Abstract
Intercellular electrical communication between smooth muscle and endothelial cells was examined in guinea-pig mesenteric arterioles using the whole-cell patch-clamp method. The time course of the current required to impose a 10 mV voltage clamp step was used to determine the extent of electrical coupling between them. Currents recorded from both smooth muscle and endothelial cells relaxed in a multi-exponential manner, indicating the existence of electrical coupling between cells.
18β-Glycyrrhetinic acid, a gap junction blocker, quickly blocked electrical communication at 40 μM, while neither heptanol nor octanol did so at concentrations of up to 1 mM.
In the current clamp mode, repetitive spikes, induced by 10 mM Ba2+ solutions, could be recorded from both kinds of cells. After blocking gap junctions, spikes could only be recorded from the smooth muscle cell layer, indicating that they had been conducted through myoendothelial junctions.
In endothelial cells, acetylcholine (ACh, 3 μM) induced hyperpolarizing responses, which had two phases (an initial fast and a second slower phase) in the current clamp condition. This ACh response persisted in the presence of 18β-glycyrrhetinic acid, although this compound seemed to make the membrane slightly leaky.
After blocking gap junctions, the membrane potential of a single cell in a multicellular preparation could be well clamped. Thus, 18β-glycyrrhetinic acid may be useful in studying the function of both arteriolar smooth muscle and endothelial cells while they remain located within a multicellular preparation.
Vascular endothelial cells play an important role in the control of vascular motor activity. Vasoactive substances such as nitric oxide can be released from endothelial cells and influence the underlying smooth muscle cells. In the vascular wall, on the other hand, coupling sites between the smooth muscle and endothelial cells exist which allow the passage of current (von der Weid & Bény, 1993; Bény, 1997) and dyes between the two layers (Little et al. 1995). These myoendothelial gap junctions may also play an important role in the control of vascular motor activity by allowing humoral and electrical communication between two cell layers.
The importance of gap junctions has often been assessed by applying substances such as heptanol, octanol and halothane which clearly block gap-junctions in cardiac muscle (for review, see Spray & Burt, 1990). However their effectiveness in smooth muscle has been questioned (Hashitani & Suzuki, 1997). In liver epithelial cells, 18β-glycyrrhetinic acid, a saponin isolated from licorice root, has been shown to block dye movements between neighbouring cells (Guan et al. 1996). The effect of this compound has not yet been examined on smooth muscle preparations. We have examined the effect of 18β-glycyrrhetinic acid on mesenteric arterioles of the guinea-pig. This preparation was chosen because it has been shown that electrical coupling between cells exists (Hirst & Neild, 1978) but the exact nature of this communication has not been determined. Our results indicate the existence of electrical coupling between smooth muscle and endothelial cells in the arteriole, coupling which is effectively inhibited by 18β-glycyrrhetinic acid.
METHODS
Tissue preparation
Male guinea-pigs, weighing 250–400 g, were killed by CO2 inhalation and exsanguination. The ileum was excised and the first-order arterioles were dissected from it (diameter, 50–100 μm). Two kinds of preparations were made from these arterioles. For the experiments where recordings were made from the smooth muscle cells, the arteriole was incubated in nominally Ca2+-free solution containing 0.5 mg ml−1 collagenase (Wako Pure Chemical Industries, Ltd, Osaka, Japan) for 15 min at 35°C, and then the adventitial layer was removed to reveal the smooth muscle layer. For recording from the endothelial cells, the tapering arteriole segment was inverted by pushing the smaller end into the lumen with a tungsten wire (20 μm in diameter); no enzymatic treatment was used. Segments of 1 mm of these preparations were pinned out in a small (0.8 mm × 1.5 mm) recording chamber. Preparations were superfused with preheated (35°C) bath solution at a constant rate (1 ml min−1).
Fluorescence microscopy
Segments of arterioles were inverted in the presence of fluorescein isothiocyanate (FITC)-labelled dextran with a molecular weight of 4000 (1 mg ml−1). The inside and outside of the vessels were washed and remaining fluorescence was observed by a fluorescence microscope with excitation of 460–490 nm and emission of > 515 nm.
