Abstract
The role of protein kinase C in controlling L-arginine transport in alveolar macrophages was investigated.
L-[3H]Arginine uptake in rabbit alveolar macrophages declined by 80 % after 20 h in culture. 4β-Phorbol 12-myristate 13-acetate (PMA), but not 4α-phorbol 12-myristate 13-acetate (α-PMA), present during 20 h culture, enhanced L-[3H]arginine uptake more than 10-fold. Staurosporine and chelerythrine opposed this effect.
L-[3H]Arginine uptake was saturable and blockable by L-lysine. After PMA treatment Vmax was increased more than 5-fold and Km was reduced from 0.65 to 0.32 mM.
Time course experiments showed that PMA increased L-[3H]arginine uptake almost maximally within 2 h. This short-term effect was not affected by cycloheximide or actinomycin D.
L-[3H]Arginine uptake and its stimulation by PMA was also observed in sodium-free medium.
L-Leucine (0.1 mM) inhibited L-[3H]arginine uptake by 50 % in sodium-containing medium, but not in sodium-free medium. At 1 mM, L-leucine caused significant inhibition in sodium-free medium also. L-Leucine showed similar effects on PMA-treated cells.
N-Ethylmaleimide (200 μm, 10 min) reduced L-[3H]arginine uptake by 70 % in control cells, but had no effect on PMA-treated (20 or 2 h) cells.
In alveolar macrophages, multiple transport systems are involved in L-arginine uptake, which is markedly stimulated by protein kinase C, probably by modulation of the activity of already expressed cationic amino acid transporters.
The amino acid L-arginine, besides being involved in protein synthesis, is the substrate of nitric oxide (NO) synthase and arginase, two enzymes which play a particular role in alveolar macrophages and other cells of the monocyte/ macrophage lineage. Arginase, which catalyses the hydrolysis of L-arginine to L-ornithine, a precursor in polyamine synthesis (Tabor & Tabor, 1984), appears to be expressed constitutively in macrophages including alveolar macrophages, although the level of its expression can be modulated by different stimuli (e.g. Hey et al. 1995; Modolell et al. 1995; Wang et al. 1995). On the other hand, NO synthase is not expressed constitutively in macrophages and alveolar macrophages, but can be induced; and typical inductive stimuli are bacterial toxins and/or pro-inflammatory cytokines (e.g. Jorens et al. 1991; Hey et al. 1995; for review see Förstermann & Kleinert, 1995). However, marked species differences exist with regard to the mechanisms controlling the induction of NO synthase in macrophages. Thus, in macrophages or alveolar macrophages of rats and mice NO synthase can easily be induced, whereas in other species, such as rabbits and humans for example, the induction of NO synthase appears to be much more complex (e.g. Hey et al. 1995; for review see Dugas et al. 1995).
In macrophages the intracellular availability of L-arginine may become rate limiting for both the NO synthase and arginase pathways, particularly under the conditions when high levels of NO synthase are induced (e.g. Hibbs et al. 1987; Hey et al. 1997). The transport of L-arginine into cells is mediated via specific cationic amino acid transporters and several systems have been differentiated, both functionally (e.g. system y+, y+L, b0,+or B0,+) and at the molecular level (e.g. White, 1985; van Winkle et al. 1985; van Winkle et al. 1988; Devés et al. 1993; for review see Closs, 1996; Malandro & Kilberg, 1996). The cellular uptake of L-arginine may be one important factor controlling the intracellular availability of this amino acid. Therefore, it appeared to be of particular interest to elucidate the mechanisms involved in the control of L-arginine transport in macrophages. In murine macrophage J774 cells it has already been observed that induction of NO synthase is associated with an increase in L-arginine transport (Bogle et al. 1992). However, induction of NO synthase, but not L-arginine transport, was inhibited by dexamethasone (Baydoun et al. 1993), suggesting that the activity of L-arginine transport in macrophages may be controlled via specific and distinct pathways.
The protein kinase C signal transduction pathway plays a key role in the activation of cells of the monocyte/ macrophage lineage (e.g. Shapira et al. 1994; Mayer et al. 1995; Tapper & Sundler, 1995). In addition, protein kinase C appears also to be one of the pathways involved in the induction of NO synthase (Paul et al. 1995). Therefore, the primary aim of the present study was to characterize L-arginine transport in alveolar macrophages and to elucidate the role of protein kinase C in the control of its activity.
In the present study, alveolar macrophages of rat and rabbit were used, as these two species represent examples in which marked differences with regard to the expression of inducible NO synthase have been described in alveolar macrophages (Hey et al. 1995). As most of the molecular knowledge about cationic amino acid transporters is currently available for the mouse species (for review see Closs, 1996; Malandro & Kilberg, 1996), a few additional comparative experiments were also carried out on mouse alveolar macrophages.
METHODS
Preparation and culture of alveolar macrophages
Mongrel rabbits (2-2.5 kg, Lammers, Euskirchen), Sprague- Dawley rats or NMRI mice (both bred at the University of Bonn) of either sex were killed by stunning followed by exsanguination. Lung and trachea were excised en bloc, washed with calcium- and magnesium-free Dulbecco's phosphate-buffered saline (D-PBS) and lavaged three times by instilling about 60 ml (rabbit lung), 15 ml (rat lung) or 1–2 ml (mouse lung) of cold (4°C) D-PBS (Hey et al. 1995). Usually for one preparation of alveolar macrophages, lavage fluids of one or two rabbit lungs, six to eight rat lungs or about forty mice lungs were pooled and centrifuged at 400 g for 5 min. The cells were washed three times with D-PBS and thereafter resuspended in Dulbecco's modified Eagle's medium-Ham's F-12 medium (DMEM-F-12 medium) supplemented with 5 % fetal calf serum, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and 5 μg ml−1 amphotericin B and plated in sterile 6-well dishes (NUNC; 3 × 106 cells well−1). The alveolar macrophages were allowed to adhere for 2 h (37°C, 5 % CO2). After 2 h the medium was renewed to remove non-adherent cells. The adherent cells consisted of more than 95 % alveolar macrophages according to morphological criteria (May Grünwald-Giemsa staining). Thereafter, cells were either used directly for uptake studies or cultured for 20 h in the absence or presence of test substances followed by the uptake studies. In some experiments, N-ethylmaleimide (NEM, 200 μm) was added for the last 10 min of the culture period followed by the uptake studies. Finally, in some experiments test substances were added to the culture medium after the 20 h culture period and cells were cultured for an additional 0.25-6 h followed by the uptake studies. Cell viability assessed by Trypan Blue exclusion was always greater than 95 %.
Measurement of L-[3H]arginine uptake in alveolar macrophages
L-[3H]Arginine uptake was measured immediately after the adherence period of the alveolar macrophages (no culture) or after different time periods in culture, mostly 20 h. Adherent alveolar macrophages were rinsed once with 2 ml of prewarmed Krebs- Hepes solution of the following composition (mM): NaH2PO4, 0.001; NaCl, 118.5; KCl, 5.57; CaCl2, 1.25; MgCl2, 1.2; Na2EDTA, 0.03; L-(+)-ascorbic acid, 0.06; Hepes, 20.0 (adjusted to pH 7.4 using NaOH); and D-(+)-glucose, 11.1.
In most experiments alveolar macrophages were incubated with Krebs-Hepes solution containing 0.1 or 100 μm L-[3H]arginine (37 kBq) for 2 or 5 min, respectively. Incubation was terminated by placing the plates on melting ice and rinsing alveolar macrophages three times with 2 ml ice-cold Krebs-Hepes solution containing 10 mM unlabelled L-arginine. Finally, alveolar macrophages were extracted in 1 ml 0.4 M HClO4 and radioactivity in the cell extracts was measured by liquid scintillation spectrometry in a Beckman LS 5000TD. External standardization was used to correct for counting efficiency. In one initial series of experiments the time of exposure to L-[3H]arginine was varied from 1 to 25 min (Fig. 1). L-[3H]arginine uptake was expressed as d.p.m. per 3 × 106 cells, as picomoles (taking into account the specific activity of the labelled amino acid) or as a percentage of the uptake observed in controls of the respective cell preparation.
