Abstract
The properties of muscarinic receptor-mediated Ca2+ mobilization were investigated in hippocampal cultures using fluorescent imaging techniques.
Somatic responses to carbachol (1-10 μm) were observed in 21 % of neurones under control conditions (5.4 mM K+, 1.8 mM Ca2+, 0.5-1 μm tetrodotoxin). Smaller responses were observed in Ca2+-free medium.
In cells where responses to carbachol were absent under control conditions, responses were often observed following depolarization with high extracellular K+ (16.2-25 mM). These responses decreased in magnitude with time after the depolarizing episode. Mobilization of Ca2+ from stores using caffeine (50 mM) exhibited similar properties.
Carbachol responses were greatly facilitated in the presence of moderate elevations in extracellular K+ or Ca2+ levels (2- or 3-fold, respectively). These conditions were usually, but not always, associated with a small increase in cytosolic Ca2+ levels (< 50 nM).
Muscarinic responses in 10.8 mM K+ were inhibited by 80–95 % in the presence of the L-type voltage-gated Ca2+ channel antagonists nitrendipine (2-5 μm) or nifedipine (10 μm). Depletion of intracellular Ca2+ stores with thapsigargin (2-10 μm) blocked responses.
Oscillatory Ca2+ mobilizing responses were observed in some cells. Their expression was facilitated by moderate cytosolic Ca2+ elevations and by increasing the duration of carbachol exposure.
Ca2+ mobilizing responses were also observed in dendritic regions. These were smaller than somatic responses, but had faster decay kinetics.
In conclusion, muscarinic receptor-mediated Ca2+ mobilization in cultured hippocampal neurones shows a strong Ca2+ dependence. Moderate intracellular Ca2+ rises greatly facilitate muscarinic responses and uncover, in some cells, oscillatory Ca2+ mobilization. These effects appear to reflect the loading state of intracellular Ca2+ stores.
The mobilization of Ca2+ from intracellular stores by activation of G-protein-linked (metabotropic) neurotransmitter receptors is an essential cell signalling mechanism. This process generally involves stimulation of phosphoinositide-specific phospholipase C (PLC) and the concomitant formation of inositol 1,4,5-trisphosphate (IP3), which binds to specific receptor-operated Ca2+ channels on intracellular vesicles (Berridge, 1993). The neurotransmitter acetylcholine can mobilize intracellular Ca2+ through activation of muscarinic receptors (Wakamori et al. 1993; Seymour-Laurent & Barish, 1995). To date five different types of muscarinic receptor (M1-M5) have been identified. In the brain, M1 and M3 receptors are thought to couple to phosphoinositide (PI) turnover (Hulme et al. 1990). A pharmacologically defined M3-like receptor is reported to mediate Ca2+ mobilization in freshly dissociated hippocampal neurones (Wakamori et al. 1993).
Many components of the inositol phosphate signalling pathway have the potential to be modulated by physiological changes in intracellular Ca2+ levels. In cultured cerebellar granule cells we have shown previously that metabotropic glutamate (mGlu), but not muscarinic, receptor-mediated Ca2+ mobilization is Ca2+ dependent (Irving et al. 1992a), possibly reflecting a Ca2+ dependence in mGlu receptor-mediated PLC activation (Eberhard & Holz, 1988). Inositol polyphosphate formation induced by both of these receptor systems is sensitive to changes in Ca2+ levels in neonatal cerebral cortical slices (Challiss et al. 1994a, b). The IP3 receptor itself can also be regulated by cytosolic Ca2+ (Bezprozvanny et al. 1991). However, the precise role of intracellular Ca2+ levels in regulating metabotropic receptor-mediated Ca2+ mobilization in hippocampal neurones remains to be determined.
In non-excitable tissue, metabotropic receptor-mediated mobilization of intracellular Ca2+ stores is commonly associated with oscillatory Ca2+ signals. Such activity is thought to be important for the selective activation of particular Ca2+-dependent processes inside the cell (Goldbeter et al. 1990). However, there is only limited evidence that metabotropic receptor activation is able to activate oscillatory Ca2+ mobilization in mammalian neurones (Murphy & Miller, 1989; Reynolds & Miller, 1989; Crawford et al. 1997).
In the present study we have used fluorescence imaging techniques to investigate further the properties of muscarinic receptor-mediated Ca2+ mobilization from intracellular stores in cultured hippocampal neurones. Specifically, we have investigated the Ca2+ dependence, oscillatory nature and localization of Ca2+ mobilizing responses induced by the cholinergic agonist carbachol (Hulme et al. 1990).
METHODS
Cell culture
Cultures of rat hippocampal neurones were prepared as described previously (Richmond et al. 1996). Briefly, rat pups (1-3 days old) were killed by cervical dislocation, and the hippocampus was subsequently removed and cut into 500 μm slices. These were washed in Hepes-buffered saline of the following composition (mM): NaCl, 130; Hepes, 10; KCl, 5.4; CaCl2, 1.8; MgCl2, 1.0; D-glucose, 25 (at pH 7.4). The slices were then treated with a mixture of pronase E and protease type X (both at 0.5 mg ml−1) for 30 min. The tissue was dissociated by trituration, washed, centifuged, then plated on to coverslips that had been pre-treated with poly-L-lysine (20 mg ml−1 for 3 h). Cultures were then incubated at 37°C in medium consisting of 90 % minimal essential medium, 10 % dialysed fetal bovine serum and 2 mM L-glutamine. Cells were maintained in a humidified atmosphere of 5 % CO2 in air at 37°C for up to 6 weeks. After 3–5 days in culture, cytosine arabinofuranoside (final concentration, 5 μm) was added to inhibit glial cell proliferation. Cells were studied between 6 and 36 days in culture.
Dye loading and subsequent experiments were performed in Hepes-buffered saline at room temperature (20-25°C). Coverslips with attached cells were loaded with the Ca2+-sensitive dye indo-1 AM (4-6 μm; 45 min), or in a few cases fura-2 AM (6 μm; 60 min). In order to block indirect actions of metabotropic receptor activation through effects on synaptically driven Ca2+ transients (Irving & Collingridge, 1995), all experiments were performed in the presence of tetrodotoxin (0.5-1 μm). Compounds were applied directly to the perfusate (1.5 ml min−1). All data were obtained from neurones, which were identified by their morphological and functional characteristics. Responses to carbachol were primarily observed in medium to large diameter neurones (> 12 μm at the soma). Experiments were performed on at least two sets of cultures obtained from different rats; up to ten neurones were analysed per experiment. Unless otherwise indicated data were compared using Student's paired t test.
