Abstract
The atypical NR2B subunit-selective NMDA receptor antagonist ifenprodil was originally believed to act as a competitive antagonist at the polyamine binding site of the NMDA receptor. However, a number of studies have suggested that ifenprodil might bind to a distinct site.
Using whole-cell voltage clamp recordings, we have studied the interaction of spermine with both ifenprodil and the related NR2B selective antagonist Ro 8–4304 at the NMDA receptor in rat cultured cortical neurones in the presence of saturating concentrations of glycine.
Ifenprodil and Ro 8-4304 inhibited steady-state currents evoked by 100 μm NMDA in the absence of spermine with IC50 values of 0.3 and 0.6 μm, respectively. In the presence of 1 and 3 mm spermine, IC50 values for ifenprodil were 1.4 and 1.8 μm and for Ro 8-4304 they were 3.0 and 7.5 μm, respectively.
In the presence of spermine, the on-time constant of receptor blockade by both antagonists was significantly slower than control and the off-time constant of recovery from receptor blockade following removal of Ro 8-4304 was significantly faster.
Fast application of spermine during an NMDA steady-state current in the continuous presence of a subsaturating concentration of either antagonist resulted in a biphasic increase in the current, consistent with a fast increase upon spermine binding and a slow increase resultant from dissociation of antagonist due to spermine binding-induced allosteric reduction in receptor antagonist affinity. In agreement with this, at higher, saturating concentrations of antagonist, the slow increase in current amplitude was markedly reduced or absent.
These observations are consistent with a non-competitive, allosteric interaction between spermine and the antagonists, such that spermine binding to the NMDA receptor results in a reduction in receptor affinity for the antagonists and vice versa.
The effects of Mg2+ on the NMDA-evoked currents and its interaction with ifenprodil were similar to those of spermine, supporting the suggestion that Mg2+ might be the physiological ligand acting at the spermine site mediating glycine-independent stimulation.
Ifenprodil is the prototypic NR2B subunit-selective NMDA receptor antagonist which exhibits a markedly higher affinity for both recombinant receptors containing NR2B compared with those containing NR2A (Williams, 1993), NR2C or NR2D (Williams, 1995a) and a subset of native NMDA receptors (Williams et al. 1993; Priestley et al. 1994; Kew et al. 1998a). Several NR2B subunit-selective antagonists have now been identified, including Ro 25-6981 (Fischer et al. 1997), CP 101–606 (Chenard et al. 1995) and Ro 8-4304 (Kew et al. 1998b). Members of this class of compounds are neuroprotective both in vitro (Graham et al. 1992; Fischer et al. 1997; Menniti et al. 1997) and in in vivo models of ischaemia (Gotti et al. 1988; Fischer et al. 1996; Di et al. 1997) but notably appear to lack many of the side effects associated with non-selective NMDA receptor antagonists in vivo (Jackson & Sanger, 1988; Perrault et al. 1989; Chenard et al. 1995; Fischer et al. 1996). Ifenprodil, Ro 25-6981 and Ro 8-4304 act via a novel state-dependent mechanism of action (Kew et al. 1996; Fischer et al. 1997; Kew et al. 1998b), which together with their subunit selectivity seems likely to underlie the desirable neuropharmacological profile of this class of compounds.
Ifenprodil was originally believed to act as a competitive antagonist at a polyamine binding site of the NMDA receptor (Carter et al. 1990), but various studies have suggested that ifenprodil binds to a distinct site (Reynolds & Miller, 1989; Gallagher et al. 1996). Polyamines, such as spermine, exert multiple effects on the NMDA receptor including a ‘glycine-dependent’ stimulation, mediated by an increase in receptor affinity for glycine, a ‘glycine-independent’ stimulation, which is observed in the presence of saturating concentrations of glycine, a decrease in affinity for glutamate site agonists and a voltage-dependent inhibition which becomes more pronounced at hyperpolarized membrane potentials (Lerma, 1992; Benveniste & Mayer, 1993; reviewed by Williams, 1995b). Accordingly, it has been proposed that NMDA receptors might contain at least three distinct spermine binding sites (Williams et al. 1994; Williams, 1995b). Notably, the effects of spermine are dependent on both the NMDAR1 splice variant and the NR2 subunit composition of the NMDA receptor (reviewed by Williams, 1995b). All four effects occur at recombinant heteromeric receptors containing the NR2B subunit, whilst only ‘glycine-dependent’ stimulation and voltage-dependent block are seen at receptors containing NR2A (Williams et al. 1994). Spermine exerts no effect on receptors containing either NR2C or NR2D (Williams, 1995a). Furthermore, the ‘glycine-independent’ potentiation occurs only at receptors containing the NMDAR1 splice variant lacking the 5′ 21-amino acid insert (Durand et al. 1993). Gallagher et al. (1996) have used site-directed mutagenesis to identify a residue on NR2B which is absolutely required for the high affinity ifenprodil, but not polyamine, interaction with the NMDA receptor. However, site-directed mutagenesis of amino acids in the NMDAR1 subunit that abolish the ‘glycine-independent’ spermine potentiation also result in a reduction of sensitivity to ifenprodil (Williams et al. 1995; Kashiwagi et al. 1996). Thus, it has been proposed that whilst ifenprodil and polyamines might bind to distinct sites there is likely to be at least an allosteric linkage between these sites (Kashiwagi et al. 1996). Interestingly, it has recently been suggested that Mg2+ might be the physiological agonist at the NR2B subunit-specific spermine site (‘glycine-independent’ potentiation) (Paoletti et al. 1995).
In this study, we have used whole-cell voltage clamp recordings from rat cultured cortical neurones to examine the interaction between spermine and both ifenprodil and Ro 8-4304. We present evidence for an allosteric, non-competitive interaction. Additionally, we have examined the interaction of Mg2+ and ifenprodil and have found that Mg2+ appears to act in a manner analogous to that of spermine.
METHODS
Cortical neuronal cultures
Cortical neurones were prepared from embryos removed from 17- to 18-day-old pregnant rats (Roro spf 120; BRL, Fullinsdorf, Switzerland) which were killed by CO2 inhalation, as approved by the local institutional animal welfare committee. CO2 was administered at 100 % and the embryos were killed by decapitation. Neurones were grown on astrocyte feeder layers as previously described for hippocampal neurones (Möckel & Fischer, 1994).
Whole-cell voltage clamp recordings
Cortical neurones were used for electrophysiological experiments after 7–14 days in vitro. Whole-cell voltage clamp recordings were performed as described previously (Kew et al. 1996). Cultures were continuously perfused with a simplified salt solution (mm: NaCl, 149; KCl, 3.25; CaCl2, 2; MgCl2, 2; Hepes, 10; and D-glucose, 11; pH adjusted to 7.35 with NaOH and osmolarity adjusted to 350 mosmol l−1 using sucrose). Patch pipettes were pulled from thin-walled borosilicate glass (GC150TF; Clark Electromedical Instruments, Reading, UK) using a DMZ universal electrode puller (Zeitz-Instrumente, Augsburg, Germany). Pipettes had resistances of approximately 2–4 MΩ when filled with patch-pipette solution (mm: CsF, 120; CsCl, 10; EGTA, 11; CaCl2, 0.5; and Hepes, 10; pH adjusted to 7.25 with CsOH and osmolarity adjusted to 330 mosmol l−1 with sucrose). Whole-cell current recordings were made from cultured neurones using an Axopatch 200A amplifier (Axon Instruments). Pipette seal resistances were typically > 10 GΩ and pipette capacitance transients were minimized both prior to and following membrane breakthrough. No series resistance compensation was applied.
