Abstract
The objective of this study was to determine potential post-pubertal gender-specific differences in the contractility of papillary muscles, the electrophysiological properties and Ca2+ transients of freshly dissociated ventricular myocytes from the rat heart.
The contractions of rat papillary muscles from 2- to 14-month-old male and female rats were studied under isometric and isotonic conditions (29 °C). While the hearts of young (2–4 months) male and female rats displayed a similar contractile profile, papillary muscles of female rats aged 6 months and older exhibited smaller isometric and isotonic contractions, smaller maximal rates of tension and shortening development and decline (±DT/dt and ±DL/dt) velocities during both the onset and relaxation phases, and shorter contractions than age-matched males.
To explore the possible cellular basis accounting for these differences, action potentials and macroscopic currents were recorded from freshly dissociated myocytes using the whole-cell patch clamp technique (35 °C). Action potentials from male and female myocytes of 3- and 9-month-old rats did not vary as a function of age or gender. Consistent with these results, the magnitude (expressed in pA pF−1), voltage-dependence and kinetics of the inward rectifier (IK1), transient outward (Ito) and sustained (IK) K+ currents displayed little, if any dependence on age or gender.
L-type Ca2+ current (ICa(L)) measured in caesium-loaded myocytes (35 °C) from male and female rats of 3, 6 and 9 months of age exhibited similar characteristics. In contrast, while Ca2+ transients measured with indo-1 were similar between 3-month-old male and female rat myocytes, Ca2+ transients of 10-month-old female myocytes were significantly reduced and showed a diminished rate of relaxation in comparison with those recorded in male rats of similar age.
These results suggest that important gender-related changes in excitation-contraction coupling occur following puberty, probably due to differences in Ca2+ handling capabilities at the level of the sarcoplasmic reticulum.
The hearts of men and women are known to respond differently to chronic or acute stress of both physiological and pathological nature. It is well documented that the incidence of and mortality rate due to coronary artery diseases are relatively lower in pre-menopausal women than men of similar age (Tobin et al. 1987; Grady et al. 1992). Although many factors may account for these gender-related differences, it is recognized that sexual hormones in women, mainly oestrogens, lower the risk of developing coronary heart disease. Indeed, hormone therapy reduces the risk for coronary heart disease in post-menopausal women, especially for those already diagnosed with the disease (Grady et al. 1992). In contrast, when comparisons are performed among groups demonstrating certain cardiovascular diseases, the relative risk of mortality may become similar, or even reverse. Although less likely to have invasive cardiac procedures, short-term morbidity and death rate of women who have experienced an acute myocardial infarction is worse than that of men (Greenland et al. 1991; Kostis et al. 1994). Interestingly the higher rate in early mortality of women with an acute myocardial infarction appears to be the result of greater death from low output, yet women surviving an acute myocardial infarction have a greater ejection fraction (Rouleau et al. 1996). Compensatory hypertrophy normalized to body surface is more prevalent in women with essential hypertension than men (Devereux et al. 1984), an observation consistently reported in animal studies (Scheuer et al. 1982).
Few experimental studies have been carried out to define specific gender differences regarding the contractile, electrical and biochemical behaviour of the cardiac muscle under physiological conditions. Investigations using the isolated working heart preparation have documented distinct cardiac adaptations to moderate (Schaible et al. 1981) or intense (Schaible & Scheuer, 1981) training exercise in male and female rats. Hearts from non-trained male rats showed greater stroke work and volume than hearts from female rats (Schaible et al. 1981). Although gonadectomy resulted in marked reduction in the mechanical function of the myocardium and alterations in the enzymatic profile (shifts in myosin isoforms, myosin ATPase and Ca2+-ATPase activities) in the two genders, the effects were more profound in the male (Schaible et al. 1984). Most of these effects could be prevented by hormone therapy in the two sexes, with testosterone being more effective in the male and female than oestrogen in the female (Scheuer et al. 1987). These studies suggest that fundamental, as well as adaptational differences exist between the mammalian hearts of males and females. The basis for these differences remains to be elucidated at the cellular and molecular levels.
The main purpose of our study was to determine the potential gender differences in the contraction of left ventricular papillary muscles measured isometrically and isotonically, and action potentials, ionic currents and Ca2+ transients recorded in freshly dissociated ventricular myocytes. As ageing is an important determinant of several cardiac parameters including contraction and action potential morphology (Capasso et al. 1983; Lakatta, 1987; Gwathmey et al. 1990; Felzen et al. 1991), ionic currents (Cohen & Lederer, 1988; Kilborn & Fedida, 1990; Wahler, 1992; Walker et al. 1993; Cerbai et al. 1994) and excitation- contraction coupling (Gwathmey et al. 1990; Xiao et al. 1994), we also examined whether these potential differences vary as a function of age from the post-pubertal period to middle age (2–14 months old). Our data indicate that the hearts of 6-month-old and older female rats exhibit significantly smaller isometric and isotonic contractions, tension and shortening velocities, and shorter contractions than age-matched males. Patch clamp experiments revealed no significant differences in the action potential and ionic currents between age-matched males and females from 3- to 9-month-old rats. In contrast, while Ca2+ transients measured with indo-1 were similar between 3-month-old male and female rat myocytes, Ca2+ transients of 10-month-old female myocytes were significantly reduced in comparison with those recorded in male rats of similar age. These results suggest that important gender-related changes in excitation-contraction coupling occur during maturation of the adult rat heart.
METHODS
Isolated papillary muscle studies
Male and female Wistar rats of 2, 3, 4, 6, 9 or 14 months of age were anaesthetized by an intramuscular injection of ketamine hydrochloride (87 mg kg−1) and xylazine (13 mg kg−1). Once the animal became unconscious (level of sedation assessed by tongue reflex), the chest was cut opened and the heart quickly removed. The left ventricle was opened and a papillary muscle was excised and mounted in a bathing chamber filled with Krebs-Henseleit solution bubbled with 95 % O2−5 % CO2 (pH 7.4) maintained at 29°C.
The base of the muscle was held by a stainless steel clamp, and the other end was tied to a lever with an electromagnetic feedback system (Brutsaert et al. 1988) to allow control of force, length and velocity. Once mounted, the muscle was field stimulated at 10 % above threshold strength at a frequency of 0.1 Hz with a Grass S-88 stimulator (Quincy, MA, USA) through platinum electrodes.
In all experiments, the preload was adjusted immediately after mounting the preparation by pulling the muscle to a length that yielded maximum developed tension (Lmax). The muscles were then equilibrated for 2 h while recording isotonic contractions at a speed of 0.05 mm s−1 on a Gould 2400 s pen recorder. At the end of the period of equilibration, isometric and isotonic contractions were measured at Lmax. For the maximum unloaded muscle velocity measurements (Vmax), after adjusting the elastic damping of the force-length-velocity lever feedback system to compensate for electromechanical transients, the velocity of shortening at 5 % of total tension (5 % load clamp) was obtained by abruptly decreasing the load on the muscle at the time of activation. The 5 % load clamp was used as an approximation of Vmax. The output of the Gould recorder was connected to an electronic differentiator and both signals were digitized at 0.5 kHz per channel by an A/D converter (model DT 2821-F-801, Data Translation Inc., Marlborough, MA, USA) relayed to a microcomputer (model 286, Compaq Computer Corp. Houston, TX, USA). Analysis of force-length properties was performed by custom-made software running under MS-DOS.
Single cell isolation procedure
Three, six, nine or ten-month-old Wistar rats of both sexes were heparizined (2000 units kg−1) at least 30 min before excising the heart from the animal under anaesthesia. The animal was anaesthetized following an identical protocol to that used in contraction experiments. After excision, the heart was quickly removed and placed in cold (4°C) physiological salt solution of the following composition (mM): 135 NaCl, 5.4 KCl, 0.33 NaH2PO, 1.0 MgCl2, 2.5 CaCl2, 5 Hepes-NaOH (pH 7.4, bubbled with 100 % O2), and 5.5 D-glucose. The connective tissue and pericardium were mechanically removed while perfusing in a retrograde fashion by gravity (65 cm column) at 37°C for 10–15 min at a flow rate of 0.8 ml min−1. After rinsing the vasculature free of blood, the perfusate was then switched to a nominally Ca2+-free perfusing solution for 5 min. To the Ca2+-free reperfusing medium, crude collagenase (Worthington, Type II, 160 U or 0.5 mg ml−1) and protease (Sigma, Type XIV, 1.35 U or 0.3 mg ml−1) were added and digestion was allowed to proceed at a flow rate of 0.5 ml min−1 for 35–45 min during which time the perfusate was recycled. The ventricles were then isolated and rinsed several times with fresh enzyme- and Ca2+-free solution, and cut into small pieces approximately 10 mm3 in size. The pieces were subsequently transferred to a 50 ml Falcon (Lincoln Park, NJ, USA) centrifuge flask containing 15 ml of an enzyme-free solution containing 0.2 mM Ca2+ (37°C). Myocytes were then dispersed by gentle agitation and the supernatant containing viable myocytes kept at room temperature. This last step of rinsing and agitating was repeated several times and the supernatant stored for each cycle. Only rod-shaped myocytes displaying clear striations and lacking spontaneous contractile activity or waves were used in this study, normally within 8 h following isolation.