Microscopy and data analysis
In most experiments a laser-scanning confocal imaging system (MRC Bio-Rad 600/UV) connected to a Zeiss Axiovert microscope (× 40, 1.3 n.a., oil immersion objective; or a × 40, 1.2 n.a., water immersion objective) was used for image acquisition and processing. Indo-1 was excited using a 351 nm line. Twin photomultiplier channels detected bands of fluorescence centred on 405 and 485 nm. Ratiometric images were usually collected at 5–20 s intervals; this was increased to 1–2 s intervals during muscarinic responses. Data were analysed both on- and off-line using COMOS TCSM software (Bio-Rad). Ratio values were calculated for each pixel in the frame for fluorescence intensities above a threshold. Numerical data were derived from somatic measurements unless otherwise indicated. In some experiments the ratio values have been converted to estimated measurements of [Ca2+]i using a method similar to that of Phenna et al. (1995) and the equations of Grynkiewicz et al. (1985). An apparent Kd value of indo-1 for Ca2+ of 236 nM was used (Phenna et al. 1995). The calibration was performed in situ, where Rmax, Rmin (the maximum and minimum signal ratios) and β (the ratio of the 485 nm signal under minimal and saturation Ca2+ conditions) values were determined using solutions containing a Ca2+ ionophore (10 μm ionomycin) with either nominally Ca2+-free saline plus 1 mM EGTA or 1.8 mM Ca2+. Due to the uncertainties associated with the accurate calibration of Ca2+ levels in cells the majority of data are presented as changes in fluorescence ratio rather than intracellular Ca2+ concentrations. In a few experiments, including all those involving caffeine exposure, Ca2+ measurements were made using a standard, conventional imaging system (Improvision, Coventry, UK) and the Ca2+-sensitive dye fura-2.
Materials
Nitrendipine was obtained from Tocris Cookson; carbachol, cytosine arabinoside, dialysed fetal bovine serum, EGTA, glutamine, Hepes, indo-1 AM, ionomycin, nifedipine, pirenzipine, poly-L-lysine, pronase E, protease type X, tetrodotoxin and thapsigargin were purchased from the Sigma Chemical Company; minimal essential medium (MEM) was purchased from Gibco.
RESULTS
Carbachol responses under control conditions
Under control conditions (5.4 mM K+, 1.8 mM Ca2+) a small proportion of neurones (21 %; 20/94 cells) exhibited intracellular Ca2+ transients on exposure to carbachol (10 μm; 90 s duration; Fig. 1). A proportion of these cells (5/21) exhibited oscillatory responses and the oscillations were not synchronized between cells (Fig. 1). Basal cytosolic Ca2+ levels ranged from 40 to 160 nM, with no significant difference between the resting Ca2+ levels of responsive and non-responsive cells.
Figure 1. Effects of Ca2+-free medium on carbachol-induced Ca2+ elevations in cultured hippocampal neurones.
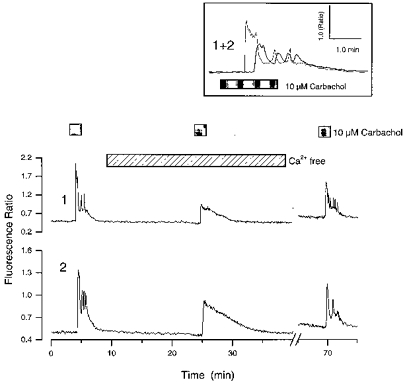
The traces show changes in the indo-1 fluorescence ratio with time from the somas of 2 cultured hippocampal neurones from the same experiment. The cells were exposed to sequential applications of carbachol; the second exposure was made in the presence of nominally Ca2+-free medium. Note the asynchronous, oscillatory activity in normal medium (inset) and the inhibition of the peak response in Ca2+-free medium. The gap in the trace indicates a period where the experiment was paused. Compounds were applied for the times indicated by the bars above the graphs.
In order to determine whether the cholinergic responses involved Ca2+ mobilization, the ability of carbachol to elevate Ca2+ in the presence of nominally Ca2+-free extracellular medium was investigated. Under these conditions responses to carbachol (10 μm) were observed in 10/12 cells that responded in control medium; however, they were often smaller in amplitude and were not oscillatory (Fig. 1). Where only cells that responded to carbachol in control medium were analysed, the mean peak intracellular Ca2+ rise induced by carbachol under control conditions was 284 ± 86 nM (mean ±s.e.m.), whereas in the absence of extracellular Ca2+ it was 75 ± 20 nM (P < 0.01; 10 cells).
Effects of prior depolarization
It has been reported previously in cultured hippocampal, nucleus cuneatus and neocortical neurones that caffeine-sensitive intracellular Ca2+ stores are empty at rest, but can be loaded by membrane depolarization (Shmigol et al. 1994). However, in other types of neuronal culture, including cerebellar granule cells (Irving et al. 1992a; Simpson et al. 1996) and sensory neurones (Thayer et al. 1988; Shmigol et al. 1994), Ca2+ mobilizing responses are present under control conditions, suggesting that their stores are sufficiently loaded to allow Ca2+ release. It was therefore investigated whether the low incidence of carbachol responses observed under these conditions reflected functionally depleted intracellular Ca2+ stores. In twenty cells that failed to respond to carbachol (10 μm) at rest, responses were observed following depolarization with high extracellular K+ (16.2-25 mM; applied for 1.5-2 min; Fig. 2A). The magnitude of the agonist-evoked responses was inversely related to the time between membrane depolarization and exposure to agonist (Fig. 2A), which is consistent with the spontaneous discharging of intracellular Ca2+ stores following removal of a depolarizing stimulus (Shmigol et al. 1994; Garaschuk et al. 1997). The rate of run-down varied between cells: in 6/12 cells studied responses to carbachol could only be obtained within 6 min of removal of the high K+-containing medium, whereas in the remaining cells responses persisted for up to 12 min following the depolarizing episode. In cells where responses to carbachol ran down slowly, the initial effect of increasing the time interval separating depolarization from carbachol exposure was often a shortening of the duration of the response to carbachol with little or no effect on the peak (Fig. 2A). In some cases an oscillatory response was observed immediately following the depolarizing episode, but not with longer periods of washout (Fig. 2B).
