Abstract
To find out whether muscle afferents influence the excitability of corticospinal projections to antagonist muscles, we studied sixteen healthy subjects and one patient with a focal brain lesion.
Using transcranial magnetic and electrical brain stimulation we tested the excitability of corticomotoneuronal connections to right forearm muscles at rest after conditioning stimulation of the median nerve at the elbow. Somatosensory potentials evoked by median nerve stimulation were also recorded in each subject.
Test stimuli delivered at 13–19 ms after median nerve stimulation significantly inhibited EMG responses elicited in forearm extensor muscles by transcranial magnetic stimulation, but did not inhibit responses to electrical stimulation. In contrast, magnetically and electrically elicited responses in forearm flexor muscles were suppressed to the same extent.
The higher the intensity of the test shocks, the smaller was the amount of median nerve-elicited inhibition. Inhibition in extensor muscles was also smaller during tonic wrist extension, or if the induced electrical stimulating current in the brain flowed from posterior to anterior over the motor strip rather than vice versa. Test responses evoked by magnetic transcranial stimulation in the first dorsal interosseous and in brachioradialis muscles were not inhibited after median nerve stimulation at the elbow. Stimulation of digital nerves failed to inhibit motor potentials in extensor muscles.
Test stimuli delivered at 15 and 17 ms after radial nerve stimulation significantly inhibited EMG responses elicited in forearm flexor muscles by magnetic transcranial stimulation.
In the patient with a focal thalamic lesion, who had dystonic postures and an absent N20 component of the somatosensory-evoked potentials but normal strength, median nerve stimulation failed to inhibit magnetically evoked responses in forearm extensor muscles.
We propose that activation of median nerve muscle afferents can suppress the excitability of cortical areas controlling the antagonist forearm extensor muscles acting on the hand. The inhibitory effect occurs at short latency and might assist spinal pathways mediating reciprocal inhibition by contrasting the co-activation of antagonistic pools of corticospinal cells.
Motor commands from many areas of the brain as well as afferent information from the body can influence the activity of corticospinal cells. Stimulation of cutaneous and proprioceptive afferents activates precentral cells at short latencies (see Porter & Lemon, 1993; Rothwell, 1994). This short-latency facilitation of corticospinal cells is the functional correlate of the long-latency component of the stretch reflex in distal muscles. In humans, studies using transcranial brain stimulation have shown that afferent input to the motor cortex increases the excitability of corticomotoneuronal connections to the homonymous muscle (Troni et al. 1988; Deuschl et al. 1991; Mariorenzi et al. 1991; Kasai et al. 1992; Wolfe & Hayes, 1995; Baldissera & Leocani, 1995; Terao et al. 1995; Nielsen et al. 1997). However, the effect that muscle input has on corticospinal outputs to antagonist muscles remains obscure. In this paper we investigated the effect of median nerve muscle afferent stimulation on cortimotoneuronal connections to antagonist muscles in humans. We found a pattern of cortical reciprocal inhibition similar to that in the spinal cord.
METHODS
Subjects
Experiments were conducted on sixteen healthy volunteers of both sexes (9 men and 7 women), aged 21–40 years (mean, 31.3 ± 4.9 years). Most subjects took part in more than one experiment. All subjects gave their informed consent. Experimental procedures conformed to the declaration of Helsinki and were approved by the local ethics committee. Participants included a patient with a focal thalamic lesion - a 61-year-old man who had a left thalamic haemorrhage in June 1996. At the time of investigation, 8 months after the stroke, the neurological examination showed normal sensation, normal strength, absence of pyramidal signs, normal vision, unimpaired speech and clumsy movement of the right arm with dystonic posturing and co-contraction during the execution of fine motor skills (writing).
Transcranial brain stimulation (TCS)
Test responses in the target muscles were evoked with magnetic and electrical TCS. Magnetic stimuli were delivered by a Novametrix Magstim 200 (The Magstim Company, Whitland, Dyfed, UK) connected to a large round coil (o.d., 13 cm). The coil was centred upon the vertex and stimuli were delivered to the left motor cortex. Unless otherwise specified, current in the coil flowed in the less favourable direction, i.e. clockwise (viewed from above). The induced currents therefore flowed out from the motor cortex in a posterior direction. Preliminary experiments comparing the two directions of flow showed that even though clockwise currents (in the coil) required a slightly higher stimulus intensity, they yielded a test shock that was more sensitive to the inhibitory effect of conditioning median nerve stimulation. Magnetic stimuli were delivered at the intensity necessary to evoke the smallest stable motor-evoked potential in the relaxed target muscles. The stimulator output was usually 15 % above the motor threshold intensity during relaxation. With the current flowing clockwise the absolute intensity used ranged between 55 and 70 % of the maximum stimulator output. In selected experiments test stimuli were delivered at different intensities, and in a separate set of control experiments the effect of current direction in the coil was studied by reversing the current flow.
Electrical TCS was delivered by a Digitimer D180 high voltage stimulator (Digitimer Ltd, Welwyn Garden City, Herts, UK), supplying a maximum output of 750 V. Surface Ag-AgCl disc electrodes (o.d., 9 mm) were placed on the scalp with the cathode on the vertex and the anode 5 cm laterally on the line between the vertex and the left auditory meatus. Single electrical pulses having a time constant of 50 μs were given and stimulus intensity was adjusted to obtain the smallest stable motor response in the relaxed target muscle. During experiments with electrical stimulation, to minimize I wave production by the electrical test shock (Rothwell et al. 1991b) and to reduce the discomfort for the subject, the motor potential in the target muscle was kept at a small size and stimulation intensities were kept low. In the experiments comparing electrical and magnetic TCS the intensity of the two stimulators was adjusted to evoke control motor responses of similar isoelectric peak amplitude. The two types of stimulation as well as control and test condition were randomly alternated in the same trial. Brain stimuli were delivered at randomly varied intervals ranging from 7 to 10 s.
