Abstract
Single mechanically skinned fibres from rat extensor digitorum longus (EDL) muscles were used to investigate the mechanisms underlying inorganic phosphate (Pi) movements between the myoplasm and the sarcoplasmic reticulum (SR). Force transients elicited by caffeine/low Mg2+ application were used to assess the rate of Pi-induced inhibition of SR Ca2+ release and the subsequent recovery of Ca2+ release following removal of myoplasmic Pi.
Myoplasmic Pi reduced SR Ca2+ release in a concentration- and time-dependent manner. A 10 s exposure to 10, 20 and 50 mm myoplasmic Pi reduced SR Ca2+ release by 12 ± 9, 29 ± 5 and 82 ± 5 %, respectively.
Removal of myoplasmic ATP at the time of Pi exposure significantly increased the rate and extent of SR Ca2+ release inhibition. For example, Ca2+ release was reduced by 86 ± 6 % (n = 6) after 20 s exposure to 20 mm Pi in the absence of ATP compared with only 47 ± 5 % (n = 5) in the presence of ATP.
The half and full recovery times for SR Ca2+ release following washout of myoplasmic Pi were 35 s and ∼7 min, respectively. Recovery of Ca2+ release was unaffected by the absence of ATP during washout of Pi but was prevented when fibres were washed in the presence of high myoplasmic Pi (30 mm). Neither the Pi transporter blocker phenylphosphonic acid (PHPA) nor the anion channel blockers anthracene-9-carboxylic acid (9-AC) and 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (DIDS) affected the rate of recovery of SR Ca2+ release.
These results show that Pi entry and exit from the SR occur primarily through a passive pathway that is insensitive to well-known anion channel blockers. Pi inhibition of SR Ca2+ release appears to be a complicated phenomenon influenced by the rate of Pi movement across the SR as well as by the rate, extent and species of Ca2+-Pi precipitate formation in the SR lumen. The more rapid inhibitory effect of Pi in the absence of myoplasmic ATP suggests that Pi may inhibit SR Ca2+ release more efficiently during the later stages of fatigue.
Fatigue in skeletal muscle is characterized by a decline in force output as a consequence of intense muscular activity. One of the proposed myoplasmic factors that contributes to the decline of force is inorganic phosphate (Pi), which accumulates to between 30 and 40 mm during fatiguing stimulation (Cady et al. 1989; Baker et al. 1993). Force reduction by Pi has primarily been attributed to an inhibition of contractile protein function (reviewed by Fitts, 1994). However, Pi has also been shown to reduce force generation in both skinned (Fryer et al. 1995) and intact (Westerblad & Allen, 1996) skeletal muscle fibres by decreasing Ca2+ release from the sarcoplasmic reticulum (SR). It was concluded in both studies that the mechanism of Ca2+ release inhibition involved the movement of Pi from the myoplasm into the SR lumen with subsequent formation of Ca2+-Pi precipitate once the solubility product was exceeded in the SR lumen.
In order to understand these results, and to clarify the role of Pi in interfering with SR Ca2+ release during fatigue, it is necessary to determine the rate and magnitude of Pi fluxes between the myoplasm and the SR and to define the pathways through which these fluxes occur. Neither of these are well understood. Previous studies on SR vesicles have suggested that Pi may enter the SR of skeletal muscle via an ATP-dependent Pi transporter that is stimulated by Ca2+ and Mg2+ (Carley & Racker, 1982) and blocked by phenylphosphonic acid (PHPA; Stefanova et al. 1991a,b). Recently, Fryer et al. (1997) estimated that Pi could rapidly enter the SR (30–170 μm s−1) at physiological concentrations of myoplasmic Pi (10–50 mm) in single skinned fibres from the rat. The partial inhibition (37 %) by PHPA of Pi diffusion into the SR in this study was taken as evidence for a component of Pi entry mediated by the previously described Pi transporter. However, the resistance of most of the Pi entry to PHPA blockade (Fryer et al. 1997) along with previous studies in SR vesicles showing inhibition of Pi fluxes by stilbene derivatives (Kasai & Kometani, 1979; Campbell & MacLennan, 1980) suggest that Pi may enter the SR by a passive pathway.
Pi movements between the myoplasm and the SR might occur through one (or several) of the various Cl− channels (Kourie et al. 1996) or voltage-dependent anion channels (VDACs; Lewis et al. 1994; Junankar et al. 1995; Shoshan-Barmatz et al. 1996) which have been found in SR membranes. Many of the former are blocked by 9-AC while VDACs and other anion channels are blocked by stilbene derivatives such as 4,4′-diisothiocyanostilbene-2,2′-di-sulphonic acid (DIDS) and 4-aceto-amido-4′-isothiocyanostilbene-2,2′-disulphonate (SITS) (Kasai & Kometani, 1979; Campbell & MacLennan, 1980).
In this study we assessed the mechanisms underlying Pi entry into and exit from the SR by characterizing the rates of onset and recovery from Ca2+-Pi precipitation-induced failure of SR Ca2+ release. The contributions of active and passive Pi movements were assessed by changing myoplasmic [ATP] as well as using known blockers of the Pi transporter and various SR anion channels. The results show that Pi can rapidly equilibrate between the myoplasm and SR and reduce SR Ca2+ release via a passive pathway that is actually inhibited by myoplasmic ATP. The time course of recovery of SR Ca2+ release appears to be consistent with the gradual resolubilization of Ca2+-Pi precipitate once Pi passively exits the SR. Preliminary results have been reported previously in abstract form (Posterino & Fryer, 1997).
METHODS
Skinned fibre preparation
The skinned fibre preparation was used as described previously (Lamb & Stephenson, 1990; Posterino & Lamb, 1996). Briefly, outbred male Wistar rats (3–6 months old) were anaesthetized with halothane (2 % v/v) in a bell jar and killed by an overdose of the anaesthetic. The extensor digitorum longus (EDL) muscles were removed and subsequently pinned at resting length under paraffin oil (Ajax Chemicals, Sydney) on a bed of Sylgard 184 (Dow Chemicals, Midland, MI, USA). Single muscle fibres were isolated and mechanically skinned with fine forceps, leaving approximately 70–90 % of the fibre bulk. A segment of the skinned fibre was then attached to an isometric force transducer (KG3, Scientific Instruments, Germany), the output of which was sent to a chart recorder. After measuring the resting length of the fibre it was stretched by 20 % to give a sarcomere length of approximately 3.1–3.2 μm (based on previous measurements). Next, the fibre diameter was measured and the preparation moved to a 2 ml Perspex bath containing a potassium hexamethylene-diamine-tetraacetate (HDTA) solution (see below) for 2 min prior to the commencement of the experimental protocol. All experiments were performed at room temperature (23 ± 2°C).
Solutions
The standard potassium (K-) HDTA solution contained (mm): K+, 126; Na+, 37; HDTA2-, 50; total ATP, 8; total Mg2+, 8.6; creatine phosphate (CP), 10; total EGTA, 0.05; Hepes, 90; azide, 1; pH 7.10 ± 0.01 and pCa (-log10[Ca2+]) 7.0–7.1. The free [Mg2+] was 1 mm. All other solutions were variants based on the standard K-HDTA composition. A solution containing lowered free [Mg2+] (0.05 mm) was used to stimulate the SR Ca2+-release channel. This solution contained only 2.15 mm total Mg2+. Maximum Ca2+-activated force was determined in each fibre by exposure to a solution (Max) containing 50 mm Ca-EGTA in place of HDTA2- and a total Mg2+ of 8.1 mm. The free calcium ion concentration in this solution (23 μm) was determined by measuring the excess [EGTA] using a titration method previously described (Stephenson & Williams, 1981). Following exposure to the Max solution, fibres were relaxed in a heavily buffered solution in which HDTA was replaced with EGTA (50 mm) and the total Mg2+ was 10.3 mm. In some experiments fibres were exposed to solutions that lacked ATP (termed rigor). Rigor solutions lacked ATP and CP and had 1.5 mm total Mg2+. Unless otherwise stated, the free [Mg2+] of all solutions was 1 mm. The osmolality of the above solutions ranged between 280 and 302 mosmol kg−1.