Figure 2. Facilitation of carbachol and caffeine responses following high K+ depolarization.
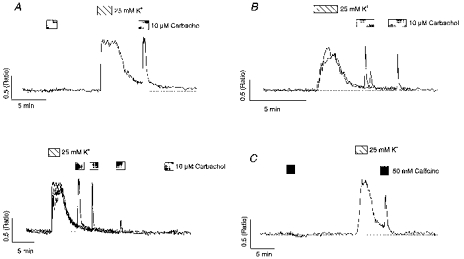
A (upper panel), the cell was exposed to carbachol prior to and following depolarization with 25 mM K+. A (lower panel) illustrates 4 overlaid traces where carbachol was applied at various time intervals following K+ depolarization in the same cell. B, 2 overlaid traces from a cell where carbachol was applied 1.5 or 4 min following depolarization with 25 mM K+. Note the oscillatory response with the shorter washout time. C, the cell was exposed to caffeine prior to and following depolarization with 25 mM K+.
It was noted that carbachol responses were enhanced maximally when the agonist was applied within a very short period of K+ depolarization, often when intracellular Ca2+ levels were above resting values. To investigate whether this residual Ca2+ elevation exerted a direct action on the transduction pathway linking carbachol with Ca2+ release (Bezprozvanny et al. 1991; Irving et al. 1992a), the properties of carbachol responses were compared with activation of a different Ca2+ mobilizing pathway, using a high concentration (50 mM) of caffeine. At low doses (0.5-2 mM) caffeine indirectly mobilizes Ca2+ by sensitizing the Ca2+-induced Ca2+-release channel to Ca2+; however, at higher concentrations (> 5 mM) it directly activates the channel (Sitsapesan & Williams, 1990). Consistent with the findings of Shmigol et al. (1994), responses to caffeine were also dependent on prior depolarization in the majority of neurones tested (Fig. 2C). In fourteen cells caffeine responses were absent under control conditions, but could be elicited following a brief period of depolarization with high K+. Furthermore, as with carbachol responses, the magnitude of responses to caffeine decreased as the time interval separating them from the depolarizing episode increased (not illustrated). These data indicate that with hippocampal neurones the loading state of intracellular stores is the primary factor in determining the magnitude of responses to Ca2+ mobilizing agonists.
Facilitation of carbachol-induced Ca2+ mobilization by moderate extracellular cation elevations
More modest conditions for the enhancement of carbachol responses were investigated. A doubling of the extracellular K+ concentration (5.4 to 10.8 mM) greatly enhanced responses to carbachol when applied in its continued presence (Fig. 3A and B). These conditions were usually associated with a small intracellular Ca2+ rise (33 ± 11 nM). Overall, the proportion of neurones responding to carbachol (applied for 90 s) increased from 19 to 40 % when the K+ concentration was doubled (58 cells where both conditions were tested). The facilitation of muscarinic responses was lost rapidly on return to normal K+-containing medium (Fig. 3A).
Figure 3. Enhancement of carbachol responses in the presence of modest elevations in extracellular cation levels.
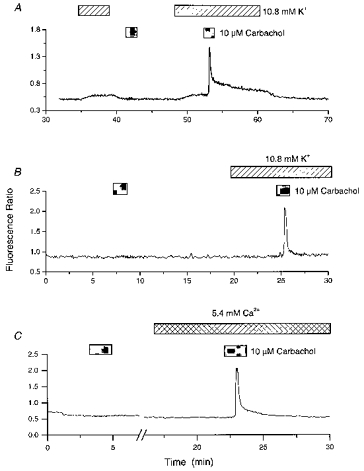
A, carbachol was applied following or in the presence of a moderate elevation in the extracellular K+ concentration (10.8 mM). Responses to carbachol were observed when combined with, but not preceded by, exposure to 10.8 mM K+. B, carbachol was applied in the absence and presence of 10.8 mM K+. C, the cell was exposed to carbachol in the presence of normal extracellular Ca2+ and K+ (1.8 and 5.4 mM, respectively) and then in the presence of high extracellular Ca2+ (5.4 mM). Note the absence of intracellular Ca2+ rises in the presence of 10.8 mM K+ in B and 5.4 mM Ca2+ in C.
Cells that responded to carbachol under control conditions, without prior depolarization, often generated larger responses in the presence of 10.8 mM K+. Where only cells that responded under both conditions were analysed, the mean peak response to carbachol in the presence of 5.4 mM K+-containing medium was 164 ± 49 nM, whereas in the presence of 10.8 mM K+ it was 306 ± 76 nM (P < 0.05; 11 cells). Furthermore, the latency between agonist exposure and response was often reduced in the presence of elevated extracellular K+, for example in four cells the time from the start of carbachol perfusion to the peak of the response was 96 ± 8 s in 5.4 mM K+ and 56 ± 8 s in 10.8 mM K+ (these values include a dead space time of approximately 40 s; P < 0.05).
In some cases treatment with 10.8 mM K+ had no detectable effect on cytosolic Ca2+ levels but still facilitated responses to carbachol when applied in its presence (Fig. 3B), suggesting that the enhancement is not simply due to increased cytosolic Ca2+ levels per se. However, such conditions could permit the loading of intracellular Ca2+ stores by increasing the rate of Ca2+ flux across the plasma membrane. Treatment of cells with high extracellular Ca2+ (5.4 mM) also facilitated carbachol responses while also having little or no effect on intracellular Ca2+ levels (Fig. 3C). In the presence of 5.4 mM Ca2+ intracellular Ca2+ levels were raised by only 10 ± 10 nM. The pooled, calibrated data from eighty-eight cells showing the effects of different extracellular K+ and Ca2+ concentrations on intracellular Ca2+ levels and Ca2+ mobilizing responses to carbachol are presented in Fig. 4.