Figure 1. Time-dependent accumulation of L-[3H]arginine in rabbit alveolar macrophages.
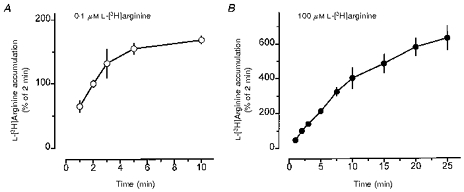
Freshly prepared alveolar macrophages were allowed to adhere in culture dishes for 2 h (3 × 106 cells well−1). Thereafter, alveolar macrophages were incubated for 1–10 min (A) or 1–25 min (B) in amino acid-free Krebs-Hepes solution to which either 0.1 μm (○) or 100 μm (•) L-[3H]arginine (37 kBq) was added. Ordinate: radioactivity in cell extracts expressed as a percentage of the mean accumulation observed during 2 min incubation of the respective cell preparation (for absolute values see text and Table 1). Values are means ±s.e.m. of 4–7 experiments.
The sodium dependency of L-[3H]arginine uptake was studied by replacing NaCl by equimolar choline chloride. When the temperature dependency of the L-[3H]arginine uptake was studied, the culture medium was removed and the cells were incubated for 10 min in Krebs-Hepes medium at 37, 30 or 23°C immediately followed by the uptake protocol at the respective temperature. The temperature dependency of L-[3H]arginine was calculated using Arrhenius's rate equation. The ln rate of arginine uptake k (pmol min−1) was plotted against the reciprocal of absolute temperature (103 T−1) using linear regression analysis. The activation energy (μ) was calculated from the regression equation, ln k = ln k0 - μR-1 T−1, which was transformed to μ=RT(ln k0 - ln k), R being the gas constant and T the absolute temperature. The temperature coefficient Q10 between the temperatures T1 and T2 was obtained from ln Q10= 10μR-1T1−1T2−1 (Muscholl & Ritzel, 1980).
For determination of the kinetic parameters of L-[3H]arginine uptake alveolar macrophages were incubated with different concentrations of L-[3H]arginine (10 nM to 3 mM) and accumulation was analysed using the computer program GraphPad Prism (GraphPad Software, San Diego, CA, USA) by fitting the data to Michaelis-Menten kinetics (Fig. 3).
Figure 3. Effect of PMA on the kinetics of L-arginine uptake in rabbit alveolar macrophages.
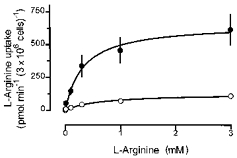
Alveolar macrophages were cultured for 20 h in the absence (○) or presence of 100 nM PMA (•). Thereafter, L-[3H]arginine uptake was studied during a 2 min incubation period in amino acid-free Krebs-Hepes solution to which L-[3H]arginine, at the concentrations indicated by the abscissa, was added. L-Arginine uptake was calculated from the accumulated radioactivity taking into account the specific activity of the tracer. Values are means ±s.e.m. of 9–14 experiments of 7 separate cell preparations.
Extraction of RNA and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from mouse and rat alveolar macrophages using Trizol reagent. The first strand cDNA was synthesized from 2 μg of total RNA using oligo(dT)18 primer and avian myoblastosis virus (AMV) reverse transcriptase under the conditions recommended by the supplier (Promega, Mannheim, Germany). The cDNA product was used for subsequent amplification by PCR. Oligonucleotide primers were constructed based on the European Molecular Biology Laboratory (EMBL) sequences for rat (r) β-actin (accession number V01217; J00691), mouse (m) β-actin (M12481; X03765), rCAT-1 (L10151; one mismatch for mCAT-1) and m/rCAT-2B (M62838; M32485; U53927; 100 % homology between the corresponding sequences mCAT-2B and rCAT-2B). Amplification of the cDNAs with the β-actin primers served as a positive control for the quality and quantity of the cDNAs. Primer pairs for rβ-actin were: 5′-GCACCAGGGTGTGATGGTGGGTA-3′ (sense; nucleotides 117-139) and 5′-AGAGGTCTTTACGGATGTCAACG-3′ (antisense; 880-858); mβ-actin: 5′-GTTACCAACTGGGACGACATGGA-3′ (sense; 148–170 partial sequence) and 5′-CTTCATGGTGCTAGGAGCCAGAG-3′ (antisense; 900–878 partial sequence); rCAT-1: 5′-GCTGCCTCAACACCTATGATCTGG-3′ (sense; 89-112) and 5′-ACGATGCCCACAGGAATGGC-3′ (antisense; 857-838); m/rCAT-2B: 5′-ATGGTGGCTGGGTTTGTGAAAG-3′ (sense; 616-637) and 5′-CAACCCATCCTCCGCCATAGC-3′ (antisense; 1116-1096). PCR amplification was performed using Taq DNA polymerase and specific primers in a programmable thermal reactor (Hybaid, Middlesex, UK) with initial heating for 3 min at 94°C, followed by thirty-five cycles of 45 s denaturation at 94°C, annealing at 56°C (30 s), extension at 72°C (1 min), and a final extension for 10 min at 72°C. PCR products were separated by 1.2 % agarose gel electrophoresis and documented by a video documentation system (MWG, Ebersberg, Germany).
Statistics
If not stated otherwise, values are means ±s.e.m. of n experiments; usually one to three determinations of the respective test protocol were carried out on one cell preparation, but at least three separate cell preparations were used for each protocol. Statistical significance of differences was evaluated by Student's t test. When multiple comparisons were performed the significance of differences was evaluated by ANOVA followed by the modified t test according to Bonferroni using the computer program GraphPad InStat (GraphPad Software). When groups of different variances were compared, Welch's test was applied. P < 0.05 was accepted as being significant.
Drugs and special chemicals
Actinomycin D, amphotericin B, L-arginine HCl, chelerythrine HCl, cycloheximide, cytochalasin B, Dulbecco's phosphate-buffered saline without calcium and magnesium (D-PBS), DMEM-F-12 medium, N-ethylmaleimide (NEM), L-leucine, L-lysine HCl, 4β-phorbol 12-myristate 13-acetate (PMA), and staurosporine were all from Sigma; AMV reverse transcriptase was from Promega (Mannheim, Germany); L-[2,3-3H]arginine HCl (36-40 Ci mmol−1) was from Sigma or Dupont; choline chloride was from Fluka; fetal calf serum was from Vitromex (Vilshofen, Germany); 4α-phorbol 12-myristate 13-acetate (α-PMA) was from Biomol (Hamburg, Germany); all primers for RT-PCR were from MWG (Ebersberg, Germany); Trizol reagent and Taq DNA polymerase were from Gibco.
Stock solutions (10 mM) were prepared in dimethyl sulphoxide (DMSO) for actinomycin D, cytochalasin B, PMA, α-PMA and staurosporine, and in ethanol for NEM. Lipopolysaccharide (LPS; 500 μg ml−1) was dissolved in D-PBS. None of the solvents applied alone at the concentration which was applied with the highest concentration of the respective drug affected L-[3H]arginine uptake (each n = 3, data not shown).