Electrophysiological techniques
The tight-seal patch-clamp method was used in conventional whole-cell clamp configuration. Current or voltage signals were acquired using an EPC-7 patch-clamp system (List-Medical), filtered with a 4-pole low-pass Bessel filter (E-3201A; NF Circuit Design Block, Yokohama, Japan), and then digitized with a Digidata 1200A data acquisition system (Axon Instruments). Voltage clamp protocols were controlled with pCLAMP software (Axon Instruments). In current clamp experiments, voltage signals were stored on a DAT tape with a bandwidth of DC-10 kHz (PC-204, Sony Magnescale Inc., Tokyo) before digitization.
Solutions and chemicals
The composition of standard bath solution was (mM): NaCl, 141.5; KCl, 5.4; CaCl2, 1.8; MgCl2, 1; Hepes, 10; glucose, 5. The pH was adjusted to 7.3 with NaOH. Nominally Ca2+-free solution was prepared by simply omitting CaCl2; 10 mM Ba2+ solution was prepared by adding BaCl2 to nominally Ca2+-free solution. The pipette solution contained (mM): KCl, 143; MgCl2, 1; EGTA, 1; Hepes, 10; glucose, 5. The pH was adjusted to 7.3 with KOH. FITC-dextran, heptanol, octanol, 18β-glycyrrhetinic acid and acetylcholine (ACh) were obtained from Sigma. Solutions containing heptanol or octanol were made using either DMSO (0.1 %; Wako Pure Chemical) or α-cyclodextrin (concentrations up to 10 mM; Wako Pure Chemical) as the solvent. 18β-Glycyrrhetinic acid was dissolved directly in the bath solution.
Statistics
Numerical data are represented as means ± standard deviation, with n referring to the number of measurements. Differences between groups of data were tested by checking both the variances (F test) and means (Student's t test).
RESULTS
Patch-clamp recordings were made on the superfused smooth muscle cells in preparations in which the adventitial layer had been removed by enzyme treatment. Alternatively endothelial cells were accessed in inverted preparations, the apical membrane of the endothelial cell being smooth enough to undertake patch-clamp recordings without enzymatic treatment (Yamamoto et al. 1992). Possible damage of the endothelial cells during the inverting process was assessed, because endothelial cells sometimes came off the vessel walls during the process. Seven arterioles were inverted in the presence of the extracellular space marker FITC-dextran (1 mg ml−1), and FITC-dextran was then washed out thoroughly. Under a fluorescence microscope, cells which had been peeled off during the inverting process were clearly observed; however, none of the endothelial cells remaining on the arterial walls were stained. As we used endothelial cells that remained on the arterial walls only, the data presented in this paper came from living cells which could exclude the extracellular space marker.
When an isolated single cell is voltage clamped, the relaxation of capacitive current due to a small voltage step can be well fitted to a single exponential function (Lindau & Neher, 1988). This is not the case, however, when multicellular preparations are used (Fig. 1A). The current produced by a 10 mV voltage step recorded from a smooth muscle cell is shown in Fig. 1Aa; that from an endothelial cell is shown in Fig. 1Ab. In the smooth muscle cell, the fast relaxation of the current was followed by smaller and slower components. In the endothelial cell, on the other hand, the current relaxed slowly in a multi-exponential manner. Quantitative analysis was not performed on the time course of relaxation; it is well documented that voltage control along multicellular preparations is poor (de Roos et al. 1996). However, only input resistance (Rin) was calculated by the following equations:
| (1) |
and
| (2) |
where Vp is the amplitude of the voltage step, Ra is the access resistance of the pipette, and I0 and Iss are the membrane current amplitude at the beginning and the end of voltage step, respectively. Calculated mean input resistance was 82.2 ± 60.8 MΩ (n = 23; range, 28.0- 308.7 MΩ) in the smooth muscle cells and 4.6 ± 2.8 MΩ (n = 16; range, 1.5-10.4 MΩ) in the endothelial cells.
Figure 1. Membrane current and resting membrane potential obtained from smooth muscle and endothelial cells in intact segments of arterioles.
