Abstract
Microinjection studies have established that both NMDA and non-NMDA excitatory amino acid (EAA) receptor subtypes are involved in the integration of baroreceptor afferent inputs within the nucleus of the solitary tract (NTS). The present study was undertaken to determine which EAA receptor subtypes are involved in baroreceptor afferent integration by second and higher order NTS neurones.
Experiments utilizing intracellular recordings or extracellular recordings with microiontophoresis were performed in pentobarbitone-anaesthetized, paralysed and artificially ventilated rats to determine the ionotropic EAA receptor subtypes involved in baroreceptor afferent integration in the NTS. NTS neurones were classified according to their responses to aortic depressor nerve (ADN) stimulation: monosynaptic neurones (MSNs), polysynaptic neurones (PSNs) and ADN-non-evoked neurones (NENs).
In the extracellular studies, the ADN-evoked discharge of most MSNs was selectively reduced by microiontophoretic application of the non-NMDA receptor antagonists 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; n = 8, P < 0.05) or 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo(f)quinoxaline-7-sulphonamide (NBQX; n = 9, P < 0.01), but not by the NMDA antagonist dl-2-amino-5-phosphonopentanoic acid (AP-5; n = 6, P = 0.28). ADN-evoked responses of PSNs were attenuated by microiontophoretic application of AP-5 (n = 12, P < 0.001), CNQX (n = 13, P < 0.001) or NBQX (n = 11, P < 0.001). All EAA antagonists inhibited the spontaneous discharge of MSNs/PSNs and NENs (P < 0.01 for each group).
In the intracellular studies, ADN stimulation evoked faster rising and shorter duration excitatory postsynaptic potentials (EPSPs) in MSNs (n = 16) than in PSNs (n = 15) (P < 0.05 for each comparison).
Our results demonstrate that synaptic inputs from ADN to MSNs have faster rise times and shorter durations than those to PSNs, suggesting that baroreceptor inputs to MSNs and PSNs are mediated by different synaptic mechanisms. These more rapid synaptic events are selectively mediated by non-NMDA receptors. In addition, synaptic integration of ADN inputs by PSNs is mediated by both NMDA and non-NMDA receptors. Finally, the ADN-evoked discharge of some MSNs and PSNs is not attenuated by ionotropic EAA antagonists, suggesting that another receptor or transmitter system may mediate synaptic excitation in these neurones.
Excitatory amino acid (EAA) receptors within the nucleus of the solitary tract (NTS) are critical in the mediation of the arterial baroreflex. Hypotension and bradycardia typically result from the microinjection of NMDA and/or non-NMDA EAA agonists into the NTS (Talman et al. 1980; Kubo & Kihara, 1988; Galloudec et al. 1989; Leone & Gordon, 1989; Ohta & Talman, 1994; Tian & Hartle, 1994), whereas the microinjection of NMDA and/or non-NMDA EAA antagonists into the NTS causes hypertension and attenuates the baroreflex (Talman et al. 1981; Guyenet et al. 1987; Talman, 1989; Leone & Gordon, 1989; Galloudec et al. 1989; Kubo & Kihara, 1991; Gordon & Leone, 1991; Tian & Hartle, 1994).
In vitro electrophysiological studies of NTS neurones have provided information regarding the role of various EAA receptor subtypes in afferent integration. NTS neurones are depolarized during perfusion of slice preparations or acutely dissociated cells with NMDA and/or non-NMDA EAA agonists (Drewe et al. 1990; Tell & Jean, 1990; Drewe & Kunze, 1994; Nabekura et al. 1994), whereas the perfusion of slice preparations with NMDA and/or non-NMDA EAA antagonists blocks the responses of NTS neurones to stimulation of the solitary tract (Miller & Felder, 1988; Andresen & Yang, 1990; Brooks & Spyer, 1993).
However, the ionotropic EAA receptor subtypes that mediate excitation evoked by identified baroreceptor afferent inputs and the role of particular receptor subtypes at various levels of afferent integration are currently unknown. Zhang & Mifflin (1997) found the responses of NTS neurones to microiontophoretic application of selective EAA receptor agonists differed depending upon whether the neurone received a monosynaptic or a polysynaptic baroreceptor input. This suggested that monosynaptic neurones (MSNs) and polysynaptic neurones (PSNs) utilize different EAA receptor subtypes in the integration of baroreceptor afferent inputs. The present study was designed to define further the synaptic mechanisms of baroreceptor afferent integration by NTS neurones, and the roles of EAA receptor subtypes in the mediation of these synaptic inputs. Based on the results of our previous study as well as the microinjection and in vitro studies described above, we focused on NMDA and non-NMDA (kainate and AMPA) ionotropic EAA receptor subtypes.
The experimental goals of the present study were threefold. (1) To describe the physiological properties of the synaptic input from baroreceptor afferents to NTS neurones. (2) To determine the EAA receptor subtypes mediating the synaptic inputs from baroreceptors to different order NTS neurones. (3) To determine whether there are tonic EAA inputs to aortic depressor nerve (ADN)-evoked and ADN-non-evoked NTS neurones. Baroreflex-related NTS neurones in vivo were identified by their responses to stimulation of the ADN, which, in rats, contains primarily baroreceptor afferent fibres (Sapru & Krieger, 1977; Sapru et al. 1981; Cheng et al. 1997). The ADN input was characterized as monosynaptic or polysynaptic using electrophysiological methods (Miles, 1986; Scheuer et al. 1996; Zhang & Mifflin, 1997). The results indicate that low frequency ADN stimulation evokes excitation in MSNs via non-NMDA EAA receptors, while in PSNs ADN-evoked excitation is mediated via both NMDA and non-NMDA EAA receptors.
METHODS
Animals
Successful experiments were performed on seventy-six male Sprague-Dawley rats (330-450 g; Charles River Laboratories, Wilmington, MA, USA). Rats were housed two per cage in a fully accredited (AAALAC and USDA) laboratory animal room with free access to food and water. All experimental rats were given at least 1 week to acclimatize before use. All experimental protocols were approved by the Institutional Animal Care and Use Committee. At the end of the experiments, animals were killed by an overdose of pentobarbitone sodium (100 mg kg−1, i.v.).
