Abstract
The effects of extracellular acidosis and Cd2+ on the transient outward current (Ito) have been investigated in rat and human ventricular myocytes, using the whole-cell patch-clamp technique.
In rat myocytes, exposure to acidic extracellular solution (pH 6.0) shifted both steady-state activation and inactivation curves to more positive potentials, by 20.5 ± 2.7 mV (mean ± s.e.m.; n = 4) and 19.8 ± 1.2 mV, respectively. Cd2+ also shifted the activation and inactivation curves in a positive direction in a concentration-dependent manner.
In human myocytes, the steady-state activation and inactivation curves were located at more positive potentials. The effect of Cd2+ was similar, but acidosis had less effect than in rat myocytes (e.g. pH 6.0 shifted activation by only 7.2 ± 2.2 mV and inactivation by 13.7 ± 0.5 mV; n = 4).
In both species, the effect of acidosis decreased with increasing concentrations of Cd2+ and vice versa, suggesting competition between H+ and Cd2+ for a common binding site.
The data indicate that acidosis and divalent cations influence Ito via a similar mechanism and act competitively in both rat and human myocytes, but that human cells are less sensitive to the effects of acidosis.
Transient outward current (Ito) has been identified in cardiac tissues of many species (Kenyon & Gibbons, 1979a; b; Josephson et al. 1984; Clark et al. 1988; Dukes & Morad, 1991;Näbauer et al. 1993). In some preparations, only one voltage-dependent Ito component was found (Dukes & Morad, 1991), whereas in others two components of Ito, one Ca2+1-dependent and one Ca2+1-independent, were identified (Coraboeuf & Carmeliet, 1982). The Ca2+1-independent component is carried by K+ ions and is sensitive to 4-aminopyridine (Kenyon & Gibbons, 1979a, b; Coraboeuf & Carmeliet, 1982). On the other hand, extensive evidence indicates that the charge carrier of the Ca2+1-dependent component is Cl− (Zygmunt & Gibbons, 1991; Sipido et al. 1993). Ito plays an important role in the early repolarization phase of the cardiac action potential and its variable regional density underlies the heterogeneity of the action potential within the ventricular wall (Antzelevitch et al. 1991).
Extracellular acidosis is known to influence many membrane currents (see Krafte & Kass, 1988; Zhang & Siegelbaum, 1991). Protons and divalent cations induce a shift of voltage-dependent channel gating which is usually explained by a screening of membrane surface charge which changes the potential sensed by the voltage sensor of the channels (see Hille, 1984). However, this theory cannot easily explain the different sensitivity of various channels to divalent ions (Mayer & Sugiyama, 1988; Talukder & Harrison, 1995) nor the wide range of potency of various divalents (Mayer & Sugiyama, 1988; Agus et al. 1991; Talukder & Harrison, 1995). Hence the existence of divalent-specific binding sites adjacent to the voltage sensor of the channel has been proposed.
In this report, we have examined and compared the effects of acidosis and the divalent cation Cd2+ on Ito in rat and human ventricular myocytes, as well as their interaction. Cd2+ was chosen as a representative of divalent cations because of its known potency to modulate Ito (Agus et al. 1991) and because it is often employed as an efficient blocker of L-type Ca2+ current (ICa,L) when studying Ito. The results show that both acidosis and Cd2+ cause a positive shift in activation and inactivation potentials and act in a competitive way; the sensitivity of Ito to acidosis is less pronounced in human than in rat ventricular myocytes.
METHODS
Rat cells
The technique for cell isolation was as described previously (Mubagwa et al. 1997). In brief, rats were anaesthetized with sodium pentobarbitone (Nembutal; 30 mg kg−1, i.p.) 10 min after having received heparin (500 U, i.p.). Afterwards, the hearts were excised and perfused on a Langendorff apparatus at 37°C. Cells were dissociated by a protocol which consisted of a constant-flow perfusion (8-10 ml min−1) for: (1) 5 min with Ca2+-free Tyrode solution; (2) 3 min with Ca2+-free Tyrode solution, containing collagenase (Type A, Boehringer Mannheim; 0.22 mg ml−1) and protease (Type XIV, Sigma; 0.12 mg ml−1); (3) 7–11 min with Ca2+-free, collagenase-containing Tyrode solution; and (4) 10 min with 0.18 mM Ca2+-containing Tyrode solution. The isolated myocytes were stored in normal Tyrode solution at room temperature (22-25°C).
Human cells
Patients
Myocytes were obtained from eight hearts, six of which were from patients with terminal heart failure due to dilated (n = 3) or ischaemic (n = 3) cardiomyopathy undergoing transplantation and two were non-failing donor hearts, one which could not be transplanted for technical reasons and a second which was rejected 2 days after transplantation. The mean age of the hearts was 56 ± 6.3 years (means ±s.e.m.). All patients (5 males, 1 female) received digoxin, diuretics and vasodilators.
The investigation was performed according to the Declaration of Helsinki and the protocol was approved by the ethical committee of the University. Informed consent was obtained before organ explantation.
