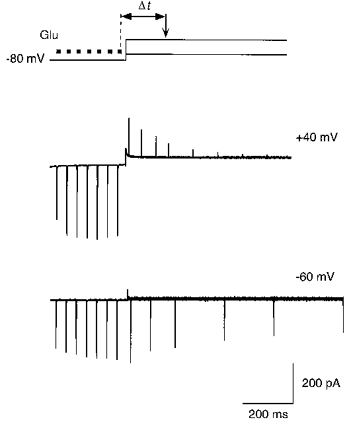
Currents in GluR-B(Q) channels recorded with 25 μM spermine added to intracellular solution ‘b’. Top panel, voltage protocol for measuring the time course of reblock of closed channels. A conditioning train of glutamate pulses (▪; 7 pulses at 14 Hz) was applied at −80 mV. Test glutamate pulses (arrow) were then applied at variable time intervals (Δt) after the end of the conditioning train, either at +40 or −60 mV. The shortest delay between the end of the last conditioning glutamate application and the test application was 70 ms. Middle and lower panels, overlaid current traces recorded as indicated in the top panel, at test potentials of +40 mV (middle) and −60 mV (lower). Note the decrease in the test current amplitudes with the longer time intervals. Capacitance transients occurring with the voltage step were cut off.
The results in Fig. 5 clarify why currents show facilitation even after a delay (e.g. inset to Fig. 4A). During the high frequency train, channels are both relieved from block and desensitized. With the delay between the end of the conditioning train of glutamate pulses and the test pulse, however, the channels recover from desensitization; for GluR-B(Q) channels, the recovery from desensitization occurs within 10–15 ms meaning that with a 180 ms delay more than 99 % of the channels have recovered. In contrast, the process of reblock takes much longer either during the conditioning potential (a 160 ms delay was present before stepping to the test potential, but for reblock τ≈ 375 ms) or during the test potential (test pulse occurred 20 ms after the step, but for reblock τ≈ 110 ms). Also, at positive potentials, open channels are reblocked much faster (see Koh et al. 1995a). We estimated the rate of reblock for open, cyclothiazide treated GluR-B(Q) channels using a similar voltage protocol as in Koh et al. 1995a (data not shown). At +30 mV, τ≈ 3.3 ms, but again this rate of block is much too slow for channels to be significantly reblocked during the 1 ms application time. Nevertheless, since some reblock does occur, the measurement of current amplitude at the test potential underestimates the degree of relief from block. Still, it represents an index of the degree to which channels are blocked by polyamines during the conditioning potential.
Relief from polyamine block occurs primarily in open channels
To examine the voltage dependence of the relief from block, we compared the amplitude of a test current at +30 mV following different conditioning potentials (Fig. 6). During the conditioning potential, channels were either closed (Fig. 6A, upper trace) or activated by a single glutamate pulse (lower trace). Without the glutamate pulse, the amplitude of the test current was small and independent of membrane potential (Fig. 6B, filled circles), suggesting that even at very negative potentials little relief of block occurs in closed channels. In contrast, a single pulse of glutamate strongly relieved channels from the block with the extent of this relief dependent on membrane potential, getting stronger at more negative potentials (open circles). Thus, relief from polyamine block occurs primarily when channels are in the open state.
Figure 6. Relief from block in open and closed AMPAR channels.
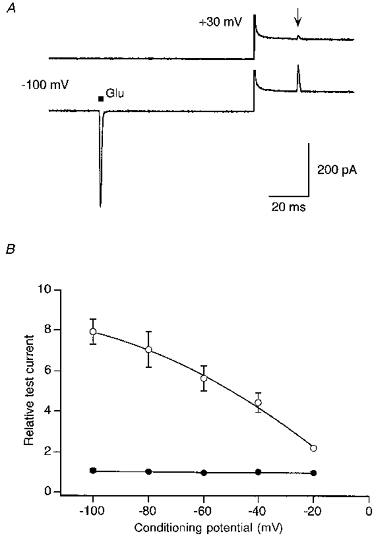
Currents in GluR-B(Q) channels recorded with 50 μM spermine added to intracellular solution ‘b’. A, current traces were generated using a voltage jump protocol similar to that shown in the inset of Fig. 4A except that the test potential was at +30 mV (outward going currents) and the conditioning potential was −100 mV. The delay between the conditioning (▪) and the test (arrow) glutamate application was 100 ms with an 80 ms delay occurring before the voltage step. Note that even with the very negative conditioning potential, closed channels are significantly blocked by spermine as indexed by the current amplitude without (upper trace) and with a single conditioning pulse of glutamate (lower trace). Capacitance transients were cut off. B, relative test currents amplitudes, measured at +30 mV, with (•) or without (○) a single glutamate pulse applied at various conditioning potentials. Mean data from 4–7 outside-out patches. To average the results across patches, we normalized all current amplitudes to the amplitude of their respective control test current at −40 mV. For the control recordings, the error bars are smaller than the symbols.