RESULTS
Characteristics of L-[3H]arginine uptake in rabbit alveolar macrophages
When freshly prepared rabbit alveolar macrophages were incubated immediately after a 2 h adherence period (‘no culture’) with L-[3H]arginine (37 kBq ml−1, 0.1 μm or 100 μm) for different time intervals, a time-dependent, saturable accumulation of radioactivity was observed (Fig. 1). Accumulation of 0.1 μm L-[3H]arginine into rabbit alveolar macrophages increased almost linearly between 1 and 3 min. Thereafter, the rate of increase decreased rapidly reaching a plateau by between 5 and 10 min (Fig. 1A). When 100 μm L-[3H]arginine was used, the linear phase of accumulation was more prolonged, up to about 10 min (Fig. 1B). Therefore, in subsequent experiments an incubation period of 2 or 5 min was used to study initial uptake of 0.1 and 100 μm L-[3H]arginine, respectively. In the kinetics study, however, a 2 min incubation period was used for all concentrations of L-[3H]arginine. The absolute amounts of radioactivity accumulated in freshly prepared, non-cultured rabbit alveolar macrophages during 2 min incubation with 0.1 μm or 5 min incubation with 100 μm L-[3H]arginine were 8714 ± 1 221 and 12 400 ± 930 d.p.m. (3 × 106 cells)−1, respectively, corresponding to 0.56 ± 0.08 and 793 ± 59 pmol L-arginine (3 × 106 cells)−1. Generally, accumulated radioactivity showed much smaller variations within one cell preparation than between different cell preparations. Therefore, data were mostly expressed as a percentage of controls or of a reference treatment group within an individual cell preparation. If not stated otherwise, the tracer concentration of L-[3H]arginine (0.1 μm) was used as the standard condition to study L-arginine uptake.
Time course experiments showed that the L-[3H]arginine uptake in alveolar macrophages changed depending on the time period in culture. After the first hours in culture L-[3H]arginine uptake was increased, maximally by about 180 % after 6–8 h (data not shown). Thereafter, it declined continuously reaching a level of about 20 % after 20 h in culture (Table 1).
Table 1.
Effect of 20 h culture in the absence or presence of PMA on l-[3H]arginine uptake in rabbit, rat and mouse alveolar macrophages
| Rabbit | Rat | Mouse | |
|---|---|---|---|
| No culture | |||
| Uptake in pmol (3 × 106 cells)−1 | 0.56 ± 0.08 (33) | 1.28 ± 0.16 (16)‡ | 3.13 ± 0.31 (6) |
| Uptake as % of mean of individual cell preparation | 100 ± 3 (33) | 100 ± 2 (16) | 100 ± 5 (6) |
| 20 h culture control | |||
| Uptake in pmol (3 × 106 cells)−1 | 0.13 ± 0.01 (116) ** | 1.05 ± 0.11 (15) | 8.84 ± 2.22 (10) * |
| Uptake as % of mean of individual ‘no culture’ | 19 ± 4 (17) ** | 54 ± 4 (15) ** | 246 ± 43 (4) ** |
| 20 h culture + PMA | |||
| Uptake in pmol (3 × 106 cells)−1 | 0.91 ± 0.07 (96) † | 2.51 ± 0.19 (22) † | 15.77 ± 3.93 (8) |
| Uptake as % of mean individual ‘20 h culture control’ | 1475 ± 157 (76) † | 268 ± 20 (22) † | 169 ± 12 (8) † |
l-[3H]Arginine uptake in rabbit, rat and mouse alveolar macrophages was studied immediately after adherence (no culture) or after 20 h culture in the absence or presence of PMA (100 nm). l-[3H]Arginine uptake was measured by determining the cellular radioactivity after 2 min incubation in amino acid-free Krebs-Hepes solution to which l-[3H]arginine (37 kBq, 0.1 μm; see Fig. 1A) was added. l-[3H]Arginine uptake per 2 min is expressed as pmol (3 × 106 cells)−1 or as a percentage of the mean uptake observed in the respective individual cell preparation ‘no culture’ or ‘20 h culture control’. Results are means ±s.e.m. with the number of experiments (n) in parentheses. Usually, 2–3 individual experiments of a treatment protocol were carried out on the same cell preparation, and one cell preparation was obtained from lungs of 1–2 rabbits, 6–8 rats or about 40 mice.
P < 0.05
P < 0.01 compared with the respective ‘no culture’ value
P < 0.01 compared with the respective ‘20 h culture control’ value.
The protein kinase C-activating phorbol ester PMA (Blumberg, 1980; Ashendal, 1985), present during the 20 h culture period, caused a concentration-dependent increase in L-[3H]arginine uptake. At 100 nM, the highest concentration of PMA tested, a large increase was observed (Fig. 2, Table 1). Thus, the presence of PMA not only opposed the culture-dependent decline in L-[3H]arginine uptake, but enhanced it above the levels observed in non-cultured alveolar macrophages (Table 1). It should be noted that the concentration response for this effect of PMA was rather steep, since 10 nM PMA caused an increase by only about 200 % (Fig. 2). It should be emphasized that in Fig. 2 a large number of experiments were pooled and that the magnitude of the effect of PMA showed considerable variance between different series of experiments ranging from an increase of about 5-fold to a more than 15-fold increase. Therefore, when the effect of PMA was further characterized (see below), the effect of PMA on the actual cell preparation had to be used as a reference. α-PMA (100 nM), a phorbol ester which does not activate protein kinase C (Blumberg, 1980), did not cause an increase, but rather reduced L-[3H]arginine uptake by 40 % (Fig. 2).
Figure 2. Effects of PMA and α-PMA on L-[3H]arginine uptake in rabbit alveolar macrophages.
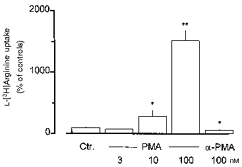
Alveolar macrophages were cultured for 20 h in the absence (Ctr.) or presence of PMA or α-PMA (at the concentrations indicated). Thereafter, L-[3H]arginine uptake was studied during a 2 min incubation period in amino acid-free Krebs-Hepes solution to which L-[3H]arginine (37 kBq, 0.1 μm) was added. Height of columns: L-[3H]arginine uptake expressed as a percentage of mean uptake observed in the controls of the respective cell preparation (Ctr.). Values are means +s.e.m. of 6–10 experiments. *P < 0.05; **P < 0.01, compared with Ctr.
Analysis of the kinetics of L-[3H]arginine uptake in rabbit alveolar macrophages (Fig. 3) revealed that Vmax was increased from 127 ± 8.9 pmol min−1 (3 × 106 cells)−1 (mean ± s.d) in control alveolar macrophages to 655 ± 32 pmol min−1 (3 × 106 cells)−1 (mean ±s.d.) after culture in the presence of 100 nM PMA (P < 0.01), whereas the Km was reduced from 0.65 ± 0.13 mM (mean ±s.d.) in controls to 0.32 ± 0.05 mM (mean ±s.d.) after culture in the presence of PMA (P < 0.05).
Staurosporine (100 nM, present during the 20 h culture period), although causing a small, but significant increase in L-[3H]arginine uptake by its own, largely inhibited the stimulatory effect of 100 nM PMA (Fig. 4). Chelerythrine alone had no effect, but when applied during the culture period at 10 and 30 μm it inhibited the stimulatory effect of 100 nM PMA by 49 ± 8 and 62 ± 7 %, respectively (each n = 8).
Figure 4. Effects of PMA and staurosporine on L-[3H]arginine uptake in rabbit alveolar macrophages.
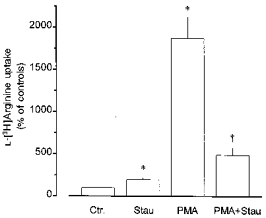
Alveolar macrophages were cultured for 20 h in the absence (Ctr.) or presence of staurosporine (Stau, 100 nM) or PMA (100 nM) alone or in combination, as indicated. Thereafter, L-[3H]arginine uptake was studied during a 2 min incubation period in amino acid-free Krebs-Hepes solution to which L-[3H]arginine (37 kBq, 0.1 μm) was added. Height of columns: L-[3H]arginine uptake expressed as a percentage of the mean uptake observed in the controls of the respective cell preparation (Ctr.). Results are means +s.e.m. of 6–22 experiments. *P < 0.01, compared with Ctr.; †P < 0.01, compared with PMA alone.