Cell isolation
Part of the left ventricular wall was excised together with its arterial branch. The segment was then perfused via the artery on a Langendorff apparatus at 37°C at constant flow (8-10 ml min−1) for: (1) 30 min with a nominally Ca2+-free Tyrode solution; (2) 30–40 min with Ca2+-free Tyrode solution containing collagenase (Type A, Boehringer Mannheim, 1 mg ml−1) and protease (Type XIV, Sigma, 0.1 mg ml−1); and (3) 15 min with 0.09 mM Ca2+ solution. After the perfusion the tissue was cut into chunks and cells were disaggregated by mechanical agitation in 0.09 mM Ca2+-containing Tyrode (Ca2+-Tyrode) solution. Subsequently, the Ca2+ concentration was gradually increased in three to four steps to the final Ca2+ concentration (1.8 mM). The cells were stored at room temperature in 1.8 mM Ca2+-Tyrode solution. The yield was approximately 10 %. Only cells with clear cross-striations without granulation were selected for experiments.
Voltage clamp
Voltage clamp was performed in the whole-cell configuration of the patch-clamp method using an Axopatch 200A amplifier (Axon Instruments) and heat-polished borosilicate glass pipettes (horizontal puller; Zeitz Instrumente, Germany) with resistances of 2–6 MΩ when filled with pipette solution. Voltage-clamp signals were low-pass filtered (5 kHz 4-pole Bessel), digitized by an A/D converter (Labmaster DMA, Scientific Solutions, Inc., Solon, OH, USA) at 1 kHz (except for measurement of cell capacitance, where the signal was digitized at 100 kHz) and stored in an IBM-AT personal computer using pCLAMP software (Axon Instruments). Series resistances and membrane capacitances were not compensated and capacitive transients decayed with a time constant of 1.0 ± 0.05 ms (n = 42) in rat and 1.2 ± 0.08 ms (n = 28) in human myocytes. Cell capacitance was calculated from the time integral of the capacitive transient elicited by a 10 mV depolarizing step from the holding potential of -80 mV and was 150.8 ± 7.2 pF (n = 42) for rat myocytes and 350.4 ± 29 pF (n = 28) for human cells. The rather high value for human cells is probably related to the fact that the cells were mostly obtained from hypertrophied, dilated hearts. Cell capacitance of human cells from healthy donor hearts averaged 212.1 ± 37.6 pF (n = 7). Series resistance was estimated by dividing the time constant of the capacitive transient by the cell capacitance and the values were 6.4 ± 0.3 MΩ (n = 42) for rat and 4.0 ± 0.4 MΩ (n = 28) for human cells. All experiments were performed at room temperature (22-25°C).
Solutions and drugs
Normal Tyrode solution contained (mM): NaCl, 135; KCl, 5.4; CaCl2, 1.8; MgCl2, 0.9; Na2HPO4, 0.33; Hepes, 10; and glucose, 10 and the pH was adjusted to 7.4 with NaOH. In the acidic solution, Mes was employed instead of Hepes and pH was adjusted to 6.0. To block ICa,L, nisoldipine (10 μm) or Cd2+ (0.1-2 mM) were added. In some experiments, Ca2+-free, 5 mM MgCl2-containing Tyrode solution was used to block ICa,L. Intracellular solution contained (mM): potassium aspartate, 130; KCl, 25; MgCl2, 1; Na2ATP, 5; EGTA, 1; Hepes, 5; Na2GTP, 0.1 (pH 7.2, adjusted with KOH). Nisoldipine was from Bayer AG and was prepared as a 20 mM stock solution in dimethyl sulphoxide. Solutions containing nisoldipine were protected from light. Other drugs or chemicals were from Sigma or Merck.
Ito measurement
Under our experimental conditions, only the Ca2+1-independent component of Ito was studied since EGTA (1 mM) was included in the pipette solution and ICa,L was blocked. In most experiments, the holding potential was set at -80 mV from which depolarizing test pulses were made to various levels every 10 s. Test pulses were always preceded by a brief (20 ms) prepulse to -40 mV to inactivate the INa. The amplitude of Ito was measured as the difference between peak current and current remaining at the end of a 900 ms test pulse. Such a method could lead to underestimation of the Ito magnitude because the current at 900 ms was still declining. Experiments with longer test pulses revealed that the Ito amplitude obtained as the difference between peak current and the current at the end of a 9 s pulse was larger by 15.4 ± 5.7 % (n = 4). Also, the amplitude of 4-aminopyridine-sensitive current was slightly greater (16.1 ± 3.7 %; n = 4) than the current obtained as the difference between peak value and value at the end of a 900 ms pulse. However, since the kinetic parameters of Ito inactivation were not influenced by either protons or divalent ions (see Results), the subtraction method appears to be reliable enough for the determination of Ito amplitude.