Surgical preparation
Rats were initially anaesthetized with pentobarbitone sodium (60 mg kg−1, i.p.) and were placed on a thermostatically controlled heating pad. Body temperature was maintained at 36-38°C throughout the experiment. After placement of a venous catheter (tail vein) and cannulation of the trachea, the animal was artificially ventilated with oxygenated room air, and subsequent anaesthetic was given as an infusion of 10–20 mg kg−1 h−1 (i.v.). Gallamine triethiodide (initially 40 mg kg−1, i.v., supplemented with 20–40 mg kg−1 h−1) was also given for neuromuscular blockade. A femoral artery was cannulated for arterial blood pressure monitoring. Arterial pressure was measured using a Cobe CDX transducer (Cobe Laboratories, Lakewood, CO, USA). Mean arterial blood pressure and heart rate were determined from the pulsatile signal using a Coulbourn blood pressure processor (Coulbourn Instruments, Allentown, PA, USA). Depth of anaesthesia was assessed by monitoring the stability of arterial blood pressure and heart rate during hindpaw pinch, and was adjusted by appropriate changes in the infusion rate. ADNs were isolated bilaterally and marked with small pieces of black suture. After all surgical procedures were performed, the rat was placed in a stereotaxic head frame and an occipital craniotomy was performed to expose the dorsal surface of the medulla in the region of the obex. The ADN ipsilateral to the central recording site was mounted on bipolar stimulating electrodes. The ADN was stimulated with single pulses of 0.5-1 ms duration, 500 μA intensity, and at an interstimulus interval of 1.5 s.
Intracellular recordings
Intracellular recordings were made using single-barrel electrodes filled with 2–4 % neurobiotin in 0.05 M Tris buffer (diameter of electrode tips, < 1 μm). All recordings were performed 1.2 mm caudal and 0.5 mm rostral to the calamus scriptorius, 0-0.8 mm lateral to the mid-line, and 0.2-1 mm below the surface. The electrode was lowered into the tissue in 1 μm steps by a stepdriver controller (Nanostepper; ALA Associates, Westbury, NY, USA). To ensure that a good penetration was made, the membrane potential had to exceed 50 mV after 1 min of observation and the membrane potential drift had to be less than 5 mV during the data collection period. Membrane potentials were amplified by a DC amplifier (World Precision Instruments), and sent to a digital oscilloscope (Nicolet Instrument Co., Madison, WI, USA), an audiomonitor (Grass Instrument Co.), a videotape recorder and a CED1401 analog-to-digital converter (CED, Cambridge, UK) interfaced with a PC. Sigavg data acquisition software (CED) was used for analysis. When a NTS neurone was successfully impaled and an ADN-evoked excitatory or inhibitory postsynaptic potential (EPSP or IPSP) was observed, the ADN input was characterized as monosynaptic or polysynaptic. The criterion for the identification of MSNs and PSNs was originally used by Miles (1986) in an in vitro study and subsequently in in vivo electrophysiological studies (Scheuer et al. 1996; Zhang & Mifflin, 1997). A recent immunocytochemical study (Scheuer & Mifflin, 1998) verified the veracity of the criterion. In MSNs, ADN-evoked EPSPs or action potentials could follow two ADN stimuli separated by 5 ms. Most recorded cells (14/16 MSNs and 12/15 PSNs) were labelled by neurobiotin and were examined later with immunocytochemical methods to verify that the recording site was within the NTS.
Extracellular recordings and microiontophoresis
Extracellular action potential discharge was recorded and microiontophoretic application of drugs performed with a five-barrel electrode (ASI Instrument, Warren, MI, USA) with a tip diameter of 1.5-2.5 μm. The recording barrel was filled with a solution of 0.5 M sodium acetate containing 2 % Chicago Sky Blue (impedance, 8–30 MΩ). One barrel of each five-barrel electrode was filled with a solution of 3 M NaCl and was used for automatic current balancing and current and pH control measurements. The remaining three barrels were filled with different drug solutions. The five-barrel electrode was lowered into the tissue in 2.0-2.5 μm steps by a stepdriver controller (Burleigh Instrument Inc., Fishers, NY, USA) and the signals from the DC amplifier were sent to an AC filter, and then to the digital oscilloscope, audiomonitor, videotape recorder, and window discriminator (World Precision Instruments). The window discriminator output was led to the oscilloscope and to an analog-to-digital converter (CED) interfaced with the PC. Spike2 data acquisition software (CED) was used for on- and off-line analysis. Peristimulus time (PST) histograms (duration, 1.5 s; bin width, 1 ms; 40 sweeps) and ratemeter histograms (duration, 180 s; bin width, 1 s) were collected to analyse extracellular evoked and spontaneous discharge, respectively. Each PST histogram took 60 s to collect (1.5 s interstimulus interval × 40 sweeps = 60 s). One to two PST histograms were collected during baseline, during drug application and after drug application to ensure return to control levels of discharge. In rate histogram collection, baseline levels of discharge were collected for at least 25–60 s before application of the drugs.
Drugs and drug administration in extracellular recordings
After baseline spontaneous discharge rate and/or ADN-evoked responses were recorded, drugs were administered by application of ejecting currents to the drug-containing barrels, using a microiontophoretic current generator (Medical System Corp., Great Neck, NY, USA). To examine drug-induced effects on spontaneous discharge, EAA antagonists were ejected for successive 10 s periods separated by 10–20 s intervals until a steady-state level of effect was achieved. To examine antagonist effects on ADN-evoked discharge, drugs were applied continuously during collection of the PST histogram. The drug solutions for microiontophoresis were: L-glutamic acid (monosodium salt, 100 mM; Sigma), NMDA (100 mM; Sigma), (RS)-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA, 10 mM; Tocris Cookson, Bristol, UK), kainic acid (10 mM; Sigma), dl-2-amino-5-phosphonopentanoic acid (AP-5, 10 mM; Tocris Cookson), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 5 mM; RBI) and 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo(f)quinoxaline-7-sulphonamide (NBQX, 4 mM; RBI). All drugs were dissolved in 150 mM saline and pH was adjusted to 8.0-8.5. All drugs were ejected as anions. Cationic retaining currents were applied to the drug barrels to retard the passive diffusion of the drug from the electrode tip during non-ejection periods.
Data analysis
Data were analysed with MANOVA (ANOVA) or ANCOVA with a repeated-measure design (StatSoft, Tulsa, OK, USA). The Newman-Keuls test was used for post hoc comparisons. PST histograms were used to analyse the evoked discharge as well as spontaneous discharge in ADN-evoked cells. Ratemeter records (spikes s−1) were used for statistical analysis of firing rate only for the spontaneous discharge of NENs. To account for different levels of basal spontaneous discharge and drug effects on spontaneous discharge, the discharge rate increase (equal to the discharge rate during the drug application minus baseline discharge rate) was used for analysis. All cells sampled in a given group were averaged for analysis, and therefore each group includes both drug-sensitive and drug-insensitive cells. An arbitrary criterion of a change in baseline and/or evoked discharge of greater than 10 % was used for adjusting the significant responses of a single cell to the drug application. Changes of less than 10 % of baseline were considered as normal baseline variation. All values are expressed as means ±s.e.m. and significance was accepted at P < 0.05.
