Abstract
We investigated La3+ effects on recombinant and native γ-aminobutyric acid A (GABAA) receptors using rapid agonist applications and on inhibitory synaptic currents (IPSCs) in granule and stellate neurons of rat cerebellar slices.
Rapid desensitization of currents elicited by 200 ms pulses of 1 mM GABA to small lifted cells transfected with α1β3γ2 cDNAs was greatly decreased by the coapplication of 100 μm LaCl3.
GABA responses were unaffected when coapplication lasted only 2 ms. In contrast, with LaCl3 pre-perfusion, a significant slowing of deactivation in response to 2 ms applications was observed. LaCl3 pre-perfusion also prolonged the duration of responses to 20 mM taurine.
Outside-out patches excised from cells transfected with α1β3γ2 subunit cDNAs were briefly exposed to a saturating concentration of GABA, eliciting a transient activation of single channel currents with a main conductance of 30 pS. Opening and burst durations increased by pre-equilibration of patches with LaCl3.
LaCl3 depressed the peak amplitude without affecting the slow deactivation and desensitization of GABA responses in cells transfected with α6β3γ2 and α6β3δ cDNAs. No significant difference in La3+ modulation of GABA-gated currents was observed between α1β3γ2 and α1β3δ receptors.
The effects of LaCl3 on deactivation and desensitization of GABA responses observed in nucleated patches excised from rat cerebellar granule and stellate neurons were comparable to those in the cells transfected with α1β3γ2 cDNAs. In addition, La3+ clearly prolonged the spontaneous IPSC time course without changing the amplitude.
Our results indicate that La3+ has a dual action on GABA-gated currents: it decreases desensitization and increases channel opening duration. These actions depend on receptor subunit composition and contribute to the prolongation of IPSCs.
Synaptic transmission mediated by GABAA receptor channels leads to a hyperpolarization of the cell membrane due to the fast activation of postsynaptic Cl− channels by a rapid non-equilibrium exposure of receptors to a high GABA concentration (MacDonald & Olsen, 1994; Mody et al. 1994; Jones & Westbrook, 1996). GABA-mediated inhibitory postsynaptic currents (IPSCs) have been characterized by double exponential decay in several preparations (Maconochie et al. 1994; Puia et al. 1994; Kaneda et al. 1995; Jones & Westbrook, 1995; Tia et al. 1996a; Galarreta & Hestrin, 1997; Mellor & Randall, 1997). This double exponential time course has been demonstrated to be related to the kinetic properties of GABAA receptors which are due to the rapid entry and exit from desensitized states as well as to the presence of distinct receptor isoforms (Gingrich et al. 1995; Jones & Westbrook, 1995, 1996; Tia et al. 1996a). Recently, a relationship between GABAA receptor desensitization, deactivation kinetics and phosphorylation has been demonstrated (Jones & Westbrook, 1997).
Previous work has shown that in the cerebellum GABAA receptor kinetics partially underlie the properties of IPSCs and that these properties change during development (Brickley et al. 1996; Tia et al. 1996a). Molecular cloning studies have demonstrated the existence of a large gene family for GABAA receptors including six α, four β, three γ, and one δ subunit(s) (MacDonald & Olsen, 1994; McKernan & Whiting, 1996). Cerebellar granule neurons express α1, α6, β2/3, γ2 and δ subunits, which has led to the proposal that α1β2/3γ2, α6β2/3γ2, α6β2/3δ and α1α6β2/3γ2 GABAA receptor subtypes are formed in these neurons (McKernan & Whiting, 1996). Additionally it has been reported that α6 as well as δ subunit expression increases with development in vivo and invitro (Laurie et al. 1992; Zheng et al. 1993; Mathews et al. 1994; Zheng et al. 1995). The distinct subunit expression of GABAA receptors in the cerebellum possibly results in the observed heterogeneity of receptor function and pharmacological modulation (Puia et al. 1994; Tia et al. 1996a; Zhu et al. 1996; Mellor & Randall, 1997).
Among the pharmacological tools that have been shown to exert a selective antagonism on GABAA receptor subtypes composed of distinct subunits is La3+ (Im et al. 1992; Saxena & Macdonald, 1994, 1997). La3+ is a trivalent rare-earth metal that has been found to increase GABAA receptor activity and it may result in cytotoxic effects (Ma & Narahashi, 1993a,b; Ma et al. 1994). In addition, it has been shown that La3+ modulation is distinct for recombinant GABAA receptor channels comprising the α6 and/or δ subunits (Saxena & Macdonald, 1994, 1997). All the La3+ studies so far reported were performed with a steady-state exposure of non-saturating GABA concentrations. Furthermore, the modulation of GABAergic synaptic transmission by La3+ has not yet been characterized in detail. Channel kinetic behaviours, such as fast activation, deactivation and desensitization, the transient profile of neurotransmitter concentration at synapses (Jonas & Spruston, 1994; Jones & Westbrook, 1996) and presynaptic effects on the synchrony of synaptic activation (Mody et al. 1994) make it difficult to predict the effect of La3+ on shaping inhibitory synaptic transmission in the cerebellum. The extrapolation of previously described La3+-induced modulation of GABA responses to modulation of synaptic transmission is limited due to the transient profile of GABA concentration at inhibitory synapses.
Therefore, we employed in our study a fast-exchange drug application system to obtain transient jumps to high GABA concentrations in order to mimic the postsynaptic component of synaptic activation (Jonas & Spruston, 1994; Jones & Westbrook, 1996). The La3+-induced modulation of GABA-gated channel fast activation, desensitization and recovery was investigated in recombinant α1β3γ2 GABAA receptors under non-equilibrium recording conditions. Previous studies have described the action of polyvalent cations on single channel currents activated by GABA continuously applied at low concentrations (Ma et al. 1994). We extended these findings to a non-stationary condition with rapid pulses of agonist. Similarly we extended the previously reported investigation of selective La3+ modulation of some recombinant GABAA receptors to an assessment of the effects of La3+ on deactivation, desensitization and recovery from desensitization of these receptors. Finally, we investigated the La3+-induced modulation of deactivation and desensitization of GABA-gated currents in the nucleated patches of granule cells and the La3+-mediated shaping of spontaneous IPSCs recorded in granule and stellate neurons of rat cerebellar slices in order to gain insight into the subunit composition of synaptic GABAA receptors in these neurons.
METHODS
HEK 293 cell line
Human embryonic kidney (HEK) 293 cells (American Type Culture Collection, Rockville, MD, USA; ATCC No. CRL1573) were grown in minimal essential medium (MEM, Gibco), supplemented with 10 % fetal bovine serum, 100 units ml−1 penicillin (Gibco) and 100 units ml−1 streptomycin (Gibco), in a 6 % CO2 incubator. Exponentially growing cells were dispersed with trypsin, seeded at 2 × 105 cells per 35 mm dish in 1.5 ml of culture medium and plated on 12 mm glass coverslips (Fisher Scientific).
cDNA transient transfection
Rat α1, β3, γ2S and δ GABAA receptor subunit cDNAs individually subcloned into the expression vector pCDM8 (Invitrogen, Carlsbad, CA, USA) and the α6 subunit cloned into the pCIS2 expression vector were provided by Dr Peter Seeburg (Centre for Molecular Biology, University of Heidelberg, Germany). HEK 293 cells were transfected using the calcium phosphate precipitation method (Chen & Okayama, 1987) with various combinations of pCDM8α1, pCIS2α6, pCDM8β3, pCDM8γ2S and pCDM8δ. The expression of cDNAs cloned into the pCDM8 and pCIS2 vectors is under the control of the same promoter/enhancer system (cytomegalovirus promoter). The following plasmid combinations were mixed; α1: β3: γ2, α1: β3: δ, α6: β3: γ2, and α6: β3: δ (1 μg each construct) and the coprecipitates were added to culture dishes containing 1.5 ml MEM for 12–16 h at 37°C under 3 % CO2. The media was removed, the cells were rinsed twice with culture media, and finally incubated in the same media for 24 h at 37°C under 6 % CO2. Cotransfection with the plasmid pGreenLantern (Gibco) encoding a fluorescent protein allowed easy recognition of transfected cells expressing this fluorescent marker. More than 90 % of the cells expressing the GreenLantern protein also expressed GABAA receptors.