Electrophysiology
Calcium tolerant cardiac ventricular myocytes were either current or voltage clamped using the standard whole-cell configuration of the patch clamp technique (Hamill et al. 1981). Large diameter micropipettes were pulled using a two-stage micropipette puller (Narishige Scientific Instruments, Tokyo, Japan; model PP-83) and polished using a microforge (Narishige Scientific Instruments; model FP-83). With tip diameters of about 1 μm, the pipette resistances were in the range of 2–4 MΩ when filled with the internal solutions described below. Voltage or current clamp protocols were computer driven using pCLAMP software (version 5.5.1) and an Axopatch-1D amplifier or Axopatch 200 integrating patch clamp amplifier (Axon Instruments Inc., Foster City, CA, USA). Pipette and stray capacitance and series resistance were compensated for in all voltage clamp experiments. Leak subtraction was carried out either on-line by adjusting the leak subtraction knob on the patch clamp amplifier or off-line by digital subtraction using scaled currents evoked by voltage clamp steps that did not elicit time-dependent current. Action potentials were evoked in current clamp mode by applying 10 ms current pulses whose amplitudes were set at ×∼1.25 above threshold strength. Membrane current or voltage was low-pass filtered at 1 or 2 kHz (4-pole bessel filter) before being acquired at a sampling rate of 2 or 5 kHz using a PC-486 computer interfaced with a 12-bit analog-to-digital acquisition board (TL-125, Axon Instruments Inc.). Once digitized, the data were temporarily stored on the computer hard disk for later analysis (using pCLAMP version 6.0, Axon Instruments Inc.) and display (Hewlett Packard LaserJet series III).
Solutions and drugs
The composition of the Krebs-Henseleit solution used to superfuse the papillary muscles in contraction studies was the following (mM): 118 NaCl, 24.9 NaHCO3, 3.5 KCl, 1.2 KH2PO4, 2.43 MgSO4, 1.25 CaCl2, and 5 dextrose (pH maintained at 7.4 by bubbling with a 95 % O2−5 % CO2 gas at 29°C).
For all electrophysiological experiments, the bathing media were delivered by gravity at a rate of ∼1 ml min−1. Except for the indo-1 experiments which were carried out at 22°C, all other experiments were performed at 35°C. After a settling time of 5–10 min on a coverslip glued onto the bottom of a small lucite chamber (total volume < 1 ml), the ventricular myocytes were superfused with a Hepes-buffered solution having the following composition (mM): 140 NaCl, 4.2 KCl, 1.2 KH2PO4, 0.5 MgCl2, 2.5 CaCl2, 5.5 dextrose, and 5 Hepes-NaOH (pH 7.4). Action potentials, K+ currents and indo-1 fluorescence were all measured while the cells were superfused with the above solution. The micropipette solution used to dialyse the cells was as follows (mM): 110 potassium gluconate, 30 KCl, 10 NaCl, 0.5 MgCl2, 5 MgATP, 0.1 EGTA, and 5 Hepes-KOH (pH 7.2). For the indo-1 experiments, EGTA was omitted from the pipette solution and indo-1 (penta-potassium salt form) was added at a final concentration of 50 μM (from a 500 μM stock in DMSO).
The L-type Ca2+ current was recorded using bathing and pipette solutions that were designed to minimize the activity of K+ channels, electrogenic ion exchangers and Ca2+-dependent currents. The composition of the superfusate was as follows (mM): 140 NaCl, 10 CsCl, 0.5 MgCl2, 2.5 CaCl2, 5.5 dextrose, and 5 Hepes-CsOH (pH 7.4). The micropipette solution used to dialyse the cells contained (mM): 105 aspartic acid, 105 CsOH, 20 CsCl, 20 tetraethylammonium chloride (TEA), 0.5 MgCl2, 5 MgATP, 5 EGTA, and 5 Hepes-CsOH (pH 7.2).
ATP, EGTA, TEA, aspartic acid, potassium gluconate, CsCl, CsOH, Hepes, DMSO and isoprenaline were purchased from Sigma Chemical Co. Indo-1 was obtained from Molecular Probes (Eugene, OR, USA). All other chemicals and salts are from Mallinckrodt Inc. (Paris, KY, USA).
Indo-1 fluorescence
In some experiments, the concentration of free intracellular Ca2+ ([Ca2+]i) was simultaneously measured with membrane potential in current clamped cells using a ratiometric technique as previously reported (Leblanc & Hume, 1990; Leblanc & Leung, 1995). In brief, the penta-potassium salt form of the fluorescent Ca2+ indicator indo-1 (50 μM) was added to the pipette solution and allowed to dialyse into the cell after gaining whole-cell access. Background and cell autofluorescence were cancelled out just before gaining whole-cell access. A mercury arc lamp was used to excite indo-1. The fluorescent dye was excited at 340 nm by means of a narrow bandwidth filter (±10 nm) and a dichroic mirror (> 380 nm). Exposure of the cell to UV light was controlled by an electronic shutter (Optikon, model T132, Vincent Associates Rochester, NY, USA) anchored between the arc lamp and the epifluorescence attachment of an inverted Nikon Diaphot epifluorescence microscope (× 40 Nikon oil-immersion fluor objective; NA, 1.3). The shutter could be either manually triggered to open to verify the level of loading of indo-1, or switched in the open position by a transistor-transistor logic (TTL) pulse delivered by the computer acquisition interface during current clamp protocols. The circular excitation beam was slightly larger than the recording window, a procedure which minimized background fluorescence contamination emanating from the dye present in the pipette solution. The emitted fluorescent light (> 380 nm) was then relayed to the lateral port of the microscope and processed by a spectral microfluorimeter (Sycamore Scientific, Santa Barbara, CA, USA) equipped with a CCD camera (Pulnix America Inc., model TM-440, Sunnyvale, CA, USA) and a TV monitor (JVC, model TM-122U, Tokyo, Japan) to view the image of the cell throughout the experiment and insure its proper alignment within the detection window. The emitted light was first split by a series of dichroic mirrors, and passed through narrow bandpass (±10 nm) filters centred at 400 and 500 nm. Light intensity was monitored by means of two matched photo multiplier tubes (Hamamatsu type R2560HA). The analogue ratio (Burr Brown Corp., model M/N DIV100HP, Allan Crawford Associates Ltd., Calgary, Alberta, Canada) of the two fluorescent signals (400 nm : 500 nm; indo-1 ratio : signal used to monitor relative changes in [Ca2+]i) was electronically filtered using a low-pass Butterworth filter set at 60 Hz (Frequency Devices model 901, Haverhill, MA, USA) and digitized on-line with membrane voltage using the same acquisition system described above (TL-1-125 LabMaster Board, Axon Instruments Inc.) and the data temporarily stored on the computer's hard disk (PC- 486/33 MHz).
Experimental protocols
In order to perform a valid comparison of the functional properties of muscles and myocytes originating from different animals, it was important to establish a sequence of protocols that was rigorously followed. For all current and voltage clamp experiments, cell capacitance was measured by integrating the mean of five capacitative current transients (using pCLAMP software, version 6.0) elicited by consecutive 20 ms test pulses from −50 to −60 mV. Membrane capacitance was calculated using the following equation: C =Q/ΔV, where C is the whole-cell capacitance (in pF), Q is the amount of charge transferred (in fC), and ΔV is the magnitude of the hyperpolarizing pulse (in mV).
For the current (except indo-1) and voltage clamp experiments carried out with K+-containing solutions, cell dialysis was allowed to proceed for 2 min. Sixteen action potentials were first recorded in current clamp mode at a frequency of 0.1 Hz; this protocol was repeated after a 2 min rest period, except that the frequency of stimulations was raised to 0.5 Hz. After 1 min rest, the amplifier was switched to voltage clamp mode and the cell maintained at a holding potential (Vh) of −80 mV. From this Vh, 250 ms steps from −120 to +50 mV were applied in 10 mV increments at a frequency of 0.5 Hz to record macroscopic currents (see Fig. 3A).
Figure 3. Age and sex differences in the mechanical properties of rat papillary muscles studied under isotonic conditions.