Steady-state activation curves were constructed in the following two ways. In most experiments, Ito currents at various test potentials were divided by the driving force (taking -70 mV as the reversal potential) and each peak conductance was subsequently normalized to the maximal conductance. The reversal potential (-70 mV) was determined experimentally using a tail current protocol and is in good accordance with the data of others (Apkon & Nerbonne, 1991;Näbauer et al. 1993). In some experiments (n = 4), Ito was activated by a brief step (20 ms) to different potentials and the relative amplitude of the tail current upon return to -20 mV was taken as a measure of Ito activation by the first step. The two protocols produced similar results. The inactivation curve was obtained using a two-step voltage-clamp protocol, with a 1 s conditioning step to different potentials followed by a 500 ms test pulse to +60 mV. Inactivation by a given potential was then determined as the ratio of the test current amplitude to the maximal test current. The test current amplitude was obtained as the difference between peak current and the current at the end of the 500 ms pulse (amplitude measured as the difference between peak current and current at 9 s was larger by 26.7 ± 4.3 %; n = 4). The voltage dependence of both activation and inactivation was fitted by a Boltzmann equation, providing V½ (the potential at which half-activation or -inactivation occurs) and slope factor.
Data presentation and statistics
Data are presented as means ± standard error (s.e.m.). Curve fitting with a least-squares method was performed using the software Origin (Microcal Software, Northampton, MA, USA). When appropriate, Student's paired or non-paired t tests were carried out using Origin.
RESULTS
Rat myocytes
Effect of acidosis
In rat ventricular myocytes, acidification (pH 6.0) of the external solution had a paradoxical effect on Ito. Acidosis caused either an increase or decrease of Ito or else had no effect at all, depending on both the holding and test potentials. When the holding potential was set to -80 mV, acidosis suppressed the current at most test potentials (Fig. 1A) except very positive ones (≥+60 mV), where acidosis did not elicit any effect (not shown). In contrast, when holding at less negative potentials (-30 mV), the current increased (Fig. 1B).
Figure 1. Effect of acidosis (pH 6.0) on Ito in rat myocytes.
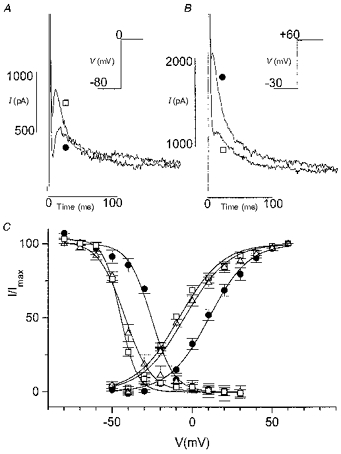
ICa,L was blocked by nisoldipine (10 μm). A, decrease of Ito by acidosis. Current activated by a step from a holding potential of -80 to 0 mV. Traces in control conditions (□) and in pH 6.0 (•) are superimposed. Inset, voltage-clamp protocol (20 ms prepulse to -40 mV to inactivate INa not shown). B, increase of Ito by acidosis. Current activated by a step to +60 mV after a 1 s prepulse to -30 mV in control (□) and in acidosis (•). Only currents during the test pulse are shown. Inset, voltage-clamp protocol. C, effect of acidosis on steady-state activation and inactivation curves. □, control; •, acidosis; ▵, second control. The effect of acidosis was reversible.
Steady-state activation and inactivation
Such a paradoxical effect could be explained if the voltage dependence of both activation and inactivation was shifted. Steady-state activation and inactivation curves were constructed as described in Methods and are shown in Fig. 1C. Acidosis induced a significant rightward shift of both steady-state activation and inactivation curves of Ito. Under conditions where ICa,L was blocked by nisoldipine (10 μm), the V½ of the steady-state activation curve was shifted from -10.6 ± 0.7 mV (n = 4) in control Tyrode solution (pH 7.4) to +9.8 ± 2.3 mV in acidic solution, and the V½ of the inactivation curve was shifted from -45.3 ± 0.9 mV to -25.5 ± 0.4 mV. The slope of both curves was not significantly changed (13 ± 0.9 in control and 12.4 ± 0.6 in acidosis for the activation curve; 4.5 ± 0.3 in control and 5.3 ± 0.1 in acidosis for the inactivation curve; n = 4). The effect of acidosis was completely reversible. In evaluating the V½ and the slope of the activation curve it should be realized that the voltage drop due to the series resistance resulted in a rightward shift of the curve and a decrease of the slope; this error was much less for the inactivation curve. The magnitude of the shift is not subject to this error because maximum currents and currents at V½ were the same in control and acidic conditions. Since dihydropyridine Ca2+ antagonists were reported to inhibit Ito and to accelerate inactivation (Gotoh et al. 1991), the effect was also examined under conditions where Ca2+-free, 5 mM Mg2+-containing external solution, instead of nisoldipine, was used to block ICa,L. Under these conditions, the shift of activation and inactivation curves was similar: V½ of activation was shifted to more positive potentials from -12.8 ± 4.5 mV by 20.9 ± 2.6 mV and V½ of inactivation from -49.8 ± 2.4 mV by 20.6 ± 0.7 mV (n = 3).
