Abstract
Secretory responses were measured in single rat pituitary melanotrophs as the relative increase in membrane capacitance (Cm) 8 min after the start of dialysis with solutions containing 0.45 μm Ca2+. In the added presence of cAMP (0.2 mM) in the patch pipette solution, capacitance responses increased 2- to 3-fold in comparison with controls.
To study whether cAMP-dependent mechanisms affect cytosolic calcium activity ([Ca2+]i), dibutyryl cyclic AMP (dbcAMP, 10 mM) was added to intact melanotrophs and [Ca2+]i was measured using fura-2 AM. Addition of dbcAMP caused a transient reduction in [Ca2+]i to 82 ± 21 nM from a resting value of 100 ± 19 nM (mean ± s.e.m., n = 32, P < 0.002), indicating that the cAMP-induced increase in secretory activity was not the result of cAMP acting to increase [Ca2+]i, which then increased secretory activity.
To investigate whether cAMP affects the secretory apparatus directly, the interaction of a single secretory granule with the plasmalemma was monitored by measuring discrete femtofarad steps in Cm. The signal-to-noise ratio of recordings was increased by pre-incubating the cells with a hydrophobic anion, dipicrylamine.
Recordings of unitary exocytic events (discrete ‘on’ steps in Cm) showed that the amplitude of ‘on’ steps - a parameter correlated to the size of exocytosing secretory granules - increased from 4.2 ± 0.2 fF (n = 356) in controls to 7.9 ± 0.2 fF in the presence of cAMP (n = 329, P < 0.001), while the frequency of unitary exocytic events was similar in controls and in the presence of cAMP.
The results suggest that a cAMP-dependent mechanism mediates the fusion of larger granules with the plasmalemma.
The production and release of peptide hormones by endocrine cells is mediated via the regulated secretory pathway (Alberts et al. 1994). Peptide prohormones arriving in the trans-Golgi network are segregated from proteins travelling via the constitutive route and packaged separately into secretory granules (Orci et al. 1987). Condensation is not the only process to which peptide hormones are subjected as the secretory granules mature. Many peptide hormones and neurotransmitters are synthesized as inactive precursors from which the active molecules have to be liberated by proteolysis. These cleavages are thought to begin in the trans-Golgi network and continue in the secretory granules (Alberts et al. 1994). Thus, when cells are stimulated to exocytose, granules containing active and inactive peptide hormones may empty their contents into the extracellular space. In order to prevent such uncontrolled loss of inactive hormone during secretory activity, a mechanism may exist that mediates preferential fusion of only those granules containing fully processed peptide hormones. In search of such a mechanism, we studied rat pituitary pars intermedia cells (melanotrophs), which secrete a number of peptides deriving from the post-translational processing of pro-opiomelanocortin (POMC), including β-endorphin, α-melanocyte stimulating hormone (α-MSH) and adrenocorticotrophin (ACTH; Mains & Eipper, 1979). Much of the processing of these peptides takes place in the secretory granules (Loh & Gritsch, 1981).
During the maturation process, the size of the secretory granules of endocrine cells increases (Farquahar et al. 1978; Salpeter & Farquahar, 1981; Tooze et al. 1991), and in rat melanotrophs this growth is thought to be associated with metabolic processing of the secretory granule contents (Tanaka et al. 1991). Is the growth of secretory granules physiologically regulated? It has been reported that the size of secretory granules in rat pars intermedia depends on the physiological state of the animal. Their size is reduced when rats are pre-treated with bromocriptine, a dopamine D2 agonist (Bäck, 1989). In contrast, in hypersecretory melanotrophs treated with the dopamine antagonist haloperidol, an increase in secretory granule size has been reported (Bäck & Soinila, 1994). In rat melanotrophs dopamine controls secretion by inhibiting adenylate cyclase, which results in a reduction in the amount of second messenger cyclic AMP (cAMP; Munemura et al. 1980). Do cAMP-dependent mechanisms regulate the growth of secretory granules in rat melanotrophs?
Previous studies have demonstrated that the secretory activity of melanotrophs is modulated by cytosolic calcium activity ([Ca2+]i) and cAMP (Yamamoto et al. 1987; Lee, 1996). However, the site of cytoplasmic action of cAMP remains unclear. Single-cell studies have indicated that cAMP may affect the Ca2+-dependent secretory machinery directly (Sikdar et al. 1990; Ämmäläet al. 1993), and that the secretory granules may be a possible site of modulation (Bäck, 1989; Bäck & Soinila, 1994). We therefore investigated whether cAMP-dependent mechanisms affect the interaction of a single secretory granule with the plasma membrane. The patch-clamp technique was used to monitor small discrete changes in membrane capacitance (Cm). Increases in capacitance are a direct measure of increases in the surface area of the plasma membrane, and small discrete changes in Cm are due to the interactions of single secretory granules with the plasma membrane (Neher & Marty, 1982). These have previously been recorded in rat melanotrophs (Zupancic et al. 1994; Kreft & Zorec, 1997), but in the present study the signal-to-noise ratio of recordings has been improved by pre-incubating the cells with the hydrophobic anion dipicrylamine (DPA; Oberhauser & Fernandez, 1995). The DPA ion, being mobile in the membrane, increases the capacitance signal observed (Oberhauser & Fernandez, 1995) when a granule fuses with the plasma membrane, so helping to resolve small steps in Cm.