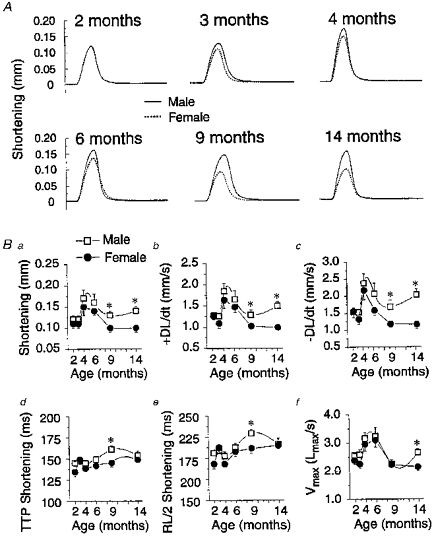
A, typical isotonic contractions recorded from male (continuous lines) and female (dotted lines) papillary muscles for all six age groups investigated. B, summary of mean isotonic contractile data. Graphs are plots of age-dependent changes of peak shortening (Ba); shortening velocity (+DL/dt, Bb); relaxation velocity (-DL/dt, Bc); time-to-peak shortening (TTP Shortening, Bd); duration of contraction (RL/2 Shortening, Be); and maximum shortening velocity measured at Lmax (Vmax, Bf) of male (□) and female (•) papillary muscles. ±DL/dt and Vmax were obtained from electronic differentiation of the tension recordings. TTP Shortening and RL/2 Shortening are the times from beginning of contraction to 50 % lengthening, respectively. * Significantly different from age-matched females with P < 0.05. Number of hearts used is identical to that described in Fig. 1.
For measurements of L-type Ca2+ current (ICa(L)) with Cs+ and TEA-based solutions, after 2 min rest period to allow dialysis while maintaining the cell at Vh=−80 mV, a two-step protocol was used: an initial 500 ms step to −45 mV to inactivate voltage-dependent Na+ channels, followed by 250 ms steps ranging from −40 to +70 mV applied in 10 mV increments (0.5 Hz) to record ICa(L) (Fig. 4A). Ca2+ transients were measured in indo-1- and K+-loaded myocytes in response to current clamp steps imposed at a frequency of 0.1 Hz as described above. The protocol was initiated 5 min after breaking the gigaohm seal. Five minutes was a reasonable compromise between sufficient loading of the dye and run-down of the membrane systems involved in excitation- contraction coupling. If loading was judged inadequate after this period, the cell was systematically discarded.
Figure 4. Analysis of the resting and action potentials of current clamped cardiac myocytes from 3- and 9-month-old male and female rats.
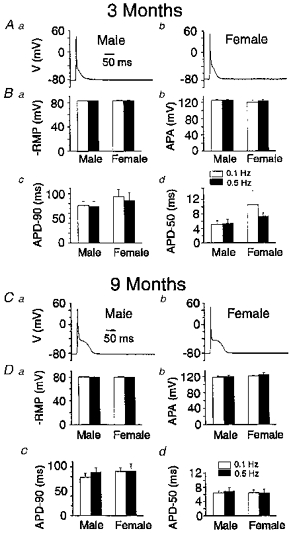
A, typical recordings of membrane action potentials from cells obtained from 3-month-old male (left trace) and female (right trace) myocytes (0.1 Hz). B, pooled data reporting the measurements of resting membrane potential (-RMP, Ba); action potential amplitude (APA, Bc); and action potential duration measured at 90 % (APD-90, Bc) and 50 % (APD-50, Bd) repolarization. The results obtained at 0.1 (open columns) and 0.5 Hz (filled columns) stimulation rates were taken from 13 and 9 myocytes isolated from 5 male and 5 female rat hearts, respectively. Statistical comparisons between gender, and frequencies of stimulation revealed no significant difference between means. Panels C and D adopt the same nomenclature except the data were obtained from 9-month-old animals. D, results were obtained from 16 and 14 myocytes isolated from 3 male and 2 female rat hearts, respectively. As for panel B, none of the comparisons revealed statistically significant differences between means.
Statistical analysis
All values presented in graphs are means ±s.e.m. Student's unpaired t test was used to assess the statistical significance of differences between age-matched male and female preparations. One-way ANOVA test was employed to determine the statistical significance when more than two groups were compared, in particular when comparing possible age differences within the same gender. A probability of P < 0.05 was accepted as the level of significance.
RESULTS
Figure 1 reports the heart weight, body weight, heart weight : body weight ratio, and estimated ventricular cell surface of male (open bars) and female (filled bars) rats of different ages. The heart and body weights of male and female rats increased with age and were smaller in female animals than those of age-matched males (Fig. 1A and B). The heart weight : body weight ratio of male and female rats displayed a steady decline from 2- to 6-month-old rats and remained fairly constant thereafter (Fig. 1C), as similarly observed in a study with female rats (Capasso et al. 1983); except for the comparison of 3- and 6-month-old animals which was just at the limit of significance, this parameter was significantly larger in females than age-matched males. Cell capacitance of isolated myocytes from 3-, 6- and 9-month-old male rats was 24, 20 and 28 % larger, respectively, than cells from age-matched female animals (Fig. 1D).
Figure 1. Age and gender differences in heart weight (A), body weight (B), heart weight to body weight ratio (C) and capacitative cell surface area (D).
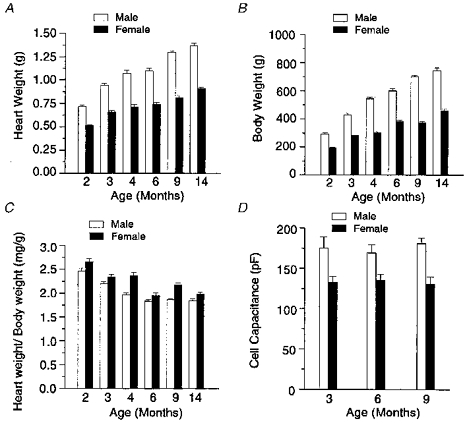
As indicated, open and filled columns are data obtained from male and female rats, respectively. For both male and female rats, panels A, B and C report the mean data derived from 8 to 14 animals. For panels A and B, all comparisons between males and females revealed highly significant differences (P < 0.001). C, comparisons between male and female rats of 3 and 6 months of age were just at the limit of significance (P = 0.065 and 0.102, respectively); all other comparisons revealed significant differences with P < 0.05. For panels A, B and C mean cross-sectional areas of male (M) and female (F) papillary muscles from animals of the different age groups (x months = x m) studied were the following (mm2): 2 m, M 0.90 ± 0.07; F 0.71 ± 0.07 (n.s., P = 0.08); 3 m, M 0.89 ± 0.09; F 0.81 ± 0.08 (n.s., P = 0.53); 4 m, M 0.80 ± 0.05; F 0.71 ± 0.07 (n.s., P = 0.28); 6 m, M 0.82 ± 0.06; F 0.87 ± 0.06 (n.s., P = 0.58); 9 m M 0.80 ± 0.05; F 0.75 ± 0.10 (n.s., P = 0.64); 14 m, M 0.83 ± 0.06; F 0.77 ± 0.04 (n.s., P = 0.43). D, membrane capacitance of myocytes of 3-, 6- and 9-month-old female rats was 133 ± 7 pF (n = 16), 135 ± 7 pF (n = 17), and 130 ± 9 pF (n = 13), respectively; the capacitance of myocytes of 3-, 6- and 9-month-old males was 175 ± 13 pF (n = 16), 169 ± 10 pF (n = 14), and 181 ± 6 pF (n = 16), respectively. A comparison of cell capacitance of age-matched males and females showed significant differences for all three age groups (P < 0.01); no significant difference was detected for each gender as a function of age (P > 0.7).
Contraction studies
The mechanical properties of left ventricular papillary muscles of male and female rats of 2–14 months of age were investigated and data are summarized in Figs 2 and 3. For all age groups, mean cross-sectional area was similar for male and female papillary muscles (see legend of Fig. 1) indicating that the muscles were comparable in size. Figure 2A illustrates typical isometric tension recordings for all age groups tested. One general observation from these recordings was that for all age groups, the contraction of female muscles (dashed lines) tended to be smaller than their male counterpart (continuous lines), and this difference was accentuated for 6-month-old animals and older.
Figure 2. Isometric contractile profile of male and female rat papillary muscles as a function of age.