Motor-evoked potentials (MEPs)
Test MEPs were generally recorded from forearm extensor and flexor muscles with standard Ag-AgCl surface disc electrodes (o.d., 9 mm). A pair of electrodes was placed 2–3 cm apart over the belly of the extensor carpi radialis muscle and over the belly of the flexor carpi radialis. Though surface electrodes mainly detect the EMG signal of the muscle beneath, they could also pick up some activity from nearby muscles. Thus electrodes over the extensor carpi radialis could also record some EMG signal from nearby synergist forearm extensor muscles, and electrodes over the flexor carpi radialis could also detect some activity from nearby synergist forearm flexor muscles. For this reason, and because various forearm muscles flexing or extending the carpus and the fingers can be considered for their reciprocal functional interactions as two unique functional units, we ascribed the signal from the electrodes over the extensor carpi radialis to forearm extensor muscles, and the signal from flexor carpi radialis to forearm flexor muscles. Throughout the text the term (forearm) flexors or extensors refers to muscles located in the forearm and flexing or extending the carpus and/or the fingers. In control experiments MEPs were recorded from the first dorsal interosseous muscle (FDI) with the active electrode placed over the motor point and the reference on the metacarpophalangeal joint. To compare the responses in FDI and forearm extensors, the intensity of TCS was adjusted to evoke MEPs with an area of about 50 % of the rectified M wave in each muscle. In several recordings in two subjects the EMG signal was derived using a pair of teflon-coated monopolar electrodes (15 mm in length) with the uninsulated tips (length, 0.1 mm) sited 10 mm or less apart. The target muscle was identified by recording the EMG activity during the movement produced by contraction of the target muscle.
Because MEPs in the relaxed forearm muscles often have a polyphasic shape that can bias measurement of their peak-to-peak size, we full-wave rectified and averaged the MEPs and expressed their size as a percentage of the area of the rectified averaged unconditioned control response (taken to be 100 %). Because electrical MEPs are shorter than magnetic MEPs at similar amplitudes, when we compared the two types of stimulation we monitored and measured the isoelectric peak amplitude (in μV), i.e. from the baseline to the highest peak of the response.
EMG signals were carefully checked for possible contamination between the two groups of antagonist muscles in the forearm. To reduce the possible contamination, the EMG signal was band passed with a high low-pass filter value (200 Hz to 5 kHz), and in preliminary experiments the volume-conducted effect was ruled out by recording an eventual M wave in each of the two channels after stimulation of the antagonist motor nerve. Finally, after each experimental session all the sweeps were reviewed off-line and trials were accepted for further analysis only if they contained several sweeps with a large potential (not less than 0.3 mV) on a channel not picked up by the other channel. For each conditioning-test interval (i.e. the interval between the conditioning stimulation of the median nerve at the elbow and the test TCS), eight conditioned and eight unconditioned responses were acquired and the relative averages were measured.
With the exception of some control experiments that required a slight voluntary isometric contraction (15 % of maximum EMG activity) of the forearm extensor muscles, subjects were asked to keep the target muscles fully relaxed during recordings. TCS strength was adjusted to elicit MEPs of similar size at rest and during contraction.
Electrical stimulation of the peripheral nerves
Conditioning electrical stimuli were delivered by a dedicated programmable insulated constant current peripheral nerve stimulator incorporated in the EMG acquisition and analysis integrated system (Phasis II, ESAOTE, Florence, Italy). Bipolar electrical stimulation (cathode proximal) was delivered to the median nerve at the elbow (interelectrode distance, 20 mm; diameter of each stimulating electrode, 9 mm) with a square pulse of 0.1 ms and at an intensity producing a minimum activation of motor axons as monitored by the presence of the M wave in forearm flexors. In three experiments we studied the opposite experimental design by stimulating the radial nerve at the spiral groove. The presence of a small M wave (generally smaller than an isolectric peak amplitude of 50 μV) was indicative of the constant condition of nerve stimulation during each experimental session. The absolute intensity of conditioning stimulation of the median nerve at motor threshold ranged between 6.0 and 12.3 mA. In most subjects these intensities also evoked an H reflex in forearm flexor muscles. The conditioning-test intervals in the principal experiments were 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 and 24 ms. In most subjects all these intervals were studied in the main experiment describing the time course of the inhibition. In control experiments, in extensors only the 15 and 17 ms conditioning-test intervals, and in flexors only the 14 and 15 ms intervals were studied (as specified for each experiment in the Results section) because they corresponded to the period of maximum inhibition.
In control experiments conditioning stimuli were given to fingers one, two and three of the right hand through a pair of metal ring electrodes (cathode proximal) at the intensity needed to produce a sensation similar to the paraesthesias evoked by median nerve stimulation at the elbow; generally 2–3 times the subject's perceptual threshold. In these experiments, conditioning-test intervals were corrected for the sensory conduction along the hand and the forearm by adding to the conditioning-test interval the latency of the orthodromic sensory action potential evoked by finger stimulation and recorded by a pair of Ag-AgCl surface disc electrodes (diameter, 9 mm) at the elbow. The signal was amplified, band passed (100 Hz to 5 kHz) and averaged on-line (16 sweeps).
Somatosensory-evoked potentials (SEPs)
SEPs evoked by median nerve stimulation at the elbow were recorded with a standard P3-ipsilateral earlobe montage (Tomberg et al. 1991) in each subject by standard Ag-AgCl skin surface disc electrodes (diameter, 9 mm). One recording electrode was placed over the left parietal cortex (P3), the other over the ipsilateral earlobe. EEG signals were amplified, band passed (5 Hz to 1.5 kHz), averaged on-line (200 sweeps) and the peak latency of the N20 component (see Results) was measured off-line.
