Abstract
The intracellular calcium concentration ([Ca2+]i) was measured at 35 °C using the fluorescent indicator indo-1 in patch-clamped, single uterine myocytes from pregnant rats to investigate the relationship between depolarization, Ca2+ current (ICa) and [Ca2+]i.
Membrane depolarization activated ICa and produced a [Ca2+]i transient. The rapid increase in [Ca2+]i occurred at the same time as the inward ICa. Both ICa and the increase in [Ca2+]i were abolished by nifedipine (10 μm).
When the membrane potential was held at -80 mV the threshold depolarization for an increase in [Ca2+]i was about -55 to -50 mV. As the magnitude of the depolarization was increased to about 0 mV there was an increase in the size of both ICa and the increase in [Ca2+]i. As the magnitude of the depolarization was further increased both ICa and the [Ca2+]i increase declined.
When the depolarizing pulses were applied at 3 Hz to mimic normal action potentials then the individual [Ca2+]i transients did not fully relax and a tetanic rise of [Ca2+]i was observed. Under these conditions, there was not a simple relationship between the magnitude of the Ca2+ response and Ca2+ entry. When pairs of depolarizing pulses were applied, the increase in [Ca2+]i produced by the second pulse was larger (in relation to the magnitude of the L-type Ca2+ current) than that produced by the first pulse. This facilitation was abolished by both ryanodine and cyclopiazonic acid suggesting a role for release from intracellular stores.
We conclude that the L-type Ca2+ current is the major source of Ca2+ ions entering the cell to produce the [Ca2+]i transient on depolarization. The magnitude of the increase in [Ca2+]i may, however, be amplified by Ca2+-induced Ca2+ release.
Timely and controlled uterine activity is important during pregnancy and parturition. Our knowledge of the mechanisms underlying uterine contraction has increased in recent years, mainly as a consequence of measurements of intracellular ions and patch-clamp studies (for a review see Wray, 1993). Thus, as with other types of smooth muscle cells, it is known that contraction is accompanied by an increase in the intracellular Ca2+ concentration ([Ca2+]i) (Szal et al. 1994; Taggart et al. 1996). The regular phasic, spontaneous contractions of the uterus occur as a result of membrane depolarization via action potentials and many agonists evoke changes in membrane potential (Parkington & Coleman, 1990). There is, however, remarkably little known about how changes in membrane potential affect [Ca2+]i and hence contraction. Both L- and T-type Ca2+ channels have been reported in myometrium of some, but not all, species (Ohya & Sperelakis, 1989; Honoréet al. 1989; Inoue et al. 1990). To date, no study has correlated Ca2+ entry through the surface membrane Ca2+ channels with changes in [Ca2+]i.
The changes in [Ca2+]i occurring with stimulation could arise from Ca2+ release by the sarcoplasmic reticulum (SR) as well as from Ca2+ entry through membrane channels. It has been suggested that a Ca2+-induced Ca2+ release (CICR) mechanism contributes significantly to the depolarization-induced [Ca2+]i elevation in some types of smooth muscle (Ganitkevich & Isenberg, 1992) but not in others (Iino, 1989; Guerrero et al. 1994). Although ryanodine receptors have been identified on the SR, it is unclear whether there is any physiological role for CICR in controlling uterine contractility (Lynn et al. 1995; Taggart & Wray, 1997). Thus, at both the cellular and tissue levels our understanding of electromechanical coupling lacks detail. Therefore, the major goal of this work was to investigate how depolarization affects intracellular Ca2+ in myometrial cells and, in particular, to establish (i) at what threshold voltage a definite rise in [Ca2+]i occurs, and (ii) what mechanisms are responsible for the rise in [Ca2+]i.
Previous studies using the sucrose-gap technique have shown that the contractile response of the uterus is dependent upon the duration, size and frequency of imposed potentials (Mironneau, 1973). As the normal physiological stimulation to the uterus involves trains of action potentials, we wished to determine the [Ca2+]i response to both single pulses and trains of pulses and, in particular, whether the [Ca2+]i transients evoked by Ca2+ entry through the surface membrane Ca2+ channels would be amplified by Ca2+ release from the SR.
An abstract of some of this work has been published (Shmigol et al. 1997).
METHODS
Cell isolation
The method of cell preparation was similar to that described previously (Amédée et al. 1986; Shmigol et al. 1995). Female Wistar rats at the end of gestation (19-21 days) were killed by cervical dislocation under CO2 anaesthesia. The intrauterine contents were quickly removed and uterine horns were collected in Ca2+-free and Mg2+-free Hanks' balanced salt solution (HBSS; see below). Strips of muscle were dissected along the natural longitudinal bands, ca 1 mm × 20–30 mm. The strips were incubated in 5 ml of Ca2+-free and Mg2+-free HBSS at 35°C for 30 min, followed by 20–30 min incubation in the enzyme-containing solution. The enzyme solution was composed of Ca2+-free and Mg2+-free HBSS to which 500 U ml−1 collagenase (Type XI), 0.05 % soybean trypsin inhibitor (Type II-S) and 0.2 % bovine serum albumin (Fraction V, essentially fatty acid free) were added. At the end of the incubation period the strips were removed from the enzyme solution and washed thoroughly in three to four changes of HBSS for at least 20 min. After the last wash, the muscle strips were transferred to Kraft-Brühe (KB) medium (Klökner & Isenberg, 1985). After 30 min incubation of the strips in KB medium, trituration was begun with a fire-polished glass Pasteur pipette of 3 mm tip orifice. The cloudy suspension from the first trituration was discarded, as it contained much debris and damaged cells. The remaining unbroken tissue fragments were transferred to fresh KB medium and triturated again. The suspension obtained was strained on an 80 nm nylon mesh. The filtrate was saved and the remaining tissue particles were resuspended in fresh KB medium and triturated again. These steps were repeated 3 or 4 times. Freshly dissociated myocytes were kept in KB medium at 4°C until use. Most data were obtained within the first 6 h after dissociation.