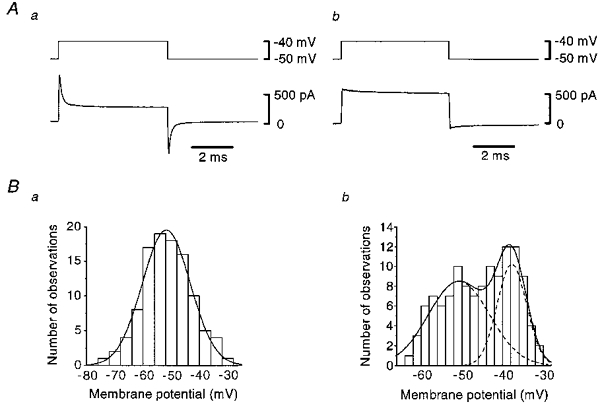
A, membrane currents (lower traces) induced by step depolarizations (upper traces) in a smooth muscle (a) and in an endothelial cell (b). Averages of ten individual traces. Signals were filtered at a cut-off frequency (−3 dB) of 10 kHz. B, distributions of resting membrane potentials in smooth muscle (a) and in endothelial cells (b). Smooth curves were fitted with Gaussian distributions calculated with the Levenberg-Marquardt method.
In the current clamp mode, the membrane potentials of both smooth muscle and endothelial cells were steady and quiescent. Figure 1B shows the distributions of resting membrane potentials recorded from smooth muscle (a) and endothelial cells (b). In the smooth muscle cells, the resting membrane potential had a Gaussian distribution, and the mean was −51.7 ± 8.5 mV (n = 105); this value is somewhat more positive than that recorded with conventional microelectrodes (Hashitani & Suzuki, 1997), perhaps reflecting removal of the adventitia. In the endothelial cells, on the other hand, the distribution of the resting membrane potential was fitted with the sum of two Gaussian distributions, the means of which were −50.7 ± 7.5 mV and −37.7 ± 3.6 mV (n = 121). Statistical analysis revealed that there was no significant difference between the more negative potentials detected in endothelial cells and smooth muscle cells (P > 0.2).
Changes in the time course of the current required to impose a 10 mV voltage clamp step were used to determine the extent of coupling between cells (see de Roos et al. 1996). The effects of heptanol or octanol were tested first. Neither changed the time course or magnitude of the clamp currents, recorded either from the smooth muscle or endothelial layer for up to 15 min of observation. Octanol (5 mM), however, on occasions reduced the step current but, as we were worried about the selectivity of this compound when applied at this high concentration, no further studies were carried out. Finally the effects of 18β-glycyrrhetinic acid were examined (Fig. 2). It can be seen in Fig. 2 that 18β-glycyrrhetinic acid almost abolished the current required to impose a 10 mV command step when recordings were made either from the smooth muscle layer (Fig. 2A) or from the endothelial layer (Fig. 2B). After application of 18β-glycyrrhetinic acid, only rapid capacitive currents and some sustained currents were observed and the relaxation of these transient currents could be well fitted with single exponential functions. Stable rapid transients were often obtained after 3 min but on occasions a period of exposure of up to 6 min was required. According to the method described by Lindau & Neher (1988), membrane current, I(t), induced by a voltage step having amplitude of Vp was expressed as:
| (3) |
where t is time after voltage step, τ is time constant of current relaxation, I0 is membrane current at t = 0 and Iss is steady-state current. Input resistance (Rin) and capacitance (Cin) were calculated by eqns (1), (2) and the following:
| (4) |
Figure 2. Effect of 18β-glycyrrhetinic acid on membrane currents recorded from smooth muscle and endothelial cells.
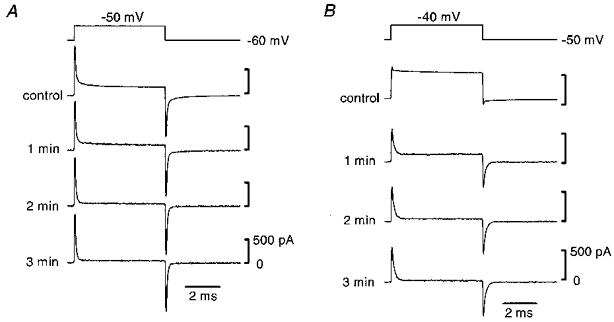
Membrane currents induced by step depolarizations (top traces) in a smooth muscle (A) and in an endothelial cell (B). Currents were recorded before (controls) and 1, 2 and 3 min after application of 18β-glycyrrhetinic acid (40 μM). Signals were filtered at a cut-off frequency of 10 kHz.