Solutions containing inorganic phosphate (Pi) were similar in composition to either the K-HDTA or rigor solutions described above except that (i) CP was completely removed and (ii) HDTA was isosmotically replaced with Pi in a ratio of 1 : 1.3. These changes mimic fatigue-like conditions and have been previously shown to have little effect on the ionic strength and osmolality of the solutions (Fryer et al. 1995). The osmolality of Pi solutions ranged between 255 and 315 mosmol kg−1 (e.g. rigor solutions with 30 mm Pi had an osmolality of 315 mosmol kg−1 compared with 302 mosmol kg−1 for the standard rigor solution). In all Pi solutions the Hepes concentration was increased to 100 mm in order to make up some of the difference in osmolality due to the removal of CP. Matching solutions in the presence and absence of Pi never varied in osmolality by more than 25 mosmol kg−1. Such differences in osmolality and ionic strength have previously been shown to have negligible effects on Ca2+ release and force production (Lamb et al. 1993).
Experimental protocols
Estimation of releasable SR Ca2+. The amount of releasable Ca2+ within the SR of a single skinned fibre was assessed by exposing the fibre to a standard K-HDTA solution containing 0.05 mm free Mg2+, 30 mm caffeine and 1 mm total EGTA (termed the caffeine solution). The peak force response evoked by the caffeine solution was used as a qualitative estimate of the amount of releasable (free) Ca2+ within the SR. Each experiment began with depletion of the SR Ca2+ using the above solution in which the released Ca2+ is effectively chelated by the EGTA present. Fibres were then washed for 1 min in a standard K-HDTA solution containing 1 mm total EGTA. The SR of fibres was subsequently reloaded with Ca2+ to different levels by exposure (for 0 s to 60 s) to a standard K-HDTA solution that contained equal amounts of the Max and relaxing solutions (pCa ∼6.7; termed the load solution). Loading of the SR was rapidly stopped by subsequent exposure to a K-HDTA solution containing 1 mm total EGTA for 6 s. The peak force response elicited upon subsequent exposure to the caffeine solution was then compared with the load time. A linear relationship was observed for load times between 0 and 30 s with no additional loading occurring at longer load times (data not shown). Thus the peak force response was found to provide an accurate qualitative estimate of the amount of releasable Ca2+.
Time course of Pi inhibition of SR Ca2+ release. The time course of Pi diffusion into the SR and its associated effect on Ca2+ release were determined using the following protocol. Single skinned fibres were initially exposed to the caffeine solution for 1 min to both induce maximum Ca2+ release and to deplete the SR of all releasable Ca2+. Fibres were then washed in a K-HDTA solution with 1 mm total EGTA for 1 min to wash away the caffeine and to prevent any Ca2+ uptake during this time and then exposed to the load solution (pCa 6.7) for 30 s. Ca2+ loading was subsequently quenched by exposure to a K-HDTA solution with 1 mm total EGTA for 6 s. Fibres were then exposed to a similar solution (equilibration solution) for between 5 and 30 s, briefly washed in a weakly buffered K-HDTA solution (150 μm total EGTA) for 6 s and then exposed to the caffeine solution again to release all the remaining Ca2+ in the SR. The protocol was repeated twice to ensure that the response to the caffeine solution remained constant (fibres were excluded if the peak height of the caffeine response was not stable within a range of 10 %). The protocol was repeated with the exception that the equilibration solution was replaced with an appropriate solution containing 10, 20 or 50 mm Pi for between 5 and 30 s. Following a brief wash (6 s) in standard K-HDTA solution containing 150 μm total EGTA, Ca2+ release was elicited with the caffeine solution. The brief wash (6 s) prior to exposure to the caffeine solution was found to be sufficient to ensure that myoplasmic Pi was largely washed away from the fibre to remove any direct effect of Pi on the contractile apparatus and to minimize the loss of Pi from the SR.
The presence of 1 mm EGTA in both the equilibration and Pi solutions prevented Ca2+ uptake into the SR, as the free [Ca2+] in these solutions was less than 10−8 M. However, due to a constant Ca2+ leak (∼10 μm Ca2+ s−1; Bakker et al. 1996; Posterino & Lamb, 1996), and the absence of any Ca2+ uptake, the SR of fibres was gradually depleted of Ca2+ with approximately 30 % of the Ca2+ content being lost after 30 s. Therefore, in order to equilibrate fibres in Pi solutions for periods > 30 s whilst ensuring the SR still had a measurable Ca2+ content, some fibres were subsequently transferred from the equilibration solution (or comparable Pi solutions) after 30 s exposure to paraffin oil for 30 s to 2.5 min. This limited the volume of fluid bathing the fibre, thus preventing any leaked Ca2+ from simply diffusing away into the bulk solution. Despite these precautions, a net Ca2+ leak from the SR still occurred, albeit at a much slower rate, suggesting that most of the leaked Ca2+ was chelated by the 1 mm EGTA, but that some of the leaked Ca2+ was also resequestered by the SR of the fibre under oil.
After obtaining a response to caffeine following Pi exposure, the protocol was repeated with the exception that the fibre was washed for 7 min in a Pi-free solution to ensure that most of the Pi was removed from the SR (see Fig. 4). In every fibre the peak force response to caffeine immediately following washout was equivalent to the response before exposure to Pi (± 5 %; cf. Fig. 1). Subsequently, the peak height of the caffeine response following Pi exposure was normalized to the average of the caffeine responses before and after Pi exposure.
Figure 4. Time course of recovery of SR Ca2+ release following removal of myoplasmic Pi.
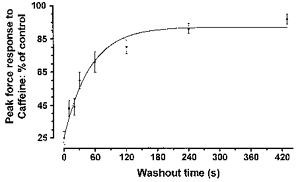
The recovery of the peak force response to caffeine following exposure to 50 mm Pi (cf. Fig. 3A) was plotted against the length of time that fibres were washed in Pi -free solution. The curve was fitted with a single exponential (R2= 0.97). The half-time to recovery was 35 s. Points on the graph indicate the mean ±s.e.m. from 4–14 fibres.
Figure 1. Effect of 20 mm Pi exposure on caffeine-induced Ca2+ release from the SR.
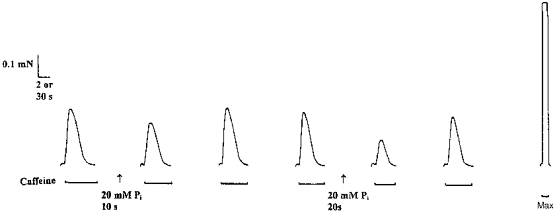
The SR of a single mechanically skinned fibre was first completely depleted of all Ca2+ by exposure for 2 min to a K-HDTA solution containing 30 mm caffeine, 0.05 mm free Mg2+ and 1 mm total EGTA (termed caffeine solution: see Methods). The fibre was then reloaded (at pCa 6.7, 1 mm total EGTA) for 30 s, quenched for 10 s in a solution containing 1 mm total EGTA, and then once again depleted of Ca2+ in the caffeine solution. The protocol was repeated with the exception that the fibre was equilibrated for 10 s in 20 mm Pi. The subsequent response to caffeine was reduced by 25 % compared with controls (2nd response). A repeat of this protocol after the fibre had been washed for 7 min completely restored the response to caffeine (3rd response). The experiment was then repeated with a different equilibration period (20 s) in the presence or absence of 20 mm Pi. The response to caffeine following 20 s equilibration in Pi was reduced by 42 % of the interpolated control (5th response). Maximum Ca2+-activated force was determined with a solution of pCa < 4.5 (termed Max). Time scale: 2 s for caffeine responses, 30 s for response to Max.