Drugs were diluted from concentrated stock solutions into a modified version of the salt solution used to perfuse the culture that lacked MgCl2 and included 30 μm glycine. Drugs were applied to cells by fast perfusion from double- or triple-barrelled capillary assemblies composed of large-tipped (approximately 350 μm) capillaries with an internal diameter of 320 μm. The control barrel was perfused with salt solution that also lacked MgCl2 and included 30 μm glycine. Ifenprodil was obtained from Synthelabo Recherche (Bagneux, France). Ro 8-4304 was synthesized at F. Hoffmann-La Roche, Basel, Switzerland. Solution equilibration times were determined by stepping from a solution of kainate (100 μm) in 10 mm NaCl to one containing 149 mm NaCl. The mean time constant of the exponential increase in membrane current after such a step was 29.2 ± 1.5 ms (n = 20 measurements from 4 cells, ±s.e.m.) (Kew et al. 1996).
Exponential curve fitting and measurement of drug on- and off-rates
Neuronal currents were filtered (cut-off frequency, 5 kHz), digitized (sampling frequency, 48 kHz) and recorded using a Digital Audio Tape (DAT) recorder (DTR-1204, Biologic, Claix, France) and were subsequently captured on-line to the hard disk of a Gateway 2000 P4D-66 computer using pCLAMP 6 software (Axon Instruments) (sampling frequency, 0.5–2 kHz).
Apparent antagonist dissociation constants (KD) were calculated from measured on- (τon) and off-rate (τoff) time constants by first deriving the estimated forward (k+) and reverse (k-) rate binding constants according to the scheme:
where R is the receptor, A is the antagonist and RA is the antagonist-bound receptor. k- is the measured 1/τoff and k+ was derived from the following function:
and
Potentiation concentration-response curves
Best fit lines were computed for potentiation concentration- response data using the Hill equation:
where Imax is the maximum response and n is the slope factor.
Inhibition curves
Inhibition curves were fitted according to either the Hill equation with baseline:
or the Hill equation without baseline:
where I∞ is the maximum inhibition level and n is the slope factor.
RESULTS
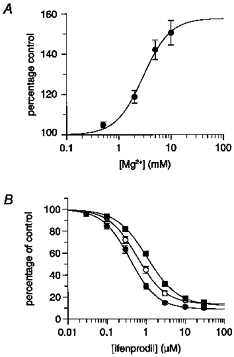
To examine the interaction between spermine and both ifenprodil and Ro 8-4304, inhibition curves were performed with these antagonists in the absence and presence of 1 and 3 mm spermine. All experiments were carried out in the presence of a saturating (30 μm) glycine concentration at a holding potential of −30 mV. Under these conditions any potentiation of the NMDA-evoked current by spermine would be expected to be the glycine-independent form. Ifenprodil inhibited steady-state currents evoked by 100 μm NMDA in the absence of spermine with an IC50 of 0.3 μm (Hill slope = 1.1) (Fig. 1A). In the presence of 1 and 3 mm spermine the IC50 values for ifenprodil were 1.4 μm (Hill slope = 1.3) and 1.8 μm (Hill slope = 0.9), respectively. Thus, although the IC50 of ifenprodil was increased in the presence of 1 mm spermine, the lack of a further significant shift of the inhibition curve to the right with 3 mm spermine was incompatible with a competitive interaction. In the absence of spermine, Ro 8-4304 inhibition curves yielded an IC50 of 0.6 μm (Hill slope = 1), whereas in the presence of 1 and 3 mm spermine the values were 3.0 μm (Hill slope = 1) and 7.5 μm (Hill slope = 1), respectively (Fig. 1B). The increase in shift of the Ro 8-4304 inhibition curve in the presence of increasing concentrations of spermine resembled a competitive interaction as predicted by Schild analysis. Notably, in the case of both ifenprodil and Ro 8-4304, the maximum percentage of the NMDA-evoked steady-state current inhibited was always greater in the presence of spermine than in control, spermine-free, conditions.
Figure 1. Ifenprodil and Ro 8-4304 inhibition curves in the absence and presence of spermine.
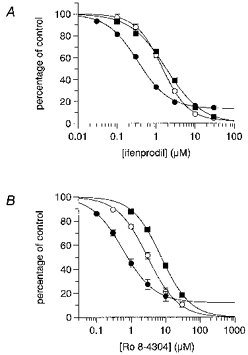
Inhibition curves for the antagonism by either ifenprodil (A) or Ro 8-4304 (B) of steady-state responses to 100 μm NMDA in the absence (•) or presence of 1 mm (○) or 3 mm (▪) spermine. The antagonism of NMDA responses by increasing concentrations of antagonist is expressed as a function of control response (i.e. pre-antagonist response amplitude = 100 %). The figure shows fitted curves from the mean data obtained from 4–5 neurones in each experiment using the Hill equation, from which IC50 values and Hill slopes were derived: A, control: 0.34 μm, slope = 1.1; +1 mm spermine: 1.4 μm, slope = 1.3; +3 mm spermine: 1.8 μm, slope = 0.9; and B, control: 0.59 μm, slope = 1.0; +1 mm spermine: 3.0 μm, slope = 1.0; +3 mm spermine: 7.5 μm, slope = 1.0. The maximum inhibition produced by both antagonists was greater in the presence of spermine than control. Where standard errors are not visible they are smaller than the symbol size.
Spermine potentiation concentration-response curves in the absence and presence of ifenprodil and Ro 8-4304 were also performed. In the absence of ifenprodil, spermine potentiated the 100 μm NMDA-evoked steady-state current to a maximum of approximately 170 %, with an EC50 of 160 μm (Hill slope = 1.3) (Fig. 2A). In the continuous presence of 1 μm ifenprodil, a maximum potentiation of approximately 350 % was achieved with an EC50 of 410 μm (Hill slope = 1.8). In the presence of 10 μm ifenprodil the maximum potentiation fell to approximately 210 % with an EC50 of 2540 μm (Hill slope = 2.7). There was considerable cell-to-cell variability in the level of potentiation achieved in the presence of spermine in all experiments. A second series of control spermine potentiation concentration- response curves, performed for the experiments with Ro 8-4304, yielded a similar maximum potentiation of 190 % with an EC50 of 141 μm (Hill slope = 1.4) (Fig. 2B). In the presence of 1 and 10 μm Ro 8-4304, spermine elicited a maximum potentiation of approximately 350 and 570 % with EC50 values of 370 μm (Hill slope = 1.4) and 1740 μm (Hill slope = 1.6), respectively.
Figure 2. Spermine concentration-response curves in the absence and presence of ifenprodil or Ro 8-4304.
