Abstract
Pancreatic HCO3− and fluid secretion were studied by monitoring luminal pH (pHL) and luminal volume simultaneously in interlobular duct segments isolated from guinea-pig pancreas. The secretory rate and HCO3− flux were estimated from fluorescence images obtained following microinjection of BCECF-dextran (70 kDa, 20 μM) into the duct lumen.
Ducts filled initially with a Cl−-rich solution swelled steadily (2.0 nl min−1 mm−2) when HCO3−2/CO was introduced, and the luminal pH increased to 8.08. When Cl− was replaced by glucuronate, spontaneous fluid secretion was reduced by 75 %, and pHL did not rise above 7.3.
Cl−-dependent spontaneous secretion was largely blocked by luminal H2DIDS (500 μM). We conclude that, in unstimulated ducts, HCO3− transport across the luminal membrane is probably mediated by Cl−-HCO3− exchange.
Secretin (10 nM) and forskolin (1 μM) both stimulated HCO3− and fluid secretion. The final value of pHL (8.4) and the increase in secretory rate (1.5 nl min−1 mm−2) after secretin stimulation were unaffected by substitution of Cl−.
The Cl−-independent component of secretin-evoked secretion was not affected by luminal H2DIDS. This suggests that a Cl−-independent mechanism provides the main pathway for luminal HCO3− transport in secretin-stimulated ducts.
Ducts filled initially with a HCO3−-rich fluid (125 mM HCO3−, 23 mM Cl−) secreted a Cl−-rich fluid while unstimulated. This became HCO3−-rich when secretin was applied.
Addition of H2DIDS and MIA (10 μM) to the bath reduced the secretory rate by 56 and 18 %, respectively. Applied together they completely blocked fluid secretion. We conclude that basolateral HCO3− transport is mediated mainly by Na+-HCO3− cotransport rather than by Na+-H+ exchange.
Studies on isolated pancreatic ducts have identified various ion channels and transporters that may be involved in transepithelial HCO3− secretion by this tissue (Case & Argent, 1993). Measurements of intracellular ion concentrations (Zhao et al. 1994; Villanger et al. 1995; Ishiguro et al. 1996a; De Ondarza & Hootman, 1997) and electrophysiological data (Gray et al. 1993; Novak & Greger, 1988a,b) indicate that the accumulation of intracellular HCO3− across the basolateral membrane of the duct cells may be achieved both by H+ extrusion via a Na+-H+ exchanger and/or H+-ATPase, and also by HCO3− uptake via a Na+-HCO3− cotransporter. HCO3− secretion across the luminal membrane is generally thought to involve the parallel operation of a Cl−-HCO3− exchanger and a cAMP-activated Cl− channel that is believed to be the cystic fibrosis transmembrane conductance regulator (CFTR).
Uncertainty, however, still remains concerning the relative contributions of these various transport proteins to secretin-stimulated HCO3− secretion. A mathematical model of the pancreatic duct epithelium, based on data obtained from the rat (Sohma et al. 1996), predicts the secretion of a pancreatic juice with a HCO3− concentration of about 75 mM - as is observed in vivo in that species - but it cannot explain the much higher HCO3− concentrations achieved in many other species (e.g. guinea-pig, dog and human). There may therefore be significant species differences in the relative contributions of the different channels and transporters to secretin-evoked secretion.
In a previous paper (Ishiguro et al. 1996b), we described a new experimental protocol for detecting HCO3− secretion across the duct cell epithelium by monitoring the luminal pH of interlobular ducts isolated from the guinea-pig pancreas. In so doing, we demonstrated the presence of an apparently Cl−-independent, luminal efflux pathway for HCO3− which is stimulated by secretin. Furthermore we showed that, in secretin-stimulated ducts, transepithelial HCO3− transport requires extracellular (basolateral) Na+ but is not exclusively dependent on either a Na+-H+ exchanger or H+-ATPase activity. This was consistent with other data suggesting that Na+-HCO3− cotransport makes a substantial contribution to HCO3− uptake across the basolateral membrane (Ishiguro et al. 1996a).
Our working hypothesis, therefore, is that pancreatic duct cells in the guinea-pig, and probably other species, are able to raise the luminal HCO3− concentration to a higher value than in the rat (a) because accumulation of HCO3− across the basolateral membrane is driven by electrogenic Na+-HCO3− cotransport in addition to Na+-H+ exchange, and (b)because they have an additional, Cl−-independent mechanism for HCO3− efflux across the luminal membrane.
To evaluate the relative contributions of these various transporters to secretion, it is necessary to quantify the net secretory flux of HCO3−. Measurements of luminal pH (pHL) only provide values for the luminal HCO3− concentration and, therefore, only a qualitative indication of HCO3− secretion up to the time where pHL reaches its steady-state value. A quantitative estimate of the HCO3− flux has to take into account the changes in luminal volume that result from fluid flow to the lumen. In this paper we describe a new method for measuring luminal volume (and therefore fluid secretory rate) and luminal pH simultaneously in isolated interlobular duct segments. This has allowed us to quantify HCO3− and fluid secretion, and examine the effects of Cl− substitution and selective inhibition of apical and basolateral transporters. From these results we conclude that secretin-stimulated fluid secretion by guinea-pig pancreatic ducts involves the participation of a Na+-HCO3− cotransporter at the basolateral membrane and a largely Cl−-independent HCO3−-efflux pathway at the luminal membrane.
METHODS
Isolation and culture of interlobular ducts
Female Hartley guinea-pigs (350–450 g) were killed by cervical dislocation. The body and tail of the pancreas were removed and interlobular ducts (diameter 100–150 μm) were isolated and cultured overnight as described previously (Ishiguro et al. 1996a).
Solutions
Bath solution
The standard Hepes-buffered solution contained (mM): 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 D-glucose and 10 Hepes, and was equilibrated with 100 % O2. The standard HCO3−-buffered solution contained (mM): 115 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 D-glucose and 25 NaHCO3, and was equilibrated with 95 % O2- 5 % CO2. Cl−-free solutions were made by replacing Cl− with glucuronate. All solutions were adjusted to pH 7.4 at 37°C.
Luminal injection solution
The standard solution injected into the duct lumen contained (mM): 139 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 D-glucose and 1 Hepes. Cl−-free solutions were made by replacing Cl− with glucuronate. These luminal injection solutions were adjusted to pH 7.2 at 37°C. The high-HCO3− solution contained (mM): 14 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 D-glucose, 125 NaHCO3 and 1 Hepes, and was equilibrated with 95 % O2- 5 % CO2 (pH 8.2) prior to microinjection. All luminal injection solutions also contained a dextran conjugate of the pH-sensitive fluoroprobe 2′7’-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF-dextran, 70 kDa) at a concentration of 20 μM.
Measurement of luminal pH (pHL)
During overnight culture both ends of the interlobular duct segments sealed spontaneously thus isolating the luminal space from the bathing medium. The lumen of the cultured interlobular ducts was micropunctured as described previously (Ishiguro et al. 1996b). The duct was first immobilized with a suction pipette within a 700 μl perfusion chamber on an inverted microscope (Olympus IX) and superfused at 3 ml min−1 at 37°C. Thereafter the lumen was punctured with a double-barrelled (theta-glass) micropipette (tip diameter 10–12 μm) and a small volume of oil was injected. The pipette tip was pushed forward through the oil droplet and the luminal fluid was withdrawn and replaced by the injection solution containing BCECF-dextran. The puncture pipette was removed once the duct had been filled sufficiently to regain its cylindrical shape (Fig. 1A). Any possible leakage of the luminal solution at the puncture site was prevented by the oil droplet.