Fluorescence measurements and voltage clamp
An aliquot of cell suspension was placed in a chamber mounted on the stage of an inverted microscope (Nikon Diaphot-200; Japan) equipped with an epifluorescence illuminator and a shutter for interrupting the excitation light (100 W xenon lamp). The cytoplasmic free Ca2+ concentration was measured by means of indo-1-based dual emission technique (Ganitkevich & Isenberg, 1991). The cells were illuminated at 340 nm. The light emitted was split by a dichroic mirror centred at 450 nm, passed through two interference filters, and collected by a pair of photomultipliers in the band between 390 and 420 nm (F400) and 490 and 520 nm (F500). Indo-1 was loaded into the cell through a patch electrode containing 100 mM K5indo-1 (Molecular Probes, Eugene, OR, USA) after the whole-cell configuration had been achieved (see below). The intensity of fluorescence at both wavelengths approached the steady-state level within the first 3–5 min of cell dialysis. During this period the cells were held at a holding potential of -50 or -80 mV and were neither stimulated nor illuminated. Autofluorescence of unloaded cells was negligible compared with indo-1-loaded cells. Background fluorescence originating mostly from the patch pipette was electronically subtracted after formation of a gigaohm seal but before rupture of the membrane. After being filtered at 100 Hz, signals from both photomultipliers were fed into an IBM-compatible computer through an ADC board (Digidata 1200B; Axon Instruments, Foster City, CA, USA). The same computer was used to record the membrane currents and to generate the voltage-clamp protocol. Membrane currents were recorded with the conventional patch-clamp technique. Patch pipettes of 3–4 MΩ resistance were fabricated from 1.5 mm glass capillaries (Clark Electromedical Instruments, Pangbourne, UK). The Axopatch-1B amplifier (Axon Instruments) was used to achieve the whole-cell voltage clamp. The fast and the slow components of the pipette capacitance were cancelled electronically after a tight seal was established between the pipette and the membrane. The membrane capacitance of the near-term uterine myocytes exceeded the range of the whole-cell capacitance compensation of the amplifier used. Thus, it was compensated incompletely. To facilitate the settling time of the voltage clamp, up to 85 % of the series resistance was compensated electronically. All current records were filtered at 1 kHz and digitally corrected for a passive leakage. The leakage current was measured at the beginning of each record by applying a -10 mV voltage pulse.
Calibration of [Ca2+]i
The relationship between [Ca2+]i and the F400/F500 ratio is given by the equation:
(Grynkiewicz et al. 1985), where Kd is the effective dissociation constant of indo-1, β is F0/Fs, i.e. the ratio of the fluorescence signals at 500 nm without Ca2+ (F0) and with saturating Ca2+ (Fs), Rmin is the F400/F500 ratio in the absence of Ca2+, and Rmax is the F400/F500 ratio at saturating Ca2+ concentration. The optical properties of indo-1 in the cytoplasm can be different from those in aqueous solution. Therefore, an intracellular calibration procedure was performed. Rmin was measured in cells dialysed with a solution containing 10 mM EGTA. The value of Kdβ was determined by superfusing the cells with a solution containing 6 mM Ca-EGTA and 3 mM free EGTA assuming a free Ca2+ concentration of 428 nM at 37°C (Benham, 1989). The mean values of Rmin and Kdβ were found to be 0.47 ± 0.03 and 1124 ± 149 nM, respectively (n = 7). Rmax was determined at the end of each experiment by stepping the command potential to -200 mV and back to 0 mV several times. This manoeuvre caused massive Ca2+ entry into the cell due to the increased membrane leakage and thus, saturation of indo-1 with Ca2+. The value of Rmax varied between 2 and 3.
Solutions
The cells were continuously superfused with an extracellular solution containing (mM): NaCl, 140; KCl, 5.4; CaCl2, 2; MgCl2, 1.2; glucose, 10; Hepes, 10; adjusted to pH 7.4 with NaOH. Ca2+-free and Mg2+-free HBSS used for cell isolation was composed of (mM): NaCl, 137; KCl, 5.1; KH2PO4, 0.44; Na2HPO4, 0.26; glucose, 5.5; Hepes, 10; adjusted to pH 7.2 with NaOH. KB medium contained (mM): KCl, 40; K2HPO4, 10; KOH, 105; taurine, 10; glucose, 11; EGTA, 0.1; Hepes, 5. Methanesulphonic acid was used to adjust the pH of this solution to 7.2. The pipette solution contained (mM): CsCl, 130; MgATP, 2; K5indo-1, 0.1; Hepes, 10; adjusted to pH 7.2 with approximately 8 mM NaOH. Thus, the sodium concentration in the pipette solution was about 8 mM. In some experiments the Ca2+ entry blocking drug nifedipine was used. The nifedipine (10 μm)-containing solution was applied onto the cell under investigation from a glass pipette positioned at a distance of about 100 μm from the cell. Ryanodine (5 μm; Calbiochem) was added to the pipette solution and several transients were elicited in its presence before any measurements were made. All experiments were performed at 35°C. All chemicals were obtained from Sigma, unless indicated otherwise.