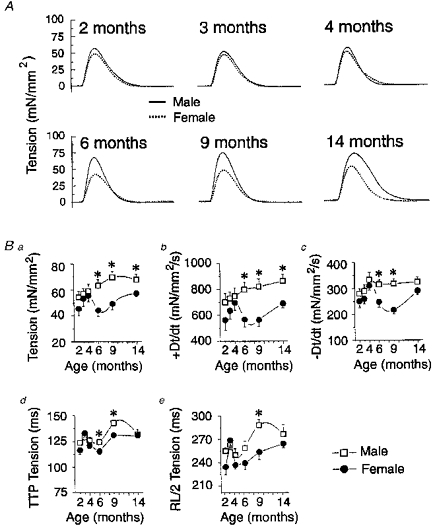
A, sample tension recordings from male (continuous lines) and female (dotted lines) papillary muscles recorded under isometric conditions for all six age groups investigated. Tension was normalized against the cross-sectional area of the muscles. B, summary of mean isometric contractile data. Graphs are plots of age-dependent changes in normalized peak tension (Ba); normalized velocity of tension development (+DT/dt, Bb); normalized velocity of tension relaxation (-DT/dt, Bc); time-to-peak tension (TTP Tension, Bd); and duration of contraction (RL/2 Tension, Be) of male (□) and female (•) papillary muscles. ±DT/dt were obtained from electronic differentiation of the tension recordings. TTP Tension and RL/2 Tension are the times from beginning of contraction to 50 % tension decline, respectively. * Significantly different from age-matched females with P < 0.05. Number of hearts used is identical to that described in Fig. 1.
This is demonstrated further in Fig. 2B which shows the age-dependent changes of several parameters which were pooled from similar experiments. Peak tension (Fig. 2Ba) normalized to cross-sectional area of male papillary muscles (open squares) increased from 2 to 9 months of age and remained stable up to 14 months. A similar behaviour was apparent for the maximum rate of tension development (+DT/dt, Fig. 2Bb), maximum rate of tension decline (-DT/dt, Fig. 2Bc), time-to-peak tension (TTP Tension, Fig. 2Bd) and duration of contraction (RL/2 Tension, Fig. 2Be). Age-dependent changes in the contractile profile of female papillary muscles was different. After an initial gradual increase between 2 and 4 months, peak tension declined between 4 and 6 months and slowly increased from 6 to 14 months; +DT/dt, -DT/dt, TTP tension and RL/2 tension followed a similar pattern to that of peak tension. Peak tension and +DT/dt were significantly reduced for muscles of 6-, 9- and 14-month-old females; -DT/dt and TTP tension were also smaller for muscles of 6- and 9-month-old females vs. males. Although a tendency for a difference was apparent for hearts from 6- to 14-month-old rats, the duration of contraction was only significantly longer in 9-month-old males.
During the same experiments, we also analysed the contractile profile of these same papillary muscles under isotonic conditions and the results are documented in Fig. 3. As in Fig. 2, panel 3A depicts sample tension traces for the six age groups investigated. Shortening amplitude increased and then decreased with age in male papillary muscles with a peak reached between 4 and 6 months. The amplitude of shortening was also larger in male vs. female muscles at 9 and 14 months of age. The duration of contraction was noticeably longer for 9-month-old male vs. female muscles.
The mean data for these experiments are displayed in Fig. 3B. As evident for both male and female hearts, shortening amplitude (Fig. 3Ba), shortening (+DL/dt, Fig. 3Bb) and relaxation (-DL/dt, Fig. 3Bc) velocities, and shortening velocity at Lmax (Vmax, Fig. 3Bf) varied in a bell-shaped manner as a function of age with a maximum reached at 4 months. Although there was a tendency for these parameters to be smaller in female hearts at 4 and 6 months, a significant difference between the two genders was only revealed at 9 and 14 months, with the exception of Vmax which was statistically different only at 14 months. A significant difference in TTP shortening (Fig. 3Bd) and duration of contraction (RL/2 shortening, Fig. 3Be) was only detected at 9 months. Taken together, the results demonstrate that the contractile properties of rat cardiac muscle vary with age and gender. Gender mechanical differences seem to occur at a post-pubertal stage and are in general maintained up to 14 months of age. The heart of male rats older than 4 months exhibits a better mechanical performance than that of females, evidenced from a more powerful contraction and faster rates of onset and relaxation.
Electrophysiological experiments in freshly isolated myocytes
Gender-related differences in the mechanical performance of rat cardiac muscle could, at least in part, be related to alterations in the basic electrical properties of the myocytes. This possibility was examined by carrying out whole-cell patch clamp experiments on freshly dispersed ventricular myocytes isolated from male and female rat hearts. The electrophysiological characteristics were compared in two age groups. Experiments were performed using 3- and 9-month-old rats as these ages revealed the least and most distinct contractile properties between males and females.
Figure 4A shows typical action potentials recorded under current clamp conditions from 3-month-old male (Aa) and female (Ab) myocytes. The myocytes were well polarized displaying a resting membrane potential (RMP) of ∼−80 mV. The morphology of the action potentials was characterized by: (1) a fast depolarizing upstroke which peaks near +50 mV, followed by (2) rapid and (3) slow repolarizing phases as previously shown by others. Figure 4B summarizes data obtained from a large number of myocytes for both genders. The resting and action potential parameters were studied at two frequencies of stimulation, 0.1 (open columns) and 0.5 Hz (filled columns). Male and female myocytes exhibited similar values of RMP (Fig. 4Ba), action potential amplitude (APA, Fig. 4Bb), and action potential durations measured at 90 % (APD-90, Fig. 4Bc) and 50 % (APD-50, Fig. 4Bd) repolarization, this at the two frequencies of stimulation studied. All these parameters were not significantly different from those measured in myocytes from 9-month-old rats of both sexes (Fig. 4C and D). These results indicate that the basic resting and action potential characteristics did not vary with age, gender or frequency of stimulations in our conditions.
Since the shape of the action potential is determined by a fine balance between inward and outward currents, it is possible that the apparent lack of changes seen in the above experiments could be related to the alterations of several ion channels leading to a similar morphology of the action potential. This question was explored by comparing K+ currents in myocytes from male and female hearts of 3- and 9-month-old rats. Three K+ currents mainly determine the shape of the rat ventricular action potential: (1) an inward rectifier K+ current (IK1) which plays a role in maintaining RMP and during the late repolarizing phase of the action potential (Wahler, 1992); (2) a large 4-aminopyridine-sensitive transient outward K+ current (Ito) which is responsible for the rapid component of repolarization (Josephson et al. 1984; Apkon & Nerbonne, 1991); and (3) a slowly inactivating delayed rectifier K+ current (IK) (Apkon & Nerbonne, 1991) which also contributes to repolarization of the action potential at depolarized membrane potentials. Figure 5A and B show typical families of currents evoked by the protocol shown at the bottom of panel A, and recorded from male (left traces) and female (right traces) myocytes from 3- and 9-month-old rats, respectively. From a holding potential of −80 mV, hyperpolarizing steps ranging from −120 to −90 mV elicited a large inward current which partially inactivated during the step. Mild depolarizations above the holding potential revealed time-independent currents that inwardly rectified. The properties of the inward and outward current components during hyperpolarizing and mild depolarizing steps, respectively, are consistent with those of IK1. Although mean currents recorded from male myocytes were larger than those of females (data not shown), when expressed as current density, there were no significant differences in current magnitude at both ages studied. Steps more positive than −40 mV elicited a transient followed by a sustained outward current. Depolarizing steps from a holding potential of −50 mV markedly reduced the size of the early transient and sustained current (data not shown). These data support the idea that the dynamic outward currents measured at positive potentials are produced by Ito and IK. As evident in Fig. 5A and B, the amplitude and kinetics of inward and outward currents in male and female myocytes at both ages were very similar. Figure 5C and D show plots of the current-voltage (I-V) relationships for peak inward and outward current (Ipeak, filled symbols) and current measured at the end of the pulse (ISS, open triangles) for the two genders at 3 (panel C) and 9 months of age (panel D). There were no 11significant differences between currents recorded from male and female myocytes at any voltage examined, nor were there any age-dependent changes in amplitude. The lack of age- and gender-related changes of the three major K+ currents in this preparation largely explains our failure to detect alterations in RMP and action potential morphology.
Figure 5. Possible age- and gender-specific changes in K+ currents of male and female myocytes from two age groups.
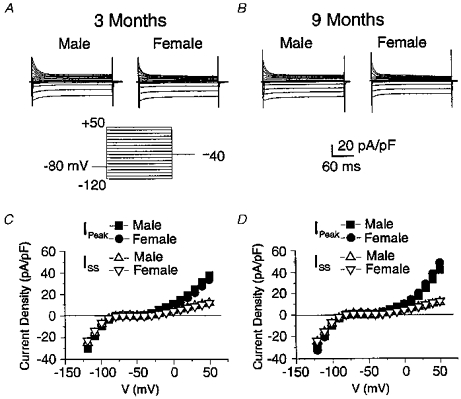
A, typical families of currents elicited by the voltage clamp protocol shown below from a 3-month-old male (left traces) and female (right traces) myocyte. B, similar families of currents recorded under identical conditions except they were obtained from 9-month-old animals. Notice for panels A and B that current is expressed as current density (in pA pF−1). C, summary of mean current-voltage relationships of peak inward and outward current density (Ipeak) and late or sustained current density during the pulse (ISS) for 3-month-old male and female myocytes as indicated. Standard errors of the mean are within the size of the symbols. Data from 18 and 25 myocytes from male and female rats, respectively. D, summary of similar data obtained from 9-month-old rats; nomenclature is identical to panel C. Data were obtained from 21 and 19 myocytes from male and female rats, respectively. For panels C and D, statistical analysis of current density between male and female myocytes revealed no significant differences at all potentials. There were also no differences detected within each gender as a function of age.
