Abstract
The perforated whole-cell configuration of patch clamp and the pH fluorescent indicator SNARF were used to determine the electrogenicity of the Na+-HCO3− cotransport in isolated rat ventricular myocytes.
Switching from Hepes buffer to HCO3− buffer at constant extracellular pH (pHo) hyperpolarized the resting membrane potential (RMP) by 2.9 ± 0.4 mV (n = 9, P < 0.05). In the presence of HCO3−, the anion blocker SITS depolarized RMP by 2.6 ± 0.5 mV (n = 5, P < 0.05). No HCO3−-induced hyperpolarization was observed in the absence of extracellular Na+. The duration of the action potential measured at 50 % of repolarization time (APD50) was 29.2 ± 6.1 % shorter in the presence of HCO3− than in its absence (n = 6, P < 0.05).
Quasi-steady-state currents were evoked by voltage-clamped ramps ranging from −130 to +30 mV, during 8 s. The development of a novel component of Na+-dependent and Cl−-independent steady-state outward current was observed in the presence of HCO3−. The reversal potential (Erev) of the Na+-HCO3− cotransport current (INa,Bic) was measured at four different levels of extracellular Na+. A HCO3−:Na+ ratio compatible with a stoichiometry of 2:1 was detected. INa,Bic was also studied in isolation in standard whole-cell experiments. Under these conditions, INa,Bic reversed at −96.4 ± 1.9 mV (n = 5), being consistent with the influx of 2 HCO3− ions per Na+ ion through the Na+-HCO3− cotransporter.
In the presence of external HCO3−, after 10 min of depolarizing the membrane potential (Em) with 45 mm extracellular K+, a significant intracellular alkalinization was detected (0.09 ± 0.03 pH units; n = 5, P < 0.05). No changes in pHi were observed when the myocytes were pre-treated with the anion blocker DIDS (0.001 ± 0.024 pH units; n = 5, n.s.), or when exposed to Na+-free solutions (0.003 ± 0.037 pH units; n = 6, n.s.).
The above results allow us to conclude that the cardiac Na+-HCO3− cotransport is electrogenic and has an influence on RMP and APD of rat ventricular cells.
The steady-state intracellular pH of heart cells is about 1 pH unit more alkaline than would be expected if H+ were in electrochemical equilibrium. The maintenance of intracellular pH (pHi) in the physiological range requires mechanisms for H+ extrusion, net OH− (HCO3−) influx or both. A Na+-H+ exchanger has been historically implicated in the maintenance and regulation of myocardial pHi (Lazdunski et al. 1985). However, over the last few years, bicarbonate-dependent mechanisms (Thomas, 1989; Grace et al. 1993) have been identified in the myocardium by studies performed under conditions in which the physiological buffer system was present in the media. Three different bicarbonate transporters have been characterized in the myocardium: an acid loader, the Cl−-HCO3− exchanger (Vaughan-Jones, 1982), and two acid extruders, the Na+-dependent Cl−-HCO3− exchanger (Liu et al. 1990) and the Na+-HCO3− cotransporter (Dart & Vaughan-Jones, 1992; Lagadic-Gossmann et al. 1992; Camilión de Hurtado et al. 1995, 1996).
Na+-HCO3− cotransport was first described by Boron & Boulpaep (1983) in the renal proximal tubule of the salamander, with a HCO3− : Na+ stoichiometry of 3:1, which generates a net flux of negative charge across the cell membrane. The same stoichiometry was reported in retinal glia of the salamander (Newman, 1991). In contrast, a cotransport stoichiometry of 2:1 has been estimated for amphibian optic nerve and mouse cerebral astrocytes (Astion & Orkand, 1988; Brookes & Turner, 1994), leech glial cells (Deitmer & Shlue, 1989), frog retinal pigment epithelium (Hughes et al. 1989), hepatic (Fitz et al. 1992) and corneal endothelial cells (Wiederholt et al. 1985). An electrically silent symport was reported to be present in sheep Purkinje fibres (Dart & Vaughan-Jones, 1992) and isolated guinea-pig ventricular myocytes (Lagadic-Gossmann et al. 1992), but the lack of electrogenicity of the symport in myocardium has been challenged by experiments performed in cat heart multicellular preparations (Camilión de Hurtado et al. 1995, 1996).
In order to obtain further insight into the possible electrogenicity of the myocardial Na+-HCO3− symport, experiments in isolated rat myocytes were carried out using the perforated whole-cell configuration of the patch-clamp technique to record membrane currents and voltage. The fluorescent indicator SNARF was used in parallel experiments for pHi measurements. In the present study, we present the first evidence for the presence of an electrogenic Na+-HCO3− symport in isolated rat cardiac myocytes and its contribution to the modulation of resting membrane potential (RMP) and action potential (AP) waveform. Part of the results has been reported in abstract form (Aiello et al. 1997).
METHODS
Cell isolation
All experiments were performed in accordance with the guidelines for Animal Care of the Scientific Committee of the University of La Plata School of Medicine. Rats (200–300 g) were anaesthetized by intra-abdominal injection of sodium pentobarbitone (35 mg (kg body weight)−1) and then killed by cervical dislocation. Their hearts were quickly removed, mounted in a Langendorff apparatus and retrogradely perfused with Krebs-Henseleit solution (K-H) containing (mm): 123 NaCl, 4.69 KCl, 1.35 CaCl2, 20 NaHCO3, 1.2 NaH2PO4, 1.2 MgSO4, 11 glucose; pH 7.35 after gassing with 95 % O2-5 % CO2. Hearts were perfused at constant pressure for a stabilization period of 10–15 min. Single ventricular myocytes were isolated using an enzymatic dispersion technique in which hearts were perfused with nominally Ca2+-free K-H for 5 min before treatment with collagenase (74.5 units ml−1, Worthington Biochemical Corp., Freehol, NJ, USA) in Ca2+-free K-H for 45 min. The left ventricle was then removed, placed in Ca2+-free K-H and cut into small pieces (2 mm × 2 mm). After a final wash, the tissue was kept in K-H at room temperature, and single myocytes were obtained by gentle trituration.
Whole-cell recordings
Isolated rat ventricular myocytes were placed in a recording chamber and superfused with bath solution at a flow rate of 1.5 ml min−1. Only rod-shaped myocytes with clear and distinct striations and an obvious marked shortening and relaxation during stimulation were used. The experiments were performed at room temperature (20–22°C).