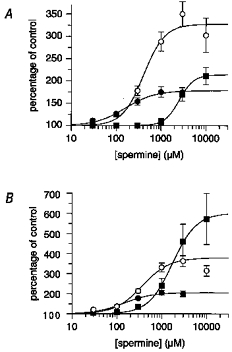
Mean steady-state currents are expressed as a function of control responses (i.e. pre-spermine response amplitude = 100 %) in the absence of antagonist (•) or in the continual presence of 1 μm (○) or 10 μm (▪) ifenprodil (A) or in the continual presence of 1 μm (○) or 10 μm (▪) Ro 8-4304 (B). The figure shows fitted curves using the Hill equation which yielded EC50 values and Hill slopes of: A, control: 160 μm, slope = 1.3; +1 μm ifenprodil: 410 μm, slope = 1.8; +10 μm ifenprodil: 2540 μm, slope = 2.7; and B, control: 141 μm, slope = 1.4; +1 μm Ro 8-4304: 370 μm, slope = 1.4; +10 μm Ro 8-4304: 1740 μm, slope = 1.6.
To examine further the nature of the spermine-antagonist interaction we examined the kinetics of antagonist block and unblock of NMDA-evoked steady-state currents in the absence and presence of spermine. Stable NMDA-evoked (100 μm) steady-state currents were obtained and a rapid jump was made into a solution containing 100 μm NMDA and either 10 μm ifenprodil or 10 μm Ro 8-4304. After a stable steady-state current was again achieved, a rapid jump was made back into an antagonist-free solution. These experiments were performed both in the absence and in the continual presence of 1 mm spermine for ifenprodil and 10 mm spermine for Ro 8-4304. In the case of ifenprodil, only the antagonist on-time constants were measured as ifenprodil exhibits a relatively slow off-rate and a long period of NMDA application is required to measure the off-time constant, which results in a degree of irreversible receptor run-down (Kew et al. 1998b). The mean on-time constant of receptor blockade by 10 μm ifenprodil in the absence of spermine was 1144 ± 50 ms (mean ±s.e.m., n = 18 from 6 cells) with a maximum inhibition of 80 ± 2 % (Fig. 3A). In the presence of 1 mm spermine, the on-time constant for ifenprodil was 3312 ± 121 ms with a maximum inhibition of 87 ± 1 %. Thus, in the presence of 1 mm spermine the on-time constant for ifenprodil was significantly slowed (P < 0.0001, Student's paired t test). The percentage of the current inhibited was also significantly greater in the presence of 1 mm spermine (P < 0.0001, Student's paired t test). The mean on- and off-time constants of receptor blockade by 10 μm Ro 8-4304 in the absence of spermine were 205 ± 16 and 4361 ± 213 ms (Fig. 3B), respectively (n = 12 from 3 cells), which yielded a calculated KD of 0.5 μm, in excellent agreement with our previous data (Kew et al. 1998b). The maximum inhibition of the steady-state current was 89 ± 1 %. In the presence of 10 mm spermine, the on- and off-time constants for Ro 8-4304 were 1512 ± 45 and 2380 ± 121 ms, respectively, and the maximum inhibition of the steady-state current was 50 ± 1 %. Thus, the presence of spermine significantly slowed the on-time constant of receptor blockade by Ro 8-4304 (P < 0.0001, Student's t test), significantly reduced the off-time constant (P < 0.0001, Student's t test) and significantly reduced the maximum inhibition of the steady-state current (P < 0.0001, Student's t test).
Figure 3. The effects of spermine on the kinetics of antagonist interaction with the NMDA receptor.
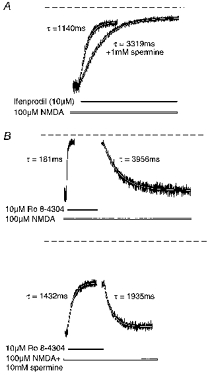
A, comparison of the kinetics of block of steady-state inward currents evoked by 100 μm NMDA, following fast application of 10 μm ifenprodil in the absence and presence of spermine. The responses have been scaled to the same amplitude to facilitate direct visual comparison (actual steady-state currents = 290 and 395 pA in the absence and presence of spermine, respectively). Single exponential curves were fitted to the digitized data (continuous lines) and yielded on-time constants for the ifenprodil block of 1140 and 3319 ms in the absence and presence of 1 mm spermine, respectively. Ifenprodil inhibited 83 and 87 % of the currents in the absence and presence of spermine, respectively. The dashed line indicates the baseline current. B, comparison of the kinetics of block and unblock of steady-state inward currents evoked by 100 μm NMDA, following fast application of 10 μm Ro 8-4304 in the absence and presence of spermine. Single exponential curves were fitted to the digitized data (continuous lines, not shown for the inhibition of the current by 10 μm Ro 8-4304 in the absence of spermine) and yielded on-time constants for the Ro 8-4304 block of 181 and 1432 ms and off-time constants for the unblock of 3956 and 1935 ms in the absence and presence of 10 mm spermine, respectively. Ro 8-4304 inhibited 90 and 49 % of the currents in the absence and presence of spermine, respectively (control steady-state currents = 365 and 730 pA in the absence and presence of spermine, respectively). The dashed lines indicate the baseline current.
We also investigated the kinetics of the potentiation of 100 μm NMDA-evoked steady-state currents by spermine in the presence of ifenprodil or Ro 8-4304. NMDA-evoked steady-state currents were obtained in the presence of the appropriate antagonist and a rapid jump was made into a solution containing spermine until a steady-state current was again achieved, at which point a rapid jump was made back into a spermine-free solution. The kinetics of the glycine-independent potentiation by spermine have been shown previously to be fast (Lerma, 1992; Benveniste et al. 1993). We measured the on- and off-time constants of the potentiation of a 100 μm NMDA-evoked steady-state current by 1 mm spermine in the absence of antagonist as 29 ± 1 and 64 ± 4 ms, respectively (n = 12 from 3 cells) with a mean potentiation of 223 ± 6 % (all potentiations are expressed as a percentage of the pre-spermine control current, i.e. pre-spermine control current = 100 %) (Fig. 4). However, the speed of solution exchange using our perfusion system (τ=∼ 30 ms) renders the accuracy of these fast measured time constants somewhat uncertain. In the presence of 1 μm ifenprodil, a jump into 10 mm spermine during a 100 μm NMDA-evoked steady-state current resulted in a two-phase potentiation (Fig. 5A and Table 1), the first being a rapid potentiation with a measured time constant of 12 ± 1 ms (n = 7 cells) and a mean maximum potentiation of 269 ± 17 %. This fast potentiation consistently rose to a peak and then decayed to a plateau level (240 ± 18 %) before the second phase of the potentiation began. The second phase was a much slower potentiation with a measured time constant of 9164 ± 642 ms, resulting in a mean final potentiation of 428 ± 34 %. The current decay following removal of spermine also consisted of two components, a fast decay with a mean time constant of 42 ± 6 ms and a slow decay with a time constant of 5191 ± 86 ms (Fig. 5A and Table 1). On the same cells, in the presence of 10 μm ifenprodil, 10 mm spermine also elicited a two-phase potentiation, the first a rapid potentiation with a measured time constant of 17 ± 2 ms and a mean peak potentiation of 247 ± 17 % which decayed to a plateau of 215 ± 14 % and the second a slow potentiation which reached a maximum of 276 ± 16 % (Fig. 5B and Table 1). An accurate assessment of the time constant of the slow potentiation was not possible in many cases due to its small amplitude in relation to the current noise. The current decay following removal of spermine again consisted of a fast component (time constant = 67 ± 12 ms) and a minor slow component (Fig. 5B and Table 1). Thus, as with the spermine potentiation concentration-response curves, the maximum potentiation was significantly greater in the presence of 1 μm than 10 μm ifenprodil. This difference appears to be the result of the markedly larger slow potentiation in the presence of 1 μm relative to 10 μm ifenprodil, whilst the extent of the fast potentiation was similar in the presence of both ifenprodil concentrations.
