Abstract
Cultured human capillary endothelial cells (HCEC) contain a large inward rectifier current, IK(IR), that can be abolished by removing external K+ or by adding 50 μm Ba2+.
We show that IK(IR) is responsible for maintaining the hyperpolarized potential (−60.6 ± 0.5 mV, n = 83) of HCEC. Blocking IK(IR) with 50 μm Ba2+ shifts the zero current level and depolarizes HCEC by 36.5 ± 1.3 mV (n = 4).
Increasing external Ca2+ concentration ([Ca2+]o) from 0.5 to 7 mm reduces the magnitude of IK(IR) by 36.5 ± 2.3 % (n = 5) and depolarizes the cells by 10.33 ± 2.4 mV (n = 3), whereas decreasing [Ca2+]o from 1.8 to 0.5 mm increases the amplitude of IK(IR) by 6.9 ± 1.9 % (n = 4). The relationship between [Ca2+]o and the percentage block of IK(IR) gives a Kd value of 5.4 ± 0.6 mm at −120 mV.
IK(IR) is also blocked by other divalent ions, with Ba2+ >> Sr2+ > Mg2+ > Mn2+= Ca2+, and the block of peak current at −120 mV being 85.3 ± 3.2 % (n = 5) for 50 μm Ba2+, 62.9 ± 2.2 % (n = 5) for 5 mm Sr2+, 40.7 ± 2.5 % (n = 9) for 5 mm Mg2+, 33.4 ± 2.1 % (n = 5) for 5 mm Mn2+ and 32.9 ± 2.1 % (n = 5) for 5 mm Ca2+.
The voltage dependence of Sr2+ block of peak IK(IR) occurred with a Kd value of 1.0 ± 0.09 mm for −140 mV, 1.9 ± 0.16 mm for −130 mV, 3.1 ± 0.28 mm for −120 mV, 4.6 ± 0.34 mm for −110 mV and 6.4 ± 0.5 mm for −100 mV (n = 5), with a calculated electrical distance (δ) of 0.44 from the outside.
Endothelial cells are not electrically excitable yet their ionic channels have been postulated to play an important role in the modulation of intracellular calcium and subsequent release of endothelial factors such as nitric oxide (NO), endothelin and prostacyclin (Adams et al. 1989). To date there have been several studies characterizing a variety of ion channels such as receptor-operated non-selective cation channels (Johns et al. 1987; Nilius et al. 1993), K+ and cation channels activated by shear stress (Olesen et al. 1988; Schwarz et al. 1992), Ca2+-dependent potassium channels (Sauve et al. 1988; Demirel et al. 1994), non-selective cation channels (Fichtner et al. 1987; Inazu et al. 1994) and inward rectifier K+ channels in bovine and porcine aortic as well as human endothelial cells (Colden-Stanfield et al. 1987; Johns et al. 1987; Takeda et al. 1987; Silver & DeCoursey, 1990; Jow et al. 1996; for review, see Nilius et al. 1997). However, the physiological roles of these channels are only beginning to be understood.
The inward rectifier channel is the dominant ion channel in whole-cell studies of endothelial cells (Himmel et al. 1994) and has been postulated to play an important role in determining the resting membrane potential in endothelial cells (Campbell et al. 1991; Voets et al. 1996). Resting membrane potential is important in determining the driving force for Ca2+ entry and in establishing the gradient for other ion channels (Voets et al. 1996). Wellman & Bevan (1995) suggest that shear stress hyperpolarizes endothelial cells by opening the inward rectifier K+ channel, KIR, thereby increasing the driving force for Ca2+ entry and stimulating NO release and relaxation of rabbit cerebral arteries. Membrane depolarization initiates the formation of H2O2 and possibly other oxidants in bovine pulmonary arterial endothelial cells (al-Mehdi et al. 1996) and inhibits endothelial relaxing factor (EDRF)/NO synthesis by inhibiting the Ca2+ influx pathway (Luckhoff & Busse, 1990; Busse et al. 1991).
One of the aims of the present study was to quantify the contribution of the inward rectifier current to the resting membrane potential of human capillary endothelial cells. The second goal was to explore the effects of extracellular Ca2+ on the inward rectifier current and to compare the blocking action of other divalent ions such as Ba2+, Sr2+, Mg2+ and Mn2+. We have chosen human capillary endothelial cells to examine these issues as they are clearly relevant to human physiology and we have a good understanding of the ion channels present in them (Jow et al. 1996).
METHODS
Cell culture
Human capillary endothelial cells (HCEC) were obtained from Cell Systems Corporation (WA, USA), and were cultured in growth medium for human capillary endothelial cells (4M1-500, Cell Systems Corporation). HCEC were fed every two days and passaged between 7 and 15 days or at 80–90 % confluence. The HCEC used for this study were from subcultures two to six.