Signal acquisition, storage and data analysis
An integrated computer-controlled four-channel system was used for processing and storage of biological signals (Phasis II, ESAOTE). Data were stored on magnetic support and analysed off-line.
Statistical analysis
Data in the text are expressed as means ± 1 s.e.m., and n is the sample size. Because the sample size in some experiments did not allow a Gaussian distribution of data, and because in some experiments the variance between the control and test experimental conditions differed, data were statistically analysed by non-parametric tests. Usually we used the two-sample Mann-Whitney U test. In some instances specified in the text, we used the one-sample Wilcoxon signed-rank test. The level of significance was P < 0.05 (two tailed).
In the first experiment studying the whole time course of median nerve-elicited MEP inhibition we statistically analysed all the relevant time intervals (14, 15, 16, 17, 18, 19 and 20 ms). In all the remaining experiments with surface recordings, once the core inhibitory phase was identified for extensor and flexor muscles we assessed only two conditioning-test intervals (15 and 17 ms in extensors, 14 and 15 ms in flexors) and pooled the data, assuming that the mechanism underlying the inhibition at the two intervals is the same. The values for these experiments reported in the Results therefore represent the means ±s.e.m. of the pooled data of the two conditioning-test intervals. In the corresponding figures, data for the two intervals are shown separately.
In needle recording experiments from several muscles conducted in two subjects, we statistically evaluated the results in each muscle of each subject by a Mann-Whitney U test (n = 8 sweeps unconditioned versus 8 sweeps conditioned).
RESULTS
The effect of conditioning stimulation of the median nerve on motor-evoked potentials evoked by brain stimulation in forearm muscles
Motor-evoked potentials (MEPs) evoked by transcranial brain stimulation (TCS), after conditioning stimulation to the median nerve, and recorded in relaxed forearm extensor muscles exhibited a pronounced inhibitory phase preceded by a short period of slight facilitation: inhibition began at the conditioning-test interval of 13 ms, peaked at the 15 ms interval, and progressively disappeared from 18 ms onwards. The most pronounced phase lasted for some 4–5 ms (Fig. 1A). Under the same experimental conditions, forearm flexor muscles behaved differently (Fig. 1B), undergoing only slight inhibition. Flexor inhibition began at 14–15 ms, and at longer conditioning-test intervals promptly recovered giving way to a facilitation. The comparison of the two time courses at the intervals disclosing the most prominent inhibition (13-20 ms), yielded a significantly smaller area under the curve in extensors than in flexors (-36.1 ± 4.3 %, P < 0.05). Analysis of single subjects showed that the conditioning-test interval inducing maximum inhibition differed significantly in extensor and flexor muscles (16.5 ± 0.4 versus 14.4 ± 0.4 ms, n = 106, P < 0.05). The mean conditioning-test intervals needed to induce the maximum excitation were far shorter in extensors than in flexors (11.5 ± 0.2 versus 17.8 ± 0.9 ms, n = 10, P < 0.05). A representative subject is shown in Fig. 1C.
Figure 1. Magnetic MEPs in forearm extensor and flexor muscles conditioned by median nerve stimulation.
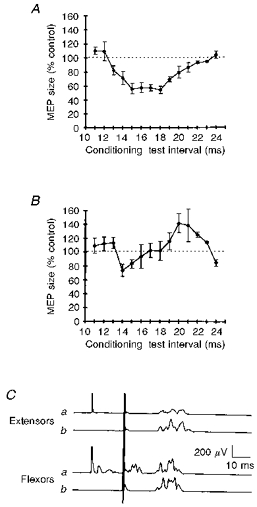
The average time course of the effect of a conditioning stimulus delivered at motor threshold intensity over the median nerve at the elbow on the size of magnetically evoked MEPs in forearm extensor (A) and flexor muscles (B). In A and B, the x-axis shows the conditioning-test intervals (ms), and the y-axis the MEP size as a percentage of the area of the unconditioned control response. The graphs show that median nerve stimulation at conditioning-test intervals of 15–18 ms produced a marked decrease in the MEP size in extensors, whereas it induced far less pronounced inhibition in flexors. Each point is the average of 10 subjects, except the 22 and 23 ms intervals, which were studied in 6 subjects. Error bars show 1 s.e.m.C, magnetic MEPs in forearm extensors and flexors conditioned (a) and unconditioned (b) by a stimulus delivered over the median nerve at the elbow at the conditioning-test interval of 18 ms in a representative subject. Note that the electrodes over forearm extensor muscles do not pick up the small M wave and the H reflex evoked in flexors by conditioning stimulation of the median nerve. Each trace is the average of 8 sweeps.
In five subjects, we assessed the effect of a slight voluntary forearm extensor contraction on inhibition of magnetic MEPs in extensors after median nerve stimulation at conditioning-test intervals of 15 and 17 ms (Fig. 2A). The size of the conditioned test MEP was 43.5 ± 5.9 % (n = 10) of the unconditioned control value at rest and 70.3 ± 7.3 % (n = 10) during contraction (P < 0.05).
Figure 2. Characteristics of MEP inhibition in extensors after median nerve stimulation.