Calculated mean input resistance was 1.0 ± 1.3 GΩ (n = 27; range, 0.3-5.6 GΩ) in the smooth muscle cells and 0.5 ± 0.2 GΩ (n = 24; range, 0.3-1.0 GΩ) in the endothelial cells. In the presence of 18β-glycyrrhetinic acid, input resistance was increased by a factor of 12.0 ± 8.6 (n = 23; range, 3.3-35.1) in smooth muscle cells and by a factor of 140.7 ± 89.4 (n = 16; range, 29.3-314.2) in endothelial cells. The mean input capacitance was 9.6 ± 2.5 pF (n = 27; range, 6.3-14.7 pF) in the smooth muscle cells and 10.0 ± 2.4 pF (n = 24; range, 5.5-14.0 pF) in the endothelial cells. After 18β-glycyrrhetinic acid was removed, the time course of the current induced by the voltage step gradually changed back; however, complete recovery could not be obtained during observations of up to 15 min.
Together these observations suggest that both smooth muscle cells and endothelial cells are coupled to other cells. The experiments do not indicate whether or not the two layers are coupled together. To examine this aspect of electrical communication, smooth muscle cells were excited with a solution containing 10 mM Ba2+ (see Hashitani & Suzuki, 1997) in the current clamp condition. As shown in Fig. 3Aa, 10 mM Ba2+ solution depolarized the smooth muscle membrane and induced repetitive spikes. A similar depolarization and discharge of action potentials was recorded after gap junctions had been blocked by 18β-glycyrrhetinic acid in each of the six preparations examined (Fig. 3Ab). After blocking gap junctions, the spike discharge induced by 10 mM Ba2+ solution was less frequent. When the same experiment was carried out, recording from the endothelial layer, 10 mM Ba2+ produced a similar depolarization and discharge of spikes to that recorded from the muscle layer (Fig. 3Ba). However after the addition of 18β-glycyrrhetinic acid, although 10 mM Ba2+ solution continued to produce a depolarization, no associated spikes were recorded in each of the four preparations examined (Fig. 3Bb).
Figure 3. Ba2+-induced spike discharges recorded from smooth muscle and endothelial cells before and after disrupting gap junctions.
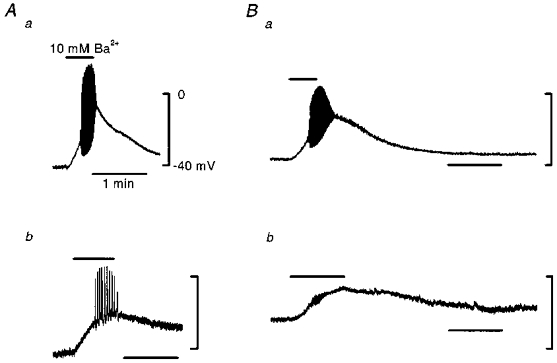
A, effect of 10 mM Ba2+ in smooth muscle cells before (a) and after (b) application of 18β-glycyrrhetinic acid (40 μM). a and b are recordings from different cells. B, effect of 10 mM Ba2+ in an endothelial cell before (a) and after (b) application of 18β-glycyrrhetinic acid (40 μM). a and b are recordings from the same cell. Calibrations given in Aa apply to all traces. Application period of 10 mM Ba2+ solution was indicated by the bar in each trace. Signals were filtered at a cut-off frequency of 100 Hz.
The effect of 18β-glycyrrhetinic acid on the resting membrane potential was also examined. In smooth muscle cells, the effect varied among cells, ranging from hyperpolarization of 11.6 mV to depolarizaton of 18.1 mV, and the mean was a depolarization of 4.7 ± 9.5 mV (n = 13). In the endothelial cells, on the other hand, 18β-glycyrrhetinic acid always depolarized the membrane, and its mean was 14.4 ± 7.5 mV (n = 8, range, 4.1-26.4 mV).
To test the responsiveness of endothelial cells in the presence of 18β-glycyrrhetinic acid, the response to ACh (3 μM) was observed before and after application of 18β-glycyrrhetinic acid in current clamp mode (Fig. 4). In control conditions, ACh induced membrane hyperpolarization with two phases, an initial fast phase followed by a slower second phase. After washing out ACh, the membrane depolarized transiently before returning to the resting membrane potential (Fig. 4A). ACh-induced hyperpolarization with comparable amplitude to the control could be observed after the disruption of gap junctions by 18β-glycyrrhetinic acid (Fig. 4B). However, the transient depolarization was not obvious after disrupting gap junctions in most cases. The ACh response persisted after application of 18β-glycyrrhetinic acid in each of the nine preparations examined.