Time course of the relief from polyamine block is voltage dependent
The control recording in Fig. 6 (filled circles) suggests that closed channels are blocked to almost the same extent at negative potentials. Hence, the negative limb of the doubly rectifying current-voltage relationship of Ca2+-permeable AMPARs, using a single pulse of glutamate at each potential, apparently reflects how many channels are relieved from the block during the glutamate pulse rather than the voltage dependence of the block of closed channels. Such a process requires, however, that the rate of unblock is also voltage dependent. Figure 7A compares the activation time course of currents in GluR-B(Q) channels at three different membrane potentials. Clearly, the time course of current activation is voltage dependent, being slower at −20 mV than at −100 mV. The voltage dependence of this action is shown in Fig. 7B (filled symbols). In contrast, in the absence of polyamines, currents were activated with the same time course at all potentials (open symbols). These results strongly support the idea that the shape of the I-V of Ca2+-permeable AMPAR channels at negative potentials reflects the potential dependence of relief from block rather than the degree of polyamine block of closed channels.
Figure 7. In the presence of spermine, the time course of current activation depends on the membrane potential.
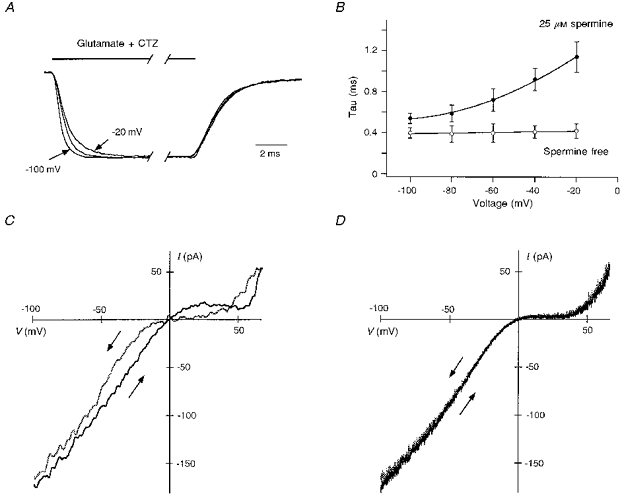
Currents in GluR-B(Q) channels recorded with 25 μM spermine added to intracellular solution ‘b’. A, currents recorded in the presence of 100 μM cyclothiazide at −20, −60 (middle trace) and −100 mV. Glutamate was applied for 50 ms, and currents were normalized to their steady-state amplitude at the end of the pulse. Note that the decay of currents is identical at all potentials. B, voltage dependence of the time course of current activation in the presence (•) or absence (○) of spermine in the recording pipette. In spermine-free conditions (recorded with intracellular solution ‘b’ without added spermine), current activation reflects the channel activation time course and the solution exchange rate. Mean data from 5–8 outside-out patches. C and D, I-V relations recorded from the same outside-out patch in the presence of 100 μM cyclothiazide and glutamate using fast (10 ms, C) or slow (1 s, D) duration voltage ramps (−100 to +60 mV or vice versa). Arrows indicate direction of the voltage change with the grey traces corresponding to the +60 to −100 mV direction.
The degree of polyamine block depends on the rate at which channels are relieved from block, a process which is strongly voltage dependent (Fig. 7A and B). One would anticipate, therefore, that the shape of currents produced by a voltage ramp would depend on the speed and directionality of the ramp. The results in Fig. 7C and D confirm this prediction. Here, voltage ramps from −100 to +60 mV or vice versa with either a duration of 10 ms (Fig. 7C) or 1 s (Fig. 7D) were applied to patches in the continuous presence of cyclothiazide and glutamate. With the 10 ms duration, the directionality of the ramp determined the shape of the I-V relation, reflecting the fact that the speed of the ramp occurred so quickly that polyamine block did not reach equilibrium and was strongly influenced by the starting voltage. Hence, when the voltage was changed from −100 mV to +60 mV, the degree of block was reduced over the entire voltage range (channels were quickly relieved from block at negative potentials and remained unblocked at all subsequent potentials). This contrasts to when the ramp was started at +60 mV. Here, channels initially blocked at positive potentials remained blocked at moderate negative potentials due to the slower rate of unblock. With a 1 s duration, I-V s obtained by either ascending or descending voltage ramps were indistinguishable (Fig. 7D) indicating that with such slow voltage changes, channel block reached equilibrium at each potential.
In summary, Ca2+-permeable AMPAR channels appear to be blocked at negative potentials to nearly the same extent. Hence, current amplitudes, induced by a single pulse of glutamate, apparently reflect the kinetics of relief from polyamine block in open channels, a process occurring more rapidly at negative potentials.