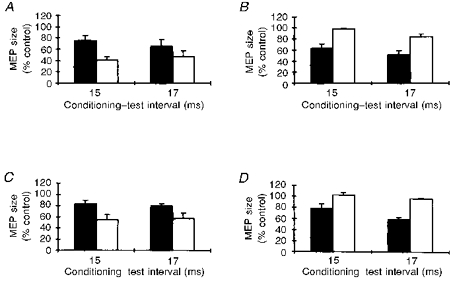
The effects of a slight voluntary contraction (A), of test stimulus intensity (B), and of the direction of the current flow in the coil (C) on the MEP inhibition in forearm extensors after median nerve stimulation at the most effective conditioning-test intervals, 15 and 17 ms. A: contraction, ▪; at rest, □. B: 60 % of stimulator output (clockwise current), ▪; 85 % of stimulator output (clockwise currents), □. C: anticlockwise currents, ▪; clockwise currents, □. The graphs show that conditioning stimulation of the median nerve produced a larger inhibition at rest than during contraction (A), with lower test stimulus intensities (B), and with current flowing clockwise in the coil. D, the effect of conditioning stimulation delivered to the median nerve at the elbow on magnetic MEPs in forearm extensor muscles (▪) and in the first dorsal interosseous muscle (□) at conditioning-test intervals of 15 and 17 ms. The graph shows that a conditioning stimulus over the median nerve selectively inhibits magnetic MEPs in the forearm extensors but leaves responses in a small hand muscle unchanged. Each bar is the mean of 5 subjects, and error bars show 1 s.e.m.
Experiments conducted in five subjects to study the function of test stimulus strength, at constant conditioning stimulus intensity and at intervals of 15 and 17 ms, showed that higher stimulus intensities invariably produced significantly less-inhibited MEPs (Fig. 2B). The test MEP was 56.7 ± 5.9 % (n = 10) at 60 % of stimulator output and 90.8 ± 3.3 % (n = 10) at 85 % of stimulator output (P < 0.05).
Experiments, in five subjects, comparing the effect of clockwise and anticlockwise current flow in the stimulating coil showed that for forearm extensor MEPs of similar size (using higher stimulator output for clockwise current), clockwise current proved significantly more effective in detecting inhibition elicited by median nerve stimulation (Fig. 2C). The test MEP was 55.4 ± 6.4 % (n = 10) with clockwise current and 81.0 ± 3.5 % (n = 10) with anticlockwise current (P < 0.05).
In experiments designed to assess whether the effects of median nerve stimulation spread over other muscles, in five subjects we recorded MEPs from the forearm extensor muscles and the first dorsal interosseous (FDI) muscle (Fig. 2D). Whereas in forearm extensors the test MEPs showed a significant inhibition (68.4 ± 5.3 %, n = 10, P < 0.05, one-sample test), in the FDI muscle we found no changes in test MEP size (99.3 ± 2.4 %, n = 10, P > 0.1, one-sample test).
The possible contribution of EMG signals arising from other muscles was evaluated in two subjects in several recordings at rest with intramuscular needle electrodes, at the most effective conditioning-test interval for detecting inhibition with surface electrodes. Needle electrodes were inserted in the extensor digitorum communis, in the extensor carpi radialis, in the extensor pollicis longus, and in the brachioradialis muscles. In both subjects a median nerve conditioning stimulus variably but significantly (P < 0.05) inhibited MEPs in extensor muscles acting across the wrist. Conversely, it left MEPs in the brachioradialis muscle unchanged (P > 0.1; data not shown).
The N20 component of the somatosensory-evoked potentials (SEPs) evoked by median nerve stimulation (at the elbow), i.e. the scalp potential arising from the sensory cortex at the arrival of the somatosensory volley from the thalamus, had a latency of 15.4 ± 0.6 ms (n = 10).
Comparison between electrical and magnetic test stimulation of the motor cortex
In five subjects, at the 15 and 17 ms intervals a conditioning stimulus over the median nerve left electrical MEPs in forearm extensors unchanged (101.9 ± 5.0 %, n = 10, P > 0.1, one-sample test) but significantly inhibited magnetic MEPs in these muscles (56.9 ± 6.3 %, n = 10, P < 0.05, one-sample test) (Fig. 3A). The isoelectric peak amplitude of magnetic control MEPs was 198.7 ± 36.3 μV (n = 10), and the amplitude of control electrical MEPs was 184.7 ± 43.6 μV (n = 10; P > 0.1).
Figure 3. Magnetic versus electrical test TCS, and mixed nerve versus cutaneous conditioning input.
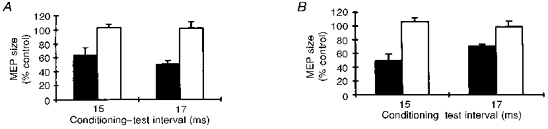
A, comparison of magnetic MEPs (▪) and electrical MEPs (□) in forearm extensor muscles after a stimulus delivered over the median nerve at the elbow at the most significant conditioning-test intervals of 15 and 17 ms. The graph shows that a conditioning stimulus over the median nerve selectively reduces the size of magnetic MEPs, whereas it does not inhibit electrical MEPs. B, comparison between stimulation of the median nerve at the elbow at motor threshold intensities (▪) and cutaneous stimulation of the fingers (□). Conditioning-test intervals for finger stimulation were corrected by adding the conduction time from the fingers to the elbow (see Methods). The graph shows that conditioning median nerve stimulation decreases the MEP size whereas finger stimulation does not. Each bar is the mean of 5 subjects, and error bars show 1 s.e.m.
In five subjects, electrical and magnetic MEPs in forearm flexors underwent similar inhibition: 81.5 ± 5.8 % (n = 10, P < 0.05, one-sample test) and 77.4 ± 10.8 % (n = 10, P > 0.5), respectively. The control magnetic MEPs had an amplitude of 180.1 ± 10.6 μV (n = 10), and the control electrical MEPs an amplitude of 165.9 ± 16.7 μV (n = 10; P > 0.1; data not shown).