Where appropriate, the results are presented as means ±s.e.m. Otherwise, the traces shown represent typical results from at least four similar experiments, obtained from different cells. Statistical differences were tested using ANOVA.
RESULTS
Cells chosen for study were spindle shaped, with smooth surfaces and sharp edges. They were healthy as judged by their ability to contract in response to application of high-potassium solution and subsequently relax on its removal. In addition, once a Ca2+ signal could be seen, there were no noticeable changes in [Ca2+]i upon further indo-1 loading and stable baselines of [Ca2+]i and current records were maintained during control conditions. The resting concentration of [Ca2+]i after loading with indo-1 varied from cell to cell in the range 55–155 nM when cells were held at a holding potential of -80 mV. This holding potential value was chosen in order to remove possible steady-state inactivation of Ca2+ channels, which could occur at more positive potentials. However, the physiological resting potential level in uterine smooth muscle cells near the end of pregnancy is about -50 mV (Parkington & Coleman, 1990). We therefore compared the resting [Ca2+]i in cells held at both potentials. On average, resting [Ca2+]i in the cells held at -50 mV was 167 ± 75 nM (n = 19). When the holding potential was set to -80 mV the resting [Ca2+]i decreased to 109 ± 45 nM (n = 28). This difference was statistically significant (P < 0.05). Since resting [Ca2+]i was affected by holding potential, it was important to determine the threshold and voltage dependence of calcium current (ICa) and [Ca2+]i in these cells.
The voltage threshold of the depolarization-induced [Ca2+]i transients
The voltage threshold for elevation of [Ca2+]i on depolarization was examined using a holding potential of -80 mV. In seven cells held at this voltage, the threshold potential of the [Ca2+]i transients was investigated by applying depolarizing pulses to various potentials. The duration of command pulses in these experiments was set to 3 s to reveal the time course of the [Ca2+]i rise caused by depolarization. In two cells measurable [Ca2+]i elevation was seen at -55 mV. In the remaining five cells the threshold was -50 mV. The same values of threshold potential were found for ICa. Figure 1A illustrates one of these experiments. Superimposed are membrane potential (top traces), [Ca2+]i transients (middle traces) and ICa (bottom traces) elicited by voltage steps to -60, -50, -40 and -30 mV. The inset in Fig. 1A shows ICa on an expanded time scale. It is clear that membrane depolarization to -50 mV activated both ICa and [Ca2+]i elevation, and that further depolarization further increased both parameters. In addition, ICa inactivation was incomplete, so that, even at the end of the command pulse there was steady-state ICa. This non-inactivating component of ICa could account for the difference in resting [Ca2+]i observed in cells held at -80 and -50 mV. To examine the possibility of continuous Ca2+ entry into the cell at holding potentials positive to the threshold potential, we measured steady-state inactivation of ICa in six different cells using a standard double-pulse protocol (Fig. 1B). In these experiments, conditioning voltage pulses between -100 and -10 mV were applied for 10 s followed by a test voltage step to 0 mV. The steady-state inactivation curve was compared with the activation characteristics of ICa calculated from the falling phase of the I-V curve. It is clear from Fig. 1B that these curves overlaped resulting in a ‘window’ at membrane potentials between -60 and -10 mV. The voltage level for half-inactivation of ICa was found to be -53 mV (see Fig. 1B). Therefore, it can be expected that conditioning hyperpolarization will augment both ICa and [Ca2+]i transients elicited by subsequent depolarization. Indeed, the voltage step to 0 mV increased [Ca2+]i by 123 ± 26 nM when the holding potential was -50 mV and by 286 ± 37 nM when the holding potential was -80 mV (n = 6). Both ICa and [Ca2+]i transients were completely abolished in Ca2+-free solution or when 10 μm nifedipine was applied (not illustrated).
Figure 1. The threshold voltage of depolarization for the depolarization-activated Ca2+ entry in uterine myocytes.
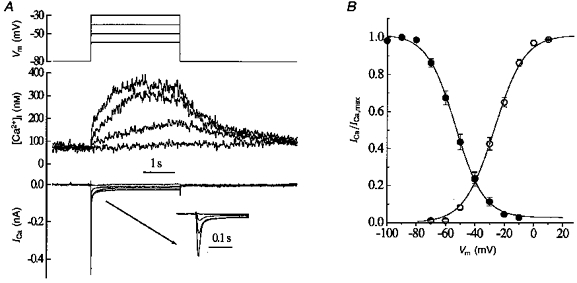
A, traces show (from top to bottom): membrane potential (Vm), [Ca2+]i and membrane current (ICa). The inset shows the current records on an expanded time scale. B, steady-state inactivation (•) and activation (○) of ICa as a function of membrane potential. The continuous lines were obtained by fitting Boltzmann equations to the experimental data. Half-inactivation occurred at -53 mV with a slope factor of 9.9 mV. The potential for half-activation was -27 mV, with a slope factor of 11 mV. Error bars are s.e.m. (n = 6).