RESULTS
Extracellular recording and microiontophoresis
General characteristics of NTS neurones
Data were obtained from 115 NTS neurones, fifty-six of which were evoked by stimulation of ADN, and fifty-nine of which were not evoked by the ADN stimulation (non-evoked neurones, NENs). These numbers do not represent the relative proportion of ADN-evoked and ADN-non-evoked neurones in the NTS because not every NEN encountered was studied. The vast majority of NTS neurones encountered (> 80 %) were NENs. Of the fifty-six ADN-evoked NTS neurones, twenty-one were characterized as MSNs and thirty-five as PSNs. The mean onset latency of MSNs (13.2 ± 2.0 ms; range, 2.0-32 ms) was significantly shorter (P < 0.001) than that of PSNs (25.4 ± 1.0 ms; range, 14–38 ms). Ten of twenty-one MSNs and twenty-five of thirty-five PSNs were spontaneously active. The mean discharge rate for the spontaneously active MSNs was 1.8 ± 0.3 Hz, and was lower (P < 0.05) than that of the spontaneously active PSNs (4.8 ± 0.9 Hz). In addition, all NENs encountered were spontaneously active with a mean firing rate of 5.6 ± 0.6 Hz.
In a few MSNs (n = 4) it was possible to examine the responses to blood pressure increases induced by i.v. injection of phenylephrine. Figure 1A illustrates a MSN that responded to ADN stimulation with a short latency response and therefore probably received myelinated ADN inputs. The increase in blood pressure produced by i.v. phenylephrine (2 μg kg−1) resulted in action potential discharge frequencies greater than 100 Hz, without a significant change in action potential amplitude. Figure 1B illustrates a longer latency MSN which probably received non-myelinated ADN inputs. Similar increases in blood pressure induced comparatively moderate increases in discharge frequency.
Figure 1. Responses of two ADN-evoked NTS neurones (MSNs) to increases in blood pressure induced by i.v. phenylephrine.

Aa, a short latency MSN. This neurone responded to single- (left, 5 sweeps) and double-pulse (right) ADN stimulation (stimulus artifacts indicated by * in this and subsequent figures). At a 5 ms interval, double pulses evoked two action potentials (right), indicating this neurone is a MSN. Ab, in the same neurone, increasing blood pressure by i.v. phenylephrine (2 μg kg−1, bottom panel; ABP, arterial blood pressure) elicited discharge of up to 100 Hz without changes in action potential amplitude (top panel is the extracellularly recorded action potential discharge, AP; middle panel is the ratemeter record of discharge frequency, 1 s bin width). Ba, a long latency MSN. This neurone responded to single- (left, 5 sweeps) or double-pulse (right) ADN stimulation. At a 5 ms interval, double pulses evoked two action potentials (right), indicating this neurone is a MSN. Bb, PST histograms (40 sweeps) of responses to single-pulse (left PST histogram) and double-pulse (right PST histogram) ADN stimulation. Bc, increasing blood pressure with phenylephrine (2 μg kg−1, i.v., lower panel) also increased the discharge of this neurone (upper panel, ratemeter record, 1 s bin width).
Demonstration of EAA antagonist selectivity
The selectivity of the various EAA antagonists was examined by their ability to block selectively discharge induced by selective EAA agonists (AMPA, kainate and NMDA). Figure 2A illustrates that microiontophoretic application of AP-5 (20 nA) selectively blocked NMDA-induced excitation in a NTS neurone (top panel), and did not alter AMPA- or kainate-induced excitation (middle and bottom panels) at even higher currents (up to 40 nA). Figure 2B illustrates that microiontophoretic application of NBQX selectively blocked AMPA- and kainate-induced excitation at low ejection current (20 nA), and did not block NMDA-induced excitation at the same or higher ejecting currents (up to 40 nA). The selectivity of CNQX was similarly tested and, like NBQX, CNQX blocked non-NMDA-induced, but not NMDA-induced, excitation (data not shown). Ejection currents of greater than 40 nA were often accompanied by non-specific antagonist effects or anomalous effects attributed to effects on nearby neurones. For these reasons, ejection currents of 40 nA or less were used.
Figure 2. Tests of the selectivity of NMDA (A) and non-NMDA (B) antagonists on EAA subtype-specific agonist-induced excitation of NTS neurones.

The sweeps are arranged with a ratemeter record of spontaneous discharge (in spikes s−1; 1 s bin width) above a trace of mean arterial blood pressure (MABP). The selective EAA agonists (iontophoretic current intensities indicated in parentheses) were applied during the periods indicated by the short bars. The periods of co-iontophoretic application of EAA antagonists (long bars) and the current intensities are indicated above the agonist application bars in each panel.
Effects of EAA antagonists on the spontaneous discharge of NTS neurones
The effects of the various EAA antagonists were first examined on the spontaneous discharge of NTS neurones. The spontaneous discharge of most NTS neurones was decreased by AP-5 (2/2 MSNs, 7/7 PSNs and 7/11 NENs), NBQX (2/5 MSNs, 8/9 PSNs and 22/28 NENs) and CNQX (2/2 MSNs, 9/10 PSNs and 16/20 NENs). Figure 3A illustrates examples of the effects of EAA antagonists on the spontaneous discharge of NENs. AP-5 (top panel), NBQX (middle panel) and CNQX (bottom panel) all inhibited spontaneous discharge in a current-dependent manner. The population responses of the effects of EAA antagonists on the spontaneous discharge of NTS neurones are shown in Fig. 3B (for NENs) and Fig. 4B (for MSNs and PSNs). In most cells, the effects of EAA antagonists on spontaneous discharge were current dependent, except for the effects of AP-5 on NENs. AP-5 decreased the spontaneous discharge of most NENs (5/7) in a non-current-dependent manner. In certain groups, as few as two neurones were spontaneously active; therefore the data regarding the effects of EAA antagonists on the spontaneous activity of MSNs and PSNs were pooled. All EAA antagonists inhibited the spontaneous discharge of MSNs/PSNs (P < 0.01 for each group) and NENs (P < 0.01 for each group). NBQX and CNQX were more potent inhibitors of the spontaneous discharge of NENs than AP-5 (P < 0.05, Fig. 3B).
Figure 3. Effects of EAA antagonists on the spontaneous discharge of NENs.