The nystatin- or gramicidin-perforated whole-cell configuration of the patch-clamp technique (Hamill et al. 1981; Korn et al. 1991) was used for voltage-clamp and current-clamp recordings with a patch-clamp amplifier (Axopatch 200A, Axon Instruments). The standard whole-cell configuration (Hamill et al. 1981) was used in some of the experiments. Patch pipettes were pulled with a PP-83 puller (Narishige, Tokyo, Japan) and fire-polished with a MF-83 Microforge (Narishige) to a final resistance of 0.5–1 MΩ when filled with a control pipette solution. The pipette tip was positioned above the cell, and the pipette potential and capacitance were nullified. Nystatin or gramicidin produced good intracellular access after 15–20 min of seal formation. Membrane voltage and whole-cell currents (filtered at 1 kHz) were digital and directly recorded onto a hard disk via an analog-to-digital convertor (Digidata 1200, Axon Instruments) interfaced with an IBM clone computer running pCLAMP and Axotape software (Axon Instruments). Data analysis was performed with pCLAMP (Clampfit). In most of the experiments, an Ag-AgCl wire directly in contact with the extracellular solution was used as the reference electrode. Since the pipette potential was nulled in external solution, all current-clamp tracings and voltage-clamp protocols required corrections for junction potential. This was accomplished by filling twenty pipettes with standard internal solution. They were then nulled in internal solution, and the difference in potential on immersion in external solution was recorded. The measured junction potential value was consistently −10 mV, and this value was used to correct the current-clamp data and the voltage-clamp protocols. Junction potential values were also calculated with JPcalc software (P. H. Barry, University of New South Wales, Sydney, Australia). There were no significant differences in the value of the junction potential among the external solutions used in the present work. A 3 m KCl-agar bridge was used as the reference electrode in the Cl−-free experiments. Under external Cl−-free conditions, junction potential values were approximately +2 mV and voltage-clamp protocols were not corrected. For each cell, the capacitative current was recorded to determine the membrane capacitance (Cm) and the currents were normalized for cell capacitance. Mean Cm was 153.15 ± 14.21 pF (n = 18).
Fluorescence measurements
Intracellular pH was measured in parallel experiments. Briefly, pHi was measured using the pH-sensitive probe SNARF-1 (Molecular Probes). The cells were loaded at room temperature for 25 min with 5 μm SNARF-1 AM. This probe was previously dissolved in dimethyl sulphoxide (DMSO) and pluronic acid. The final DMSO concentration was 0.15 mm. Cells attached to laminin-coated cover-slips were then transferred to the stage of an inverted microscope. The myocytes were continuously superfused with external solutions. The pHi of a single cell was recorded by microfluorometry using a dual-emission wavelength system. The excitation light was provided by a xenon lamp and attenuated by two neutral density filters. The fluorescent probe was excited at 510 nm, and the emitted light from the specimen was collected using a Nikon Diaphot inverted epifluorescence microscope, split by a series of dichroic mirrors and passed through narrow band-pass (10 nm) optical filters centred at 580 and 640 nm. The light was measured simultaneously at 580 and 640 nm by means of two matched photomultiplier tubes (Hamamatsu type R2560HA, Japan). The voltages from the two photomultiplier tubes, and an analog ratio of the two fluorescence signals (used to monitor relative changes in pHi) were digitized on-line.
At the end of each experiment, the fluorescence emission was calibrated using the high-K+ nigericin technique (Thomas et al. 1979; Blank et al. 1992; Spitzer & Bridge, 1992). The calibration solution contained (mm): 140 KCl, 1 MgCl2, 2 EGTA, 12 Hepes, 0.01 nigericin, 11 glucose, 20 2,3-butanedionemonoxime (BDM) (the latter to prevent muscle contracture) (Spitzer & Bridge, 1992). The pH was adjusted with KOH to different values ranging from 7.5 to 6.5. Such a calibration gave a non-linear relation between pH and the fluorescence ratio (F580/F640). At any given pHi, [HCO3−]i was calculated from the Henderson-Hasselbach equation.
Solutions
The HCO3−-free external solution (Hepes-buffered) contained (mm): 133 NaCl, 5 KCl, 1.2 MgSO4, 0.8 MgCl2, 10 glucose, 1 CaCl2, 10 Hepes; pH adjusted to 7.35 with 5 mm NaOH (total Na+ 138 mm). The HCO3−-buffered solution contained (mm): 118 NaCl, 5 KCl, 1.2 MgSO4, 0.8 MgCl2, 10 glucose, 1 CaCl2, 10 Hepes, 15 choline chloride, 20 NaHCO3; pH adjusted to 7.35 with Tris base after gassing with 95 % O2-5 % CO2. Choline chloride was added to the HCO3−-buffered solution in order to have the same Cl− concentration as in the Hepes-buffered solution and avoid differences in junction potential values among solutions.
In the Na+-free experiments, the external NaCl was replaced completely with an equimolar concentration of choline chloride in both Hepes-buffered and HCO3−-buffered solutions. The Na+-free Hepes-buffered solution was adjusted to pH 7.35 with Tris base. NaHCO3 was replaced completely with choline bicarbonate in the HCO3−-buffered solution. Experiments at different levels of extracellular Na+ were also performed. In these experiments, NaCl was replaced with choline chloride in order to obtain extracellular concentrations of 30, 60 and 90 mm NaCl. The pH was adjusted to 7.35 with Tris base. Sacarose (20 mm) was included in the low extracellular Na+, Hepes-buffered solution to equilibrate the total amount of ions with the HCO3−-buffered solution.
In the Cl−-free experiments, NaCl, KCl, MgCl2 and CaCl2 were replaced with equimolar concentrations of sodium glucuronate, potassium gluconate, MgSO4 and CaSO4, respectively. Choline chloride was not added to the HCO3−-buffered solution in the Cl−-free experiments.
The nystatin or gramicidin pipette solutions contained (mm): 130 potassium gluconate, 10 KCl, 8 NaCl, 0.5 MgCl2, 1 EGTA, 10 Hepes, and 0.3 mg ml−1 nystatin or 0.6 mg ml−1 gramicidin; pH adjusted to 7.15 with KOH. NaCl was not added to the pipette solution in the Na+-free experiments.
In the standard whole-cell experiments the HCO3−-free solution contained (mm): 5 caesium methanesulphonate, 133 sodium gluconate, 1.2 MgSO4, 1.8 MgCl2, 0.05 BaCl2, 10 Hepes, 10 TEA, 0.01 TTX, 0.01 nifedipine; pH adjusted to 7.35 with 5 mm NaOH (total Na+ 138 mm). The HCO3−-containing solution contained (mm): 5 caesium methanesulphonate, 118 sodium gluconate, 1.2 MgSO4, 1.8 MgCl2, 0.05 BaCl2, 10 Hepes, 20 NaHCO3, 10 TEA, 0.01 TTX, 0.01 nifedipine; pH adjusted to 7.35 with Tris base after gassing with 95 % O2-5 % CO2. The pipette solution used in the standard whole-cell experiments contained (mm): 140 caesium methanesulphonate, 1 MgCl2, 5 EGTA, 4 Na2ATP, 10 Hepes, 10 TEA; pH adjusted to 7.15 with CsOH.
The HCO3−-buffered solution used in the K+-induced depolarization experiments contained (mm): 100 NaCl, 5 KCl, 1 MgSO4, 0.35 NaH2PO4, 10 glucose, 40 choline chloride, 20 NaHCO3; pH 7.35 after gassing with 95 % O2-5 % CO2. K+-induced depolarization was assessed by replacing 40 mm choline chloride with 40 mm KCl. In the Na+-free experiments, NaHCO3 was replaced by choline bicarbonate.
Nystatin, gramicidin, TTX, TEA, nifedipine, BDM and SITS were purchased from Sigma Chemical Co. DIDS was purchased from Calbiochem.