Electrophysiological measurements
HCEC were recorded in whole-cell voltage clamp with the patch-clamp technique (Hamill et al. 1981). Patch electrodes were pulled from borosilicate glass (outer diameter, 1.5 mm; inner diameter, 1 mm; Sutter Instrument Co., CA, USA) and fire polished using an MF-83 Microforge (Narishigi Inc., Tokyo, Japan), and had resistances of 3–4 MΩ. We found that fire polishing was necessary in order to obtain the very high > 30 GΩ seal resistances required to observe the negative resting membrane potential of these cells. The input resistance of cells at −60 mV measured near 10 GΩ and seal resistance in cell-attached modes was greater than 30 GΩ. Membrane potentials were corrected for liquid junction potentials (< 5 mV). The resting membrane potential of single endothelial cells averaged −60.6 ± 0.5 mV (n = 83). The mean cell capacitance was 51.4 ± 6.9 pF (n = 34). All recordings were done at room temperature (25 ± 3°C). An EPC9 amplifier with the acquisition program Pulse-PulseFit (HEKA, Lambrecht, Germany) was used for recording and data acquisition, with Igor Pro (WaveMetrics, Inc., Lake Oswego, OR, USA) and Origin (Microcal Software, Inc., Northampton, MA, USA) used for analysis. Currents were filtered at 3 kHz. All results are expressed as mean values ±s.e.m. The standard pipette solution for whole-cell recordings was (in mmol l−1): 120 potassium aspartate, 20 KCl, 0.5 EGTA, 5 Hepes, 5 phosphocreatine (disodium salt) and 1 MgCl2 (pH 7.2 with KOH). In some experiments 5 mm MgATP and 10 mm EGTA were included in the pipette solution. We saw no difference in the current with or without MgATP and with high or low EGTA. The standard bath recording solution consisted of (in mmol l−1): 140 NaCl, 5.4 KCl, 5 Hepes, 15 dextrose, 1.8 CaCl2, 0.4 KH2PO4 and 0.3 Na2HPO4 (pH 7.2 with NaOH). Additional Ca2+, Mg2+, Mn2+ and Sr2+ were added to the standard bath solution by equimolar replacement of NaCl.
RESULTS
Ba2+-sensitive inward rectifier K+ current
In whole-cell patch recordings, the mean resting membrane potentials of human capillary endothelial cells were tightly grouped around −60.6 ± 0.5 mV, although more depolarized potentials were seen when the seal resistance of the patch electrode was less than 30 GΩ. A large inward current elicited by hyperpolarizing voltage steps was exhibited in 92 % (n = 118) of HCEC. During hyperpolarizing pulses negative to −100 mV, the inward current activated rapidly and decayed with time (Fig. 1A). The current-voltage relationship in Fig. 1E shows a strong inward rectification. The mean peak current amplitude of the inward current at −120 mV was −360.8 ± 49.2 pA or 8.4 ± 0.87 pA pF−1 (n = 38). Application of Ba2+ (50 μm) to the outside of the cell inhibited both the peak and the steady-state inward current at −120 mV, by 85.3 ± 3.1 and 87.5 ± 2.3 %, respectively (n =6, Fig. 1A and B). Removing external K+ completely eliminated the inward current, which confirms that this inward current is carried by external K+ (Fig. 1D and E). From these data, we conclude that the inward rectifier K+ current (IK(IR)) is the dominant inward current in human capillary endothelial cells. Figure 1 also demonstrates the depolarizing shift in the zero current level seen upon block of IK(IR).
Figure 1. Ba2+ block and dependence on external K+ of inward rectifier K+ current in HCEC.
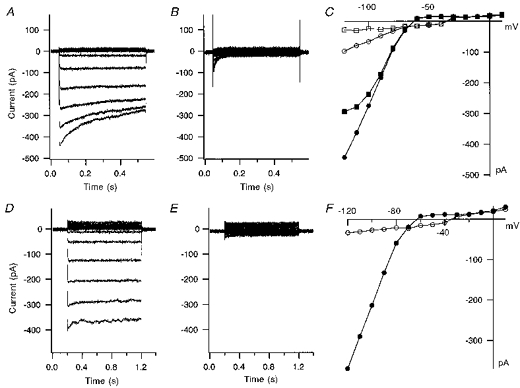
A, current traces from a HCEC recorded in response to voltage step pulses from −120 to +10 mV in 20 mV intervals at a holding potential of −60 mV. B, current traces from the same voltage pulse protocol after application of 50 μm Ba2+. C, current-voltage plot of peak (•) and steady-state (▪) current in standard bath solution, and peak (○) and steady-state (□) current after application of Ba2+ for the same cell. D, current traces from a HCEC response to voltage steps of −120 mV to +10 mV from a holding potential of −60 mV with 5.4 mm K+ in the standard bath solution. E, current traces from the same voltage protocol in the absence of external K+. F, peak current-voltage plot from the currents in D and E before (•) and after (○) removing all external K+. Elimination of external K+ completely blocks the inward current.