Figure 4. The relationship between extracellular cation levels, the intracellular Ca2+ concentration and responses to carbachol (CCh).
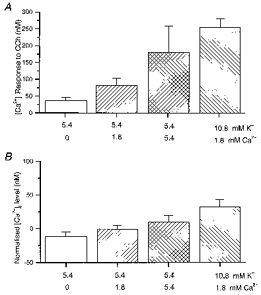
A, mean calibrated change in [Ca2+]i on exposure to carbachol (10 μm; 90 s) for a range of extracellular Ca2+ and K+ concentrations. B, normalized intracellular Ca2+ level (relative to that in 5.4 mM K+, 1.8 mM Ca2+) at the time of the carbachol exposure under the various conditions. Pooled data were obtained from experiments where carbachol was applied under at least 2 separate conditions, without prior depolarization, and includes all cells that were responsive to carbachol.
Exposure of cells to carbachol in the presence of 10.8 mM K+ allowed reproducible responses to be obtained without the problems associated with the gradual run-down of responses following high K+ depolarization. Such conditions were therefore used to characterize carbachol responses in more detail. The involvement of muscarinic receptors in mediating the enhanced responses to carbachol in the presence of 10.8 mM K+ was verified using the muscarinic antagonist pirenzipine. Responses to carbachol (1-10 μm) were abolished in its presence (3 μm; 9 cells; data not shown). It is unlikely that nicotinic receptor activation contributed to the observed responses as nicotine (50-100 μm) did not elevate Ca2+ in the presence of tetrodotoxin. Furthermore, selective activation of another G-protein-linked receptor system with mGlu receptor agonists exhibited similar properties to carbachol (data not shown).
Oscillatory carbachol responses in elevated K+
The expression of oscillatory responses to carbachol were also enhanced in the presence of 10.8 mM K+. Under control conditions, without prior depolarization, 24 % of responses to a brief period (90 s) of carbachol exposure were oscillatory (5/23 responses), whereas in the presence of 10.8 mM K+ the proportion was increased to 40 % (15/37 responses). Moreover, in 3/5 cells that showed a single spike Ca2+ elevation in response to carbachol under control conditions, an oscillatory response was observed in the presence of 10.8 mM K+ (Fig. 5A).
Figure 5. Effects of 10.8 mM K+ on the duration of carbachol responses and oscillatory activity.
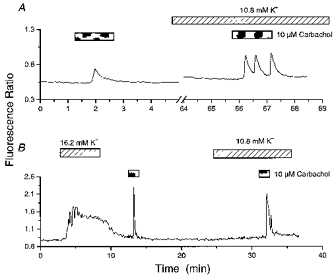
A shows a cell that generated a single spike in response to carbachol in 5.4 mM K+, but exhibited an oscillatory response in 10.8 mM K+. B, a cell was exposed to carbachol following a period of high K+ (16.2 mM) exposure and then in the presence of a moderate elevation in extracellular K+ levels (10.8 mM). The cell depicted failed to respond under control conditions (not illustrated). Note the broadening of the carbachol response in 10.8 mM K+.
A comparison of carbachol responses following depolarization with 16.2-25 mM K+ with those in the presence of 10.8 mM K+ showed some differences. Responses to carbachol in the presence of 10.8 mM K+ were more likely to be oscillatory and were of a longer duration (Fig. 5B). The time for the initial peak response to decay by 50 % in 10.8 mM K+ was 43 ± 1 s, whereas following depolarization with high K+ (16.2 or 25 mM; 2–4 min between depolarization and carbachol exposure) it was 10 ± 2 s (P < 0.05; 7 cells).
Effects of thapsigargin
To investigate whether the enhanced muscarinic responses in 10.8 mM K+ involved Ca2+ mobilization we used the compound thapsigargin, which depletes intracellular Ca2+ stores by inhibition of the sarcoplasmic/endoplasmic Ca2+ ATPase (SERCA) (Law et al. 1990) and prevents Ca2+ mobilization following their spontaneous emptying (Irving et al. 1992a). In the presence of 10.8 mM K+, treatment of cells with thapsigargin (1-5 μm; 5–10 min) either abolished or substantially reduced subsequent responses to carbachol (Fig. 6A) in young (1-2 week old) cultures. In most instances where thapsigargin was applied in 10.8 mM K+-containing medium, carbachol responses were blocked without an associated increase in cytosolic Ca2+ levels, suggesting that any thapsigargin-induced net efflux of Ca2+ from stores is rapidly cleared from the cytosol. In some cells, particularly in older cultures, higher doses of thapsigargin (10-20 μm; 5–10 min) were required to abolish completely metabotropic receptor responses (data not shown). Thapsigargin has been reported to inhibit voltage-gated Ca2+ channel (VGCC) activity (Rossier et al. 1993); however, such an action remains to be demonstrated in central neurones (Simpson et al. 1995). In the present investigation thapsigargin had no obvious inhibitory action on VGCC-mediated Ca2+ elevations at the doses used: exposure of cells to thapsigargin (2-10 μm) in the presence of 25 mM K+ often increased intracellular Ca2+ levels but never decreased them (Fig. 6B; see also Irving et al. 1992a). The uncovering of thapsigargin-mediated Ca2+ elevations under these conditions may reflect enhanced Ca2+ store loading and/or partial saturation of Ca2+ buffering mechanisms.
Figure 6. Thapsigargin blocks carbachol responses.
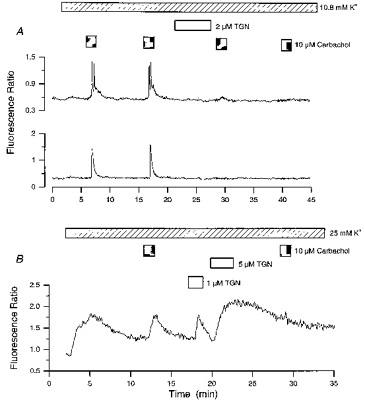
A, 2 cells (8 days in culture) in the same field were exposed to successive applications of carbachol in the presence of 10.8 mM K+. Cells were exposed to thapsigargin (TGN; 6 min) between the second and third carbachol application. Note that the thapsigargin-mediated inhibition of responses was not associated with an increase in cytosolic Ca2+ levels. B, a cell was exposed to 2 applications of carbachol in the presence of 25 mM K+. Thapsigargin was applied after the first carbachol exposure. Note the thapsigargin-induced Ca2+ elevations.