To investigate the putative modulation of unitary exocytic events by cAMP, discrete ‘on’ steps in Cm were recorded in the presence and absence of cAMP. We report that in the presence of added cytosolic cAMP, the amplitude, but not the frequency, of discrete ‘on’ steps in Cm is increased compared with controls. This result indicates a novel cAMP-dependent step in the regulated secretory pathway of rat melanotrophs mediating the fusion of larger granules with the plasma membrane.
METHODS
Cell preparation
Male rats (Wistar, 180–200 g) were killed by asphyxiation using an anaesthetic chamber with a raised CO2 atmosphere followed by decapitation, a procedure approved by the Veterinary Administration of the Slovenian Ministry for Agriculture and Forestry according to the Law for Animal Health Protection and the Instructions for Granting Permits for Animal Experimentation for Scientific Purposes. Melanotrophs from the pituitary pars intermedia were prepared as described (Rupnik & Zorec, 1992) and kept in cell culture for between 1 and 7 days before they were used for experiments.
Fura-2 measurements
Intracellular [Ca2+] was measured after loading cells with 4 μm fura-2 AM (Molecular Probes) in culture medium at 37°C for 30 min. The cells were rinsed three times with the extracellular recording solution and measurements were made at room temperature (23°C). Fura-2 fluorescence was excited at two different wavelengths (340 and 380 nm) using a rotating filter wheel (Sutter Instruments) fitted to the xenon-arc lamp housing of a Nikon Diaphot inverted microscope with a Fluor × 40 objective lens. Images of the emission were passed through a 430 nm dichroic mirror, filtered at 510 nm and collected by a CCD camera (DIC, TE, World Precision Instruments, Sarasota, FL, USA). The digital images were stored and processed by a Miracal system (Life Science Resources, Cambridge, UK). The 340 nm: 380 nm ratio images were converted into [Ca2+]i using the formula:
where Kd, the dissociation constant of fura-2, was taken as 150 nM, β is the 380 nm fluorescence ratio in Ca2+-free and saturating Ca2+ conditions, and Rmin and Rmax are the fluorescence ratios in Ca2+-free and saturating Ca2+ conditions, respectively; these were determined at the end of the experiment by exposing the cells to 10 μm ionomycin in external solution containing 10 mM EGTA and 10 mM Ca2+, respectively. For a set of experiments these values were pooled, and used for calibration. The mean values of Rmin, Rmax and β were 1.91, 9.54 and 4.79, respectively. Differences in [Ca2+]i were analysed using a Student's paired t test.
Electrophysiology
The non-compensated method of whole-cell recording of membrane capacitance (Cm) was employed to measure large (pF) changes in Cm as described (Zorec et al. 1991). Briefly, the cells were voltage clamped at a holding potential of -70 mV. Membrane capacitance was recorded using a two-phase lock-in amplifier (1600 Hz, 1 mV peak-to-peak) incorporated into a patch-clamp amplifier (SWAM IIC, Henigman, Piran, Slovenia; see Zorec et al. 1991). A DC current (low-pass filtered, 1–10 Hz, -3 dB), holding potential and real and imaginary admittance signals (low-pass filtered, 1 Hz, -3 dB) were used in calculations (Zorec et al. 1991). The reversal potential used in the calculation was -50 mV, which did not change throughout a recording. The plots of the passive cell parameters, the access conductance (Ga), the parallel combination of leak and membrane conductance (Gm) and the Cm were derived by a computer-aided reconstruction following an analog-to-digital conversion (CED 1401, Cambridge Electronic Design, Cambridge, UK) using an IBM-compatible PC. The software (CAP3) was written by Dr J. Dempster (University of Strathclyde, Glasgow, UK). Recordings were made at room temperature with pipette resistances between 1 and 4 MΩ. The standard pipette solution contained (mM): KCl, 150.0; MgCl2, 2.0; EGTA, 0.5; CaEGTA (Ca2+-saturated EGTA), 1.5; Hepes, 10; pH 7.2 adjusted with KOH. The external solution contained (mM): NaCl, 131.8; CaCl2, 1.8; KCl, 5.0; MgCl2, 2.0; D-glucose, 5; NaH2PO4, 0.5; NaHCO3, 5; Hepes, 10; pH 7.2 adjusted with NaOH. EGTA and CaEGTA was prepared as 100 mM stock solutions as described (Neher, 1988). Total EGTA concentration was 2 mM, which exceeds the buffering capacity of melanotrophs (Thomas et al. 1990). The free cytosolic calcium concentration ([Ca2+]i) was estimated to be 0.45 μm from an apparent dissociation constant for the CaEGTA complex of 0.15 μm (Grynkiewicz et al. 1985), and assuming that the cytosol equilibrates with the pipette solution upon the establishment of whole-cell recording. The pipette and bath solutions were of similar osmolarity (within 5 %) measured by freezing-point depression (Camlab, Cambridge, UK).