IK(IR) is blocked by external Ca2+
Figure 2A shows typical IK(IR) traces obtained from HCEC bathed in standard external bath solution containing 1 mm Ca2+. Application of the standard external solution with elevated [Ca2+] (9 mm) decreased IK(IR) by 42 % in this cell (Fig. 2B). The residual IK(IR) was further blocked by 50 μm Ba2+ indicating that Ca2+ block is less potent than Ba2+ block (Fig. 2C). Figure 2D shows the current traces after washout of external Ca2+ and Ba2+ from the bath with standard external solution containing 1 mm Ca2+. Figure 2E shows the current-voltage plot of the currents in 1 mm Ca2+, 9 mm Ca2+, 50 μm Ba2+ and after washout (< 5 min) of external Ca2+ and Ba2+. This result indicates that the reduction in IK(IR) caused by external Ca2+ and Ba2+ is completely reversible.
Figure 2. Ca2+ block of IK(IR) is reversible.
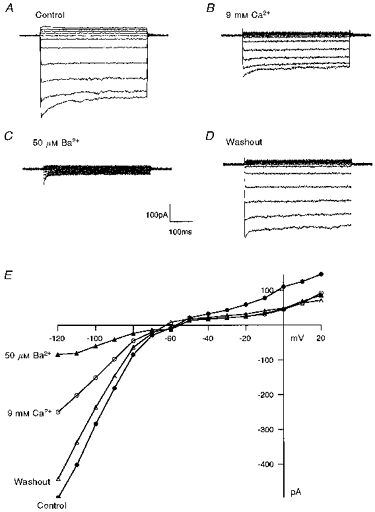
A, inward rectifier current traces of a HCEC bathed in standard bath solution. The cell was held at −60 mV and step potentials were applied from −120 to +20 mV, in 20 mV steps. B, the same cell after superfusion with standard bath solution containing 9 mm Ca2+. C, addition of 50 μm Ba2+ blocked the remaining current. D, current traces taken after washout of high external Ca2+ and Ba2+ with standard bath solution. E, current-voltage plots for control, 9 mm Ca2+, 50 μm Ba2+ and after washout.
Voltage dependence of IK(IR) and effect of external Ca2+
The voltage dependence of IK(IR) was examined by applying hyperpolarization voltage pulses to potentials more negative than −100 mV. The current shows a biphasic time course with very fast activation that then relaxes to a steady-state level (Fig. 3A). The peak inward current (Fig. 3B, ▪) increased with more negative test potentials, whereas the steady-state current (□) reached a maximum at −120 mV and then declined at more hyperpolarizing potentials. Elevation of [Ca2+]o from the normal 1 mm to 10 mm reduced the magnitude of IK(IR). Figure 3A shows paired current traces for the same hyperpolarizing voltage pulses before and after increasing [Ca2+]o from 1 to 10 mm. The mean reduction of IK(IR) peak current at −120 mV was 46.9 ± 1.9 % (n = 5). The reduction in inward rectifier current by [Ca2+]o did not appear to alter the kinetic properties of the current or the rectification of the sustained current at hyperpolarizing potentials.
Figure 3. Block of IK(IR) by elevated external Ca2+.
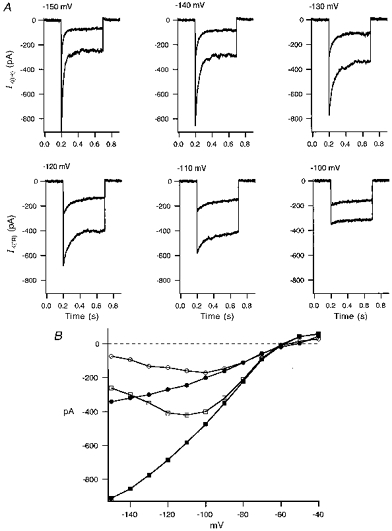
A, superimposed current traces of the cell bathed in standard bath solution with 1 mm Ca2+ and after application of bath solution containing 10 mm Ca2+. The current traces were elicited from −150 to −100 mV with a holding potential of −60 mV and pulses of 500 ms duration. B, current-voltage plot of peak current (▪) and steady-state current (□) for control, and peak current (•) and steady-state current (○) after application of bath solution containing 10 mm Ca2+.
The external Ca2+ concentration-dependent block of IK(IR) was assessed by measuring the decrease in peak current at −120 mV with various external Ca2+ concentrations (Fig. 4). Each cell was exposed to external solutions with various [Ca2+]o from 0.5 to 30 mm. The percentage decrease in current was then plotted against [Ca2+]o (Fig. 4A). The mean data are fitted by the logistic equation:
where Imax is the maximum current block, Kd is the concentration at half-block, and p is the slope of the dose-response curve or the Hill coefficient. This gives a Kd of 5.4 ± 0.6 mm and a p value of 1.07 ± 0.08 (n = 5, −120 mV) for Ca2+ block of peak IK(IR). Figure 4B shows the current traces of a HCEC at −120 mV with various external Ca2+ concentrations.