The voltage dependence of the [Ca2+]i transient
The experiments described above are consistent with the L-type Ca2+ current having a major role in the generation of the [Ca2+]i transient on depolarization. In subsequent experiments, we investigated whether the voltage dependence of the [Ca2+]i transient was consistent with its origin in the L-type current. The effects of depolarizing pulses of different magnitude on ICa and [Ca2+]i are shown in Fig. 2A. In this experiment, a holding potential of -50 mV was used. As the magnitude of the depolarizing step was increased the size of both the inward ICa and the rise in [Ca2+]i increased. However, beyond a certain voltage, both the current and [Ca2+]i rise decreased. The inset in Fig. 2A shows superimposed current traces on an expanded time scale. Mean data from six cells are shown in Fig. 2B; both current and [Ca2+]i had a bell-shaped dependence on voltage with maxima at 0 mV. This kind of voltage dependence would be expected if Ca2+ entry through the surface membrane Ca2+ channel is the only source for the depolarization-induced [Ca2+]i elevation. In our experiments, most of the Ca2+ channels inactivated within the first 100 ms of the depolarizing voltage pulse (see insets in Figs 1A and 2A) leaving only a small non-inactivating component of ICa. Therefore, a series of brief depolarizations might be expected to be more effective in delivering Ca2+ into the cell than a single prolonged voltage pulse. It is interesting to note that the action potential of smooth muscle cells from pregnant uterus comprises slow depolarization with superimposed bursts of simple spikes (Parkington & Coleman, 1988). The Ca2+ signal generated by uterine myocytes in vivo may therefore be a result of summation of the individual [Ca2+]i transients evoked by repetitive action potentials. The next series of experiments was designed to investigate the change in [Ca2+]i elicited by repetitive stimulation with trains of voltage steps. We will refer to this change as the [Ca2+]i response, to distinguish it from [Ca2+]i transients elicited by single pulses.
Figure 2. Effects of varying the magnitude of the depolarization on [Ca2+]i and ICa.
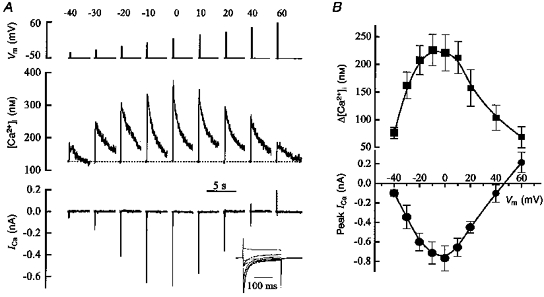
A, depolarizing steps to the voltages indicated (top) of 200 ms duration were applied as shown from a holding potential of -50 mV. The inset shows the currents traces on an expanded time scale. B, the dependence of [Ca2+]i (top) and peak inward ICa (bottom) on membrane potential. Values are means ±s.e.m. (n = 6).
[Ca2+]i responses during repetitive depolarization
The effects of repetitive depolarization on ICa and [Ca2+]i were investigated in twenty-four cells held at a holding potential of -80 mV. The trains of voltage pulses evoked changes in [Ca2+]i of larger magnitude than those evoked by a single pulse due to summation of individual Δ[Ca2+]i. Typical results obtained are illustrated in Fig. 3A. The [Ca2+]i responses (top trace) and ICa (bottom trace) were evoked by a train of ten 100 ms voltage pulses to 0 mV from a holding potential of -80 mV. Such voltage trains were repeated 3 times at 1 min intervals. In these experiments, the interval between successive pulses in the burst was 330 ms. The individual [Ca2+]i transients did not recover between pulses but superimposed to form a ‘tetanus’-like response. This is despite the fact that ICa gradually declined during the burst presumably due to lack of recovery from the combination of Ca2+- and voltage-dependent inactivation. During a 1 min resting period, ICa recovered almost completely, so that the difference in current amplitude between three successive voltage trains was almost negligible. To analyse in more detail the relationship between ICa and [Ca2+]i during the repetitive stimulation, we compared normalized values of ICa and individual [Ca2+]i transients in the train of ten voltage pulses. This is illustrated in Fig. 3B. It is clear that the normalized amplitude of ICa (•) and Δ[Ca2+]i (○) showed identical negative staircase effects in the second and third trains but not in the first train (Fig. 3B).
Figure 3. Effects of repetitive stimulation on [Ca2+]i.