Kinetics of activation and inactivation
The time course of Ito inactivation was best fitted by two exponentials (Fig. 2A, inset). Both the fast time constant (24.7 ± 2.2 ms at +40 mV with Ca2+-free, 5 mM Mg2+-containing Tyrode solution; n = 3) and the slow (364.2 ± 17.7 ms at +40 mV) time constant were voltage independent (in the range of membrane potentials from 0 to +60 mV) and acidosis did not influence them (Fig. 2A and B) regardless of the ICa,L blocker used (nisoldipine, 0 mM Ca2+-5 mM Mg2+ or Cd2+). Inactivation was, however, accelerated in the presence of nisoldipine as reported previously by Gotoh et al. (1991). The fast time constant was 12.3 ± 1.7 ms and the slow one 85.4 ± 13.7 ms at +40 mV (n = 4).
Figure 2. Effect of acidosis on activation and inactivation kinetics in rat myocytes.
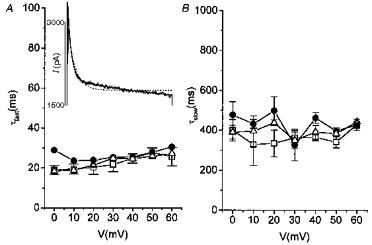
ICa,L was blocked by 0 mM Ca2+o-5 mM Mg2+o. □, control; •, acidosis; ▵, second control. A, voltage dependence of the fast time constant of inactivation (τfast) under control and acidic conditions. Inset, the Ito inactivation time course was not satisfactorily fitted by a single exponential (dotted line), but by a double exponential (continuous line, superimposed on the tracing). Current was activated by a 900 ms step to +40 mV from a holding potential of -80 mV. B shows the voltage dependence of the slow time constant of inactivation (τslow) under control and acidic conditions.
Effect of divalent cations
Since divalent cations are known to shift the voltage dependence of various membrane currents (Hille, 1984) in the same way as acidosis, the effect of Cd2+ on Ito was also explored. Under conditions where ICa,L was blocked by 0 mM Ca2+-5 mM Mg2+, addition of Cd2+ (0.3 mM) induced an effect similar to that of acidosis. Depending on the holding and test potentials, Cd2+ caused either a decrease (Fig. 3A) or an increase (Fig. 3B) of Ito, or had no effect at all (not shown). As with acidosis, the effect was due to a shift of steady-state activation and inactivation curves to more positive potentials (Fig. 3C). The V½ of the activation curve was shifted from -13.7 ± 2.6 mV (n = 3) to +8.3 ± 1.5 mV and the V½ of the inactivation curve from -45.9 ± 1.2 mV to -21.2 ± 3.3 mV. The slope of both curves was not affected (16.6 ± 1.2 in the absence and 15.8 ± 1.1 in the presence of Cd2+ for the activation curve; 4.6 ± 0.4 in the absence and 4.4 ± 0.4 in the presence of Cd2+ for the inactivation curve; n = 3). The kinetics of Ito were changed by Cd2+ in the same way as with acidosis. The voltage dependence of the time to peak Ito activation was shifted to more positive potentials, consistent with the shift of the steady-state activation curve. The voltage-independent inactivation time constants were not influenced by the presence of Cd2+ (data not shown).
Figure 3. Effect of Cd2+ on Ito in rat myocytes.
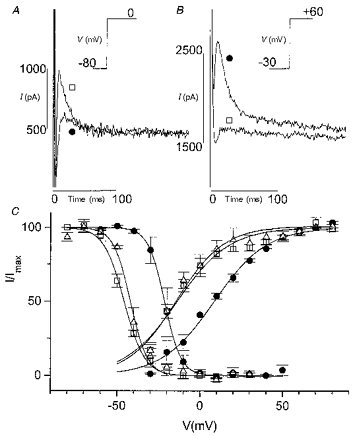
ICa,L was blocked by 0 mM Ca2+o-5 mM Mg2+o. A, decrease of Ito by Cd2+ (0.3 mM). Current activated by a step from a holding potential of -80 to 0 mV. Traces in the absence (□) and in the presence of Cd2+ (•) are superimposed. Inset, voltage-clamp protocol (20 ms prepulse to -40 mV to inactivate INa not shown). B, increase of Ito by Cd2+. Current activated by a step to +60 mV after a 1 s prepulse to -30 mV in the absence (□) and presence of Cd2+ (•). Only currents during the test pulse are shown. Inset, voltage-clamp protocol. C, effect of Cd2+ on steady-state activation and inactivation curves. □, control; •, Cd2+; ▵, second control. The effect of Cd2+ was reversible.
Interaction between the effects of pH and of Cd2+
Since the effects of acidosis and divalent Cd2+ on Ito appeared similar, we next examined their interaction. Figure 4 shows the effect of acidosis in the presence of Cd2+ (0.3 mM). Acidosis could either decrease, increase (Fig. 4A and B) or not change Ito (not shown) depending on the holding and test potentials, but the underlying shift of steady-state activation and inactivation curves was less marked in the presence of Cd2+, i.e. the V½ of the activation curve was shifted from +12.2 ± 3.2 to +18.9 ± 3.2 mV (n = 8) and the V½ of the inactivation curve from -24.9 ± 1.5 to -15.2 ± 1.3 mV (Fig. 4C). With increasing concentrations of Cd2+ (up to 5 mM) the positions of the activation and inactivation curves in control conditions (pH 7.4) were shifted to more positive potentials, and simultaneously the effect of acidosis was decreasing and disappearing at a Cd2+ concentration of 5 mM (Fig. 5). On the other hand, the effect of Cd2+ was less pronounced in acidic conditions, suggesting a competition for common binding site(s).