When PMA (100 nM) was added after the 20 h culture period for 0.25-6 h, it caused a rapid increase in L-[3H]arginine uptake, which was significantly different from control levels by 0.5 h (149 ± 44 %, n = 6; P < 0.05vs. respective time controls) and almost maximal after about 1–2 h (Fig. 5). The magnitude of this short-term stimulatory effect of PMA was in the range of that observed after PMA had been present for 20 h (compare Fig. 5vs. Figs 2 and 6; Fig. 8). This short-term effect of 100 nM PMA was largely inhibited by 100 nM staurosporine (Fig. 5). Likewise, chelerythrine present at 10 and 30 μm together with 100 nM PMA for 2 h inhibited the short-term stimulatory effect of PMA by 56 ± 7 and 72 ± 6 %, respectively (each n = 8). However, this short-term effect of 100 nM PMA was not affected by cycloheximide or by actinomycin D (Fig. 5). Neither drug, when present alone for up to 6 h, affected L-[3H]arginine uptake significantly (data not shown). On the other hand, when cycloheximide (30 μm) or actinomycin D (5 μg ml−1) had been present during the 20 h culture period, L-[3H]arginine uptake was reduced by 54 ± 13 % (n = 6) and 79 ± 6 % (n = 8), respectively. When present for 20 h together with 100 nM PMA, cycloheximide and actinomycin D reduced L-[3H]arginine uptake by 68 ± 14 % (n = 10) and 98 ± 2 % (n = 4), respectively, of the PMA-stimulated controls. Considering the about 10-fold elevated levels in the PMA-stimulated controls, it is evident that PMA still caused a significant (about 7-fold) increase in the presence of cycloheximide, but failed to enhance L-[3H]arginine uptake in the presence of actinomycin D.
Figure 5. Characterization of short-term effects of PMA on L-[3H]arginine uptake in rabbit alveolar macrophages.
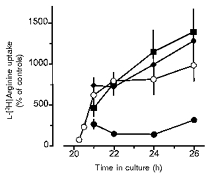
After alveolar macrophages had been cultured for 20 h in the absence of test substances, PMA alone (100 nM, ○) or in combination with cycloheximide (30 μm, ♦), actinomycin D (5 μg ml−1, ▪) or staurosporine (100 nM, •) was added to the medium and the cells were cultured for an additional 0.25-6 h as indicated by the abscissa. Thereafter, L-[3H]arginine uptake was studied during a 2 min incubation period in amino acid-free Krebs-Hepes solution to which L-[3H]arginine (37 kBq, 0.1 μm) was added. Ordinate: L-[3H]arginine uptake expressed as percentage of the mean uptake observed in the respective time controls of the individual cell preparation. Values are means ±s.e.m. of 3–13 experiments.
Figure 6. Effect of removal of sodium ions on L-[3H]arginine uptake in rabbit alveolar macrophages and its modulation by PMA.
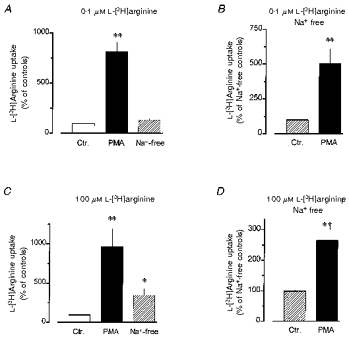
Alveolar macrophages were cultured for 20 h in the absence (Ctr.) or presence of 100 nM PMA, as indicated. Thereafter, L-[3H]arginine uptake was studied during a 2 min (A and B) or 5 min (C and D) period of incubation in amino acid-free Krebs-Hepes solution to which 0.1 μm (A and B) or 100 μm (C and D) L-[3H]arginine (37 kBq) was added. In the experiments shown in parts B and D as well as in the ones given by the ‘Na+-free’ columns of parts A and C, sodium ions of the incubation medium were replaced by equimolar choline. Height of columns: L-[3H]arginine uptake expressed as a percentage of the mean uptake observed in the controls of the respective cell preparation. Note the different scales. Results are means +s.e.m. of 9–27 experiments. *P < 0.05, **P < 0.01 compared with respective Ctr.; †P < 0.05, compared with the respective observations in sodium-containing medium.
Figure 8. Effects of N-ethylmaleimide (NEM) on L-[3H]arginine uptake in rabbit alveolar macrophages.
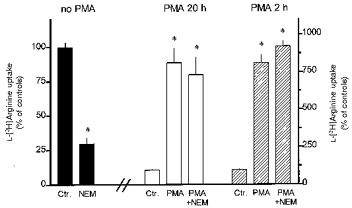
Alveolar macrophages were cultured for 20 h in the absence or presence of 100 nM PMA (PMA 20 h) or cultured for 20 h in the absence of PMA followed by 2 h culture in the presence of 100 nM PMA (PMA 2 h). In the respective experiments NEM (200 μm) was added for the last 10 min of the culture period. Ctr., control. Thereafter, L-[3H]arginine uptake was studied during a 2 min period of incubation in amino acid-free Krebs-Hepes solution to which 0.1 μm L-[3H]arginine (37 kBq) was added. Height of columns: L-[3H]arginine uptake expressed as percentage of the mean uptake observed in the controls of the respective cell preparation. Results are means +s.e.m. of 9–27 experiments. *P < 0.01 compared with respective Ctr.
L-Lysine (10 mM) inhibited the uptake of 0.1 and 100 μm L-[3H]arginine by 96 ± 1 and 91 ± 2 %, respectively, in alveolar macrophages cultured in the absence of PMA and by 97 ± 1 % and 89 ± 3 %, respectively, in alveolar macrophages cultured in the presence of PMA (n = 6-9). Replacing the sodium ions in the incubation medium by choline had no significant effect on uptake of 0.1 μm L-[3H]arginine (Fig. 6A), but enhanced uptake of 100 μm L-[3H]arginine by about 280 % (Fig. 6C). After treatment with PMA L-[3H]arginine uptake was also enhanced under sodium-free conditions (Fig. 6B and D), but at 100 μm L-[3H]arginine the magnitude of the stimulatory effect of PMA was significantly smaller than that observed in sodium-containing medium (Fig. 6C and D). However, the uptake in sodium-free controls was already markedly elevated so that the maximum level of L-arginine uptake reached after PMA treatment appeared to be similar in sodium-free and sodium-containing media. This is also indicated by the absolute amounts of L-arginine taken up in these experiments (169 ± 21 pmol (3 × 106 cells)−1 (5 min)−1 (n = 18) and 215 ± 39 pmol (3 × 106 cells)−1 (5 min)−1 (n = 27) after PMA treatment in sodium-containing and sodium-free medium, respectively).
In order to further characterize L-arginine transport, the effect of the neutral amino acid L-leucine on the uptake of 0.1 and 100 μm L-[3H]arginine was investigated (Fig. 7). Inhibition of L-[3H]arginine by L-leucine was sodium dependent. Thus, in the absence of sodium ions, 0.1 mM L-leucine failed to affect L-[3H]arginine uptake under all conditions studied (0.1 and 100 μm L-[3H]arginine, each in macrophages cultured in the absence and presence of PMA, Fig. 7B and D). However, in the presence of sodium ions, 0.1 mM L-leucine inhibited the uptake of 0.1 μm L-[3H]arginine by 43 % in control alveolar macrophages and by 59 % in cells cultured in the presence of PMA (Fig. 7A). Likewise, in the presence of sodium ions, 0.1 mM L-leucine inhibited the uptake of 100 μm L-[3H]arginine by 56 and 63 % in control and PMA-treated macrophages, respectively (Fig. 7C). At the higher concentration of 1 mM, the inhibitory effect of L-leucine in the presence of sodium ions was somewhat greater, particularly in the experiments with 100 μm L-[3H]arginine (Fig. 7C), but, in addition, 1 mM L-leucine also caused a clear inhibition in the absence of sodium ions, under all conditions studied (Fig. 7B and D).
Figure 7. Sodium-dependent effects of L-leucine on L-[3H]arginine uptake in rabbit alveolar macrophages.