We next sought to determine whether there are any age- or sex-related differences of L-type Ca2+ current (ICa(L)) which contributes to the early phase of repolarization (Mitchell et al. 1984b; Yuan et al. 1996) and plays an important role in triggering Ca2+-induced Ca2+ release (CICR) in this preparation (Cannell et al. 1987; Nabauer et al. 1989). Figure 6A and B show typical families of transient inward Ca2+ currents recorded in Cs+ and TEA conditions from myocytes of 3- (panel A) and 9-month-old (panel B) male and female rats as indicated. The currents were elicited by the protocol shown at the bottom of Fig. 6A. Ca2+current density was similar in the two genders at either age. However, there was a clear tendency for ICa(L) to increase as a function of age. Panels C, D and E of Fig. 6 report the mean I-V relationships of ICa(L) measured in myocytes from male (open circles) and female (filled circles) rats of 3, 6 and 9 months of age, respectively. Ca2+ currents of male and female myocytes displayed nearly identical bell-shaped I-V relationships at all three ages examined. As mentioned above, peak ICa(L) density increased with age in the two genders. This is better reflected in Fig. 6F which depicts a plot of the changes in peak ICa(L) density measured at 0 mV as a function of age for the two sexes. These results indicate that the smaller ventricular contractility of female rats cannot be attributed to a change in ICa(L).
Figure 6. Effect of gender and age on L-type Ca2+ current.
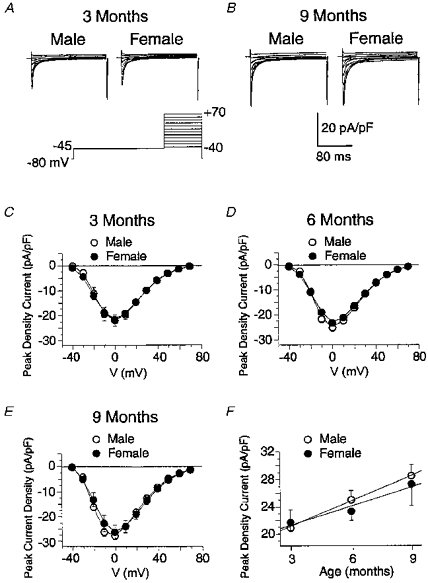
A, two sample families of L-type Ca2+ currents recorded from myocytes of 3-month-old male (left traces) and female (right traces) rats. These currents were evoked by the double pulse protocol displayed at the bottom. Note that current calibration is in picoamps per picofarad. B, same nomenclature as panel A except that the traces were recorded from 9-month-old animals. Panels C, D and E show mean I-V relationships of peak inward Ca2+ current density measured from male (○) and female (•) myocytes of 3-, 6- and 9-month-old rats, respectively. These results were obtained from pooled experiments identical to those of panels A and B. In panels C, D and E, data are taken from 16 and 19, 14 and 19, and 16 and 13 myocytes from male and female rats, respectively. Statistical analysis revealed no significant differences between male and female myocytes at any voltage examined for the three age groups. F, plot of changes in peak ICa(L) density measured at 0 mV as a function of age for the two genders. Lines are linear least-squares fits to the means.
One possibility to explain the smaller level of contractility of female papillary muscles of older animals may relate to distinct excitation-contraction coupling properties to those of male muscles. To examine this question, we simultaneously measured action potentials and Ca2+ transients with patch clamp and indo-1 fluorescence techniques in myocytes dialysed and superfused with K+-containing solutions. Figure 7A and B show sample recordings of action potentials (top traces) and indo-1 ratio (bottom traces) from cells of male (left) and female (right) 3- and 10-month-old rats. The two age groups were chosen because no differences in isometric (Fig. 2) and isotonic (Fig. 3) contractions were observed for 3-month-old animals, whereas 6- to 14-month-old rats exhibited the largest differences in contraction. The 1st and 16th traces of a train of action potentials (0.1 Hz) are shown. Rat ventricular myocytes are known to exhibit post-rest potentiation after a period of rest and a negative force-frequency relationship (Capogrossi et al. 1986; Borzak et al. 1991; Bassani & Bers, 1994). Both male and female myocytes showed a large initial Ca2+ transient (after a resting period of 5 min) whose amplitude diminished during stimulation. Calcium transients recorded in myocytes from 3-month-old male and female rats displayed very similar characteristics (Fig. 7A). In contrast, both the initial and steady-state Ca2+ transients were found to be smaller in cells from 10-month-old female vs. male rats (Fig. 7B), in spite of comparable levels of resting [Ca2+]i (data not shown). This is further demonstrated in Fig. 7C where the mean data for the two genders and age groups are represented in bar graphs.
Figure 7. Effects of age and gender on intracellular Ca2+ transients recorded simultaneously with action potentials in whole-cell current clamped myocytes at room temperature.
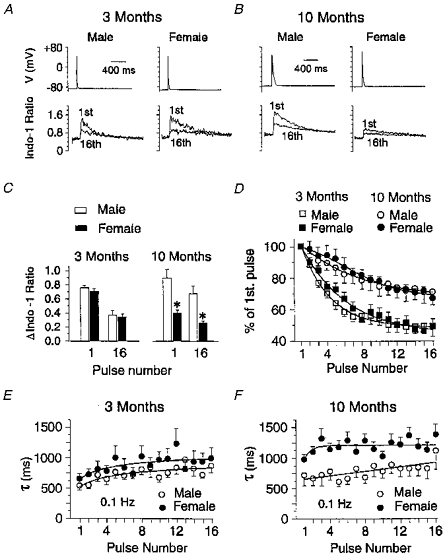
A, sample recordings of action potentials (top traces) and Ca2+ transients (lower traces) from myocytes of 3-month-old male (left) and female (right) rats. Only the 1st and 16th traces of a train at 0.1 Hz are shown for the sake of clarity. B, similar nomenclature to panel A except the data were obtained from myocytes of 10-month-old rats. C, bar graphs reporting mean data of the first (pulse number 1) and last (pulse number 16) Δindo-1 ratio (peak - rest) recorded during a 0.1 Hz train for the two genders and age groups as indicated. The train was imposed after a 5 min rest period. * Significantly different from male with P < 0.001. D, pulse dependence of normalized Ca2+ transient amplitude of male and female myocytes of the two age groups as labelled. The amplitude of each Ca2+ transient during the train was normalized against that evoked during the 1st pulse and thus expressed as a percentage of 1st pulse. The lines are single exponential or polynomial fits to the means. E, plot of the pulse dependence of the time constant of relaxation of the Ca2+ transient (τ) of myocytes of 3-month-old male (○) and female (•) rats. The two lines are mono-exponential fits to the means. F, same nomenclature except the data were derived from 10-month-old animals. The straight and curved lines are least-squares linear and single exponential fits to the means. The data shown in panels C-F were obtained from 13 to 20 myocytes in each group.
Figure 7D shows a plot where the mean amplitude of all Ca2+ transients during each pulse of the train was normalized against the first Ca2+transient and plotted as a function of pulse number. The decline of Ca2+ transients of male and female myocytes of each age group followed a similar time course. An interesting observation was the distinct steady-state level reached by the two age groups; Ca2+ transients of myocytes from 3- and 10-month-old rats declined to ∼50 % and 72 % of their initial control levels, respectively.
The time constant of relaxation of the Ca2+ transient (τ) was also analysed in each group. For males and females, τ mildly increased during stimulation (Fig. 7E and F). While τ measured in myocytes from 3-month-old males and females were comparable (Fig. 7E), τ estimated in cells from 10-month-old females were larger than those measured in males (Fig. 7F), indicating slower rates of relaxation. Taken together, the results suggest that the observed differences in mechanical performance of papillary muscles of older female and male rats may be attributed, at least in part, to fundamental differences in intracellular Ca2+ handling capabilities in the two genders.
DISCUSSION
The main purpose of this study was to determine whether basic functional differences exist between male and female hearts. A second objective was to compare the cardiac performance of the two genders as a function of age. The contractile parameters of rat papillary muscles increased for both genders and were not significantly different from one another between the ages of 2 and 4 months. In contrast, the hearts of females were found to exhibit relatively weaker properties than those of males after 4–6 months of age, a condition that was maintained up to 14 months of age. Current clamp experiments in single ventricular myocytes revealed no significant differences in action potential morphology between the two genders for 3- and 9-month-old animals; consistent with this, L-type Ca2+, inward rectifier K+, and transient as well as sustained outward current densities were found to be independent of gender and age. However, while intracellular Ca2+ transients (indo-1) were similar in myocytes from 3-month-old male and female rats, 10-month-old female myocytes generated much smaller Ca2+ transients than age-matched male cells. Our results show for the first time that fundamental gender differences exist in Ca2+ signalling mechanisms of myocytes from mature animals, probably at the level of the sarcoplasmic reticulum (SR), and may explain, at least in part, the smaller contractility of the hearts of older female rats.