Relief from polyamine block of transiently open channels is use dependent
In Fig. 4A, a constant number of glutamate pulses (10 at 100 Hz) was applied to the patch to generate facilitated currents. Figure 8 illustrates that the degree of relief from block depends on the number of glutamate pulses. In Fig. 8A, the patch was held at −40 mV during which a variable number of glutamate pulses (0, 1–5 at 100 Hz) was applied prior to stepping to the +30 mV test potential. In the absence of any glutamate pulses (upper panel), the amplitude of the test current at +30 mV was small. However, a single pulse of glutamate enhanced the test current amplitude nearly fourfold (Fig. 8B). Additional pulses further enhanced the test current amplitude, an effect that reached a maximum after about five pulses. Thus, relief from polyamine block occurs in a use-dependent manner. Further, since we have defined current facilitation as an increase in the current amplitude relative to that of the first amplitude in the train, the process of current facilitation reflects a use-dependent relief of channels from block.
Figure 8. Relief from polyamine block is use dependent.
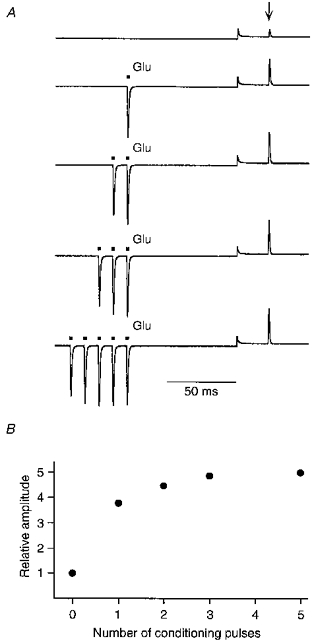
Currents in GluR-B(Q) channels were recorded with 25 μM spermine added to intracellular solution ‘b’. A, current traces recorded using a voltage jump protocol similar to that shown in Fig. 6 except that the conditioning potential was −40 mV. During the conditioning potential, glutamate was either not applied (upper trace; control) or applied (▪) a variable number of times (1–5 pulses at 100 Hz). Arrow indicates test glutamate application. B, relative test current amplitudes from the experiment in A plotted against the number of glutamate pulses during the conditioning potential. Current amplitudes were normalized to the amplitude of the control test current. A similar pattern of use dependence was found in 5 other cells.
Transmembrane electric field rather than ion flow is the primary determinant of relief from polyamine block
Relief from polyamine block at negative membrane potentials occurred primarily in the open state. In open channels, polyamines might be repelled from their blocking site either by transmembrane electric field and/or by inward-directed current flow. To test the contribution of these two actions, we measured relief from block under ionic conditions where the driving force for permeating ions was altered (Fig. 9). In 135 mM Na+, the reversal potential was around 0 mV, and a train of glutamate pulses at −30 mV activated inwardly directed currents. Similar to previous results, the amplitude of the test current at +30 mV (Fig. 9A, upper trace) was strongly enhanced, being on average 3.7 ± 0.2 (n = 3) times larger than the control current amplitude (lower trace). In reduced extracellular Na+ (30 mM NaCl in 105 mM NMDG to maintain osmolarity; Fig. 9B-D), the reversal potential was around −30 mV. Correspondingly, a train of glutamate pulses applied at −30 mV (Fig. 9B) elicited no net measurable current. This zero net current is composed of an inward- and outward-directed component of equal magnitude, but the inward-directed component is considerably smaller than that in 135 mM Na+ at the same potential. Nevertheless, the train of glutamate pulses enhanced the test current at +30 mV by about 4.3 ± 0.2 (n = 5) times, comparable with the enhancement when the inward-directed current was larger (cf. Fig. 9A). Moreover, when glutamate was applied at −20 mV in 30 mM Na+, eliciting outwardly directed currents, the test current measured at +30 mV was still enhanced relative to the control current (3.3 times; Fig. 9C). On the other hand, at 0 mV the test current amplitude was essentially the same regardless of the absence or presence of the conditioning pulses of glutamate. These results suggest that in open channels, the absolute transmembrane electric field rather than ionic currents is the primary mechanism underlying relief from polyamine block.
Figure 9. Membrane potential rather than ion flow relieves AMPAR channels from spermine block.
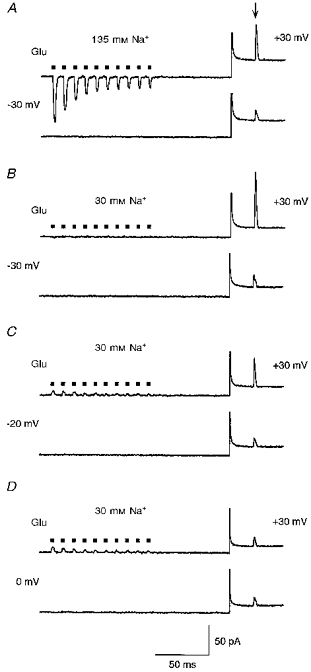
Currents in GluR-B(Q) channels were recorded with 25 μM spermine added to intracellular solution ‘b’. A, the extracellular solution contained 135 mM NaCl and 1.8 mM CaCl2. Upper trace, test current amplitude at +30 mV was measured 100 ms after a 100 Hz train of glutamate pulses (▪) at −30 mV. Lower trace, in control the same voltage protocol was used, but glutamate was applied only during the test potential. Arrow indicates test glutamate application. B, same experimental protocol as in A, but the extracellular solution contained 30 mM NaCl, 105 mM NMDG and 1.8 mM CaCl2. Note the absence of current during the glutamate applications at −30 mV (upper trace). C and D, same experimental protocol and extracellular solution as in B except that the conditioning potential was −20 mV (C) or 0 mV (D). Note that currents during the train are now outward. Capacitance transients were cut out.
