Abstract
Extracellular recording techniques have been used to study nerve impulses in single sensory nerve terminals in guinea-pig cornea isolated in vitro.
Nerve impulses occurred spontaneously and were evoked by electrical stimulation of the ciliary nerves.
The nerve impulses were identified as originating in polymodal receptors, mechano-receptors or ‘cold’ receptors. All three types are believed to be nociceptors.
Tetrodotoxin (TTX, 1 μm) blocked nerve impulses evoked by electrical stimulation of the ciliary nerves. However, ongoing and/or naturally evoked nerve impulses persisted in the presence of TTX in all three types of receptors. Lignocaine (lidocaine; 1 mm) blocked all electrical activity.
TTX-resistant sodium channels therefore play a major role in generating the action potentials that signal pain to the brain.
Nociceptor neurones are sensory neurones that are activated by tissue damage and potentially noxious thermal stimuli. Activation of the nociceptive nerve terminals in tissues such as skin and joints generates action potentials that propagate both centrally to cause painful sensations and locally, in the nerve terminal axons, to trigger the release of neuropeptides producing neurogenic inflammation. The mechanism for the generation of action potentials in the nerve terminals of nociceptor neurones may differ from that in the majority of other nerve cells. In dorsal root ganglia (DRGs) and trigeminal ganglia, the cell bodies of small sensory neurones thought to be mainly nociceptive express tetrodotoxin-resistant (TTX-R) Na+ channels as well as the TTX-sensitive Na+ channels that are responsible for action potential generation and propagation elsewhere in the nervous system (Yoshida & Matsuda, 1979; Caffrey et al. 1992; Elliot & Elliot, 1993; Jeftinija, 1994; Rizzo et al. 1994; Akopian et al. 1996; Sangameswaran et al. 1996; Villiere & McLachlan, 1996). The level of expression of TTX-R Na+ channels is high enough in the cell bodies of small sensory neurones that they support the discharge of action potentials in the presence of TTX (Yoshida & Matsuda, 1979; Caffrey et al. 1992; Elliot & Elliot, 1993; Jeftinija, 1994; Villiere & McLachlan, 1996). Furthermore, agents like prostaglandin E2 and adenosine, which are released in damaged tissues and induce sensitization of nociceptors, increase the TTX-R Na+ current in isolated DRG cell bodies (Gold et al. 1996; England et al. 1996). TTX-R Na+ channels accumulate in the peripheral axons at a site of nerve injury demonstrating that they are anterogradely transported (Novakovic et al. 1998). However, the density of TTX-R Na+ channels along the peripheral axons is normally insufficient to support action potential propagation (Yoshida & Matsuda, 1979; Caffrey et al. 1992; Villiere & McLachlan, 1996). These channels may therefore not contribute to action potential generation in the peripheral terminals of nociceptive neurones.
The mechanisms by which tissue damage initiates action potentials in nociceptor terminals are largely a matter of speculation because of the small size of the terminals (<0.5 μm diameter) and their indeterminate location in intact tissues like skin. What is known has been inferred indirectly from recordings from afferent axons when the environment of the receptors is pharmacologically manipulated (Kress & Reeh, 1996). Here we report for the first time electrical activity recorded directly from single identified nociceptor nerve endings in the cornea of the eye.
METHODS
All experimental procedures conformed to the Australian National Health and Medical Research Council guidelines and were approved by the University of New South Wales Animal Care and Ethics Committee.