Figure 4. Inhibition of IK(IR) by a range of external Ca2+ concentrations.
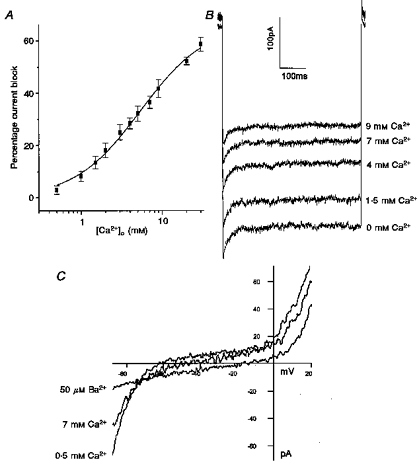
A, effect of [Ca2+]o from 0.5 to 30 mm on the mean percentage block of peak inward rectifier current at −120 mV (n = 5). Inhibition of the current at different concentrations of external Ca2+ is well fitted by a logistic equation with an EC50 value of 5.4 ± 0.6 mm. B, representative current traces of a HCEC at −120 mV with [Ca2+]o from 0 to 9 mm. C, shifts in zero current level of a HCEC exposed to divalent cations. The cell was held at −60 mV and voltage ramps were applied from −120 to +20 mV. The zero current level occurred at −62 mV with 0.5 mm Ca2+, −47 mV with 7 mm Ca2+, and −18 mV with 50 μm Ba2+ in the bath solution.
The membrane potential is modulated by external Ca2+
Elevations of external Ca2+ concentration decreased IK(IR) in all the endothelial cells examined (n = 34). IK(IR) has been shown by Voets et al. (1996) to be a primary component in establishing the resting membrane potential of bovine pulmonary artery endothelial cells. Therefore, block of IK(IR) in HCEC should shift the resting membrane potential. Indeed, we found that HCEC membrane potential depolarized as [Ca2+]o was increased from 0.5 to 7 mm. The reversal potential was determined in voltage clamp by noting the zero current level from averages of voltage ramps (−120 to +100 mV). When external Ca2+ was increased from 0.5 to 7 mm, the reversal potential shifted 10.33 ± 2.4 mV (n = 3) positive from the initial reversal potential of −59.7 ± 1.8 mV (n = 3). Ba2+ at 50 μm is a more potent blocker of the inward rectifier current and, not surprisingly, depolarized the endothelial cells to a much greater extent. Ba2+ depolarized the cells by 36.5 ± 1.3 mV from a resting potential of −59 ± 1.1 mV (n = 4). Figure 4C shows data from a single HCEC with the shifts in zero current level after application of 7 mm Ca2+ or 50 μm Ba2+.
Figure 5 shows the effects of 5 mm Mn2+ or Mg2+ on IK(IR). Mn2+ (5 mm) in the presence of 1.8 mm external Ca2+ blocked 33.4 ± 2.1 % of IK(IR) in five cells tested. Mg2+ (5 mm) with 1.8 mm external Ca2+ blocked IK(IR) by 40.7 ± 2.5 % in nine cells tested. This block did not change the kinetics of the IK(IR) current.
Figure 5. External Mn2+ and Mg2+ block of HCEC IK(IR).
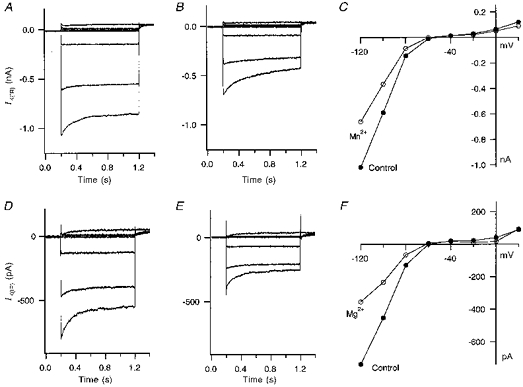
A, current traces from a HCEC in Mn2+-free standard bath solution. The cell was held at −60 mV and pulsed from −120 mV to +20 mV in 20 mV steps. B, current traces after switching to a bath solution containing 5 mm Mn2+. C, the current-voltage plot of the same cell in standard bath solution and in bath solution containing 5 mm Mn2+. D, current traces (same voltage protocol as A) of another HCEC in standard bath solution without Mg2+. E, current traces after application of 5 mm Mg2+ in the standard bath solution to the same HCEC. F, plot of the current-voltage relationship of the HCEC before and after application of Mg2+.