High-resolution capacitance measurements (in the fF range) were made using the compensated technique as described previously (see Zorec et al. 1991; Zupancic et al. 1994), using a sinusoidal voltage (1600 Hz, 30 mV, peak-to-peak). Peak-to-peak noise was measured in whole-cell mode at the beginning of the recordings, filtered at 30 Hz (-3 dB, low-pass filtered, Bessel 4-pole). The cells were voltage clamped at -50 to -60 mV. Measurements of step amplitudes were made manually from the out-of-phase lock-in signals (capacitance signal, Cm) using the cursor option in the WCP software (Dr J. Dempster, University of Strathclyde) on a PC-compatible computer, and the appearance of a step was ascertained by progressive filtering. A step-like event in the capacitance signal was considered as a step when there was no projection of the step in the conductance signal (in-phase lock-in signal). The frequency of exocytic events was determined in segments of records (10-20 s duration) with more than five events and with a linear increase in Cm with time. Resting Cm was determined following establishment of whole-cell recording by nullifying the capacitative currents evoked by sinusoidal stimulation. Cell diameters were measured using an eye-piece micrometer. Cyclic AMP and ATP were prepared as stock solutions and diluted into the standard pipette solution prior to the experiments. Dipicrylamine (DPA; courtesy of Dr J. M. Fernandez, Mayo Clinic, Rochester, NY, USA), dissolved in DMSO (100 mM), was diluted to a final concentration of 10 μm in the bathing solution. The cells were incubated in this DPA-containing bathing solution for 2 min at 37°C. DMSO as a vehicle did not affect the recorded responses of Cm. Unless stated otherwise, all salts were from Sigma. Experiments were performed at room temperature (23°C). Statistics are in the format means ±s.e.m. and differences between samples were tested using Student's unpaired t test, ANOVA and the F test.
RESULTS
To study whether the Ca2+-dependent secretory activity of a single rat melanotroph is enhanced by cAMP as has been reported previously (Sikdar et al. 1990; Lee, 1996), we added cAMP-ATP (0.2 mM cAMP and 1 mM ATP) to the Ca2+-containing patch pipette solution (0.45 μm Ca2+). Using the non-compensated method of recording, secretory responses were measured as the increase in Cm 8 min after the establishment of whole-cell recording, relative to resting Cm. Secretory responses increased 2- to 3-fold in comparison with responses in cells dialysed with a Ca2+-containing pipette solution, or in the presence of 1 mM ATP only (Figs 1 and 2). The rise in Cm over several minutes is indicative of net exocytosis, suggesting that cAMP-dependent mechanisms potentiate exocytosis in single rat melanotrophs, consistent with previous reports (Yamamoto et al. 1987; Sikdar et al. 1990; Ämmäläet al. 1993; Lee, 1996). These effects could occur because either (i) cAMP increases the levels of [Ca2+]i (for example by increasing Ca2+ release from intracellular stores) which further stimulates fusion, or (ii) cAMP directly affects granule fusion with the plasma membrane.
Figure 1. Effect of intracellular cAMP-ATP on the Ca2+-dependent secretory response of rat melanotrophs measured as a change in membrane capacitance (Cm).
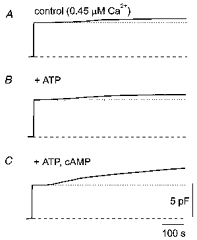
The time courses of Cm following intracellular dialysis with 0.45 μm Ca2+ alone in control (A), in the added presence of 1 mM ATP (B) and in the added presence of 0.2 mM cAMP and 1 mM ATP (C) are shown. The membrane holding potential was -70 mV in all these experiments.
Figure 2. Effect of intracellular ATP and cAMP-ATP on the Ca2+-dependent capacitance response of rat melanotrophs.
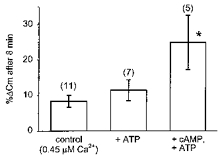
Changes in Cm (%ΔCm), measured 8 min after the start of whole-cell recording, were determined relative to the Cm immediately following patch rupture. Control cells were dialysed with 0.45 μm Ca2+ alone. Note that in the presence of cAMP (0.2 mM), secretory responses are significantly enhanced (*P < 0.02). Heights of columns represent the mean, while the vertical bars are the s.e.m. Numbers in parentheses are the numbers of observations, in this and in Figs 5B and 6.