Morphological changes during development
The body and heart weights increased in parallel during development in female and male rats, with females displaying lower heart and body masses than males. In contrast, the heart weight : body weight ratio was significantly higher in females than males, this at all ages examined. Although it is tempting to suggest that female rats may compensate their relatively weaker heart by increasing their heart mass, this hypothesis is not supported by the fact that in spite of larger heart weight : body weight ratios, there was no significant difference in contractility between the two genders for 2- to 4-month-old rats. Cell capacitance of myocytes from male hearts, a parameter commonly used as an index of cell surface, was found to be ∼25 % larger than that measured from age-matched female myocytes. Although not systematically studied, male myocytes also appeared larger than female cells when visually examined under the microscope. Surprisingly, this parameter did not vary as a function of age for 3- to 9-month-old rats in the two genders. Although we cannot exclude the possibility that this type of measurement may be somewhat biased by subjectively selecting cells, this appears unlikely in view of: (1) the very homogeneous distributions of cells in the different age and gender groups (notice the small error bars in Fig. 1D); and (2) the fact that regardless of age, the cell capacitance of female myocytes was consistently smaller than that of male cells. Since the difference in cell capacitance of myocytes from male and female rats remained constant for animals between 3 and 9 months of age, it seems unlikely that the smaller contractile profile of female hearts of 6 months and older is related to differences in cell morphology. More experiments will be necessary to address this question in more detail.
Tension studies in papillary muscles
A remarkable feature of our papillary muscle tension studies is that significant gender differences in cardiac contractility appeared relatively late during development, that is between 4 and 9 months of age for isometric and isotonic contractions. Peak isometric contraction of male papillary muscles increased linearly from 2 to 14 months; this was accompanied by similar time-dependent changes in the rates of contraction and relaxation. Although female papillary muscles displayed a similar contractile profile to that of males from 2 to 4 months, this was followed by a sudden drop in contraction between 4 and 6 months, an effect which was maintained up to 14 months of age. Although Capasso et al. (1983) did not find an early decline in contractility from 4 to 6 months in a study performed only with female rats, they nevertheless reported little change in peak isometric contraction for animals between 1 and 4 months of age, and a progressive increase, as we did, from 6 to 10 months. More complex age-dependent changes in isotonic contractility were observed in the two genders with bell-shaped relationships for peak contraction, and contraction and relaxation rates. Our results with female rats are consistent with the reported decline in shortening velocity from 4- to 10-month-old female animals (Capasso et al. 1983). Qualitatively similar results were noticed between male and female rats with significant differences being detected at 9 months and tendencies of weaker contractions at earlier stages of growth. For both isotonic and isometric contractions, the more powerful contractions of male papillary muscles at later stages of growth tended to be longer than those of females.
One possibility that might explain the gender differences seen at later developmental stages is that they may occur as a consequence of the physiological changes imposed by puberty. In the rat, puberty is known to take place 50 to 60 days after birth in the two sexes. Even though sexual maturity may be reached after ∼2 months of age, post-pubertal changes related to alterations in the hormonal status of the two genders (testosterone in the male and oestrogens in the female) may last for several months, as seen by analogy in humans. Our results do not provide an explanation for the fact that the female rat heart becomes relatively weaker than that of males during ageing. This aspect will require further investigation.
Membrane action potentials and ionic currents in single cells
Patch clamp experiments were carried out in enzymatically dispersed ventricular myocytes to determine if the gender-related changes in contractility of multicellular cardiac preparations were due to altered membrane electrical properties that may directly or indirectly influence Ca2+ entry across the sarcolemma. Current clamp experiments at two frequencies of stimulation (0.1 and 0.5 Hz) failed to reveal any significant change in action potential morphology. Consistent with these data were the results of voltage clamp experiments which showed that the inward rectifier K+ current or IK1, dominant conductance near the resting membrane potential (Wahler, 1992), and transient outward K+ (Ito) and delayed rectifier K+ (IK, sustained current) outward currents that are mainly responsible for the repolarization of the action potential in this preparation (Josephson et al. 1984; Apkon & Nerbonne, 1991) were similar in the two genders and age groups studied. Although early developmental changes in IK1 have been reported (Wahler, 1992), evidence suggests that these channels undergo little change in the adult and ageing heart (Wahler, 1992; Walker et al. 1993). Opposite to our findings, Walker et al. (1993) reported an increase in action potential duration of male cells from 2- to 25-month-old rats which was attributed to an age-dependent reduction of Ito. The decrease of Ito and associated increase in APD has been suggested to compensate for the well-known reduced rate of Ca2+ refilling by the sarcoplasmic reticulum and desensitization to β-adrenergic stimulation of the senescent heart which is known to develop slower contractions (Lakatta, 1987). Besides differences in experimental conditions and isolation procedures, we have no explanation for this discrepancy. Only two age groups (3 and 9 months of age) were studied in the hope of highlighting possible differences in ion channel activities between the two genders. We cannot rule out the possibility that significant changes could have been detected at later stages of life.
Although L-type Ca2+ current (ICa(L)) tended to increase from 3- to 9-months of age in the two genders, this effect was found to lie just at the limit of significance. Age-matched myocytes from the two genders exhibited similar current densities and kinetics. Our results are consistent with those of Walker et al. (1993) who reported a lack of changes in the magnitude of ICa(L) as a function of age. The similar magnitude and kinetics of ICa(L) of male and female myocytes from age-matched rats can thus not explain the weaker contraction of the female heart seen in older animals. Our data suggest that the gender and age differences in contractility are probably due to events that are downstream of the action potential and sarcolemmal ion channels.
Intracellular Ca2+ transients
In cardiac muscle, the action potential triggers a complex cascade of events that leads to a build-up of intracellular Ca2+ which then activates actomyosin cross-bridge cycling and contraction. Although it has been proposed that reverse-mode Na+-Ca2+ exchange (Leblanc & Hume, 1990; Kohmoto et al. 1994) or a voltage-dependent mechanism (Ferrier & Howlett, 1995) may participate in this event, it is now widely accepted that the resulting Ca2+ transient is mainly due to the fusion of localized Ca2+ transients or ‘Ca2+ sparks’. These membrane-delimited events are believed to be mediated by the opening of clusters of SR Ca2+ release channels (ryanodine receptors) responding to Ca2+ entry through single L-type Ca2+ channels (dihydropyridine receptors), to which they are co-localized at the transverse tubular membrane (Cheng et al. 1993). In rat ventricular myocytes, a very small fraction of the Ca2+ entering the cell through ICa(L) can directly stimulate the myofilaments; thus the majority of the calcium ions sensed by fluorescent Ca2+ probes comes from SR stores.
To explore the possibility that intracellular ionic mechanisms might be involved, we simultaneously measured membrane potential and intracellular concentration of Ca2+ ([Ca2+]i, indo-1) in male and female myocytes from 3- and 10-month-old rats. Ca2+ transients were elicited by action potentials imposed at a frequency of 0.1 Hz following a 5 min rest period allowing cell dialysis of indo-1. No significant age and gender differences in the indo-1 ratio at rest were observed between all groups examined. Similar to our study, Xiao et al. (1994) found little age-dependent (2–24 months) differences in Ca2+ current, Ca2+ transient and contraction of male cardiac myocytes. However, whereas peak Ca2+ transient and rate of relaxation of the indo-1 ratio were similar in the two genders for 3-month-old rats, significant differences were apparent at 10 months of age.