Sr2+ produces a voltage-dependent block of IK(IR)
We observed a profound block of IK(IR) by Sr2+ relative to Ca2+, Mg2+ and Mn2+. The degree of block by 5 mm Sr2+ was of roughly the same magnitude as that seen with 50 μm Ba2+. Sr2+ at 5 mm (−120 mV) blocked 63 ± 2.2 % of peak IK(IR) in five cells compared with 85.3 ± 3.2 % for 50 μm Ba2+ in six cells (−120 mV). Figure 6 shows the effect of 5 mm Sr2+ on both peak and steady-state IK(IR) in HCEC. Sr2+ blocked the two components of IK(IR) to comparable levels (63 ± 2.2 % of peak current and 68 ± 2.1 % of sustained current at −120 mV), with increased block occurring at more negative potentials. Figure 7 further investigates the voltage dependence of this Sr2+ block. Figure 7A shows dose-response curves for Sr2+ block of fractional peak inward rectifier current for voltages ranging from −140 to −110 mV. The EC50 values were: 1.0 ± 0.09 mm for −140 mV, 1.9 ± 0.16 mm for −130 mV, 3.1 ± 0.28 mm for −120 mV, and 4.6 ± 0.34 mm for −110 mV (n = 5).
Figure 6. Sr2+ blocks IK(IR) in a voltage-dependent manner.
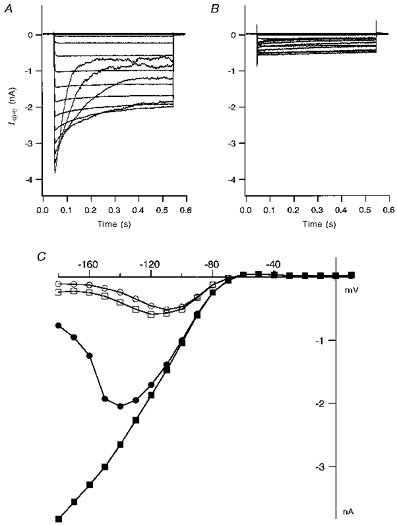
A, IK(IR) traces during strong hyperpolarizing pulses (−180 to +20 mV) from a holding potential of −60 mV from a HCEC perfused with standard external solution. B, current traces from the same cell in the presence of 5 mm Sr2+. Both the transient and steady-state components of the inward current were blocked. C, current-voltage plot for the peak current (▪) and the current at the end of the pulse (•) in standard bath solution, and the peak current (□) and the steady-state current (○) in 5 mm Sr2+.
Figure 7. EC50 values for block of IK(IR) by Sr2+ over a range of test potentials (−140 to −100 mV).
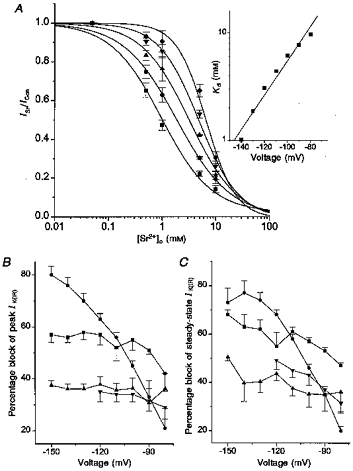
A, fractional inhibition of peak inward current vs. external Sr2+ concentration at −140 mV (▪), −130 mV (•), −120 mV (▴), −110 mV (▾) and −100 mV (♦). ICon is the peak current in standard bath solution and ISr is the current in the presence of 0.05–10 mm Sr2+. The voltage dependence of the Sr2+ block was well fitted by the logistic equation. The inset shows a plot of EC50 values vs. test potential for the same cells. The data points were well fitted with a linear regression yielding a slope such that an e-fold change in EC50 occurs over a 27 mV range. B, percentage block of peak IK(IR)vs. the test potentials for 10 mm Ca2+ (▪), 5 mm Mg2+ (▴), 5 mm Mn2+ (▾) and 5 mm Sr2+ (•). C, percentage block of steady-state IK(IR) by 10 mm Ca2+ (▪), 5 mm Mg2+ (▴), 5 mm Mn2+ (▾) and 5 mm Sr2+ (•) for the various test potentials shown.
The Sr2+ dissociation constant at a given test potential is described as:
where δ is the fractional electrical distance between the external mouth of the channel and the Sr2+ binding site, z is the valency of the blocking ion (Sr2+), and V, F, R and T are the potential across the membrane, the Faraday constant, the gas constant and the absolute temperature, respectively. The δ value was calculated using the above equation (Woodhull, 1973) and the slope value of the Kdvs. voltage is shown in the inset of Fig. 7A. The value of δ is 0.44, as measured from the outside, such that Sr2+ senses 44 % of the membrane field in reaching its blocking site in physiologically normal internal and external solutions. Alternatively, one can describe this as an e-fold change in EC50 over a 27 mV range. The voltage dependences for the other divalent cations examined are quantified in Fig. 7B and C. Sr2+ block of IK(IR) was highly voltage dependent for both peak and steady-state IK(IR), suggesting that the ion blocks within the voltage field. Ca2+, Mn2+ and Mg2+ were all largely voltage independent in their block of IK(IR), although Ca2+ showed some weak voltage dependence at the more positive potentials of −80 and −90 mV. These ions are therefore presumably blocking from the outside of the channel.