Afferents responsible for antagonist MEP inhibition
To assess the possible contribution of cutaneous input, in five subjects we compared the effects induced on magnetic MEPs in forearm extensors by cutaneous stimuli delivered to the first three fingers and by median nerve stimulation at the elbow (Fig. 3B). At the two corrected conditioning-test intervals (see Methods) for testing the core of the inhibitory phase, cutaneous stimulation of the fingers left test MEPs in extensor muscles unchanged (102.3 ± 4.6 %, n = 10, P > 0.1, one-sample test), whereas median nerve stimulation at the elbow significantly depressed test MEPs (59.4 ± 6.1 %, n = 10, P < 0.05, one-sample test).
The effect of conditioning stimulation of the radial nerve on MEPs in forearm flexors
In three subjects we assessed whether the inhibitory effect described above also operates in the opposite direction. Radial nerve stimulation (at the 15 and 17 ms intervals) significantly inhibited the test MEPs in forearm flexors (74.1 ± 6.2 %, n = 6, P < 0.05, one-sample test; data not shown).
The effect of a focal thalamic lesion
In the patient with a focal thalamic lesion (Fig. 4), test MEPs in forearm extensors of both sides had similar amplitudes, latencies and durations. A conditioning shock delivered at 14–17 ms intervals to the median nerve contralateral to the lesion before a test shock over the ipsilateral motor cortex failed to evoke inhibition (Fig. 4A). Conversely, a conditioning shock given to the ipsilateral median nerve before a test shock to the contralateral motor cortex inhibited MEPs in forearm extensors; the time course of inhibition overlapped that in healthy subjects. Stimulation of the median nerve at the elbow elicited a normal N20 component of the SEPs on the clinically healthy side of the brain but no N20 on the side of the lesion (Fig. 4B).
Figure 4. Time course of magnetic MEP inhibition in forearm extensors after a conditioning stimulus delivered to the median nerve in a patient with a thalamic lesion.
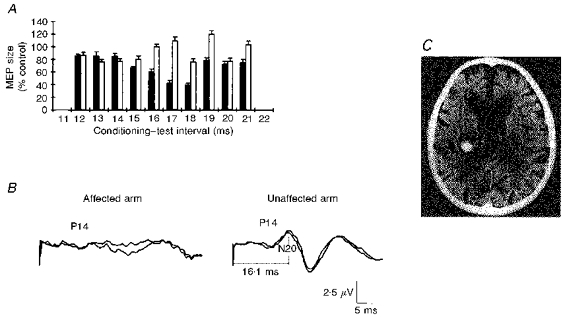
A, test responses in the affected upper limb contralateral to the lesion (□) and in the sound upper limb ipsilateral to the lesion (▪). Each bar is the mean size of 8 conditioned MEPs, and error bars show 1 s.e.m.B, SEPs evoked by median nerve stimulation contralateral (on the left) and ipsilateral (on the right) to the lesion. In A, note that when conditioning stimulation and test responses are in the (affected) forearm contralateral to the lesion and the N20 component is absent there is no magnetic MEP inhibition. Conversely, when conditioning stimulation and test responses are in the (sound) forearm ipsilateral to the thalamic lesion and the N20 is regularly present, test responses are regularly inhibited. The P14 component (generated in the brainstem) is bilaterally present. C, the brain CT scan showing a left thalamic haemorrhage.
DISCUSSION
Low-intensity stimulation of the median nerve at the elbow can suppress the EMG responses evoked in forearm extensor muscles by magnetic TCS delivered to the motor cortex some 13–19 ms later. A similar inhibition of responses elicited in flexors occurs after radial nerve stimulation. We argue that forearm flexor muscle afferents specifically inhibit the corticospinal outputs to antagonist muscles at the cortical level. Conversely, the inhibition of test motor responses in agonists may depend on spinal mechanisms.
Inhibition of MEPs in forearm extensors after median nerve stimulation: evidence for cortical involvement
Several lines of evidence in this study indicate that inhibition of MEPs in forearm extensors arises from interaction within the motor cortex. First, the latency of the inhibition was appropriate. The input arrival at the cortex coincided with the onset of the N20 component - which is 1-1.5 ms earlier than the peak and therefore at a latency of about 14 ms in our subjects. Inhibition of the extensor MEPs began at a conditioning-test interval of 13 ms, surprisingly suggesting that inhibition occurs if the test magnetic stimulus is given to the brain 1 ms before the conditioning input arrives. The probable explanation of this apparently contradictory finding is that a single magnetic shock produces a series of I waves outlasting the stimulus for 5 ms or more (Rothwell et al. 1991b). Thus the conditioning-test interval is probably shorter than the actual time lag between the arrival of the afferent volley at the cortex and the trans-synaptic corticospinal activation elicited by the test TCS shock. The delayed trans-synaptic activation leaves at least 4 ms for the inhibition of the later I waves, thus reducing the size of the test MEP. In addition, the finding from the patient with a thalamic lesion abolishing both the N20 component of the SEPs (i.e. the afferent input to the cerebral cortex) and the median nerve inhibition of the extensor MEPs, supports the idea of a cortical inhibition.
A second point favouring cortical involvement is that, because the sites of activation differ (Rothwell et al. 1991b), magnetic MEPs are more sensitive than electrical MEPs to changes in cortical excitability (Datta et al. 1989; Day et al. 1991; Ugawa et al. 1991; Nielsen et al. 1997). Hence the uninhibited electrical MEPs in our experiments suggest that the inhibitory effects on magnetic MEPs in extensors arise at the cortical level.
A third point arguing in favour of a cortical origin of the inhibition comes from our experiments in which the MEP was elicited by anterior-posterior-induced current flow in the brain (clockwise in the coil) rather than the posterior- anterior flow (anticlockwise in the coil). Both the flows presumably activate the same population of corticospinal elements through different sets of cortical interneurones (Day et al. 1989). The larger effect of median nerve stimulation on anterior-posterior-induced current is therefore explained by a differential effect on the two sets of circuits within the cortex.