Figure 4. Effect of acidosis on Ito in the presence of Cd2+ in rat myocytes.
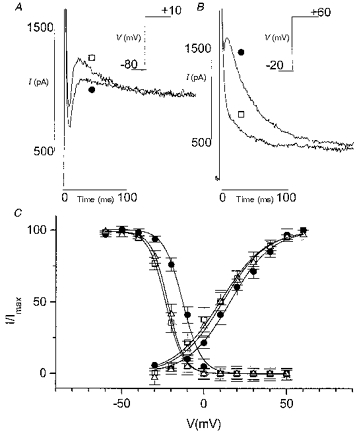
ICa,L was blocked by Cd2+ (0.3 mM). A, decrease of Ito by acidosis. Current activated by a step from a holding potential of -80 to +10 mV. Traces in control conditions (□) and in pH 6.0 (•) are superimposed. Inset, voltage-clamp protocol (20 ms prepulse to -40 mV to inactivate INa not shown). B, increase of Ito by acidosis. Current activated by a step from a holding potential of -20 to +60 mV in control (□) and in acidosis (•). Inset, voltage-clamp protocol. C, effect of acidosis on steady-state activation and inactivation curves. □, control; •, acidosis; ▵, second control. The effect of acidosis was less marked than in the absence of Cd2+ (with nisoldipine or 0 mM Ca2+o-5 mM Mg2+o to block ICa,L).
Figure 5. Interaction of acidosis and Cd2+ in rat myocytes.
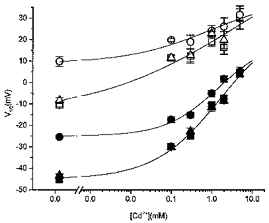
Concentration dependence of the effect of Cd2+ on the V½ of activation (open symbols) or inactivation (filled symbols) in control (squares), acidosis (circles) and second control (triangles). With increasing Cd2+ concentration, the V½ of both activation and inactivation was shifted to more positive potentials. The effect of acidosis consisted of a further shift of V½ to a more positive value but the magnitude of this additional shift decreased with increasing Cd2+ concentration. At 5 mM Cd2+ acidosis failed to induce a further shift. The values at 0 mM Cd2+ were obtained with nisoldipine as the ICa,L blocker. Different Cd2+ concentrations were applied in different groups of cells; n varied between 3 and 8. Data points were fitted by eye (continuous lines).
Human myocytes
Comparison of outward currents in rat and human myocytes
Outward currents in human myocytes (Fig. 6A) were a bit different from those in rat cells (Fig. 6B). The sustained current at the end of a 900 ms test pulse was smaller than in the rat (26.7 ± 4.7 % of peak value in human; n = 16; and 46.7 ± 2.5 % in rat at a Vm of +60 mV; n = 29; P < 0.001). The inactivation kinetics were also different since the time course of inactivation was reasonably fitted by a single exponential (time constant of 79.3 ± 11.4 ms; ICa,L blocked by Cd2+; n = 6). Figure 7 shows that both steady-state activation and inactivation curves were at potentials more positive than in the rat. The V½ of the activation curve was +0.75 ± 2.9 mV (n = 4) and theV½ of the inactivation curve was -39.4 ± 0.2 mV in the absence of Cd2+, with nisoldipine as the ICa,L channel blocker.
Figure 6. Comparison of outward current in human and rat myocytes.
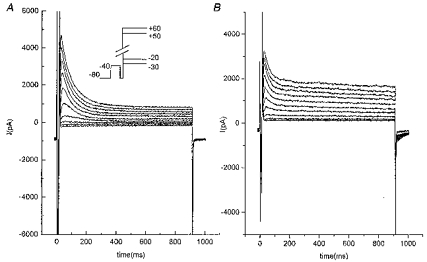
A, depolarization-induced outward current in human ventricular cell. Inset, voltage-clamp protocol: depolarizing pulses were preceded by a 20 ms prepulse to -40 mV to inactivate the INa current. B, depolarization-induced outward current in rat myocytes. Same voltage-clamp protocol as in A.
Figure 7. Effect of acidosis on Ito in human myocytes.
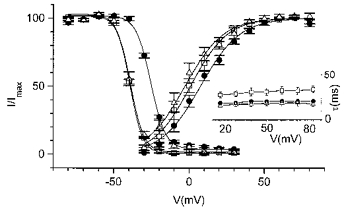
ICa,L was blocked by nisoldipine (10 μm). Steady-state activation and inactivation curves of Ito in control and acidosis are shown. Inset, voltage dependence of the Ito inactivation time constant in control and acidosis. □, control; •, acidosis; ▵, second control.