Statistics
Data were expressed as means ±s.e.m. and were compared with Students's t test for paired and unpaired values, and repeated-measures ANOVA followed by Dunnett's test. A value of P < 0.05 was considered statistically significant (two-tailed test).
RESULTS
Figure 1A shows a representative continuous recording of pHi before and after replacing the extracellular Hepes-buffered solution (nominally HCO3−-free) with a CO2/HCO3−-buffered one (HCO3− 20 mm) at constant extracellular pH (pHo). Figure 1B shows the average changes in pHi after the acid load induced by replacement of the Hepes-buffered superfusate with HCO3−-buffered solution. An early decrease in pHi, followed by a recovery, can be seen. Initially, CO2 entry causes intracellular acidification but, within 1–3 min, pHi recovers towards control values. After 7 min, pHi recovers completely to values not different from those obtained before the replacement of the extracellular solution. Myocytes exposed to extracellular Hepes had an average pHi of 7.16 ± 0.01 (n = 7) and, after 7 min of exposure to HCO3−, the pHi was 7.16 ± 0.05 (n = 7).
Figure 1. pHichanges after switching the extracellular solution from Hepes-buffered solution to HCO3−-buffered solution.
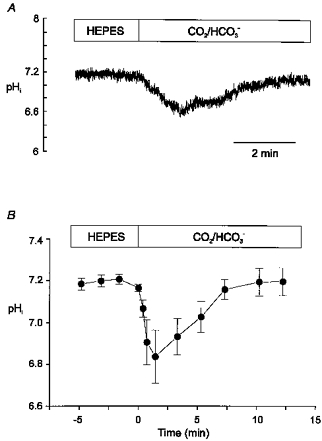
A, continuous recording of pHi from a rat cardiac myocyte loaded with SNARF-1 AM before and after changing the superfusate from a Hepes-buffered solution to a HCO3−-buffered one. B, mean pHi data recorded from 7 myocytes subjected to the experimental protocol shown in A. The change from Hepes-buffered to CO2/HCO3−-buffered superfusate (at constant pHo) induced a transient acidification due to CO2 permeation that was followed by a recovery to values similar to control.
Figure 2A shows a representative continuous recording of resting membrane potential (RMP) before and after changing the extracellular Hepes-buffered solution for a HCO3−-buffered superfusate. RMP hyperpolarized rapidly by 3.5 mV in the presence of the physiological buffer. This hyperpolarization of RMP remained constant despite the changes of pHi that occurred during a similar period of time in the myocytes in which pHi was measured (Fig. 1). The washout of the HCO3−-containing solution with Hepes-buffered superfusate reversed this hyperpolarization. On average, after 7 min in the presence of HCO3−, RMP hyperpolarized by 2.9 ± 0.4 mV (n = 9, P < 0.05). Figure 2B shows a continuous recording of RMP from a myocyte exposed successively to Hepes- and HCO3−-buffered Na+-free solutions. Changing the superfusate from Hepes- to HCO3−-buffered solution in the absence of extracellular Na+ did not produce significant changes in RMP. In the absence of extracellular Na+, the difference between the values of RMP recorded after 7 min of exposure of the myocytes to HCO3− and those recorded in Hepes was 1.2 ± 1.3 mV (n = 4, n.s.). Figure 2C shows the effect of the anion blocker SITS (Boyarski et al. 1988; Fitz et al. 1992; Nakanishi et al. 1992; Kusuoka et al. 1994; Camilión de Hurtado et al. 1995) on a representative recording of RMP. In the presence of HCO3−, SITS (0.1 mm) depolarized RMP by 2.6 ± 0.5 mV (n = 5, P < 0.05). In the representative experiment shown in Fig. 2C the onset rate of SITS-induced depolarization was faster than that of HCO3−-induced hyperpolarization. The change from one solution to another of different composition may give a different onset rate to that produced by the addition of pharmacological drugs. Nevertheless, it is important to stress that the steady-state average values of HCO3−-induced hyperpolarization and SITS-induced depolarization were both of the same magnitude. SITS did not affect RMP when the myocytes were exposed to extracellular HCO3−-free solution: in four cells superfused with Hepes-buffered solution the average values of RMP were −75.8 ± 1 mV in control and −75.3 ± 1.1 mV after adding 0.1 mm SITS to the bath solution (n.s.). The latter group of myocytes was then exposed to HCO3−-buffered solution in the continuous presence of SITS. Under these conditions, the mean value of RMP was −75.9 ± 1.6 mV (n = 4), indicating that the anion blocker was able to prevent the HCO3−-induced hyperpolarization. Figure 2D shows representative action potential (AP) recordings in the absence of extracellular HCO3− and after 7 min of exposure of the myocytes to 20 mm HCO3−. The physiological buffer shortened the duration of the AP. This effect was reversed after washout with Hepes-buffered solution (data not shown). On average, AP duration measured at 50 % of repolarization time (APD50) was 29.2 ± 6.1 % shorter in the presence of HCO3− than in its absence (n = 6, P < 0.05).
Figure 2. Hyperpolarization of RMP and action potential duration shortening induced by external HCO3−.
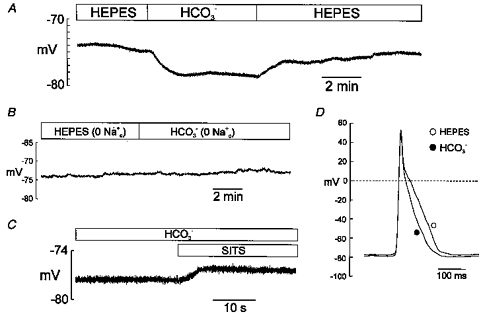
Perforated whole-cell configuration. A, continuous recording of RMP from a rat cardiac myocyte exposed successively to Hepes- and HCO3−-buffered solutions and then back to the Hepes-buffered solution. B, representative recording of RMP in a myocyte during successive treatment with Hepes- and HCO3−-buffered Na+-free solutions. C, continuous recording of RMP in a myocyte exposed to HCO3− before and after addition of 0.1 mm SITS to the extracellular solution. D, action potential recordings under current-clamp mode before and after superfusion of a myocyte with external HCO3−. APD50 and APD90 of this myocyte were 80 and 144 ms in Hepes, and 44 and 108 ms in HCO3−, respectively. In the presence of HCO3−, a SITS-sensitive and Na+-dependent hyperpolarization of RMP was observed. Action potential duration shortening was also detected in the presence of HCO3− in the extracellular solution.
Figure 3 shows the effects of changing the extracellular superfusate from the Hepes-buffered to the HCO3−-buffered solution on steady-state currents evoked by 8 s duration voltage-clamped ramps from −130 to +30 mV, from a holding potential of −75 mV. A novel component of a steady-state outward current was detected in the presence of HCO3− (Fig. 3A), consistent with an electrogenic influx of this anion. The current evoked at +30 mV increased 1.76 ± 0.33 (n = 4, P < 0.05) times 7 min after switching the extracellular superfusate from a Hepes-buffered to a HCO3−-buffered solution. The inset shows the Na+ dependence of this novel current: in the absence of Na+, HCO3− failed to induce the appearance of any steady-state outward current. Under these conditions the amplitudes of the outward currents evoked at +30 mV were 1.65 ± 0.32 pA pF−1 in Hepes-buffered superfusate and 1.43 ± 0.33 pA pF−1 in HCO3−-buffered solution (n = 5, n.s.). Figure 3B shows that the HCO3−-sensitive current, obtained by subtracting the current registered in Hepes from that measured after 7 min in the presence of HCO3−, reversed at around −95 mV (−87 ± 4.8 mV; n = 4).