Rigor solution protocols. The role of passive diffusion of Pi into the SR was determined using the protocol described above with the exception that fibres were equilibrated for 10–30 s in a solution that lacked ATP (rigor solution) in the presence or absence of 10 and 20 mm Pi. Rigor solutions were heavily buffered with 1 mm total EGTA even though SR Ca2+ uptake would be eliminated by the absence of ATP. Fibres were also pre-exposed to a Pi-free rigor solution prior to equilibration for 6 s to wash away ATP bound to the fibre. Following equilibration, fibres were washed in a standard K-HDTA solution (with 125 μm total EGTA) for 6 s to restore ATP to the fibre.
Time course of recovery from Pi-inhibited SR Ca2+ release. The protocol used was similar to that described above with the exception that after the SR was reloaded with Ca2+, skinned fibres were exposed to either a standard K-HDTA solution or a 50 mm Pi solution (both containing 125 μm total EGTA (pCa < 7.4)) for 20 s and then equilibrated in paraffin oil for a further 2 min. The concentration of Pi used (50 mm) was chosen to maximize the rate of Pi entry into the SR whilst the length of time in oil ensured that Pi had completely equilibrated to a steady-state level in the SR. Fibres were then washed for 6 s to 7 min in a standard K-HDTA solution that was weakly buffered with 125 μm total EGTA. This concentration of EGTA ensured that net Ca2+ leak from the SR was small over several minutes of washout and prevented a net accumulation of Ca2+. Fibres were always washed for a minimum of 6 s to remove the bulk of the myoplasmic Pi from the contractile proteins before Ca2+ release was evoked with the caffeine solution. Thus, the minimum wash time (6 s) was designated as the 0 s time point (see Fig. 4). All other time points took into account the initial 6 s washout period.
Effects of ATP removal on the recovery of SR Ca2+ release. The washout protocol described above was used with the exception that following equilibration in oil (2 min) with or without previous Pi exposure, fibres were initially washed in a rigor solution for 6 s to remove the bulk of myoplasmic ATP from the fibre, and then exposed to an identical rigor solution for an additional 24, 54 or 114 s (30 s, 1 min or 2 min total washout taking into account the 6 s pre-rigor wash). During this time fibres produced rigor-force due to the absence of ATP. Furthermore, ATP-dependent Ca2+ uptake or any other ATP-dependent process was eliminated and net Ca2+ leak from the SR was also somewhat depressed (previously estimated at 6 μm Ca2+ s−1 in mechanically skinned EDL fibres; G. S. Posterino, unpublished observations). Fibres were then briefly exposed to a K-HDTA solution containing 125 μm total EGTA (6 s) to restore ATP and relax the fibre before Ca2+ release was elicited by exposure to the caffeine solution.
Effects of raised myoplasmic [Pi] on the recovery of SR Ca2+ release. The protocol used was identical to the one described above with the exception that following the initial equilibration period in Pi and paraffin oil (2 min), fibres were initially exposed to a rigor solution containing either 5 or 30 mm Pi (6 s) to wash ATP from the myoplasm and then exposed to an identical solution for an additional 24, 54 or 114 s, respectively. During this time rigor force developed although it was depressed (compared with controls) due to the presence of Pi in the solution. Fibres were then briefly washed for 6 s in a standard K-HDTA solution with 125 μm total EGTA to restore ATP, relax the fibre and to wash away the myoplasmic Pi and Ca2+ release was again elicited with the caffeine solution.
Chemicals and data analysis
All chemicals were obtained from Sigma-Aldrich (Australia) except for HDTA (Fluka, Buchs, Switzerland). Stock solutions of anthracene-9-carboxylic acid (9-AC), DIDS and phenylphosphonic acid (PHPA) were prepared by dissolving the compounds in double distilled water at concentrations of 100, 10 and 500 mm, respectively. Aliquots of these stock solutions were then added directly to the skinned fibre solutions. None of the blockers altered the pH or osmolality of the solutions.
All results are expressed as the mean ±s.e.m. of n observations. Statistical significance between the results was determined using the Student's t test for paired or unpaired observations where appropriate. Results were considered significantly different if P < 0.05.
RESULTS
Time course of failure of SR Ca2+ release
When Pi enters the SR it may precipitate with Ca2+ once the Ca2+-Pi solubility product is exceeded in the SR lumen (Beil et al. 1977). If this occurs, the free [Ca2+] within the SR declines leading to a reduction in the amount of Ca2+ that can be released upon stimulation (Fryer et al. 1995; Westerblad & Allen, 1996). In the first set of experiments the time course of Pi inhibition of SR Ca2+ release was estimated by measuring the reduction in the peak force response elicited by the caffeine solution after exposure to different Pi concentrations for different lengths of time.
Figure 1 illustrates the time-dependent effect of Pi exposure on Ca2+ release from the SR in a single EDL fibre. Equilibrating the fibre in 20 mm myoplasmic Pi for just 10 s reduced the force response elicited by the caffeine solution by 25 % (see 2nd response). Longer exposure to Pi (20 s) caused a further reduction in the force response (by 42 %, see 5th response). This effect was completely reversed by subsequently washing the fibre in a Pi-free equilibration solution for 7 min between Pi exposures. The results of such experiments are summarized in Fig. 2A which shows both the concentration and time dependence of Pi inhibition of SR Ca2+ release. It can be seen that the rate of inhibition of Ca2+ release initially increases linearly with increasing myoplasmic [Pi] in the 10–50 mm range. In contrast, the total degree of inhibition of SR Ca2+ release varied in a more complex manner with both time and [Pi]. None of the [Pi] completely suppressed Ca2+ release. Ca2+ release was only reduced by 27 ± 9 % (n = 5) at 10 mm Pi after 20 s and remained at this level for longer exposure times. At higher myoplasmic [Pi] (20 and 50 mm) the response to caffeine was reduced by a maximum of 78 ± 4 % (n = 5) and 82 ± 5 % (n = 4), respectively, which were not significantly different (P > 0.2) indicating that Ca2+-Pi precipitation mechanisms are saturated at [Pi] > 20 mm Pi. The inhibitory effects of exposure to 50 mm[Pi] appeared to partially reverse after a period of 20–90 s (Fig. 2A), but this effect was not statistically significant at the 0.05 level. The cause of the variability of Pi effects in this range is not known. One possibility is that it reflects complicated time-dependent changes in the species of Ca2+-Pi precipitate formed (Walton et al. 1967) within the SR lumen.
Figure 2. Effect of Pi exposure on caffeine-induced Ca2+ release in the presence and absence of myoplasmic ATP.
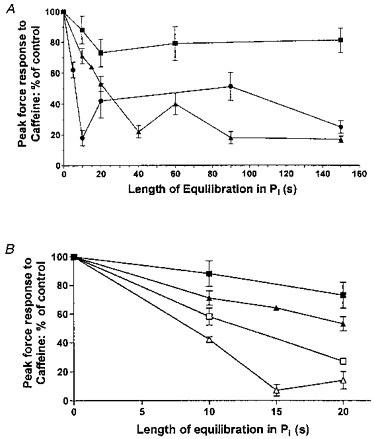
A, concentration and time dependence of caffeine response inhibition in the presence of myoplasmic ATP and varying myoplasmic [Pi]: (▪) 10 mm, (▴) 20 mm, (•) 50 mm. B, inhibition of caffeine responses by 10 mm Pi (▪) and 20 mm Pi (▴) in the presence of myoplasmic ATP compared with the effect of exposure to the same solutions in the absence of myoplasmic ATP (□, ▵). There was a significant reduction (P < 0.05) in the peak force response to caffeine following Pi exposure in the absence of ATP compared with the presence of ATP at all times examined. All values are expressed as the mean ±s.e.m. from 3–8 fibres.