Rat ventricular myocytes are known to exhibit post-rest potentiation and negative staircase behaviours following resumption of stimulation after a period of rest (Capogrossi et al. 1986; Borzak et al. 1991; Bassani & Bers, 1994). Potentiation of the Ca2+ transient and twitch contraction after a long diastolic interval has been attributed to enhanced Ca2+ loading of the SR as a consequence of relatively smaller Ca2+ efflux (or perhaps Ca2+ influx produced by a small but finite reverse-mode activity) at rest by the Na+-Ca2+ exchanger in this species (Shattock & Bers, 1989) or time-dependent recovery of the SR Ca2+ process (Orchard & Lakatta, 1985). In contrast to other species such as rabbit and guinea-pig which exhibit positive staircase force-frequency relations, the negative staircase relationship of the rat ventricle has been suggested to occur as a consequence of: (1) the very short plateau of the action potential which limits Ca2+ entry through ICa(L) (Szigligeti et al. 1996), (2) enhanced Ca2+ efflux through forward-mode Na+-Ca2+ exchange during each action potential, and (3) the strong re-uptake activity of the SR Ca2+-ATPase (Bassani & Bers, 1994). The net effect is a time-dependent reduction in SR Ca2+ content until a steady-state is reached where Ca2+ extrusion via the Na+-Ca2+ exchanger perfectly balances Ca2+ entry through ICa(L). In our study, the amplitudes of the first (P1; post-rest) and last (P16; steady-state) Ca2+ transients of a train of sixteen action potentials were both smaller in a 10-month-old female vs. an age-matched male myocytes. Whether the age-dependent increases in steady-state peak Ca2+ transient in the two genders are related to alterations in SR Ca2+ and/or sarcolemmal membrane transporters cannot be deduced from our data, and will require more experiments. An interesting corollary observation is the fact that when the Ca2+ transients during the train were normalized against that elicited during P1, the negative staircase relationships in the two genders were virtually identical. These results suggest that in spite of absolute gender differences in magnitude, the relative importance of the Ca2+ handling systems of age-matched male and female myocytes is probably relatively similar.
Two systems are mainly responsible for the extrusion of Ca2+ from the cytoplasmic compartment and thus determine the relaxation rate of the Ca2+ transient: (1) the SR Ca2+-ATPase, and (2) the Na+-Ca2+ exchanger; in adult rat myocytes, the former and latter transporters account for 92 and 7 %, respectively, with minor contributions from the sarcolemmal Ca2+-ATPase and mitochondria (Bassani et al. 1994). The smaller Ca2+ transients seen in older female cells were also accompanied by slower rates of relaxation as evident from the larger time constant τ. These data support the contention that the reduced contractility of adult female compared with male papillary muscles may be, at least in part, related to alterations in intracellular Ca2+ handling capabilities. The larger τ of Ca2+ transients could partly account for the reduced rate of isometric (-DT/dt) and isotonic (-DL/dt) relaxation of papillary muscles of > 6-month-old female rats. Based on the following arguments, we speculate that it is likely that the activity of the Na+-Ca2+ exchanger was similar in the two genders: (1) in cardiac myocytes, Ca2+ entering the cell through voltage-gated Ca2+ channels is matched by an equivalent efflux of Ca2+ through the Na+-Ca2+ exchanger (Bridge et al. 1990), a process that maintains Ca2+ homeostasis within tight limits; since ICa(L) density and kinetics were similar in male and female myocytes, one can anticipate that with a similar load of intracellular Na+ (both cell types were dialysed with 10 mM Na+) and provided that other systems still play a relatively small role in Ca2+ homeostasis (Bassani et al. 1994) the activity of the exchanger would remain relatively constant; (2) since the final phase of repolarization of the rat ventricular action potential is extremely sensitive to conditions that affect Na+-Ca2+ exchange activity (Mitchell et al. 1984a; Schouten & ter Keurs, 1991), one would expect a major gender difference in APD-90, which was not observed, even at two frequencies of stimulation.
An alternative and more attractive possibility that might explain the gender differences observed in our study is that the SR of middle-aged male and female cardiac cells each display distinctive behaviour. Whether the smaller Ca2+ transient is due to a reduced efficacy of the Ca2+-induced Ca2+ release triggering mechanism, or changes in the properties of the ryanodine receptor and SR Ca2+ release process (generation of Ca2+ sparks, inactivation, recovery, etc.), SR Ca2+ re-uptake mechanism or a combination of these factors, will necessitate further investigation. One possible explanation might be that the SR Ca2+ content of female myocytes is reduced relative to that of male cells, this related to reduced activity from the SR Ca2+-ATPase. The SR Ca2+ content has a profound effect on the efficacy of the triggering mechanism, that is, fractional Ca2+ release for a given amplitude of ICa(L) increases with enhanced loading of the SR (Bassani et al. 1995). However, one must be careful in interpreting these results as the relatively smaller rate of relaxation of the Ca2+ transient seen in 10-month-old female myocytes cannot necessarily be taken as proof that SR Ca2+ re-uptake is relatively weaker. Indeed the rate of decline of [Ca2+]i during a Ca2+ transient elicited under physiological conditions is known to increase as a function of peak [Ca2+]i (Bers & Berlin, 1995).
Physiological significance of our findings
To our knowledge, this is the first systematic study designed to unravel gender-specific differences in cardiac mechanical function at the cellular level. Our data indicate that the myocardium of male and female rats undergoes distinct post-pubertal changes that confer to the latter a relatively weaker contractile profile that appears to be related to a reduced ability of the myocytes to generate intracellular Ca2+ transients, probably at the level of the SR. Our results extend previous experimental studies that reported that the heart of sedentary male rats displayed greater stroke work and volume than the heart of female rats (Schaible et al. 1981) and further advance our knowledge related to gender-specific adaptations (Schaible et al. 1984; Scheuer et al. 1987) of the heart muscle to physiological stimuli such as exercise (Schaible et al. 1981; Schaible & Scheuer, 1981) and to pathological stimuli such as myocardium loss due to myocardial infarction, a problem that results in morbidity and mortality in a large percentage of the human population.
Acknowledgments
This work was supported by grants awarded to N. L. and J.-L. R. from the Heart and Stroke Foundation of Québec, the Medical Research Council of Canada, and funds from the Fonds pour la Formation de Chercheurs et l'Aide à la Recherche (FCAR) and Montréal Heart Institute. N. L. is a Fonds de Recherche en Santé du Québec (FRSQ) scholar.
References
- Apkon M, Nerbonne JM. Characterization of two distinct depolarization-activated K+ currents in isolated adult rat ventricular myocytes. Journal of General Physiology. 1991;97:973–1011. doi: 10.1085/jgp.97.5.973. 10.1085/jgp.97.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani JWM, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells - species-dependent differences in cellular mechanisms. The Journal of Physiology. 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani JWM, Yuan WL, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. American Journal of Physiology. 1995;268:C1313–1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- Bassani RA, Bers DM. Na-Ca exchange is required for rest-decay but not for rest-potentiation of twitches in rabbit and rat ventricular myocytes. Journal of Molecular and Cellular Cardiology. 1994;26:1335–1347. doi: 10.1006/jmcc.1994.1152. [DOI] [PubMed] [Google Scholar]
- Bers DM, Berlin JR. Kinetics of [Ca]i decline in cardiac myocytes depend on peak [Ca]i. American Journal of Physiology. 1995;268:C271–277. doi: 10.1152/ajpcell.1995.268.1.C271. [DOI] [PubMed] [Google Scholar]
- Borzak S, Murphys S, Marsh JD. Mechanisms of rate staircase in rat ventricular cells. American Journal of Physiology. 1991;260:H884–892. doi: 10.1152/ajpheart.1991.260.3.H884. [DOI] [PubMed] [Google Scholar]
- Bridge JHB, Smolley JR, Spitzer KW. The relationship between charge movements associated with ICa and INa-Ca in cardiac myocytes. Science. 1990;248:376–378. doi: 10.1126/science.2158147. [DOI] [PubMed] [Google Scholar]
- Brutsaert DL, Meulemans AL, Sipido KR, Sys SU. Effects of damaging the endocardial surface on the mechanical performance of isolated cardiac muscle. Circulation Research. 1988;62:358–366. doi: 10.1161/01.res.62.2.358. [DOI] [PubMed] [Google Scholar]
- Cannell MB, Berlin JR, Lederer WJ. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 1987;238:1419–1423. doi: 10.1126/science.2446391. [DOI] [PubMed] [Google Scholar]