Figure 1. Bright-field and fluorescence images of an interlobular duct segment microinjected with BCECF-dextran.
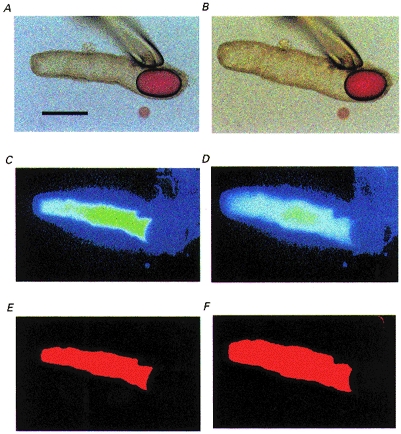
A, bright-field image of a sealed duct segment after micropuncture and replacement of the luminal fluid with a solution containing BCECF-dextran. The oil droplet prevents leakage of the luminal solution after withdrawal of the pipette. The bar indicates 200 μm. B, the same duct after 10 min of stimulation with 10 nM secretin showing the increase in luminal volume resulting from fluid secretion. C and D, fluorescence images of the luminal space corresponding to A and B, respectively. E and F, binary images obtained by transformation of C and D, respectively.
The pH of the duct lumen (pHL) was estimated by microfluorometry using a modification of the method described previously (Ishiguro et al. 1996b). Briefly, the ducts were illuminated alternately at excitation wavelengths of 430 and 480 nm. Luminal pH was calculated from the fluorescence ratio (F480/F430) measured at 530 nm. Calibration was performed in situ by varying the pH of the superfusate after first permeabilizing the ducts with digitonin (2.5 mM for 2 min).
Measurement of luminal volume and fluid secretory rate
Along with the measurement of pHL, images of BCECF-dextran fluorescence were obtained at 1 min intervals using a CCD camera. The fluorescence images were subsequently transformed to binary images using ARGUS-50 software (Hamamatsu Photonics, Hamamatsu, Japan). Figure 1 shows bright-field images taken at the beginning of a representative experiment (Fig. 1A) and after stimulation with secretin (Fig. 1B). Panels C and D are the corresponding images of BCECF-dextran fluorescence from the lumen, and panels E and F are the binary images obtained from panels C and D. The threshold intensity for the transformation to the binary image was set automatically by the software.
In order to determine the secretory rate, the initial values for the length (L0), diameter (2R0) and projected area (A0) of the duct lumen were measured in the first image of the series. The initial volume of the duct lumen (V0) was calculated, assuming cylindrical geometry, as πR02L0. The luminal surface area of the epithelium (E) was taken to be approximately 2πR0L0 (neglecting the ends of the cylinder since L0 >> R0). In subsequent images of the series, the luminal image area A was expressed as relative area (A/A0). A representative experiment is illustrated in Fig. 2A. Relative volume (V/V0) was calculated from relative area (A/A0) using V/V0= (A/A0)3/2. This assumes that R/R0=L/L0, i.e. that the duct swells radially and longitudinally by the same fraction. This assumption was supported by more detailed measurements of R and L in several duct image series. Measurements from the experiment in Fig. 2A are shown in Fig. 2B. The rate of fluid secretion was then calculated at 1 min intervals from the increment in duct volume and expressed as the secretory rate per unit luminal area of epithelium (nl min−1 mm−2) (Fig. 2C).
Figure 2. Measurement and calibration of fluid secretory rate in interlobular ducts.
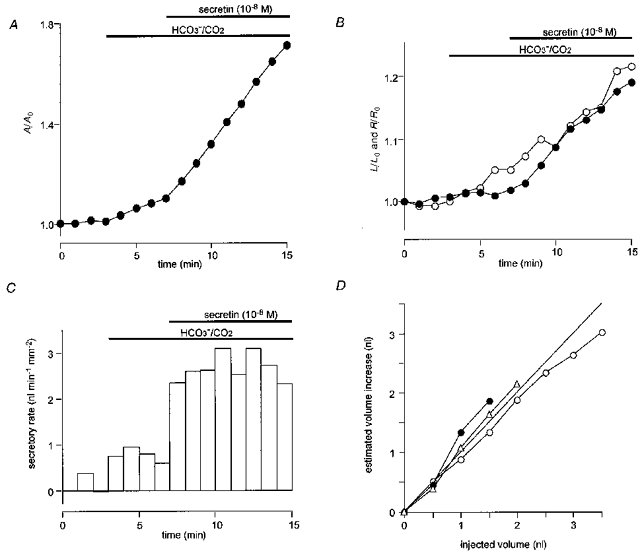
A, changes in the relative area (A/A0) of the luminal space in a duct exposed first to HCO3−/CO2 and then stimulated with 10 nM secretin in Cl−-free conditions. B, changes in relative length (L/L0, •) and relative radius (R/R0, ○) corresponding to the changes in A/A0 shown in A. C, changes in secretory rate calculated from the changes in A/A0 shown in A. The values for R0, L0, E, V0 and the final volume of this duct were 70 μm, 692 μm, 0.30 mm2, 10.6 nl and 17.5 nl respectively. D, calibration of fluid secretory rate showing the relationship between the cumulative injected volume and the calculated increase in volume in three individual ducts. Initial volumes of the ducts were 5.2 nl (○), 8.6 nl (•) and 11.2 nl (▵). The straight line is the line of identity.
In order to validate the calculation of absolute duct volumes from fluorescence images, direct calibration of luminal volume was performed using a nanolitre-syringe driver (Sutter Instruments, San Francisco, CA, USA). In these experiments, the tip of the micropuncture pipette was retained in the duct lumen. The duct was superfused with a Hepes-buffered, HCO3−-free solution (under which conditions no change of luminal volume was observed), and then 0.5 nl volumes of the BCECF-dextran solution were injected repeatedly. Figure 2D shows representative calibration data from three individual ducts in which the volume increase calculated from the change in the fluorescence image area is plotted against the cumulative injected volume. The data points lie close to the line of identity thus confirming the calibration of the system.
Since fluid secretion was negligible in the absence of HCO3−, it was also possible to determine the coefficient of variation in the duct volumes obtained by this method from series of images of the same duct. For three ducts, each examined over a 5 min period, the values were 2.2, 0.3 and 0.9 %.
Materials
BCECF-dextran and dihydro-4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (H2DIDS) were obtained from Molecular Probes; ouabain, forskolin, NaCN and sodium iodoacetate from Sigma; secretin (porcine) from Peptide Institute (Osaka, Japan); and N-methyl-N-isobutylamiloride (MIA) from Research Biochemicals International (Natick, MA, USA).
Statistics
Averaged data are presented as the means ±s.e.m. unless otherwise indicated. Tests for statistically significant differences were made with Student's t test for paired or unpaired data as appropriate.
RESULTS
Luminal pH and secretory rate in the presence of Cl−
The luminal fluid that had accumulated during overnight culture of the ducts was removed by micropuncture and replaced by a weakly buffered, HCO3−-free, Cl−-rich solution containing the pH-sensitive fluoroprobe BCECF-dextran. The collapsed ducts were filled until they had just regained a cylindrical shape after which the puncture pipette was removed in order to isolate the duct lumen.