Electrophysiological studies
Sagittal slices of cerebellum (150-200 μm) were prepared from 7- to 20-day-old Sprague-Dawley rats decapitated whilst under ether anaesthesia, using a protocol approved by the Georgetown University Animal Care and Use Committee. Cerebellar neurons and transfected cells were visualized with an Axioskop FS microscope (Zeiss) equipped with fluorescent and Nomarski optics and an electrically insulated water immersion × 63 objective lens with a long working distance. Stellate neurons were identified as previously reported (Nusser et al. 1997). Nucleated and outside-out patches from granule or transfected cells as well as small lifted HEK cells were voltage clamped at -60 mV. Series resistance was typically less than 15 MΩ, and was checked for constancy throughout the experiment with a 10 mV negative pulse applied every minute. Experiments were performed at room temperature (22-24°C) using an extracellular medium composed of (mM): NaCl, 120; KCl, 3.1; K2HPO4, 1.25; NaHCO3, 26; dextrose, 5.0; MgCl2, 1.0; and CaCl2, 2.0; which contained 2 mM kynurenic acid (Aldrich) to block excitatory amino acid-mediated synaptic transmission. The solution was maintained at pH 7.4 by bubbling with 5 % CO2-95 % O2. Osmolarity was adjusted to 325 mosmol l−1 with sucrose. The recording chamber was continuously perfused at a rate of 5 ml min−1 and completely submerged in a total volume of 500 μl. LaCl3 (Sigma) was dissolved and bath perfused in a Hepes-buffered solution containing (mM) NaCl, 145; KCl, 5; CaCl2, 2; MgCl2, 1; and Hepes, 5; adjusted to pH 7.2 with NaOH to avoid precipitation in carbonate buffer. Typical borosilicate glass pipette resistance was 5–7 MΩ (Wiretrol II, Drummond, Broomall, PA, USA). The internal pipette solution contained (mM): CsCl, 145; EGTA, 5.0; MgATP, 5.0; and Hepes, 10; adjusted to pH 7.2 with CsOH.
Drug application
A piezoelectric translator (P-245.30, Physik Instrumente, Waldbronn, Germany) was used to switch quickly from control Hepes-buffered solution to the same solution with added 1 mM GABA or GABA plus 100 μm LaCl3. After each patch recording, on- and off-rates as well as pulse duration, were measured from the open tip currents generated by the liquid junction potential due to a 50: 1 dilution of the GABA-containing solution. We studied GABA-activated currents elicited by rapid agonist applications with a piezoelectric translator on small (capacitance < 7 pF) lifted HEK 293 cells expressing recombinant GABA receptor heteromers and on nucleated patches excised from granule neurons. Brief pulses (2 ms) of GABA in the extracellular solution elicited currents with relatively fast rise times and exponential decay both in small cells and in patches. A concern of our experimental strategy was that the speed of application in small lifted cells might have been slowed down by the larger cell surface compared with patches. An investigation of the average current produced by 2 ms applications of extracellular solution with 10 mM KCl added while recording from a small lifted cell indicated that these responses were not as fast as those observed in excised patches or nucleated patches but they were sufficiently rapid to produce exchange times of less than 0.5 ms.
Data acquisition and analysis
Currents were filtered at 1–2 kHz with an 8-pole low-pass Bessel filter, digitized using a PC-compatible microcomputer equipped with a Digitata 1200 data acquisition board (Axon Instruments). Off-line data analysis, curve fitting, and figure preparation were performed with Origin (MicroCal Software, Northampton, MA, USA) and pCLAMP 6.03 (Axon Instruments) software. Peak amplitudes were measured at the absolute maximum of the currents, taking into account the noise of the baseline and noise around the peak. Rise times were measured as the time elapsed from 20 to 80 % of the peak amplitude of the response. Curve fitting was performed using simplex algorithm least squares exponential fitting routines with double exponential equations of the form I(t) =If exp(-t/τf) +Is exp(-t/τs), where If and Is are the amplitudes of the fast and slow decay components, and τf and τs are their respective decay time constants. For step applications, a constant term describing the steady state was added. To compare decay time between different experiments we used a weighted mean decay time constant:
Averages of 100 spontaneous IPSCs (sIPSCs) in granule neurons or miniature IPSCs (mIPSCs) in stellate neurons were collected with dedicated software, courtesy of Dr S. Traynelis (Emory University, Atlanta, GA, USA). Single channel current records were low-pass filtered at 10 kHz and stored on magnetic tape by pulse code modulation (sampling frequency, 94 kHz). For analysis, analog signals were sampled at 5 kHz and filtered at 1 kHz (8-pole Bessel filter). Events lists and mean open time estimates were prepared using the pCLAMP 6.03 software suite (Axon Instruments). Subconductance levels were excluded from the dwell time analysis. Optimum burst interval was determined by examining the response of various burst parameters to a series of burst intervals of increasing duration. Unless otherwise indicated, data are expressed as means ±s.e.m.; P values represent the results of independent Student's t tests, with prior analysis of variance, as appropriate.
RESULTS
La3+ inhibits rapid desensitization of GABA-gated currents in α1β3γ2-transfected cells
To determine the effect of La3+ on GABAA receptor desensitization, LaCl3 was added to 1 mM GABA solution to achieve a final concentration of 100 μm. We initially investigated the effects of LaCl3 applied simultaneously with GABA without allowing pre-equilibration. We elicited Cl− currents in HEK 293 cells transfected with GABAA receptor subunit cDNAs (α1β3γ2) by rapid applications of 1 mM GABA in isolated, lifted cells, at a holding potential of -60 mV, using a piezo-driven double-barrelled application pipette. As shown in Fig. 1, a 200 ms application of GABA (1 mM) induced a rapidly developing inward current (20-80 % rise time = 0.25-0.8 ms), followed by a fast desensitization in the continued presence of the agonist. In eighteen cells tested, the average ratio between the amplitude at the end of the 200 ms application and the peak amplitude (S/P) was 41.6 ± 2.7 % and the deactivation time course after removal of GABA was well described by biexponential functions with time constants of 21.7 ± 9.1 ms (τoff,f) and 242 ± 28 ms (τoff,s), respectively (%Fast, relative contribution of the fast desensitization component to peak amplitude, 36.1 ± 2.7 %). La3+ markedly reduced the extent of rapid desensitization of GABAA receptors in these cells, increasing the ratio of S/P to 89.9 ± 2.8 %. The relaxation time constants following the removal of GABA were not significantly altered (τoff,f= 19.8 ± 1.6 ms and τoff,s= 191.3 ± 10.3 ms), but the relative contribution of the fast component was significantly large (%Fast = 65.1 ± 1.9 %). GABA-gated peak current was increased by the presence of La3+ from 358.9 ± 77.0 to 465.6 ± 86.4 pA, although this increase was not statistically significant.
Figure 1. La3+ coapplication inhibits rapid desensitization of GABA-gated currents in α1β3γ2-transfected cells.