Although intracellular [Ca2+] measurements have been made in intact single rat melanotrophs in culture (Nemeth et al. 1990), to date there are no reports to suggest that cAMP affects [Ca2+]i. To resolve this question, single melanotrophs were stimulated with dibutyryl cyclic AMP (dbcAMP), a membrane-permeable cAMP analogue, while [Ca2+]i was monitored using the Ca2+ indicator, fura-2 AM. Figure 3A shows the response of a single cell. Addition of 10 mM dbcAMP caused a slight decrease in [Ca2+]i, which was not sustained and recovered to the basal level. The [Ca2+]i values before, 1 min and 10 min after addition of dbcAMP were 100 ± 19, 82 ± 21 and 104 ± 13 nM (mean ± s.e.m, n = 32), respectively (see Fig. 3B). Thus, while cAMP enhanced the secretory response (Figs 1 and 2), it paradoxically reduced [Ca2+]i (Fig. 3). It is therefore unlikely that the potentiation of the secretory response with cAMP-ATP in the pipette in the presence of 0.45 μm[Ca2+]i (Figs 1 and 2; see also Lee, 1996) occurs by affecting [Ca2+]i.
Figure 3. Effect of dibutyryl cAMP on [Ca2+]i in rat melanotrophs.
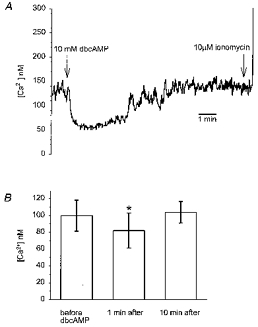
A, time course of the effect of dibutyryl cAMP (dbcAMP) on [Ca2+]i in a single rat melanotroph. The points of application of dbcAMP and ionomycin as concentrated boluses are indicated by the arrows (10 μl of 0.4 M dbcAMP was added to 0.4 ml of bath solution to raise the concentration to 10 mM. A 10 μm concentration of ionomycin in the bath was similarly achieved by adding 0.8 ml of 5 mM ionomycin). B, histograms of [Ca2+]i before, 1 min and 10 min after dbcAMP application. The data are the means ±s.e.m. for 32 cells. *P < 0.002 (Student's paired t test), significantly different to control data obtained before dbcAMP addition.
The cAMP-mediated increase in Cm (Figs 1 and 2) could be due to the increased amplitude and/or frequency of unitary exocytic events. Using the compensated mode of recording we measured changes in Cm at high resolution (fF range) after pretreatment with DPA. Initially, the effect of DPA pretreatment (2 min, 10 μm) on Cm was studied. The resting Cm of cells with a mean diameter of 13 μm was 5.5 ± 0.1 pF (n = 9) in controls and 6.2 ± 0.2 pF (n = 14) after DPA pretreatment, a significant difference (P < 0.05). Comparison of Fig. 4A and B reveals that discrete steps in Cm are clearly detected when cells are pre-incubated with DPA. The amplitude of these steps was increased from control values of 3.4 ± 0.2 fF (n = 144, recorded in 6 cells) to 4.2 ± 0.2 fF (n = 356, recorded in 20 cells), which was significantly different (P < 0.01). The increase in amplitude of the steps was not associated with an increase in the peak-to-peak noise level, since the noise of whole-cell recordings in controls was 1.8 ± 0.2 fF (5 cells), and not significantly different from the noise recorded after DPA pretreatment (1.8 ± 0.1 fF, 15 cells), indicating that DPA pretreatment resulted in improved signal-to-noise ratio, as reported by Oberhauser & Fernandez (1995).
Figure 4. Amplification of discrete capacitance steps by dipicrylamine (DPA) and the effect of intracellular cAMP-ATP.
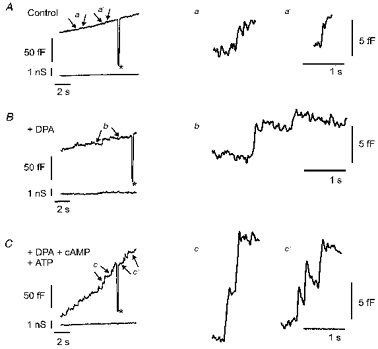
A, epoch of a recording of Cm as a function of time in a cell not treated with DPA. The standard patch pipette solution (see Methods) contained 0.45 μm Ca2+. B, time-dependent changes in Cm from a cell dialysed with standard intracellular solution but pretreated with DPA (2 min, 10 μm). C, time course of Cm in a DPA-pretreated cell dialysed with a standard internal solution containing 0.2 mM cAMP and 1 mM ATP. The asterisks in A, B and C indicate the addition of a 100 fF capacitance calibration signal, which shows that there was no cross-talk between the capacitance and conductance signals (lower traces in A, B and C; see Zorec et al. 1991; Zupancic et al. 1994). The epochs of records marked by arrows (a, a' in A, b in B and c, c' in C) are expanded on the right side of the figure to indicate the relative amplitudes of the capacitance steps. The size of the steps in B is almost twice that shown in A. The resting Cm values for the cells shown in A, B and C were 5.1, 6.7 and 6.7 pF, respectively. Holding potential, -55 mV. Traces were low-pass filtered (30 Hz, -3 dB, Bessel 4-pole).