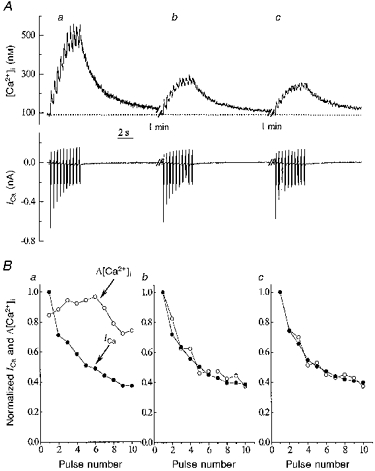
A, traces show: top, [Ca2+]i; bottom, ICa. During the periods of stimulation (shown by the spikes in the current record) 100 ms duration depolarizing pulses were applied from a holding potential of -80 to 0 mV at 3 Hz. B, changes in ICa and Δ[Ca2+]i during repetitive stimulation. ICa was normalized to the value of the first ICa in the burst. [Ca2+]i transients were normalized to the maximal Δ[Ca2+]i occurring during the train of voltage steps. Graphs labelled a-c correspond to transients a-c shown in A.
The effects of ryanodine
This discrepancy between ICa and the [Ca2+]i response could occur if the extracellular Ca2+ entry caused Ca2+ release from the SR, i.e. CICR. We therefore next tested this hypothesis using a specific CICR blocker, ryanodine. In these experiments, the interval between successive voltage pulses was set to 500 ms and the number of voltage pulses in a train was restricted to two (paired-pulse stimulation). Typical records are shown in Fig. 4A. Under control conditions, the increment in [Ca2+]i elicited by a second voltage pulse was the same or larger than that evoked by the first pulse, while ICa decreased (the negative staircase, see above). We calculated the ratio between Δ[Ca2+]i and the total Ca2+ entry during each particular voltage pulse by dividing Δ[Ca2+]i by the integral of ICa. This, so-called, unit [Ca2+]i transient should have a constant value if there is no intracellular Ca2+ release and the cytoplasmic Ca2+ buffers are far from saturation. It was, however, found that the unit [Ca2+]i transient in response to a second voltage pulse (UT2) significantly exceeded the first unit transient (UT1). The mean values of control UT1 and UT2 were 33 ± 5.9 and 66 ± 17 nM pC−1, respectively. The difference was statistically significant (P < 0.05, n = 7). In another group of cells, when 5 μm ryanodine was present in the pipette solution the amplitude of the second [Ca2+]i transient was smaller than that of the first. There was also some augmentation of the first [Ca2+]i transient. Accordingly, the difference between UT1 and UT2 became insignificant. This is summarized in Fig. 4B, in which the unit [Ca2+]i transients during paired-pulse stimulation under control conditions and in the presence of ryanodine are compared (n = 7). This finding is consistent with the idea that the initiation of CICR requires an increased Ca2+ concentration in the vicinity of the ryanodine receptors.
Figure 4. The effects of ryanodine on the [Ca2+]i transients elicited by a pair of voltage pulses.
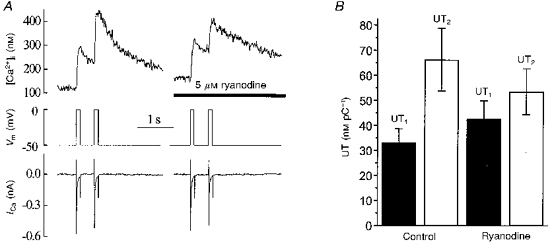
A, traces show (from top to bottom): [Ca2+]i, membrane potential and ICa elicited under control conditions (left) and in the presence of 5 μm ryanodine in the pipette solution (right, different cell). B, ryanodine abolished the difference between the mean value of calculated unit [Ca2+]i transients in response to the first (UT1) and second (UT2) voltage pulses. Values are means ±s.e.m. (n = 7).
The effects of cyclopiazonic acid (CPA)
Given the above results we concluded that the difference in magnitude between the first and subsequent [Ca2+]i transients evoked by trains of depolarizations can be attributed to CICR contributing to the [Ca2+]i elevation during the first train. If this is the case, one would expect that other inhibitors of SR function should also eliminate this difference. We examined this by inhibiting the SR Ca2+-ATPase with CPA. Under these conditions, the SR Ca2+ content will be decreased due to spontaneous leakage and no further Ca2+ release will occur in response to stimulation. We examined the effects of CPA on the [Ca2+]i responses evoked by repetitive stimulation. Typical records obtained are shown in Fig. 5. The application of 10 μm CPA caused an elevation of [Ca2+]i, which comprised both a transient and a sustained component. When two trains of ten voltage pulses were applied in the presence of CPA, the amplitudes of the first and second [Ca2+]i responses were almost identical (see Fig. 5). ICa elicited by ten voltage steps during each train is shown superimposed in the bottom trace (note the different time scale). On average, the amplitudes of the first and second [Ca2+]i responses obtained in the presence of CPA were similar: 357 ± 34 and 313 ± 42 nM, respectively (n = 7). In contrast, under control conditions they differed significantly from each other: 762 ± 116 and 333 ± 72 nM, respectively (n = 17, P < 0.05; compare, e.g. the [Ca2+]i responses in Fig. 3A with those in Fig. 5). It can also be seen that there was no significant difference between the amplitude of the second [Ca2+]i response under control conditions and either the first or second response in the presence of CPA. This result favours the idea that CICR contributes to the [Ca2+]i increase evoked by a train of voltage pulses provided the SR contains enough Ca2+ for release.
Figure 5. The effect of cyclopiazonic acid (CPA) on the [Ca2+]i responses elicited by two trains of ten voltage steps.