Electrophysiology
Eyes from guinea-pigs (150–300 g, killed with 100 mg kg−1 pentobarbitone i.p.) were mounted in a recording chamber and superfused with physiological saline of the following composition (mm): Na+, 151; K+, 4.7; Ca2+, 2; Mg2+, 1.2; Cl−, 144; H2PO3−, 1.3; HCO3−, 16.3; and glucose, 9.8. This solution was gassed with 95 % O2-5 % CO2 (to pH 7.4) and maintained at 31–33°C.. The optic nerve and associated ciliary nerves were drawn into a suction stimulating electrode. The stimulus parameters were modified as required throughout the experiment (pulse width, 0.1–0.5 ms, 5–30 V). A glass recording electrode (tip outer diameter, ∼50 μm) filled with physiological saline was applied to the surface of the corneal epithelium with slight suction. Electrical activity was recorded through an AC amplifier (Neurolog NL104, Digitimer Ltd, Welwyn Garden City, UK; gain, ×2000; high pass filter set at 0.1 Hz) and the output digitized at 44 kHz and stored on magnetic tape using a PCM recorder (A. R. Vetter Co. Inc., Rebersburg, PA, USA). Recordings were only made from sites where the nerve impulses were readily distinguished from the noise (∼10 μV peak-to-peak when low pass filtered at 3–5 kHz). At many sites on the cornea, no evoked or spontaneous electrical activity was recorded or the signals were too small to be analysed. All drugs were supplied by Sigma and were applied by their addition to the superfusion solution and in some experiments by internal perfusion of the recording electrode with drug-containing solutions. Internal perfusion of the recording electrode was achieved by inserting a fine plastic tube to within 200 μm of the electrode tip (see Brock & Cunnane, 1995).
A MacLab data acquisition system (ADInstruments Pty Ltd, Castle Hill, NSW, Australia) was used to digitize (sampling frequencies, 10–20 kHz) electrophysiological signals previously recorded on tape. Prior to digitizing, the signals were filtered using a low pass filter (cut-off, 3–5 kHz). Subsequent analysis was made with the computer program Igor Pro (Wavemetrics, Lake Oswego, OR, USA). Unless otherwise stated the averaged traces shown in the figures are the average of 100 records. Prior to averaging, all nerve terminal impulses (NTIs) were aligned at their point of maximum rate of rise or fall.
Immunohistochemistry
Corneas were fixed overnight in Zamboni's solution and rinsed in dimethylsulphoxide prior to incubation in the universal marker for nervous tissue, anti-protein-gene-product 9.5 (PGP 9.5) antibody (Ultraclone, 1:1000; Lundberg et al. 1988) followed by Cy3-labelled anti-rabbit antibody (Jackson, 1:200). They were then mounted flat in buffered glycerol and viewed with an Olympus confocal microscope. The micrograph in Fig. 1 is of the central region of whole-mounted guinea-pig cornea showing immunoreactivity to PGP 9.5 in four superimposed optical sections (each 3.3 μm thick). Images were analysed using NIH Image v. 1.6.1 (NIH, Bethesda, MD, USA).
Figure 1. Recording from the corneal epithelium.
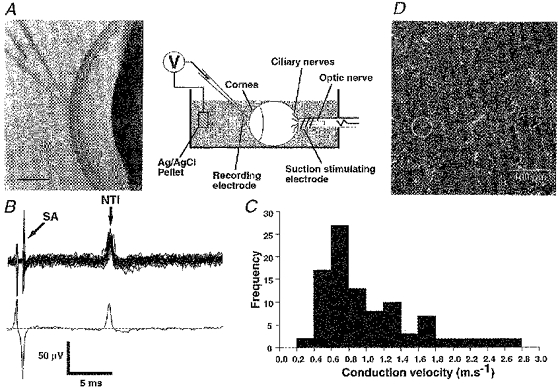
A, schematic diagram of recording set-up and photomicrograph showing the location of the recording electrode (scale bar, 1 mm). B, a single nerve terminal impulse (NTI) evoked by stimulation of the ciliary nerves. The upper part shows 50 overlaid traces recorded during a train of stimuli at 1 Hz and the lower part shows the average of these traces (SA, stimulation artefact). C, frequency distribution of conduction velocities for all single NTIs recorded. D, confocal micrograph of nerve terminals in the guinea-pig cornea. Most nerve terminals approach the surface of the epithelium at right angles and appear as single dots. On average, 2 terminals lie beneath the opening of a 50 μm pipette (circle).