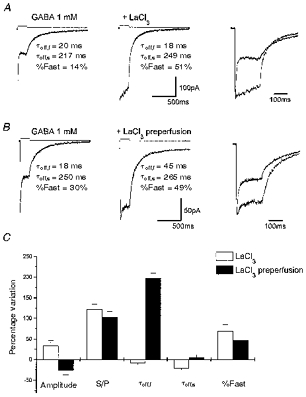
A, averaged response induced by 200 ms application of 1 mM GABA and 1 mM GABA + 100 μm LaCl3 in a lifted small cell transfected with α1β3γ2 cDNAs. Also shown is the biexponential fitting to the offset currents measured at the end of GABA application with an indication of the fitting parameters. The removal of fast desensitization can be better observed in the right panel where the traces are shown superimposed. B, averaged response in another lifted small cell transfected with α1β3γ2 cDNAs induced by 200 ms application of 1 mM GABA and 1 mM GABA + 100 μm LaCl3. Applications of GABA + LaCl3 were preceded by perfusion with LaCl3 (100 μm). Holding potential, -60 mV. In the right panel the traces are shown superimposed. Above each trace are shown the currents generated by the liquid junction potential due to a 50: 1 dilution of the GABA-containing solution measured after ‘blowing out’ the patch, to give an indication of the duration of the pulse application. Calibration bars apply to A and B, but not to the current pulse. C, summary of the kinetic analysis of the LaCl3 effects on responses to 200 ms GABA applications. S/P, ratio of the current at the beginning and at the end of the 200 ms application; τoff,f and τoff,s, deactivation time constants; %Fast, percentage contribution of the fast component of biexponential functions fitted to the offset currents measured at the end of GABA application. Each bar represents the mean ±s.e.m. of 18 patches studied.
We then compared the action of La3+ on lifted cells pre-exposed for at least 1 min to control solution containing 100 μm La3+ before coapplication of GABA and La3+. As shown in Fig. 1B, pre-equilibration of the receptors with La3+ reduced the extent of rapid desensitization. In contrast to the experimental protocol without La3+ pre-perfusion, the deactivation at the end of the GABA step was prolonged. In eighteen cells tested, the average S/P ratio increased from 41.4 ± 3.0 to 80.0 ± 4.6 % and the deactivation time constants after removal of GABA went from 23.8 ± 8.1 ms (τoff,f) and 265 ± 27 ms (τoff,s) to 71.0 ± 18.4 ms (τoff,f) and 277 ± 29 ms (τoff,s), respectively. The increase of fast component was statistically significant (40 ± 3.2 %vs. 62.3 ± 3.3 %, P < 0.05). Average GABA-gated peak current was not significantly decreased by the presence of La3+, from 262 ± 56 to 194 ± 49 pA. In Fig. 1C, we report the average percentage variation of the parameters characterizing responses to 200 ms GABA applications.
La3+ prolongs deactivation of GABA responses in the α1β3γ2-transfected cells
To investigate La3+ shaping of deactivation following brief application of agonist, 2 ms pulses of saturating GABA concentrations were applied to the HEK 293 cells transfected with α1β3γ2 subunits by a piezo-driven double-barrelled application pipette. The currents elicited by 2 ms applications of GABA showed a rapid activation in the range of that observed with 200 ms applications, and the time course of deactivation could be fitted with biexponentional functions with time constants of 11.8 ± 0.9 ms (τf) and 235.0 ± 19.7 ms (τs), respectively (%Fast = 68.8 ± 2.1 %). As shown in Fig. 2, compared with control, the coapplication of La3+ (100 μm) with GABA in eighteen cells, did not alter the decay time course of the GABA response (τf= 12.8 ± 0.6 ms, τs= 212.5 ± 15.6 ms, %Fast = 69.5 ± 1.9 %) and we observed a non-significant increase of peak currents (from 301.8 ± 54.2 to 328.3 ± 77.0 pA). Similarly, the peak currents recorded from eighteen cells with La3+ pre-equilibration were not significantly different (229.4 ± 39.7 pA vs. 193.8 ± 48.7 pA). However, La3+ pre-equilibration led to a significant prolongation of the current decay (τf= 26.9 ± 2.5 ms, τs= 352.8 ± 46.6 ms, %Fast = 54.7 ± 5 %). As illustrated in Fig. 2C, compared with control, the fast and slow components of current deactivation were increased by 147.1 ± 25.4 % and 45.9 ± 24.1 %, respectively, and the relative contribution of the fast component to peak amplitude was reduced by 19.8 ± 8.8 %. Since brief GABA pulses can cause substantial desensitization (Jones & Westbrook, 1995), we investigated the actions of La3+ on the speed of recovery from desensitization. As demonstrated by paired-pulse applications of agonist, the recovery of channels from desensitization was gradually increased with the increase of the application interval and the recovery time course of desensitization extended over several hundred milliseconds. Coapplication of La3+ (100 μm) with GABA (1 mM) failed to alter the recovery from desensitization (Fig. 3A). However, recovery of GABA-gated channels from desensitization was clearly increased when La3+ was pre-perfused (Fig. 3B). It should be noted (Fig. 3B) that when La3+ was pre-perfused, the responses to both applications in paired pulses were greatly prolonged. In Fig. 3C and D are summarized the results obtained comparing recovery from desensitization between coapplication (Fig. 3C) and pre-perfusion (Fig. 3D). These data further suggest that the desensitization of GABAA receptor channels is significantly removed by La3+ but this effect requires pre-equilibration.
Figure 2. La3+ pre-perfusion prolongs GABA current deactivation in α1β3γ2-transfected cells.
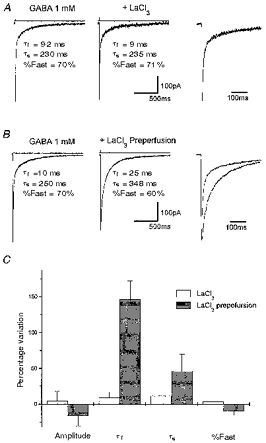
A, averaged response induced by 2 ms applications of 1 mM GABA and 1 mM GABA + 100 μm LaCl3 in a lifted small cell transfected with α1β3γ2 cDNAs. Also shown is the biexponential fitting to the current decay with an indication of the fitting parameters. The traces are shown superimposed in the right panel. B, averaged response in another lifted small cell transfected with α1β3γ2 cDNAs induced by 2 ms application of 1 mM GABA and 1 mM GABA + 100 μm LaCl3. Applications of GABA + LaCl3 were preceded by perfusion with LaCl3 (100 μm). Holding potential, -60 mV. Prolongation of the current decay can be better observed in the right panel where the traces are shown superimposed. Above each trace are shown the currents generated by the liquid junction potential due to a 50:1 dilution of the GABA-containing solution measured after ‘blowing out’ the patch, to give an indication of the duration of the pulse application. Calibration bars apply to A and B, but not to the current pulse. C, summary of the kinetic analysis of the LaCl3 effects on responses to 2 ms GABA applications. τf and τs, deactivation time constants; %Fast, percentage contribution of the fast component of biexponential functions fitted to the current decay. Each bar represents the mean ±s.e.m. of 18 patches studied.
Figure 3. La3+ speed-up recovery from desensitization in α1β3γ2-transfected cells.