Figure 4. ACh-induced membrane hyperpolarization recorded from an endothelial cell before and after disrupting gap junctions.
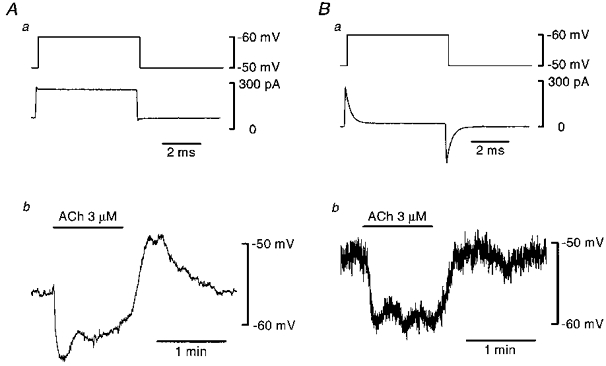
Aa, membrane current (lower trace) induced by a step depolarization (upper trace) in an endothelial cell under control conditions. Ab, change in membrane potential induced by ACh (3 μM) applied during a period indicated at the top. Current clamp recording. Ba, membrane current (lower trace) induced by a step depolarization (upper trace) in the same endothelial cell in the presence of 18β-glycyrrhetinic acid (40 μM). Bb, change in membrane potential induced by ACh (3 μM) applied during a period indicated at the top. Current clamp recording. Current signals were filtered at a cut-off frequency of 10 kHz and voltage signals at 10 Hz. Input resistance of this particular cell was 0.4 GΩ and capacitance was 6.8 pF measured in the presence of 18β-glycyrrhetinic acid.
If 18β-glycyrrhetinic acid had indeed produced substantial uncoupling between cells then it should be possible to control the membrane potential of the patched cell in the intact vessel segment. Figure 5A shows that, in the presence of 18β-glycyrrhetinic acid, this was the case. The characteristic inward Ca2+ and outward K+ currents were induced by applying a range of depolarizing steps to the smooth muscle cell. In the endothelial cell, time-dependent currents were not evoked by step changes of membrane potential (Fig. 5Ba); the current-voltage relationship observed in this cell, when a ramp pulse was applied, was almost linear and the input resistance of this cell was 0.7 GΩ (Fig. 5Bb).
Figure 5. Membrane currents recorded from smooth muscle and endothelial cells in the presence of 18β-glycyrrhetinic acid (40 μM).
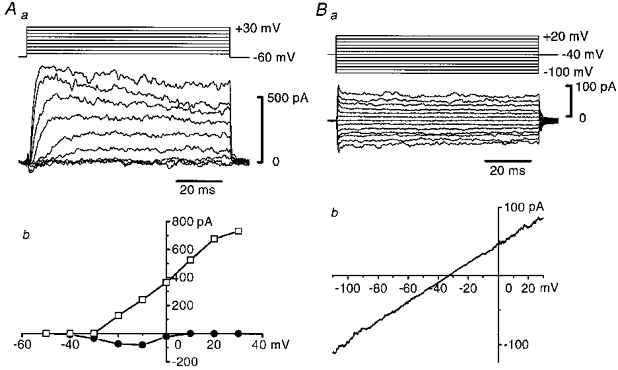
Aa, a family of membrane currents (lower traces) induced by voltage steps (upper traces) in a smooth muscle cell. Signals were filtered at a cut-off frequency of 1 kHz, and were digitally corrected for capacitive and leakage currents. Ab, current-voltage relationship of peak inward currents (•) and peak outward currents (□) measured in Aa. Ba, a family of membrane currents (lower traces) induced by voltage steps (upper traces) in an endothelial cell. Signals were filtered at a cut-off frequency of 1 kHz and were not corrected for capacitive and leakage currents. Bb, current-voltage relationship obtained with a ramp protocol in the same cell. Average of three traces. Actual duration of the ramp pulse was 1.6 s and filter cut-off frequency was 100 Hz.