Role of L-type voltage gated Ca2+ channels
It was determined whether the ability of elevated extracellular K+ to enhance responses to carbachol was dependent on Ca2+ influx through L-type VGCCs. Responses to carbachol (10 μm) in 10.8 mM K+ were strongly inhibited in the presence of the L-type Ca2+ channel antagonists nifedipine (10 μm; 83 ± 9 % inhibition; n = 9; P < 0.01; Fig. 7A) or nitrendipine (5 μm; 95 ± 5 % inhibition; n = 5; P < 0.01; not illustrated). Conversely, in the presence of the selective L-type Ca2+ channel agonist Bay K 8644 (0.5 μm; 5.4 mM K+) responses to carbachol were enhanced (Fig. 7B). Exposure of cells to nifedipine or nitrendipine in the presence of 10.8 mM K+ lowered intracellular Ca2+ levels to near-control values. Exposure of cells to Bay K 8644 in control medium was associated with a moderate increase in cytosolic Ca2+ levels.
Figure 7. Dependence of carbachol responses on Ca2+ influx through L-type VGCCs.
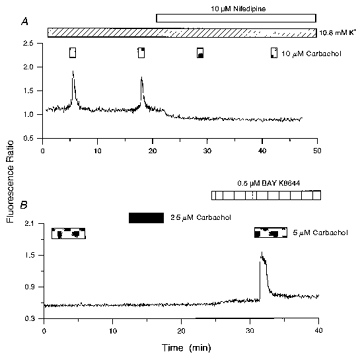
A, the cell was exposed to 4 successive applications of carbachol (CCh) in the presence of 10.8 mM K+. The third and fourth applications were made in the presence of nifedipine. B, carbachol was applied in normal K+-containing medium, and in the presence of 0.5 μm Bay K 8644. Note that increasing the dose of agonist failed to elicit a response under control conditions.
Carbachol-induced oscillations during prolonged agonist exposure
Neurones that displayed a single spike response to brief application (90 s) of carbachol in 10.8 mM K+ were often able to sustain oscillatory activity during prolonged exposure to the agonist (15 min; Fig. 8A). In addition, cells that showed oscillations during a brief agonist exposure exhibited a greater number of oscillations during prolonged agonist application. Of nineteen cells that responded to a brief application of carbachol (10 μm; 90 s; 10.8 mM K+) with a single Ca2+ transient, six cells exhibited Ca2+ oscillations (2 or more spikes) during prolonged agonist exposure (6-20 min). The pattern of oscillatory activity varied between cells, both in terms of frequency of oscillation (0.4-5 Hz) and rate of run-down of responses. This oscillatory activity was usually superimposed on top of a sustained elevation in intraneuronal Ca2+ levels. The majority of non-oscillatory cells also exhibited a sustained Ca2+ elevation during prolonged agonist exposure which was often, but not always, preceded by a Ca2+ transient. Of thirteen non-oscillatory cells, seven exhibited an initial Ca2+ transient followed by a sustained Ca2+ elevation and three displayed only a sustained elevation in intraneuronal Ca2+ levels (Fig. 8B).
Figure 8. Metabotropic receptor-mediated Ca2+ responses during prolonged agonist exposure.
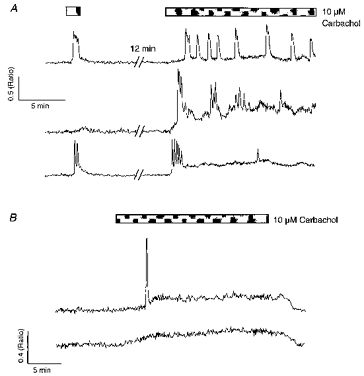
A, 3 cells in 10.8 mM K+ were exposed to a brief (1.5 min) application of carbachol and then to a prolonged period of agonist exposure (15 min). The upper cells only showed oscillations during prolonged exposure. The lower cell showed 2 oscillations during the brief exposure and a burst of 5, rapidly desensitizing oscillations during prolonged carbachol application. B shows 2 non-oscillatory cells exhibiting different types of response to prolonged carbachol exposure in 10.8 mM K+. The upper cell showed an initial Ca2+ transient followed by a sustained Ca2+ plateau; the lower cell displayed only a sustained Ca2+ elevation.
Localization of Ca2+-mobilizing responses
The spatial localization of responses to carbachol was investigated. Carbachol raised intracellular Ca2+ levels throughout the somatodendritic compartment, but larger responses were observed in the soma (Fig. 9). Oscillatory and single spike responses had a similar spatial profile. By scaling responses in large dendrites and soma it was apparent that the Ca2+ signals associated with Ca2+ mobilization in these regions had different decay kinetics (Fig. 9). In five cells where the initial response to carbachol in 10.8 mM K+ was analysed, the mean time for the peak somal Ca2+ signal to decay by 50 % was 21 ± 4 s, whereas the dendritic Ca2+ signal decayed in 13 ± 4 s (P < 0.01). It was also noted that dendritic basal Ca2+ levels were often lower than those of the soma.
Figure 9. Localization of Ca2+-mobilizing responses.
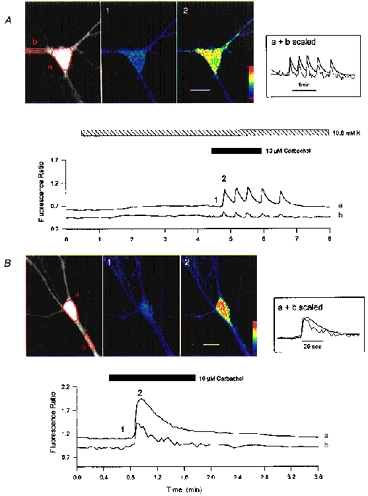
A and B illustrate 2 cells exposed to carbachol in the presence of 10.8 mM K+. A showed an oscillatory response to carbachol; note the small elevation in the fluorescence ratio on perfusion with 10.8 mM K+. B is a cell that exhibited a single Ca2+ spike response to carbachol. Traces from somatic (a) and selected dendritic (b) regions and pseudocoloured Ca2+ images at times indicated by 1 and 2 are illustrated. Regions of interest are defined in the left-hand, single emission wavelength image (485 nm). Insets show scaled somatic and dendritic responses (matching peaks); note the broadening of the somatic Ca2+ signal. The pseudocolour bars are 0–2 ratio units in A and 0-2.5 ratio units in B. The scale bars are 20 μm.