Ducts were initially superfused with a Hepes-buffered HCO3−-free solution. During this equilibration period, pHL did not change significantly and no fluid secretion was observed (0.01 ± 0.17 nl min−1 mm−2, mean ±s.e.m., n = 5; Fig. 3A and B). Switching the bath solution from the HCO3−-free solution to one containing 25 mM HCO3− (equilibrated with 5 % CO2) caused pHL first to decline rapidly as a result of CO2 diffusing from the bath to the luminal fluid. Thereafter pHL increased and after 4 min it had reached 8.08 ± 0.06 and was still increasing. During this period, a constant secretory rate (2.02 ± 0.52 nl min−1 mm−2) was observed. These results indicate that, with 148 mM Cl− initially present in the lumen, there was a significant spontaneous secretion of HCO3−-rich fluid. The net HCO3− flux, JHCO3, which was calculated from the secretory rate Jv and the rate of change of pHL (see Appendix), was 1.38 ± 0.15 nmol min−1 mm−2 during the final minute of this period. From the ratio of JHCO3 and Jv, the effective concentration of HCO3− in the secreted fluid was estimated to be 790 ± 74 mM. Since salt-water coupling in the pancreatic duct is believed to generate a near-isosmotic fluid (Case et al. 1968) the HCO3− concentration in the secreted fluid would not be expected to exceed about 150 mM. Because the calculated value was so much greater than this, we conclude that more than 80 % of the HCO3− flux during the early part of the experiment was due to Cl−-HCO3− exchange and did not therefore generate any flow of fluid to the lumen.
Figure 3. Luminal pH and secretory rate in the presence of Cl−.
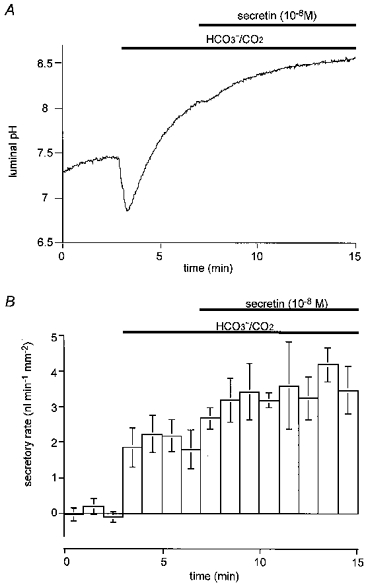
Ducts filled with a weakly buffered, HCO3−-free, Cl−-rich (148 mM) solution containing 20 μM BCECF-dextran were initially superfused with a Hepes-buffered HCO3−-free solution. After a 3 min period, the bath solution was switched to a solution containing 25 mM HCO3− equilibrated with 5 % CO2. After a further 4 min, 10 nM secretin was applied. A, changes in luminal pH; one of five experiments. B, changes in secretory rate; means ±s.e.m. of all five experiments. One-way analysis of variance showed significance at the P < 0.005 level.
When 10 nM secretin was then added to the bath, pHL increased further and reached 8.37 ± 0.06 after 8 min. The fluid secretory rate increased by a further 1.50 ± 0.19 nl min−1 mm−2 to a value of 3.52 ± 0.41 nl min−1 mm−2 and then remained constant during the 8 min period of secretin stimulation (Fig. 3A and B). The HCO3− flux calculated for the final minute of this period was 0.96 ± 19 nmol min−1 mm−2. By this stage, the effective concentration of HCO3− in the secreted fluid was 277 ± 26 mM, indicating that at least 40 % of the HCO3− flux was probably still the result of Cl−-HCO3− exchange rather than net secretion.
In four similar experiments, spontaneous fluid secretion was followed for 20 min before secretin was applied (Fig. 4B). As in Fig. 3B, the secretory rate increased rapidly when the bath solution was switched from the Hepes solution to the HCO3−/CO2 solution. However, while the luminal pH remained at a high value (Fig. 4A), the rate of spontaneous fluid secretion declined slowly over the 20 min period from 1.93 ± 0.26 nl min−1 mm−2 during the first 5 min to 1.28 ± 0.12 nl min−1 mm−2 in the final 5 min (P < 0.05). Despite the accumulation of fluid in the lumen during this period, stimulation with 10 nM secretin was still able to evoke secretory rates comparable with the highest values obtained in the shorter experiments.
Figure 4. Time course of luminal pH and secretory rate during spontaneous secretion in the presence of Cl−.
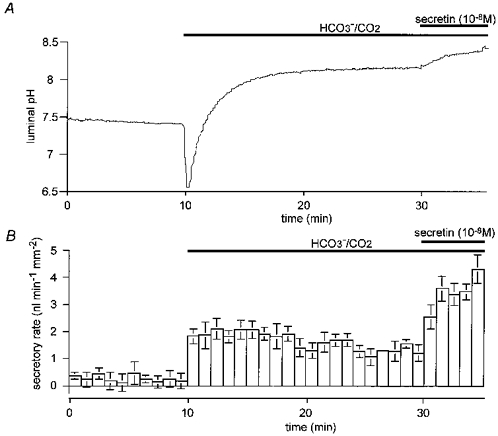
Under the same experimental conditions as in Fig. 3, changes in luminal pH and fluid secretory rate were monitored for 20 min in the HCO3−/ CO2 bath solution before secretin (10 nM) was applied. A, changes in luminal pH; one of four experiments. B, changes in secretory rate; means ±s.e.m. of all four experiments.
Figure 7A (•) shows the concentration-response curve for the effects of secretin on the fluid secretory rate. The concentration of secretin required for a half-maximal increase in fluid secretion was approximately 0.9 nM (log[secretin]=−9.07 ± 0.94). Stimulation with forskolin (1 μM) also increased the fluid secretory rate, to 2.83 ± 0.17 nl min−1 mm−2 (n = 5; Fig. 7B), supporting our previous observation that an elevation of the intracellular cAMP concentration is sufficient to stimulate a full secretory response.
Figure 7. Effects of secretin and forskolin on fluid secretion.
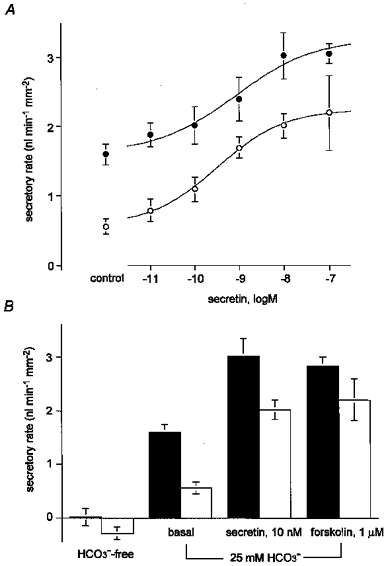
A, concentration-response curve for the effects of secretin on the secretory rate in isolated interlobular ducts in the presence (•) and absence (○) of Cl−. Data are shown as the means ±s.e.m. of at least five experiments. One-way analysis of variance showed significant variations with concentration at the P < 0.0001 level in both sets of data. B, secretory rate in the absence of HCO3− (HCO3−-free), under unstimulated conditions (spontaneous), during stimulation with secretin (10 nM) and in the presence of forskolin (1 μM). ▪, secretory rate in the presence of Cl−; □, secretory rate in the absence of Cl−. Means ±s.e.m. of at least five experiments.