Figure 10 summarizes the relative increase of test currents measured at +30 mV as a function of the conditioning potential. In these experiments, the same protocol was used as in Fig. 9, but the extracellular solution consisted of 10 mM NaCl and 125 mM NMDG to further minimize possible influence of inward currents (reversal potential (Vrev) was around −40 mV). The relative increase in test current amplitude was strongly dependent on membrane potential, being larger at more negative potentials. At positive potentials (up to +60 mV), relative current amplitudes were unchanged or even slightly reduced. However, at +80 mV the relative current amplitude was enhanced again suggesting a relief from block (Fig. 10, inset). This relief from block probably reflects the fact that spermine can permeate through AMPAR channels at extremely positive potentials, as suggested previously for kainate receptor channels (Bähring et al. 1997).
Figure 10. Voltage and concentration dependence of relief from spermine block.
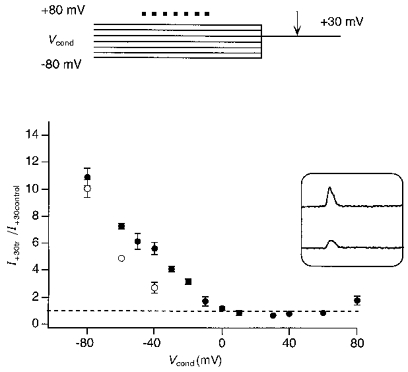
Ratio of the test current amplitudes, measured at +30 mV with (I+30tr) and without (I+30control) a conditioning train of glutamate pulses, plotted against the conditioning potential (Vcond) at which the patches were held before stepping to +30 mV. Currents in GluR-B(Q) channels were recorded with either 25 μM (•) or 100 μM (○) spermine added to intracellular solution ‘b’. The voltage protocol (top) was the same as in Fig. 9 but the extracellular solution contained 10 mM NaCl and 125 mM NMDG (Vrev about −40 mV). Each point represents the mean of 3–8 experiments. Inset, test currents measured at +30 mV with (upper trace) and without (lower trace) a train of glutamate pulses at a conditioning potential of +80 mV.
The above experiments (Fig. 10, filled circles) were made with 25 μM spermine added to the pipette solution ‘b’. With a higher concentration of spermine (100 μM), relief from block still occurred (Fig. 10, open circles). However, a stronger hyperpolarization was required to obtain the same relative increase in the test current amplitude.
Facilitation of native polyamine-sensitive AMPARs
Native AMPARs are heteromeric channels composed of different subunits. Ca2+ permeability and polyamine sensitivity are determined by the relative abundance of the edited GluR-B(R) subunit (Geiger et al. 1995; Koh et al. 1995a). AMPAR channels in hippocampal dentate gyrus basket cells are Ca2+ permeable and polyamine sensitive. In nucleated patches from these cells, the I-V was doubly rectifying as expected for polyamine-sensitive channels (Fig. 11A, left). Nucleated patches had the advantage in that they retained the endogenous intracellular content, including polyamines, for long periods of time. In these patches, glutamate pulses at 12 Hz induced a sustained current facilitation at 1.25 Hz (Fig. 11A, middle). Facilitation was also observed in outside-out patches isolated from dentate gyrus basket cells (Fig. 11A, right, 8 Hz) recorded within 5 min after patch formation. In patches from ten basket cells, the currents facilitated in varying degrees from 15 to 30 %. AMPARs in certain neocortex layer II nonpyramidal cells are also Ca2+ permeable and polyamine sensitive (A. Rozov & N. Burnashev, unpublished data). Currents in these channels also facilitated (four nucleated patches; data not shown). In contrast, AMPARs in hippocampal CA1 pyramidal cells are Ca2+ impermeable (Jonas & Sakmann, 1992) and polyamine insensitive (linear I-V in Fig. 11B, left), and facilitation was not observed (Fig. 11B, middle and right) under the same experimental conditions (n = 12). Thus, activity-dependent current facilitation is also found in native AMPARs which are Ca2+ permeable and polyamine sensitive. These results also suggest that endogenous levels of polyamines can mediate activity-dependent current facilitation.
Figure 11. Facilitation of currents in native Ca2+-permeable, polyamine-sensitive AMPAR channels.