A, ratemeter records from 3 NENs illustrate inhibition of spontaneous discharge by microiontophoretic application of AP-5 (top panel), NBQX (middle panel) and CNQX (bottom panel). Periods of drug application are indicated by the bars, with the current intensity given above each bar. B, the effects of the EAA antagonists on the spontaneous discharge of NENs for the population studied. The concentration of each drug and the number (n) of cells in each group are given in parentheses. All three EAA antagonists inhibited the spontaneous discharge of NENs, with NBQX and CNQX exhibiting an apparently higher potency than AP-5 (P < 0.05).
Figure 4. The inhibition of ADN-evoked discharge and spontaneous discharge in MSNs and PSNs by selective EAA antagonists.

A, inhibition of ADN-evoked discharge of MSNs (□) and PSNs (▪) during iontophoresis of EAA antagonists. The iontophoretic current intensities are given in parentheses below each drug. The ADN-evoked responses of PSNs were attenuated by both NMDA and non-NMDA antagonists, while those of MSNs were only attenuated by non-NMDA antagonists. Number of cells (n) in each group for MSNs and PSNs, respectively: AP-5, n = 6 and 12; NBQX, n = 9 and 11; CNQX, n = 8 and 13. B, the spontaneous discharge of MSNs and PSNs was inhibited by all 3 EAA antagonists. □, AP-5 (10 mM; n = 9, 7 PSNs, 2 MSNs);  , NBQX (4 mM; n = 14, 9 PSNs, 5 MSNs); ▪, CNQX (5 mM; n = 12, 10 PSNs, 2 MSNs). †P < 0.05; ††P < 0.01.
, NBQX (4 mM; n = 14, 9 PSNs, 5 MSNs); ▪, CNQX (5 mM; n = 12, 10 PSNs, 2 MSNs). †P < 0.05; ††P < 0.01.
Effects of EAA antagonists on ADN-evoked responses
AP-5
Microiontophoretic application of AP-5 (20 and 40 nA) reduced ADN-evoked responses in most PSNs (11/12, P < 0.001), but in only one of six MSNs (P = 0.28) (Fig. 4A). At 40 nA ejection current, eleven PSNs responded to AP-5 with a reduction of 12–100 % of the baseline, and one PSN did not show any obvious response to AP-5 (change of less than 10 % of baseline). In the MSN group, however, only one cell was sensitive to AP-5, with a reduction of 82.5 % of baseline at the 40 nA ejection current. Group comparisons indicated that there was a significant difference between MSN and PSN groups (P < 0.05) in their responses to AP-5. Figure 5A illustrates the lack of effect of AP-5 on a MSN. Compared with the control response (a), AP-5 at 20 nA (b) and 40 nA (c) did not decrease the ADN-evoked discharge. In Fig. 6Aa-f, microiontophoretic application of AP-5 at 20 and 40 nA decreased the spontaneous discharge and the ADN-evoked responses of a PSN in a current-dependent manner. As illustrated in the population responses of ADN-evoked neurones to AP-5 in Fig. 4A, both ejection currents of AP-5 decreased ADN-evoked discharge in PSNs, but not in MSNs.
Figure 5. Effects of EAA antagonists on the ADN-evoked responses of MSNs.

A, lack of effect of AP-5 on the ADN-evoked discharge of a MSN. This MSN was identified by its ability to follow double-pulse ADN stimulation (top left). The ADN-evoked discharge of this neurone was not attenuated by AP-5 (up to 40 nA) as illustrated on 4 PST histograms (a-d). One sweep of raw data corresponding to each of the 4 PST histograms is presented in the top right panel with the number of the corresponding PST histogram given above each sweep. B, responses of a MSN to microiontophoretic application of CNQX. Each panel consists of 10 sweeps of the response to ADN stimulation, with the sweeps offset by an arbitrary amount to aid visualization. Compared with the control (a), the somato-dendritic component of the ADN-evoked action potential was inhibited by application of CNQX at intensities of 20 nA (b); however, this intensity did not inhibit the initial segment component of the evoked action potentials. At higher ejection currents (40 nA, c) some of the evoked initial segment components were inhibited.
Figure 6. Potent inhibition of ADN-evoked and spontaneous discharge of PSNs by both NMDA and non-NMDA antagonists.

A, top left panel illustrates inability to follow double-pulse ADN stimulation, therefore the neurone was classified as a PSN. PST histograms (a-f) illustrate inhibition of ADN-evoked and spontaneous discharge during iontophoresis of AP-5 at the current intensities indicated. One sweep of raw data corresponding to a PST histogram is presented in the top right panel with the number of the corresponding PST histogram given above each sweep. B, inhibition of spontaneous and evoked discharge of a PSN by NBQX. Panels arranged as in A.
NBQX and CNQX
The population responses of the ADN-evoked discharge of NTS neurones to NBQX and CNQX are illustrated in the middle and bottom panels of Fig. 4A. NBQX decreased the ADN-evoked discharge of six of nine MSNs (range, 15.4-74.4 % reduction at 40 nA ejection current for 6 responding cells; P < 0.05 for all cells at both 20 and 40 nA ejection currents compared with the baseline) and ten of eleven PSNs (range, 11.0-100 % reduction at 40 nA ejection current; P < 0.001 for all cells at both ejection currents). CNQX similarly decreased the ADN-evoked discharge of five of eight MSNs (range, 14.0-73.7 % reduction at 40 nA ejection current; P < 0.05 for all cells at 40 nA ejection current, but not at 20 nA ejection current) and all thirteen PSNs (range, 34.4-100 % reduction at 40 nA ejection current; P < 0.001 for all cells at both ejection currents). Because the ADN-evoked discharge of some MSNs was resistant to blockade by NBQX and CNQX, when viewed as populations the blockade of ADN-evoked discharge by these non-NMDA antagonists was weaker in MSNs than in PSNs (P < 0.05 for each comparison). Therefore, the population of MSNs appeared to be composed of two groups of neurones with distinct types of response to non-NMDA antagonists. In the first group (n = 11), ADN-evoked discharge was attenuated by CNQX or NBQX in a manner indistinguishable from the PSNs. In the second group (n = 6), ADN-evoked discharge was resistant to CNQX and NBQX.
It is worth noting that although the ADN-evoked discharge of some NTS neurones was not decreased by these non-NMDA antagonists, these cells were not totally unresponsive to the non-NMDA antagonists. In some MSNs (see example in Fig. 7), NBQX or CNQX decreased spontaneous discharge without altering the ADN-evoked discharge. Of thirteen ADN-evoked NTS neurones with an evoked discharge not sensitive to EAA antagonists (6 to AP-5, 4 to NBQX and 3 to CNQX), eight were spontaneously active. The spontaneous discharge, but not the evoked discharge, of seven neurones (2 MSNs to AP-5, 2 MSNs and 1 PSN to NBQX, and 2 MSNs to CNQX) was inhibited by EAA antagonists, with the exception of one MSN in which the spontaneous activity was also insensitive to NBQX.