Luminal pH and secretory rate in the nominal absence of Cl−
Our previous study demonstrated that ducts stimulated with secretin could alkalinize the luminal fluid, presumably as a result of HCO3− secretion, in the virtual absence of luminal Cl− (Ishiguro et al. 1996b). The next experiment was designed to investigate whether fluid secretion could also be observed under these conditions. Ducts were superfused first with a HCO3−-free, Cl−-free solution (in which all of the Cl− was replaced by glucuronate) for at least 15 min. The lumen was then punctured and filled with a weakly buffered, Cl−-free solution containing BCECF-dextran. Switching the bath solution to a HCO3−-buffered, Cl−-free solution acidified the lumen as before. But under these conditions (with Cl− absent from both basolateral and luminal solutions) pHL recovered only partially (Fig. 5A), and after 4 min the value was still less than 7.4 (7.30 ± 0.04, n = 5). The rate of fluid secretion (0.47 ± 0.17 nl min−1 mm−2; Fig. 5B) was also significantly smaller than in the presence of Cl−. The HCO3− flux was greatly diminished under these conditions (0.22 ± 0.02 nmol min−1 mm−2). These results therefore suggest that the spontaneous secretion of HCO3− and fluid into the lumen of the unstimulated ducts is largely dependent on the presence of Cl−.
Figure 5. Luminal pH and secretory rate in the absence of Cl−.
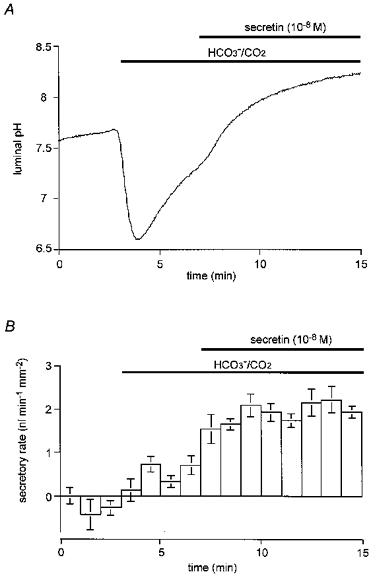
Ducts filled with a weakly buffered, HCO3−-free, Cl−-free solution (replaced with glucuronate) containing 20 μM BCECF-dextran were initially superfused with a Hepes-buffered, HCO3−-free, Cl−-free solution. After a 3 min equilibration period, the bath solution was switched to a solution containing 25 mM HCO3− equilibrated with 5 % CO2. After a further 4 min, 10 nM secretin was applied. A, changes in luminal pH; one of five experiments. B, changes in secretory rate; means ±s.e.m. of all five experiments.
In the absence of extracellular Cl−, 10 nM secretin still caused pHL to increase rapidly, reaching 8.27 ± 0.09 after 8 min (Fig. 5A). The secretory rate also increased by 1.54 ± 0.22 nl min−1 mm−2 (Fig. 5B). Thus, the absence of Cl− did not affect the final value of pHL or the increase in fluid secretory rate following secretin stimulation. The HCO3− flux during the final minute of this period was 0.38 ± 0.04 nmol min−1 mm−2. The latter value is somewhat smaller than that obtained during stimulation in the presence of Cl−. However, the effective concentration of HCO3− in the secreted fluid in the absence of Cl− was estimated to be 167 ± 16 mM, confirming that the calculated HCO3− flux probably reflected a true secretory flux, accompanied by water in near-isosmotic proportions, with relatively little, if any, contribution from anion exchange.
The low rate of spontaneous secretion that was observed when the bath solution was switched to the HCO3−/CO2 solution in the absence of Cl− continued for 20 min in the longer experiment shown in Fig. 6B. While the luminal pH continued to rise slowly during this period (Fig. 6A), there was a slow decline in the secretory rate (from 0.48 ± 0.10 nl min−1 mm−2 in the first 5 min to 0.32 ± 0.07 nl min−1 mm−2 in the last 5 min; P≈ 0.05) as also observed in the presence of Cl− (Fig. 4B). The secretory response to 10 nM secretin, however, was undiminished compared with the shorter experiments illustrated in Fig. 5.
Figure 6. Time course of luminal pH and secretory rate during spontaneous secretion in the absence of Cl−.
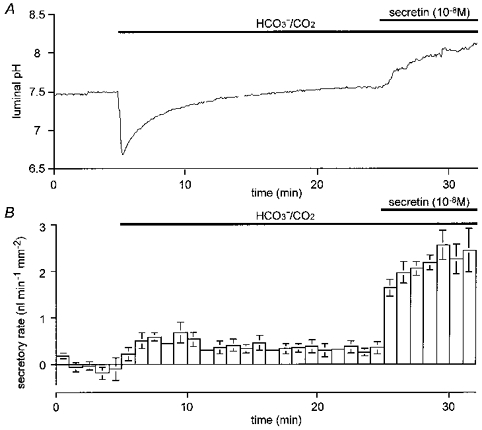
Under the same experimental conditions as in Fig. 5, changes in luminal pH and fluid secretory rate were monitored for 20 min in the HCO3−/ CO2 bath solution before secretin (10 nM) was applied. A, changes in luminal pH; one of four experiments. B, changes in secretory rate; means ±s.e.m. of all four experiments.
Figure 7A (○) shows the concentration-response curve for the effects of secretin on the fluid secretory rate. The concentration of secretin required for a half-maximal increase in fluid secretion was approximately 0.3 nM (log[secretin]= (−9.49 ± 0.45), which was not significantly different from the value obtained in the presence of Cl−. Stimulation with forskolin (1 μM) also increased the fluid secretory rate in the absence of extracellular Cl−, to 2.21 ± 0.39 nl min−1 mm−2 (n = 5; Fig. 7B), although this was not significantly different from the rate observed in the presence of Cl−. These results indicate that both secretin and forskolin can evoke the secretion of a HCO3−-rich fluid in the nominal absence of Cl− from the bath and with, at most, a very low concentration of Cl− in the lumen (as judged by our previous measurements of the luminal Cl− concentration under comparable conditions; Ishiguro et al. 1996b).
Luminal pH and fluid secretory rate with high luminal HCO3−
Although the HCO3− concentration in the lumen of guinea-pig interlobular ducts in vivo is not known, the concentration of HCO3− in the secretion collected from the main pancreatic duct of anaesthetized guinea-pigs can reach 150 mM during secretin stimulation (Padfield et al. 1989). Therefore, to study the flux of HCO3− and water into the lumen under more physiological conditions, in the following experiments the duct lumen was filled with a solution initially containing 125 mM HCO3− and 23 mM Cl− (pH 8.2 when equilibrated with 5 % CO2). Figure 8 shows the changes in pHL and fluid secretory rate under such conditions. Prior to the application of secretin, there was a slight decrease in pHL (from 8.12 ± 0.05 to 8.04 ± 0.03, P < 0.05) accompanied by a sustained secretion of fluid at a rate of 1.03 ± 0.20 nl min−1 mm−2 (n = 4). The calculated HCO3− flux in the final minute of this control period was very small (0.02 ± 0.01 nmol min−1 mm−2) and the estimated concentration of HCO3− in the secreted fluid was only 24 ± 9 mM. This suggests either that the principal anion in the spontaneously secreted fluid was Cl− rather than HCO3−, or that spontaneous HCO3− secretion was accompanied by a net exchange of luminal HCO3− for cytosolic Cl−.
Figure 8. Luminal pH and secretory rate with high luminal HCO3−.
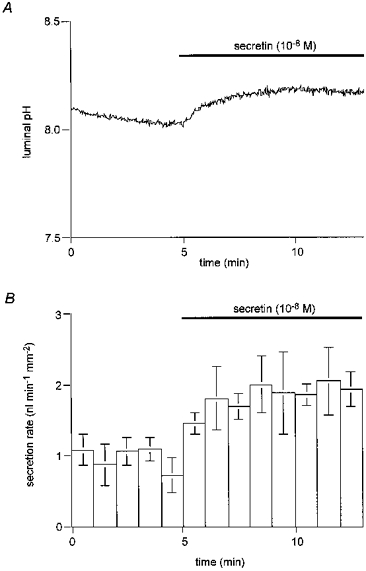
Ducts filled with a BCECF-dextran solution containing 125 mM HCO3− and 23 mM Cl− (pH 8.2) were superfused with the standard HCO3−-buffered solution and 10 nM secretin was applied after 5 min. A, changes in luminal pH; one of four experiments. B, changes in fluid secretory rate; means ±s.e.m. of all four experiments.