The complex time dependence of Pi effects on SR Ca2+ release is unlikely to represent direct effects of Pi on the contractile proteins. Nevertheless, the time course of Pi washout on the maximum Ca2+-activated force was examined in three fibres (not shown). These fibres were initially exposed to a 50 mm Pi solution containing 1 mm EGTA for 2 min and were then either immediately exposed to a Pi-free maximum activating solution (pCa 4.5) or washed for 6 or 30 s in a Pi-free solution before maximum force was elicited. There was no difference in the peak force response obtained at either washout time compared with controls, demonstrating that the effects of myoplasmic Pi can be rapidly washed away from a skinned fibre in less than 1 s.
Time course of failure of SR Ca2+ release in the absence of myoplasmic ATP
It has previously been suggested that Pi enters the SR via an ATP-dependent Pi transporter (Carley & Racker, 1982; Stefanova et al. 1991a, b). However, Pi has also been shown to accumulate in SR vesicles in the apparent absence of ATP (Kasai & Kometani, 1979) indicating the additional presence of a passive diffusion mechanism. The role of passive diffusion of Pi into the SR was assessed in the present experiments by exposing fibres to 10 and 20 mm myoplasmic Pi in the absence of ATP (rigor solutions). A surprising result was that the rate of Pi inhibition of Ca2+ release was significantly increased in the absence of ATP when compared with Pi exposure in the presence of ATP (Fig. 2B). For example, the caffeine response was suppressed by 47 ± 5 % (n = 5) after 20 s exposure to 20 mm Pi in the presence of ATP but was inhibited by 86 ± 6 % (n = 6) when exposed to the same solution in the absence of ATP. These results indicate that the rate of onset of precipitation-induced Ca2+ release inhibition is much faster when myoplasmic ATP is absent. This effect cannot be explained by direct inhibitory effects of the rigor solution on the contractile proteins as prior exposure of skinned fibres to rigor solutions temporarily increased the maximum Ca2+-activated force by approximately 5–10 % (examined in three fibres; not shown).
Time course of recovery of SR Ca2+ release
If Ca2+-Pi precipitate formation is an important factor underlying the reduction in force and Ca2+ release seen during fatigue, then it is important to determine the time course of recovery from precipitation in order to understand the time course of recovery from fatigue.
Figure 3A shows the recovery time course of caffeine responses in a single fibre washed for different lengths of time after establishment of Ca2+-Pi-induced precipitation. Longer wash periods in Pi-free solutions (e.g. 30 s on left-hand side of Fig. 3A) were clearly associated with a greater recovery of SR Ca2+ release than shorter periods (e.g. 6 s on right-hand side of Fig. 3A). Summarized data from four to fourteen fibres at each recovery time point are shown in Fig. 4. The time course of recovery of SR Ca2+ release was well described (R2= 0.97) by a single exponential curve with a half-time (T½) of 35 s (Fig. 4). It should be noted that a relatively large amount of Ca2+ (∼25 %) could still be released when fibres were washed for the minimum period (6 s, designated as 0 s in Fig. 4) whereas full recovery required ∼7 min of washing in a Pi-free solution (Fig. 4).
Figure 3. Recovery from Pi-induced Ca2+ release inhibition under different myoplasmic conditions.
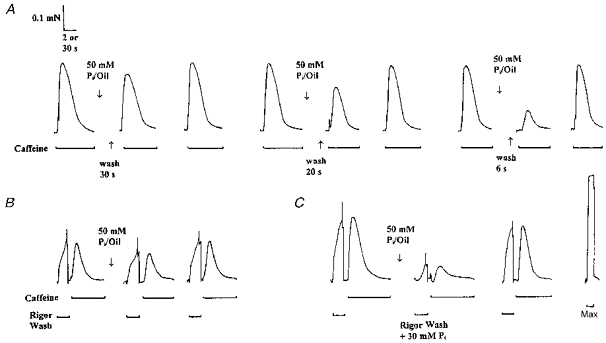
The time course of Pi removal from the SR under different myoplasmic conditions was examined in a single mechanically skinned fibre. A, following a Ca2+-deplete/Ca2+-load cycle the fibre was exposed to a solution containing 50 mm Pi for 30 s and then equilibrated in paraffin oil for a further 2 min (see Methods for details). The fibre was then washed in a standard (Pi-free) K-HDTA solution for 30, 20 and 6 s before a force response to caffeine was elicited (see 2nd, 5th and 8th response, respectively). Note the reduction in the recovery of the response to caffeine with shorter washout periods. B, a similar protocol was employed with the exception that the same fibre was washed in the absence of myoplasmic ATP (rigor solution) for 30 s which resulted in the production of rigor force. Following the brief reapplication of ATP (6 s), Ca2+ release was evoked with the caffeine solution. The recovery of the caffeine response was unaffected for the same washout period. C, however, when the same fibre was washed for 30 s in rigor solution containing 30 mm Pi, the response to caffeine did not recover. Maximum Ca2+-activated force was induced with a heavily buffered high Ca2+ solution (Max). Time scale: 2 s for caffeine responses and 30 s for responses to the rigor solutions and Max.
Time course of recovery of SR Ca2+ release in the absence of myoplasmic ATP
Pi fluxes between the myoplasm and the SR lumen have not been well characterized. In particular, the mechanism by which accumulated Pi is removed from the SR is unknown and could potentially involve an ATP-dependent transporter or ATP-gated channel. In this group of experiments (example shown in Fig. 3B) fibres were washed in a rigor solution (no ATP) for 30 s following equilibration in 50 mm Pi in oil for 2 min. The response to caffeine subsequently recovered to 71 % of the control responses, a result which was very similar to the response obtained after washout in a standard K-HDTA solution (with ATP) for 30 s in the same fibre (81 % recovery, see Fig. 3A). On average, the response to caffeine obtained after a wash period of 30 s to 2 min in the absence of ATP was greater than comparable responses in fibres washed in the presence of ATP (Fig. 5). This effect was only significant at the 1 min time point (P < 0.05; Fig. 5) and can be explained by a rigor-induced increase in the maximum Ca2+-activated force (5–10 % measured in two fibres; not shown) and to a small increase in the Ca2+ sensitivity of the contractile apparatus. The mean response to caffeine following exposure to 50 mm Pi and washout for 30 s under various myoplasmic conditions is also summarized in Table 1. Clearly, the absence of myoplasmic ATP did not inhibit the rate of recovery of SR Ca2+ release, suggesting that ATP-dependent processes are not important for the removal of lumenal Pi to the myoplasm.
Figure 5. Recovery of SR Ca2+ release is not inhibited by myoplasmic ATP removal but is inhibited by the presence of myoplasmic Pi.
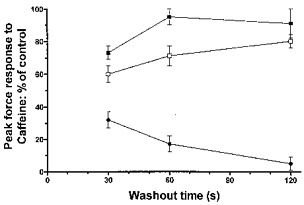
The recovery of the peak force response to caffeine after prior inhibition by 50 mm Pi was plotted against the length of time fibres were washed (30 s to 2 min) under various myoplasmic conditions: □, presence of myoplasmic ATP, cf. Fig. 3A; ▪, absence of myoplasmic ATP, cf. Fig. 3B; and •, absence of myoplasmic ATP and presence of 30 mm myoplasmic Pi, cf. Fig. 3C. Note that the response to caffeine recovered to a similar extent if fibres were washed in either the presence or absence of ATP, but did not recover when 30 mm Pi was also present during the washout. Points represent mean data ±s.e.m. from 3–14 fibres.
Table 1.