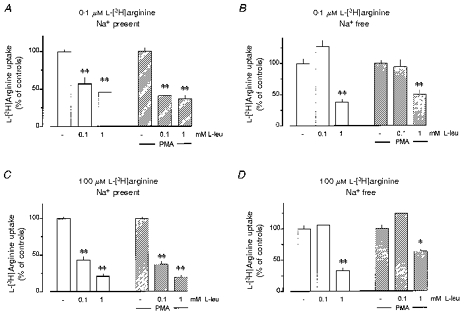
Alveolar macrophages were cultured for 20 h in the absence (Ctr.) or presence of 100 nM PMA, as indicated. Thereafter, L-[3H]arginine uptake was studied during a 2 min (A, B) or 5 min (C, D) period of incubation in amino acid-free Krebs-Hepes solution to which 0.1 μm (A, B) or 100 μm (C, D) L-[3H]arginine (37 kBq) was added. In the experiments shown in B and D, sodium ions of the incubation medium were replaced by equimolar choline. The values below the columns indicate the millimolar concentrations of L-leucine (L-leu) added to incubation medium. Height of columns: L-[3H]arginine uptake expressed as a percentage of the mean uptake observed in the controls of the respective cell preparation. Results are means +s.e.m. of 9–27 experiments. *P < 0.05, **P < 0.01 compared with respective experiments in the absence of L-leucine.
When NEM (200 μm) was added for the last 10 min of the culture period, subsequent uptake of L-[3H]arginine was reduced by 71 % (Fig. 8). On the other hand, pretreatment with NEM failed to affect the uptake of L-[3H]arginine in alveolar macrophages which had been exposed for either 20 or 2 h to PMA (Fig. 8).
Finally, L-arginine transport into alveolar macrophages showed a marked temperature dependency. Thus, in control alveolar macrophages L-[3H]arginine uptake was reduced by 58 ± 3 and 87 ± 1 % (each n = 9), when the incubation temperature was reduced from 37°C to 30 and 23°C, respectively. In alveolar macrophages cultured in the presence of PMA the temperature dependency of L-arginine transport was significantly attenuated. Thus, after reduction of the incubation temperature to 30 and 23°C, L-[3H]arginine uptake was reduced by only 33 ± 5 % and 76 ± 3 % (each n = 9), respectively (each P < 0.01vs. controls). The activation energy (determined by performing Arrhenius plots), was reduced from 110.4 kJ mol−1 in control alveolar macrophages to 81.9 kJ mol−1 in PMA-treated alveolar macrophages, resulting in Q10 values of 4.16 and 2.88, respectively.
In order to test whether microfilament-associated intracellular transport mechanisms might be involved in the PMA-mediated activation of L-arginine transport the effect of cytochalasin B was studied. When cytochalasin B (30 μm) was present between 19.5 and 21 h of culture L-[3H]arginine uptake was reduced by 26 ± 6 % (n = 6, P < 0.05). When L-[3H]arginine uptake was enhanced by PMA (100 nM, present from 20–21 h of culture), cytochalasin B (30 μm, present 30 min before PMA, i.e. from 19.5 to 21 h of culture) caused a reduction by a similar fraction (30 ± 2 %, n = 5; P < 0.01).
L-[3H]Arginine uptake in rat and mouse alveolar macrophages
Since the number of alveolar macrophages recovered from lungs of rats, and particularly of mice, is rather small (compared with rabbit lungs) only some selected experiments were performed on alveolar macrophages of these species. In comparison to rabbit alveolar macrophages, L-[3H]arginine uptake in freshly prepared rat alveolar macrophages was about twice as high and it declined less when the cells were cultured for 20 h (Table 1). L-[3H]Arginine uptake in freshly prepared mouse alveolar macrophages was even higher and further increased when the cells were cultured for 20 h (Table 1).
As in rabbit alveolar macrophages, PMA (100 nM, present during the 20 h culture period) caused a significant increase in L-[3H]arginine uptake, both in rat and mouse alveolar macrophages. However, due to the higher control levels in rat and mouse alveolar macrophages, the relative increases were considerably smaller (Table 1). However, the absolute level of L-[3H]arginine uptake reached in PMA-treated rat alveolar macrophages was about three times higher than in rabbit alveolar macrophages, and in PMA-treated mouse alveolar macrophages it was markedly higher.
In contrast to observations in rabbit alveolar macrophages, staurosporine (100 nM, present during the 20 h culture period) caused a reduction in L-[3H]arginine uptake by about 60 % in rat alveolar macrophages and by 70 % in mouse alveolar macrophages (Fig. 9). Likewise, chelerythrine (10 and 30 μm, present during the 20 h culture period) caused a reduction in L-[3H]arginine uptake in rat alveolar macrophages by 36 ± 5 and 56 ± 6 %, respectively (each n = 9). Furthermore, 100 nM staurosporine (present together with 100 nM PMA for 20 h) almost abolished the stimulatory effect of PMA in both rat and mouse alveolar macrophages (Fig. 9).
Figure 9. Role of protein kinase C in the regulation of L-[3H]arginine uptake in rat and mouse alveolar macrophages.
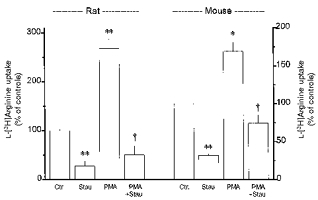
Alveolar macrophages were cultured for 20 h in the absence (Ctr.) or presence of staurosporine (Stau, 100 nM) or PMA (100 nM) alone or in combination, as indicated. Thereafter, L-[3H]arginine uptake was studied during a 2 min incubation period in amino acid-free Krebs-Hepes solution to which L-[3H]arginine (37 kBq, 0.1 μm) was added. Height of columns: L-[3H]arginine uptake expressed as a percentage of the mean uptake observed in the controls of the respective cell preparation (Ctr.). Results are means +s.e.m. of 6–22 experiments. *P < 0.05, **P < 0.01, compared with respective controls (Ctr.); †P < 0.01, compared with respective value in PMA alone.
Finally, significant effects on L-[3H]arginine uptake in rat alveolar macrophages could also be demonstrated after short-term exposure to PMA or staurosporine. When PMA (100 nM) or staurosporine (100 nM) had been present for only 1 h (from 20–21 h of culture), L-[3H]arginine uptake was increased by 67 ± 9 % (n = 4, P < 0.01) and inhibited by 52 ± 9 % (n = 7, P < 0.01), respectively.
Expression of mRNA for CAT proteins in rat and mouse alveolar macrophages
Using specific primers based on the published sequences for rCAT-1 and mCAT-2B (the primers for mCAT-2B showed 100 % homology to the corresponding rat sequences whereas that of the sense primer of the primer pair for rCAT-1 showed a single mismatch to the corresponding sequence of mCAT-1; see Methods and Discussion) RT-PCRs were carried out with RNA isolated from mouse and rat alveolar macrophages which had been cultured for 20 h (i.e. the conditions under which the uptake studies had been performed). Figure 10 shows that PCR products of the expected size were obtained for CAT-1 and even slightly stronger signals for CAT-2B from both rat and mouse alveolar macrophages. Similar results were obtained in three independent cell preparations for each species. Although RT-PCR is only a semiquantitative technique it may be mentioned that neither the expression of mRNA for CAT-1 nor that for CAT-2B appeared to be reduced in three different cell preparations of rat alveolar macrophages cultured for 20 h in the presence of 100 nM staurosporine.
Figure 10. Detection of mRNA for CAT proteins by RT-PCR in mouse and rat alveolar macrophages.
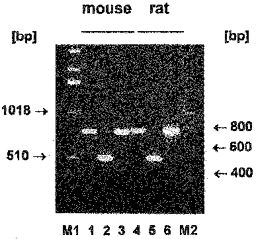
Alveolar macrophages of mouse and rats were cultured for 20 h in 60 mm culture dishes (6 × 106 cells dish−1) followed by RNA isolation. RT-PCR was performed as described in Methods using RNA from mouse (lanes 1-3) or rat (lanes 4-6) alveolar macrophages and primers specific for CAT-1 (lanes 1 + 4); CAT-2B (lanes 2 + 5); m-β-actin (lane 3) and r-β-actin (lane 6). M1 and M2 represent markers for numbers of base pairs (bp).