Upon application of 10 nM secretin, pHL rose to 8.21 ± 0.04 and the fluid secretory rate increased to 1.89 ± 0.33 nl min−1 mm−2. The calculated HCO3− flux at the end of this period was 0.32 ± 0.07 nmol min−1 mm−2 and the concentration of HCO3− in the secreted fluid was estimated to be 149 ± 13 mM, indicating a minimal contribution from anion exchange. It also appears that, under these relatively physiological conditions, the composition of the secreted fluid changed upon stimulation with secretin from being Cl−-rich to HCO3−-rich.
Inhibition of basolateral membrane transporters and metabolism
The methodology described above allows us to continuously monitor fluid and HCO3− secretion in isolated interlobular pancreatic ducts. Using this methodology, we have also studied the effects of transport inhibitors added either basolaterally (i.e. to the bathing fluid) or to the luminal fluid.
In pancreatic ducts of rat and cat, Na+,K+-ATPase activity is restricted to the basolateral membrane (Bundgaard et al. 1981; Madden & Sarras, 1987). Ouabain, a specific inhibitor of Na+,K+-ATPase strongly inhibits fluid secretion in perfused cat pancreas (Case & Scratcherd, 1974) and in isolated rabbit pancreas (Kuijpers et al. 1984). Consequently, HCO3− secretion in pancreatic duct cells is thought to require the indirect participation of Na+,K+-ATPase in order to generate a sodium gradient which favours the operation of Na+-H+ exchange and Na+-HCO3− cotransport.
In the experiment shown in Fig. 9A, 1 mM ouabain was added to the bath solution during stimulation with 10 nM secretin in the presence of Cl− (the same conditions as in Fig. 3). After 7 min, ouabain had reduced the secretory rate by 74 %: from 3.50 ± 0.15 to 0.92 ± 0.11 nl min−1 mm−2 (n = 4). In the experiments shown in Fig. 9B, intracellular ATP was depleted by adding both cyanide, an inhibitor of cytochrome oxidase, and iodoacetate, a glycolytic inhibitor, to the perfusate during stimulation with 10 nM secretin in the absence of Cl− (the same conditions as in Fig. 5). The combination of 2 mM cyanide and 0.1 mM iodoacetate caused the secretory rate to fall by 83 %: from 2.18 ± 0.31 to 0.36 ± 0.04 nl min−1 mm−2 (n = 4). These results show that secretin-evoked fluid secretion across the pancreatic duct epithelium requires the activity of Na+,K+-ATPase and intracellular ATP as an energy source.
Figure 9. Effects of ouabain and ATP depletion on fluid secretion.
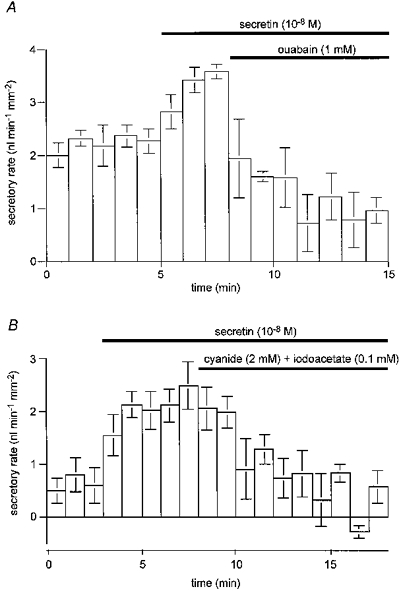
A, the effect of 1 mM ouabain on fluid secretory rate. Ducts filled with a Cl−-containing BCECF-dextran solution were superfused with the standard HCO3−-buffered solution and then stimulated with 10 nM secretin; mean ±s.e.m. of four experiments. B, the effect of 2 mM cyanide and 0.1 mM iodoacetate on secretory rate. Ducts filled with a Cl−-free BCECF-dextran solution were superfused with a Cl−-free HCO3−-buffered solution and stimulated with 10 nM secretin; means ±s.e.m. of four experiments.
In a previous paper (Ishiguro et al. 1996a) we proposed (on the basis of a variety of evidence) that the accumulation of HCO3− across the basolateral membrane of the guinea-pig pancreatic duct cell was achieved largely (about 75 %) through the activity of a Na+-HCO3− cotransporter which could be inhibited by H2DIDS. In order to evaluate the contribution of the cotransporter to fluid secretion, we have now examined the effect of basolateral application of H2DIDS on secretory rate. These experiments were carried out in the absence of Cl− using the same protocol as those shown in Fig. 5. During stimulation with 10 nM secretin, 500 μM H2DIDS was added to the bath solution for a period of 4 min (Fig. 10A). After 2 min of H2DIDS application, the secretory rate had decreased by 56 ± 12 % (n = 5, P < 0.01). However, after 4 min of application, the secretory rate appeared to have recovered partially and the reduction was only 26 ± 18 %, which was not significantly different from the value prior to the application of H2DIDS. Assuming that inhibition of the Na+-HCO3− cotransporter was complete, this suggests that a H2DIDS-insensitive transport mechanism, most probably Na+-H+ exchange, was supporting HCO3− accumulation across the basolateral membrane under these conditions.
Figure 10. Effects of basolateral inhibitors on fluid secretion.
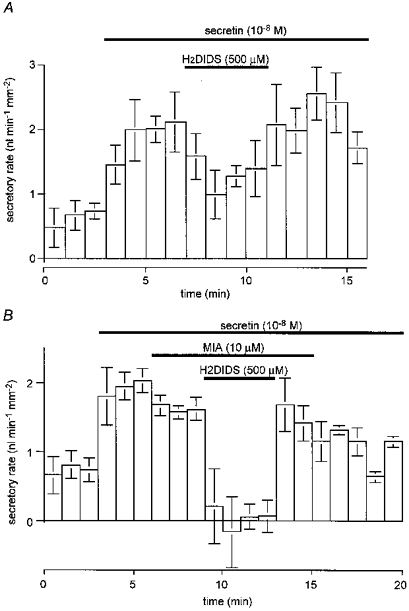
Ducts filled with a Cl−-free BCECF-dextran solution were superfused with a Cl−-free HCO3−-buffered solution and then stimulated with 10 nM secretin. A, the effect of basolateral H2DIDS (500 μM) on secretory rate; mean ±s.e.m. of five experiments. B, the effect of basolateral MIA (10 μM) and H2DIDS (500 μM) on secretory rate; means ±s.e.m. of four experiments.
In another experiment (Fig. 10B), 10 μM MIA, a specific and potent inhibitor of Na+-H+ exchange (Dixon et al. 1987), was added to the bath to inhibit the basolateral exchanger. MIA had rather less effect on secretory rate than H2DIDS and only reduced the secretory rate by 18 ± 4 % (n = 4, P < 0.05). However, when 500 μM H2DIDS was also added to the bath, the combined effect of MIA and H2DIDS was to completely block fluid secretion. Furthermore, when H2DIDS but not MIA was removed from the bath, the secretory rate showed a rapid and substantial recovery. These results support our hypothesis that the H2DIDS-sensitive Na+-HCO3− cotransporter makes a larger contribution to the basolateral accumulation of HCO3− in secretin-stimulated ducts than the MIA-sensitive Na+-H+ exchanger.