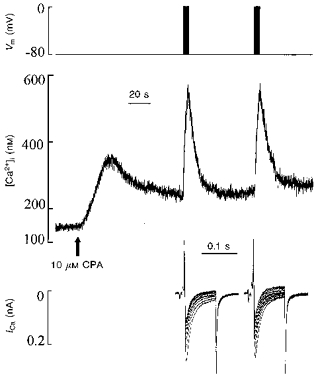
Traces show (from top to bottom): membrane potential, [Ca2+]i and superimposed ICa. The top trace shows the point at which the depolarizing trains were applied. The arrow indicates the beginning of bath application of CPA (10 μm). Note that the long time scale of this figure means that the individual [Ca2+]i transients produced by each depolarization in the train were not resolved.
Refilling of the store
As illustrated in Fig. 3, with trains of voltage pulses, the [Ca2+]i response to the second and subsequent trains under control conditions showed no CICR. These trains of voltage pulses were applied at 1 min intervals, which could be too rapid to allow the refilling of the SR, and hence the [Ca2+] in the SR may have been too low for CICR to occur. If this assumption is true, then CICR should occur in the subsequent trains if a longer period between voltage trains is used to allow store recovery and refilling. This was therefore investigated by allowing a longer period for SR refilling. Typical records obtained in these experiments are illustrated in Fig. 6. The stimulation protocol used consisted of three triplets of voltage trains. Each subsequent train in a triplet was applied when values during the previous train had recovered to baseline. The triplets were separated by a 3 min resting period. As expected, the amplitude of the [Ca2+]i response progressively decreased within the triplet of voltage trains. It is clear, however, that a 3 min resting period was sufficient for partial restoration of the [Ca2+]i response (marked by double-headed arrows). Since the amplitude of ICa did not increase after the 3 min resting period, the augmentation of the [Ca2+]i response observed could be attributed to partial restoration of CICR.
Figure 6. Changes in [Ca2+]i (top) and ICa (bottom) elicited by trains of voltage pulses.
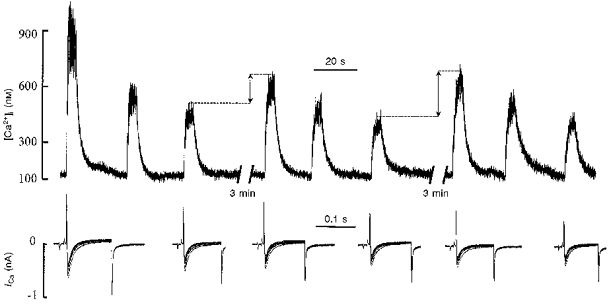
Three trains of ten 100 ms voltage pulses were applied at 30 s intervals. These trains were followed by a 3 min resting period after which the same stimulation protocol was repeated. ICa (superposition of ten traces) corresponding to the first and third trains in each triplet is shown. Note the decrease in the amplitude of the [Ca2+]i response within the triplet of voltage trains and its partial restoration following the 3 min resting period (indicated by double-headed arrows). The magnitude of ICa in each burst showed the ‘negative staircase’ phenomenon.
DISCUSSION
The major aim of the work in this paper was to investigate the mechanisms by which the membrane potential affects [Ca2+]i in myometrial cells and to relate these to the surface membrane ICa and CICR from the SR. Depolarization activated an inward current, which was classified as an L-type current based on its sensitivity to nifedipine and its voltage dependence. The mean maximal ICa density was calculated to be 6.3 ± 0.8 pA pF−1 (n = 10) when depolarization was applied from a holding potential of -80 mV. This value is similar to that reported by Miyoshi et al. (1991) and Inoue & Sperelakis (1991), also in pregnant rat myometrial cells. To date, no data on simultaneous ICa and [Ca2+]i recordings from uterine myocytes under voltage-clamp conditions have been published, though much work has been done on other smooth muscles (Becker et al. 1989; Ganitkevich & Isenberg, 1991; Kamishima & McCarron, 1996).
Mechanism of Ca2+ entry
Our data suggest that the L-type Ca2+ current is the major source of the entry of Ca2+ ions into the uterine smooth muscle cell. This is supported by the following observations: (i) the threshold voltage of [Ca2+]i is the same as that of ICa, (ii) the rise in [Ca2+]i on depolarization is abolished by nifedipine, and (iii) the voltage dependence of the increase in [Ca2+]i is similar to that of ICa. It is not, however, what would be expected from Ca2+ entry via voltage-dependent Na+-Ca2+ exchange as this would increase with further depolarization (Philipson et al. 1982). Comparing the steady-state inactivation and activation characteristics of ICa we found that at a physiological level of membrane potential there is a ‘window current’ flowing into the cell, which might contribute to the maintenance of resting [Ca2+]i.
The effect of repetitive depolarization
In vivo uterine contractions are triggered by trains of action potentials arising from spontaneous, slow wave depolarizations (Parkington & Coleman, 1990). We have simulated this by applying trains of depolarizing pulses. Depolarization of the cell with trains of voltage pulses at 3 Hz frequency resulted in a clear summation of the rise in [Ca2+]i. This summation is presumably due to the fact that the mechanisms which remove Ca2+ from the cell do so at a slower rate than those delivering Ca2+. During the train of voltage pulses we found a discrepancy between pulse-to-pulse changes of ICa and Δ[Ca2+]i, which was attributed to Ca2+ release from the SR caused by Ca2+ entry from the extracellular space (CICR).