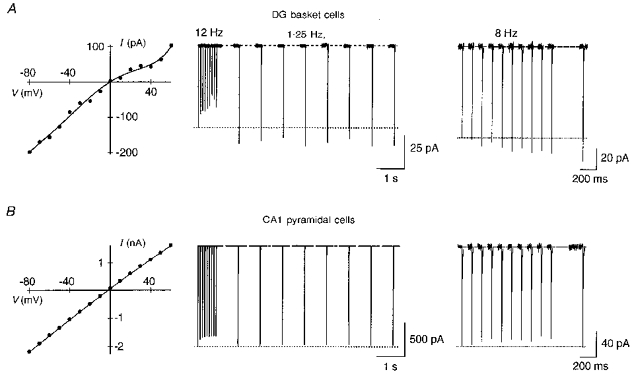
Glutamate-activated currents in patches isolated from neurones in a brain slice. The pipette solution was ‘a’. A, hippocampal dentate gyrus (DG) basket cells express polyamine-sensitive AMPARs. Currents in a nucleated patch, which retains endogenous polyamines, show a doubly rectifying I-V (left). After a 12 Hz train, currents were facilitated by 20 % at 1.25 Hz stimulation (middle). Currents in an outside-out patch measured within 5 min after patch formation also facilitated (8 Hz, right). A current delayed by 380 ms from the train was facilitated by 28 %. B, hippocampal CA1 pyramidal cells express polyamine-insensitive AMPARs as verified by the linear I-V (left). Currents did not facilitate in nucleated patches (middle) or outside-out patches (right) using the same experimental protocols as in A. Membrane potential −60 mV in A and B.
Use-dependent polyamine unblock counteracts channel desensitization
During repetitive stimulation, channel desensitization and activity-dependent relief from polyamine block presumably proceed simultaneously. Thus, polyamine-dependent current facilitation may be masked, especially in AMPARs possessing a slow recovery from desensitization. Indeed, in Ca2+-permeable GluR-A channels, currents strongly declined at 33 Hz stimulation (Fig. 12A, left trace), but after partial removal of desensitization by 10 μM cyclothiazide currents now facilitated (Fig. 12A, right trace). To assess the contribution of polyamine unblock to current amplitudes during repetitive activation in channels without cyclothiazide treatment, we compared the steady-state current amplitudes (Iss) to that of the first current (I1) in a train in the presence or absence of intracellular spermine (Fig. 12B). In both instances, the steady-state currents were depressed. However, the extent of depression was much stronger in the absence of spermine. On average, Iss/I1 at 14 Hz was 0.48 ± 0.04 (n = 5) with and 0.28 ± 0.02 (mean ±s.d., n = 3) without spermine in the pipette. Thus, use-dependent polyamine unblock substantially counteracts current depression caused by a slow recovery from desensitization.
DISCUSSION
At many synapses in the brain, Ca2+-permeable AMPAR channels mediate fast excitatory transmission (McBain & Dingledine, 1993; Otis et al. 1995; Gu et al. 1996; Isa et al. 1996). These channels are blocked by endogenous polyamines in a voltage-dependent manner (Koh et al. 1995a; Kamboj et al. 1995; Barnes-Davies & Forsythe, 1996). The functional significance of this block has been suggested to be a voltage-dependent mechanism regulating Ca2+ influx (Bowie & Mayer, 1995). Our results indicated that during repetitive activation currents through Ca2+-permeable AMPAR channels facilitated and that this facilitation arose by a use-dependent relief of polyamine block. Even when currents are depressed by a slow recovery from desensitization, the process of polyamine-dependent facilitation still occurred, counteracting desensitization.
Mechanism of activity-dependent facilitation of AMPARs
Activity-dependent current facilitation was found only in AMPAR channels that were Ca2+ permeable and sensitive to intracellular polyamines (Figs 1 and 11). Ca2+ flux, however, is not necessary for facilitation to occur since it still occurred in Ca2+-free solutions and was stronger at intermediate rather than more negative potentials. In contrast, facilitation does not occur when intracellular polyamines are washed out (Fig. 3A), and the I-V of facilitated currents is almost linear and deviates from the control I-V in a manner consistent with a relief from polyamine block (Fig. 4). Thus, given that steady-state facilitation is reached only after multiple glutamate applications, the underlying mechanism controlling facilitation in Ca2+-permeable AMPARs appears to be a use-dependent relief of the block by polyamines.
To characterize the mechanism underlying the activity- dependent relief from block, we primarily used a voltage protocol consisting of a conditioning potential followed by an application of glutamate at a test potential, typically at +30 mV, where channels are most strongly blocked by polyamines in the control condition. During the conditioning potential either no (control) or a variable number of glutamate pulses were applied, and the current amplitude of the glutamate pulse during the test step was used as an index of the degree to which channels were relieved from block during the conditioning potential. From this approach, we found some surprising insights into the mechanism of polyamine block of AMPAR channels. Perhaps most surprising was that polyamines are not ‘classical’ open channel blockers since they reblock channels in the closed state (though block and unblock occur much more rapidly in the open state). In addition, closed channels were blocked by polyamines to nearly the same extent (Fig. 6) regardless of the membrane potential (−100 to −20 mV). The lack of relief from block during the conditioning potential in closed channels could reflect the fact that they are rapidly reblocked either following the step to the test potential and/or during the 1 ms glutamate application, but such an alternative seems unlikely (see Results: Polyamines block closed AMPAR channels). It is not clear yet how closed AMPAR channels are blocked by polyamines. One possibility is that the reblock occurs during spontaneous openings of the channel in the absence of agonist but given the rapid rate of reblock such an effect seems unlikely. Alternatively, polyamines could interact with negative charges in the pore which are accessible in the closed state but which due to a conformational change underlying channel gating are not accessible in the open state of the channel. Such charges, if they exist, remain unidentified.