When DPA-pretreated cells were dialysed with a solution containing cAMP, the mean amplitude of the discrete steps in Cm was 7.9 ± 0.2 fF (n = 329, recorded in 10 cells), significantly higher (P < 0.001) in comparison with values obtained in cells treated with DPA only (4.2 ± 0.2 fF, n = 356, 20 cells; Fig. 5). The cAMP-enhanced secretory responses observed in Figs 1 and 2 may thus be explained by an increased amplitude of discrete exocytic Cm steps. This could indicate that secretory granules undergoing exocytosis have a larger diameter in the presence of cAMP, which is consistent with earlier reports (Bäck, 1989; Bäck & Soinila, 1994).
Figure 5. Amplitude of capacitance steps measured in DPA-pretreated cells dialysed with standard pipette solution (control) or cAMP-ATP.
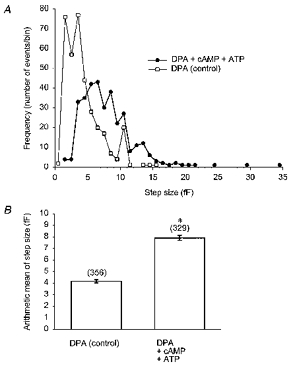
A, amplitude histogram (frequency polygon) of capacitance steps denoting unitary exocytic steps in control cells (□) and in the presence of cAMP-ATP (0.2 mM, 1 mM; •). The bin width used for the histogram was 1 fF. B, bar chart showing the mean step size (error bars denote ±s.e.m.) of capacitance steps in control cells and significant increase (*P < 0.001) in the presence of cAMP-ATP. The data from A and B are from experiments performed at a holding potential of -55 mV.
On the other hand, the cAMP-enhanced secretory responses shown on Figs 1 and 2 may also be due to an increased frequency of unitary events. The frequency of appearance of discrete steps in Cm was measured in linearly changing epochs of Cm records (see Zupancic et al. 1994). The frequency of discrete exocytic steps in Cm was variable (Fig. 4), but statistically similar in controls (pretreated with DPA only, 34 ± 7 min−1, 14 epochs in 11 cells) and in cells dialysed with cAMP-ATP (37 ± 8 min−1, 15 epochs in 8 cells, Fig. 6). Thus it is likely that cAMP-enhanced secretory responses recorded in Figs 1 and 2 are due to an increased amplitude of unitary exocytic events.
Figure 6. Frequency of occurrence of unitary exocytic events in control and in the presence of cAMP-ATP.
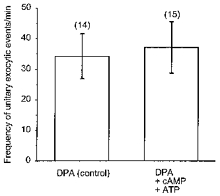
The frequency of appearance of discrete steps in Cm was measured in linearly changing epochs of Cm records (see Fig. 4 and Methods). Note that the frequency of discrete exocytic steps in Cm was statistically similar in controls (treated with DPA only) and in cells dialysed with cAMP-ATP (0.2 mM cAMP, 1 mM ATP).
In support of this, it is to be expected that the mean rate of Cm rise by cAMP should increase in proportion to the cAMP-mediated increase in the amplitude of unitary exocytic events. The rate of change in Cm was therefore measured from sections of the record that showed a linear increase in Cm with time (see Fig. 4). In cells pretreated with DPA only (n = 11), the mean rate of increase in Cm was 4.7 ± 1.6 fF s−1 (n = 44), whereas it was 11.2 ± 0.5 fF s−1 (n = 42) in the presence of cAMP-ATP (see also Fig. 4B and C), which was significantly different (P < 0.05). The enhanced mean rate of rise in Cm in the presence of cAMP-ATP was close to the predicted 2-fold increase in the amplitude of unitary exocytic events in the presence of cAMP-ATP (Fig. 5).
DISCUSSION
In this work we investigated whether the late stages of the regulated secretory pathway leading to exocytosis are regulated by cAMP-dependent mechanisms. For this the interaction of a single granule with the plasma membrane was monitored by capacitance measurements, while the cytosol of a single rat melanotroph was dialysed with Ca2+-containing solutions with or without cAMP-ATP.