Recovery of the response to caffeine after 30 s washout of Pi under various myoplasmic conditions
| Washout conditions | Mean response to caffeine (% of controls) |
|---|---|
| ATP | 60 ± 5 (14) |
| No ATP | 73 ± 4 (7) |
| No ATP + 30 mm Pi | 32 ± 5 (4)**** |
| No ATP + 2 mmPHPA | 65±3(3) |
| ATP + 100 μm DIDS | 79 ± 4 (4)*** |
| ATP + 100 μm 9-AC | 74 ± 7 (3) |
The mean response to caffeine indicates the peak force response to caffeine after washout of Pi for 30 s (as detailed in Fig. 3) as a percentage of the control responses before and after Pi exposure. Washout conditions: ATP refers to a standard K-HDTA solution; No ATP refers to a rigor solution with or without various modifications. All solutions contained 125 μm total EGTA. Values represent the mean response ±s.e.m.with the sample size noted in parentheses. Asterisks represent a significant difference from the response to caffeine following washout under normal myoplasmic conditions (ATP)
P < 0.01
P < 0.001
Time course of recovery of SR Ca2+ release in the presence of myoplasmic Pi
The possibility that Pi moves from the SR lumen to the myoplasm via passive diffusion was tested by looking at the time course of recovery of SR Ca2+ release in the presence of raised myoplasmic [Pi]. Myoplasmic ATP was omitted in these experiments to rule out any complicating effects resulting from SR Ca2+ pump activity or other potential ATP-dependent Pi-extrusion processes.
Figure 3C shows an example of the effect of washing a fibre in the absence of ATP and in the presence of 30 mm myoplasmic Pi for 30 s. Compared with washout alone (Fig. 3A) or washout in the rigor solution (Fig. 3B), washout in the presence of Pi prevented recovery of the force response to caffeine at the same time interval. This can be seen more clearly in the summarized data plotted in Fig. 5 and listed in Table 1. In contrast to rigor wash alone, 1–2 min wash in rigor solution plus 30 mm Pi induced a time-dependent decrease in the caffeine response (Fig. 5), an effect consistent with additional Pi entry from myoplasm to SR lumen during this time. These results support the previous contention that Pi exit from the SR, like its entry (Fig. 3B), occurs via a passive diffusion pathway (see Fig. 3B) which depends on the Pi concentration gradient across the SR.
The effect of anion channel and transporter blockers on the recovery of Ca2+ release
We next tested whether the pathway for Pi exit from the SR is either (i) a DIDS- or 9-AC-sensitive anion channel or (ii) some type of pore associated with the SR Pi transporter protein. The protocol used to determine the rate of Pi exit from the SR was modified such that fibres were washed for 30 s in a standard K-HDTA solution containing either 100 μm 9-AC or 100 μm DIDS. For experiments with PHPA, fibres were washed in a rigor solution containing 2 mm PHPA in order to prevent the interference of PHPA binding by ATP (Fryer et al. 1997).
Retardation of Pi exit from the SR by any of the drugs should have been manifested as an impairment in the rate of recovery of the caffeine response. None of the tested compounds had this effect (Table 1), In fact, the response to caffeine after washout of Pi in the presence of DIDS was actually greater than the force response obtained after washout under standard myoplasmic conditions (P < 0.01), an effect which may be due to DIDS ability to increase the open probability of the ryanodine receptor/SR Ca2+ release channels (Oba et al. 1996). Overall, these results suggest that neither Cl− channels nor the VDACs are primarily responsible for the passage of Pi from the SR lumen to the myoplasm. However, these data do not rule out the potential role of all types of Cl− channel (some of which may not be modulated by myoplasmic ATP and/or which are not affected by these blockers) or other non-specific anion channels residing within the SR membrane.
DISCUSSION
The key findings of this study are: (1) myoplasmic Pi inhibits SR Ca2+ release in a concentration- and time-dependent manner, (2) the rate of onset of this inhibition is faster in the absence of myoplasmic ATP, and (3) recovery from Pi-induced inhibition has a T½ of ∼35 s, is ATP independent, and is dependent upon the [Pi] gradient across the SR. These findings provide further insight into the nature of Pi movements across the SR and are relevant to the understanding of the time course of metabolic fatigue and recovery from fatigue in skeletal muscle.
Inhibition of SR Ca2+ release by myoplasmic Pi
A number of mechanisms could potentially account for the inhibitory effects of prior myoplasmic Pi elevation on SR Ca2+ release. These include: (i) direct effects of Pi on the myofilaments due to insufficient washout of Pi; (ii) decreased SR Ca2+ content due to Pi activation of SR Ca2+-release channels or reduced activity/reversal of the SR Ca2+ ATPase pump; or (iii) movement of Pi into the SR with subsequent formation of Ca2+-Pi precipitate.
A residual effect of myoplasmic Pi on the myofilaments is unlikely to account for the decline in caffeine response for several reasons. Firstly, this study has shown, and previous studies have calculated (Fryer et al. 1995) that most of the Pi can diffuse out of a 50 μm diameter fibre and into the bulk solution in < 1 s. Such a time course is incompatible with the complex concentration and time dependence seen over many minutes in the present study.
It has been previously shown that 10 mm Pi can increase the open probability of the skeletal muscle ryanodine receptor by ∼2-fold (Fruen et al. 1994). In addition, it has been suggested that myoplasmic Pi can inhibit or even reverse the SR Ca2+-ATPase pump (Barlogie et al. 1971; Zhu & Nosek, 1991; Xiang & Kentish, 1995; Steele et al. 1996). As Pi is present in the equilibration solutions in our experimental protocols, it is possible that the above actions of Pi would lead to a reduced SR Ca2+ content and hence caffeine response. Recovery of the caffeine response in such a situation would require a period of SR Ca2+ re-loading, a process that requires the presence of myoplasmic ATP. However, our results clearly demonstrated that the caffeine response could recover just as quickly in the absence of ATP as it did in its presence (Fig. 5), indicating that the releasable Ca2+ freed up during the recovery period was already present within the SR lumen.
Given the arguments above, the most likely mechanism to explain the inhibition of the caffeine response involves the movement of Pi from the myoplasm to the SR lumen with subsequent reduction of SR Ca2+ release once the Ca2+-Pi solubility product is exceeded (Fryer et al. 1995). In these circumstances the rate of decline of the caffeine response should be limited by either the rate of Pi entry or by the subsequent rate of formation of Ca2+-Pi complexes and precipitates.
Pi entry into the SR and Ca2+-Pi precipitate formation
The results (Figs 1, and 2A and B) suggested that the process responsible for the decline in releasable Ca2+ after Pi exposure has the following properties: (i) an initial rapid phase, the rate of which is almost linearly related to myoplasmic [Pi] in the 10 to 50 mm range; (ii) an even faster initial rate in the absence of myoplasmic ATP; and (iii) a complicated time dependence.
If the process described above is rate limited by Pi entry into the SR, then its properties should be compatible with known Pi channel and/or transport mechanisms. A Pi transporter that is dependent on myoplasmic Ca2+, Mg2+ and ATP (Carley & Racker, 1982; Stefanova et al. 1991b) and largely blocked by phosphonic acid derivatives such as PHPA (Stefanova et al. 1991a) has been described in SR vesicles. In skinned fibres, Fryer et al. (1997) found that Pi entry into the SR could be partially blocked by PHPA (37 %); however, this was only found under conditions where the blocker was applied in the absence of both myoplasmic Pi and ATP, which presumably compete with PHPA at a binding site on the Pi transporter (Stefanova et al. 1991a). These previous results, plus the present observation showing an apparent stimulation of the rate of Pi entry in the absence of myoplasmic ATP (Fig. 2B) suggest that the ATP-dependent Pi transporter is not a main pathway for Pi entry into the SR of skinned fibres, implying that some other pathway is primarily responsible.
This leaves the possibility that Pi enters the SR passively through either ion channels or pores in the SR membrane. It is known that Pi can pass through certain SR Cl− channels (Tanifuji et al. 1987; D. Laver, unpublished observations) as well as through the SR VDAC/porin pathway that has been previously characterized in mitochondria (Hodge & Colombini, 1997). However, given that Pi does not appear to pass through the 87 pS conductance Cl− channel incorporated into artificial bilayers (Stefanova et al. 1991b), and that neither 9-AC nor DIDS had much effect on the rate of Pi exit from the SR (Table 1), we can only suggest that Pi fluxes in the present study occur through an as yet unknown pathway that is quite insensitive to DIDS and 9-AC.