Effect of acidosis
The response of Ito in human cells to acidosis was qualitatively similar to that in rat myocytes, but less pronounced (Fig. 1C and Fig. 7). In the absence of Cd2+ (with nisoldipine as the ICa,L blocker), acidosis shifted the V½ of the activation curve only by 7.2 ± 2.2 mV (n = 4) and the V½ of the inactivation curve by 13.7 ± 0.5 mV. The kinetics of inactivation were both potential (in the range from +20 to +80 mV) and pH insensitive and an acceleration of inactivation was again observed in the presence of nisoldipine (time constant of 31.1 ± 4.4 ms; n = 4).
Effect of divalent cations
Application of Cd2+ (0.3 mM) induced an effect qualitatively and quantitatively similar to that in rat myocytes. Under conditions where ICa,L was blocked by nisoldipine, Cd2+ shifted both steady-state activation and inactivation curves to more positive potentials by 16.1 ± 1.3 mV (n = 3) and 23.2 ± 1.5 mV, respectively (Fig. 8). The kinetics of inactivation were both potential and Cd2+ insensitive and decay was again accelerated by nisoldipine (Fig. 8, inset).
Figure 8. Effect of Cd2+ on Ito in human myocytes.
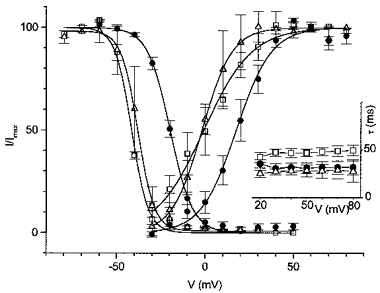
ICa,L was blocked by nisoldipine (10 μm). Steady-state activation and inactivation curves of Ito in the absence and presence of Cd2+. Inset, voltage dependence of the Ito inactivation time constant in the absence and presence of Cd2+. □, control; •, 0.3 mM Cd2+; ▵, second control.
Interaction of protons and Cd2+
In the presence of Cd2+ (0.3 mM) the activation and inactivation curves were located at more positive potentials (V½: +22.2 ± 2.3 mV and -17.0 ± 0.7 mV, respectively; n = 5) and acidosis did not induce any further shift of these curves (Fig. 9), a marked difference to rat myocytes, where a significant shift induced by acidosis was still observable at 2 mM Cd2+ (see Figs 4 and 5).
Figure 9. Effect of acidosis on Ito in the presence of Cd2+ in human myocytes.

ICa,L was blocked by Cd2+ (0.3 mM). Steady-state activation and inactivation curves of Ito in control and acidosis. Inset, voltage dependence of the Ito inactivation time constant in control and acidosis. □, control; •, acidosis; ▵, second control.
DISCUSSION
We have demonstrated that acidosis can either increase or decrease Ito or have no effect at all, depending on the holding and test potentials. The depression of Ito when activated from potentials negative to -70 mV to potentials less positive than +60 mV is explained by a rightward shift of the activation curve. The absence of an effect of acidosis on Ito activated by steps from -80 mV to potentials more positive than +60 mV makes the possibility of a channel block unlikely and further supports the hypothesis that a shift in channel gating underlies the observed Ito modulation. On the other hand, acidosis induced a stimulation of Ito activated from holding potentials positive to -50 mV (potentials where inactivation is significant) as a result of a positive shift of the steady-state inactivation curve. Therefore, under physiological conditions when the resting membrane potential is negative to -70 mV and the action potential overshoot less positive than +60 mV (as in all ventricular or atrial working cells), acidosis should inhibit Ito. However, in preparations such as nodal tissue where the resting membrane potential is under physiological conditions between -50 and -60 mV, or in any cardiac cell when, under pathological conditions, the membrane is depolarized (e.g. during ischaemia by accumulation of external potassium), acidosis will stimulate Ito because the negative effect of the rightward activation shift will be overridden by the increased availability of channels due to the shift of the inactivation curve. The stimulation of Ito by acidosis when activated from a depolarized potential (-40 mV) was recently reported by Hulme & Orchard (1996). Taken together, acidosis will influence Ito differently in different regions of the heart according to the actual resting membrane potential, an action which can alter action potential dispersion and contribute to arrhythmogenesis under ischaemic conditions.
Similar shifts of the voltage dependence of Na+ and Ca2+ channel gating induced either by extracellular protons or by divalent cations have been described in a variety of tissues (Schauf, 1975; Ohmori & Yoshii, 1977; Kostyuk et al. 1982; Krafte & Kass, 1988; Zhang & Siegelbaum, 1991; Huang et al. 1993; Davidson et al. 1995). The phenomenon is usually explained by the surface charge theory, according to which the titration of negative external surface charges leads to changes in surface potential and therefore to changes in the potential sensed by the voltage sensor of the channel. In the original Gouy-Chapman screening model (see Hille, 1984) only the charge of the screening ion was important whereas the improved Gouy-Chapman-Stern model (see Hille, 1984) also takes into account the specific binding of some ions. However, one limitation of this model remains that it assumes a uniform charge (or binding site) distribution, as expected if negatively charged phospholipids were involved. In contrast, studies of Green et al. (1987) and of Cukierman et al. (1988) in planar lipid bilayers suggest that the negative charge on the channel protein itself is the relevant one, while the membrane phospholipid charge is less important. This was further supported by a report of Mayer & Sugiyama (1988), where manganese induced a significant shift of both steady-state activation and inactivation curves of IA in cultured rat sensory neurones, but in contrast did not influence voltage dependence of gating of delayed rectifier K+ current. Similar data were obtained by Talukder & Harrison (1995) in cultured rat hippocampal neurons for various di- and trivalent cations. Whereas our data do not allow us to distinguish between these possibilities, they indicate that the binding sites are accessible for both protons and divalent cations and that protons and divalent cations compete for them.