RESULTS
Extracellular recording from the nerve terminals of corneal nociceptors
Three types of nociceptor (polymodal, mechano-sensitive and ‘cold’ sensitive) are found in many ectodermal tissues, including the cornea (Belmonte et al. 1997). The nociceptor terminals end in the most superficial layer of the corneal epithelium. When an electrode (∼50 μm tip diameter) was applied with light suction to the surface of the corneal epithelium of guinea-pig eyes isolated in vitro (Fig. 1A), electrical stimulation of the ciliary nerves sometimes evoked antidromic stimulus-locked nerve terminal impulses (NTIs; Fig. 1B). In thirty-five of these recordings, two or more NTIs (2 NTIs, n = 24; 3 NTIs, n = 3; > 3 NTIs, n = 12) were evoked at different latencies as the stimulus strength was increased. However, in eighty-nine recordings only a single all-or-none diphasic NTI was elicited by electrical stimulation of the parent axons (Fig. 1B). The conduction velocities for the antidromically propagated nerve impulses ranged from 0.3 to 2.7 m s−1, consistent with the recordings arising from both C fibres and thin myelinated Aδ fibres (see Fig. 1C).
The spacing of nerve terminals at the surface of the corneal epithelium is compatible with the recorded unitary NTIs arising in single nerve terminals. Single nerve terminals visualized immunohistochemically in whole-mount preparations of guinea-pig cornea turned at right angles from the stromal nerve bundles to terminate close to the surface of the corneal epithelium (Fig. 1D). The minimum distance between 154 ± 14 (mean ± s.e.m) terminals in seven fields (of mean area (132 ± 9) × 103μm2) sampled near the centre of four corneas ranged from 16.6 to 20.6 μm (mean, 18.6 ± 0.5 μm). This means that on average 12 ± 0.8 terminals end in an area 100 μm × 100 μm and, on average, about two terminals lie beneath the opening of a pipette 50 μm in diameter (Fig. 1D). Thus the probability that only one terminal is appropriately positioned for its activity to be recorded alone is high.
Spontaneous and evoked activity in single nerve terminals
Only recordings in which the activity of a single nerve terminal could be clearly identified were further analysed (n = 119). In 57 % of these recordings, NTIs of similar amplitude and shape to the electrically evoked potentials appeared spontaneously (Fig. 2A and B). It was confirmed that ongoing NTIs arose in the same axons as those elicited by electrical stimulation because the antidromically propagated NTIs silenced the spontaneous activity for about 10–20 ms (Fig. 2A, upper trace), presumably because the nerve terminals were refractory to excitation. Conversely, when a spontaneous NTI occurred just before or after the stimulus artefact, the electrical stimulus failed to evoke a response (Fig. 2A, lower trace). This would be expected if the orthodromically propagated spontaneously generated NTI collided with the antidromically propagated electrically evoked nerve action potential.
Figure 2. Spontaneous and evoked NTIs.
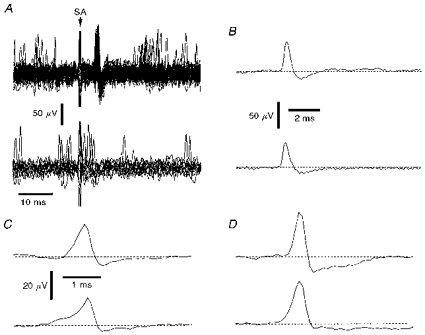
A, upper part shows overlaid traces in which electrical stimulation of the ciliary nerves evoked a stimulus locked NTI and lower part shows traces in which the occurrence of a spontaneous NTI just before or after the stimulus artefact caused failure of the electrically evoked NTI (SA, stimulation artefact). B, averaged evoked (upper) and spontaneous (lower) NTIs recorded in the same attachment as in A. C and D, averages of electrically evoked (upper traces) and spontaneously occurring (lower traces) NTIs recorded from a mechano-nociceptor (C) and a polymodal receptor (D). The scale bars in C also apply in D.
All the diphasic NTIs recorded from the corneal surface had a predominant positive-going component (Figs 1C, 2A–D and 4A–D). Similarly shaped signals have been recorded extracellularly from the terminals of motor nerves, where either passive invasion of the nerve terminal from a point of action potential propagation failure (Dudel, 1963) or active invasion of the nerve terminal by the action potential (Katz & Miledi, 1965) could explain their configuration. Thus the shape of the NTIs is consistent with their being recorded close to the terminations of sensory axons.
Figure 4. Effects of TTX (1 μm for 30 min).