De Ondarza & Hootman (1997) have recently demonstrated the participation of a vacuolar-type H+-ATPase in the recovery of intracellular pH following an acid load in guinea-pig pancreatic duct cells. This transporter, working in concert with carbonic anhydrase, could theoretically contribute to the accumulation of intracellular HCO3− and thus to HCO3− and fluid secretion. In order to assess this possibility, we examined the effect of bafilomycin A1, a specific inhibitor of vacuolar H+-ATPases, on secretory rate. When applied at a concentration of 1 μM during stimulation with 10 nM secretin (in the presence of Cl−), the secretory rate was 3.41 ± 0.22 nl min−1 mm−2 (n = 4). This was not significantly different from the rate prior to the addition of bafilomycin A1 (3.45 ± 0.21 nl min−1 mm−2, n = 4) and it suggests that the contribution of this transporter to secretion is probably small.
Inhibition of luminal membrane transporters
To study the luminal transport mechanisms involved in HCO3− secretion, we also introduced transport inhibitors into the luminal space. In our preliminary attempt to identify these mechanisms (Ishiguro et al. 1996b), three anion channel blockers were tested for their ability to inhibit the increase in pHL following stimulation with forskolin. In Cl−-free conditions we found that luminal glibenclamide, H2DIDS and 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) all failed to inhibit the increase in pHL following stimulation. These results suggested the existence of a novel HCO3− efflux pathway at the luminal membrane. However, it was also evident that, when Cl− was present in the lumen, alkalinization occurred spontaneously even in the absence of stimulation. We suggested that this spontaneous component of HCO3− secretion might be mediated by Cl−-HCO3− exchange; if so, stilbenes such as H2DIDS would be expected to block it. To test this prediction, we have now investigated the effect of H2DIDS on luminal alkalinization and secretory rate.
In the presence of Cl−, H2DIDS (500 μM) injected into the duct lumen markedly inhibited the increase in pHL when the bath solution was switched to the HCO3−-buffered solution (Fig. 11A). Luminal pH increased only modestly to 7.34 ± 0.05 (n = 5) after 4 min compared with 8.00 ± 0.05 (n = 4; P < 0.001) in the absence of H2DIDS. Over the same time period, luminal H2DIDS reduced the secretory rate by 59 ± 21 % compared with the controls (P < 0.05, Fig. 11B). These results indicate that, in the presence of Cl−, a significant fraction of the spontaneous HCO3− efflux and the flow of fluid to the lumen is sensitive to H2DIDS and therefore probably involves Cl−-HCO3−exchange.
Figure 11. Effects of luminal H2DIDS on fluid secretion.
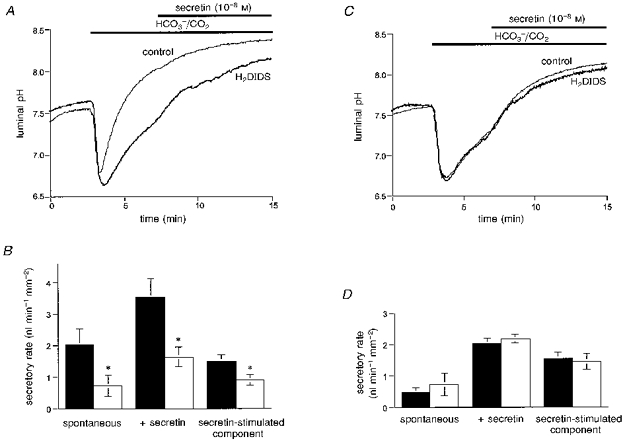
A and B, ducts filled with a Cl−-containing BCECF-dextran solution with or without H2DIDS (500 μM) were superfused with the standard HCO3−-buffered solution and then stimulated with 10 nM secretin. A, the effect of luminal H2DIDS (500 μM) on luminal pH. Control data are represented by the thin line, and data from ducts containing H2DIDS by the thick line. Mean traces of five and four experiments respectively. B, the effect of luminal H2DIDS (500 μM) on secretory rate. ▪, control data; □, data from ducts containing H2DIDS. The secretin-stimulated component of the secretory rate is the difference between the secretin-evoked and spontaneous secretory rates. Means ±s.e.m. of five and four experiments respectively. * Statistical differences (P < 0.05). C and D, ducts filled with a Cl−-free BCECF-dextran solution with or without H2DIDS (500 μM) were superfused with the Cl−-free HCO3−-buffered solution and stimulated with 10 nM secretin. C, the effect of luminal H2DIDS (500 μM) on luminal pH. Control data are represented by the thin line, and data from ducts containing H2DIDS by the thick line. Mean traces of five and four experiments respectively. D, the effect of luminal H2DIDS (500 μM) on secretory rate. ▪, control data; □, data from ducts containing H2DIDS. Means ±s.e.m. of five and four experiments respectively.
When the ducts containing H2DIDS were then stimulated with secretin, the rise in pHL became more rapid and after 8 min pHL had approached a steady-state value that was not significantly lower than in the secretin-stimulated control ducts (Fig. 11A). Furthermore, the increase in fluid secretion evoked by secretin (Fig. 11B) was only reduced by 33 ± 16 % (P < 0.05) by luminal H2DIDS.
In the absence of Cl−, neither the rise in pHL nor the secretory rate was affected by luminal H2DIDS under resting or secretin-stimulated conditions (Fig. 11C and D). Furthermore the pattern of changes in pHL observed in Cl−-free conditions (with or without H2DIDS) was similar to those observed in Cl−-containing conditions when H2DIDS was present in the lumen (thick line, Fig. 11A). These results support our hypothesis that, while spontaneous secretion is Cl−-dependent and inhibited by H2DIDS, and therefore probably involves Cl−-HCO3− exchange, the secretion of HCO3−-rich fluid evoked by secretin in the nominal absence of Cl− is not inhibited by H2DIDS and must therefore be mediated by a novel channel or transporter.
DISCUSSION
Measurement of luminal pH and secretory rate
The rate of fluid secretion by the pancreatic duct epithelium (uncontaminated by acinar fluid) was first measured by Argent et al. (1986) in interlobular ducts isolated from rat pancreas. Using a micropuncture technique, they collected total fluid output over 1 h periods and determined the mean secretory rate under a variety of experimental conditions. To examine ductal HCO3− secretion in guinea-pig pancreatic ducts, we modified the micropuncture technique and used it to inject pH-sensitive BCECF-dextran into the luminal space thereby allowing changes in luminal pH to be measured (Ishiguro et al. 1996b). The limitations of this approach were that (a) it was not possible to quantify HCO3− secretion since changes in luminal volume were not taken into account, and (b) we could not determine whether the flux of HCO3− into the lumen was due to net secretion or due to exchange with luminal Cl− via a Cl−-HCO3− exchanger. To overcome these problems, in the present study we have used fluorescence images of BCECF-dextran in the luminal space to monitor simultaneously changes in luminal volume (and hence fluid secretion) and luminal pH (which reflects the HCO3− flux).