Figure 4. Kinetics of the glycine-independent potentiation of the NMDA current by spermine.
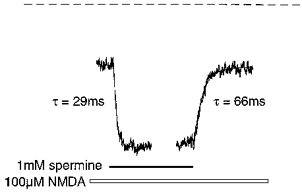
Kinetics of potentiation and recovery of a steady-state inward current evoked by 100 μm NMDA, following fast application and removal of 1 mm spermine. Single exponential curves were fitted to the digitized data (continuous lines) and yielded on- and off-time constants of 29 and 66 ms, respectively. Application of spermine resulted in a potentiation of the steady-state current to 234 % of control level (pre-spermine response amplitude = 265 pA). The dashed line indicates the baseline current.
Figure 5. Effects of fast application and removal of spermine during a 100 μm NMDA-evoked steady-state current in the continuous presence of 1 or 10 μm ifenprodil.
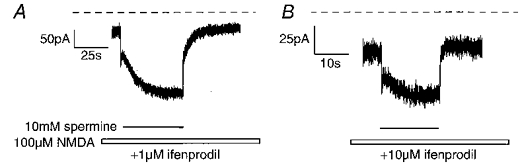
Comparison of the effects of fast application and removal of 10 mm spermine during a 100 μm NMDA steady-state current evoked in the continuous presence of 1 (A) or 10 μm (B) ifenprodil. Application of spermine resulted in a fast potentiation of the current which decayed to a plateau before a second slow potentiation began. Note the relative amplitude of the slow potentiation is greater in the presence of 1 μm (A) than 10 μm (B) ifenprodil. Upon removal of spermine, the current decay exhibited an initial fast phase followed by a slower decay to steady state. The dashed lines indicate the baseline current.
Table 1.
Comparison of the effects of 10 mm spermine application during a 100 μm NMDA-evoked steady-state current in the presence of 1 or 10 μm ifenprodil
| Fast potentiation | Slow potentiation | ||||||
|---|---|---|---|---|---|---|---|
| τ (ms) | Peak (% control) | Plateau (% control) | τ (ms) | Maximum (% control) | Fast decay τ (ms) | Slow decay τ(ms) | |
| 1 μm ifenprodil | 12 ± 1 | 269 ± 17*1 | 240 ± 18*2 | 9164 ± 642 | 428 ± 34† | 42 ± 6 | 5191 ± 86 |
| 10 μm ifenprodil | 17 ± 2 | 247 ± 17*1 | 215 ± 14*2 | n.a. | 276 ± 16† | 67 ±12 | n.a. |
*Not significantly different (*1 P > 0.34 *2 P > 0.17, Student's paired t test † Significantly different (P < 0.01, Student's paired t test). n.a., not available.
Due to the relatively fast off-rate kinetics of Ro 8-4304 in comparison to ifenprodil (Kew et al. 1998b), it was possible to make four applications of spermine at each Ro 8-4304 concentration for each cell and to analyse the averaged responses. In the presence of 10 μm Ro 8-4304, rapid application of 10 mm spermine again resulted in a two-phase potentiation. The mean total potentiation was 800 ± 77 %. There was no obvious separation between the two components or any intermediate plateau phase as seen with ifenprodil but the potentiation was well fitted by a double exponential with time constants and relative amplitudes of 100 ± 15 ms (30 ± 3 %) and 984 ± 92 ms (70 ± 3 %) for the fast and slow components, respectively (n = 4) (Fig. 6A and Table 2). The current decay following removal of spermine was also well fitted by a double exponential with time constants and relative amplitudes of 84 ± 6 ms (77 ± 8 %) and 464 ± 92 ms (23 ± 9 %) (Fig. 6A and Table 2). Jumping out of spermine resulted in a consistent rapid increase in the current amplitude prior to the current relaxation (Fig. 6A). In the presence of 100 μm Ro 8-4304, the potentiation following rapid application of spermine was well fitted with a single exponential yielding a time constant of 79 ± 5 ms and a maximum potentiation of 359 ± 24 % (Fig. 6B and Table 2). The current decay following removal of spermine was also well fitted with a single exponential with a time constant of 43 ± 4 ms (Fig. 6B and Table 2).
Figure 6. Effects of fast application and removal of spermine during a 100 μm NMDA-evoked steady-state current in the continuous presence of 10 or 100 μm Ro 8-4304.
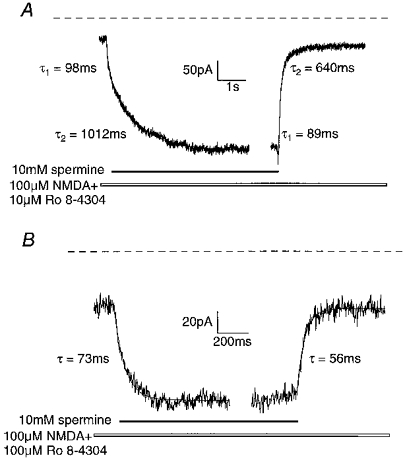
A, application of 10 mm spermine in the presence of 10 μm Ro 8-4304 resulted in a two-phase potentiation which was well fitted by a double exponential curve (continuous line) with time constants and relative amplitudes of 98 ms (29 %) and 1012 ms (71 %) of the fast and slow components, respectively. Upon removal of spermine, the current decay was again well fitted by a double exponential curve with time constants and relative amplitudes of 89 ms (56 %) and 640 ms (44 %) of the fast and slow components, respectively. Note that jumping out of spermine consistently resulted in a rapid potentiation of the current prior to the decay. The dashed line indicates the baseline current. B, application of 10 mm spermine in the presence of 100 μm Ro 8-4304 resulted in a single-phase potentiation that was well fitted by a single exponential curve (continuous line) with a time constant of 73 ms. The current decay upon removal of spermine was also well fitted by a single exponential curve with a time constant of 56 ms. The dashed line indicates the baseline current. Note the relatively smaller maximum potentiation elicited by spermine in the presence of 100 μm compared with 10 μm Ro 8-4304.
Table 2.