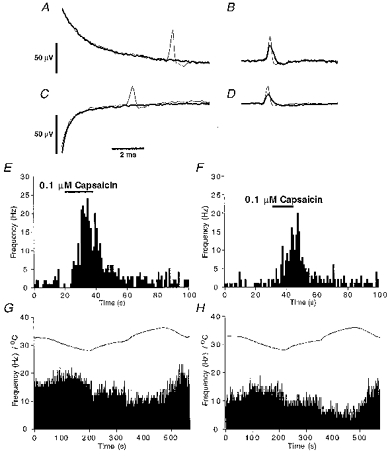
A-D, averaged electrically evoked (A and C) and spontaneously occurring (B and D) NTIs recorded before (thin line) and in the presence of TTX (thick line) from a mechano-nociceptor (A and B) and a polymodal receptor (C and D). E and F, the effects of capsaicin (0.1 μm) on the frequency of occurrence of NTIs recorded from a polymodal receptor before (E) and during (F) application of TTX. G and H, the effects of temperature changes (upper curve) on the frequency of occurrence of NTIs recorded from a cold-sensitive receptor before (G) and during (H) application of TTX. Histograms of ongoing activity have bin widths of 1 s.
In nine recordings, the initial positive-going phase of the spontaneously occurring NTIs rose more slowly than that of the electrically evoked NTI and the negative-going component was smaller (see Fig. 2C and D). The simplest explanation for these shape differences is that a transient slow outward current which reflects a generator potential precedes the initiation of spontaneous impulses.
Identification of subtypes of nociceptor
In 30 % of the recordings, ongoing NTIs appeared at low, irregular frequencies (0–4.5 Hz; mean, 0.61 ± 0.16 Hz; n = 36) that increased upon application of 0.05–0.5 μm capsaicin (Fig. 3Aa). When tested, these terminals could also be activated by mechanical stimulation (n = 12, Fig. 3Ab), performed by pushing the recording electrode gently against the corneal surface with a displacement of the micromanipulator. We conclude that these NTIs arose from corneal polymodal nociceptors (Belmonte & Giraldez, 1981; Belmonte et al. 1991; Belmonte et al. 1997) with properties similar to those in skin and other tissues (Kumazawa, 1996).
Figure 3. Identification of subtypes of nociceptor.
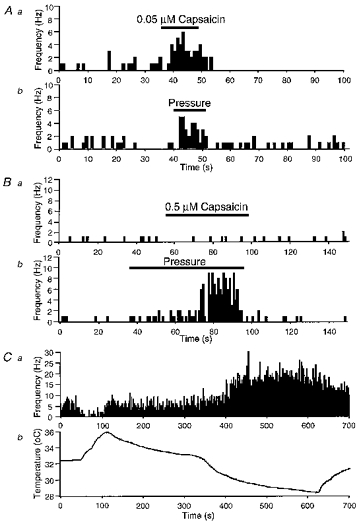
A and B, the effects of capsaicin (Aa and Ba) and mechanical stimulation (Ab and Bb) on the frequency of NTIs in a polymodal receptor (A) and a mechano-nociceptor (B). Ca, the effects of temperature on the frequency of NTIs in a cold-sensitive receptor. Cb, organ bath temperature during the period of recording shown in Ca. In A, B and C the abscissa scale in panel b also applies in panel a. Histograms of ongoing activity have bin widths of 1 s.
In a smaller proportion of cases (11 %), NTIs occurred at 0–2.7 Hz (mean frequency, 0.85 ± 0.24 Hz; n = 13) and their frequency of discharge was increased exclusively by mechanical stimulation (Fig. 3B). This behaviour is typical of corneal mechano-nociceptor terminals (Belmonte et al. 1997).
In another 19 % of the recordings, the ongoing NTIs discharged at higher frequencies (range, 1.4–17 Hz; mean firing frequency, 6.1 ± 0.7 Hz; n = 22), sometimes in bursts. In these nerve terminals cooling and warming the bathing solution increased and decreased the firing frequency respectively (Fig. 3C). These firing properties are like those of cold-sensitive corneal receptors (Tanelian & Beuerman, 1984; Gallar et al. 1993).