Validity of the experimental model
When isolated pancreatic ducts were stimulated with secretin or forskolin for 15 min, the luminal volume usually increased by 40–50 % in total. The fluid secretory rate during this period was quite constant, indicating that the accompanying rise in luminal hydrostatic pressure - which would tend to oppose duct swelling - was negligible. We routinely stopped the experiments after 15 min because, by that stage, the ionic composition of the luminal solution would probably differ significantly from that of the solution initially injected. However, in the longer experiments shown in Fig. 4, where secretin was applied after 20 min of spontaneous secretion, the response to secretin was undiminished despite the substantial accumulation of luminal fluid during the control period. Other studies, in which changes in luminal volume have been induced osmotically, have shown that the volume of isolated ducts can double without any significant restriction due to limited compliance (M. C. Steward & R. M. Case, in preparation).
Our assessment of the validity of this preparation as an experimental model for pancreatic duct fluid and HCO3− secretion is based on the following observations. First, fluid secretion was almost completely blocked by ouabain or by depletion of intracellular ATP. This shows that fluid secretion was achieved by primary or secondary active ion transport, and not by passive diffusion or osmotic imbalance. Secondly, secretin caused a concentration-dependent increase in fluid secretion which was mimicked by forskolin, an activator of adenylate cyclase. The concentration of secretin required for a half-maximal increase in fluid secretion was approximately 0.9 nM - similar to the Km for cyclic AMP production (2 nM) evoked by secretin in the same preparation (Fayeed et al. 1995).
Luminal mechanisms for HCO3− secretion in unstimulated ducts
In the anaesthetized guinea-pig a significant amount of spontaneous fluid secretion is observed and the concentration of HCO3− in the juice (ca. 95 mM) is moderately high (Padfield et al. 1989). In our experiments on isolated duct segments, spontaneous fluid secretion was observed when HCO3−/CO2 was added to the perfusate but the rate depended upon the initial ionic composition of the luminal fluid. When the luminal fluid initially contained 148 mM Cl−, spontaneous fluid secretion was fairly rapid, being equivalent to 57 % of the maximal secretory rate obtained with secretin (Fig. 3). At the same time pHL increased to a value above pH 8.0. This clearly indicates that spontaneous secretion is dependent on the presence of HCO3−/CO2 in the bath and that the secreted fluid is itself rich in HCO3− - consistent with the results of the in vivo studies. It is interesting to note, however, that in the isolated ducts the rate of spontaneous secretion in the presence of a high luminal Cl− concentration is much higher, relative to the secretin-evoked response, than is observed in vivo where the luminal Cl− concentration is much lower (Padfield et al. 1989). In agreement with this interpretation, we observed that the spontaneous secretion declines with time (Fig. 4), presumably as the initially high luminal Cl− concentration falls, whereas the response to secretin is maintained.
When the Cl− in the luminal fluid was replaced with glucuronate, both the secretory rate and the increase in pHL were greatly reduced (Fig. 5). With a more physiological luminal fluid, initially containing 23 mM Cl− and 125 mM HCO3−, the situation was similar. We therefore conclude that, in our experimental model, the spontaneous secretion of HCO3−-rich fluid is dependent on a moderately high luminal Cl− concentration. Furthermore, it is almost completely blocked by luminal H2DIDS (Fig. 11). This suggests that the luminal membrane mechanism responsible for spontaneous secretion may be analogous to that proposed for the rat pancreatic duct, namely a H2DIDS-blockable Cl−/HCO3− exchanger linked to a Cl− conductance (Gray et al. 1988; Novak & Greger, 1988b). In ducts containing 148 mM Cl−, the initially steep gradients for Cl− and HCO3− across the luminal membrane accelerate the passive exchange of these ions thus accounting for the rapid increase in pHL. The recycling of Cl− across the luminal membrane via the Cl− conductance, driven by the outward electrochemical gradient for Cl−, results in net solute transfer to the lumen and thus net fluid secretion.
Luminal mechanisms for HCO3− secretion in secretin-stimulated ducts
Although Cl−-free conditions inhibited responses in unstimulated ducts, the secretin-evoked increase in secretory rate and the final value of pHL were hardly altered when Cl− was replaced by glucuronate (Figs 3 and 5). This is in contrast to results from micropunctured rat ducts (Ashton et al. 1991) where secretin-evoked fluid secretion was reduced by 70 % when the Cl− in the bath solution was replaced by gluconate.
The secretion of HCO3−-rich fluid in response to secretin in the guinea-pig ducts was also unaffected by luminal H2DIDS under these conditions (Fig. 11). Although the mechanism by which HCO3− is transported into the lumen in the absence of Cl− is still unknown, our present data strongly suggest that this is the dominant pathway for luminal HCO3− transport in secretin-stimulated guinea-pig ducts.
Although the magnitude of the secretin-evoked component of fluid secretion in the guinea-pig ducts (shown in Fig. 11) appeared to be unaffected by replacement of Cl−, we nonetheless obtained some evidence of a contribution from luminal Cl−-HCO3− exchange when Cl− was present. Under these conditions, the secretin-stimulated increase in fluid secretion was partially but significantly inhibited (33 %) by luminal H2DIDS (Fig. 11B). This suggests that the luminal anion exchanger may play a role in secretin-stimulated fluid secretion when Cl− concentrations permit, but is not an essential component of the mechanism. This would be consistent with our own preliminary observations suggesting that secretin appears to activate Cl−-HCO3− exchange in guinea-pig pancreatic ducts (Ishiguro et al. 1995) - an observation which has also been reported in bile duct epithelial cells (Alvaro et al. 1993).
Accumulation of intracellular HCO3− across the basolateral membrane
The data in the present paper support our hypothesis (Ishiguro et al. 1996b) that intracellular accumulation of HCO3− in secretin-stimulated ducts is achieved largely by HCO3− uptake via a H2DIDS-sensitive Na+-HCO3− cotransporter and to a lesser extent by H+ extrusion via an MIA-sensitive Na+-H+ exchanger. When added to the bath separately, H2DIDS and MIA reduced the secretory rate during stimulation with secretin by 56 and 18 %, respectively. When applied simultaneously, however, they completely blocked secretion. The observation that the inhibitory effects of the two blockers are not additive suggests that inhibition of one pathway may lead to a compensatory increase in the activity of the other. This may simply be a consequence of sharing the same inward Na+ gradient as their primary driving force - a reduction in the Na+ influx through one transporter will increase the Na+ gradient driving the other one.
Although the existence of a vacuolar-type H+-ATPase has been demonstrated in rat, pig and also guinea-pig pancreatic duct cells (Zhao et al. 1994; Villanger et al. 1995; De Ondarza & Hootman, 1997), the lack of effect of bafilomycin A1 on secretin-evoked fluid secretion, as compared with the complete inhibition that we observed when MIA and H2DIDS were applied simultaneously, suggests that the contribution of the H+-ATPase is minimal in this species.
In summary, we have developed an experimental protocol for monitoring HCO3− and fluid secretion simultaneously in isolated interlobular duct segments from guinea-pig pancreas. The results obtained using this new methodology support our hypothesis that, in secretin-stimulated pancreatic duct cells, HCO3− uptake across the basolateral membrane is achieved largely by Na+-HCO3− cotransport, and that HCO3− efflux across the luminal membrane, and hence fluid secretion, may involve a novel, Cl−-independent mechanism.
Acknowledgments
This study was supported by the Pancreatic Research Foundation of Japan, the Ministry of Health and Welfare (Japan), The Wellcome Trust, the British Council (Tokyo) and a Monbusho international scientific research program grant from the Ministry of Education, Science, and Culture (Japan). We thank Dr G. Varga, Dr J. I. San Román, Mr T. F. Lang and Miss K. Kordas for helpful discussions.