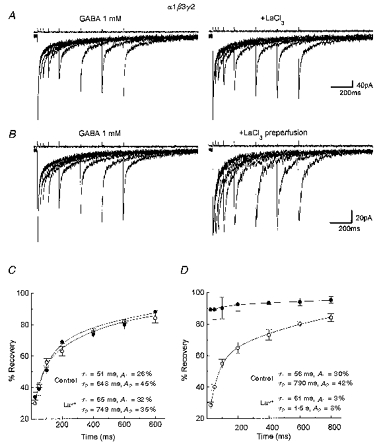
Left panel in A, superimposed traces evoked by two successive applications in lifted small cells transfected with α1β3γ2 cDNAs of 2 ms GABA pulses separated by 25, 50, 100, 200, 400, 600 and 800 ms intervals. Right panel in A, superimposed traces evoked by two successive applications of 2 ms GABA + LaCl3 (100 μm) pulses separated by increasing intervals. Holding potential, -60 mV. B, same as in A except that LaCl3 (100 μm) perfusion preceded the combined GABA + LaCl3 applications. C and D, comparison of the recovery time course of the second response from the desensitization in the various experimental conditions in a lifted small cell transfected with α1β3γ2 cDNAs. ○, control; •, + La3+. In C, La3+ was coapplied with GABA, while in D it was also pre-perfused. The percentage recovery from desensitization at each interval between two brief GABA pulses is calculated according to the formula ([Peak2 - onset]/[peak1 - onset]× 100), and is plotted against the interpulse interval. Note the apparent prevention of desensitization with LaCl3 pre-perfusion. Each data point represents the mean ±s.e.m. of 16 patches studied. The continuous lines are fitted to the double exponential equation: %recovery = 100 - A1exp(t/τ1) - A2exp(t/τ2) where τ1, τ2, A1 and A2 are the time constant and the amplitude of the two components. The τ1, τ2, A1 and A2 values in the respective experimental conditions are indicated. Above each trace are shown currents to give an indication of the duration of the pulse applications. Vertical calibration does not apply to these traces.
Channel kinetics in response to La3+ by a brief, non-equilibrium exposure to a high concentration of GABA
To examine the action of La3+ on non-steady-state properties of GABAA receptor channels, outside-out patches were isolated from the HEK 293 cells transfected with α1β3γ2 subunit cDNAs. Figure 4A illustrates the kinetics of individual GABAA channels elicited by fast jumps to 1 mM GABA lasting 2 ms at a holding potential of -70 mV. The rapid application of GABA led to a series of brief openings, followed by long closures and some reopenings before the relaxation of agonist from the binding site. As shown in Fig. 4 and summarized in Table 1, the channel current amplitude in fourteen patches was unchanged by the pre-application of La3+ (100 μm) (conductance: control, 29.2 ± 3.2 pS; La3+, 29.7 ± 2.4 pS). In contrast, La3+ significantly increased the measured open time, accompanied by a non-significant increase in the number of channel openings measured in each sweep. An example of the distribution of the open times is shown in Fig. 4 with a superimposed double exponential fitting. In Table 1 we report the weighted time constants of similar distributions in all patches studied in control conditions and in the presence of La3+. As demonstrated in Fig. 4A and B, the longer openings occurred rarely in control conditions whereas they frequently appeared by the addition of La3+. The individual time constants describing the open time distribution were changed by the presence of La3+ (τ1, 1.2 ± 0.4 ms vs. 1.8 ± 0.5 ms; τ2, 2.6 ± 0.3 ms vs. 9.9 ± 0.9 ms, respectively). The burst duration as well as the number of closings per burst increased while the number of bursts per sweep decreased (Table 1) as previously reported for the effects of lanthanides on native GABAA receptor (Ma et al. 1994).
Figure 4. La3+ prolongs channel opening durations in α1β3γ2-transfected cells.
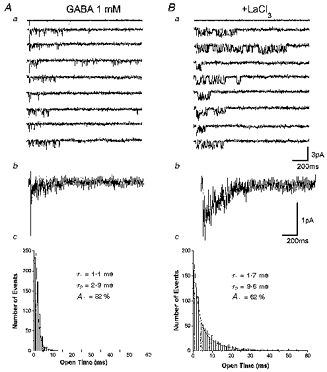
Aa, channel activity evoked by 2 ms pulses of 1 mM GABA as indicated in the current above the traces in an outside-out patch excised from a cell transfected with α1β3γ2 cDNAs. Holding potential, -70 mV. Ab, ensemble averages derived from thirty applications as those shown above. Ac, open time distribution of GABA-activated channel currents for the patch shown above with superimposed double exponential fitting and an indication of the fitting parameters derived. Because of the high level of channel activity as a consequence of the GABA applications we analysed channel currents selecting sweeps with limited channel activity. τ1 and τ2 are the decay time constants and A1 is the percentage contribution of the fast component of biexponential functions fitted to the open time distribution. B, same as A except that GABA was coapplied with LaCl3 (100 μm) after LaCl3 pre-perfusion. Summary of the data obtained in 14 patches is given in Table 1.
Table 1.
La3+ alters kinetics of channel currents in α1β3γ2-transfected cells
| Parameter | Control | La3+ (100 μm) |
|---|---|---|
| Channel current (pA) | 2.0 ± 0.7 | 2.0 ± 0.5 |
| Openings sweep−1 | 85 ± 21 | 134 ± 15 |
| Weighted open time (ms) | 1.3 ± 0.2 | 5.6 ± 0.4* |
| Average open probability sweep−1 | 0.011 ± 0.02 | 0.044 ± 0.063 |
| Bursts sweep−1 | 14.1 ± 2.2 | 9.8 ± 1.1 |
| Closings burst−1 | 2.6 ± 0.6 | 7.1 ± 1.4* |
| Burst duration (ms) | 8.8 ± 1.5 | 31.4 ± 2.1* |
Channel activity was evoked by 2 ms pulses of 1 mM GABA in outside-out patches excised from cells transfected with α1β3γ2 cDNAs. Outside-out membrane patches were voltage clamped at a holding potential of −70 mV. Because of the high level of channel activity as a consequence of the GABA applications we selected sweeps with limited channel activity for the analysis. At least 30 sweeps per patch were considered. LaCl3 (100 μm) was pre-perfused and applied together with GABA. Data derived from 14 patches studied are expressed as means ± s.e.m.
P < 0.01, Student's t test. Weighted open time derives from the weighted decay time constants of biexponential functions fitted to the open time as illustrated in Fig. 4.
La3+-induced modulation of deactivation with taurine as an agonist of GABAA receptors
Taurine, a low affinity GABAA receptor agonist, at high concentration has been found to open Cl− channels, with little desensitization (Zhu & Vicini, 1997). To further examine the role of desensitization in La3+-induced modification of GABAA receptor kinetics, taurine was applied with brief pulses to HEK 293 cells transfected with α1β3γ2 subunit cDNAs. As shown in the example in Fig. 5, applications (2 ms) of taurine at a 20 mM concentration elicited Cl− currents with a fast rise time (less than 0.5 ms), followed by a rapid single exponential decay (τ= 4.2 ± 0.5 ms, n = 12). Application with 100 μm La3+ by pre-perfusions significantly slowed down (τ= 9.8 ± 1.5 ms, P < 0.05, n = 12) the decay time course of currents induced by taurine; however, the peak current was not changed significantly (243.4 ± 40 vs. 189 ± 28 pA, P > 0.05, n = 12). In Fig. 5B, we report a summary of percentage changes of time constant and peak amplitude of taurine currents by the presence of La3+.
Figure 5. La3+ pre-perfusion prolongs deactivation of taurine-activated currents in α1β3γ2-transfected cells.
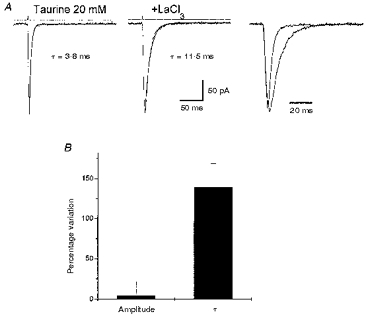
A, averaged response induced by 2 ms applications of 20 mM taurine and 20 mM taurine + 100 μm LaCl3 in a lifted small cell transfected with α1β3γ2 cDNAs. Application of GABA + LaCl3 was preceded by perfusion with LaCl3 (100 μm). Holding potential, -60 mV. Prolongation of the current decay can be better observed in the right panel where the traces are shown superimposed. B, summary of the LaCl3 effects on responses to 2 ms taurine applications. τ, deactivation time constants of the monoexponential functions fitted to the current decay. Each bar represents the mean ±s.e.m. of 12 cells studied.