DISCUSSION
Ca2+ mobilization induced by carbachol or caffeine was facilitated by conditions that functionally charge intracellular Ca2+ stores. These observations are in broad agreement with previous studies on metabotropic (Murphy & Miller, 1989) and caffeine (Shmigol et al. 1994; Garaschuk et al. 1997) responses.
The absence of responses to Ca2+ mobilizing agonists under control conditions does not necessarily mean that the Ca2+ stores are physically empty, as the endoplasmic reticulum luminal Ca2+ concentration may affect the sensitivity of Ca2+ stores to caffeine (Shmigol et al. 1996) and to IP3 (Missiaen et al. 1992). In addition, an apparent absence of Ca2+ mobilizing responses may result from low rates of release from stores that are rapidly excluded from the cytosol (Garaschuk et al. 1997).
In the present investigation the Ca2+ stores spontaneously depleted following removal of a store-loading stimulus. This run-down of responses was removed by exposure to carbachol in the presence of a modest elevation of extracellular cation levels. Such conditions allowed reproducible responses to be obtained without intervening periods of strong depolarization as used in previous protocols to investigate Ca2+ mobilizing responses (Murphy & Miller, 1989; Irving et al. 1992b). The primary effect of this new protocol is presumably to charge intracellular Ca2+ stores, given that they are functionally empty at rest. Part of the facilitation of carbachol responses by raised cytosolic Ca2+ levels may, however, involve effects of Ca2+ on the IP3 receptor (Bezprozvanny et al. 1991) and/or other aspects of the signal transduction mechanism (Irving et al. 1992a). The reduction in the response latency to carbachol in elevated K+ relative to that in control medium observed in the present study may indicate higher levels of IP3 formation (Carter & Ogden, 1997).
Loading Ca2+ stores via activation of L-type Ca2+ channels
The low apparent Ca2+ content of intracellular stores observed in the majority of hippocampal neurones under control conditions is not consistent with the presence of a ‘capacative’ Ca2+ influx pathway (Putney, 1986), where the physical emptiness of the stores would be expected to trigger refilling. Rather, the Ca2+ stores are charged by depolarization, which stimulates Ca2+ influx through VGCCs. Carbachol responses were highly dependent on Ca2+ influx through L-type channels, suggesting that IP3-sensitive Ca2+ stores are loaded by this pathway. It has been reported previously that caffeine-sensitive Ca2+ stores can be ‘overloaded’ by Ca2+ influx through VGCCs during depolarization; however, L-type Ca2+ channels were not implicated in this effect (Garaschuk et al. 1997). A linkage between L-type Ca2+ channels and mGlu, but not muscarinic, receptors has been suggested to exist in cerebellar granule cells (Irving et al. 1992a; Chavis et al. 1996); however, in these studies they were not directly implicated in loading Ca2+ stores.
In a small proportion of cells carbachol responses were observed under control conditions, indicating that some cells have functionally loaded Ca2+ stores at rest. Such effects may reflect differences in basal levels of Ca2+ influx between cells. It has been reported by Magee et al. (1996) that a population of dihydropyridine-sensitive Ca2+ channels are active at resting membrane potentials in hippocampal neurones. Such activity could provide a pathway for loading Ca2+ stores under these conditions. The present data are consistent with the existence of basal L-type Ca2+ channel activity, given that Bay K 8644 enhances activity by affecting the mode of channel gating (Nowycky et al. 1985) and that Bay K 8644 elevated Ca2+ levels in the absence of depolarization.
The present results can be explained by a simple scheme (Fig. 10) whereby the loading state of the intracellular Ca2+ stores is regulated dynamically by the membrane potential of the neurone. Under control conditions, most cells did not respond to carbachol, suggesting that the stores were functionally ‘empty’. The model assumes that the loading state is governed by the net flux of Ca2+ across the store walls which is, in turn, determined by the activity of the release channels and the Ca2+ pump. As neurones depolarize, more Ca2+ enters through L-type Ca2+ channels and this accelerates store loading by driving the pump. For modest depolarization the Ca2+ pumps (store and plasma membrane) can effectively handle the increase in Ca2+ entering the cell and so luminal Ca2+ is elevated but the cytoplasmic Ca2+ concentration does not necessarily rise. The ability of carbachol to mobilize Ca2+ is then simply related to the level of Ca2+ contained within the stores. The effects of thapsigargin are consistent with this scheme: the absence of a thapsigargin-induced increase in cytosolic Ca2+ would only require that the level of Ca2+ leak is sufficiently low that it is effectively handled by the Ca2+ buffering capacity of the plasma membrane and cytosol. In the present scheme, L-type Ca2+ channels are shown as the important source of external Ca2+ based on the potent effects of Bay K 8644, nifedipine and nitrendipine. However, hippocampal neurones contain a plethora of channels, both voltage and ligand gated, which can elevate cytosolic Ca2+ and a role for certain of these under different conditions cannot be excluded.
Figure 10. Scheme illustrating the effects of depolarization on IP3-sensitive Ca2+ stores in cultured hippocampal neurones.