DISCUSSION
In vascular tissue, three kinds of intercellular connections could exist: junctions between smooth muscle cells, between endothelial cells and between smooth muscle and endothelial cells. Evidence for the existence of each of these pathways has been provided by experiments which have examined the movements of dyes within the two cell layers of a range of blood vessels. In the pig coronary artery, dyes move from endothelial cell to endothelial cell and from smooth muscle cell to smooth muscle cell (Bény & Connat, 1992; Bény & Pacicca, 1994). In hamster cheek pouch arterioles, Lucifer Yellow only diffuses between endothelial cells (Segal & Bény, 1992; Little et al. 1995) whereas dyes with smaller molecular size diffuse between the two cell layers (Little et al. 1995). Morphological studies indicate there are gap junctions between both like and unlike cells in a range of blood vessels (Spagnoli et al. 1982; Bény & Connat, 1992).
Intercellular communication between the endothelial and muscle layers has also been inferred from electrophysiological studies. In the pig coronary artery, changes in the membrane potential of smooth muscle cells induced by β-receptor stimulation (Bény & Pacicca, 1994), by tetrabutylammonium (von der Weid & Bény, 1993) or by current application (Bény, 1997) were conducted to endothelial cells and were inhibited by halothane (Bény & Pacicca, 1994). In the present experiments, we found that repetitive spikes induced by 10 mM Ba2+ solution could be recorded from both kinds of cells. As the endothelial cells did not exhibit time-dependent currents in the voltage clamp experiments, these spikes must have originated in the smooth muscle cell layer, a clear indication for the existence of functional myoendothelial electrical coupling. This connection was disrupted by 18β-glycyrrhetinic acid. The spikes recorded from the endothelial cells had fast rising and falling phase, like those recorded from the smooth muscle cells. Clearly if the junctions showed rectification, it must be very slight.
This being the case, one would expect smooth muscle and the endothelial cells to have the same membrane potential. In the present experiments, the endothelial cells could be divided into two populations in terms of resting membrane potential. One population of cells had identical resting membrane potentials to those of the smooth muscle cells but the other had more depolarized potentials than those of the smooth muscle cells. We suggest that in the arterioles, the smooth muscle and the endothelial cells are all isopotential and that in some of our preparations we mechanically disrupted a few gap junctions when we inverted the arteriole, although the cells were not so damaged as to include the extracellular space marker during the process. In the absence of electrical coupling to the muscle layer, endothelial cells will exhibit their own resting membrane potential, which in isolated cells has been found to be less negative than that of smooth muscle cells (Popp et al. 1996). This idea is supported by the finding that endothelial cells were depolarized when they were uncoupled and several smooth muscle cells were hyperpolarized. It is not clear why a proportion of smooth muscle cells depolarized when the preparations were exposed to 18β-glycyrrhetinic acid.
The most direct evidence for the importance of electrical coupling in arterioles came from the electrical measurements made from either the muscle cell layer or from the endothelial cell layer. Large currents flowed in both cell types when a voltage step was applied. Even small steps required currents which did not readily reach steady state values. Such observations are entirely predicted by cable theory for current flow in syncytia (Jack et al. 1975). However when 18β-glycyrrhetinic acid was applied, only small currents were required. Furthermore when a range of clamp steps were applied, the membrane potential was controlled and current-voltage relationships like those described for single cells were obtained. Daut et al. (1988) reported that input resistance of single endothelial cells was 200-fold higher than that measured in a confluent layer. In the present experiments, 18β-glycyrrhetinic acid increased input resistance of endothelial cells by a factor of 140. Since 18β-glycyrrhetinic acid has been shown to disrupt gap junctions in other tissues, the simplest explanation is that it is doing so in this tissue. Clearly the electrical properties of individual cells are almost entirely dominated by their connections with neighbouring cells.
In rat liver epithelial cells, 18β-glycyrrhetinic acid blocks dye coupling within 30 min (a short-term effect), and dephosphorylates connexin 43 causing morphological disassembly of the gap junction (a long-term effect; Guan et al. 1996). As electrical coupling was disrupted within several minutes in guinea-pig arterioles, the dephosphorylation of the connexin and the disassembly of gap junctions are unlikely to have occurred.