La3+-induced effects in α1β3δ-, α6β3γ2-, and α6β3δ-transfected cells
We wished to verify further the subunit dependence of La3+-induced modification of GABAA receptors, particularly with regard to the reported alteration of the La3+ effects caused by the expression of the δ and α6 subunits (Saxena & Macdonald, 1994, 1997) and to investigate the effect of La3+ on deactivation and desensitization observed in GABAA receptors containing these subunits. We therefore studied the kinetics of GABA-gated currents of cells transfected with α1β3δ, α6β3γ2 and α6β3δ subunit cDNAs. As shown in Fig. 6A and B, currents elicited by 200 ms GABA applications in cells expressing α6β3δ subunits show a characteristic response with rapid activation, slow deactivation and little desensitization. Similar results were observed for α6β3γ2 receptors as previously reported (Tia et al. 1996b). Figure 6C compares the amplitude of responses of α1β3γ2, α1β3δ, α6β3γ2 and α6β3δ GABAA receptors to rapidly applied GABA with and without La3+ pre-perfusion. In Fig. 6D and E are reported, respectively, the weighted time constant τw deriving from the double exponential fitting of deactivation and the S/P ratio for different subunit combinations. These results indicate that, as with α1β3γ2 receptors, La3+ significantly slowed deactivation in cells transfected with α1β3δ subunit cDNAs but not in cells transfected with α6β3γ2 and α6β3δ cDNAs. Furthermore, La3+ significantly reduced the peak currents in α6β3γ2 and α6β3δ receptors (Fig. 6C), which were much smaller than those in α1β3γ2 or α1β3δ receptors. Lastly, La3+ increased the S/P ratio only with those subunit combinations where significant fast desensitization was observed.
Figure 6. La3+ inhibits GABA-gated currents in α6β3δ- and α6β3γ2-transfected cells.
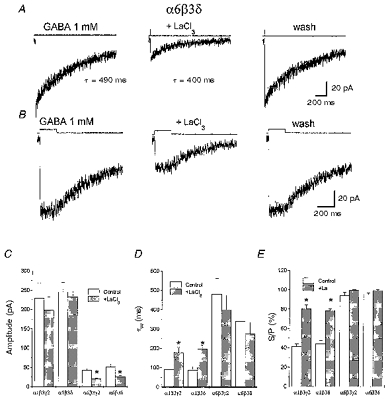
A, averaged responses induced in a lifted small cell transfected with α6β3δ cDNAs by 2 ms applications of 1 mM GABA together with the exponential fitting to the current decay and the decay time constant. Fitting was extended to baseline. LaCl3 (100 μm) coapplied with GABA and pre-perfused, reversibly reduced the peak current. B, averaged responses induced in a lifted small cell transfected with α6β3δ cDNAs by 200 ms applications of 1 mM GABA together with the exponential fitting to the offset currents measured at the end of GABA application and the respective time constant. Fitting was extended to baseline. LaCl3 (100 μm), coapplied with GABA and pre-perfused, reversibly reduced the peak current. Holding potential, -60 mV. C-E, summary of the LaCl3 effects on amplitude and kinetics of responses to 2 ms and 200 ms GABA applications in cells transfected with distinct GABA receptor subunit combinations. C, amplitude; D, weighted deactivation time constants (τw); E, ratio of the current at the beginning and at the end of a 200 ms application (S/P). Each bar represents the mean ±s.e.m. of 18 patches studied. * Statistical significance with respect to control (P < 0.05 ANOVA followed by t test).
La3+-induced effects on GABAA receptor of nucleated patches from cerebellar granule cells
Since native GABAA receptors of cerebellar granule cells express the combinations of α1, α6, β2/3, γ2 and δ subunits (McKernan & Whiting, 1996), we studied La3+-induced modifications of GABA-gated currents in nucleated patches from granule neurons in postnatal days 7–8 (P7-P8) rat cerebellar slices visually identified by their location and morphological characteristics. Exposure of nucleated patches (200 ms) to 1 mM GABA using a piezo-driven double-barrelled application pipette at a holding potential of -60 mV evoked fast gated, rapidly desensitizing inward currents as previously reported (Tia et al. 1996a; Zhu & Vicini, 1997). Pre-perfusion of La3+ increased the S/P ratio from 30.8 ± 4.7 to 71.0 ± 4.5 (P < 0.01, n = 18) and prolonged the weighted time constant of the offset deactivation due to the fast removal of agonist as observed in the recombinant α1β3γ2 receptors (not shown). La3+, however, did not significantly change the peak amplitude. In Fig. 7A is illustrated the current produced in a nucleated patch by 2 ms GABA pulses and the effect of La3+. As illustrated in Fig. 7C, the peak GABA current recorded in eighteen patches was not affected by La3+ pre-perfusion and the current decay was markedly prolonged. Compared with control, the fast component of current deactivation increased by 420 ± 160 % and the slow component by 38 ± 30 %. Furthermore, as demonstrated by paired-pulse applications of GABA (Fig. 7B), the recovery of channels from desensitization was increased by La3+ (Fig. 7D).
Figure 7. Effects of La3+ on GABA responses in nucleated patches from cerebellar granule cells.
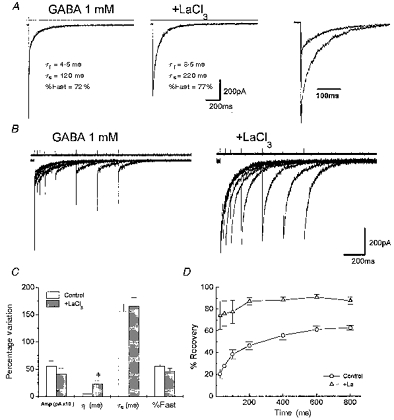
A, averaged response in a nucleated outside-out patch isolated from a cerebellar granule neuron of a rat at the age of P7, induced by 2 ms applications of 1 mM GABA together with the exponential fitting to the current decay and the weighted time constant. LaCl3 (100 μm) coapplied with GABA and pre-perfused (middle), produced prolongation of the current decay as it can be better observed in the right panel where the traces are shown superimposed. Left panel in B, superimposed traces evoked by two successive applications of 2 ms GABA pulses separated by 25, 50, 100, 200, 400, 600 and 800 ms intervals in a nucleated patch isolated from a P7 rat cerebellar granule neuron. Right panel in B, superimposed traces evoked in the same patch by two successive applications of 2 ms GABA (1 mM) + LaCl3 (100 μm) pulses separated by increasing intervals. LaCl3 was also pre-perfused. C, summary of the effects of LaCl3 on GABA responses in nucleated patches excised from cerebellar granule cells in rats at P7-P8. Amp, amplitude; τf and τs, deactivation time constants; %Fast, percentage contribution of the fast component of biexponential functions fitted to the current decay. Each bar represents the mean ±s.e.m. of 18 patches studied. * Statistical significance with respect to control (P < 0.05 ANOVA followed by t test). D, comparison of the recovery time course of the second response from the desensitization in the two experimental conditions in nucleated patches excised from cerebellar granule cells in rats at P7-P8. The percentage recovery from desensitization at each interval between two brief GABA pulses is calculated and plotted as in Fig. 3. The τ1, τ2, A1 and A2 values in the respective experimental conditions are indicated. Note the apparent prevention of desensitization with LaCl3 pre-perfusion. Each data point represents the mean ±s.e.m. of 11 patches studied.