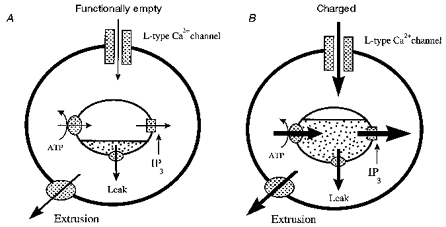
A, at rest, the Ca2+ stores are functionally empty, with little or no release in response to IP3 formation. B, in the presence of a modest depolarization Ca2+ influx via L-type VGCCs loads the Ca2+ stores and allows a strong Ca2+ signal on activation of IP3 receptors. See text for further details.2
Oscillatory Ca2+ mobilization
In the present study we observed oscillatory Ca2+ mobilization, which could be modulated by both intracellular Ca2+ levels and the duration of carbachol exposure. One previous report (Murphy & Miller, 1989) has indicated that mGlu and adrenergic receptor activation could induce oscillatory Ca2+ mobilization in a proportion of cultured hippocampal neurones. In light of the present data, the ability of raised cytosolic Ca2+ levels to facilitate oscillatory activity is likely to reflect the ability of such conditions to continuously replenish intracellular Ca2+ stores. However, other effects, such as the ability of elevated Ca2+ to enhance the generation of IP3 and alter the sensitivity of the IP3 receptor itself (Bezprozvanny et al. 1991), may also have a role.
Spatial localization of metabotropic responses
Ca2+ mobilizing responses were preferentially associated with the somatic compartment, with smaller responses, but faster decay kinetics, observed in dendritic regions. Given that the plasma membrane is an important regulator of cytosolic Ca2+ levels, differences in the rates of decay between the two compartments may simply result from differences in their respective surface area to volume ratios. The proportionately larger somatic signal may be due to enhanced Ca2+ mobilization associated with perinuclear regions, as reported previously using acutely dissociated hippocampal neurones (Phenna et al. 1995).
The observation that dendritic Ca2+ levels were in general regulated at a lower level than those of the soma is consistent with the findings of Segal & Manor (1992) using fluo-3. However, these observations must be treated with caution given the potential for compartmentalization of dye during ester loading (Connor, 1993), for contributions from presynaptic elements which are proportionately larger in dendritic regions and for axial chromatic aberrations associated with confocal imaging of indo-1 (Niggli et al. 1994).
Physiological implications
The facilitation of metabotropic receptor-induced Ca2+ mobilization by Ca2+ influx could enable these receptors to act as coincidence detectors, whereby their activation is enabled by membrane depolarization. Such properties might underlie the role of these receptors in synaptogenesis and synaptic plasticity as discussed previously (Irving et al. 1992a; Bliss & Collingridge, 1993).
Acknowledgments
The authors are grateful to the Wellcome Trust, grants 034404 and 047368, for financial support.
References
- Berridge MJ. Inositol triphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Bliss T, Collingridge G. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Carter TD, Ogden D. Kinetics of Ca2+ release by InsP3 in pig single aortic endothelial cells: evidence for an inhibitory role of cytosolic Ca2+ in regulating hormonally evoked Ca2+ spikes. The Journal of Physiology. 1997;504:17–33. doi: 10.1111/j.1469-7793.1997.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challiss RAJ, Mistry R, Gray DW, Nahorski SR. Modulation of muscarinic cholinoceptor-stimulated inositol 1,4,5-trisphosphate accumulation by N-methyl-D-aspartate in neonatal rat cerebral cortex. Neuropharmacology. 1994a;33:15–25. doi: 10.1016/0028-3908(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Challiss RAJ, Mistry R, Gray DW, Nahorski SR. Modulatory effects of NMDA on phosphoinositide responses evoked by the metabotropic glutamate receptor agonist 1S,3R-ACPD in neonatal rat cerebral cortex. British Journal of Pharmacology. 1994b;112:231–239. doi: 10.1111/j.1476-5381.1994.tb13057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavis P, Fagni L, Lansman JB, Bockaert J. Functional coupling between ryanodine receptors and L-type calcium channels in neurons. Nature. 1996;382:719–722. doi: 10.1038/382719a0. [DOI] [PubMed] [Google Scholar]
- Connor JA. Intracellular Ca2+ mobilisation by inositol 1,4,5-trisphosphate: intracellular movements and compartmentalisation. Cell Calcium. 1993;14:185–200. doi: 10.1016/0143-4160(93)90066-f. [DOI] [PubMed] [Google Scholar]
- Crawford JH, Wootton JF, Seabrook GR, Scott RH. Activation of Ca2+-dependent currents in cultured dorsal root ganglion neurons from neonatal rats by metabotropic glutamate receptor activation and intracellular βNAD+ and cGMP, the precursors to cyclic ADP-ribose formation. Journal of Neurophysiology. 1997;77:2573–2584. doi: 10.1152/jn.1997.77.5.2573. [DOI] [PubMed] [Google Scholar]
- Eberhard DA, Holz RW. Intracellular Ca2+ activates phospholipase C. Trends in Neurosciences. 1988;11:517–520. doi: 10.1016/0166-2236(88)90174-9. 10.1016/0166-2236(88)90174-9. [DOI] [PubMed] [Google Scholar]
- Garaschuk O, Yaari Y, Konnerth A. Release and sequestration of calcium by ryanodine-sensitive stores in rat hippocampal neurons. The Journal of Physiology. 1997;502:13–30. doi: 10.1111/j.1469-7793.1997.013bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbeter A, Dupont G, Berridge MJ. Minimal model for signal-induced Ca2+ oscillations and for their frequency encoding through protein phosphorylation. Proceedings of the National Academy of Sciences of the USA. 1990;87:1461–1465. doi: 10.1073/pnas.87.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hulme EC, Birdsall NJM, Buckley NJ. Muscarinic receptor subtypes. Annual Review of Pharmacology and Toxicology. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- Irving AJ, Collingridge GL. Properties of metabotropic receptor-mediated Ca2+ oscillations in cultured rat hippocampal neurons. The Journal of Physiology. 1995;487.P:56. doi: 10.1111/j.1469-7793.1998.747bg.x. P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving AJ, Collingridge GL, Schofield JG. L-Glutamate and acetylcholine mobilise Ca2+ from the same intracellular pool in cerebellar granule cells using transduction mechanisms with different Ca2+ sensitivities. Cell Calcium. 1992a;13:293–301. doi: 10.1016/0143-4160(92)90064-y. 10.1016/0143-4160(92)90064-Y. [DOI] [PubMed] [Google Scholar]