Comparison of the effects of 10 mm spermine application during a 100 μm NMDA-evoked steady-state current in the presence of 10 or 100 μm Ro8-4304
| Potentiation | Relaxation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| τ1 (ms) | Amplitude (%) | τ2 (ms) | Amplitude (%) | Maximum (% control) | τ1 (ms) | Amplitude (%) | τ2 (ms) | Amplitude (%) | |
| 10 μm Ro 8-4304 | 100 ± 15 | 30 ± 3 | 984 ± 92 | 70 ± 3 | 800 ± 77 | 84 ± 6 | 77 ± 8 | 464 ± 92 | 23 ± 9 |
| 100 μm Ro8-4304 | 79 ± 5 | — | — | — | 359 ± 24 | 43 ± 4 | — | — | — |
We also examined the interaction between Mg2+ and ifenprodil at the NMDA receptor. All experiments with Mg2+ were carried out at a holding potential of +30 mV to avoid the voltage-dependent Mg2+ block. From Mg2+ concentration-effect curves, Mg2+ potentiated 100 μm NMDA-evoked steady-state currents to a fitted maximum of approximately 160 % of control with an EC50 of 2.9 mm (Hill slope = 1.7). (Fig. 7A). As with spermine, the potentiation of a 100 μm NMDA-evoked steady-state current by 10 mm Mg2+ was rapid with mean measured on- and off-time constants of 17 ± 1 and 34 ± 1 ms, respectively (n = 21, from 3 cells), and a mean potentiation of 221 ± 2 % (Fig. 8A). As with spermine, the extent of the potentiation varied considerably (see Fig. 7Avs.8A). We performed inhibition curves with ifenprodil in the presence of 2 and 10 mm Mg2+. In the absence of Mg2+, ifenprodil inhibited steady-state currents evoked by 100 μm NMDA with an IC50 of 0.39 μm (Hill slope = 1.2) (Fig. 7B). In the presence of 2 and 10 mm Mg2+ the IC50 values for ifenprodil were 0.59 (Hill slope = 1.2) and 1.1 μm (Hill slope = 1.1), respectively. We also compared the kinetics of inhibition of 100 μm NMDA-evoked stable steady-state currents by 10 μm ifenprodil in the absence and presence of 10 mm Mg2+. The mean on-time constant of receptor blockade by 10 μm ifenprodil in the absence of Mg2+ was 1052 ± 94 ms with a maximum inhibition of 86 ± 2 % (n = 7). In the presence of 10 mm Mg2+ the on-time constant for ifenprodil was 2106 ± 117 ms with a maximum inhibition of 88 ± 1 % (Fig. 8B). Thus, in the presence of Mg2+ the on-time constant of receptor blockade by ifenprodil was significantly slowed (P < 0.0001, Student's paired t test).
Figure 7. Potentiation of NMDA currents by Mg2+: interaction with ifenprodil.
A, Mg2+ potentiation concentration-response relationship, showing potentiation of steady-state outward currents elicited by 100 μm NMDA application. Mean steady-state currents (n = 6) are expressed as a percentage of control responses (i.e. pre-Mg2+ response amplitude = 100 %). The figure shows a fitted curve using the Hill equation which yielded an EC50 and Hill slope of 2.9 mm and 1.7, respectively. B, inhibition curves for the antagonism by ifenprodil of steady-state responses to 100 μm NMDA in the absence (•) or presence of 2 mm (○) or 10 mm (▪) Mg2+. The antagonism of NMDA responses by increasing concentrations of antagonist is expressed as a function of control response (i.e. pre-antagonist response amplitude = 100 %). The figure shows fitted curves from the mean data obtained from 4 neurones in each experiment using the Hill equation, from which IC50 values and Hill slopes were derived: control: 0.39 μm, slope = 1.2; +2 mm Mg2+: 0.59 μm, slope = 1.2; +10 mm Mg2+: 1.1 μm, slope = 1.1. Where standard errors are not visible they are smaller than the symbol size.
Figure 8. The effects of Mg2+ on the kinetics of ifenprodil interaction with the NMDA receptor.
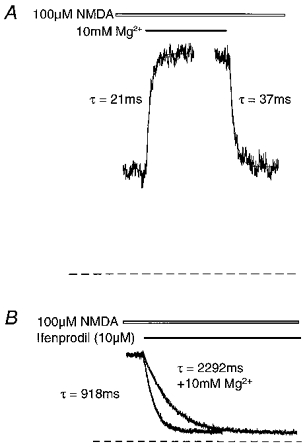
A, kinetics of potentiation and recovery of a steady-state outward current evoked by 100 μm NMDA, following fast application and removal of 10 mm Mg2+. Single exponential curves were fitted to the digitized data (continuous lines) and yielded on- and off-time constants of 21 and 37 ms, respectively. Application of Mg2+ resulted in a potentiation of the steady-state current to 216 % of control level (pre-Mg2+ response amplitude = 255 pA). The dashed line indicates the baseline current. B, comparison of the kinetics of inhibition of steady-state outward currents evoked by 100 μm NMDA, following fast application of 10 μm ifenprodil in the absence or presence of Mg2+. The responses have been scaled to the same amplitude to facilitate direct visual comparison (actual steady-state currents = 425 pA and 620 pA in the absence and presence of Mg2+, respectively). Single exponential curves were fitted to the digitized data (continuous lines) and yielded on-time constants for the ifenprodil block of 918 and 2292 ms in the absence and presence of 10 mm Mg2+, respectively. Ifenprodil inhibited 89 and 90 % of the currents in the absence and presence of Mg2+, respectively. The dashed line indicates the baseline current.
We also examined the potentiation of NMDA-induced steady-state currents by 10 mm Mg2+ in the presence of ifenprodil. Steady-state currents were evoked by 100 μm NMDA application in the continuous presence of either 1 or 10 μm ifenprodil and a rapid jump was made into an identical solution containing 10 mm Mg2+ until steady state was achieved, at which point a rapid jump was made back into a Mg2+-free solution. In the presence of both 1 and 10 μm ifenprodil, application of 10 mm Mg2+ resulted in a rapid potentiation of the current to 187 ± 12 and 168 ± 9 % of control, respectively (n = 7 and 11 from 5 cells, respectively) (Table 3). In the presence of 1 μm, but not 10 μm, ifenprodil this rapid potentiation was followed by a secondary, slow potentiation to 249 ± 21 % of control. Likewise, upon removal of Mg2+ in the presence of 1 μm ifenprodil a two-phase current relaxation with a fast component and a smaller slow component was evident, whilst in the presence of 10 μm ifenprodil jumping out of Mg2+ resulted in a monophasic, fast, current relaxation. Thus, as with spermine, a larger potentiation was evident in the presence of 1 μm relative to 10 μm ifenprodil, apparently resulting from the presence of a secondary, slow potentiation.
Table 3.
Comparison of the effects of 10 mm Mg2+ application during a 100 μm NMDA-evoked steady-state current in the presence of 1 or 10 μm ifenprodil
| Fast potentiation | Slow potentiation | |||||
|---|---|---|---|---|---|---|
| τ (ms) | Peak (% control) | τ (ms) | Maximum (% control) | Fast decay τ (ms) | Slow decay τ (ms) | |
| 1 μm ifenprodil | 12 ± 1 | 187 ± 12*1†1 | 7230 ± 1167 | 249 ± 21†1,2 | 23 ± 3 | 6104 ± 1157 |
| 10 μm ifenprodil | 18 ± 3 | 168 ± 9*1,*2 | — | 175±7†2*2 | 19 ± 2 | — |
*Not significantly different *1 P > 0.22 *2 P > 0.11, Student's t test † Significantly different †1,2 P < 0.002, Student's t test
DISCUSSION
In this study we have examined the interaction between spermine and the NR2B subunit-selective antagonists, ifenprodil and Ro 8-4304. Our observations, using a largely kinetic based approach, demonstrate the simultaneous binding to the NMDA receptor of both spermine and either ifenprodil or Ro 8-4034 and clearly illustrate an allosteric interaction between the two binding sites. In addition, we have shown that Mg2+ appears to act in a manner analogous to spermine, as previously suggested (Paoletti et al. 1995), and also exhibits a non-competitive interaction with ifenprodil.