One possible explanation for the results shown in Figs 1 and 2 is that the rate and extent of Ca2+-Pi precipitate formation within the SR lumen is rate limiting for the decline in SR releasable Ca2+ after myoplasmic Pi exposure. If higher concentrations of myoplasmic Pi lead to faster Pi entry then the Ca2+-Pi solubility product is attained more quickly and Ca2+ release is attenuated accordingly (Fig. 2A). Even though the rate of decline of SR Ca2+ release varied almost linearly with myoplasmic Pi in the 10 to 50 mm range, the maximum extent of inhibition saturated between 20 and 50 mm Pi, consistent with the previous suggestion that the SR [Pi] within mechanically skinned rat EDL fibres saturates at around 30–35 mm (Fryer et al. 1997). Saturation of the SR [Pi] places a limit on the total amount of lumenal [Ca2+] that can be precipitated and thus the lowest level at which the releasable [Ca2+] may be clamped (∼20 % of control values in these fibres).
In order to more fully understand the complex time-dependent changes in releasable [Ca2+] (Fig. 2A) as well as its modulation by myoplasmic [ATP], it is necessary to appreciate that the rate and extent of such a process is not only governed by the Ca2+-Pi solubility product but also by the rates at which various Ca2+-Pi precipitate species are formed and with which they are mutually interconverted (Walton et al. 1967; Beil et al. 1977). The final effect of precipitate formation on releasable Ca2+ will ultimately be determined by the proportion of stable (i.e. relatively insoluble) crystal species to metastable (i.e. relatively soluble) crystal species. Time-dependent transformations between such species may explain the complex time dependence of changes in releasable Ca2+ at longer Pi exposure times (Fig. 2A). It is also interesting and important to note that the rate of Ca2+-Pi crystal formation is markedly inhibited in the presence of millimolar levels of Mg-ATP (Feher & Lipford, 1985), a phenomenon which may explain the results in Fig. 2B and also have implications for precipitate formation during the late stages of fatigue (further discussed in a separate section below). Feher & Lipford (1985) suggested a number of possible mechanisms including (i) prevention of crystal nucleation, (ii) prevention of crystal growth by adsorption to nuclei, or (iii) complexation of Mg2+ with Pi and Ca2+ with ATP to lower the effective concentration of precipitating anions. As precipitation occurs in the SR lumen, one way ATP may regulate this mechanism would be if the lumenal [ATP] falls as a consequence of ATP removal from the myoplasm, a process which could occur by ATP equilibration through VDACs in the SR membrane (Shoshan-Barmatz et al. 1996). Alternatively, the results in Fig. 2B could be explained if ATP simply blocked Pi entry into the SR through certain channels. One such channel could be the small Cl− channel (conductance of 75 pS) found in the SR membrane of rabbit skeletal muscle which is inhibited by high myoplasmic [ATP] (Kourie, 1997). Preliminary experiments on these channels incorporated into bilayers show that they can conduct Pi (D. Laver, unpublished data). Irrespective of the exact mechanism of Pi entry and its possible modulation by ATP, it is known that Ca2+-Pi precipitation is modulated by myoplasmic ATP, perhaps making this the ATP-sensitive step.
Recovery of Pi-inhibited SR Ca2+ release
The results suggested that the process responsible for the recovery of releasable Ca2+ after Pi exposure: (i) has a T½ of ∼35 s (Fig. 4), (ii) is active in the absence of myoplasmic ATP (Fig. 3B, Table 1), (iii) is inhibited by 30 mm myoplasmic Pi (Fig. 3C, Table 1), and (iv) is not blocked by either 9-AC, DIDS or PHPA (Table 1). As previously mentioned, the recovery of releasable Ca2+ in the absence of myoplasmic ATP indicates that the process involves a freeing up of calcium that is already available in the SR lumen rather than a re-accumulation of Ca2+ that has been lost from the SR. Thus, the recovery process must require solubilization of Ca2+-Pi precipitate that has previously been formed in the SR lumen.
Ca2+-Pi precipitate solubilization and Pi exit from the SR
Solubilization of Ca2+-Pi precipitate can only occur if either lumenal [Ca2+] or [Pi] drops such that the solubility product for crystal formation is no longer exceeded. The time-dependent recovery of the caffeine response in myoplasmic solutions that are Pi free implies that lumenal [Ca2+] is actually increasing. Therefore, solubilization of Ca2+-Pi precipitate in the present experiments is likely to result from the reduction of lumenal [Pi] due to its movement into the myoplasm. This contention is supported by the close agreement between our measured half-recovery time (35 s) and that seen for the rate of efflux of Pi from passively loaded SR vesicles (∼30 s, Campbell & MacLennan, 1980). The present data suggest that Pi passively exits the SR down a concentration gradient because this process was substantially inhibited in the presence of 30 mm myoplasmic Pi (Figs 3C and 5, Table 1) and was not inhibited by the absence of myoplasmic ATP (Figs 3B and 5, Table 1). In fact, the recovery of releasable Ca2+ appeared to be slightly faster in the absence of myoplasmic ATP, a result which would appear to contradict the precipitate-stabilizing effect of low Mg-ATP discussed above. However, Feher & Lipford (1985) found that the re-introduction of Mg-ATP had little effect on Ca2+-Pi crystals once they were already formed. The apparent speeding of recovery in the absence of myoplasmic ATP could potentially be explained by an increase in the maximum Ca2+-activated force (5–10 % measured in two fibres, not shown) and to a small increase in the Ca2+ sensitivity of the contractile apparatus following rigor-induced force. Alternatively, the presence of myoplasmic ATP might impede the exit of Pi into the myoplasm after its passage through the SR membrane in the same way it might impede Pi diffusion into the SR.
The lack of effect of PHPA on the recovery time course suggests that the SR Pi transporter characterized by Stefanova et al. (1991a, b)is not responsible for trans-SR Pi movement. Similarly, both 9-AC (100 μm) and DIDS (100 μm) failed to attenuate the rate of Pi exit, indicating that the pathway involved is not a 9-AC-sensitive Cl− channel nor is it the VDAC/porin in the SR membrane. The reason for the significant speeding up of recovery in the presence of DIDS (Table 1) is not known, but could involve some type of residual effect of DIDS on either the SR Ca2+ release channel (Oba et al. 1996) or the contractile proteins.
Relevance to intact skeletal muscle function at rest and during fatigue
This study confirms and extends previous work (Fryer et al. 1995, 1997) showing that physiological levels of myoplasmic Pi can equilibrate across the SR and lead to alterations in SR Ca2+ handling. Typically, the myoplasmic [Pi] estimated in resting mammalian fibres ranges from ≤ 1 mm in fast-twitch fibres to ∼6 mm in slow-twitch fibres (Kushmerick et al. 1992). Given that the free Ca2+ in the SR lumen is ∼1 mm (Gonzalez-Serratos et al. 1978), and that the solubility product of Ca2+-Pi is ∼ 6 mm2 (Fryer et al. 1995), then it is possible that slow-twitch fibres may already contain calcium in the form of Ca2+-Pi precipitates. This may help explain why the SR Ca2+ content of slow-twitch fibres responds very differently to myoplasmic Ca2+ loads when compared with fast-twitch fibres (Fryer & Stephenson, 1996).