Median nerve inhibition of MEPs in forearm flexors
The different shape and time course of the MEP inhibition argues against a possible effect of electrical cross-talk between flexors and extensors.
In contrast to the results in the extensors, we found that median nerve stimulation suppressed MEPs elicited in flexors by electrical and magnetic TCS to the same extent. Thus, following the reasoning above, this suggests that flexor inhibition was produced by interactions at a spinal rather than cortical level. Such spinal inhibition could, for example, be the result of antidromic activation of motor axons and of orthodromic volleys in muscle afferents, both set up by peripheral nerve stimulation. These could produce after-hyperpolarization of motoneurones and activate Renshaw cell circuits within the spinal cord.
Afferents responsible for the antagonist cortical inhibition
Median or radial nerve stimulation at the elbow at threshold intensities for activating motor axons probably excites large diameter cutaneous, joint, muscle spindle and tendon afferents. Our observation that cutaneous afferents did not inhibit forearm MEPs suggests that cutaneous input does not contribute to antagonist cortical inhibition. Of the remaining afferents, the most likely candidate for involvement would be muscle spindle afferents since they have a rapidly conducting pathway to the motor cortex (Wiesendanger & Miles, 1982). Other slower muscle afferents (for example group III) seem unlikely to intervene, first because the inhibition elicited after their activation has a longer latency, and second because stimulation of slower afferents leaves the size of the MEPs unchanged in extensors (Priori et al. 1998).
Relationship of the present findings to previously described effects of peripheral nerve stimulation on voluntary EMG activity and responses to TCS
We have argued above that forearm muscle afferents inhibit the areas of the motor cortex controlling antagonist muscles. This differs from previously published findings. Several investigators have used magnetic TCS to show that the cerebral cortex is involved in long-latency reflexes. Most experiments have focused on hand muscles (Troni et al. 1988; Mariorenzi et al. 1991; Deuschl et al. 1991), where the evidence for transcortical reflexes is strongest (Thilmann et al. 1991; Rothwell et al. 1991a). Deletis et al. (1992) noted that stimulation of the median nerve at the wrist (at a higher intensity than that used here) could inhibit MEPs evoked by electrical TCS in forearm flexors. However, they did not investigate whether it was a cortical or spinal phenomenon.
Investigations of possible transcortical inhibitory reflexes have been limited to the I1 reflex, a short EMG inhibition best observed in hand muscles after digital nerve stimulation. Jenner & Stephens (1982) speculated that it was of spinal origin but dependent on cortical facilitation. Several investigators testing I1 with electrical and magnetic TCS (Maertens de Noordhout et al. 1992) and with H and F responses (Clouston et al. 1995), found no evidence for a cortical origin of I1, confirming the spinal nature of the response. Thus, because in our experiments cutaneous stimulation failed to elicit inhibition, it seems unlikely that our extensor inhibition, for which cortical involvement is strong, could be related to the I1 reflex. In addition, it is difficult to elicit an I1 reflex in forearm muscles with weak stimulation. An I1 reflex appears here only if strong single stimuli (Caccia et al. 1970) or trains of three to four stimuli are used (Clouston et al. 1995). We conclude that the inhibitory mechanism described here differs from I1.
The following observations allow us to exclude an inhibitory mechanism involving the propriospinal system (Pierrot-Deseilligny, 1996). First, no studies have described inhibition from median afferents onto propriospinal neurones. Second, the onset latency of propriospinal inhibition of extensor MEPs is about 6 ms after radial nerve stimulation at the wrist (Mazevet et al. 1996), equivalent to 2–3 ms at the elbow. Finally, propriospinal inhibition has an equal effect on responses evoked by electrical and magnetic TCS.
Our experiments with electrical TCS confirm previous findings that conditioning stimulation of the mixed nerve supplying antagonist muscles does not inhibit forearm muscle MEPs (Berardelli et al. 1987). In addition, our experiments using magnetic TCS showed that a radial nerve shock inhibited MEPs in flexors. The different behaviour of magnetic and electrical MEPs supports the cortical origin of the inhibition we noted in forearm flexors after radial nerve stimulation.
The origin of the inhibition of corticospinal outputs to antagonist muscles after median nerve stimulation
Muscle input is traditionally assumed to excite areas of motor cortex controlling the agonist muscle (see Porter & Lemon, 1993). This is the basis for the long loop reflexes. Our results now suggest that, in addition, at least some muscle groups are organized inversely, so that muscle afferents also inhibit areas of motor cortex controlling the antagonist(s). This organization could be similar to the reciprocal inhibition seen in the spinal cord. But if it is, why did extensor inhibition in our experiments not have an accompanying facilitation of flexor MEPs? The absence of flexor facilitation (and the presence even of inhibition) was unexpected but may have been due to the variable distribution of transcortical responses in the flexors. For example, Day et al. (1991) showed that the stretch reflex in superficial finger flexors has a transcortical component, whereas Rothwell et al. (1991a) suggested that the transcortical reflexes in wrist flexors do not. Because our data were recorded over the flexor carpi radialis muscle, the transcortical facilitation might have been small or absent. A small transcortical facilitation might also have been obscured by spinal inhibition. Our calculations suggest that inhibition of corticospinal projections to extensors began about 4 ms after the arrival of the afferent volley at the sensory cortex. We do not know how this input reaches the motor cortex. It may take several additional milliseconds to travel from the sensory cortex through cortico-cortical connections. Alternatively, a direct input from the thalamus to the motor cortex, slower than the pathway to the sensory cortex, arrives some 2 ms later (Mauguiere et al. 1983). Both possibilities suggest that inhibition begins almost immediately after the afferent volley reaches the motor cortex. It is difficult to compare this with estimates of the cortical delay for facilitatory effects since the latter have not been made in such detail. For example, in a study on forearm muscles, to produce the afferent input Day et al. (1991) used muscle stretch (which initiates a dispersed discharge with a variable delay) and tested for facilitation of MEPs at 10 ms intervals only. In patients with typical cortical myoclonus, estimates of the cortical delay between arrival of afferent input at the cortex and motor output vary between 4 and 10 ms (Obeso et al. 1985). We tentatively conclude that cortical facilitation and inhibition would have similar latencies.