Figure 7. Example of a MSN in which NBQX selectively attenuated spontaneous discharge without changing the ADN-evoked response.
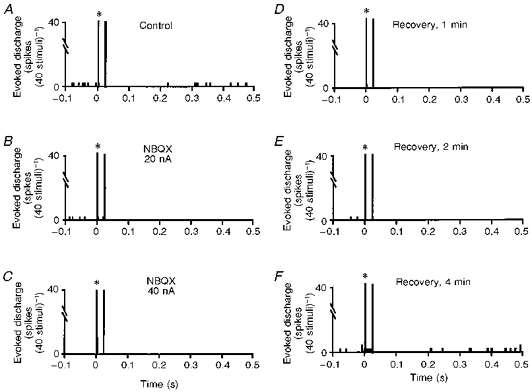
A, control PST histogram. B and C, microiontophoretic application of NBQX (20 and 40 nA, respectively) decreased the spontaneous discharge of this neurone in a current-dependent manner, but did not affect the ADN-evoked responses. D-F, the spontaneous discharge took 4 min to recover to baseline.
In addition, two other characteristics were observed. First, the EAA antagonist-induced attenuation of either spontaneous or ADN-evoked discharge was not accompanied by changes in the amplitude or duration of the action potential. This indicates that the EAA antagonists had little effect on the membrane potential or excitability of the neurones. In contrast, GABA, an inhibitory amino acid that reduces the excitability of NTS neurones, produces pronounced changes in action potential amplitude and duration while inhibiting the evoked and spontaneous discharge of NTS neurones (Mifflin & Zhang, 1996). Second, lower ejection currents of NBQX or CNQX decreased the ADN-evoked somato-dendritic action potential in some neurones (5 MSNs and 1 PSN) without decreasing the ADN-evoked initial segment spike (see examples in Fig. 5Bb3). At higher ejection currents (40 nA), the ADN-evoked initial segment spike (see example in Fig. 5Bc3) was also inhibited.
Intracellular studies
The responses of thirty-one ADN-evoked NTS neurones were successfully recorded. Sixteen of these were characterized as MSNs and fifteen as PSNs (Fig. 8A). The ADN-evoked responses of the majority of NTS neurones consisted of an EPSP which, in some cells, was followed by an IPSP. In MSNs, ADN stimulation evoked solely an EPSP in eleven cells and an EPSP/IPSP in five cells. In PSNs, ADN stimulation evoked solely an EPSP in eleven cells and an EPSP/IPSP in four cells. Between one and four action potentials were often generated by the EPSP. Several electrophysiological parameters in MSNs were different from those in PSNs (Fig. 8B). First, the onset latencies of ADN-evoked responses of MSNs (6.6 ± 1.3 ms; range, 1.9-16 ms) were significantly shorter (P < 0.001) than those of PSNs (20.7 ± 1.7 ms; range, 9–35 ms). Second, the duration of the ADN-evoked EPSP in MSNs (10.5 ± 1.2 ms; range, 3–23 ms) was much shorter (P < 0.001) than that in PSNs (21.8 ± 2.0 ms; range, 6–35 ms). Third, the rising phase of the EPSP in MSNs (1.5 ± 0.2 V s−1) was faster (P < 0.05) than that in PSNs (0.8 ± 0.2 V s−1). Resting membrane potential (59.5 ± 2.0 mV for MSNs and 61.2 ± 2.2 mV for PSNs) and the amplitude of the ADN-evoked EPSP (6.2 ± 0.8 mV for MSNs and 7.8 ± 1.0 mV for PSNs) were not significantly different (P = 0.56 and P = 0.22, respectively) between MSNs and PSNs.
Figure 8. Intracellular studies of ADN-evoked NTS neurones.

A, examples of a PSN (top panel of sweeps) and a MSN (middle panel of sweeps) receiving ADN inputs. ADN stimulation evoked an EPSP which occasionally generated an action potential in each cell. Only the MSN followed double-pulse ADN stimulation (compare the right-hand sweeps in the top and middle panels). The EPSPs from these 2 cells are superimposed in the bottom panel. The EPSP in the MSN has a faster rise time and a shorter duration than the EPSP from the PSN. B, various properties of MSNs (□, n = 16) and PSNs (▪, n = 15), and ADN-evoked EPSPs in the population of NTS neurones studied. The EPSP in PSNs had a significantly longer duration (upper left histogram) and slower rate of rise (upper right histogram) than that in MSNs (†P < 0.05, ††P < 0.01).
DISCUSSION
The present study is the first to compare, in vivo at the single cell level, the effects of selective EAA receptor antagonists on NTS neurones involved in baroreceptor regulation of cardio-respiratory function, to determine the role of the different ionotropic EAA receptor subtypes in the mediation of baroreceptor-evoked excitation of second and higher order neurones. The results demonstrate that, within the NTS, NMDA and non-NMDA receptors play a critical role in the integration of arterial baroreceptor afferent inputs. However, the relative contribution of each receptor subtype varies depending upon the level of afferent integration.
Ionotropic EAA receptors and baroreceptor afferent integration
The present study found that both NMDA and non-NMDA receptor subtypes were involved in ADN, and thereby baroreceptor, afferent integration in the NTS. These receptor subtypes are utilized at different levels of afferent processing in response to low frequency afferent stimulation. The ADN-evoked discharge of most second order neurones was selectively attenuated by non-NMDA antagonists, but not by an NMDA antagonist. The ADN-evoked discharge of most higher order neurones was attenuated by antagonists for both ionotropic receptor subtypes. In most MSNs, the non-NMDA receptor subtype(s), rather than the NMDA receptor subtype, plays the major role in transmission across the first synapse from ADN afferent fibre to second order neurone in the NTS during low frequency afferent stimulation. Similar findings have been reported in other brain regions. For example, monosynaptic responses of tectal neurones to optic tract stimulation are attenuated by non-NMDA antagonists, but not by NMDA antagonists. In contrast, polysynaptic responses of tectal neurones to optic tract stimulation are reduced by both NMDA and non-NMDA antagonists (Hickmott & Constantine-Paton, 1993). Non-NMDA receptors, not NMDA receptors, are involved in the neurotransmission of cardiopulmonary C fibre inputs to NTS neurones (Wilson et al. 1996).