Contribution of CICR
We suggest that CICR occurs during the train of voltage steps provided: (i) the SR contains a sufficient amount of Ca2+ for release and, (ii) [Ca2+]i in the vicinity of ryanodine receptors is high enough to initiate their opening. It is likely that the threshold for CICR activation is relatively high in uterine myocytes and that this is the reason why CICR does not occur during the single voltage-clamp pulse but requires more substantial Ca2+ entry, achievable during the repetitive stimulation. The role of increased [Ca2+]i for initiation of CICR was most easily observed during paired-pulse stimulation. In these experiments, we calculated the unit [Ca2+]i transients triggered by ICa and found that the unit [Ca2+]i transient elicited by the second voltage pulse in a pair significantly exceeded that elicited by the first pulse, revealing the contribution of CICR. In the presence of ryanodine, the difference between the first and second unit transients was not significant. These changes in the unit transients are consistent with the known mechanism of ryanodine action. At concentrations lower than 10 μm, ryanodine interacts with SR Ca2+-release channels in a use-dependent manner resulting in the channels being in a permanently open low-conductance state (Rousseau et al. 1987; Bezprozvanny et al. 1991). In accordance with this the augmentation of the first unitary transient and the decrease in the second in the presence of 5 μm ryanodine reflects the long-lasting, though low-conductance, opening of ryanodine receptors. In other words, CICR in the presence of low concentrations of ryanodine is actually not inhibited, but rather it is dissipated and finally disappears upon SR depletion of releasable Ca2+.
One could argue that the difference in the amplitudes of the first and second [Ca2+]i responses elicited by trains of ten voltage steps might be explained by increasing Ca2+-buffering capacity, perhaps as a result of a continuous diffusion of indo-1 into the cell. This possibility can be excluded, however, based on the results of the experiments with CPA and on the partial restoration of decreased [Ca2+]i during a prolonged resting period (see Figs 5 and 6, respectively). When CICR was excluded by inhibition of the SR Ca2+ pump, the magnitudes of the first and second [Ca2+]i responses were identical. This strongly suggests that the difference in magnitude seen under control conditions was due to SR Ca2+ release, and not to an increase in Ca2+ buffering. These data are therefore compatible with the idea that under certain circumstances CICR can contribute to the depolarization-mediated Ca2+ signalling in uterine smooth muscle cells. It should be noted that previous work on uterine strips also found some evidence for CICR in pregnant but, interestingly, not in non-pregnant uterus (Taggart & Wray, 1997).
Physiological significance
Depolarization is one of the most important mechanisms underlying myometrial contractions. Many agonists which alter contractility of the uterus do so by affecting membrane potential (as well as stimulating inositol 1,4,5-trisphosphate production (Marc et al. 1992)). From the present study, it is now clear that the depolarization opens L-type Ca2+ channels in the surface membrane and permits Ca2+ entry. The threshold voltage for both the current and [Ca2+]i increase was found to be between -55 and -50 mV. These values are similar to the reported normal physiological resting potential for rat myometrium (Parkington & Coleman, 1990). This agreement between our studies and those from intact preparations suggests that the single cell data will be applicable to intact preparations. It remains to be established how the presence of agonists will affect the relationship between depolarization, ICa and [Ca2+]i.
Acknowledgments
We are grateful to the MRC for financial support.
References
- Amédée T, Mironneau C, Mironneau J. Isolation and contractile responses of single pregnant rat myometrial cells in short-term primary culture and the effects of pharmacological and electrical stimuli. British Journal of Pharmacology. 1986;88:873–880. doi: 10.1111/j.1476-5381.1986.tb16261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker PL, Singer JJ, Walsh JV, Fay FS. Regulation of calcium concentration in voltage clamped smooth muscle cells. Science. 1989;244:211–214. doi: 10.1126/science.2704996. [DOI] [PubMed] [Google Scholar]
- Benham CD. ATP-activated channels gate calcium entry in single smooth muscle cells dissociated from rabbit ear artery. The Journal of Physiology. 1989;419:689–701. doi: 10.1113/jphysiol.1989.sp017893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Erlich BE. Bell-shaped calcium response curves of Ins(1,4,5)P3-gated and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–752. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Depolarization-mediated intracellular calcium transients in isolated smooth muscle cells of guinea-pig urinary bladder. The Journal of Physiology. 1991;435:187–205. doi: 10.1113/jphysiol.1991.sp018505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Contribution of Ca2+-induced Ca2+ release to the [Ca2+]i transients in myocytes from guinea-pig urinary bladder. The Journal of Physiology. 1992;458:119–137. doi: 10.1113/jphysiol.1992.sp019409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Guerrero A, Singer JJ, Fay RS. Simultaneous measurement of Ca2+ release and influx into smooth muscle cells in response to caffeine. A novel approach for calculating the fraction of current carried by calcium. Journal of General Physiology. 1994;104:395–422. doi: 10.1085/jgp.104.2.395. 10.1085/jgp.104.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E, Amédée T, Martin C, Dacquet C, Mironneau C, Mironneau J. Calcium channel current and its sensitivity to (+)isradipine in cultured pregnant rat myometrial cells. Pflügers Archiv. 1989;414:477–483. doi: 10.1007/BF00585060. [DOI] [PubMed] [Google Scholar]