We can only speculate on the mechanism of this inhibition. As far as we know, no animal study has directly addressed the question of whether muscle input inhibits corticospinal antagonist projections. However, this is the mechanism we favour to account for the present results. Possible support for this view comes from preliminary anatomical data (Bertrand et al. 1996). The fact that we observed reduced inhibition during contraction of extensors suggests that the arrangement may be similar to spinal reciprocal inhibition. In other words, besides inhibiting antagonist motoneurons (via inhibitory interneurons) at the spinal level, muscle input from contracting agonist muscle inhibits the pyramidal cells controlling the antagonist muscle(s) at the cortical level. Finally, the inhibition produced by radial nerve stimulation in flexors, and the lack of inhibition in other muscles (FDI and brachioradialis), indicates specific bidirectional antagonist cortical inhibition involving pairs of antagonists but not functionally unrelated muscles.
Functional implications
Our findings show that some of the actions of the afferent muscle input to human motor cortex resemble those seen at the spinal level: not only may there be facilitation of cortical projections to agonist muscle, but there may also be inhibition of cortical projections to antagonist muscles. Cortical antagonist inhibition presumably acts in parallel with spinal reciprocal inhibition. For example, if corticospinal activity decreases presynaptic inhibition (Rudomin, 1990) in the forearm as in the leg (Iles, 1996), suppression of corticospinal input to extensor muscles might increase presynaptic inhibition from median nerve afferents on radial nerve muscle afferents, thus boosting spinal inhibitory circuits. Functionally, as our data from the patient with the thalamic lesion also suggest, the antagonist cortical inhibition may prevent the inappropriate co-activation of antagonist corticospinal ouputs and assist the inhibitory spinal pathways.
Acknowledgments
Dr A. Priori was supported by a post-doctoral fellowship from the University of Rome ‘La Sapienza’. The authors thank Dr G. Cruccu for statistical advice, Dr M. Inghilleri for helpful suggestions, and Dr N. Smania for referring the patient.
References
- Baldissera F, Leocani L. Afferent excitation of human motor cortex as revealed by enhancement of direct cortico-spinal actions on motoneurones. Electroencephalography and Clinical Neurophysiology. 1995;97:394–401. doi: 10.1016/0924-980x(95)00125-5. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Day BL, Marsden CD, Rothwell JC. Evidence favouring presynaptic inhibition between antagonist muscle afferents in the human forearm. The Journal of Physiology. 1987;391:71–83. doi: 10.1113/jphysiol.1987.sp016726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand L, Capaday C, Devanne H, Lavoie BA. Intracortical connections between motor cortical zones controlling antagonist muscles. Society for Neuroscience Abstracts. 1996;22:1083. [Google Scholar]
- Caccia MR, McComas AJ, Upton ARM, Blog T. Cutaneous reflexes in small muscles of the hand. Journal of Neurology Neurosurgery and Psychiatry. 1970;36:960–977. doi: 10.1136/jnnp.36.6.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouston PD, Kiers L, Menkes D, Sander H, Chiappa K, Cros D. Inhibition of motor evoked potential by digital electrical stimulation. Electroencephalography and Clinical Neurophysiology. 1995;97:114–125. doi: 10.1016/0924-980x(94)00310-4. [DOI] [PubMed] [Google Scholar]
- Datta AK, Harrison LM, Stephens JA. Task-dependent changes in the size of response to magnetic brain stimulation in human first dorsal interosseous muscle. The Journal of Physiology. 1989;418:13–23. doi: 10.1113/jphysiol.1989.sp017826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens de Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD. Electric and magnetic stimulation of the human motor cortex: surface EMG and single motor unit response. The Journal of Physiology. 1989;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Riescher H, Struppler A, Rothwell JC, Marsden CD. Changes in the response to magnetic and electric stimulation of the motor cortex following muscle stretch in man. The Journal of Physiology. 1991;433:41–58. doi: 10.1113/jphysiol.1991.sp018413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deletis V, Schild JH, Beric A, Dimitrijevic MR. Facilitation of motor evoked potentials by somatosensory afferent stimulation. Electroencephalography and Clinical Neurophysiology. 1992;85:302–310. doi: 10.1016/0168-5597(92)90106-l. 10.1016/0168-5597(92)90106-L. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Michels R, Berardelli A, Schenck E, Inghilleri M, Lucking CH. Effects of electric and magnetic transcranial stimulation on long latency reflexes. Experimental Brain Research. 1991;83:403–410. doi: 10.1007/BF00231165. [DOI] [PubMed] [Google Scholar]
- Iles JF. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of I a afferents from human lower limb. The Journal of Physiology. 1996;491:197–207. doi: 10.1113/jphysiol.1996.sp021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner JR, Stephens JA. Cutaneous reflex responses and their central neural pathway studied in man. The Journal of Physiology. 1982;333:405–419. doi: 10.1113/jphysiol.1982.sp014461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai T, Hayes KC, Wolfe DL, Allatt RT. Afferent conditioning of motor evoked potentials following transcranial magnetic stimulation of motor cortex in normal subjects. Electroencephalography and Clinical Neurophysiology. 1992;85:95–101. doi: 10.1016/0168-5597(92)90074-l. 10.1016/0168-5597(92)90074-L. [DOI] [PubMed] [Google Scholar]