The IC50 values of both ifenprodil and Ro 8-4304 for NMDA receptor blockade were increased in the presence of 1 mm spermine, which produced a relatively parallel shift to the right of their inhibition curves. However, increasing the spermine concentration to 3 mm resulted in relatively little increased shift in the ifenprodil inhibition curve, whilst the IC50 of Ro 8-4304 was increased further. In the presence of spermine the maximum percentage of the NMDA-evoked current inhibited by both ifenprodil and Ro 8-4304 was greater than in control. The increased percentage inhibition might result from ‘depotentiation’ of the receptor due to spermine unbinding following application of antagonist, as a result of either a competitive interaction or more probably from an antagonist binding-induced allosteric reduction in affinity for spermine, resulting in spermine dissociation from the receptor (see below). NMDA receptor affinity for spermine was also reduced in the presence of increasing concentrations of both ifenprodil and Ro 8-4304. The maximum percentage potentiation achieved with spermine increased with increasing concentrations of Ro 8-4304. However, although the maximum spermine-induced potentiation was elevated in the presence of 1 μm ifenprodil relative to control, in the presence of 10 μm ifenprodil it fell to close to control levels. The increase in spermine-induced potentiation in the presence of antagonist relative to controls is likely to represent the unblocking of the NMDA receptor due to antagonist unbinding that most probably results from a spermine binding-induced allosteric reduction in antagonist affinity, which causes its dissociation from the receptor. All experiments were carried out at a holding potential of −30 mV in an attempt to minimize the voltage-dependent inhibitory effects of spermine, but it should be noted that, particularly at high concentrations of spermine, a degree of such inhibition is likely to occur. These concentration-response experiments suggested that whilst the interaction between spermine and Ro 8-4304 at the NMDA receptor was consistent with a competitive interaction as predicted by Schild analysis, the interaction between ifenprodil and spermine was not. The lack of any significant increase in shift of the ifenprodil inhibition curve in the presence of 3 mm relative to 1 mm spermine, and particularly the reduction in the maximum spermine-induced potentiation in the presence of 10 μm relative to 1 μm ifenprodil, suggested that the spermine-ifenprodil interaction was allosteric rather than competitive.
In agreement with previous studies (Lerma, 1992; Benveniste et al. 1993), we have found that the kinetics of spermine binding to and unbinding from the NMDA receptor were rapid. In the presence of spermine the on-time constant of receptor blockade by both ifenprodil and Ro 8-4304 was significantly slower than in its absence. If the interaction between the antagonists and spermine was competitive then, since the rate of spermine unbinding from the NMDA receptor is very fast relative to the rate of antagonist binding, the on-time constant for block by the antagonists would be predicted to remain unaffected by the presence of spermine. Thus the slowing of the rate of antagonist binding in the presence of spermine is, rather, compatible with an allosteric interaction such that spermine binding to the NMDA receptor results in a reduction in receptor affinity for both antagonists, resulting in a slowing of the rate of binding. In agreement, the current recovery following removal of Ro 8-4304, which reflects the rate of Ro 8-4304 unbinding from the NMDA receptor, was significantly faster in the presence of 10 mm spermine than in control. This observation is incompatible with a competitive interaction and implies the simultaneous occupancy of the NMDA receptor by both Ro 8-4304 and spermine, with the presence of spermine resulting in a reduction in receptor affinity for Ro 8-4304 and a resultant faster rate of dissociation from the receptor. Interestingly, whilst the percentage of the steady-state current inhibited by the antagonist was actually significantly increased for ifenprodil in the presence of 1 mm spermine, it was significantly reduced for Ro 8-4304 in 10 mm spermine. If antagonist binding to the receptor resulted in an allosteric reduction in affinity for spermine, then in the presence of the lower concentration of spermine (1 mm), although ifenprodil affinity is reduced, ifenprodil binding to the receptor might result in a sufficient reduction in affinity for spermine to induce spermine dissociation from the receptor. The loss of spermine-induced potentiation in combination with the ifenprodil-mediated antagonism would result in a relatively larger inhibition of the current than in spermine-free conditions. In contrast, in the presence of 10 mm spermine the percentage of the current inhibited by Ro 8-4304 was significantly less than control. Thus, in this case, although Ro 8-4304 binding to the NMDA receptor might result in an allosteric reduction in receptor affinity for spermine, it is not sufficient to induce the dissociation of spermine at this higher concentration. The reduced size of the inhibition relative to control probably results from the spermine binding-induced reduction in affinity for Ro 8-4304. Importantly, the relative levels of inhibition by the antagonists in the presence and absence of spermine corresponded well with the data from the inhibition curves.
As with the spermine concentration-response analysis, fast application of spermine during an NMDA steady-state current in the continuous presence of 1 μm ifenprodil resulted in a significantly larger relative potentiation than in the presence of 10 μm ifenprodil. In both cases, the potentiation consisted of two distinct phases: a very rapid phase that resulted in potentiations of similar magnitude; and a slow phase that was of a substantially larger amplitude with 1 μm than 10 μm ifenprodil, which resulted in the significantly larger final potentiation in the presence of 1 μm ifenprodil. The measured time course of the slow potentiation in 1 μm ifenprodil is faster than the measured off-time constant of ifenprodil from the NMDA receptor in the absence of spermine (Kew et al. 1998b), suggesting that the slow potentiation is not the result of a simple competitive interaction between spermine and ifenprodil, where the rate of spermine binding would be dependent on the rate of ifenprodil unbinding from the receptor. The observed results are, again, more compatible with an allosteric interaction between the ifenprodil and spermine binding sites. Accordingly, spermine is able to bind to an ifenprodil-occupied receptor, which results in at least a portion of the initial fast potentiation produced by spermine and, upon binding, it induces an allosteric reduction in receptor affinity for ifenprodil which results in ifenprodil unbinding from the receptor at a rate faster than in the absence of spermine, as already demonstrated with Ro 8-4304 (Fig. 3B), thus resulting in the slow potentiation. Notably, 1 μm ifenprodil is not saturating at the NR2B-containing receptor population, therefore a small portion of the initial fast potentiation upon application of spermine might result from spermine binding to, and potentiation of, receptors not occupied by ifenprodil. However, 10 μm ifenprodil produces a maximal high-affinity inhibition and, accordingly, all NR2B-containing receptors would be expected to be ifenprodil bound. Thus, the rapid potentiation upon application of spermine in the presence of 10 μm ifenprodil is likely to result almost entirely from spermine binding to ifenprodil-occupied receptors. This suggests that an NMDA receptor that is ifenprodil-bound but which is still capable of opening at a reduced probability (Kew et al. 1996) can be potentiated upon spermine binding. In the presence of 10 μm ifenprodil, the spermine binding-induced allosteric reduction in receptor affinity for ifenprodil results in the dissociation of substantially less ifenprodil and, thus, a smaller-amplitude slow potentiation. The decay of the current following removal of spermine also consisted of distinct fast and slow components. The fast component probably reflects the unbinding of spermine from the receptor which, in turn, would result in a return to a higher receptor affinity for ifenprodil and, thus, the slow current decay is likely to represent the inhibition of the current as ifenprodil rebinds. Accordingly the measured time course of the slow decay in the presence of 1 μm ifenprodil (τ= 5.2 s) is in good agreement with the measured on-time constant of block of a 100 μm NMDA-evoked steady-state current in the absence of spermine by 1 μm ifenprodil (τ= 5.8 s; J. N. C. Kew and J. A. Kemp, unpublished observations).