La3+ prolongs decay of sIPSCs in granule and stellate neurons
To study the modulation of inhibitory synaptic strength by La3+, GABA-gated sIPSCs were recorded from granule neurons in cerebellar slices from rats at different developmental ages at the holding potential of -60 mV. Figure 8A illustrates sIPSCs from a P18 rat cerebellar granule cell recorded in the presence of kynurenic acid (2 mM), which were completely abolished by 5 μm bicuculline (not shown, Puia et al. 1994). We analysed the inhibitory synaptic activities recorded in the absence and presence of 100 μm La3+. La3+ failed to alter sIPSC amplitude as illustrated by the cumulative amplitude distributions reported in Fig. 8B. The major effect of La3+, as shown in Fig. 8C, was the rightward shift of the cumulative distribution of the weighted time constants deriving from the double exponential fitting of sIPSC decay. In Fig. 8D, we report the summary of the average values of parameters characterizing sIPSCs in ten cells at P7-P8 and ten cells at P18-P20. As we previously reported (Tia et al. 1996a), a developmental decrease of the amplitude and time course was observed. However, at both ages La3+ (100 μm) produced a marked increase in both fast and slow decay time constants without significant change in amplitude of spontaneously occurring IPSCs (Fig. 8D). Compared with controls in rats at P7-P8, the fast component of current deactivation was increased by 192 ± 45 % and the slow component of deactivation was increased by 124 ± 19 %. In neurons in the P18-P20 group, the percentage increases for the fast and slow deactivation components were 283 ± 54 % and 104 ± 20 %, respectively.
Figure 8. La3+ prolongs sIPSCs from cerebellar granule cells.
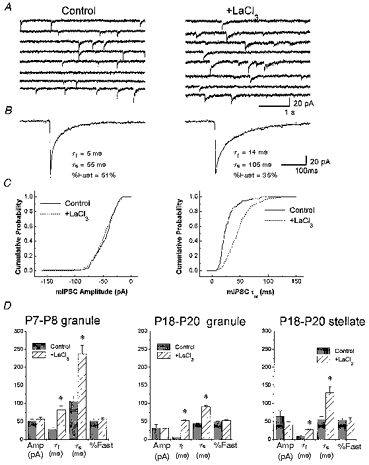
A, representative sIPSCs recorded from cerebellar granule neurons at P18. The traces in the left panel are segments of recordings of sIPSCs after 3 min bath perfusion with LaCl3 (100 μm). B, averages of 100 sIPSCs from the cerebellar granule neurons in A with the biexponential fitting to the current decay and the fitting parameters. C, the cumulative relative frequency of the amplitude and weighted time constants of the sIPSCs shown in panel A is illustrated for control, and LaCl3 treatment. D, summary of the effects of LaCl3 on sIPSCs recorded from cerebellar granule neurons at P7-P8 (left) and P18-P20 (middle) and P18-P20 stellate neurons (right). The y-axis labels of panel D are indicated below the x-axis under each pair of histogram columns. Amp, amplitude; τf and τs, deactivation time constants and percentage contribution of the fast component (%Fast) of biexponential functions fitted to the current decay. Each bar represents the mean ±s.e.m. of 10 cells studied. At least 100 sIPSCs were measured before and during LaCl3 bath perfusion. * Statistical significance with respect to control (P < 0.05 ANOVA followed by t test).
Presynaptic effects of La3+ have been well established (reviewed in Van der Loot & Molgó 1994). Indeed in some granule cells La3+ increased the frequency of occurrence of sIPSCs although this effect was not always observed and it was not quantified. It is possible therefore that the prolongation of sIPSC decay in cerebellar granule neurons could be due to a presynaptic effect of the cation. One way to reduce the extent of presynaptic effects is to study sIPSCs in the presence of TTX (mIPSCs). Unfortunately in cerebellar granule neurons in slices, TTX completely abolishes the occurrence of sIPSCs (Puia et al. 1994; Brickley et al. 1996). Therefore we performed additional experiments on the action of La3+ on mIPSCs in cerebellar stellate neurons. In these neurons, TTX-insensitive mIPSCs can be easily recorded and the composition of postsynaptic GABAA receptor is prevalently α1β2/3γ2 (Nusser et al. 1997; Auger & Marty, 1997). In nine stellate neurons in rats at P18-P20 La3+ (100 μm) produced a marked increase in both fast and slow decay time constants without significant change in amplitude of mIPSCs recorded in the presence of TTX (1 μm) and kynurenic acid (2 mM) (data summarized in Fig. 8D). Compared with control, the fast component of current deactivation was increased by 170 ± 35 % and the slow component of deactivation was increased by 133 ± 23 %. These changes were not significantly different from those observed in granule neurons in both age groups, indicating that the La3+ action is not due to alterations of presynaptic Ca2+ entry.
DISCUSSION
With fast agonist concentration jumps, we demonstrated that La3+ decreases desensitization and slows deactivation by increasing opening durations of GABA-gated channels and these effects are concomitant with the prolongation of the decay time course of GABAergic synaptic currents in cerebellar granule neurons. In addition, we confirm previous findings that La3+ modulation is dependent on GABAA receptor subunit composition, leading to the possible use of this polyvalent cation to distinguish between subtypes of native GABAA receptors.
La3+ removes fast GABAA receptor desensitization
GABAA receptors composed of the α1β3γ2 subunits are the most common receptor throughout the brain (Fritschy et al. 1992; McKernan & Whiting, 1996). The time course of responses with this subunit combination, as measured from current decay during 200 ms applications of saturating GABA concentration, reflects the rate of entry into a fast desensitized state. Both coapplication and pre-perfusion of La3+ resulted in a predominant decrease of this entry rate.
Previous reports of La3+ potentiation of currents produced by submaximal GABA concentrations with native and recombinant receptors demonstrated a decreased EC50 of GABA dose responses (Ma & Narahashi, 1993a,b; Ma et al. 1994; Saxena & Macdonald, 1997). This has been proposed to be due to an increased affinity of the GABAA receptor (Ma & Narahashi, 1993a,b; Ma et al. 1994; Saxena & Macdonald, 1997). An alternative interpretation of the decreased EC50 for GABA by La3+ is related to the inhibition of fast desensitization rather than increased receptor affinity. In fact, as discussed in Jones & Westbrook (1996), with application techniques of slow rising agonist concentration, the peak response results from the equilibrium between open and desensitized states. As shown from our study of recovery from desensitization measured by paired GABA pulses, La3+ dramatically reduced the accumulation of desensitized GABAA receptors. Since accumulation of desensitized receptors results in longer deactivation (Jones & Westbrook, 1995, 1996) we were expecting faster deactivation, with a greater proportion of fast components in the presence of La3+. Indeed, we observed faster deactivation at the end of 200 ms pulses when La3+ was coapplied with GABA. However, GABA responses elicited by brief applications characterized by fast and slow decay components were unaltered by La3+ coapplication, probably because during the brief time of coapplication there was not sufficient time for the trivalent cation to act.