- Maertens de Noordhout A, Rothwell JC, Day BL, Dressler D, Nakashima K, Thompson PD, Marsden CD. Effect of digital nerve stimuli on responses to electrical and magnetic stimulation of the human brain. The Journal of Physiology. 1992;447:535–548. doi: 10.1113/jphysiol.1992.sp019016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariorenzi R, Zarola F, Caramia MD, Paradiso C, Rossini PM. Non-invasive evaluation of central motor tract excitability changes following peripheral nerve stimulation in healthy humans. Electroencephalography and Clinical Neurophysiology. 1991;81:90–101. doi: 10.1016/0168-5597(91)90002-f. 10.1016/0168-5597(91)90002-F. [DOI] [PubMed] [Google Scholar]
- Mauguiere F, Desmedt JE, Courjon J. Astereognosis and dissociated loss of frontal and parietal components of somatosensory evoked potentials in hemispheric lesions. Brain. 1983;106:271–311. doi: 10.1093/brain/106.2.271. [DOI] [PubMed] [Google Scholar]
- Mazevet D, Pierrot-Deseilligny E, Rothwell JC. A propriospinal-like contribution to electromyographic responses evoked in wrist extensor muscles by transcranial magnetic stimulation of the motor cortex. Experimental Brain Research. 1996;109:495–499. doi: 10.1007/BF00229634. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Fedirchuk B. Evidence suggesting a transcortical pathway from cutaneous foot afferents to tibialis anterior motoneurones in man. The Journal of Physiology. 1997;501:473–484. doi: 10.1111/j.1469-7793.1997.473bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JA, Rothwell JC, Marsden CD. The spectrum of cortical myoclonus. Brain. 1985;108:193–224. doi: 10.1093/brain/108.1.193. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Transmission of the cortical command to move to cervical propriospinal premotoneurones. Progress in Neurobiology. 1996;48:489–517. doi: 10.1016/0301-0082(96)00002-0. 10.1016/0301-0082(96)00002-0. [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon R. Corticospinal Function and Voluntary Movement. Oxford: Oxford University Press; 1993. [Google Scholar]
- Priori A, Berardelli A, Inghilleri M, Pedace F, Giovannelli M, Manfredi M. Electrical stimulation over muscle tendons in humans. Evidence favouring presynaptic inhibition of Ia fibres due to activation of group III tendon afferents. Brain. 1998;121:373–380. doi: 10.1093/brain/121.2.373. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. Control of Human Voluntary Movement. London: Chapmann & Hall; 1994. [Google Scholar]
- Rothwell JC, Colebatch J, Britton TC, Priori A, Thompson PD, Day BL, Marsden CD. Physiological studies in a patient with mirror movements and agenesis of the corpus callosum. The Journal of Physiology. 1991a;438:34. P. [Google Scholar]
- Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. Stimulation of the human motor cortex through the scalp. Experimental Physiology. 1991b;76:159–200. doi: 10.1113/expphysiol.1991.sp003485. [DOI] [PubMed] [Google Scholar]
- Rudomin P. Presynaptic inhibition of muscle spindle and tendon organ afferents in the mammalian spinal cord. Trends in Neurosciences. 1990;13:499–505. doi: 10.1016/0166-2236(90)90084-n. 10.1016/0166-2236(90)90084-N. [DOI] [PubMed] [Google Scholar]
- Terao Y, Ugawa Y, Uesaka Y, Hanajima R, Gemba-Shimizu K, Ohki Y, Kanazawa I. Input-output organization in the hand area of the human motor cortex. Electroencephalography and Clinical Neurophysiology. 1995;97:375–381. doi: 10.1016/0924-980x(95)00179-o. 10.1016/0924-980X(95)00179-O. [DOI] [PubMed] [Google Scholar]
- Thilmann AF, Schwarz M, Topper R, Fellow SJ, Noth J. Different mechanisms underlie the long-latency stretch reflex response of active human muscle at different joints. The Journal of Physiology. 1991;444:631–643. doi: 10.1113/jphysiol.1991.sp018898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomberg C, Desmedt JE, Ozaki I. Right or left ear reference changes the voltage of frontal and parietal somatosensory evoked potentials. Electroencephalography and Clinical Neurophysiology. 1991;80:504–512. doi: 10.1016/0168-5597(91)90132-h. 10.1016/0168-5597(91)90132-H. [DOI] [PubMed] [Google Scholar]
- Troni W, Cantello R, De Mattei M, Bergamini L. Muscle responses elicited by cortical stimulation in the hand: differential conditioning by activation of the proprioceptive and exteroceptive fibers of the median nerve. In: Rossini PM, Marsden CD, editors. Non-invasive Stimulation of the Brain and Spinal Cord. New York: A. Liss Inc.; 1988. pp. 73–83. [Google Scholar]
- Ugawa Y, Day BL, Rothwell JC, Thompson PD, Merton PA, Marsden CD. Modulation of motor cortical excitability by electrical stimulation over the cerebellum in intact man. The Journal of Physiology. 1991;441:57–72. doi: 10.1113/jphysiol.1991.sp018738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesendanger M, Miles TS. Ascending pathway of low threshold muscle afferents to the cerebral cortex and its possible role in motor control. Physiological Reviews. 1982;62:1234–1270. doi: 10.1152/physrev.1982.62.4.1234. [DOI] [PubMed] [Google Scholar]
- Wolfe DL, Hayes KC. Conditioning effects of sural nerve stimulation on short and long latency motor evoked potentials in lower limb muscles. Electroencephalography and Clinical Neurophysiology. 1995;97:11–17. doi: 10.1016/0924-980x(94)00239-4. [DOI] [PubMed] [Google Scholar]