DISCUSSION
In the present study uptake of L-arginine into alveolar macrophages was characterized and a regulatory role of protein kinase C demonstrated. Most of the present experiments were carried out on cells obtained from rabbits, but some key experiments were repeated on alveolar macrophages of rats and mice in order to allow more general conclusions.
In macrophages of all three species arginase appears to be expressed constitutively (see Introduction), whereas marked species differences exist with regard to the expression of NO synthase. Thus, under all the present experimental conditions, no NO synthase activity was detectable in alveolar macrophages of rabbits (Hey et al. 1995, 1996). On the other hand, rat alveolar macrophages showed some basal NO synthase activity when the cells had been cultured in the absence of any particular inductive stimulus, and showed a substantial further increase in NO synthase activity after exposure to LPS (Hey et al. 1995, 1997). Likewise, in several murine macrophage cell types (bone marrow, different cell lines) the induction of marked NO synthase activity was demonstrated (e.g. Bogle et al. 1992; Baydoun et al. 1993; Modolell et al. 1995), although we are not aware of any study on mouse alveolar macrophages.
L-Arginine uptake in rabbit alveolar macrophages
In mammalian cells several systems mediating the transport of cationic amino acids (L-arginine, L-lysine and L-ornithine) have been described (for review see Closs, 1996). They have been differentiated by their affinity for the cationic amino acids, by their dependence on sodium ions and their ability to also transport neutral amino acids. In the present study, L-arginine uptake in rabbit alveolar macrophages was saturable and showed a Km value in the higher micromolar range (Fig. 3). It was blockable by L-lysine, another cationic amino acid, indicating that it was mediated via cationic amino acid transport systems. The involvement of specific transport mechanisms is further indicated by the marked temperature dependency. Finally, L-arginine uptake did not depend on the presence of extracellular sodium ions (Fig. 6) excluding a role of the system B0,+ which has been described as the only system to transport cationic amino acids in a sodium-dependent manner (van Winkle et al. 1985). In contrast, sodium ions appear to exert some inhibitory effect on L-arginine uptake, an effect which was, however, only seen at the higher concentrations of L-arginine (100 μm), but not at the low concentration of 0.1 μm (Fig. 6). An inhibitory effect of sodium ions on cationic amino acid transport has been described for the system b0,+ (van Winkle et al. 1988). System b0,+ is additionally characterized by the ability to transport large neutral amino acids in a sodium-independent manner, and in the present experiments L-leucine (1 mM) inhibited L-arginine uptake in the absence of sodium ions by about 50 % (Fig. 7B and D). However, the situation is more complex, since in the presence of sodium ions, L-leucine markedly inhibited L-arginine uptake even at the low concentration of 100 μm (Fig. 7A and C). This suggests the additional involvement of system y+L which has been reported to transport cationic amino acids independently of sodium ions and concomitantly to show high affinity for large neutral amino acids in a sodium-dependent manner (Devés et al. 1993). Finally, NEM has been shown to inactivate system y+ without affecting system y+L (Devés et al. 1993). In the present experiments, pretreatment of the alveolar macrophages for 10 min with NEM resulted in a marked reduction of L-arginine uptake (Fig. 8). Altogether it becomes evident that in rabbit alveolar macrophages a rather heterogeneous population of transport systems appears to be involved in the transport of cationic amino acids. However, it is not clear at present whether the different transporter characteristics reflect separate transporter entities or different functional states of the same transporter. The fact that pretreatment with PMA caused a rapid change in some of the characteristics of L-arginine transport (see below) may support the latter possibility.
The activity of L-arginine transport in alveolar macrophages markedly changed depending on the time in culture. Compared with freshly prepared cells, there was a transient increase followed by a reduction by about 80 % below the initial values after 20 h in culture. It remains unclear which of the activation states may represent the basic situation in vivo, as ‘freshly isolated cells’ are not naive cells, because they had just experienced the isolation and adherence protocol which is certainly a particular cellular stress.
The presence of the phorbol ester PMA during the 20 h culture period not only prevented the decline in the activity of L-arginine transport, but enhanced it substantially above initial levels (Table 1, Fig. 2). Two additional observations indicate that this effect of PMA was mediated via specific activation of protein kinase C. (i) The phorbol ester α-PMA, which does not activate protein kinase C, had no effect on L-arginine transport. (ii) Staurosporine and chelerythrine, inhibitors of protein kinase C (Tamaoki et al. 1986; Herbert et al. 1990), opposed the effect of PMA. Following activation by phorbol esters, protein kinase C often shows rapid downregulation, but in the present study the effect of PMA did not vanish after 20 h of exposure to the phorbol ester. However, there exist several isoenzymes of protein kinase C and only some of them show rapid downregulation whereas others can maintain sustained responses to phorbol esters or diacylglycerol (for review see Dekker & Parker, 1994). A role for protein kinase C as a stimulatory pathway in the control of cationic amino acid transporters has already been demonstrated in human lymphocytes (Yoshimoto et al. 1992), human endothelial cells (Pan et al. 1995) and Caco-2 cells, an intestinal epithelial cell line (Pan & Stevens, 1995), whereas in mouse astrocytes staurosporine failed to affect L-arginine transport upregulated by LPS (Schmidlin & Wiesinger, 1995).
The mechanism by which staurosporine on its own caused a slight activation of L-arginine transport (Fig. 4) is unclear at present. However, it is unlikely that this effect involves protein kinase C, as chelerythrine was without effect. Likewise, staurosporine had stimulatory effects on the expression of NO synthase in endothelial cells (Li et al. 1996) and vascular smooth muscle (Hecker et al. 1997) by mechanisms unlikely to involve inhibition of protein kinase C.
Analysis of the kinetics of L-arginine uptake revealed that the stimulatory effect of PMA on L-arginine transport was mainly caused by an increase in Vmax (Fig. 3), indicating that activation of protein kinase C caused an increase in the amount of active transporter and/or enhanced the transporter activity. The fact that an effect of PMA was already evident after 30 min and was almost maximal after about 2 h (Figs 5 and 8) suggests that protein kinase C can cause an activation of pre-existing silent transporters. Alternatively, activation of protein kinase C may increase the activity of already functional transporters. The following observations support these assumptions.
(i) The short term effects of PMA were also seen in the presence of actinomycin D or cycloheximide, i.e. when the de novo synthesis of proteins was inhibited (Fig. 5).
(ii) Some characteristics of the L-arginine transport changed after exposure to PMA. (a) The inhibitory effect of NEM was lost after pretreatment with PMA, and this effect was identical independently of whether the cells were treated with PMA for only 2 or for 20 h (Fig. 8). Since NEM has been shown to inactivate system y+, but not system y+L (Debés et al. 1993), it may be concluded that transporters with properties of system y+ lost their significance in PMA-treated cells. One explanation could be that system y+ contributes significantly to the L-arginine uptake in untreated cells but the large increase in transport activity after PMA is caused by activation of transporters with characteristics of system y+L. However, in conflict with such an interpretation are the observations that sensitivity to the inhibitory effects of L-leucine (another discriminator between system y+ and y+L) was similar in PMA-treated and untreated cells. An alternative explanation for the loss of NEM sensitivity could be that the transporters representing system y+ may have changed some of their characteristics following activation by PMA. Thus, PMA could have caused structural changes in the transporter proteins with the consequence that either alkylation of sulfhydryl groups lost the functional signifiance or critical sulfhydryl groups may be less accessible for NEM. Whether this is caused by direct protein kinase C-mediated phosphorylation of transporter proteins (see also below) or whether protein kinase C affects modulatory proteins remains an open question which has to be addressed in future studies. (b) The Km was reduced after treatment with PMA, although only by a factor of two. Again, this could have been caused by changes in already active transporters or by the activation of a different transporter entity which then accounted for most of the L-arginine transport after PMA stimulation. (c) The observation that the Q10 value of the L-arginine uptake was reduced (i.e. the activation energy of the transporter was lower) after treatment with PMA further supports the assumption that protein kinase C can mediate alterations in the transporter proteins which may facilitate the translocation of L-arginine across the cell membrane.