In 9 % of the recordings, NTIs discharged spontaneously but capsaicin, mechanical stimulation and/or cooling failed to modify their frequency of discharge and, in the remaining 31 % of the recordings, NTIs were only evoked by electrical stimuli. These latter might represent silent nociceptors that only become able to be excited when the tissue is inflamed (Schmidt et al. 1995).
Effects of Na+ channel blockers on NTIs
Application of TTX (1 μm) abolished the electrically evoked antidromic NTI in all receptors tested (13 polymodal, 7 mechano- and 13 cold receptors) (Fig. 4A and C) within 15 min. However, the ongoing NTIs (Fig. 4B and D) and/or those evoked by chemical (Fig. 4E and F), mechanical or thermal stimulation (Fig. 4G and H) (depending on the type of receptor) always persisted during the period of TTX application (>30 min).
Although TTX did not prevent the occurrence of NTIs, it reduced their amplitude and prolonged their time course (Fig. 4B and D). This indicates that the action potentials propagating in the terminals are partly supported by TTX-sensitive Na+ channels. In the presence of TTX, the frequency of ongoing NTIs decreased in cold receptors (control, 7.0 ± 1.5 Hz; 30 min in TTX, 3.5 ± 0.8 Hz; n = 13; P < 0.01, Wilcoxon signed rank test) but there was no consistent effect on NTI frequency in the polymodal and mechano-nociceptors (control, 0.93 ± 0.28 Hz; 30 min in TTX, 0.91 ± 0.42 Hz; n = 20).
In seventeen experiments (4 polymodal, 4 mechano- and 9 cold receptors), the ongoing NTIs and/or those evoked by chemical, mechanical or thermal stimulation were not blocked when the solution both in the bath and in the recording electrode (see Methods) contained TTX (1 μm) together with the inorganic Ca2+ channel blocker Cd2+ (0.1 mm). This finding demonstrates that voltage-dependent Ca2+ entry is not responsible for impulse activity in nociceptor terminals.
The effects of the local anaesthetic lignocaine (lidocaine; 1–5 mm), which is known to block TTX-R Na+ channels in cell bodies isolated from dorsal root ganglia (Roy & Narahashi, 1992), were also investigated. In nine experiments (4 polymodal and 5 cold receptors), this agent abolished both the electrically evoked and the ongoing NTIs within 10 min. Furthermore, in the presence of lignocaine, NTIs could no longer be evoked by chemical or thermal stimulation.
DISCUSSION
In the present study, an extracellular recording approach has been used to monitor electrical activity occurring in the nerves supplying the surface layer of the guinea-pig cornea. It could be confirmed that this activity arose in the axons of sensory nerves because the level of ongoing nerve activity was increased by capsaicin, and mechanical and/or thermal stimulation in most cases. This conclusion is supported by previous reports that the corneal epithelium is very densely supplied by the thin myelinated or unmyelinated axons of sensory neurones, most of which terminate at a few micrometres from the corneal surface (Belmonte et al. 1997). It is likely that the recorded activity originated in the terminals of sensory nerves for the following reasons. (1) The majority of axons supplying the most superficial layer of the guinea-pig cornea terminate abruptly as they approach the surface; a few axons run transversely for short distances (up to 100 μm) within the surface layer of the corneal epithelium before terminating. (2) The configuration of the recorded nerve impulses is consistent with the recordings being made from axon terminations.
The recorded electrical activity could be shown to originate in a single unit by several criteria: (1) a single stimulus-locked nerve impulse was evoked in an all-or-none manner by increasing the stimulation strength; (2) when ongoing electrical activity was recorded, the evoked and spontaneously occurring nerve impulses appeared to be conducted in the same axons (see above); and (3) the morphological studies showed that on average two axons terminated below the opening of a 50 μm tipped electrode. It would be expected that, in a relatively large proportion of experiments, two or more axon terminals would be enclosed by the recording electrode, so that the proportion of recordings with two or more units might have been expected to be higher than was observed. However, neighbouring nerve terminals have a relatively high probability of originating from a single parent axon, as the terminals of individual sensory neurones are concentrated within particular regions of the cornea (Belmonte et al. 1997). It is also possible that, because of marked spatial attenuation of the signals recorded with the extracellular electrode, only a proportion of nerve terminals are located close enough to the surface of the cornea for their electrical activity to be detected. In support of the latter suggestion, there were many sites on the cornea surface where no electrical activity could be detected (see Methods).