- Iino M. Calcium induced calcium release mechanism in guinea pig taenia caeci. Journal of General Physiology. 1989;94:363–383. doi: 10.1085/jgp.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y, Nakao K, Okabe K. Some electrical properties of human pregnant myometrium. American Journal of Obstetrics and Gynecology. 1990;162:1090–1098. doi: 10.1016/0002-9378(90)91322-4. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Sperelakis N. Gestational change in Na+ and Ca2+ channel current densities in rat myometrial smooth muscle cells. American Journal of Physiology. 1991;260:C658–663. doi: 10.1152/ajpcell.1991.260.3.C658. [DOI] [PubMed] [Google Scholar]
- Kamishima T, McCarron JG. Depolarization-evoked increases in cytosolic calcium concentration in isolated smooth muscle cells of rat portal vein. The Journal of Physiology. 1996;492:61–74. doi: 10.1113/jphysiol.1996.sp021289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klökner U, Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig) Pflügers Archiv. 1985;405:329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- Lynn S, Morgan JM, Lamb HK, Meissner G, Gillespie JI. Isolation and partial cloning of ryanodine-sensitive Ca2+ release channel protein isoforms from human myometrial smooth muscle. FEBS Letters. 1995;372:6–12. doi: 10.1016/0014-5793(95)00924-x. [DOI] [PubMed] [Google Scholar]
- Marc S, Leiber D, Harbon S. Carbachol and oxytocin stimulate the generation of inositol phosphates in the guinea pig myometrium. FEBS Letters. 1992;201:9–14. doi: 10.1016/0014-5793(86)80561-0. [DOI] [PubMed] [Google Scholar]
- Mironneau J. Excitation-contraction coupling in voltage clamped uterine smooth muscle. The Journal of Physiology. 1973;233:127–141. doi: 10.1113/jphysiol.1973.sp010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H, Urabe T, Fujiwara A. Electrophysiological properties of membrane currents in single myometrial cells isolated from pregnant rats. Pflügers Archiv. 1991;419:386–393. doi: 10.1007/BF00371121. [DOI] [PubMed] [Google Scholar]
- Ohya Y, Sperelakis N. Fast Na+ and slow Ca2+ channels in single uterine muscle cells from pregnant rats. American Journal of Physiology. 1989;257:C409–412. doi: 10.1152/ajpcell.1989.257.2.C408. [DOI] [PubMed] [Google Scholar]
- Parkington HC, Coleman HA. Ionic mechanisms underlying action potentials in myometrium. Clinical andExperimental Pharmacology and Physiology. 1988;15:657–665. doi: 10.1111/j.1440-1681.1988.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Parkington HC, Coleman HA. The role of membrane potential in the control of uterine motility. In: Carsten ME, Miller JD, editors. Uterine Function: Molecular and Cellular Aspects. New York: Plenum Press; 1990. pp. 195–248. [Google Scholar]
- Philipson KD, Bersohn MM, Nishimoto AY. Effects of pH on Na+-Ca2+ exchange in canine cardiac sarcolemmal vesicles. Circulation Research. 1982;50:287–293. doi: 10.1161/01.res.50.2.287. [DOI] [PubMed] [Google Scholar]
- Rousseau E, Smith JS, Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. American Journal of Physiology. 1987;253:C364–368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Shmigol AV, Smith RD, Taggart MJ, Wray S, Eisner DA. Changes of pH affect calcium currents but not outward potassium currents in rat myometrial cells. Pflügers Archiv. 1995;431:135–137. doi: 10.1007/BF00374388. [DOI] [PubMed] [Google Scholar]
- Shmigol AV, Wray S, Eisner DA. Depolarization-induced Ca2+ transients in single smooth muscle cells isolated from pregnant rat uterus. The Journal of Physiology. 1997;505.P:105. doi: 10.1111/j.1469-7793.1998.803bg.x. P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szal SE, Repke JT, Seely EW, Graves SW, Parker CA, Morgan KG. [Ca2+]i signalling in pregnant human myometrium. American Journal of Physiology. 1994;267:E77–87. doi: 10.1152/ajpendo.1994.267.1.E77. [DOI] [PubMed] [Google Scholar]
- Taggart MJ, Burdyga Th, Heaton RC, Wray S. Stimulus-dependent modulation of smooth muscle intracellular calcium and force by altered intracellular pH. Pflügers Archiv. 1996;432:803–811. doi: 10.1007/s004240050202. [DOI] [PubMed] [Google Scholar]
- Taggart MJ, Wray S. Effects of modulators of sarcoplasmic reticular function on intracellular Ca2+ ([Ca2+]i) and force of rat isolated myometrium. The Journal of Physiology. 1997;499.P:8. P. [Google Scholar]
- Wray S. Uterine contraction and physiological mechanisms of modulation. American Journal of Physiology. 1993;264:C1–18. doi: 10.1152/ajpcell.1993.264.1.C1. [DOI] [PubMed] [Google Scholar]