The characteristics of Ito in human and rat myocytes were similar but not identical. One major difference was the position of steady-state activation and inactivation curves. In rat, our observations are consistent with those of others, depending on the ICa,L channel blocker used (Apkon & Nerbonne, 1991; Lefevre et al. 1991; Wettwer et al. 1993). The activation and inactivation curves in human cells were found at more positive membrane potentials. This observation corresponds well with the report by Wettwer et al. (1993). The difference is probably not related to the fact that human cells were isolated mostly from diseased hearts, since similar activation and inactivation parameters of Ito were found in myocytes isolated from ischaemic (n = 3), dilated (n = 3) and non-failing donor (n = 2) hearts. Similarly, Wettwer et al. (1993) also did not find any difference between human myocytes isolated from diseased and non-diseased hearts. Interestingly, human cells were also much less sensitive to protons while human and rat cells seemed to be equally sensitive to Cd2+. One could speculate that human myocytes from diseased hearts are chronically exposed to ischaemia and therefore they become less sensitive to protons. However, in our experiments similar results were obtained with myocytes from ischaemic, dilated and even from two non-failing donor hearts. Therefore it seems more probable that low sensitivity to protons is a basic property of human cardiac cells, both from diseased and non-diseased hearts. Such a different behaviour should have a morphological substrate and, indeed, it has been reported that Kv4.3 and Kv4.2 channel isoforms are essential components of Ito in rat myocytes (Dixon et al. 1996; Fiset et al. 1997), while only the Kv4.3 isoform underlies the bulk of Ito in canine and human heart (Dixon et al. 1996). However, further investigation is required to elucidate whether the different sensitivity to external pH is actually due to different channel isoforms underlying Ito.
Acknowledgments
We thank Mr Peter Matejovic for the preparation of rat cells.
References
- Agus ZS, Dukes ID, Morad M. Divalent cations modulate the transient outward current in rat ventricular myocytes. American Journal of Physiology. 1991;261:C310–318. doi: 10.1152/ajpcell.1991.261.2.C310. [DOI] [PubMed] [Google Scholar]
- Antzelevitch C, Sicouri S, Litovsky SH, Lukas A, Krishnan SC, Di Diego JM, Gintant GA, Liu D. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circulation Research. 1991;69:1427–1449. doi: 10.1161/01.res.69.6.1427. [DOI] [PubMed] [Google Scholar]
- Apkon M, Nerbonne JM. Characterization of two distinct depolarization-activated K+ currents in isolated adult rat ventricular myocytes. Journal of General Physiology. 1991;97:973–1011. doi: 10.1085/jgp.97.5.973. 10.1085/jgp.97.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RB, Giles WR, Imaizumi Y. Properties of the transient outward current in rabbit atrial cells. The Journal of Physiology. 1988;405:147–168. doi: 10.1113/jphysiol.1988.sp017326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraboeuf E, Carmeliet E. Existence of two transient outward currents in sheep cardiac Purkinje fibers. Pflügers Archiv. 1982;392:352–359. doi: 10.1007/BF00581631. [DOI] [PubMed] [Google Scholar]
- Cukierman S, Zinkand WC, French RJ, Krueger BK. Effects of membrane surface charge and calcium on the gating of rat brain sodium channels in planar bilayers. Journal of General Physiology. 1988;92:431–447. doi: 10.1085/jgp.92.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson KL, Kehl SJ. Changes of activation and inactivation gating of the transient potassium current of rat pituitary melanotrophs caused by micromolar Cd2+ and Zn2+ Canadian Journal of Physiology and Pharmacology. 1995;73:36–42. doi: 10.1139/y95-005. [DOI] [PubMed] [Google Scholar]
- Dixon JE, Shi W, Wang H, McDonald C, Yu H, Wymore RS, Cohen IS, McKinnon D. Role of the Kv4.3 K+ channel in ventricular muscle. A molecular correlate for the transient outward current. Circulation Research. 1996;79:659–668. doi: 10.1161/01.res.79.4.659. [DOI] [PubMed] [Google Scholar]
- Dukes ID, Morad M. The transient K+ current in rat ventricular myocytes: evaluation of its Ca2+ and Na+ dependence. The Journal of Physiology. 1991;435:395–420. doi: 10.1113/jphysiol.1991.sp018516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiset C, Clark RB, Shimoni Y, Giles WR. Shal-type channels contribute to the Ca2+-independent transient outward K+ current in rat ventricle. The Journal of Physiology. 1997;500:51–64. doi: 10.1113/jphysiol.1997.sp021998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y, Imaizumi Y, Watanabe M, Shibata EF, Clark RB, Giles WR. Inhibition of transient outward K+ current by DHP Ca2+ antagonists and agonists in rabbit cardiac myocytes. American Journal of Physiology. 1991;260:H1737–1742. doi: 10.1152/ajpheart.1991.260.5.H1737. [DOI] [PubMed] [Google Scholar]