Our finding that NMDA receptors within the NTS are involved in the baroreflex is consistent with in vivo microinjection studies (Kubo & Kihara, 1988, 1991; Galloudec et al. 1989; Ohta & Talman, 1994) with the exception of one study which observed that the microinjection of an NMDA antagonist did not block the baroreflex (Gordon & Leone, 1991). Our results indicate that the blockade of NMDA receptors by NTS microinjections will only affect that component of the reflex mediated by the NMDA-sensitive discharge of PSNs in the NTS. The component transmitted through non-NMDA receptors could be sufficient to maintain reflex function.
Our finding that NMDA receptors do not play a major role in transmission from primary afferent to second order neurone during low frequency afferent stimulation is consistent with the results of most in vitro studies (Andresen & Yang, 1990; Drewe et al. 1990; Drewe & Kunze, 1994). As discussed in the Results, the lack of an NMDA component to ADN-evoked discharge was not related to the fact that the spontaneous discharge of MSNs was lower than that of PSNs. Further support for this finding is the observation that most MSNs were insensitive to microiontophoretic application of NMDA (Zhang & Mifflin, 1997). In addition, a preliminary study has found that the vast majority of MSNs do not possess NMDA-R1 receptor subunit immunoreactivity on the soma and proximal dendrites (Scheuer et al. 1997). However, a recent in vitro study reported that there was an NMDA component to the inward current observed during stimulation of the tractus in NTS neurones which presumably received a monosynaptic tractus input (Luz Aylwin et al. 1997). One explanation for the difference between the results of this study and ours is that the primary criterion used in the in vitro study to determine whether an input was monosynaptic was a short, invariant onset latency. The ability to follow two stimuli separated by 5 ms was confirmed in only five of twenty-eight cells studied. In contrast, we relied primarily on this latter criterion as, in our experience, many of our PSNs exhibited minimal onset latency variability (Fig. 6B) while some of our longer latency MSNs exhibited greater variability (Fig. 1B). A second explanation for the difference could be that in vitro stimulation of the tractus could directly stimulate MSNs which lie close to the tractus and then project to PSNs. Thus, some MSNs identified in vitro could well be PSNs.
A potential caveat to the observations made during application of EAA antagonists arises if one considers whether the reduction in response to ADN stimulation was the result of an attenuation of ADN synaptic transmission, or merely a reflection of a reduced background excitability. We do not believe that our results reflect a reduction in spontaneous discharge or neuronal excitability for the following reasons. First, in our analysis the background level of spontaneous discharge was subtracted from the discharge classified as evoked, so that the reported responses are those mediated by ADN stimulation and do not include the ongoing, spontaneous discharge. Second, during the application of EAA antagonists, the amplitude and duration of evoked active potentials did not change. In contrast, the inhibition of ADN-evoked discharge by the inhibitory amino acid GABA, which increases membrane potential and conductance, is associated with large changes in action potential amplitude and duration (Mifflin & Zhang, 1996). This indicates that the membrane potential was not changed during EAA antagonist application. Thus, excitability changes do not appear to be a component of the EAA antagonist-induced reduction of the evoked responses in the NTS. Third, the blockade of ADN-evoked responses by EAA antagonists was not selective for neurones with spontaneous activity. The spontaneous activity recorded possibly represents non-ADN inputs to these neurones. For example, NBQX reduced the evoked responses of ten of eleven PSNs, including two PSNs with no spontaneous activity. The one PSN in which the evoked responses were not sensitive to NBQX was spontaneously active. AP-5 attenuated the evoked responses of only one MSN, and this MSN was not spontaneously active. The evoked responses of the remaining five MSNs, which included two spontaneously active ones, were not sensitive to AP-5. The spontaneous discharge, but not the ADN-evoked discharge, of these two MSNs was reduced by AP-5, further indicating a dissociation between drug effects on spontaneous and evoked discharge. Fourth, our intracellular results strongly correlate with our extracellular results regarding an NMDA involvement in synaptic transmission at different afferent levels. In conclusion, our results and interpretation are consistent with those of the previously discussed in vitro studies which have also reported that EAA antagonists block EPSPs evoked by tractus stimulation independently of changes in neuronal excitability.
MSNs insensitive to EAA antagonists
Consistent with our previous finding that some NTS neurones were not sensitive to NMDA or non-NMDA EAA agonists (Zhang & Mifflin, 1997), the ADN-evoked discharge of some NTS neurones was not attenuated by either NMDA or non-NMDA antagonists. To ensure this was not a technical problem, electrodes that recorded insensitive NTS neurones were carefully checked. We frequently found that, in the same rat, using the same electrode and the same ejecting currents, ADN-evoked discharge was attenuated by microiontophoretic application of CNQX or NBQX in one NTS neurone (for example, in a PSN), but was unaltered in another NTS neurone (for example, in a MSN). Of our total population of seventeen MSNs tested by non-NMDA antagonists, the ADN-evoked discharge of six was totally insensitive to these antagonists. One explanation for this insensitivity could be that the ADN synaptic inputs to these MSNs are located on distal dendrites and not on the soma or proximal dendritic area; therefore, our iontophoretically applied EAA antagonists could not reach these remote synapses. Why this arrangement would mainly occur in MSNs and not in PSNs is unclear, and both the soma and proximal dendrites of NTS neurones are densely innervated by ADN afferent fibre terminals (Mendelowitz et al. 1992). The fast rise time of ADN-evoked EPSPs in MSNs also suggests that these inputs are located close to the soma. Another explanation could be that the ADN input to EAA-insensitive neurones is not mediated by ionotropic EAA receptors. Consistent with this hypothesis is the previously discussed insensitivity of some NTS neurones to EAA agonists (Zhang & Mifflin, 1997). The existence of another transmitter capable of mediating fast transmission from some ADN fibres to second order NTS neurones should be considered.
Intracellular results
Consistent with the microiontophoretic study, our intracellular study confirmed that the synaptic mechanisms underlying transmission from baroreceptor to MSNs are different from those from baroreceptors to PSNs. Following ADN stimulation, faster rising and shorter duration EPSPs were observed in MSNs than in PSNs. This more rapid and transient form of synaptic transmission is consistent with mediation by non-NMDA receptors, which have been reported to mediate fast synaptic neurotransmission in most other brain regions (Collingridge et al. 1988; Hablitz & Sutor, 1990; Nakanishi et al. 1992; Hickmott & Constantine-Paton, 1993). However, it is important to keep in mind that we have no information on the relative convergence either of ADN afferent fibres onto second order neurones within the NTS or between higher order neurones within the NTS. Such factors could also contribute to the relative differences in EPSP duration observed in the present study.
The fast EPSP mediating transmission from ADN primary afferent to the NTS neurone may have important functional consequences. Both a frequency-dependent and a time-dependent inhibition of synaptic transmission in the NTS have been described (see Mifflin & Felder, 1994, for review). However, it has recently been demonstrated that most MSNs do not exhibit time-dependent inhibition (Scheuer et al. 1996). The rapid onset, short duration EPSP in MSNs could provide a high safety factor for transmission across the first synapse, which makes MSNs less susceptible to time-dependent inhibition.