APPENDIX
Calculation of the secretory HCO3− flux
The HCO3 content of the duct lumen Q is given by:
where C is the luminal concentration of HCO3 and V is the luminal volume. Since both C and V change with time, the rate of change of the HCO3 content of the duct lumen is given by:
since
where JHCO3 and Jv are the fluxes of bicarbonate and fluid volume expressed per unit area of epithelium (luminal membrane), we obtain:
To calculate dC/dt from the rate of change of luminal pH (dpHL/dt) we use:
If we assume that the luminal concentration of H2CO3 is constant, i.e. that there is rapid exchange of CO2 across the duct wall and sufficient carbonic anhydrase activity in the lumen, then the HCO3/CO2 buffering system acts as an open buffer with buffering capacity:
This is obtained by differentiating the Henderson- Hasselbalch equation:
where pK’ is the effective pK for the CO2-H2CO3-HCO3− buffering system (6.1 at 37°C) and s is the solubility of CO2 (3 × 10−5 mol l−1 mmHg−1 at 37°C). Rearranging this gives:
Differentiating at constant PCO2, and neglecting the small amount of buffering capacity due to the luminal Hepes (which will be minimal at the higher pHL values reached in these experiments), gives:
Thus:
Hence the flux of HCO3 can be calculated using:
and the effective HCO3 concentration of the secreted fluid is JHCO3/Jv.
References
- Alvaro D, Cho WK, Mennone A, Boyer JL. Effect of secretin on intracellular pH regulation in isolated rat bile duct epithelial cells. Journal of Clinical Investigation. 1993;92:1314–1325. doi: 10.1172/JCI116705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argent BE, Arkle S, Cullen MJ, Green R. Morphological, biochemical and secretory studies on rat pancreatic ducts maintained in tissue culture. Quarterly Journal of Experimental Physiology. 1986;71:633–648. doi: 10.1113/expphysiol.1986.sp003023. [DOI] [PubMed] [Google Scholar]
- Ashton N, Argent BE, Green R. Characteristics of fluid secretion from isolated rat pancreatic ducts stimulated with secretin and bombesin. The Journal of Physiology. 1991;435:533–546. doi: 10.1113/jphysiol.1991.sp018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundgaard M, Møller M, Poulsen JH. Localization of sodium pump sites in cat pancreas. The Journal of Physiology. 1981;313:405–414. doi: 10.1113/jphysiol.1981.sp013673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case RM, Argent BE. Pancreatic duct cell secretion: control and mechanisms of transport. In: Go VLW, Dimagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele GA, editors. The Pancreas: Biology, Pathophysiology, and Disease. 2. New York: Raven Press; 1993. pp. 301–350. [Google Scholar]
- Case RM, Harper AA, Scratcherd T. Water and electrolyte secretion by the perfused pancreas of the cat. The Journal of Physiology. 1968;196:133–149. doi: 10.1113/jphysiol.1968.sp008499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case RM, Scratcherd T. The secretion of alkali metal ions by the perfused cat pancreas as influenced by the composition and osmolality of the external environment and by inhibitors of metabolism and Na+,K+-ATPase activity. The Journal of Physiology. 1974;242:415–428. doi: 10.1113/jphysiol.1974.sp010715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ondarza J, Hootman SR. Confocal microscopic analysis of intracellular pH regulation in isolated guinea pig pancreatic ducts. American Journal of Physiology. 1997;272:G124–134. doi: 10.1152/ajpgi.1997.272.1.G124. [DOI] [PubMed] [Google Scholar]
- Dixon SJ, Cohen S, Cragoe EJ, Grinstein S. Estimation of the number and turnover rate of Na+/H+ exchangers in lymphocytes. Journal of Biological Chemistry. 1987;262:3625–3632. [PubMed] [Google Scholar]
- Fayeed OMH, Potter A, Musa OA, Lindsay ARG, Lau KR, Case RM. Intracellular cyclic AMP in interlobular pancreatic ducts isolated from guinea-pigs. The Journal of Physiology. 1995;489.P:129P.. [Google Scholar]
- Gray MA, Greenwell JR, Argent BE. Secretin-regulated chloride channel on the apical plasma membrane of pancreatic duct cells. Journal of Membrane Biology. 1988;105:131–142. doi: 10.1007/BF02009166. [DOI] [PubMed] [Google Scholar]
- Gray MA, Plant S, Argent BE. cAMP-regulated whole cell chloride currents in pancreatic duct cells. American Journal of Physiology. 1993;264:C591–602. doi: 10.1152/ajpcell.1993.264.3.C591. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Lindsay ARG, Steward MC, Case RM. Secretin stimulation of Cl−-HCO3− exchange in interlobular ducts isolated from guinea-pig pancreas. The Journal of Physiology. 1995;482.P:22P.. [Google Scholar]
- Ishiguro H, Steward MC, Lindsay ARG, Case RM. Accumulation of intracellular HCO3− by Na+-HCO3− cotransport in interlobular ducts from guinea-pig pancreas. The Journal of Physiology. 1996a;495:169–178. doi: 10.1113/jphysiol.1996.sp021582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Steward MC, Wilson RW, Case RM. Bicarbonate secretion in interlobular ducts from guinea-pig pancreas. The Journal of Physiology. 1996b;495:179–191. doi: 10.1113/jphysiol.1996.sp021583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers GAJ, Van Nooy IGP, De Pont JJHHM, Bonting SL. The mechanism of fluid secretion in the rabbit pancreas studied by means of various inhibitors. Biochimica et Biophysica Acta. 1984;778:324–331. doi: 10.1016/0005-2736(84)90376-6. [DOI] [PubMed] [Google Scholar]
- Madden ME, Sarras JRMP. Distribution of Na+, K+-ATPase in rat exocrine pancreas as monitored by K+-NPPase cytochemistry and [3H]-ouabain binding: a plasma membrane protein found primarily to be ductal cell associated. Journal of Histochemistry and Cytochemistry. 1987;35:1365–1374. doi: 10.1177/35.12.2824600. [DOI] [PubMed] [Google Scholar]
- Novak I, Greger R. Electrophysiological study of transport systems in isolated perfused pancreatic ducts: properties of basolateral membrane. Pflügers Archiv. 1988a;411:58–68. doi: 10.1007/BF00581647. [DOI] [PubMed] [Google Scholar]
- Novak I, Greger R. Properties of luminal membrane of isolated rat pancreatic ducts: effect of cyclic AMP and blockers of chloride transport. Pflügers Archiv. 1988b;411:546–553. doi: 10.1007/BF00582376. [DOI] [PubMed] [Google Scholar]
- Padfield PJ, Garner A, Case RM. Patterns of pancreatic secretion in the anaesthetized guinea pig following stimulation with secretin, cholecystokinin octapeptide, or bombesin. Pancreas. 1989;4:204–209. doi: 10.1097/00006676-198904000-00009. [DOI] [PubMed] [Google Scholar]
- Sohma Y, Gray MA, Imai Y, Argent BE. A mathematical model of the pancreatic duct epithelium. Journal of Membrane Biology. 1996;154:53–67. doi: 10.1007/s002329900132. [DOI] [PubMed] [Google Scholar]
- Villanger O, Veel T, Ræder MG. Secretin causes H+/HCO3− secretion from pig pancreatic ductule by vacuolar-type H+-adenosine triphosphatase. Gastroenterology. 1995;108:850–859. doi: 10.1016/0016-5085(95)90460-3. [DOI] [PubMed] [Google Scholar]
- Zhao H, Star RA, Muallem S. Membrane localization of H+ and HCO3− transporters in the rat pancreatic duct. Journal of General Physiology. 1994;104:57–85. doi: 10.1085/jgp.104.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]