- Green WN, Weiss LB, Andersen OS. Batrachotoxin-modified sodium channels in planar lipid bilayers. Journal of General Physiology. 1987;89:841–872. doi: 10.1085/jgp.89.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic Channels Of Excitable Membranes. Sunderland, MA, USA: Sinauer Associates, Inc.; 1984. [Google Scholar]
- Huang R-C, Peng Y-W, Yau KW. Zinc modulation of a transient potassium current and histochemical localization of the metal in neurons of the suprachiasmatic nucleus. Proceedings of the National Academy of Sciences of the USA. 1993;90:11806–11810. doi: 10.1073/pnas.90.24.11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme JT, Orchard CH. Acidosis increases transient outward potassium current in rat cardiac muscle. The Journal of Physiology. 1996;496.P:46. P. [Google Scholar]
- Josephson IR, Sanchez-Chapula J, Brown AM. Early outward current in rat single ventricular cells. Circulation Research. 1984;54:157–162. doi: 10.1161/01.res.54.2.157. [DOI] [PubMed] [Google Scholar]
- Kenyon JL, Gibbons WR. Influence of chloride, potassium and tetraethylammonium on the early outward current of sheep cardiac Purkinje fibers. Journal of General Physiology. 1979a;73:117–138. doi: 10.1085/jgp.73.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon JL, Gibbons WR. 4-Aminopyridine and the early outward current of sheep cardiac Purkinje fibers. Journal of General Physiology. 1979b;73:139–157. doi: 10.1085/jgp.73.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk PG, Mironov SL, Doroshenko PA, Ponomarev VN. Surface charges on the outer side of mollusc neuron membrane. Journal of Membrane Biology. 1982;70:171–179. [Google Scholar]
- Krafte DS, Kass RS. Hydrogen ion modulation of Ca channel current in cardiac ventricular cells. Evidence for multiple mechanisms. Journal of General Physiology. 1988;91:641–657. doi: 10.1085/jgp.91.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre IA, Coulombe A, Coraboeuf E. The calcium antagonist D600 inhibits calcium-independent transient outward current in isolated rat ventricular myocytes. The Journal of Physiology. 1991;432:65–80. doi: 10.1113/jphysiol.1991.sp018376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Sugiyama K. A modulatory action of divalent cations on transient outward current in cultured rat sensory neurones. The Journal of Physiology. 1988;396:417–433. doi: 10.1113/jphysiol.1988.sp016970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubagwa K, Stengl M, Flameng W. Extracellular divalent cations block a cation non-selective conductance unrelated to calcium channels in rat cardiac muscle. The Journal of Physiology. 1997;502:235–247. doi: 10.1111/j.1469-7793.1997.235bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näbauer M, Beuckelmann DJ, Erdmann E. Characteristics of transient outward current in human ventricular myocytes from patients with terminal heart failure. Circulation Research. 1993;73:386–394. doi: 10.1161/01.res.73.2.386. [DOI] [PubMed] [Google Scholar]
- Ohmori H, Yoshii M. Surface potential reflected in both gating and permeation mechanisms of sodium and calcium channels of the tunicate egg cell membrane. The Journal of Physiology. 1977;267:429–463. doi: 10.1113/jphysiol.1977.sp011821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf CL. The interaction of calcium with Myxicola giant axons and a description in terms of a simple surface charge model. The Journal of Physiology. 1975;248:613–624. doi: 10.1113/jphysiol.1975.sp010991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipido KR, Callewaert G, Carmeliet E. [Ca2+]i transients and [Ca2+]i-dependent chloride current in single Purkinje cells from rabbit heart. The Journal of Physiology. 1993;468:641–667. doi: 10.1113/jphysiol.1993.sp019793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukder G, Harrison NL. On the mechanism of modulation of transient outward current in cultured rat hippocampal neurons by di- and trivalent cations. Journal of Neurophysiology. 1995;73:73–79. doi: 10.1152/jn.1995.73.1.73. [DOI] [PubMed] [Google Scholar]
- Wettwer E, Amos G, Gath J, Zerkowski H, Reidemeister J, Ravens U. Transient outward current in human and rat ventricular myocytes. Cardiovascular Research. 1993;27:1662–1669. doi: 10.1093/cvr/27.9.1662. [DOI] [PubMed] [Google Scholar]
- Zhang J, Siegelbaum SA. Effects of external protons on single cardiac sodium channels from guinea pig ventricular myocytes. Journal of General Physiology. 1991;98:1065–1083. doi: 10.1085/jgp.98.6.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt AC, Gibbons WR. Calcium-activated chloride current in rabbit ventricular myocytes. Circulation Research. 1991;68:424–437. doi: 10.1161/01.res.68.2.424. [DOI] [PubMed] [Google Scholar]