Figure 3. Effects of external HCO3− on steady-state currents.
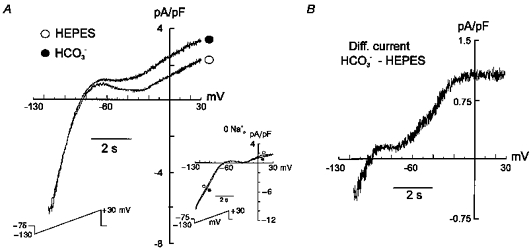
Perforated whole-cell configuration. A, steady-state currents evoked by 8 s duration voltage-clamp ramps ranging from −130 to +30 mV, from a holding potential of −75 mV, before and after exposure of a rat cardiac myocyte to external HCO3−. Inset: steady-state currents recorded in the absence of intracellular and extracellular Na+. B, difference current obtained after subtracting the currents recorded in the absence of HCO3− from those recorded in its presence. The appearance of a steady-state outward current was observed in the presence of HCO3−. The HCO3−-sensitive difference current reversed at −95 mV.
The HCO3−-sensitive difference current (Fig. 3B) showed a slight rectification between −90 and −70 mV (2 out of 4 cells) and a strong rectification above −20 mV (4 out of 4 cells). The origin of rectification within these two ranges of voltage is not apparent to us. However, we could speculate that changes in a CO2/HCO3−-sensitive K+ current could account for the rectification at very negative potentials, since K+ channels (specially inward rectifier channels) are the main charge carriers over this range of voltage. The possibility that the observed rectification above −20 mV could be due to saturation of Na+-HCO3− cotransporter activity may also be considered.
The HCO3−-induced hyperpolarization observed in this study was reversed and prevented by SITS, a non-specific anion flux blocker which includes targets such as Cl− currents (Hume & Harvey, 1991), Na+-dependent and -independent Cl−-HCO3− exchangers (Vaughan-Jones, 1982; Boyarski et al. 1988; Liu et al. 1990) and the Na+-HCO3− symport (Fitz et al. 1992; Nakanishi et al. 1992; Kusuoka et al. 1994; Camilión de Hurtado et al. 1995). Among these mechanisms, the only one which would remain active in the absence of external Cl− is the Na+-HCO3− symport. In order to show unambiguously that Cl− is not involved in the process studied in the present work, experiments in the absence of external Cl− were performed. Gramicidin, an antibiotic complex that makes pores in the cell membrane which are not permeable to anions, was used as a perforating agent in these 0 [Cl−]o whole-cell experiments. Figure 4 shows steady-state currents recorded in the absence of external Cl−, before and after 7 min of exposure of the myocytes to HCO3−-containing solution. Under 0 [Cl−]o conditions, a similar fraction of steady-state outward current to that registered in the presence of external Cl− was detected. The amplitude of the current evoked at +30 mV was 1.97 ± 0.43 times greater in the presence of HCO3− than in its absence. The HCO3−-sensitive current, shown in Fig. 4B, reversed at −85 mV (−82.5 ± 2.2 mV; n = 4), a value close to that measured with external Cl−-containing solutions.
Figure 4. Na+-HCO3− cotransport current in the absence of external Cl−.
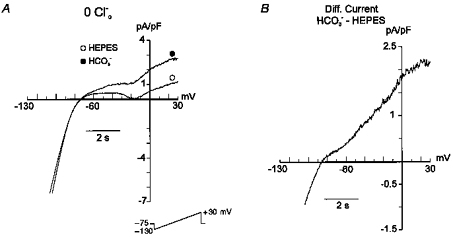
A, steady-state currents, evoked by the same voltage protocol used in Fig. 3, recorded in the absence of extracellular Cl−, before and after 7 min of exposure of the myocyte to HCO3−-containing solution. B, difference current obtained after subtracting the currents recorded in the absence of HCO3− from those recorded in its presence. The HCO3−-sensitive current has similar characteristics to the current registered with Cl−-containing solutions.
The reversal potential (Erev) of the HCO3−-sensitive difference current was measured at different levels of extracellular Na+. The Erev of the Na+-HCO3− cotransport current (INa,Bic) with a HCO3−:Na+ stoichiometry of 2:1 was calculated using the following equation (Newman, 1991):
If we consider that the experiments were performed at 20–22°C and we assume that [Na+]i (8 mm) and [HCO3−]o (20 mm) are constants, the INa,BicErev will be a function of [Na+]o and [HCO3−]i. In order to analyse the [Na+]o dependence of the INa,BicErev, experiments were undertaken in which the Erev was measured under different [Na+]o. The dotted line in Fig. 5 shows the theoretical relationship by which a symport current with stoichiometry of n = 2and [HCO3−]i of 13.9 mm (pHi 7.1) alter its Erev as a function of [Na+]o. The four experimental points obtained in our experiments are shown superimposed on the theoretical line (n = 5for [Na+]o of 30 and 60 mm and n = 4for [Na+]o of 90 and 138 mm).
Figure 5. Mean reversal potential data for Na+-HCO3− cotransport currents recorded at different concentrations of extracellular Na+.
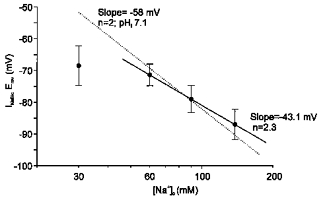
•, mean values of INa,BicErev registered at 30 mm (n = 5), 60 mm (n = 5), 90 mm (n = 4) and 138 mm (n = 4) extracellular Na+. The dotted line represents the INa,BicErev calculated using the equation described in the text and assuming a HCO3− :Na+ ratio of 2:1. [HCO3−]i was assumed to be 13.9 mm (pHi 7.1). The means ±s.e.m. of the INa,BicErev recorded at 60, 90 and 138 mm extracellular Na+ were fitted to a regression line with a slope of −43 mV mm−1. This value corresponds to a Na+-HCO3− symport with a stoichiometry n value of 2.3. The results are consistent with the presence of a Na+-HCO3− cotransport current with a HCO3−:Na+ stoichiometry ratio of 2:1.