DISCUSSION
In the present study we have shown that human capillary endothelial cells contain a large inward rectifier current whose block by divalent cations produces significant depolarizations in the HCEC resting membrane potential. This depolarization by divalent cations would be expected to reduce the driving force for agonist-induced Ca2+ entry, and as such may play a physiological role in pathological situations in which the concentrations of divalent ions such as Ca2+ and Mg2+ may be varying, as in hypo- or hyperparathyroidism (Elin et al. 1990), kidney failure (Henning et al. 1968), or treatment with diuretics (Dyckner & Wester, 1984). Modulation of agonist-induced Ca2+ entry would alter the endothelial cell sensitivity to the agonists with respect to the Ca2+-dependent signals they mediate, such as NO release and intracellular Ca2+ release (Adams et al. 1989). Divalent ion modulation of the level of inward rectifier current would also be expected to have effects upon the amount of Ca2+ influx elicited by growth factors such as vascular endothelial growth factor (VEGF) that trigger sustained Ca2+ entry (Bates & Curry, 1997).
The peak inward rectifier current (IK(IR)) in these HCEC increases with hyperpolarizing potentials; however, the steady-state current reaches a maximum at about −120 to −130 mV and decreases with more hyperpolarizing potentials (Fig. 3B and 6C). This voltage dependence of IK(IR) has also been demonstrated in guinea-pig ventricular myocytes (Biermans et al. 1987) and rabbit osteoclasts (Hammerland et al. 1994). The reduction of the steady-state current with extreme hyperpolarizations has been shown to be largely due to block by external Na+ (Standen & Stanfield, 1979; Biermans et al. 1987; Voets et al. 1996). In agreement with this we found that in experiments in which external Na+ was replaced with NMDG+, the voltage-dependent block of IK(IR) at negative potentials was largely removed (not shown). The present studies were conducted with normal extracellular Na+ and K+ as we are interested in the physiological role of divalent ion block of IK(IR). The Na+ block of IK(IR) only occurs at potentials negative to −120 mV and would therefore not be expected to have a large physiological effect on IK(IR).
The inward rectifier current of these human capillary endothelial cells is blocked by low concentrations (50 μm) of Ba2+. Ba2+ is known to block inward rectifier K+ current at low concentrations in a variety of preparations (Hagiwara et al. 1978; Standen & Standfield, 1978; Kubo et al. 1993). Voets et al. (1996) showed that block of the inward rectifier current by 100 μm Ba2+ in bovine pulmonary artery endothelial cells greatly depolarized these cells. We also show that external Ca2+ as well as Ba2+ depolarize human endothelial cells by blocking IK(IR). Several groups have reported external Ca2+ block of IK(IR) in guinea-pig cardiac myocytes (Biermans et al. 1987; Shioya et al. 1993), in rabbit osteoclasts (Hammerland et al. 1994), and in rat coronary artery smooth muscle cells (Robertson et al. 1996). However, depolarization by external Ca2+ has not been studied in any of these reports.
External divalent cations such as Mg2+ and Sr2+ reduce the unitary current amplitude of inward rectifier channels in guinea-pig cardiac myocytes, as reported by Shioya et al. (1993). An external Mg2+ concentration of 5 mm blocked inward rectifier current by 41.2 % at −80 mV in rat coronary artery smooth muscle cells (Robertson et al. 1996), and 4 mm Mg2+ reduced the steady-state IK(IR) by 85 % at −120 mV in guinea-pig ventricular myocytes (Biermans et al. 1987). In HCEC, both 5 mm Mg2+ and 5 mm Mn2+ reduced IK(IR), by 40.7 ± 2.5 % (n = 9) and 33.4 ± 2.1 % (n = 5) at −120 mV, respectively (Fig. 5).
Standen & Stanfield (1978) have previously reported on the block of inward rectifier current in frog skeletal muscle fibres by 10 mm Sr2+. They describe the voltage-dependent Sr2+ block of the steady-state inward rectifier current as being similar to that of Ba2+; in contrast to us they do not see any voltage dependence to the block of the peak inward current in the presence of Sr2+. This difference is unlikely to be due to the Sr2+ concentration used as we see our effect over a range of Sr2+ concentrations (50 μm to 10 mm). We do, however, use physiologically normal 5.4 mm extracellular K+ in our bath solution, whereas they use 115 mm K+. It is possible that the elevated K+ slows the blockade of the channel by Sr2+ such that the voltage-dependent block of the peak current is not observed but the block is seen at the end of the pulse. However, they also do not observe a block by extracellular Ca2+ in their studies, whereas we do. It therefore seems more likely that there are different inward rectifier channels in these two preparations. Sequence alignment of cloned inward rectifier channels overall shows a high degree of homology in the putative pore-forming region; however, differences do occur and some of these may account for the varying results seen in the literature for this class of channel.