La3+ slows deactivation of GABAA channels
A La3+ binding site different from GABA has been proposed (Ma & Narahashi, 1993a,b; Ma et al. 1994; Saxena & Macdonald, 1994, 1997). The binding of La3+ may lead to conformational changes that destabilize the desensitized state but it may also produce additional actions on GABAA receptors. Previous investigation on the action of La3+ on native GABA channel open time distribution demonstrated an increased proportion of openings at longer open states and increased burst durations supporting the proposal that La3+ produces an alteration of binding/unbinding rates. (Ma & Narahashi, 1993a,b; Ma et al. 1994). Indeed, La3+ pre-perfusion prolonged deactivation at the end of a 200 ms GABA application and deactivation of responses elicited by brief GABA applications. The requirement for pre-perfusion to observe prolongation of deactivation suggests that the polyvalent cation takes a longer time to produce this effect than to remove fast desensitization, indicating distinct mechanisms underlying these effects. To further investigate La3+ prolongation of deactivation, we performed analysis of single channel currents activated by brief GABA application in the presence and the absence of the polyvalent cation. La3+ increased the channel opening durations by increasing the mean dwell time of each open state, suggesting a decrease in the channel closing rate. An alternative explanation for longer open durations is that removal of desensitization could produce longer openings if one postulates the possibility of transitions from the open to the desensitized state. However, our data show that La3+ also significantly slows taurine-induced currents. Since taurine shows little accumulation of desensitized receptors at saturating concentrations (Zhu & Vicini, 1997), desensitization is not involved in the open time prolongation by La3+ observed with both taurine and GABA.
In addition to the observation of longer open durations, we confirmed previous results obtained with native channel currents (Ma et al. 1994) which showed increased burst duration and increased number of openings per burst, indicating that polyvalent cations can also affect unbinding. These data, taken together, lead us to propose that La3+ has a dual mode of action on GABAA receptors: it decreases channel closing/unbinding rates and destabilizes the fast desensitized state. This hypothesis would be consistent with the greater effect of the polyvalent cation on the fast component rather than on the slow component of deactivation. In fact, since the slow component reflects the reopening after desensitization (Jones & Westbrook, 1996) and our data suggest that La3+ decreases desensitization as well as channel closing, these two effects acting in opposite directions will produce less variation of the slow component of deactivation.
GABAA receptor heterogeneity and La3+ modulation
The potentiation of the amplitude of GABA responses measured with applications of submaximal GABA doses in recombinant receptors has been demonstrated to be subunit-dependent (Im et al. 1992, Saxena & Macdonald, 1994, 1997). In these studies La3+ potentiated the GABA-gated currents to a greater extent with α1β2γ2 subunits than with α1β2-containing GABAA receptors (Im et al. 1992; Saxena & Macdonald, 1994). Also, with α1β2δ receptors, the polyvalent cation failed to potentiate GABA responses (Saxena & Macdonald, 1994) and GABA-gated currents were inhibited more strongly with α6β2δ than α6β2γ2 receptors (Saxena & Macdonald, 1997). Our results confirm that La3+ inhibited GABA-gated currents from receptors containing α6. However, we did not observe significantly different effects between α1β3γ2 and α1β3δ receptors nor between α6β3γ2 and α6β3δ receptors. We do not know the reasons for these discrepancies, but they may relate to differences concerning the presence of the β3 subunit and/or the use of rapid application of saturating GABA concentrations. As previously reported for GABAA receptors comprising α6β2γ2 subunits (Tia et al. 1996b), we observed a slow deactivation time course and lack of desensitization for GABA responses from α6β3γ2 and α6β3δ receptors. Furthermore, deactivation and desensitization with these receptors were largely unaltered by La3+, while the peak amplitude of GABA responses was inhibited. This suggests a link between La3+ inhibition, slow deactivation and lack of desensitization related to subunit composition.
Our result implies that knowledge of deactivation and desensitization in combination with the effects of La3+ may allow one to predict the subunit composition of native GABAA receptors. Cerebellar granule neurons show functional responses with distinct physiological and pharmacological characteristics and at the same time the expression of α1, α6, β2/3, γ2/γ3, and δ subunits has been well confirmed in these neurons (Fritschy et al. 1992; Laurie et al. 1992; McKernan & Whiting, 1996). In particular, a significant developmental change in the decay time course of sIPSCs has previously been reported in granule cells (Brickley et al. 1996; Tia et al. 1996a). With ageing, the kinetics of these currents became faster and at the same time the inhibition by furosemide, an α6 subunit selective antagonist, was increased (Korpi et al. 1995). These changes are probably related to the increased α6 subunit contribution to postsynaptic receptors in cerebellar glomeruli. The present study, which compares sIPSCs in granule neurons at two age groups (P7-P8 and P18-P20), confirmed these results and showed that the effect of La3+ on sIPSCs was to significantly slow down the time course without altering the amplitude of these currents in both age groups. Similar modulation by La3+ was also observed in nucleated patches excised from granule neurons at P7-P8. Therefore, although as discussed below presynaptic action of La3+ on sIPSCs should also be considered, the native GABAA receptor in these neurons showed a similar modulation by La3+ to that observed with recombinant α1β3γ2 or α1β3δ receptors. These results, together with the characteristic slow deactivation kinetics and La3+ inhibition of α6β3δ and α6β3γ2 receptors, indicate that the contribution of these subtypes to inhibitory synaptic transmission of cerebellar granule neurons is negligible. This result is in apparent contrast with our previous data showing a progressive increase in the furosemide antagonism of synaptic GABAA receptors. We believe that a possible explanation for these findings is that α1α6β3γ2 subtypes are formed at cerebellar glomeruli with development. This is further supported by our previous finding of faster deactivation kinetics in α1α6β3γ2 than α6β3γ2 receptors (Tia et al. 1996a,b) and by the anatomical findings that the α6 and α1 subunits were colocalized in many GABAergic Golgi synapses in granule neurons, demonstrating that the two subunits are involved in synaptic transmission in the same synapse (Nusser et al. 1995, 1996). In addition, anatomical evidence has been recently presented that receptors comprising the δ subunit are exclusively extrasynaptic in cerebellar granule neurons (Nusser et al. 1998). Removal of fast desensitization can efficiently potentiate steady-state extrasynaptic receptor activation by submaximal GABA ambient concentrations (Brickley et al. 1996). On the other hand, if extrasynaptic GABAA receptors comprise α6β2δ subunits (Nusser et al. 1998) the polyvalent cation should rather inhibit ambient GABA-evoked noise.
Modulation of inhibitory neurotransmission by La3+ and functional implications
Our results have shown that La3+ prolongs IPSCs. The duration of the postsynaptic currents depends critically on both presynaptic and postsynaptic factors. Lanthanides have a demonstrated presynaptic action that increases neurotransmitter release at the neuromuscular junction (reviewed in Van der Loot & Molgó, 1994). La3+ can also evoke excitatory amino acid release (Copenhagen & Jahr, 1989), block voltage-gated calcium channels and potentiate excitatory amino acid-mediated responses (Reichling & MacDermott, 1991). The evidence that La3+ increased IPSC duration in stellate cells even in the presence of TTX rules out an action potential-dependent presynaptic effect. In addition, our experiments demonstrated that La3+ slowed down the deactivation kinetics, reduced the desensitization and increased the duration of GABA-gated channel currents in excised patches. These results, taken together, would suggest that the effects of La3+ on IPSC decay time result from a postsynaptic action. However, lanthanides enter presynaptic terminals and act directly to promote action potential-independent spontaneous neurotransmitter release (Van der Loot & Molgó, 1994), and therefore the interpretation of our evidence cannot be conclusive and the use of lanthanum as a neurobiological tool to modify GABA-mediated inhibitory synaptic transmission should be carefully considered.
Acknowledgments
We are grateful to Dr Karl E. Krueger for critical reading of the manuscript. This work was supported by NINDS grants R01 NS32759 and K04 NS01680.