Three of the four points (those corresponding to [Na+]o of 138, 90 and 60 mm) were fitted to a linear regression line. A correlation coefficient of 0.99 and a slope of −43 mV mm−1 was obtained. This slope corresponds to a Na+-HCO3− symport with a stoichiometry n value of 2.3. The fourth point, in which the INa,BicErev was examined at [Na+]o of 30 mm, fell below the line. Although we obtained a full recovery of pHi 7–10 min after changing solutions in our experiments, as shown in Fig. 1, the possibility that a reduction of [Na+]o to 30 mm could influence the [HCO3−]i and alter the INa,BicErev for a given [Na+]o was considered. The slow recovery of pHi in very low [Na+]o has been known since the report of Ellis & McLeod (1985) and could explain the incomplete recovery of [HCO3−]i after changing to CO2/HCO3−-containing solutions. The value of INa,BicErev obtained in our experiments at 30 mm[Na+]o could be explained if [HCO3−]i was 10 mm, corresponding to a pHi of 6.96. In order to gain support for this hypothesis, parallel experiments were performed in isolated myocytes in which the recovery of pHi after switching from Hepes-buffered to HCO3−-buffered solutions was measured after a 7–8 min period. The pHi obtained was 6.91 ± 0.05, which corresponds to a [HCO3−]i of 9 mm and would represent an INa,BicErev of −73.7 mV, a value close to that obtained (−68.5 ± 6.2, n = 5). The [Na+]o dependence of the INa,BicErev thus seems to support a Na+-HCO3− symport stoichiometry of n = 2.
In order to study INa,Bic in isolation we performed experiments using the standard whole-cell configuration to get a more accurate control of the ionic composition of the intracellular solution. Ca2+, K+ and Cl− were replaced with Mg2+, Cs+ and methanesulphonate, respectively, in both the pipette and the bath solution. Ba2+, TEA, nifedipine and TTX were added to the extracellular solution. TEA was also added to the intracellular solution. Under these conditions we were able to record a HCO3−-sensitive outward current that was blocked by SITS (0.1 mm) (Fig. 6A). The HCO3−-sensitive difference current shown in Fig. 6B reversed at approximately −100 mV (−96.4 ± 1.9, n = 5), a value close to that obtained using the perforated whole-cell configuration and compatible with a HCO3−: Na+ stoichiometry of 2:1. INa,Bic recorded using the standard whole-cell configuration was about 8 times smaller than the HCO3−-sensitive current recorded using the perforated whole-cell mode. Internal cell dialysis with pipette solution probably caused the loss of a potential intracellular modulator of Na+-HCO3− cotransport activity; i.e. basal intracellular levels of cAMP could be modulating the cotransport since β-adrenoceptor activation has been reported to induce stimulation of this mechanism (Lagadic-Gossmann & Vaughan-Jones, 1993). Whereas no rectification was observed at very negative potentials, consistent with the absence of K+ in the intra- and extracellular solutions, the rectification above −20 mV was still present when INa,Bic was studied in isolation.
Figure 6. Na+-HCO3− cotransport current in isolation.
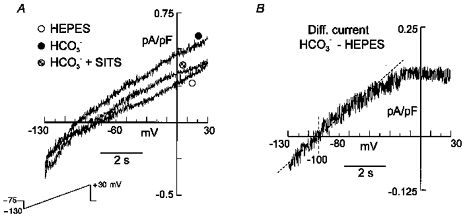
Standard whole-cell configuration. A, steady-state currents, evoked by the same voltage protocol used in Fig. 3, recorded from a rat cardiac myocyte exposed successively to external Hepes, HCO3−, and HCO3− in the presence of 0.1 mm SITS. External and internal K+, Cl− and Ca2+ were replaced with Cs+, methanesulphonate and Mg2+, respectively. TTX, nifedipine and TEA were added to the extracellular solution. TEA was also included in the pipette solution. B, HCO3−-sensitive difference current (HCO3− - Hepes) with a reversal potential of −101 mV.
Figure 7A and B shows continuous recordings of pHi in two myocytes exposed to external HCO3− in the absence and presence of the anion blocker DIDS (0.5 mm) (Newman, 1991; Lagadic-Gossmann et al. 1992; Stahl et al. 1992; Dart & Vaughan-Jones, 1992), respectively. In both cases, the cell membrane potential (Em) was depolarized by approximately 50 mV (Em would be approximately −25 mV) by isosmotically increasing the extracellular K+ concentration ([K+]o) from 5 to 45 mm. Depolarization of Em caused a significant DIDS-sensitive increase in pHi. Figure 7C shows mean data from five myocytes exposed to the same conditions of those in panels A and B. In the absence of DIDS, pHi increased from 7.17 ± 0.05 in control [K+]o to 7.25 ± 0.04 (n = 5, P < 0.05; repeated-measures ANOVA) after 10 min of enhancing [K+]o ten times. In the presence of the anion blocker, pHi was not affected after K+-induced depolarization (7.31 ± 0.01 in 5 mm[K+]o and 7.32 ± 0.02 after 10 min in 45 mm[K+]o; n = 5, n.s.). pHi was also monitored in five myocytes exposed to HCO3− in the absence of extracellular Na+, before and after K+-induced depolarization. Under Na+-free conditions, pHi was not altered after 10 min of isosmotically increasing [K+]o tenfold (7.03 ± 0.05 in control [K+]o and 7.02 ± 0.08 in 45 mm[K+]o; n = 6, n.s.). The experiments described above demonstrated that the enhancement of pHi upon depolarization of Em was DIDS sensitive, and dependent on voltage and Na+. An electrogenic Na+-HCO3− cotransport is the most plausible system for fulfilling these requirements.
Figure 7. pHi changes induced by K+-induced depolarization.
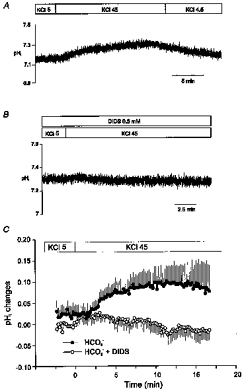
Representative recordings of pHi of two myocytes superfused with HCO3−-buffered solution in the absence (A) and presence of 0.5 mm DIDS (B). Both myocytes were subjected to a 45 mm extracellular K+-induced depolarization of Em. C, mean changes in pHi induced by the K+-induced depolarization (n = 5). After increasing the extracellular K+ concentration from 5 to 45 mm, a significant pHi alkalinization was observed. This change in pHi was prevented by pre-treatment of the myocytes with the anion blocker DIDS.
Initial rates of pHi change induced by K+ depolarization were calculated for each experiment by fitting the values to an exponential curve of the form:
where pHi,t and pHi,∞ are the pHi values at time t and after a steady state is reached, respectively. k is the rate coefficient. From these curve fits dpH/dti at time zero was calculated. K+-induced depolarization increased pHi at a rate of 0.029 ± 0.005 pH units min−1 (n = 5, P < 0.05), which, considering a total buffer capacityof 60 mm (Lagadic-Gossmann et al. 1992), would represent a HCO3− influx of approximately 1.8 mm min−1. In the cell of Fig. 3B, the amplitude of the HCO3−-sensitive difference current evoked at −25 mV was 120 pA (Cm 148 pF). Assuming a specific membrane capacitance of 1 μF cm−2, the total membrane surface area of this myocyte was estimated as 14800 μm2. Taking into account this value of surface area and assuming a smooth-surfaced, brick-shaped myocyte where the length is 6 times the width while the width is twice the thickness (Bishop & Drummond, 1979; Powel et al. 1980; Lagadic-Gossmann et al. 1992), a cell volume of 65 pl could be calculated for this myocyte. We cannot rule out the possibility that this volume could be overestimated by the assumption of a smooth surface for the myocyte. The total surface area (sarcolemmal and T-tubules) of rat myocytes was reported to be 1.5 times the sarcolemmal area (Keung, 1989). As the apparent surface area of the myocytes was not measured in the present study and the proportion of T-tubules conserved after the isolation procedure is not exactly known, the capacitative surface area was used. Accordingly, a HCO3− influx of 2.3 mm min−1 through an electrogenic Na+-HCO3− cotransport with a HCO3−:Na+ stoichiometry of 2:1 could be estimated for the representative HCO3−-sensitive current recorded at −25 mV in the perforated whole-cell configuration. This value of HCO3− influx is similar to that registered using the fluorescent indicator SNARF to measure pHi in the K+-induced depolarization experiments.