Further support for the idea that 18β-glycyrrhetinic acid disrupted coupling to effectively leave isolated cells comes from the capacitance measurements. The cells had capacitances of about 10 pF; assuming a membrane capacitance of 1 μF cm−2, the cells would have a surface area of 1000 μm2. The approximate surface area of individual vascular smooth muscle cells can be obtained by assuming that they consist of two cones, each of diameter 5 μm and height 50 μm, so giving a surface area of 800 μm2. Clearly these values are very similar. Furthermore, in rat coronary artery, single isolated smooth muscle cells have a capacitance of 13 pF (Robertson et al. 1996), a value almost identical to that determined in these experiments. In cultured bovine pulmonary artery endothelial cells, Silver et al. (1994) reported that cell capacitances were 10–15 pF, values close to those obtained in the present experiments. However the measured input resistance was lower than expected for a single cell (7.7 GΩ in rabbit basilar artery smooth muscle cells: Langton & Standen, 1993; 1.7 GΩ in cultured guinea-pig coronary endothelial cells: Daut et al. 1988; 5.6 GΩ in guinea-pig coronary endothelial cells: von Beckerath et al. 1996). This could either mean that, although most gap junctions were blocked, some electrical connections remained or that 18β-glycyrrhetinic acid caused the membrane to become slightly leaky. Small fluctuations in the membrane potential were observed in the presence of 18β-glycyrrhetinic acid (see Figs 3 and 4). This might indicate that 18β-glycyrrhetinic acid activates some channels which are not open in the control condition. Alternatively, disrupting gap junctions might reveal unsynchronized fluctuations which have offset each other in the syncytium. Even if 18β-glycyrrhetinic acid had some side effect that makes the membrane become slightly leaky, the endothelial cells did not seem to be too leaky to exhibit spikes conducted from underlying smooth muscle cells, because these cells could respond to ACh by producing membrane hyperpolarization in the presence of 18β-glycyrrhetinic acid.
In summary, 18β-glycyrrhetinic acid at 40 μM altered the electrical properties of cells in a way that was consistent with its ability to disrupt gap junctions in other tissues. When it did so it did not appear to disturb the functions of other ion channels. Thus in the presence of this compound, smooth muscle cells exhibited inward Ca2+ and outward K+ currents. Endothelial cells showed an almost linear current-voltage relationship similar to those described for human endothelial cells (Gericke et al. 1993). In contrast neither heptanol nor octanol appeared to block gap junctions in concentrations of up to 1 mM. With nitroarginine- and indomethacin-resistant hyperpolarization produced by acetylcholine or substance P in smooth muscle of guinea-pig mesenteric arteriole, assessment of involvement of myoendothelial gap junctions using heptanol was unsuccessful (Hashitani & Suzuki, 1997). Thus, 18β-glycyrrhetinic acid appears to be a very useful blocker of gap junctions in vascular smooth muscle and endothelial cells, opening up the possibility of studying intact blood vessels at the level of the single cells.
Acknowledgments
We thank Professor G. D. S. Hirst and Dr D. F. Van Helden for their valuable comments on the manuscript. We also thank Professor H. Nishino for the facility of the fluorescence microscope.
References
- Bény J-L. Electrical coupling between smooth muscle cells and endothelial cells in pig coronary arteries. Pflügers Archiv. 1997;433:364–367. doi: 10.1007/s004240050289. [DOI] [PubMed] [Google Scholar]
- Bény J-L, Connat J-L. An electron-microscopic study of smooth muscle cell dye coupling in the pig coronary arteries. Role of gap junctions. Circulation Research. 1992;70:49–55. doi: 10.1161/01.res.70.1.49. [DOI] [PubMed] [Google Scholar]
- Bény J-L, Pacicca C. Bidirectional electrical communication between smooth muscle and endothelial cells in the pig coronary artery. American Journal of Physiology. 1994;266:H1465–1472. doi: 10.1152/ajpheart.1994.266.4.H1465. [DOI] [PubMed] [Google Scholar]