Inhibition of the de novo synthesis of tranporter proteins during the 20 h culture period caused a large reduction in L-arginine uptake, both in the absence and presence of PMA, indicating that a continuous protein synthesis is required for the maintenance of L-arginine transport and its stimulation by PMA. However, stimulation of de novo synthesis of transporter proteins or of modulatory proteins appears not to be the primary effect of PMA in alveolar macrophages, whereas in the above-mentioned studies on lymphocytes, endothelial cells and Caco-2 cells (Yoshimoto et al. 1992; Pan et al. 1995; Pan & Stevens, 1995) an enhanced expression of transporter proteins was caused by activation of protein kinase C.
Since the cytoskeleton is known to be a particular target for protein kinase C (see Kiley et al. 1992; Dekker & Parker, 1994), it appeared to be an interesting idea to test whether the protein kinase C-mediated acute activation of L-arginine transport might involve a translocation of ‘silent’ transporter proteins to the cell surface. However, cytochalasin B, a toxin which inhibits microfilament-associated intracellular transport processes by inhibiting actin polymerization (Wessells et al. 1971) did not prevent the acute stimulatory effect of PMA. After 90 min exposure to cytochalasin B, L-arginine transport in the absence and presence of PMA was inhibited by about the same fraction (25-30 %), indicating that a cytochalasin B-sensitive trafficking of transporter proteins may be of significance in alveolar macrophages. However, the relatively similar reduction by cytochalasin B of L-arginine transport, independent of the state of activation of protein kinase C, suggests that the acute activiation of L-arginine transport via protein kinase C may not involve a cytochalasin B-sensitive mechanism, such as translocation. Therefore, it may be concluded that protein kinase C may cause activation of L-arginine transport by an action on transporter proteins (or modulators) already incorporated into the cell surface membrane.
L-Arginine uptake in rat and mouse alveolar macrophages
In rat and mouse alveolar macrophages, protein kinase C appears to play a similar role in the control of L-arginine transport to that in rabbit alveolar macrophages. However, the activity of L-arginine transport in alveolar macrophages of rat and mouse was higher than that in rabbit alveolar macrophages and this appeared to be caused mainly by the fact that protein kinase C was already considerably activated under basal culture conditions. This is indicated by the marked inhibitory effect of staurosporine and chelerythrine alone. The reason for these different states of activation of protein kinase C and of the L-arginine transport in alveolar macrophages of rabbits on the one side and of rat and mouse on the other side remains unclear at present, particularly as there were no differences in the way the cells were treated. Nonetheless, it can be seen that the overall magnitude of the modulatory effects of protein kinase C-mediated mechanisms on L-arginine transport is similar in alveolar macrophages from all three species. Though the effect of PMA alone is relatively smaller in rat and mice alveolar macrophages, for the estimation of the whole range of protein kinase C-mediated modulation of L-arginine transport, the difference between the reduced levels after staurosporine and the enhanced levels after PMA has to be considered (Fig. 9), and this difference is similar to what is seen in rabbit alveolar macrophages (compare with Fig. 4). Finally, it is interesting to note that a marked inhibitory effect of staurosporine on L-arginine uptake in rat alveolar macrophages was already seen after 1 h, indicating that in this species also protein kinase C is involved in the acute modulation of the activity of cationic amino acid transporters.
Although the present experiments do not allow a final conclusion about the molecular mechanisms by which protein kinase C may activate L-arginine transport in alveolar macrophages, it is worth noting that currently several proteins with properties of a cationic amino acid transporter (CAT) have been cloned, initially from murine cells (see Closs, 1996). They were originally named MCAT-1, MCAT-2A and MCAT-2B, the latter being a splicing variant of MCAT-2A. MCAT-2A is a low affinity transporter (Km > 2 mM) which appears to be expressed only in the liver and may therefore not be of significance for the present observations. On the other hand, the kinetic properties of MCAT-1 and MCAT-2B are indistinguishable and their Km values (between 0.14 and 0.38 mM) are comparable to those observed in the present study (between 0.32 and 0.65 mM). It should be mentioned that murine macrophages (RAW 264.7 cell line) expressed the MCAT-2B (Closs et al. 1993) and, in the present study, both in mouse and rat alveolar macrophages, mRNA for both CAT-1 and CAT-2B could be demonstrated by RT-PCR (Fig. 10). Although the limitation of RT-PCR with regard to quantitative statements should be kept in mind, the expression of mRNA for CAT-1 and CAT-2B appeared not to be reduced in rat alveolar macrophages cultured in the presence of staurosporine. This is at least in line with the above conclusions that protein kinase C may modulate the activity of pre-existing transporters. It is worth noting that the sequences of all CAT proteins possess several putative protein kinase C phosphorylation sites. Therefore, it will be an interesting task for future studies to characterize in more detail the CATs in alveolar macrophages, in particular to elucidate whether the large activation of the L-arginine transport is related to a direct phosphorylation of CAT proteins.
In conclusion, the present experiments showed multiple transport systems are involved in the uptake of L-arginine in alveolar macrophages and that protein kinase C represents an important stimulatory pathway in the acute control of the activity of L-arginine transport in these cells, probably by modulation of the activity of already expressed cationic amino acid transporters.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (Ra 400/9-1). We acknowledge the technical assistence of G. Ketterer for some of the initial experiments of this study. This paper contains part of the Dr. rer. nat. thesis of J. M.