- Capasso JM, Malhotra A, Remily RM, Scheuer J, Sonneblick EH. Effects of age on mechanical and electrical performance of rat myocardium. American Journal of Physiology. 1983;245:H72–81. doi: 10.1152/ajpheart.1983.245.1.H72. [DOI] [PubMed] [Google Scholar]
- Capogrossi MC, Kort AA, Spurgeon HA, Lakatta EG. Single adult rabbit and rat cardiac myocytes retain the Ca2+ and species-dependent systolic and diastolic contractile properties of intact muscle. Journal of General Physiology. 1986;88:589–613. doi: 10.1085/jgp.88.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerbai E, Barbieri M, Li Q, Mugelli A. Ionic basis of action potential prolongation of hypertrophied cardiac myocytes isolated from hypertensive rats of different ages. Cardiovascular Research. 1994;28:1180–1187. doi: 10.1093/cvr/28.8.1180. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks - elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Cohen NM, Lederer WJ. Changes in the calcium current of rat heart ventricular myocytes during development. The Journal of Physiology. 1988;406:115–146. doi: 10.1113/jphysiol.1988.sp017372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux RB, Lutas EM, Casale PN, Kligfield P, Eisenberg RR, Hammond IW, Miller DH, Reiss G, Alderman MH, Laragh JH. Standardization of M-mode echocardiographic left ventricular anatomic measurements. Journal of the American College of Cardiology. 1984;4:1222–1230. doi: 10.1016/s0735-1097(84)80141-2. [DOI] [PubMed] [Google Scholar]
- Felzen B, Rubinstein I, Lotan R, Binah O. Developmental changes in ventricular action potential properties in guinea-pigs are modulated by age-related changes in the thyroid state. Journal of Molecular and Cellular Cardiology. 1991;23:787–794. doi: 10.1016/0022-2828(91)90212-5. 10.1016/0022-2828(91)90212-5. [DOI] [PubMed] [Google Scholar]
- Ferrier GR, Howlett SE. Contractions in guinea-pig ventricular myocytes triggered by a calcium-release mechanism separate from Na+ and L-currents. The Journal of Physiology. 1995;484:107–122. doi: 10.1113/jphysiol.1995.sp020651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B, Ernster VL, Cummings SR. Hormone therapy to prevent disease and prolong life in postmenopausal women. Annals of Internal Medicine. 1992;117:1016–1037. doi: 10.7326/0003-4819-117-12-1016. [DOI] [PubMed] [Google Scholar]
- Greenland P, Reicher-Reiss H, Goldbourt U, Behar S Israeli Sprint Investigators. In-hospital and 1-year mortality in 1524 women after myocardial infarction: comparison with 4315 men. Circulation. 1991;83:484–491. doi: 10.1161/01.cir.83.2.484. [DOI] [PubMed] [Google Scholar]
- Gwathmey JK, Slawsky MT, Perreault CL, Briggs GM, Morgan JP, Wei JY. Effect of exercise conditioning on excitation-contraction coupling in aged rats. Journal of Applied Physiology. 1990;69:1366–1371. doi: 10.1152/jappl.1990.69.4.1366. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Josephson IR, Sanchez-Chapula J, Brown AM. Early outward current in rat single ventricular cells. Circulation Research. 1984;54:157–162. doi: 10.1161/01.res.54.2.157. [DOI] [PubMed] [Google Scholar]
- Kilborn MJ, Fedida D. A study of the developmental changes in outward currents of rat ventricular myocytes. The Journal of Physiology. 1990;430:1937–1960. doi: 10.1113/jphysiol.1990.sp018280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohmoto O, Levi AJ, Bridge JHB. Relation between reverse sodium-calcium exchange and sarcoplasmic reticulum calcium release in guinea pig ventricular cells. Circulation Research. 1994;74:550–554. doi: 10.1161/01.res.74.3.550. [DOI] [PubMed] [Google Scholar]
- Kostis JB, Wilson AC, O'Dowd K, Gregory P, Chelton S, Cosgrove NM, Chirala A, Cui T. Sex differences in the management and long-term outcome of acute myocardial infarction. A statewise study. Circulation. 1994;90:1715–1730. doi: 10.1161/01.cir.90.4.1715. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Cardiac muscle changes in senescence. Annual Review of Physiology. 1987;49:519–531. doi: 10.1146/annurev.ph.49.030187.002511. [DOI] [PubMed] [Google Scholar]
- Leblanc N, Hume JR. Sodium current induced release of calcium from cardiac sarcoplasmic reticulum. Science. 1990;248:372–376. doi: 10.1126/science.2158146. [DOI] [PubMed] [Google Scholar]
- Leblanc N, Leung PM. Indirect stimulation of Ca2+-activated Cl− current by Na+-Ca2+ exchange in rabbit portal vein smooth muscle. American Journal of Physiology. 1995;268:H1906–1917. doi: 10.1152/ajpheart.1995.268.5.H1906. [DOI] [PubMed] [Google Scholar]
- Mitchell MR, Powell T, Terrar DA, Twist VW. The effects of ryanodine, EGTA and low-sodium on action potentials in rat and guinea-pig ventricular myocytes: evidence for two inward currents during the plateau. British Journal of Pharmacology. 1984a;81:543–550. doi: 10.1111/j.1476-5381.1984.tb10107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Powell T, Terrar DA, Twist VW. Strontium, nifedipine, and 4-aminopyridine modify the time course of the action potential in cells from rat ventricular muscle. British Journal of Pharmacology. 1984b;18:551–556. doi: 10.1111/j.1476-5381.1984.tb10108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabauer M, Callewaert G, Cleemann L, Morad M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989;244:800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- Orchard CH, Lakatta EG. Intracellular calcium transients and developed tension in rat heart muscle: a mechanism for the negative interval-strength relationship. Journal of General Physiology. 1985;86:637–651. doi: 10.1085/jgp.86.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau J-L, Talajic M, Sussex B, Potvin L, Warnica W, Davies RF, Gardner M, Stewart D, Plante S, Dupuis R, Lauzon C, Ferguson J, Mikes E, Balnozan V, Savard P. Myocardial infarction patients in the 1990s - Their risk factors, stratification and survival in Canada: the Canadian Assessment of Myocardial Infarction (CAMI) Study. Journal of the American College of Cardiology. 1996;27:1119–1127. doi: 10.1016/0735-1097(95)00599-4. [DOI] [PubMed] [Google Scholar]
- Schaible TF, Malhotra A, Ciambrone G, Scheuer J. The effects of gonadectomy on left ventricular function and cardiac contractile proteins in male and female rats. Circulation Research. 1984;54:38–49. doi: 10.1161/01.res.54.1.38. [DOI] [PubMed] [Google Scholar]
- Schaible TF, Penpargkul S, Scheuer J. Cardiac responses to exercise training in male and female rats. Journal of Applied Physiology. 1981;50:112–117. doi: 10.1152/jappl.1981.50.1.112. [DOI] [PubMed] [Google Scholar]
- Schaible TS, Scheuer J. Cardiac function in hypertrophied hearts from chronically exercised female rats. Journal of Applied Physiology. 1981;50:1140–1145. doi: 10.1152/jappl.1981.50.6.1140. [DOI] [PubMed] [Google Scholar]
- Scheuer J, Malhotra A, Hirsch A, Capasso J, Schaible TF. Physiological cardiac hypertrophy corrects contractile protein abnormalities associated with pathologic hypertrophy in rats. Journal of Clinical Investigation. 1982;70:1300–1305. doi: 10.1172/JCI110729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuer J, Malhotra A, Schaible TF, Capasso J. Effects of gonadectomy and hormonal replacement on rat hearts. Circulation Research. 1987;61:12–19. doi: 10.1161/01.res.61.1.12. [DOI] [PubMed] [Google Scholar]
- Schouten VJA, ter Keurs HEDJ. Role of ICa and Na+/Ca2+ exchange in the force-frequency relationship of rat heart muscle. Journal of Molecular and Cellular Cardiology. 1991;23:1039–1050. doi: 10.1016/0022-2828(91)91639-9. [DOI] [PubMed] [Google Scholar]
- Shattock MJ, Bers DM. Rat vs. rabbit ventricle: Ca flux and intracellular Na assessed by ion-selective microelectrodes. American Journal of Physiology. 1989;256:C813–822. doi: 10.1152/ajpcell.1989.256.4.C813. [DOI] [PubMed] [Google Scholar]
- Szigligeti P, Pankucsi C, Banyasz T, Varro A, Nanasi PP. Action potential duration and force frequency relationship in isolated rabbit, guinea-pig and rat cardiac muscle. Journal of Comparative Physiology B. 1996;166:150–155. doi: 10.1007/BF00301179. [DOI] [PubMed] [Google Scholar]
- Tobin JN, Wassertheil-Smoller S, Wexler JP, Steingart RM, Budner N, Lense L, Wachspress J. Sex bias in considering coronary bypass surgery. Annals of Internal Medicine. 1987;107:19–25. doi: 10.7326/0003-4819-107-1-19. [DOI] [PubMed] [Google Scholar]
- Wahler GM. Developmental increases in the inwardly rectifying potassium current of rat ventricular myocytes. American Journal of Physiology. 1992;262:C1266–1272. doi: 10.1152/ajpcell.1992.262.5.C1266. [DOI] [PubMed] [Google Scholar]
- Walker KE, Lakatta EG, Houser SR. Age-associated changes in membrane currents in rat ventricular myocytes. Cardiovascular Research. 1993;27:1968–1977. doi: 10.1093/cvr/27.11.1968. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Spurgeon HA, O'Connor F, Lakatta EG. Age-associated changes in β-adrenergic modulation on rat cardiac excitation-contraction coupling. Journal of Clinical Investigation. 1994;94:2051–2059. doi: 10.1172/JCI117559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan WL, Ginsburg KS, Bers DM. Comparison of sarcolemmal calcium channel current in rabbit and rat ventricular myocytes. The Journal of Physiology. 1996;493:733–746. doi: 10.1113/jphysiol.1996.sp021418. [DOI] [PMC free article] [PubMed] [Google Scholar]