The primary result of this study is the demonstration that TTX-R Na+ channels play a role in generating nerve impulses recorded from the terminals of the corneal sensory nerves. It was discovered that TTX blocks the antidromic propagation of electrically evoked action potentials in the main nerve axon which transmits information to the central nervous system but does not inhibit spontaneous impulses in the polymodal, mechano-sensitive and cold-sensitive nerve terminals or their responsiveness to natural stimulation. In contrast, bath application of the local anaesthetic lignocaine blocks all electrical activity. The findings indicate that action potentials propagating in the sensory axons are triggered by the opening of sodium channels. However, more importantly, they demonstrate that TTX-R Na+ channels, which have been described in the cell bodies and axons of small sensory neurones (Yoshida & Matsuda, 1979; Caffrey et al. 1992; Elliot & Elliot, 1993; Jeftinija, 1994; Rizzo et al. 1994; Akopian et al. 1996; Sangameswaran et al. 1996; Villiere & McLachlan, 1996; Novakovic et al. 1998), are present at sufficiently high densities in the peripheral nerve terminals to be the primary determinant of their excitability.
In summary, we have recorded for the first time nerve impulses in single identified nociceptive nerve terminals. We have obtained evidence that polymodal, mechano-sensitive and cold-sensitive nerve terminals can produce action potentials by activation only of TTX-R Na+ channels. The presence of TTX-R Na+ channels in nociceptive endings has often been suggested. We have now provided direct support for the hypothesis that they participate in the initial steps of nociceptor activation. The three types of nociceptor present in the cornea are typical of those in other tissues including skin. For example, polymodal nociceptors similar to those in the cornea are found in joints, skeletal muscle and bladder mucosa (Kumazawa, 1996). As the TTX-R channels are present in sufficient quantity to support regenerative action potentials in nociceptive terminals, selective block of these channels might abolish painful signals arising from damaged tissue without interfering with other neural functions. In addition, blockade of these channels may selectively inhibit neurogenic inflammation of local origin.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia and in part by an Alcon Research Award to C. B. C. B. thanks the Co-operative Research Centre for Eye Research and Technology for travel support. We thank Paul Halasz of the School of Anatomy, University of New South Wales, for help with confocal microscopy.
References
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed in sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Gallar J, Pozo MA, Rebollo I. Excitation by irritant chemical substances of sensory afferent units in the cat's cornea. The Journal of Physiology. 1991;437:709–725. doi: 10.1113/jphysiol.1991.sp018621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte C, Garcia-Hirschfeld J, Gallar J. Neurobiology of ocular pain. Progress in Retinal and Eye Research. 1997;16:117–156. [Google Scholar]
- Belmonte C, Giraldez F. Responses of cat corneal sensory receptors to mechanical and thermal stimulation. The Journal of Physiology. 1981;321:355–368. doi: 10.1113/jphysiol.1981.sp013989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock JA, Cunnane TC. Effects of Ca2+ and K+ channel blockers on nerve impulses recorded from postganglionic sympathetic nerve terminals. The Journal of Physiology. 1995;489:389–402. doi: 10.1113/jphysiol.1995.sp021060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey JM, Eng DL, Black JA, Waxman SG, Kocsis JD. Three types of sodium channels in adult dorsal root ganglion neurons. Brain Research. 1992;592:283–297. doi: 10.1016/0006-8993(92)91687-a. [DOI] [PubMed] [Google Scholar]
- Dudel J. Presynaptic inhibition of the excitatory nerve terminal in the neuromuscular junction of the crayfish. Pflügers Archiv. 1963;277:537–557. [PubMed] [Google Scholar]