References
- Auger C, Marty A. Heterogeneity of functional synaptic parameters among single release sites. Neuron. 1997;19:139–150. doi: 10.1016/s0896-6273(00)80354-2. 10.1016/S0896-6273(00)80354-2. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. The Journal of Physiology. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Molecular Cell Biology. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhagen DR, Jahr CE. Release of endogenous excitatory amino acids from turtle photoreceptors. Nature. 1989;341:536–539. doi: 10.1038/341536a0. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Benke D, Mertens S, Oertel WH, Bachi T, Möhler H. Five subtypes of type A γ-aminobutyric acid receptors identified in neurons by double and triple immunofluorescence staining with subunit specific antibodies. Proceedings of the National Academy of Sciences of the USA. 1992;89:6726–6730. doi: 10.1073/pnas.89.15.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Properties of GABAA receptors underlying inhibitory synaptic currents in neocortical pyramidal neurons. Journal of Neuroscience. 1997;17:7220–7227. doi: 10.1523/JNEUROSCI.17-19-07220.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the α-subunit isoform: implications for structure-function relations and synaptic transmission. The Journal of Physiology. 1995;489:529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im MS, Hamilton BJ, Carter DB, Im WB. Selective potentiation of GABA-mediated Cl− current by La3+ ion in subtypes of cloned GABAA receptors. Neuroscience Letters. 1992;144:165–168. doi: 10.1016/0304-3940(92)90741-o. 10.1016/0304-3940(92)90741-O. [DOI] [PubMed] [Google Scholar]
- Jonas P, Spruston N. Mechanisms shaping glutamate-mediated excitatory postsynaptic currents in the CNS. Current Opinion in Neurobiology. 1994;4:366–372. doi: 10.1016/0959-4388(94)90098-1. 10.1016/0959-4388(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. Desensitization states prolong GABAA channel response to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. The impact of receptor desensitization on fast synaptic transmission. Trends in Neurosciences. 1996;19:96–101. doi: 10.1016/s0166-2236(96)80037-3. 10.1016/S0166-2236(96)80037-3. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. Shaping of inhibitory postsynaptic currents by endogenous calcineurin activity. Journal of Neuroscience. 1997;17:7626–7633. doi: 10.1523/JNEUROSCI.17-20-07626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M, Farrant M, Cull-Candy SG. Whole-cell and single-channel currents activated by GABA and glycine in granule cells of the rat cerebellum. The Journal of Physiology. 1995;485:419–435. doi: 10.1113/jphysiol.1995.sp020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Kuner T, Seeburg PH, Lüddens H. selective antagonist for the cerebellar granule cell-specific γ-aminobutyric acid type A receptor. Molecular Pharmacology. 1995;47:283–289. [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. Journal of Neuroscience. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JY, Narahashi T. Differential modulation of GABAA receptor-channel complex by polyvalent cations in rat dorsal root ganglion neurons. Brain Research. 1993a;607:222–232. doi: 10.1016/0006-8993(93)91510-y. 10.1016/0006-8993(93)91510-Y. [DOI] [PubMed] [Google Scholar]
- Ma JY, Narahashi T. Enhancement of γ-aminobutyric acid activated chloride channel currents by La3+ in rat dorsal root ganglion neurons. Journal of Neuroscience. 1993b;13:4872–4879. doi: 10.1523/JNEUROSCI.13-11-04872.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JY, Reuveny E, Narahashi T. Terbium modulation of single γ-aminobutyric acid-activated chloride channels in rat dorsal root ganglion neurons. Journal of Neuroscience. 1994;14:3835–3841. doi: 10.1523/JNEUROSCI.14-06-03835.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald RL, Olsen RW. GABAA receptor channels. Annual Review of Neuroscience. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Maconochie DJ, Zempel JM, Steinbach JH. How quickly can GABAA receptors open. Neuron. 1994;12:61–71. doi: 10.1016/0896-6273(94)90152-x. 10.1016/0896-6273(94)90152-X. [DOI] [PubMed] [Google Scholar]
- Mckernan RM, Whiting PJ. Which GABAA receptor subtypes really occur in the brain. Trends in Neurosciences. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. 10.1016/S0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Mellor JR, Randall AD. Frequency-dependent actions of benzodiazepines on GABAA receptors in cultured murine cerebellar granule cells. The Journal of Physiology. 1997;503:353–369. doi: 10.1111/j.1469-7793.1997.353bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, Dekoninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends in Neurosciences. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Cull-Candy S, Farrant M. Differences in synaptic GABAA receptor number underlie variation in GABA mini amplitude. Neuron. 1997;19:697–709. doi: 10.1016/s0896-6273(00)80382-7. 10.1016/S0896-6273(00)80382-7. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Roberts JDB, Baude A, Richards JG, Somogyi P. Relative densities of synaptic and extrasynaptic GABAA receptors on cerebellar granule cells as determined by a quantitative immunogold method. Journal of Neuroscience. 1995;15:2948–2960. doi: 10.1523/JNEUROSCI.15-04-02948.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. Journal of Neuroscience. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Stephenson FA, Somogyi P. The α6 subunit of the GABAA receptor is concentrated in both inhibitory and excitatory synapses on cerebellar granule cells. Journal of Neuroscience. 1996;16:103–114. doi: 10.1523/JNEUROSCI.16-01-00103.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia G, Costa E, Vicini S. Functional diversity of GABA-activated Cl− currents in Purkinje versus granule neurons in rat cerebellar slices. Neuron. 1994;12:117–126. doi: 10.1016/0896-6273(94)90157-0. 10.1016/0896-6273(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Reichling DB, MacDermott AB. La3+ actions on excitatory amino acid-gated currents and voltage-gated calcium currents in rat dorsal horn neurons. The Journal of Physiology. 1991;441:199–218. doi: 10.1113/jphysiol.1991.sp018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NC, MacDonald RL. Assembly of GABAA receptor subunits: role of the δ-subunit. Journal of Neuroscience. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NC, MacDonald RL. Contrasting actions of La3+ on different recombinant γ-aminobutyric acid receptor isoforms expressed in L929 fibroblasts. Molecular Pharmacology. 1997;51:328–335. doi: 10.1124/mol.51.2.328. [DOI] [PubMed] [Google Scholar]
- Tia S, Wang JF, Kotchabhakdi N, Vicini S. Developmental change of inhibitory synaptic currents in cerebellar granule neurons: Role of GABAA receptor α6 subunit. Journal of Neuroscience. 1996a;16:3630–3640. doi: 10.1523/JNEUROSCI.16-11-03630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tia S, Wang JF, Kotchabhakdi N, Vicini S. Distinct deactivation and desensitization kinetics of recombinant GABAA receptors. Neuropharmacology. 1996b;35:1375–1382. doi: 10.1016/s0028-3908(96)00018-4. 10.1016/S0028-3908(96)00018-4. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W, Molgó J. Quantal acetylcholine release at the vertebrate neuromuscular junction. Physiological Reviews. 1994;74:899–991. doi: 10.1152/physrev.1994.74.4.899. [DOI] [PubMed] [Google Scholar]
- Zheng T, Santi MR, Bovolin P, Marlier LN, Grayson DR. Developmental expression of the α6 GABAA receptor occurs only after cerebellar granule cell migration. Brain Research Developmental Brain Research. 1993;75:91–103. doi: 10.1016/0165-3806(93)90068-l. [DOI] [PubMed] [Google Scholar]
- Zheng T, Zhu WJ, Puia G, Vicini S, Grayson DR, Costa E, Caruncho HJ. Changes in γ-aminobutyrate type A receptor subunits mRNAs, translation product expression, and receptor function during neuronal maturation in vitro. Proceedings of the National Academy of Sciences of the USA. 1995;91:10952–10956. doi: 10.1073/pnas.91.23.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WJ, Vicini S. Neurosteroid prolongs GABAA channel deactivation by altering kinetics of desensitized states. Journal of Neuroscience. 1997;17:4022–4031. doi: 10.1523/JNEUROSCI.17-11-04022.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WJ, Wang JF, Krueger KE, Vicini S. δ Subunit inhibits neurosteroid modulation of GABAA receptors. Journal of Neuroscience. 1996;16:6648–6656. doi: 10.1523/JNEUROSCI.16-21-06648.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