- Daut J, Mehrke G, Nees S, Newman WH. Passive electrical properties and electrogenic sodium transport of cultured guinea-pig coronary endothelial cells. The Journal of Physiology. 1988;402:237–254. doi: 10.1113/jphysiol.1988.sp017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roos ADG, Van Zoelen EJJ, Theuvenet APR. Determination of gap junctional intercellular communication by capacitance measurements. Pflügers Archiv. 1996;431:556–563. doi: 10.1007/BF02191903. [DOI] [PubMed] [Google Scholar]
- Gericke M, Droogmans G, Nilius B. Thapsigargin discharges intracellular calcium stores and induces transmembrane currents in human endothelial cells. Pflügers Archiv. 1993;422:552–557. doi: 10.1007/BF00374001. [DOI] [PubMed] [Google Scholar]
- Guan X, Wilson S, Schlender KK, Ruch RJ. Gap-junction disassembly and connexin 43 dephosphorylation induced by 18β-glycyrrhetinic acid. Molecular Carcinogenesis. 1996;16:157–164. doi: 10.1002/(SICI)1098-2744(199607)16:3<157::AID-MC6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Suzuki H. K+ channels which contribute to the acetylcholine-induced hyperpolarization in smooth muscle of the guinea-pig submucosal arteriole. The Journal of Physiology. 1997;501:319–329. doi: 10.1111/j.1469-7793.1997.319bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Neild TO. An analysis of excitatory junctional potentials recorded from arterioles. The Journal of Physiology. 1978;280:87–104. doi: 10.1113/jphysiol.1978.sp012374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack JJB, Noble D, Tsien RW. Electric Current Flow in Excitable Cells. Oxford: Clarendon Press; 1975. [Google Scholar]
- Langton PD, Standen NB. Calcium currents elicited by voltage steps and steady voltages in myocytes isolated from the rat basilar artery. The Journal of Physiology. 1993;469:535–548. doi: 10.1113/jphysiol.1993.sp019828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M, Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflügers Archiv. 1988;411:137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Little TL, Xia J, Duling BR. Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circulation Research. 1995;76:498–504. doi: 10.1161/01.res.76.3.498. [DOI] [PubMed] [Google Scholar]
- Popp R, Bauersachs J, Hecker M, Fleming I, Busse R. A transferable, β-naphthoflavone-inducible, hyperpolarizing factor is synthesized by native and cultured porcine coronary endothelial cells. The Journal of Physiology. 1996;497:699–709. doi: 10.1113/jphysiol.1996.sp021801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson BE, Bonev AD, Nelson MT. Inward rectifier K+ currents in smooth muscle cells from rat coronary arteries: block by Mg2+, Ca2+, and Ba2+ American Journal of Physiology. 1996;271:H696–705. doi: 10.1152/ajpheart.1996.271.2.H696. [DOI] [PubMed] [Google Scholar]
- Segal SS, Bény J-L. Intracellular recording and dye transfer in arterioles during blood flow control. American Journal of Physiology. 1992;263:H1–7. doi: 10.1152/ajpheart.1992.263.1.H1. [DOI] [PubMed] [Google Scholar]
- Silver MR, Shapiro MS, DeCoursey TE. Effects of external Rb+ on inward rectifier K+ channels of bovine pulmonary artery endothelial cells. Journal of General Physiology. 1994;103:519–548. doi: 10.1085/jgp.103.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnoli LG, Villaschi S, Neri L, Palmieri G. Gap junctions in myo-endothelial bridges of rabbit carotid arteries. Experientia. 1982;38:124–125. doi: 10.1007/BF01944566. [DOI] [PubMed] [Google Scholar]
- Spray DC, Burt JM. Structure-activity relations of the cardiac gap junction channel. American Journal of Physiology. 1990;258:C195–205. doi: 10.1152/ajpcell.1990.258.2.C195. [DOI] [PubMed] [Google Scholar]
- von Beckerath N, Dittrich M, Klieber H-G, Daut J. Inwardly rectifying K+ channels in freshly dissociated coronary endothelial cells from guinea-pig heart. The Journal of Physiology. 1996;491:357–365. doi: 10.1113/jphysiol.1996.sp021221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid P-Y, Bény J-L. Simultaneous oscillations in the membrane potential of pig coronary artery endothelial and smooth muscle cells. The Journal of Physiology. 1993;471:13–24. doi: 10.1113/jphysiol.1993.sp019888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Chen G, Miwa K, Suzuki H. Permeability and Mg2+ blockade of histamine-operated cation channel in endothelial cells of rat intrapulmonary artery. The Journal of Physiology. 1992;450:395–408. doi: 10.1113/jphysiol.1992.sp019133. [DOI] [PMC free article] [PubMed] [Google Scholar]