- Elliot AA, Elliot JR. Characterization of TTX-sensitive and TTX-resistant sodium current in small cells from adult sensory ganglia. The Journal of Physiology. 1993;463:39–56. doi: 10.1113/jphysiol.1993.sp019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England S, Bevan S, Docherty RJ. PGE2 modulates the tetrodotoxin-resistant current in neonatal rat dorsal root ganglion neurones via the cyclic AMP-protein kinase A cascade. The Journal of Physiology. 1996;495:429–440. doi: 10.1113/jphysiol.1996.sp021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallar J, Pozo MA, Tuckett RP, Belmonte C. Response of sensory units with unmyelinated fibres to mechanical, thermal and chemical stimulation on the cat's cornea. The Journal of Physiology. 1993;468:609–622. doi: 10.1113/jphysiol.1993.sp019791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Reichling DB, Shuster MJ, Levine JD. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proceedings of the National Academy of Sciences of the USA. 1996;93:1108–1112. doi: 10.1073/pnas.93.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeftinija S. The role of tetrodotoxin-resistant sodium channels of small primary afferent fibres. Brain Research. 1994;639:125–134. doi: 10.1016/0006-8993(94)91772-8. [DOI] [PubMed] [Google Scholar]
- Katz B, Miledi R. Propagation of electrical activity in motor nerve terminals. Proceedings of the Royal Society B. 1965;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- Kress M, Reeh PW. Chemical excitation and sensitization in nociceptors. In: Belmonte C, Cervero F, editors. Neurobiology of Nociceptors. Oxford: Oxford University Press; 1996. pp. 258–297. [Google Scholar]
- Kumazawa T. Sensitization of polymodal receptors. In: Belmonte C, Cervero F, editors. Neurobiology of Nociceptors. Oxford: Oxford University Press; 1996. pp. 325–345. [Google Scholar]
- Lundberg LM, Alm P, Wharton J, Polak JM. Protein-gene-product 9.5. A new neuronal marker visualizing the whole uterine innervation and pregnancy-induced and developmental changes in the guinea pig. Histochemistry. 1988;90:9–17. doi: 10.1007/BF00495700. [DOI] [PubMed] [Google Scholar]
- Novakovic SD, Tzoumaka E, McGivern JG, Haraguchi M, Sangameswaran L, Gogas KR, Eglen RM, Hunter JC. Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions. Journal of Neuroscience. 1998;18:2174–2187. doi: 10.1523/JNEUROSCI.18-06-02174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo MA, Kocsis JD, Waxman SG. Slow sodium conductances of dorsal root ganglion neurons: intraneuronal homogeneity and interneuronal heterogeneity. Journal of Neurophysiology. 1994;72:2796–2815. doi: 10.1152/jn.1994.72.6.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy ML, Narahashi T. Differential properties of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. Journal of Neuroscience. 1992;12:2104–2111. doi: 10.1523/JNEUROSCI.12-06-02104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanelian DL, Beuerman RW. Responses of rabbit corneal nociceptors to mechanical and thermal stimulation. Experimental Neurology. 1984;84:165–178. doi: 10.1016/0014-4886(84)90013-x. [DOI] [PubMed] [Google Scholar]
- Sangameswaran L, Delgado SG, Fish LM, Koch BD, Jakeman LB, Stewart GR, Sze P, Hunter JC, Eglen RM, Herman RC. Structure and function of a novel voltage-gated, tetrodotoxin-resistant sodium channel specific to sensory neurons. Journal of Biological Chemistry. 1996;271:5953–5956. doi: 10.1074/jbc.271.11.5953. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmelz M, Forster C, Ringkamp M, Torebjork E, Handwerker H. Novel classes of responsive and unresponsive C nociceptors in human skin. Journal of Neuroscience. 1995;15:333–341. doi: 10.1523/JNEUROSCI.15-01-00333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villiere V, McLachlan EM. Electrophysiological properties of neurons in intact rat dorsal root ganglia classified by conduction velocity and action potential duration. Journal of Neuroscience. 1996;76:1924–1941. doi: 10.1152/jn.1996.76.3.1924. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Matsuda Y. Studies on sensory neurons of the mouse with intracellular-recording and horseradish peroxidase-injection techniques. Journal of Neurophysiology. 1979;42:1134–1145. doi: 10.1152/jn.1979.42.4.1134. [DOI] [PubMed] [Google Scholar]