DISCUSSION
The experiments reported here demonstrate that rat cardiac ventricular cells possess an electrogenic Na+-HCO3− cotransport system. Changing the myocytes' bathing superfusate from a HCO3−-free (Hepes-buffered) to a HCO3−-containing solution at constant pHo induces RMP hyperpolarization, APD shortening, and development of a steady-state outward current, consistent with the influx of HCO3− into the cell. These changes are blunted by anionic blockade or by sodium deprivation. Although we measured pHi and currents in parallel experiments and not simultaneously, the possible differences in proton handling between the two experimental protocols were minimized by employing the nystatin- or gramicidin-perforated patch, which reduces significantly the intracellular milieu disturbance due to dialysis with pipette solution. Taking the perforated cell as representative of the intact cell, the time of recovery of pHi after switching from Hepes-buffered to HCO3−-buffered solution was analysed, and the measurements of currents and voltage were performed at that time (with the exception of the very low [Na+]o experiments in which incomplete recovery of pH was detected at the time in which measurements were performed). Moreover, in our experiments, the transient acidosis caused after changing the superfusate from Hepes-buffered to HCO3−-buffered solution did not seem to affect the membrane currents that control RMP, since in the absence of extracellular Na+ exposure of the myocytes to CO2/HCO3− did not alter RMP or whole-cell currents. It is well known that the RMP of ventricular cells is mainly maintained by the inward rectifier current (IK1) (Katz, 1992). Above the equilibrium potential for K+ (EK), any enhancement of IK1 would lead to RMP hyperpolarization. However, when pHi decreases, inhibition rather than enhancement of IK1 has been reported (Ito et al. 1992).
Exposure of rat ventricular myocytes to external HCO3− caused significant APD shortening in addition to RMP hyperpolarization. In the present study, rat ventricular APs were recorded at room temperature. Thus, we cannot disregard the possibility that the APs at physiological temperature may have shorter durations than those registered by us and APD may not be significantly changed after exposure of the myocytes to HCO3−. Nevertheless, these results are in agreement with early experiments performed in canine Purkinje fibres by Spitzer & Hogan (1979), although the fact that the long plateau type of the Purkinje fibre AP is different from the spike-like AP of rat ventricle should be recognized. The authors reported that lowering extracellular HCO3− at constant pHo produced depolarization of RMP and APD lengthening. These authors suggested that these effects were due to changes in a background HCO3− current. Under the conditions of the present study, a pure HCO3− current should reverse at −11 mV and therefore generate an inward current below that potential rather than the outward current observed. Furthermore, the data herein presented, showing that this current is coupled to Na+ ions as the reflection of an electrogenic symport, are in disagreement with the possibility of a HCO3− flux through Cl− channels or through potential HCO3− channels.
Previous studies performed in our laboratory had shown evidence of a cardiac electrogenic Na+-HCO3− cotransport at tissue level (Camilión de Hurtado et al. 1995, 1996). Cat papillary muscles exposed to external HCO3− at 30°C exhibited a SITS-sensitive ouabain-insensitive 6 mV hyperpolarization of RMP (Camilión de Hurtado et al. 1995, 1996). Species differences, changes in Na+-HCO3− cotransport function due to the process of cell isolation, or the lower temperature used in this study (20°C) could account for the lower degree of hyperpolarization observed in the isolated rat myocytes. The HCO3−-induced hyperpolarization was small but consistent and, with the obvious cell-to-cell variability, it was present in all the experiments performed. Moreover, only a small hyperpolarization is expected because, in addition to the small currents generated by the symport, at RMP values the driving force for HCO3− influx through the Na+-HCO3− cotransport is not great. An Ag- AgCl wire was used most of the time as reference electrode in the present work. This type of electrode, which mainly responds to Cl− activity, can give large and variable junction potential values and could raise concerns about the significance of the slight HCO3−-induced hyperpolarization observed in this study. However, we have carefully designed the composition of solutions to avoid differences in junction potential values among them (see Methods). In addition, junction potentials were measured experimentally as described in Methods and were also calculated with JPcalc software. Finally, if junction potentials were influencing the measured values of HCO3−-induced hyperpolarization, then there would be no reason for these effects to be totally reversed by SITS and completely prevented by SITS and Na+ deprivation.
In the presence of external HCO3− we were able to record steady-state outward currents which reverse at values close to the estimated Erev for INa,Bic with a HCO3−:Na+ stoichiometry ratio of 2:1. The same stoichiometry was reported for amphibian optic nerve and mouse cerebral astrocytes (Astion & Orkand, 1988; Brookes & Turner, 1994), leech glial cells (Deitmer & Shlue, 1989), frog retinal epithelium (Hughes et al. 1989) and cat papillary muscle (Camilión de Hurtado et al. 1995, 1996). In contrast, a stoichiometry of 3:1 has been estimated for mammalian proximal tubule (Boron & Boulpaep, 1983) and salamander retinal glia (Newman, 1991). This variation in stoichiometry may reflect differences in cotransporter function in different systems. Newman (1991) suggested that a stoichiometry of 3:1 is needed to supply sufficient energy to oppose the inwardly directed Na+ gradient in tissues where HCO3− is normally transported out of the cell. In contrast, when HCO3− is transported into a cell, the Na+ gradient favours this transport and a stoichiometry of 2:1 is sufficient to transport the physiological buffer. A cotransporter stoichiometry of 2:1 in rat ventricular cells suggests that HCO3− is normally transported in an inward direction in these cells, generating an outward current that contributes to hyperpolarization of RMP and shortening of APD.
The initial decrease in pHi caused by the change from Hepes- to HCO3−-buffered solution is followed by a gradual recovery due to the activation of the acid-extrusion mechanisms. During the phase of pHi recovery, the myocardial [Na+]i rises (Harrison et al. 1992; Pérez et al. 1995) and this increase in [Na+]i might, in turn, increase Na+-K+-ATPase activity, thereby producing the hyperpolarization of RMP and the acceleration of AP repolarization. However, this is unlikely to be the cause of the present observations, since in our patch-clamp experiments [Na+]i should be controlled by the concentration of Na+ in the pipette solution. Moreover, the SITS sensitivity of the HCO3−-induced hyperpolarization and the complete absence of K+ in the solutions used in the standard whole-cell experiments allow us to rule out the potential contribution of Na+-K+-ATPase to the results observed in the present study.