Physiological significance
Single, myelinated baroreceptor afferent fibres in the ADN can fire up to 100 Hz in response to an increase in pressure (Thoren et al. 1977; Brown et al. 1978). Stimulation of the baroreceptors by an increase in blood pressure can cause MSNs to discharge at comparable frequencies (Fig. 1A). These high discharge frequencies occur in the absence of changes in action potential amplitude, suggesting that depolarization inactivation is not occurring. To follow high frequency synaptic inputs faithfully, the evoked EPSPs must be of short duration to avoid EPSP summation and depolarization inactivation. Non-NMDA EAA receptor subtypes can generate such fast EPSPs, and this is exactly what we have observed in the MSNs. Higher stimulus frequencies (e.g. using a pair of pulses separated by 5 ms to identify MSNs, equivalent to a frequency of 200 Hz), do appear to be associated with some degree of depolarization inactivation. The second of the two action potentials is invariably of smaller amplitude than the first action potential (Figs 1A and B, and 5A), and occasionally the second pulse evokes only the initial segment component of the action potential.
In contrast, the increase in discharge of most PSNs in response to blood pressure increases is typically less than 20 Hz (J. Zhang & S. W. Mifflin, unpublished data), and, by definition, PSNs do not follow short interval, paired-pulse ADN stimulation (Scheuer et al. 1996). Therefore, there is a significant low-pass filtering of the input transmitted from MSNs to PSNs. Due to the involvement of NMDA receptors, which mediate slower EPSPs (Collingridge et al. 1988; Nakanishi et al. 1992; Hickmott & Constantine-Paton, 1993), in the transmission between higher order neurones, this low-pass filtering may serve to limit EPSP summation and prevent depolarization inactivation.
In conclusion, our results indicate the following. (1) EAAs are involved in the integration of baroreceptor inputs within the NTS at multiple levels of afferent processing. (2) Non-NMDA receptor subtypes play a major role in the transmission of inputs across the first synapse from baroreceptor afferent to second order NTS neurone. (3) Both NMDA and non-NMDA receptors are involved in the integration of baroreceptor inputs by higher order NTS neurones. (4) Some transmitter(s) other than EAAs could also be involved in the transmission of baroreceptor inputs across the first synapse from baroreceptor afferent to second order NTS neurone.
Acknowledgments
The authors thank Dr D. A. Scheuer for helpful comments on an earlier draft, and M. Vitela and M. Herrera-Rosales for expert technical assistance. This work was supported by NIH grant HL-41894.
References
- Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. American Journal of Physiology. 1990;259:H1307–1311. doi: 10.1152/ajpheart.1990.259.4.H1307. [DOI] [PubMed] [Google Scholar]
- Brooks PA, Spyer KM. Evidence for NMDA receptor-mediated events in the rat nucleus tractus solitarii in vitro. American Journal of Physiology. 1993;467:21. P. [Google Scholar]
- Brown AM, Saum WR, Yasui S. Baroreceptor dynamics and their relationship to afferent fibre type and hypertension. Circulation Research. 1978;42:694–702. doi: 10.1161/01.res.42.5.694. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Powley TL, Schwaber JS, Doyle FJ., III A laser confocal microscopic study of vagal afferent innervation of rat aortic arch: Chemoreceptors as well as baroreceptors. Journal of the Autonomic Nervous System. 1997;67:1–4. doi: 10.1016/s0165-1838(97)00085-4. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Herron CE, Lester RAJ. Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral-commissural pathway of rat hippocampus. American Journal of Physiology. 1988;399:283–300. doi: 10.1113/jphysiol.1988.sp017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewe JA, Kunze DL. Synaptic modulation of isolated neurons of the solitary tract nucleus. In: Barraco R, editor. Nucleus of the Solitary Tract. Boca Raton, FL, USA: CRC Press, Inc.; 1994. pp. 225–229. [Google Scholar]
- Drewe JA, Miles R, Kunze DL. Excitatory amino acid receptors of guinea pig medial nucleus tractus solitarius neurons. American Journal of Physiology. 1990;259:H1389–1395. doi: 10.1152/ajpheart.1990.259.5.H1389. [DOI] [PubMed] [Google Scholar]
- Galloudec EL, Merahi N, Laduzzi R. Cardiovascular changes induced by the local application of glutamate-related drugs in the rat nucleus tractus solitarii. Brain Research. 1989;503:322–325. doi: 10.1016/0006-8993(89)91683-1. 10.1016/0006-8993(89)91683-1. [DOI] [PubMed] [Google Scholar]
- Gordon FJ, Leone C. Non-NMDA receptors in the nucleus of the tractus solitarius play the predominant role in mediating aortic baroreceptor reflexes. Brain Research. 1991;568:319–322. doi: 10.1016/0006-8993(91)91418-z. 10.1016/0006-8993(91)91418-Z. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Filtz TM, Donaldson SR. Role of excitatory amino acids in rat vagal and sympathetic baroreflexes. Brain Research. 1987;407:272–284. doi: 10.1016/0006-8993(87)91105-x. 10.1016/0006-8993(87)91105-X. [DOI] [PubMed] [Google Scholar]
- Hablitz JJ, Sutor B. Excitatory postsynaptic potentials in rat neocortical neurons in vitro, III. Effects of a quinoxalinedione non-NMDA receptor antagonist. Journal of Neurophysiology. 1990;64:1282–1290. doi: 10.1152/jn.1990.64.4.1282. [DOI] [PubMed] [Google Scholar]
- Hickmott PW, Constantine-Paton M. The contribution of NMDA, non-NMDA and GABA receptors to postsynaptic responses in neurons of the optic tectum. Journal of Neuroscience. 1993;13:4339–4353. doi: 10.1523/JNEUROSCI.13-10-04339.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Kihara M. Evidence of N-methyl-D-aspartate receptor-mediated modulation of the aortic baroreceptor reflex in the rat nucleus tractus solitarii. Neuroscience Letters. 1988;87:69–74. doi: 10.1016/0304-3940(88)90147-4. 10.1016/0304-3940(88)90147-4. [DOI] [PubMed] [Google Scholar]