Similar rapid jumps into spermine during a 100 μm NMDA-evoked steady-state current in the presence of either 10 or 100 μm Ro 8-4304 produced very similar results. Presumably due to the faster kinetics of binding to the NMDA receptor of Ro 8-4304 relative to ifenprodil (Kew et al. 1998b), there was no clear separation between the fast and the slow phases of the potentiation. In the presence of ifenprodil the initial decay to a plateau level following the fast potentiation is likely to represent receptor desensitization. In the presence of 10 μm Ro 8-4304, application of 10 mm spermine resulted in a biphasic potentiation that was well fitted by a double exponential with fast and slow components, whereas in the presence of 100 μm Ro 8-4304 the potentiation was well fitted by a single exponential with fast kinetics. As with ifenprodil, the time course of the slow phase of the potentiation in 10 μm Ro 8-4304 was markedly faster than the measured off-time constant of Ro 8-4304 from the NMDA receptor in the absence of spermine (Kew et al. 1998b) and is, thus, compatible with an allosteric rather than a competitive interaction between spermine and Ro 8-4304. The time course of the slow phase of the current decay (τ= 0.5 s) is also in good agreement with the measured time course of block of 100 μm NMDA-evoked steady-state currents in the absence of spermine (τ= 0.7 s; Kew et al. 1998b) and is, thus, compatible with the rebinding of ‘displaced’ Ro 8-4304 to the spermine-free receptors. Spermine concentration-response curves performed in the presence of Ro 8-4304 differed from those in ifenprodil in that the maximum potentiation produced by spermine continued to rise with increasing Ro 8-4304 concentration. However, the kinetic analysis of the potentiation by spermine revealed a significantly reduced maximum potentiation in the presence of 100 μm relative to 10 μm Ro 8-4304, presumably as a result of reduced receptor unblocking due to spermine binding-induced dissociation of Ro 8-4304. Thus, it appears that the concentration of Ro 8-4304 necessary to prevent unbinding from the NMDA receptor as a result of the spermine binding-induced allosteric reduction in affinity is higher than that of ifenprodil. Since the affinity of Ro 8-4304 and ifenprodil for the NMDA receptor are similar, it seems likely that the relative changes in affinity for antagonists upon spermine binding are different, with spermine inducing a more marked reduction in receptor affinity for Ro 8-4304 than ifenprodil, which is also compatible with the greater shift of the Ro 8-4304 inhibition curve by spermine.
Rapid application of Mg2+ during a 100 μm NMDA-evoked steady-state outward current produced a rapid potentiation to a level similar to that achieved with spermine. Thus, in agreement with the observations of Paoletti et al. (1995), Mg2+ appears to mimic the glycine-independent potentiating effects of spermine at the NMDA receptor. Concentration- response analysis of the potentiating effects of Mg2+ yielded an EC50 of 2.9 mm, similar to the value obtained by Paoletti et al. (1995) and close to the physiological concentration of extracellular Mg2+. The presence of Mg2+ resulted in a reduction in apparent receptor affinity for ifenprodil, although to a lesser degree than observed with spermine. As with spermine, the kinetics of Mg2+ binding to and unbinding from the NMDA receptor are very fast relative to the rate of ifenprodil binding to the receptor and, thus, the slowing of the on-time constant of receptor blockade by ifenprodil in the presence of Mg2+ is more compatible with an allosteric, rather than a competitive interaction between the Mg2+ and ifenprodil binding sites. Also as with spermine, the maximum potentiation following rapid application of Mg2+ was significantly larger in the presence of 1 μm than 10 μm ifenprodil as a result of the presence of the secondary, slow potentiation. The time course of the slow potentiation in the presence of 1 μm ifenprodil was again markedly faster than the measured off-time constant of ifenprodil from the NMDA receptor in the absence of spermine (Kew et al. 1998b) and the time course of the slow current decay following removal of spermine (τ= 6.1 s) was again compatible with the inhibition of the current by ‘displaced’ ifenprodil. Thus, Mg2+ also exhibits an allosteric, non-competitive interaction with ifenprodil.
In conclusion, using kinetic analysis of both the glycine-independent potentiation of NMDA-evoked currents by both spermine and Mg2+ and current inhibition by the NR2B subunit-selective antagonists ifenprodil and Ro 8-4304, we have demonstrated an allosteric, non-competitive interaction between the spermine/Mg2+ binding site(s) and the antagonist binding sites. We have also found that the effects of Mg2+ on the NMDA-evoked currents and its interaction with ifenprodil at the NMDA receptor are very similar to those of spermine, thus supporting the suggestion of Paoletti et al. (1995) that Mg2+ may be the physiological ligand acting at the spermine site mediating glycine-independent stimulation. These observations may have important consequences regarding the use of NR2B selective antagonists in vivo.
Acknowledgments
We would like to thank Dr Günther Fischer and Ms Véronique Graf for the provision of rat cortical neuronal cultures and Dr Gerhard Trube for helpful discussion.