An electrically silent Na+-HCO3− cotransport was reported to be present in sheep Purkinje fibres and isolated guinea-pig myocytes by Dart & Vaughan-Jones (1992) and Lagadic-Gossmann et al. (1992), respectively. The authors reported that after switching from Hepes- to HCO3−-buffered superfusate, hyperpolarization of RMP was observed in some preparations, but was not reversed after treatment with DIDS. We cannot rule out the possibility that species differences could be the reason for this discrepancy. Lagadic-Gossmann et al. (1992) failed to record INa,Bic using the standard whole-cell configuration of patch-clamp technique in guinea-pig ventricular myocytes. Under their conditions, INa,Bic might have been present, though barely detectable, since in the present study, when the standard whole-cell configuration was used to examine INa,Bic in isolation, the measured INa,Bic amplitude was 8 times smaller than that recorded under the less altered intracellular milieu obtained with the perforated patch configuration. In any case, we cannot deny the possibility that Na+-HCO3− cotransporters with Na+:HCO3− ratios different from that reported in the present study could be present in other cardiac tissues from different species or even in rat ventricle. Moreover, it could be possible that Na+-HCO3− symports with different stoichiometries might be present in the same tissue. Currently available electrophysiological techniques limit the investigation of a potential combination of both electroneutral and electrogenic symports in the same single cell. Recently, a clone of a renal electrogenic Na+-HCO3− symport with a stoichiometry of n = 3was expressed in Xenopus oocytes (Romero et al. 1997). Cloning, expression and characterization of cardiac Na+-HCO3− cotransporters should be assessed in order to provide new insight into this issue.
In the presence of extracellular HCO3−, the depolarization of Em by increasing [K+]o shifted pHi to more alkaline values, as would be predicted by the increase in HCO3− driving force if the transporting system were carrying net negative charges inside the cells. These findings are consistent with the presence of an electrogenic HCO3−-sensitive mechanism that regulates pHi. The change in pHi caused by the K+-induced depolarization was DIDS sensitive and Na+ dependent, reflecting a HCO3− influx through an electrogenic Na+-HCO3− symport. Similar results were previously obtained in our laboratory in experiments performed in cat papillary muscles (Camilión de Hurtado et al. 1995, 1996), where K+-induced depolarization produced SITS- and Na+-sensitive pHi alkalinization. In the present study, K+-induced depolarization of Em caused a HCO3− influx which was close to but slightly smaller than the HCO3− influx estimated from the amplitude of INa,Bic. In addition to cell-to-cell variability, another interpretation of this small difference could be that HCO3− influx through Na+-HCO3− cotransport in the K+-induced depolarization experiments would be underestimated by the participation of other pH regulating mechanisms that play a role when pHi is shifted to more alkaline values. The most likely mechanisms that could be involved in this effect are the acid loaders: the Cl−-HCO3− exchanger (Vaughan-Jones, 1982) and the novel Cl−-OH− exchanger (Sun et al. 1996). Since these mechanisms are electrically silent, no interference with the estimation of HCO3− influx through the Na+- HCO3− cotransport calculated from INa,Bic would be expected.
In summary, the present study demonstrates the presence of an electrogenic Na+-HCO3− cotransporter in isolated cardiac myocytes. This electrogenic mechanism contributes to shape the AP configuration. These effects are underlain by a steady-state outward current that can be seen only when HCO3− and Na+ are present in the media. Although HCO3− is the physiological buffer, HCO3−-buffered solutions are not usually employed in patch-clamp experiments, masking the observation of this mechanism and its possible physiological role in cardiac cells. An increase in the electrogenic Na+-HCO3− cotransport driving force resulting from continuous depolarization after increments in heart rate has been suggested (Camilión de Hurtado et al. 1996). This mechanism would lead to an enhancement of HCO3− influx that counteracts the decrease in myocardial pHi induced by the increase in CO2 production. Further physiological implications of INa,Bic in the electrical and mechanical properties of cardiac muscle need to be addressed in future research.
Acknowledgments
The authors are grateful to Mónica Rando, Cristina Taraborrelli and Javier Moreta for their excellent technical assistance and to Patricio Morgan for his collaboration in the pHi measurements of one of the experimental series. The authors are Established Investigators of the Consejo Nacional de Investigaciones y Técnicas (CONICET), Argentina.
References
- Aiello EA, Vila Petroff MG, Cingolani HE. Evidence for a cardiac electrogenic Na+/HCO3− cotransport in isolated rat ventricular myocytes. Circulation. 1997;96:I-121. [Google Scholar]
- Astion MK, Orkand RK. Electrogenic Na+/HCO3− cotransport in neuroglia. Glia. 1988;1:355–357. doi: 10.1002/glia.440010508. [DOI] [PubMed] [Google Scholar]
- Bishop SP, Drummond JL. Surface morphology and cell size measurement of isolated rat cardiac myocytes. Journal. of Molecular and Cellular Cardiology. 1979;11:423–433. doi: 10.1016/0022-2828(79)90467-x. [DOI] [PubMed] [Google Scholar]
- Blank PS, Silverman HS, Chung OY, Hogue BA, Stern MD, Hansford RG, Lakatta EG, Capogrossi MC. Cytosolic pH measurements in single cardiac myocytes using carboxy-seminaphthorhodafluor-1. American Journal of Physiology. 1992;263:H276–284. doi: 10.1152/ajpheart.1992.263.1.H276. [DOI] [PubMed] [Google Scholar]
- Boron WF, Boulpaep EL. Intracellular pH regulation in the renal proximal tubule of the salamander. Journal of General Physiology. 1983;8:53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyarski G, Ganz MB, Sterzel RB, Boron WF. pH regulation in single glomerular mesangial cells. II. Na+-dependent and independent Cl−-HCO3− exchangers. American Journal of Physiology. 1988;225:C857–869. doi: 10.1152/ajpcell.1988.255.6.C857. [DOI] [PubMed] [Google Scholar]
- Brookes N, Turner RJ. K+-induced alkanization in mouse cerebral astrocytes mediated by reversal of electrogenic Na+-HCO3− cotransport. American Journal of Physiology. 1994;267:C1633–1640. doi: 10.1152/ajpcell.1994.267.6.C1633. [DOI] [PubMed] [Google Scholar]
- Camilión de Hurtado MC, Alvarez BV, Pérez NG, Cingolani HE. Role of an electrogenic Na+/HCO3− cotransport in determining myocardial pHi after an increase in heart rate. Circulation Research. 1996;79:698–704. doi: 10.1161/01.res.79.4.698. [DOI] [PubMed] [Google Scholar]