A second observation, which is critical to understanding the mechanism of current facilitation, was that a single pulse of glutamate during the conditioning potential strongly relieved channels from block. Our interpretation of this result is that open AMPAR channels are rapidly relieved from block with the rate of unblock being voltage dependent, occurring more rapidly at negative potentials. Therefore, we proposed that at negative potentials the inwardly rectifying shape of the current-voltage relationship of Ca2+-permeable AMPAR channels primarily reflects the voltage dependence of the rate of relief from block during channel opening, rather than the voltage dependence of the block. This contrasts to previous studies on polyamine block of non-NMDAR channels where it was assumed that at very negative potentials current amplitudes reflect the voltage dependence of the polyamine block (Bowie & Mayer, 1995; Koh et al. 1995a; Kamboj et al. 1995; Barnes-Davies & Forsythe, 1996). In part, this difference could reflect differences in the affinity of polyamines for the GluR subtypes since kainate receptor subtypes have a much lower affinity than Ca2+-permeable AMPAR subtypes (Bowie & Mayer, 1995) and therefore may not be blocked at negative potentials. On the other hand, for Ca2+-permeable AMPAR subtypes, these studies did not recognize the possibility that unblock occurs primarily in the open state.
The above observations reconcile an apparent paradox of the current facilitation mechanism. In particular, relief from polyamine block was stronger at very negative potentials (Figs 6 and 10) yet the degree of facilitation, which presumably reflects activity-dependent relief from block, was larger at intermediate negative potentials (in GluR-B(Q) channels at 33 Hz, current facilitation was 16 % at −80 mV and 44 % at −40 mV). Our explanation for this apparent discrepancy is that closed Ca2+-permeable AMPAR channels are completely or nearly completely blocked by polyamines at all negative potentials with the first current amplitude in a train reflecting the rapid relief from block of open channels, a process that occurs more rapidly at negative potentials. Hence, at −80 mV, most of the channels are relieved from block during the first glutamate application. Fewer channels are therefore available to be relieved from block with subsequent applications, producing only a weak facilitation relative to the first amplitude (as we have defined it in Fig. 1). In contrast, at −40 mV, the first application relieves fewer channels from the block leaving a larger pool of blocked channels available to be relieved from block with subsequent applications producing a stronger relative facilitation.
The relief from block depended more on the absolute transmembrane electric field rather than on current flow (Figs 9 and 10). Evidence consistent with the idea that the transmembrane electric field is the primary mechanism underlying relief from block is that, in the presence of permeant divalent cations, which would produce a stronger electrostatic interaction than monovalent cations with the multivalent polyamines, the degree of facilitation was not enhanced (Fig. 2). Thus at negative potentials, the voltage drop across open channels repels polyamines from their blocking site. However, a contribution of inward-directed current flow to the relief mechanism cannot be completely ruled out, but appeared not to contribute significantly. Also, at high positive potentials the voltage drop is strong enough to push polyamines through the channel again relieving the block.
Polyamines are normal intracellular constituents present in numerous cell types at concentrations up to 1 mM (Watanabe et al. 1991). However, the free polyamine concentration within a cell is not known since it can be buffered by ATP and nucleic acids. Our data show that current facilitation of native Ca2+-permeable AMPAR channels occurred in nucleated patches where the polyamine concentration is comparable with the endogenous concentration. In most of our experiments on HEK 293 cells, the free intracellular spermine concentration was estimated to be 2 μM (see Methods). With a 4-fold increase in the free spermine concentration, relief from block still occurred, but required more hyperpolarized potentials to achieve the same relative effect. Thus, variations in the free polyamine concentration based on the relative rate of polyamine synthesis and the metabolic state of the cells may affect the potency of the relief from the block and correspondingly the facilitation pattern.