References
- Ashendal CL. The phorbol ester receptor: a phospholipid-regulated protein kinase. Biochimia et Biophysica Acta. 1985;882:219–242. doi: 10.1016/0304-4157(85)90009-7. [DOI] [PubMed] [Google Scholar]
- Baydoun AR, Bogle RG, Pearson JD, Mann GE. Selective inhibition by dexamethasone of induction of NO synthase, but not of induction of L-arginine transport, in activated murine macrophage J774 cells. British Journal of Pharmacology. 1993;110:1401–1406. doi: 10.1111/j.1476-5381.1993.tb13976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg PM. In vitro studies on the mode of action of the phorbol esters, potent tumor promoters: part 1. Critical Reviews in Toxicology. 1980;8:153–197. doi: 10.3109/10408448009037493. [DOI] [PubMed] [Google Scholar]
- Bogle RG, Baydoun AR, Pearson JD, Moncada S, Mann GE. L-Arginine transport is increased in macrophages generating nitric oxide. Biochemical Journal. 1992;284:15–18. doi: 10.1042/bj2840015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs EI. CATs, a family of three distinct mammalian cationic amino acid transporters. Amino Acids. 1996;11:193–208. doi: 10.1007/BF00813860. [DOI] [PubMed] [Google Scholar]
- Closs EI, Lyons CR, Kelly C, Cunningham JM. Characterization of the third member of the MCAT family of cationic amino acid transporters. Journal of Biological Chemistry. 1993;268:20796–20800. [PubMed] [Google Scholar]
- Dekker LV, Parker PJ. Protein kinase C - a question of specificity. Trends in Biochemical Sciences. 1994;19:73–77. doi: 10.1016/0968-0004(94)90038-8. 10.1016/0968-0004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Devés R, Angelo S, Chávez P. N-Ethylmaleimide discriminates between two lysine transport systems in human erythrocytes. The Journal of Physiology. 1993;468:753–766. doi: 10.1113/jphysiol.1993.sp019799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas B, Mossalayi MD, Damais C, Kolb J-P. Nitric oxide production by human monocytes: evidence for a role of CD23. Immunology Today. 1995;16:574–580. doi: 10.1016/0167-5699(95)80080-8. 10.1016/0167-5699(95)80080-8. [DOI] [PubMed] [Google Scholar]
- Förstermann U, Kleinert H. Nitric oxide synthase: expression and expressional control of the three isoforms. Naunyn-Schmiedeberg's Archives of Pharmacology. 1995;352:351–364. doi: 10.1007/BF00172772. [DOI] [PubMed] [Google Scholar]
- Hecker M, Preiss C, Schinikerth VB. Induction by staurosporine of nitric oxide synthase expression in vascular smooth muscle cells: Role of NF-κB, CREB and C/EBPβ. British Journal of Pharmacology. 1997;120:1067–1074. doi: 10.1038/sj.bjp.0701026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochemical and Biophysical Research Communications. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Hey C, Boucher JL, Vadon-Le Goff S, Ketterer G, Wessler I, Racké K. Inhibition of arginase in rat and rabbit alveolar macrophages by Nω-hydroxy-D,L-indospicine, effects on L-arginine utilization by nitric oxide synthase. British Journal Pharmacology. 1997;121:395–400. doi: 10.1038/sj.bjp.0701143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hey C, Cebulla G, Stichnote C, Wessler I, Racké K. Differential control of L-arginine uptake, arginase and nitric oxide synthase in rabbit alveolar macrophages. British Journal Pharmacology. 1996;119:3. P. [Google Scholar]
- Hey C, Wessler I, Racké K. Nitric oxide synthase activity is inducible in rat, but not rabbit alveolar macrophages, with a concomitant reduction in arginase activity. Naunyn-Schmiedeberg's Archives of Pharmacology. 1995;351:651–659. doi: 10.1007/BF00170166. [DOI] [PubMed] [Google Scholar]
- Hibbs JB, Jr, Vavrin Z, Taintor RR. L-Arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. Journal of Immunology. 1987;138:550–565. [PubMed] [Google Scholar]
- Jorens PG, Van Overfeld FJ, Bult H, Vermeire PA, Herman AG. L-Arginine-dependent production of nitrogen oxides by rat pulmonary macrophages. European Journal of Pharmacology. 1991;200:205–209. doi: 10.1016/0014-2999(91)90573-9. 10.1016/0014-2999(91)90573-9. [DOI] [PubMed] [Google Scholar]
- Kiley SC, Parker PJ, Fabbro D, Jaken S. Hormone- and phorbol ester-activated protein kinase C isozymes mediate a reorganization of the actin cytoskeleton associated with prolactin secretion in GH4C1 cells. Molecular Endocrinology. 1992;6:120–131. doi: 10.1210/mend.6.1.1738365. 10.1210/me.6.1.120. [DOI] [PubMed] [Google Scholar]
- Li H, Kleinert H, Wallerath T, Förstermann U. Phorbol 12-myristate 13-acetate and staurosporine enhance mRNA expression of the endothelial nitric oxide synthase (NOS III) Naunyn-Schmiedeberg's Archives of Pharmacology. 1996;354:R39. [Google Scholar]
- Malandro MS, Kilberg MS. Molecular biology of mammalian amino acid transporters. Annual Review of Biochemistry. 1996;65:305–336. doi: 10.1146/annurev.bi.65.070196.001513. 10.1146/annurev.bi.65.070196.001513. [DOI] [PubMed] [Google Scholar]
- Mayer AM, Brenic S, Stocker R, Glaser KB. Modulation of superoxide generation in in vivo lipopolysaccharide-primed rat alveolar macrophages by arachidonic acid and inhibitors of protein kinase C, phospholipase A2, protein serine-threonine phosphatase(s), protein tyrosine kinase(s) and phosphatase(s) Journal of Pharmacology and Experimental Therapeutics. 1995;274:427–436. [PubMed] [Google Scholar]
- Modolell M, Corraliza IM, Link F, Soler G, Eichmann K. Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow-derived macrophages by TH1 and TH2 cytokines. European Journal of Immunology. 1995;25:1101–1104. doi: 10.1002/eji.1830250436. [DOI] [PubMed] [Google Scholar]
- Muscholl E, Ritzel H. The mechanism of neuronal noradrenaline and dopamine β-hydroxylase release by a sodium-deficient solution substituted with urea. Neuroscience. 1980;5:441–452. doi: 10.1016/0306-4522(80)90119-0. 10.1016/0306-4522(80)90119-0. [DOI] [PubMed] [Google Scholar]
- Pan M, Stevens BR. Protein kinase C-dependent regulation of L-arginine transport activity in Caco-2 intestinal cells. Biochimica et Biophysica Acta. 1995;1239:27–32. doi: 10.1016/0005-2736(95)00136-q. [DOI] [PubMed] [Google Scholar]
- Pan M, Wasa M, Lind DS, Gertler J, Abbott W, Souba WW. TNF-stimulated arginine transport by human vascular endothelium requires activation of protein kinase C. Annals of Surgery. 1995;221:590–601. doi: 10.1097/00000658-199505000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A, Pendreigh RH, Plevin R. Protein kinase C and tyrosine kinase pathways regulate lipopolysaccharide-induced nitric oxide synthase activity in RAW 264.7 murine macrophages. British Journal of Pharmacology. 1995;114:482–488. doi: 10.1111/j.1476-5381.1995.tb13252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidlin A, Wiesinger H. Stimulation of arginine transport and nitric oxide production by lipopolysaccharide is mediated by different signaling pathways in astrocytes. Journal of Neurochemistry. 1995;65:590–594. doi: 10.1046/j.1471-4159.1995.65020590.x. [DOI] [PubMed] [Google Scholar]
- Shapira L, Takashiba S, Champagne C, Amar S, Vandyke TE. Involvement of protein kinase C and protein tyrosine kinase in lipopolysaccharide-induced TNF-alpha and IL-1 beta production by human monocytes. Journal of Immunology. 1994;153:1818–1824. [PubMed] [Google Scholar]
- Tabor CW, Tabor H. Polyamines. Annual Review of Biochemistry. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tamaoki T, Nomoto H, Takahashi I, Kato M, Marimoto Y, Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca2+ dependent protein kinase. Biochemical and Biophysical Research Communications. 1986;135:397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Tapper H, Sundler R. Protein kinase C and intracellular pH regulate zymosan-induced lysosomal enzyme secretion in macrophages. Journal of Leukocyte Biology. 1995;58:485–494. doi: 10.1002/jlb.58.4.485. [DOI] [PubMed] [Google Scholar]
- van Winkle LJ, Campione AL, Gorman JM. Na+-independent transport of basic and zwitterionic amino acids in mouse blastocytes by a shared system and by processes which distinguish between these substrates. Journal of Biological Chemistry. 1988;263:3150–3163. [PubMed] [Google Scholar]
- van Winkle LJ, Christensen HN, Campione AL. Na+-dependent transport of basic, zwitterionic, and bicyclic amino acids by a broad scope system in mouse blastocytes. Journal of Biological Chemistry. 1985;260:12118–12123. [PubMed] [Google Scholar]
- Wang WW, Jenkinson CP, Griscavage JM, Kern RM, Arabolos NS, Byrns RE, Cederbaum SD, Ignarro LJ. Co-induction of arginase and nitric oxide synthase in murine macrophages activated by lipopolysaccharide. Biochemical and Biophysical Research Communications. 1995;210:1009–1016. doi: 10.1006/bbrc.1995.1757. 10.1006/bbrc.1995.1757. [DOI] [PubMed] [Google Scholar]
- Wessells NK, Spooner BS, Ash JF, Bradley MO, Luduena MA, Taylor EL, Wrenn JR, Yamada KM. Microfilaments in cellular and developmental processes. Science. 1971;171:135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]
- White MF. The transport of cationic amino acids across the plasma membrane of mammalian cells. Biochimica et Biophysica Acta. 1985;822:355–374. doi: 10.1016/0304-4157(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Yoshimoto E, Meruelo D. Enhanced gene expression of the murine ecotropic retroviral receptor and its human homolog in proliferating cells. Journal of Virology. 1992;66:4377–4381. doi: 10.1128/jvi.66.7.4377-4381.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