The results demonstrate that the Ca2+-dependent secretory activity of a single rat melanotroph is enhanced by cAMP-ATP (Figs 1 and 2), consistent with published data (Lee, 1996). We also tested the possibility that the cAMP-ATP-mediated effects on the recorded secretory activity of melanotrophs are indirect via an increase in [Ca2+]i (Fig. 3). For this, dibutyryl cAMP, a membrane-permeable analogue of cAMP, was applied to intact cells while [Ca2+]i was monitored with fura-2. As shown on Fig. 3, application of dbcAMP resulted in a small transient reduction in resting [Ca2+]i, which could be due to an inhibition of Ca2+ influx (Nemeth et al. 1990). It is unlikely that the cAMP-ATP-mediated effects on secretory activity seen in Figs 1 and 2 (and also reported previously by Lee, 1990) are mediated through the modulation of [Ca2+]i. Further, any possible change in [Ca2+]i by cAMP in the experiments shown would be effectively buffered by the internal pipette solution containing 2 mM EGTA, a concentration exceeding the buffering capacity of a single melanotroph (Thomas et al. 1990; see Methods). In a study on insulin-secreting pancreatic β-cells, Ämmäläet al. (1993) found that increases in ΔCm produced by cAMP and forskolin were associated with only a marginal increase in [Ca2+]i through voltage-gated Ca2+ channels, which led them to suggest a Ca2+-independent potentiation mechanism of insulin release by cAMP in these cells. In previous studies the co-ordinated action of Ca2+ and cAMP on the release of α-MSH from the intermediate lobe of the rat pituitary gland has been suggested (Tsuruta et al. 1982), such that cAMP in some way enhances the effects of Ca2+ upon the release process. This has been confirmed by the experiments of Lee (1996), and by the results shown in Figs 1 and 2. In rat melanotrophs cAMP alone, in the absence of Ca2+, probably cannot affect the release process (Tsuruta et al. 1982), and similar findings have been reported in bovine pituitary lactotrophs (Sikdar, Zorec & Mason, 1990). It has been shown previously that stimulation of intact pituitary cells by dbcAMP causes phosphorylation of specific proteins in intact pituitary cells (Brattin & Portanova, 1981). Thus, the results presented in Figs 1 and 2 favour a direct effect of cAMP on the fusion of secretory granules with the plasma membrane.
Interestingly, the Ca2+-dependent rise in Cm was not affected by the presence of cytosolic ATP (Fig. 2), which contrasts with previous reports (Okano et al. 1993; Parsons et al. 1995). The difference may be explained by the higher [Ca2+]i used to stimulate secretory activity (1.8 μm in Okano et al. 1993; 2 μm in Parsons et al. 1995).
The macroscopic change in Cm due to exocytosis alone can be considered to be proportional to (i) the number of secretory vesicles (N), (ii) the probability of single granule fusion (p), and (iii) the amplitude of an elementary fusion event (cm), such that ΔCm=Npcm. To resolve whether the cAMP-ATP-enhanced rise in Cm (Figs 1 and 2) is due to altered elementary exocytic activity, the high-resolution compensated mode of Cm measurement (Neher & Marty, 1982; Zupancic et al. 1994) was used. Moreover, to further increase the signal-to-noise ratio cells were pretreated with DPA (Oberhauser & Fernandez, 1995). An important finding of this work was that the amplitude of unitary exocytic events was increased by cAMP-ATP in rat melanotrophs (Figs 3 and 5). Thus, it is likely that the cAMP-ATP-mediated increase in Cm recorded in Figs 1 and 2 is due to the increased amplitude of unitary exocytic events (Figs 4 and 5) and not to their increased frequency of occurrence. Measurements showed that the frequency of appearance of discrete steps was similar in the presence and absence of cAMP-ATP (Fig. 6).
The increase in the amplitude of unitary exocytic events indicates that the size of secretory granules undergoing exocytosis is increased by cAMP-ATP, which is in agreement with morphometric studies (Bäck, 1989; Bäck & Soinila, 1994). Among many possible mechanisms, two may account for this. One possibility is cAMP-dependent intergranule homotypic fusion. It was reported previously that granules emerging from the Golgi apparatus undergo aggregation (Farquhar et al. 1978) and intergranule fusion has been proposed to be the mechanism responsible for the increased size of mature secretory granules (Fumagalli & Zanini, 1985; Tooze et al. 1991). Intergranule fusion has been observed in rat melanotrophs with electron microscopy (K. Kosmelj, A. Cedilnik, P. Veranic, G. Zupancic, M. Rupnik, L. Kocmur-Bobanovic & R. Zorec, unpublished observations). The other possibility is that cAMP mediates the fusion of larger granules. Either of these possibilities would require proteins in the membrane of secretory granules that are phosphorylated. Phosphorylation of such proteins has been shown previously in pituitary cells (Labrie et al. 1971) and the regulatory subunit of cAMP-dependent protein kinase, the target of cAMP action, has been identified in secretory granules (Hand & Meidnieks, 1989). Protein kinase A phosphorylation sites are known to exist in proteins involved in the recruitment of vesicles, such as synapsins and rabphilin (Südhof, 1995). The observation of larger single fusion events in the presence of cAMP compared with controls suggests that the phosphorylation sites may be associated with larger secretory granules. This speculation, however, needs to be experimentally validated by immunogold labelling and electron microscopy.
Hormone processing in rat melanotrophs involves several enzymatic steps that appear to be confined to different types of secretory granules: pro-hormone is usually present in smaller, electron-dense granules, whereas the end products, such as α-MSH, are contained in larger electron-lucent granules (Tanaka et al. 1991). Therefore a cAMP-ATP-mediated increase in the amplitude of elementary exocytic events may be physiologically relevant: it may promote the exocytosis of granules containing fully processed peptide hormone. This speculation will have to be tested in the future. But if correct, it can be expected that in other peptide secretory cells a similar mechanism may operate to mediate the exocytosis of secretory granules. In support of this notion, although in contrast to our results, it has been documented that the newly synthesized form of certain hormones such as insulin appears to undergo exocytosis in preference to the stored form of the hormone (see Rhodes & Halban, 1987, and references therein).