Myoplasmic [Pi] and the onset of fatigue
Many skinned fibre studies have shown that myoplasmic Pi can depress force generation by inhibiting both the calcium sensitivity and the maximum force-generating capacity of the myofilaments (reviewed by Fitts, 1994). These effects of Pi mean that the changes in force observed during the onset and recovery from fatigue do not necessarily reflect corresponding changes in SR Ca2+ release. The validity of the Ca2+-Pi precipitate hypothesis of fatigue can therefore only be discussed with reference to studies in which myoplasmic [Pi] is elevated during the simultaneous measurement of both force and [Ca2+]i. For example, Westerblad & Allen (1996) found that microinjection of Pi to a myoplasmic concentration of 10–20 mm caused a large decrease in SR Ca2+ release within 4–12 min. Whilst no detailed analysis of the time course of onset of this effect was presented, they concluded that a Ca2+-Pi precipitation mechanism was the only one compatible with the observed results. Nevertheless, a bolus injection of Pi from a micropipette would be expected to produce a very different [Pi] transient to that seen during prolonged, intermittent muscle activity. During the early and intermediate stages of this type of fatigue the decline in force appears to be at least partly due to Pi effects on the contractile proteins, with SR Ca2+ release staying relatively unchanged until late in fatigue (Allen et al. 1995). Thus, Westerblad & Allen (1996) point out that Ca2+-Pi precipitation is unlikely to explain the failure of SR Ca2+ release in late fatigue because this occurs long after Pi has been substantially elevated, and coincides more readily with the time when myoplasmic Mg2+ rises and [ATP] falls (Westerblad & Allen, 1992). However, it is possible to reconcile both observations as follows: in early and intermediate fatigue myoplasmic [Pi] rapidly accumulates to ∼10–20 mm and starts to equilibrate across the SR via an unknown pathway. Even though myoplasmic Pi is elevated there are a number of processes operating within intact muscle fibres which delay and inhibit any rapid formation of SR lumenal precipitate at this stage. Firstly, intact fibres contain other known Pi sinks (such as mitochondria) which could compete with the SR for Pi. Secondly, our data suggest that Pi equilibration will introduce a necessary delay between [Pi] changes in the myoplasm and those in the SR, the duration of which will shorten as myoplasmic [Pi] increases. Thirdly, the rate of formation of stable precipitate will be strongly inhibited by normal levels of MgATP (Feher & Lipford, 1985) and could potentially be inhibited by other myoplasmic factors such as phosphocreatine.
We propose that rapid formation of precipitate within the SR is more likely to occur in late fatigue because myoplasmic [Pi] has had sufficient time to equilibrate across the SR. An attractive (though speculative) hypothesis based on our results (Fig. 2B) is that the rapid fall in SR Ca2+ release seen in late fatigue (Allen et al. 1995) coincides with a fall in local [ATP] within the SR lumen that greatly enhances the rate of formation of stable Ca2+-Pi precipitate. Further experiments beyond the scope of the present study are clearly required to validate this proposal.
Myoplasmic [Pi] and the recovery from fatigue
With regard to recovery from fatigue, many previous studies have generally found a good correlation between the rate of decline of myoplasmic [Pi] and the rate of force recovery. Baker et al. (1993) noted a good correlation between the recovery of maximal voluntary contractions of human muscles and the decline in myoplasmic [Pi] after short duration exercise, but found that force recovery lagged well behind [Pi] after long duration exercise.
For short duration exercise, our results would predict a transient formation of Ca2+-Pi precipitate, which will re-solubilize at a rate determined by Pi exit from the SR (Fig. 4) which itself depends on the myoplasmic [Pi] (Fig. 5). The drop in myoplasmic Pi in this situation is probably determined by the rate of its incorporation into the organic phosphate pool, and might explain why a portion of recovery from fatigue is sensitive to the rate of glycogen re-synthesis (Chin & Allen, 1997). Thus, after short duration activity, there is generally a good correlation between recovery of [Pi], force and SR Ca2+ release.
The dissociation between the time course of recovery of myoplasmic [Pi] and force after longer duration exercise (Baker et al. 1993) means that Pi effects on the contractile proteins can be ruled out, suggesting the presence of a prolonged inhibition of SR Ca2+ release. This finding can be explained by the formation of greater proportions of more stable (less soluble) forms of Ca2+-Pi crystal species over time (Walton et al. 1967). Such species would be expected to re-solubilize extremely slowly in response to a reduction of SR lumenal [Pi], leading to an apparent dissociation between [Pi] (myoplasmic and lumenal) and the recovery of SR Ca2+ release. This mechanism would also explain why the recovery of SR Ca2+ release following intracellular Pi injections was extremely slow (∼1 h), despite indications that the myoplasmic [Pi] had returned to normal (Westerblad & Allen, 1996). In addition, the formation of highly insoluble Ca2+-Pi species might explain the component of prolonged reduction of SR Ca2+ release which was found to be independent of glycogen re-synthesis (Chin & Allen, 1997).
Concluding remarks
This study has provided further insight into the mechanisms of Pi inhibition of SR Ca2+ release. We have shown that both Pi entry into and exit from the SR occurs largely by a passive mechanism that is insensitive to well-known anion channel blockers and SR Pi transport inhibitors. Such a mechanism must involve ion channels or pores; however, little is known regarding their identity. From our results we conclude that Pi inhibition of SR Ca2+ release is a complicated phenomenon influenced by the rate of Pi movement across the SR and the rate, extent and species of Ca2+-Pi precipitate formation in the SR lumen. Interestingly, the more rapid inhibitory effect of Pi in the absence of myoplasmic ATP suggests that Pi may inhibit SR Ca2+ release more efficiently during the later stages of fatigue.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia.
References
- Allen DG, Lännergren J, Westerblad H. Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Experimental Physiology. 1995;80:497–527. doi: 10.1113/expphysiol.1995.sp003864. [DOI] [PubMed] [Google Scholar]
- Baker AJ, Kostov KG, Miller RG, Weiner MW. Slow force recovery after long-duration exercise: metabolic and activation factors in muscle fatigue. Journal of Applied Physiology. 1993;74:2294–2300. doi: 10.1152/jappl.1993.74.5.2294. 10.1063/1.354713. [DOI] [PubMed] [Google Scholar]
- Bakker AJ, Lamb GD, Stephenson DG. The effect of 2,5-di-(tert-butyl)-1,4-hydroquinone on force responses and the contractile apparatus in mechanically-skinned fibres of the rat and toad. Journal of Muscle Research and Cell Motility. 1996;17:55–68. doi: 10.1007/BF00140324. [DOI] [PubMed] [Google Scholar]
- Barlogie B, Hasselbach W, Makinose M. Activation of calcium efflux by ADP and inorganic phosphate. FEBS Letters. 1971;12:265–268. doi: 10.1016/0014-5793(71)80194-1. [DOI] [PubMed] [Google Scholar]
- Beil FU, von Chak D, Hasselbach W, Weber HH. Competition between oxalate and phosphate during active Ca2+ accumulation by sarcoplasmic vesicles. Zeitschrift für Naturforschung. 1977;32:281–287. doi: 10.1515/znc-1977-3-421. [DOI] [PubMed] [Google Scholar]
- Cady EB, Jones DA, Lynn J, Newham DJ. Changes in force and intracellular metabolites during fatigue of human skeletal muscles. The Journal of Physiology. 1989;418:311–325. doi: 10.1113/jphysiol.1989.sp017842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KP, MacLennan DH. DIDS inhibition of sarcoplasmic reticulum anion efflux and calcium transport. Annals of the New York Academy of Sciences. 1980;358:328–331. doi: 10.1111/j.1749-6632.1980.tb15406.x. [DOI] [PubMed] [Google Scholar]