Polyamine-dependent facilitation as a possible postsynaptic mechanism
Ca2+-permeable AMPARs are present in a number of CNS neurons (Gilbertson et al. 1991; McBain & Dingledine, 1993; Geiger et al. 1995; Otis et al. 1995; Koh et al. 1995b; Kyrozis et al. 1995; Barnes-Davies & Forsythe, 1995; Gu et al. 1996; Isa et al. 1996; Götz et al. 1997). We found activity-dependent current facilitation in somatic patches from non-pyramidal cells in the hippocampus (Fig. 11) and neocortex indicating that this is a property of native Ca2+-permeable AMPAR channels and that endogenous levels of polyamines can mediate facilitation of AMPAR currents. Presumably, subsynaptic AMPARs also show an activity-dependent relief from polyamine block, but future experiments will be necessary to test this hypothesis directly. Nevertheless, synaptic measurements have shown that Ca2+-permeable AMPAR channels directly participate in synaptic transmission (McBain & Dingledine, 1993; Otis et al. 1995; Isa et al. 1996; Gu et al. 1996), and somatic and dendritic AMPAR channels show similar properties (Spruston et al. 1995). Further, the use of brief agonist applications to outside-out patches simulates postsynaptic events (Colquhoun et al. 1992). Taken together these observations suggest that polyamine-dependent facilitation of AMPARs is a likely postsynaptic mechanism affecting synaptic signalling.
During processing of rapid synaptic input, postsynaptic responses are depressed by desensitization but enhanced by facilitation. Since the contribution of both processes varies depending on stimulation frequency and subunit type, resulting net currents may show either facilitation or depression (see Fig. 1). Nevertheless, even in the case of current depression, use-dependent polyamine unblock may substantially counteract it, augmenting current amplitude. Thus, the contribution of polyamine unblock to synaptic currents during high frequency stimulation may be to facilitate currents as well as to maintain current amplitudes in the face of a slow recovery from desensitization or presynaptic depression.
Unlike facilitation arising from a presynaptic increase in glutamate release, polyamine-dependent facilitation of Ca2+-permeable AMPARs provides an entirely postsynaptic mechanism for the control of synaptic gain. Furthermore, facilitated AMPARs may mediate substantial transient Ca2+ entry (Schneggenburger et al. 1993; Koh et al. 1995b; Burnashev et al. 1995) during intense synaptic activity, a process that may be important for further changes in synaptic plasticity (Malenka et al. 1992; Bliss & Collingridge, 1993).
Acknowledgments
We thank Professor B. Sakmann for continuous support and comments on the manuscript, Professor P.H. Seeburg for providing clones of AMPAR subunits, and Ms Grünewald for cell culture and transfections. We are also grateful to Drs F. A. Edwards, J.G.G. Borst and J. Bekkers for critically reading the manuscript.
References
- Barnes-Davies M, Forsythe ID. Pre- and postsynaptic glutamate receptors at a giant excitatory synapse in rat auditory brainstem slices. The Journal of Physiology. 1995;488:387–406. doi: 10.1113/jphysiol.1995.sp020974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes-Davies M, Forsythe ID. AMPA receptor-mediated synaptic currents rectify with internal spermine in rat MNTB neurones in vitro. Journal of Physiology. 1996;495.P:44P. [Google Scholar]
- Bähring R, Bowie D, Benveniste M, Mayer ML. Permeation and block of rat GluR6 glutamate receptor channels by internal and external polyamines. The Journal of Physiology. 1997;502:575–589. doi: 10.1111/j.1469-7793.1997.575bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Zhou Z, Neher E, Sakmann B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. The Journal of Physiology. 1995;485:403–418. doi: 10.1113/jphysiol.1995.sp020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Jonas P, Sakmann B. Action of brief pulses of glutamate on AMPA/kainate receptors in patches from different neurones of rat hippocampal slices. The Journal of Physiology. 1992;458:261–287. doi: 10.1113/jphysiol.1992.sp019417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curutchet P, Bochet P, Prado de Carvalho L, Lambolez B, Stinnakre J, Rossier J. In the GluR1 glutamate receptor subunit a glutamine to histidine point mutation suppresses inward rectification but not calcium permeability. Biochemical and Biophysical Research Communications. 1992;182:1089–1093. doi: 10.1016/0006-291x(92)91843-f. [DOI] [PubMed] [Google Scholar]
- Fisher SA, Fischer TM, Carew TJ. Multiple overlapping processes underlying short-term synaptic enhancement. Trends in Neurosciences. 1997;20:170–177. doi: 10.1016/s0166-2236(96)01001-6. 10.1016/S0166-2236(96)01001-6. [DOI] [PubMed] [Google Scholar]
- Frausto Da Silva JJR, Williams RJP. The Biological Chemistry of the Elements: the Inorganic Chemistry of Life. Oxford: Clarendon Press; 1993. [Google Scholar]
- Geiger JRP, Melcher T, Koh D-S, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Scobey R, Wilson M. Permeation of calcium ions through non-NMDA glutamate channels in retinal bipolar cells. Science. 1991;251:1613–1615. doi: 10.1126/science.1849316. [DOI] [PubMed] [Google Scholar]