In the present study, DPA pretreatment of cells improved the signal-to-noise ratio of recorded Cm signals by 20–30 %. Thus, it could be argued that the cAMP-ATP-mediated increase in the amplitude of discrete steps in Cm is due to the increased accumulation of DPA in the granule. This is unlikely, since similar results were obtained in a preliminary study (Zupancic & Zorec, 1994) in the absence of DPA. Interestingly, in the presence of DPA ions the time course following the exocytic fusion of some discrete steps in Cm was characterized by a decay (Fig. 4). A similar time course in discrete ‘on’ steps in Cm was recorded in DPA-pretreated mast and chromaffin cells, and this phenomenon was interpreted to be due to the difference in physicochemical properties between the plasma and the granule membrane, such that DPA ions would have a higher probability of responding to a change in voltage. The decay in the Cm after exocytic fusion may thus reflect the equilibration of the lipids of the granule with the lipids of the plasma membrane (Oberhauser & Fernandez, 1995).
In summary, the results provide evidence for a novel cAMP-dependent control step in the regulated secretory pathway of peptide-releasing secretory cells, a step mediating fusion of larger granules with the plasma membrane.
Acknowledgments
We thank S. Grilc for cell cultures and D. Sket, Z. Grubic and B. Hille for comments on an earlier version of the manuscript. This work was supported by a grant (J3-6207-381) and a bilateral Slovenia-India collaborative programme in Science and Technology awarded to S. K. S. and R. Z. from the Ministry of Sciences and Technology of the Republic of Slovenia.
References
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson J. Molecular Biology of the Cell. 3. New York: Garland Publishing, Inc.; 1994. [Google Scholar]
- Ämmälä C, Ashcroft F, Rorsman P. Calcium-independent potentiation of insulin release by cyclic AMP in single β-cells. Nature. 1993;363:356–358. doi: 10.1038/363356a0. 10.1038/363356a0. [DOI] [PubMed] [Google Scholar]
- Bäck N. The effect of bromocriptine on the intermediate lobe of the rat pituitary: an electron microscopic, morphometric study. Cell and Tissue Research. 1989;255:405–410. doi: 10.1007/BF00224124. [DOI] [PubMed] [Google Scholar]
- Bäck N, Soinila S. Regulation of secretory granule formation in chronically hypersecretory melanotrophs in the rat pituitary. Cell and Tissue Research. 1994;275:339–344. doi: 10.1007/BF00319432. [DOI] [PubMed] [Google Scholar]
- Brattin WJ, Jr, Portanova R. Dibutyryl cyclic AMP-induced phosphorylation of specific proteins in adenohypophyseal cells. Molecular and Cellular Endocrinology. 1981;23:77–90. doi: 10.1016/0303-7207(81)90118-0. [DOI] [PubMed] [Google Scholar]
- Farquhar MG, Reid JJ, Daniell LW. Intracellular transport and packaging of prolactin: a quantitative electron microscope autoradiographic study of mammotrophs dissociated from rat pituitaries. Endocrinology. 1978;102:296–311. doi: 10.1210/endo-102-1-296. [DOI] [PubMed] [Google Scholar]
- Fumagalli G, Zanini A. In cow anterior pituitary, growth hormone and prolactin can be packaged in separate granules of the same cell. Journal of Cell Biology. 1985;100:2019–2024. doi: 10.1083/jcb.100.6.2019. 10.1083/jcb.100.6.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hand AR, Meidnieks MIJ. Regulatory subunit of cyclic AMP-dependent protein kinase: presence in granules and secretion by exocrine and endocrine cells. Journal of Cell Science. 1989;93:675–681. doi: 10.1242/jcs.93.4.675. [DOI] [PubMed] [Google Scholar]
- Kreft M, Zorec R. Cell-attached measurements of attofarad capacitance steps in rat melanotrophs. Pflügers Archiv. 1997;434:212–214. doi: 10.1007/s004240050387. [DOI] [PubMed] [Google Scholar]
- Labrie F, Lemaire S, Poirer G, Pelletier G, Boucher R. Adenohypophyseal secretory granules: I. Their phosphorylation and association with protein kinase. Journal of Biological Chemistry. 1971;246:7311–7317. [PubMed] [Google Scholar]
- Lee AK. Dopamine (D2) receptor regulation of intercellular calcium and membrane capacitance changes in rat melanotrophs. The Journal of Physiology. 1996;495:627–640. doi: 10.1113/jphysiol.1996.sp021621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YP, Gritsch HA. Evidence for intragranular processing of proopiocortin in the mouse pituitary intermediate lobe. European Journal of Cell Biology. 1981;26:177–183. [PubMed] [Google Scholar]
- Mains RE, Eipper BA. Synthesis and secretion of corticotropins, melanotropins and endorphins by rat intermediate pituitary cells. Journal of Biological Chemistry. 1979;254:7885–7894. [PubMed] [Google Scholar]