We demonstrate in this study the block of human capillary endothelial inward rectifier current by Ba2+, Sr2+, Ca2+, Mg2+ and Mn2+. We also quantitatively show the role of IK(IR) in establishing the resting membrane potential of these human capillary endothelial cells. The block by Sr2+ was highly voltage dependent for both the peak and steady-state current. Fits of the data in Fig. 7 show an e-fold change in current blocked over a 27 mV range. This translates into a δ value of 0.44 for Sr2+. The strong voltage dependence of Sr2+ block relative to Ca2+, Mn2+ and Mg2+ suggests that Sr2+ may be binding to a site within the voltage field whereas Ca2+, Mg2+ and Mn2+ appear to block outside the pore. This has been suggested by others for Mg2+ and Ca2+ block relative to Cs+ and Sr2+ (Shioya et al. 1993).
References
- Adams DJ, Barakeh J, Laskey R, van Breemen C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB Journal. 1989;3:2389–2400. doi: 10.1096/fasebj.3.12.2477294. [DOI] [PubMed] [Google Scholar]
- al-Mehdi AB, Ischiropoulos H, Fisher AB. Endothelial cell oxidant generation during K+-induced membrane depolarization. Journal of Cellular Physiology. 1996;166:274–280. doi: 10.1002/(SICI)1097-4652(199602)166:2<274::AID-JCP4>3.0.CO;2-M. 10.1002/(SICI)1097-4652(199602)166:2<274::AID-JCP4>3.3.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Bates DO, Curry FE. Vascular endothelial growth factor increases microvascular permeability via a Ca2+-dependent pathway. American Journal of Physiology. 1997;273:H687–694. doi: 10.1152/ajpheart.1997.273.2.H687. [DOI] [PubMed] [Google Scholar]
- Biermans G, Vereecke J, Carmeliet E. The mechanism of the inactivation of the inward-rectifying K current during hyperpolarizing steps in guinea-pig ventricular myocytes. Pflügers Archiv. 1987;410:604–613. doi: 10.1007/BF00581320. [DOI] [PubMed] [Google Scholar]
- Busse R, Luckhoff A, Mulsch A. Cellular mechanisms controlling EDRF/NO formation in endothelial cells. Basic Research in Cardiology. 1991;86:7–16. doi: 10.1007/978-3-642-72461-9_2. [DOI] [PubMed] [Google Scholar]
- Campbell DL, Strauss HC, Whorton AR. Voltage dependence of bovine pulmonary artery endothelial cell function. Journal of Molecular and Cellular Cardiology. 1991;23:133–144. doi: 10.1016/0022-2828(91)90032-h. [DOI] [PubMed] [Google Scholar]
- Colden-Stanfield M, Schilling WP, Ritchie AK, Eskin SG, Navarro LT, Kunze DL. Bradykinin-induced increases in cytosolic calcium and ionic currents in cultured bovine aortic endothelial cells. Circulation Research. 1987;61:632–640. doi: 10.1161/01.res.61.5.632. [DOI] [PubMed] [Google Scholar]
- Demirel E, Rusko J, Laskey RE, Adams DJ, van Breemen C. TEA inhibits ACh-induced EDRF release: endothelial Ca(2+)-dependent K+ channels contribute to vascular tone. American Journal of Physiology. 1994;267:H1135–1141. doi: 10.1152/ajpheart.1994.267.3.H1135. [DOI] [PubMed] [Google Scholar]
- Dyckner T, Wester PO. Intracellular magnesium loss after diuretic administration. Drugs. 1984;28(suppl. 1):161–166. doi: 10.2165/00003495-198400281-00016. [DOI] [PubMed] [Google Scholar]
- Elin RJ, Hosseini JM, Fitzpatrick L, Bliziotes MM, Marx SJ. Blood magnesium status of patients with parathyroid disease. Magnesium and Trace Elements. 1990;9:119–123. [PubMed] [Google Scholar]
- Fichtner H, Frobe U, Busse R, Kohlhardt M. Single nonselective cation channels and Ca2+-activated K+ channels in aortic endothelial cells. Journal of Membrane Biology. 1987;98:125–133. doi: 10.1007/BF01872125. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Miyazaki S, Moody W, Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. The Journal of Physiology. 1978;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hammerland LG, Parihar AS, Nemeth EF, Sanguinetti MC. Voltage-activated potassium currents of rabbit osteoclasts: effects of extracellular calcium. American Journal of Physiology. 1994;267:C1103–1111. doi: 10.1152/ajpcell.1994.267.4.C1103. [DOI] [PubMed] [Google Scholar]
- Henning HV, Quellhorst E, Wolfram D, Jakob G, Scheler F. Changes of serum calcium and serum phosphate level in chronic kidney failure and their significance the diagnosis of hyperparathyroidism. Verhandlungen der Deutschen Gesellschaft fur Innere Medizin. 1968;74:442–445. [PubMed] [Google Scholar]