- Camilión de Hurtado MC, Pérez NG, Cingolani HE. An electrogenic sodium-bicarbonate cotransport in the regulation of myocardial intracellular pH. Journal of Molecular and Cellular Cardiology. 1995;27:231–242. [PubMed] [Google Scholar]
- Dart C, Vaughan-Jones RD. Na+-HCO3− symport in the sheep cardiac Purkinje fibre. The Journal of Physiology. 1992;451:365–385. doi: 10.1113/jphysiol.1992.sp019169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer JW, Schlue WR. An inwardly directed electrogenic sodium-bicarbonate co-transport in leech glial cells. The Journal of Physiology. 1989;411:179–194. doi: 10.1113/jphysiol.1989.sp017567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D, MacLeod KT. Sodium-dependent control of intracellular pH in Purkinje fibres of sheep heart. The Journal of Physiology. 1985;359:81–105. doi: 10.1113/jphysiol.1985.sp015576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz JG, Lidofsky SD, Xie MH, Scharschmidt BF. Transmembrane electrical potential difference regulates Na+/HCO3− cotransport and intracellular pH in hepatocytes. Proceedings of the National Academy of Sciences of the USA. 1992;89:4197–4201. doi: 10.1073/pnas.89.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Kirschenlohr HL, Metcalfe JC, Smith GA, Weissberg PL, Cragoe EJ, Jr, Vandenberg JI. Regulation of intracellular pH in the perfused heart by external HCO3− and Na+/H+ exchange. American Journal of Physiology. 1993;265:H289–298. doi: 10.1152/ajpheart.1993.265.1.H289. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harrison SM, Frampton JE, McCall E, Boyer ME, Orchard CH. Contraction and intracellular Ca2+, Na+ and H+ during acidosis in rat ventricular myocytes. American Journal of Physiology. 1992;262:C348–357. doi: 10.1152/ajpcell.1992.262.2.C348. [DOI] [PubMed] [Google Scholar]
- Hughes BA, Adorante JS, Miller SS, Lin H. Apical electrogenic NaHCO3 cotransport. A mechanism for HCO3 absorption across the retinal pigment epithelium. Journal of General Physiology. 1989;94:125–150. doi: 10.1085/jgp.94.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume JR, Harvey RD. Chloride conductance pathways in heart. American Journal of Physiology. 1991;261:C399–412. doi: 10.1152/ajpcell.1991.261.3.C399. [DOI] [PubMed] [Google Scholar]
- Ito H, Vereecke J, Carmeliet E. Intracellular protons inhibit inward rectifier K+ channel of guinea-pig ventricular cell membrane. Pflügers Archiv. 1992;422:280–286. doi: 10.1007/BF00376214. [DOI] [PubMed] [Google Scholar]
- Katz AM. Physiology of the Heart, chap. 19. New York: Raven Press; 1992. The cardiac action potential; pp. 438–472. [Google Scholar]
- Keung EC. Calcium current is increased in isolated adult myocytes from hypertrophied rat myocardium. Circulation Research. 1989;64:753–763. doi: 10.1161/01.res.64.4.753. [DOI] [PubMed] [Google Scholar]
- Korn SJ, Marty A, Connor JA, Horn R. Perforated patch recording. Methods in Neurosciences. 1991;4:364–373. [Google Scholar]
- Kusuoka H, Marban E, Cingolani HE. Control of steady-state intracellular pH in intact perfused ferret hearts. Journal of Molecular and Cellular Cardiology. 1994;25:821–829. doi: 10.1006/jmcc.1994.1099. [DOI] [PubMed] [Google Scholar]
- Lagadic-Gossmann D, Buckler KJ, Vaughan-Jones RD. Role of bicarbonate in pH recovery from intracellular acidosis in the guinea-pig ventricular myocyte. The Journal of Physiology. 1992;458:361–384. doi: 10.1113/jphysiol.1992.sp019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagadic-Gossmann D, Vaughan-Jones RD. Coupling of dual acid extrusion in the guinea-pig isolated ventricular myocyte to α1- and β-adrenoceptors. The Journal of Physiology. 1993;464:49–73. doi: 10.1113/jphysiol.1993.sp019624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazdunski M, Frelin C, Vigne P. The sodium/ hydrogen exchange system in cardiac cells: its biochemical and pharmacological properties and its role in regulating internal concentrations of sodium and internal pH. Journal of Molecular and Cellular Cardiology. 1985;17:1029–1042. doi: 10.1016/s0022-2828(85)80119-x. [DOI] [PubMed] [Google Scholar]
- Liu SL, Piwnica-Worms D, Lieberman M. Intracellular pH regulation in cultured embryonic chick heart cells. Journal of General Physiology. 1990;96:1247–1268. doi: 10.1085/jgp.96.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi T, Gu H, Seguchi M, Cragoe E, Momma K. HCO3− dependent intracellular pH regulation in the premature myocardium. Circulation Research. 1992;71:1314–1323. doi: 10.1161/01.res.71.6.1314. [DOI] [PubMed] [Google Scholar]
- Newman EA. Sodium-bicarbonate cotransport in retinal glia of the salamander. Journal of Neuroscience. 1991;11:3972–3983. doi: 10.1523/JNEUROSCI.11-12-03972.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez NG, Mattiazzi AR, Camilión de Hurtado MC, Cingolani HE. Myocardial contractility recovery during hypercapnic acidosis: its dissociation from recovery in pHi by ryanodine. Canadian Journal of Cardiology. 1995;11:553–560. [PubMed] [Google Scholar]
- Powell TD, Terrar A, Twist VW. Electrical properties of individual cells isolated from adult rat ventricular myocardium. The Journal of Physiology. 1980;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MF, Hediger MH, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/HCO3− cotransporter. Nature. 1997;387:409–413. doi: 10.1038/387409a0. [DOI] [PubMed] [Google Scholar]
- Spitzer KW, Bridge JHB. Relationship between intracellular pH and tension development in resting ventricular muscle and myocytes. American Journal of Physiology. 1992;262:C316–327. doi: 10.1152/ajpcell.1992.262.2.C316. [DOI] [PubMed] [Google Scholar]
- Spitzer KW, Hogan PM. The effects of acidosis and bicarbonate on action potential repolarization in canine cardiac Purkinje fibers. Journal of General Physiology. 1979;73:199–218. doi: 10.1085/jgp.73.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl F, Lepple-Wienhues A, Kuppinger M, Tamm E, Wiederholt M. Electrogenic sodium-bicarbonate cotransport in human ciliary cells. American Journal of Physiology. 1992;262:C427–435. doi: 10.1152/ajpcell.1992.262.2.C427. [DOI] [PubMed] [Google Scholar]
- Sun B, Hun Leem C, Vaughan-Jones RD. Novel chloride-dependent acid loader in the guinea-pig ventricular myocyte: part of a dual acid-loading mechanism. The Journal of Physiology. 1996;495:65–82. doi: 10.1113/jphysiol.1996.sp021574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Thomas RC. Bicarbonate and pHi response. Nature. 1989;337:601. doi: 10.1038/337601a0. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones RD. Intracellular pH, its Measurements, Regulation and Implications in Cellular Function. New York: Alan R. Liss Inc.; 1982. Chloride-bicarbonate exchange in sheep cardiac Purkinje fibre; pp. 239–252. [Google Scholar]
- Wiederholt M, Jentsch TJ, Keller SK. Electrogenic sodium-bicarbonate symport in cultured corneal endothelial cells. Pflügers Archiv. 1985;405:S167–171. doi: 10.1007/BF00581801. [DOI] [PubMed] [Google Scholar]