- Munemura M, Cote TE, Tsuruta K, Eskay RL, Kebabian JW. The dopamine receptor in the intermediate lobe of the rat pituitary gland: pharmacological characterization. Endocrinology. 1980;107:1676–1683. doi: 10.1210/endo-107-6-1676. [DOI] [PubMed] [Google Scholar]
- Neher E. The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. The Journal of Physiology. 1988;395:193–214. doi: 10.1113/jphysiol.1988.sp016914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proceedings of the National Academy of Sciences of the USA. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth EF, Taraskevich PS, Douglas WW. Cytosolic Ca2+ in melanotrophs: pharmacological insights into regulatory influences of electrical activity and ion channels. Endocrinology. 1990;126:754–758. doi: 10.1210/endo-126-2-754. [DOI] [PubMed] [Google Scholar]
- Oberhauser AF, Fernandez JM. Hydrophobic ions amplify the capacitive currents used to measure exocytotic fusion. Biophysical Journal. 1995;69:451–459. doi: 10.1016/S0006-3495(95)79918-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano K, Monck JR, Fernandez JM. GTPγS stimulates exocytosis in patch-clamped rat melanotrophs. Neuron. 1993;11:165–172. doi: 10.1016/0896-6273(93)90280-5. [DOI] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Amherdt M, Perrelet A, Powell SK, Quinn DL, Moore HP. The trans-most cisternae of the Golgi complex: a compartment for sorting of secretory and plasma membrane proteins. Cell. 1987;51:1039–1051. doi: 10.1016/0092-8674(87)90590-3. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Coorsen JR, Horstmann H, Almers W. Docked granules, the exocytic burst, and the need for ATP hydrolysis in endocrine cells. Neuron. 1995;15:1085–1096. doi: 10.1016/0896-6273(95)90097-7. [DOI] [PubMed] [Google Scholar]
- Rhodes CJ, Halban PA. Newly synthesized proinsulin/insulin and stored insulin are released from pancreatic B cells predominantly via a regulated, rather than a constitutive pathway. Journal of Cell Biology. 1987;105:145–153. doi: 10.1083/jcb.105.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupnik M, Zorec R. Cytosolic chloride ions stimulate Ca2+-induced exocytosis in melanotrophs. FEBS Letters. 1992;303:221–223. doi: 10.1016/0014-5793(92)80524-k. [DOI] [PubMed] [Google Scholar]
- Salpeter MM, Farquhar MG. High resolution analysis of the secretory pathway in mammotrophs of the rat pituitary. Journal of Cell Biology. 1981;91:240–246. doi: 10.1083/jcb.91.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikdar SK, Zorec R, Mason WT. cAMP directly facilitates Ca-induced exocytosis in bovine lactotrophs. FEBS Letters. 1990;273:150–154. doi: 10.1016/0014-5793(90)81072-v. [DOI] [PubMed] [Google Scholar]
- Südhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Nomizu M, Kurosumi K. Intracellular sites of proteolytic processing of pro-opiomelanocortin in melanotrophs and corticotrophs in rat pituitary. Journal of Histochemistry and Cytochemistry. 1991;39:809–821. doi: 10.1177/39.6.1851777. [DOI] [PubMed] [Google Scholar]
- Thomas P, Surprenant A, Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990;5:723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]
- Tooze SA, Flatmark T, Tooze J, Huttner WB. Characterization of the immature secretory granule, an intermediate in granule biogenesis. Journal of Cell Biology. 1991;115:1491–1503. doi: 10.1083/jcb.115.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta K, Grewe CW, Cote TE, Eskay RL, Kebabian JW. Coordinated action of calcium ion and adenosine 3′,5′-monophosphate upon release of α-melanocyte-stimulating hormone from the intermediate lobe of the rat pituitary gland. Endocrinology. 1982;110:1133–1140. doi: 10.1210/endo-110-4-1133. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Furuki Y, Guild S, Kebabian JW. Adenosine 3′,5′-cyclic monophosphate stimulates secretion of α-melanocyte-stimulating hormone from permeabilized cells of the intermediate lobe of the rat pituitary gland. Biochemical and Biophysical Research Communications. 1987;143:1076–1084. doi: 10.1016/0006-291x(87)90362-7. [DOI] [PubMed] [Google Scholar]
- Zorec R, Henigman F, Mason WT, Kordas M. Electrophysiological study of hormone secretion by single adenohypophyseal cells. Methods in Neurosciences. 1991;4:194–210. [Google Scholar]
- Zupancic G, Kocmur L, Veranic P, Grilc S, Kordas M, Zorec R. The separation of exocytosis from endocytosis in rat melanotroph membrane capacitance records. The Journal of Physiology. 1994;480:539–552. doi: 10.1113/jphysiol.1994.sp020382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupancic G, Zorec R. Cyclic AMP increases the size of unitary exocytotic events in cultured rat melanotrophs. The Journal of Physiology. 1994;475.P:136. P. [Google Scholar]