References
- Benveniste M, Mayer ML. Multiple effects of spermine on N-methyl-D-aspartic acid receptor responses of rat cultured hippocampal neurones. The Journal of Physiology. 1993;464:131–163. doi: 10.1113/jphysiol.1993.sp019627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CJ, Lloyd KG, Zivkovic B, Scatton B. Ifenprodil and SL 82.0715 as cerebral antiischemic agents. III. Evidence for antagonistic effects at the polyamine modulatory site within the N-methyl-D-aspartate receptor complex. Journal of Pharmacology and Experimental Therapeutics. 1990;253:475–482. [PubMed] [Google Scholar]
- Chenard BL, Bordner J, Butler TW, Chambers LK, Collins MA, De Costa DL, Ducat MF, Dumont ML, Fox CB, Mena EE, Menniti FS, Nielson J, Pagnozzi MJ, Richter KEG, Ronau RT, Shalaby IA, Stemple JZ, White WF. (1S,2S)-1-(4-hydroxphenyl)-2-(4-hydroxy-4-phenylpiperidino)-1-propanol: a potent new neuroprotectant which blocks N-methyl-D-aspartate responses. Journal of Medicinal Chemistry. 1995;38:3138–3145. doi: 10.1021/jm00016a017. [DOI] [PubMed] [Google Scholar]
- Di X, Bullock R, Watson J, Fatouros P, Chenard B, White F, Corwin F. Effect of CP101,606, a novel NR2B subunit antagonist of the N-methyl-D-aspartate receptor, on the volume of ischemic brain damage and cytotoxic brain edema after middle cerebral artery occlusion in the feline brain. Stroke. 1997;28:2244–2251. doi: 10.1161/01.str.28.11.2244. [DOI] [PubMed] [Google Scholar]
- Durand GM, Bennett MV, Zukin RS. Splice variants of the N-methyl-D-aspartate receptor NR1 identify domains involved in regulation by polyamines and protein kinase C. Proceedings of the National Academy of Sciences of the USA. 1993;90:6731–6735. doi: 10.1073/pnas.90.14.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G, Bourson A, Kemp JA, Lorez HP. The neuroprotective activity of RO 25–6981, a NMDA receptor NR2B subtype selective blocker. Society for Neuroscience Abstracts. 1996;693:695. [Google Scholar]
- Fischer G, Mutel V, Trube G, Malherbe P, Kew JNC, Mohacsi E, Heitz M-P, Kemp JA. Ro 25-6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. Journal of Pharmacology and Experimental Therapeutics. 1997;283:1285–1292. [PubMed] [Google Scholar]
- Gallagher MJ, Huang H, Pritchett DB, Lynch DR. Interactions between ifenprodil and the NR2B subunit of the N-methyl-D-aspartate receptor. Journal of Biological Chemistry. 1996;271:9603–9611. doi: 10.1074/jbc.271.16.9603. 10.1074/jbc.271.16.9603. [DOI] [PubMed] [Google Scholar]
- Gotti B, Duverger D, Bertin J, Carter C, Dupont R, Frost J, Gaudilliere B, MacKenzie ET, Rousseau J, Scatton B, Wick A. Ifenprodil and SL 82.0715 as cerebral anti-ischemic agents. I. Evidence for efficacy in models of focal cerebral ischemia. Journal of Pharmacology and Experimental Therapeutics. 1988;247:1211–1221. [PubMed] [Google Scholar]
- Graham D, Darles G, Langer SZ. The neuroprotective properties of ifenprodil, a novel NMDA receptor antagonist, in neuronal cell culture toxicity studies. European Journal of Pharmacology. 1992;226:373–376. doi: 10.1016/0922-4106(92)90056-2. 10.1016/0922-4106(92)90056-2. [DOI] [PubMed] [Google Scholar]
- Jackson A, Sanger DJ. Is the discriminative stimulus produced by phencyclidine due to an interaction with N-methyl-D-aspartate receptors? Psychopharmacoloy. 1988;96:87–92. doi: 10.1007/BF02431538. [DOI] [PubMed] [Google Scholar]
- Kashiwagi K, Fukuchi J-I, Chao J, Igarashi K, Williams K. An aspartate residue in the extracellular loop of the N-methyl-D-aspartate receptor controls sensitivity to spermine and protons. Molecular Pharmacology. 1996;49:1131–1141. [PubMed] [Google Scholar]
- Kew JNC, Richards JG, Mutel V, Kemp JA. Developmental changes in NMDA receptor glycine affinity and ifenprodil sensitivity reveal three distinct populations of NMDA receptors in individual rat cortical neurons. Journal of Neuroscience. 1998a;18:1935–1943. doi: 10.1523/JNEUROSCI.18-06-01935.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew JNC, Trube G, Kemp JA. A novel mechanism of activity-dependent NMDA receptor antagonism describes the effect of ifenprodil in rat cultured cortical neurones. The Journal of Physiology. 1996;497:761–772. doi: 10.1113/jphysiol.1996.sp021807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew JNC, Trube G, Kemp JA. State-dependent NMDA receptor antagonism by Ro 8-4304, a novel NR2B selective, non-competitive, voltage-independent antagonist. British Journal of Pharmacology. 1998b;123:463–472. doi: 10.1038/sj.bjp.0701634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J. Spermine regulates N-methyl-D-aspartate receptor desensitization. Neuron. 1992;8:343–352. doi: 10.1016/0896-6273(92)90300-3. 10.1016/0896-6273(92)90300-3. [DOI] [PubMed] [Google Scholar]
- Menniti F, Chenard B, Collins M, Ducat M, Shalaby I, White F. CP-101,606, a potent neuroprotectant selective for forebrain neurons. European Journal of Pharmacology. 1997;331:117–126. doi: 10.1016/s0014-2999(97)10092-9. 10.1016/S0014-2999(97)10092-9. [DOI] [PubMed] [Google Scholar]
- Möckel V, Fischer G. Vulnerability to excitotoxic stimuli of cultured rat hippocampal neurons containing the calcium-binding proteins calretinin and calbindin D28k. Brain Research. 1994;648:109–120. doi: 10.1016/0006-8993(94)91911-9. 10.1016/0006-8993(94)91911-9. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Neyton J, Ascher P. Glycine-independent and subunit-specific potentiation of NMDA responses by extracellular Mg2+ Neuron. 1995;15:1109–1120. doi: 10.1016/0896-6273(95)90099-3. 10.1016/0896-6273(95)90099-3. [DOI] [PubMed] [Google Scholar]
- Perrault G, Morel E, Sanger DG, Zivkovic B. Comparison of the pharmacological profiles of four NMDA antagonists, ifenprodil, SL 82.0715, MK-801 and CPP, in mice. British Journal of Pharmacology. 1989;97:580P. [Google Scholar]
- Priestley T, Ochu E, Kemp JA. Subtypes of NMDA receptor in neurones cultured from rat brain. NeuroReport. 1994;5:1763–1765. doi: 10.1097/00001756-199409080-00019. [DOI] [PubMed] [Google Scholar]
- Reynolds IJ, Miller RJ. Ifenprodil is a novel type of N-methyl-D-aspartate receptor antagonist: interaction with polyamines. Molecular Pharmacology. 1989;36:758–765. [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Molecular Pharmacology. 1993;44:851–859. [PubMed] [Google Scholar]
- Williams K. Pharmacological properties of recombinant N-methyl-D-aspartate (NMDA) receptors containing the ɛ 4 (NR2D) subunit. Neuroscience Letters. 1995a;184:181–184. doi: 10.1016/0304-3940(94)11201-s. 10.1016/0304-3940(94)11201-S. [DOI] [PubMed] [Google Scholar]
- Williams K. Modulation of NMDA receptors by polyamines. In: Casero RA, editor. Polyamines: Regulation and Molecular Interaction. Austin, TX, USA: R. G. Landes Co.; 1995b. pp. 129–170. [Google Scholar]
- Williams K, Kashiwagi K, Fukuchi J-I, Igarashi K. An acidic amino acid in the N-methyl-D-aspartate receptor that is important for spermine stimulation. Molecular Pharmacology. 1995;48:1087–1098. [PubMed] [Google Scholar]
- Williams K, Russell SL, Shen YM, Molinoff PB. Developmental switch in the expression of NMDA receptors occurs in vivo and in vitro. Neuron. 1993;10:267–278. doi: 10.1016/0896-6273(93)90317-k. 10.1016/0896-6273(93)90317-K. [DOI] [PubMed] [Google Scholar]
- Williams K, Zappia AM, Pritchett DB, Shen YM, Molinoff PB. Sensitivity of the N-methyl-D-aspartate receptor to polyamines is controlled by NR2 subunits. Molecular Pharmacology. 1994;45:803–809. [PubMed] [Google Scholar]