- Irving AJ, Collingridge GL, Schofield JG. Interactions between Ca2+ mobilising mechanisms in cultured rat cerebellar granule cells. The Journal of Physiology. 1992b;456:667–680. doi: 10.1113/jphysiol.1992.sp019360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law GJ, Pachter JA, Thastrup OM, Hanley R, Dannies PS. Thapsigargin, but not caffeine, blocks the ability of thyrotropin-releasing hormone to release Ca2+ from an intracellular store in GH4C1 pituitary cells. Biochemical Journal. 1990;267:359–364. doi: 10.1042/bj2670359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Avery RB, Christie BR, Johnston D. Dihydropyridine-sensitive, voltage-gated Ca2+ channels contribute to the resting intracellular Ca2+ concentration of hippocampal CA1 pyramidal neurons. Journal of Neurophysiology. 1996;76:3460–3470. doi: 10.1152/jn.1996.76.5.3460. [DOI] [PubMed] [Google Scholar]
- Missiaen L, De Smedt H, Droogmans G, Casteels R. Ca2+ release induced by inositol 1,4,5-trisphosphate is a steady-state phenomenon controlled by luminal Ca2+ in permeabilized cells. Nature. 1992;357:599–602. doi: 10.1038/357599a0. 10.1038/357599a0. [DOI] [PubMed] [Google Scholar]
- Murphy SN, Miller RJ. Two distinct quisqualate receptors regulate Ca2+ homeostasis in hippocampal neurons in vitro. Molecular Pharmacology. 1989;35:671–680. [PubMed] [Google Scholar]
- Niggli E, Piston DW, Kirby MS, Cheng H, Sandison DR, Webb WW, Lederer WJ. A confocal laser scanning microscope designed for indicators with ultraviolet excitation wavelengths. American Journal of Physiology. 1994;266:C303–310. doi: 10.1152/ajpcell.1994.266.1.C303. [DOI] [PubMed] [Google Scholar]
- Nowycky MC, Fox AP, Tsien RW. Long-opening mode of gating of neuronal Ca2+ channels and its promotion by the dihydropyridine Ca2+ agonist Bay K8644. Proceedings of the National Acadamy of Sciences of the USA. 1985;82:2178–2182. doi: 10.1073/pnas.82.7.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phenna S, Jane SD, Chad JE. Increased perinuclear Ca2+ activity evoked by metabotropic glutamate receptor activation in rat hippocampal neurones. The Journal of Physiology. 1995;486:149–161. doi: 10.1113/jphysiol.1995.sp020799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Reynolds IJ, Miller RJ. Muscarinic agonists cause Ca2+ influx and Ca2+ mobilisation in forebrain neurons in vitro. Journal of Neurochemistry. 1989;53:226–233. doi: 10.1111/j.1471-4159.1989.tb07318.x. [DOI] [PubMed] [Google Scholar]
- Richmond SA, Irving AJ, Molnár E, McIlhinney RAJ, Michelangeli F, Henley JM, Collingridge GL. Localisation of the glutamate receptor subunit GluR1 on the surface of living and within cultured hippocampal neurones. Neuroscience. 1996;75:69–82. doi: 10.1016/0306-4522(96)00217-5. 10.1016/0306-4522(96)00217-5. [DOI] [PubMed] [Google Scholar]
- Rossier MF, Python CP, Burnay MM, Schlegel W, Valloton MB, Capponi AM. Thapsigargin inhibits voltage-activated calcium channels in adrenal glomerulosa cells. Biochemical Journal. 1993;296:309–312. doi: 10.1042/bj2960309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M, Manor D. Confocal microscopic imaging of [Ca2+]i in cultured rat hippocampal neurons following exposure to N-methyl-D-aspartate. The Journal of Physiology. 1992;448:655–676. doi: 10.1113/jphysiol.1992.sp019063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour-Laurent KJ, Barish ME. Inositol 1,4,5-trisphosphate and ryanodine receptor distributions and patterns of acetylcholine- and caffeine-induced calcium release in cultured mouse hippocampal neurons. Journal of Neuroscience. 1995;15:2592–2608. doi: 10.1523/JNEUROSCI.15-04-02592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmigol A, Kirischuk S, Kostyuk P, Verkhratsky A. Different properties of caffeine-sensitive Ca2+ stores in peripheral and central mammalian neurons. Pflügers Archiv. 1994;426:174–176. doi: 10.1007/BF00374686. [DOI] [PubMed] [Google Scholar]
- Shmigol A, Svichar N, Kostyuk P, Verkhratsky A. Gradual caffeine-induced Ca2+ release in mouse dorsal root ganglia neurons is controlled by cytoplasmic and luminal Ca2+ Neuroscience. 1996;73:1061–1067. doi: 10.1016/0306-4522(96)00108-x. [DOI] [PubMed] [Google Scholar]
- Simpson PB, Challiss RAJ, Nahorski SR. Divalent cation entry in cultured rat cerebellar granule cells measured using Mn2+ quench of fura 2 fluorescence. European Journal of Neuroscience. 1995;7:831–840. doi: 10.1111/j.1460-9568.1995.tb01070.x. [DOI] [PubMed] [Google Scholar]
- Simpson PB, Nahorski SR, Challiss RAJ. Agonist-evoked Ca2+ mobilisation from stores expressing inositol 1,4,5-trisphosphate receptors and ryanodine receptors in cerebellar granule neurones. Journal of Neurochemistry. 1996;67:364–373. doi: 10.1046/j.1471-4159.1996.67010364.x. [DOI] [PubMed] [Google Scholar]
- Sitsapesan R, Williams AJ. Mechanisms of caffeine activation of single Ca2+ release channels of sheep cardiac sarcoplasmic reticulum. The Journal of Physiology. 1990;423:425–439. doi: 10.1113/jphysiol.1990.sp018031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer SA, Perney TM, Miller RJ. Regulation of calcium homeostasis in sensory neurons by bradykinin. Journal of Neuroscience. 1988;8:4089–4097. doi: 10.1523/JNEUROSCI.08-11-04089.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamori M, Hidaka H, Akaike N. Hyperpolarizing muscarinic responses of freshly dissociated rat hippocampal CA1 neurones. The Journal of Physiology. 1993;463:585–604. doi: 10.1113/jphysiol.1993.sp019612. [DOI] [PMC free article] [PubMed] [Google Scholar]