- Götz T, Kraushaar U, Geiger J, Lübke J, Berger T, Jonas P. Functional properties of AMPA and NMDA receptors expressed in identified types of basal ganglia neurons. Journal of Neuroscience. 1997;17:204–215. doi: 10.1523/JNEUROSCI.17-01-00204.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JG, Albuquerque C, Lee CJ, MacDermott AB. Synaptic strengthening through activation of Ca2+-permeable AMPA receptors. Nature. 1996;381:793–796. doi: 10.1038/381793a0. 10.1038/381793a0. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp technique for high resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. 2. Sunderland, MA, USA: Sinauer Associates Inc.; 1992. [Google Scholar]
- Isa T, Itazawa S, Iino M, Tsuzuki K, Ozawa S. Distribution of neurones expressing inwardly rectifying and Ca2+-permeable AMPA receptors in rat hippocampal slices. The Journal of Physiology. 1996;491:719–733. doi: 10.1113/jphysiol.1996.sp021252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Burnashev N. Mechanisms controlling calcium entry through AMPA-type glutamate receptor channels. Neuron. 1995;15:987–990. doi: 10.1016/0896-6273(95)90087-x. [DOI] [PubMed] [Google Scholar]
- Jonas P, Sakmann B. Glutamate receptor channels in isolated patches from CA1 and CA3 pyramidal cells of rat hippocampal slices. The Journal of Physiology. 1992;455:143–171. doi: 10.1113/jphysiol.1992.sp019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboj SK, Swanson GT, Cull-Candy SG. Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. The Journal of Physiology. 1995;486:297–303. doi: 10.1113/jphysiol.1995.sp020812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh D-S, Burnashev N, Jonas P. Block of native Ca2+- permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. The Journal of Physiology. 1995a;486:305–312. doi: 10.1113/jphysiol.1995.sp020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh D-S, Geiger JRP, Jonas P, Sakmann B. Ca2+-permeable AMPA and NMDA receptor channels in basket cells of rat hippocampal dentate gyrus. The Journal of Physiology. 1995b;485:383–402. doi: 10.1113/jphysiol.1995.sp020737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrozis A, Goldstein PA, Heath MJS, MacDermott AB. Calcium entry through a subpopulation of AMPA receptors desensitized neighbouring NMDA receptors in rat dorsal horn neurons. The Journal of Physiology. 1995;485:373–381. doi: 10.1113/jphysiol.1995.sp020736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ. Heterogeneity of synaptic glutamate receptors on CA3 stratum radiatum interneurones of rat hippocampus. The Journal of Physiology. 1998;511:331. doi: 10.1113/jphysiol.1993.sp019560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Dingledine R. Heterogeneity of synaptic glutamate receptors on CA3 stratum radiatum interneurones of rat hippocampus. The Journal of Physiology. 1993;462:373–392. doi: 10.1113/jphysiol.1993.sp019560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Lancaster B, Zucker RS. Temporal limits on the rise in postsynaptic calcium required for the induction of long-term potentiation. Neuron. 1992;9:121–128. doi: 10.1016/0896-6273(92)90227-5. [DOI] [PubMed] [Google Scholar]
- Otis TS, Raman IM, Trussell LO. AMPA receptors with high Ca2+ permeability mediate synaptic transmission in the avian auditory pathway. The Journal of Physiology. 1995;482:309–315. doi: 10.1113/jphysiol.1995.sp020519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozov A, Zilberter Y, Wollmuth LP, Burnashev N. Relief from intracellular polyamine block underlies activity-dependent facilitation of currents through Ca2+-permeable AMPAR channels. The Journal of Physiology. 1997;501.P:18P. doi: 10.1111/j.1469-7793.1998.361bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather W, Dieudonné S, MacDonald JF, Ascher P. Activation and desensitization of N-methyl-D-aspartate receptors in nucleated outside-out patches from mouse neurones. The Journal of Physiology. 1992;450:643–672. doi: 10.1113/jphysiol.1992.sp019148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Zhou Z, Konnerth A, Neher E. Fractional contribution of calcium to the cation current through glutamate receptor channels. Neuron. 1993;11:133–143. doi: 10.1016/0896-6273(93)90277-x. [DOI] [PubMed] [Google Scholar]
- Sommer B, Keinänen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Köhler M, Tagaki T, Sakmann B, Seeburg PH. Flip and flop: A cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990;249:1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Spruston N, Jonas P, Sakmann B. Dendritic glutamate receptor channels in rat hippocampal CA3 and CA1 pyramidal neurons. The Journal of Physiology. 1995;482:325–352. doi: 10.1113/jphysiol.1995.sp020521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GJ, Dodt H-U, Sakmann B. Patch-clamp recordings from the soma and dendrite of neurons in brain slices using infrared video microscopy. Pflügers Archiv. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Trussell LO, Zhang S, Raman IM. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron. 1993;10:1185–1196. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Kusama-Eguchi K, Kobayashi H, Igarashi K. Estimation of polyamine binding to macromolecules and ATP in bovine lymphocytes and rat liver. Journal of Biological Chemistry. 1991;266:20803–20809. [PubMed] [Google Scholar]
- Zucker RS. Calcium and short-term synaptic plasticity. Netherlands Journal of Zoology. 1994;44:495–512. [Google Scholar]