- Carley WW, Racker E. ATP-dependent phosphate transport in sarcoplasmic reticulum and reconstituted proteoliposomes. Biochimica et Biophysica Acta. 1982;680:187–193. doi: 10.1016/0005-2728(82)90010-x. [DOI] [PubMed] [Google Scholar]
- Chin ER, Allen DG. Effects of reduced glycogen concentration on force, Ca2+ release and contractile protein function in intact mouse skeletal muscle. The Journal of Physiology. 1997;498:17–29. doi: 10.1113/jphysiol.1997.sp021838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher JJ, Lipford GB. Calcium oxalate and calcium phosphate capacities of cardiac sarcoplasmic reticulum. Biochimica et Biophysica Acta. 1985;818:373–385. doi: 10.1016/0005-2736(85)90012-4. [DOI] [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiological Reviews. 1994;74:49–93. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Fruen BR, Mickelson JR, Shomer NH, Roghair TJ, Louis CF. Regulation of the sarcoplasmic reticulum ryanodine receptor by inorganic phosphate. Journal of Biological Chemistry. 1994;269:192–198. [PubMed] [Google Scholar]
- Fryer MW, Owen VJ, Lamb GD, Stephenson DG. Effects of creatine phosphate and Pi on Ca2+ movements and tension development in rat skinned skeletal muscle fibres. The Journal of Physiology. 1995;482:123–140. doi: 10.1113/jphysiol.1995.sp020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MW, Stephenson DG. Total and sarcoplasmic reticulum calcium contents of skinned fibres from rat skeletal muscle. The Journal of Physiology. 1996;493:357–370. doi: 10.1113/jphysiol.1996.sp021388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MW, West JM, Stephenson DG. Phosphate transport into the sarcoplasmic reticulum of skinned fibres from rat skeletal muscle. Journal of Muscle Research and Cell Motility. 1997;18:161–167. doi: 10.1023/a:1018605605757. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Serratos H, Somlyo AV, McClellan G, Shuman H, Borrero LM, Somlyo AP. Composition of vacuoles and sarcoplasmic reticulum in fatigued muscle: Electron probe analysis. Proceedings of the National Academy of Sciences of the USA. 1978;75:1329–1333. doi: 10.1073/pnas.75.3.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge T, Colombini M. Regulation of metabolic flux through voltage-gating of VDAC channels. Journal of Membrane Biology. 1997;157:271–279. doi: 10.1007/s002329900235. [DOI] [PubMed] [Google Scholar]
- Junankar PR, Dulhunty AF, Curtis SM, Pace SM, Thinnes FP. Porin-Type 1 proteins in plasmalemmal and sarcoplasmic reticulum of striated muscle fibres. Journal of Muscle Research and Cell Motility. 1995;16:595–610. doi: 10.1007/BF00130241. [DOI] [PubMed] [Google Scholar]
- Kasai M, Kometani T. Inhibition of anion permeability of sarcoplasmic reticulum vesicles by 4-acetoamido-4′-isothiocyano-stilbene-2,2′-disulfonate. Biochimica et Biophysica Acta. 1979;557:243–247. doi: 10.1016/0005-2736(79)90106-8. [DOI] [PubMed] [Google Scholar]
- Kourie JI. ATP-sensitive voltage- and calcium-dependent chloride channels in sarcoplasmic reticulum vesicles from rabbit skeletal muscle. Journal of Membrane Biology. 1997;157:39–51. doi: 10.1007/s002329900214. [DOI] [PubMed] [Google Scholar]
- Kourie JI, Laver DR, Junankar PR, Gage PW, Dulhunty AF. Characteristics of two types of chloride channel in sarcoplasmic reticulum vesicles from rabbit skeletal muscle. Biophysical Journal. 1996;70:202–221. doi: 10.1016/S0006-3495(96)79564-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick MJ, Moerland TS, Wiseman RW. Mammalian skeletal muscle fibres distinguished by contents of phosphocreatine, ATP and Pi. Proceedings of the National Academy of Sciences of the USA. 1992;89:7521–7525. doi: 10.1073/pnas.89.16.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Calcium release in skinned muscle fibres of the toad by transverse tubular depolarization or by direct stimulation. The Journal of Physiology. 1990;423:495–517. doi: 10.1113/jphysiol.1990.sp018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG, Stienen GJM. Effects of osmolality and ionic strength on the mechanism of Ca2+ release in skinned skeletal muscle fibres of the toad. The Journal of Physiology. 1993;464:629–648. doi: 10.1113/jphysiol.1993.sp019655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TM, Roberts ML, Bretag AH. Immunolabelling for VDAC, the mitochondrial voltage-dependent anion channel, on sarcoplasmic reticulum from amphibian skeletal muscle. Neuroscience Letters. 1994;181:83–86. doi: 10.1016/0304-3940(94)90565-7. [DOI] [PubMed] [Google Scholar]
- Oba T, Koshita M, Van Helden DF. Modulation of skeletal muscle Ca2+ release channel gating by anion channel blockers. American Journal of Physiology. 1996;271:C819–824. doi: 10.1152/ajpcell.1996.271.3.C819. [DOI] [PubMed] [Google Scholar]
- Posterino GS, Fryer MW. Time course of inorganic phosphate (Pi) removal from the sarcoplasmic reticulum of rat fast-twitch skeletal muscle. Proceedings of the Australian Physiological and Pharmacological Society. 1997;28:85P. [Google Scholar]
- Posterino GS, Lamb GD. Effects of reducing agents and oxidants on excitation-contraction coupling in skeletal muscle fibres of rat and toad. The Journal of Physiology. 1996;496:809–825. doi: 10.1113/jphysiol.1996.sp021729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Hadad N, Feng W, Shafir I, Orr I, Varsanyi M, Heilmeyer LMG. VDAC/porin is present in sarcoplasmic reticulum from skeletal muscle. FEBS Letters. 1996;386:205–210. doi: 10.1016/0014-5793(96)00442-5. [DOI] [PubMed] [Google Scholar]
- Steele DS, McAinsh AM, Smith GL. Comparative effects of inorganic phosphate and oxalate on uptake and release of Ca2+ from the sarcoplasmic reticulum in saponin skinned rat cardiac trabeculae. The Journal of Physiology. 1996;490:565–576. doi: 10.1113/jphysiol.1996.sp021167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova HI, East JM, Lee AG. Covalent and non-covalent inhibitors of the phosphate transporter of sarcoplasmic reticulum. Biochimica et Biophysica Acta. 1991a;1064:321–328. doi: 10.1016/0005-2736(91)90318-3. [DOI] [PubMed] [Google Scholar]
- Stefanova HI, Jane ST, East JM, Lee AG. Effects of Mg2+ and ATP on the phosphate transporter of sarcoplasmic reticulum. Biochimica et Biophysica Acta. 1991b;1064:329–334. doi: 10.1016/0005-2736(91)90319-4. [DOI] [PubMed] [Google Scholar]
- Stephenson DG, Williams DA. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. The Journal of Physiology. 1981;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanifuji M, Sokabe M, Kasai M. An anion channel of sarcoplasmic reticulum incorporated into planar lipid bilayers: Single-channel behavior and conductance properties. Journal of Membrane Biology. 1987;99:103–111. doi: 10.1007/BF01871230. [DOI] [PubMed] [Google Scholar]
- Walton AG, Bodin WJ, Furedi H, Schwartz A. Nucleation of calcium phosphate from solution. Canadian Journal of Chemistry. 1967;45:2695–2701. [Google Scholar]
- Westerblad H, Allen DG. Myoplasmic free Mg2+ concentration during repetitive stimulation of single fibres from mouse skeletal muscle. The Journal of Physiology. 1992;453:413–434. doi: 10.1113/jphysiol.1992.sp019236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. The effects of intracellular injections of phosphate on intracellular calcium and force in single fibres of mouse skeletal muscle. Pflügers Archiv. 1996;431:964–970. doi: 10.1007/s004240050092. [DOI] [PubMed] [Google Scholar]
- Xiang J-Z, Kentish JC. Effects of inorganic phosphate and ADP on calcium handling by the sarcoplasmic reticulum in rat skinned cardiac muscles. Cardiovascular Research. 1995;29:319–400. [PubMed] [Google Scholar]
- Zhu Y, Nosek TM. Intracellular milieu changes associated with hypoxia impair sarcoplasmic reticulum Ca2+ transport in cardiac muscle. American Journal of Physiology. 1991;261:H620–626. doi: 10.1152/ajpheart.1991.261.3.H620. [DOI] [PubMed] [Google Scholar]