- Himmel HM, Rasmusson RL, Strauss HC. Agonist-induced changes of [Ca2+]i and membrane currents in single bovine aortic endothelial cells. American Journal of Physiology. 1994;267:C1338–1350. doi: 10.1152/ajpcell.1994.267.5.C1338. [DOI] [PubMed] [Google Scholar]
- Inazu M, Zhang H, Daniel EE. Different mechanisms can activate Ca2+ entrance via cation currents in endothelial cells. Life Sciences. 1995;56:11–17. doi: 10.1016/0024-3205(94)00402-e. [DOI] [PubMed] [Google Scholar]
- Johns A, Lategan TW, Lodge NJ, Ryan US, van Breemen C, Adams DJ. Calcium entry through receptor-operated channels in bovine pulmonary artery endothelial cells. Tissue and Cell. 1987;19:733–745. doi: 10.1016/0040-8166(87)90015-2. [DOI] [PubMed] [Google Scholar]
- Jow F, Numann R, Colatsky TJ. Ion channels in human aorta endothelial cells and human capillary endothelial cells. Biophysical Society (Abstracts) 1996;70:W-pos130. [Google Scholar]
- Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993;362:127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Luckhoff A, Busse R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by the membrane potential. Pflügers Archiv. 1990;416:305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- Nilius B, Schwartz G, Oike M, Droogmans G. Histamine-activated, non-selective cation currents and Ca2+ transients in endothelial cells from human umbilical vein. Pflügers Archiv. 1993;424:285–293. doi: 10.1007/BF00384354. [DOI] [PubMed] [Google Scholar]
- Nilius B, Viana F, Droogmans G. Ion channels in vascular endothelium. Annual Review of Physiology. 1997;59:145–170. doi: 10.1146/annurev.physiol.59.1.145. [DOI] [PubMed] [Google Scholar]
- Olesen SP, Clapham DE, Davies PF. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- Robertson BE, Bonev AD, Nelson MT. Inward rectifier K+ currents in smooth muscle cells from rat coronary arteries: block by Mg2+, Ca2+, and Ba2+ American Journal of Physiology. 1996;271:H696–705. doi: 10.1152/ajpheart.1996.271.2.H696. [DOI] [PubMed] [Google Scholar]
- Sauve R, Parent L, Simoneau C, Roy G. External ATP triggers a biphasic activation process of a calcium-dependent K+ channel in cultured bovine aortic endothelial cells. Pflügers Archiv. 1988;412:469–481. doi: 10.1007/BF00582535. [DOI] [PubMed] [Google Scholar]
- Schwarz G, Callewaert G, Droogmans G, Nilius B. Shear stress-induced calcium transients in endothelial cells from human umbilical cord veins. The Journal of Physiology. 1992;458:527–538. doi: 10.1113/jphysiol.1992.sp019432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioya T, Matsuda H, Noma A. Fast and slow blockades of the inward-rectifier K+ channel by external divalent cations in guinea-pig cardiac myocytes. Pflügers Archiv. 1993;422:427–435. doi: 10.1007/BF00375067. [DOI] [PubMed] [Google Scholar]
- Silver MR, DeCoursey TE. Intrinsic gating of inward rectifier in bovine pulmonary artery endothelial cells in the presence or absence of internal Mg2+ Journal of Geneneral Physiology. 1990;96:109–133. doi: 10.1085/jgp.96.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen NB, Stanfield PR. A potential and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. The Journal of Physiology. 1978;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen NB, Stanfield PR. Potassium depletion and sodium block of potassium currents under hyperpolarization in frog sartorius muscle. The Journal of Physiology. 1979;294:497–520. doi: 10.1113/jphysiol.1979.sp012943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Schini V, Stoeckel H. Voltage-activated potassium, but not calcium currents in cultured bovine aortic endothelial cells. Pflügers Archiv. 1987;410:385–393. doi: 10.1007/BF00586515. [DOI] [PubMed] [Google Scholar]
- Voets T, Droogmans G, Nilius B. Membrane currents and the resting membrane potential in cultured bovine pulmonary artery endothelial cells. The Journal of Physiology. 1996;497:95–107. doi: 10.1113/jphysiol.1996.sp021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman GC, Bevan JA. Barium inhibits the endothelium-dependent component of flow but not acetylcholine-induced relaxation in isolated rabbit cerebral arteries. Journal of Pharmacology and Experimental Therapeutics. 1995;274:47–53. [PubMed] [Google Scholar]
- Woodhull AM. Ionic blockage of sodium channels in nerve. Journal of General Physiology. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]