- Kubo T, Kihara M. Unilateral blockage of excitatory amino acid receptors in the nucleus tractus solitarii produces an inhibition on baroreflex in rats. Naunyn-Schmiedeberg's Archives of Pharmacology. 1991;343:317–322. doi: 10.1007/BF00251133. [DOI] [PubMed] [Google Scholar]
- Leone C, Gordon FJ. Is L-glutamate a neurotransmitter of baroreceptor information in the nucleus of the tractus solitarius. Journal of Pharmacology and Experimental Therapeutics. 1989;250:953–962. [PubMed] [Google Scholar]
- Luz Aylwin M, Horowitz JM, Bonham AC. NMDA receptors contribute to primary visceral afferent transmission in the nucleus of the solitary tract. Journal of Neurophysiology. 1997;77:2539–2548. doi: 10.1152/jn.1997.77.5.2539. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D, Yang M, Andresen MC, Kunze DL. Localization and retention in vitro of fluorescently labeled aortic baroreceptor terminals on neurons from the nucleus tractus solitarius. Brain Research. 1992;581:339–343. doi: 10.1016/0006-8993(92)90729-s. 10.1016/0006-8993(92)90729-S. [DOI] [PubMed] [Google Scholar]
- Mifflin SW, Felder RB. Baroreceptor and chemoreceptor afferent processing in the solitary tract nucleus. In: Barraco R, editor. Nucleus of the Solitary Tract. Boca Raton, FL, USA: CRC Press, Inc.; 1994. pp. 169–186. [Google Scholar]
- Mifflin SW, Zhang J. Modulation of baroreceptor inputs in rat NTS by GABA receptor subtypes. FASEB Journal. 1996;10:A337. [Google Scholar]
- Miles R. Frequency dependence of synaptic transmission in nucleus of the solitary tract in vitro. Journal of Neurophysiology. 1986;55:1076–1089. doi: 10.1152/jn.1986.55.5.1076. [DOI] [PubMed] [Google Scholar]
- Miller BD, Felder RB. Excitatory amino acid receptors intrinsic to synaptic transmission in nucleus tractus solitarii. Brain Research. 1988;456:333–343. doi: 10.1016/0006-8993(88)90236-3. 10.1016/0006-8993(88)90236-3. [DOI] [PubMed] [Google Scholar]
- Nabekura J, Kawamoto I, Akaike N. Developmental change in voltage dependency of NMDA receptor-mediated responses in nucleus tractus solitarii neurons. Brain Research. 1994;648:152–156. doi: 10.1016/0006-8993(94)91915-1. 10.1016/0006-8993(94)91915-1. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Yamamoto K, Kita H. Lateral hypothalamus and local stimulation induced postsynaptic responses in zona incerta neurons in an in vitro slice preparation of the rat. Brain Research. 1992;583:287–291. doi: 10.1016/s0006-8993(10)80035-6. [DOI] [PubMed] [Google Scholar]
- Ohta H, Talman WT. Both NMDA and non-NMDA receptors in the NTS participate in the baroreceptor reflex in rats. American Journal of Physiology. 1994;267:R1065–1070. doi: 10.1152/ajpregu.1994.267.4.R1065. [DOI] [PubMed] [Google Scholar]
- Sapru HN, Gonzalez E, Krieger AJ. Aortic nerve stimulation in the rat: cardiovascular and respiratory responses. Brain Research Bulletin. 1981;6:393–398. doi: 10.1016/s0361-9230(81)80009-3. [DOI] [PubMed] [Google Scholar]
- Sapru HN, Krieger AJ. Carotid and aortic chemoreceptor function in the rat. Journal of Applied Physiology. 1977;42:344–348. doi: 10.1152/jappl.1977.42.3.344. [DOI] [PubMed] [Google Scholar]
- Scheuer DA, Herrera-Rosales M, Mifflin SW. Monosynaptic aortic nerve (AN) inputs to NTS neurons: Correlation with NMDA receptor immunocytochemistry. Society for Neuroscience Abstracts. 1997;23:147. [Google Scholar]
- Scheuer DA, Mifflin SW. Identification of nucleus of the solitary tract neurons receiving monosynaptic inputs from the vagus nerve. FASEB Journal. 1998;12:A60. [Google Scholar]
- Scheuer DA, Zhang J, Toney GM, Mifflin SW. Temporal processing of aortic nerve evoked activity in the nucleus of the solitary tract. Journal of Neurophysiology. 1996;76:3750–3757. doi: 10.1152/jn.1996.76.6.3750. [DOI] [PubMed] [Google Scholar]
- Talman WT. Kynurenic acid microinjected into the nucleus tractus solitarius of rat blocks the arterial baroreflex but not response to glutamate. Neuroscience Letters. 1989;102:247–252. doi: 10.1016/0304-3940(89)90086-4. 10.1016/0304-3940(89)90086-4. [DOI] [PubMed] [Google Scholar]
- Talman WT, Perrone MH, Reis DJ. Evidence for L-glutamate as the neurotransmitter of baroreceptor afferent nerve fibres. Science. 1980;209:813–814. doi: 10.1126/science.6105709. [DOI] [PubMed] [Google Scholar]
- Talman WT, Perrone MH, Scher P, Kwo S, Reis DR. Antagonism of the baroreceptor reflex by glutamate diethyl ester, an antagonist to glutamate. Brain Research. 1981;217:186–191. doi: 10.1016/0006-8993(81)90198-0. 10.1016/0006-8993(81)90198-0. [DOI] [PubMed] [Google Scholar]
- Tell F, Jean A. Rhythmic bursting patterns induced in the neurons of the rat nucleus tractus solitarii, in vitro, in response to N-methyl-D-aspartate. Brain Research. 1990;533:152–156. doi: 10.1016/0006-8993(90)91809-u. 10.1016/0006-8993(90)91809-U. [DOI] [PubMed] [Google Scholar]
- Thoren P, Saum WR, Brown AM. Characteristics of rat aortic baroreceptors with nonmedulated afferent nerve fibres. Circulation Research. 1977;40:231–237. doi: 10.1161/01.res.40.3.231. [DOI] [PubMed] [Google Scholar]
- Tian B, Hartle DK. Cardiovascular effects of NMDA and MK-801 infusion at area postrema and mNTS in rat. Pharmacology Biochemistry and Behavior. 1994;49:489–495. doi: 10.1016/0091-3057(94)90060-4. 10.1016/0091-3057(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Wilson CG, Zhang Z, Bonham AC. Non-NMDA receptors transmit cardiopulmonary C fibre input in nucleus tractus solitarii in rats. The Journal of Physiology. 1996;496:773–785. doi: 10.1113/jphysiol.1996.sp021726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Mifflin SW. Influences of excitatory amino acid receptor agonists on nucleus of the solitary tract neurons receiving aortic depressor nerve inputs. Journal of Pharmacology and Experimental Therapeutics. 1997;282:639–647. [PubMed] [Google Scholar]


