Abstract
Effects of noradrenaline (NA) and the α2 agonists tizanidine and clonidine were tested on extracellularly recorded responses of γ-motoneurones in deeply anaesthetized cats. Two types of responses were used; firstly, short latency phasic responses evoked by electrical stimulation of group II afferents in a muscle nerve and, secondly, tonic background discharges.
Responses evoked by group II muscle afferents were depressed when NA and tizanidine were applied ionophoretically close to a γ-motoneurone and when clonidine was applied systemically. The number of spike potentials evoked by stimulation of these afferents decreased and their latencies increased. Responses evoked by flexor or extensor afferents in γ-motoneurones innervating flexors or extensors were similarly depressed.
Tonic discharges were inconsistently and/or insignificantly affected by locally applied NA and tizanidine but were depressed by systemically applied clonidine.
Control tests indicate specific effects of NA and tizanidine application since similarly ionophoresed H+ ions did not change responses of γ-motoneurones to stimulation of group II afferents, or only weakly enhanced their background discharges. Furthermore, serotonin ejected from a solution with a similar pH facilitated rather than depressed responses of γ-motoneurones.
The results indicate that some antispastic effects of clonidine and tizanidine may be due to the depression of group II-evoked responses of γ-motoneurones, resulting in weaker responses of muscle spindles to muscle stretches.
Exaggeration of stretch reflexes after injuries of the nervous system might be due to several factors. Hyperactivity of fusimotor neurones, and therefore abnormally strong responses of muscle spindle afferents, were repeatedly considered as one of these factors (see e.g. Hagbarth et al. 1973; Burke, 1980, 1983; Noth, 1991; Pierrot-Deseilligny et al. 1993). However, when responses of primary afferents to muscle stretches were compared, no evidence was found for a consistently stronger fusimotor drive in spastic patients than in healthy subjects (see Hagbarth et al. 1973). There has therefore been a tendency to consider that fusimotor neurones do not contribute to spasticity in a significant way (see Pierrot-Deseilligny et al. 1993). Some recent observations might nevertheless revive this possibility. Particularly relevant are observations that the noradrenaline (NA) agonist clonidine not only weakens stretch reflexes (in spastic patients: Stewart et al. 1991; Nance, 1994; B. Bussel, personal communication; in animals: K. Pearson, personal communication) but also depresses the dynamic sensitivity of muscle spindle afferents (Bennett et al. 1996). This last effect was thought to be caused by a reduction in the fusimotor drive. However, there are several ways of reducing the dynamic sensitivity. Clonidine might depress activity of γd- or βd-fusimotor neurones, it might act on these neurones themselves, or it might depress activity of neurones which provide input to them (e.g. those mediating the flexion/ extension reflexes, Andén et al. 1966). If the antispastic actions of the NA agonists clonidine and tizanidine (see Emre, 1993), and L-β-3,4-dihydroxyphenylalanine (l-DOPA, the NA precursor; Eriksson et al. 1996) involve depression at the level of γ-motoneurones, one might reconsider a contribution by these neurones to the exaggerated stretch reflexes in spastic patients.
Even if the background activity of fusimotor neurones is similar in spastic and healthy subjects, reflex activation of these neurones might be more effective in spastic patients, as pointed out by Burke (1980, 1983). γ-Motoneurones might be abnormally strongly activated by group II muscle afferents which are one of the main sources of peripheral input to them (see Appelberg et al. 1983; Gladden et al. 1998). β-Motoneurones should be as strongly activated by group II afferents as α-motoneurones (Lundberg et al. 1987a, b). Both γ- and β-motoneurones might therefore induce more potent responses of muscle spindle primary and secondary afferents than normal. The primaries and secondaries would in turn contribute to a more effective excitation of α- and β-motoneurones, primaries mainly by their direct actions and secondaries via interneuronal pathways (see Eriksson et al. 1996), but also directly (Kirkwood & Sears, 1974; Stauffer et al. 1976; Munson et al. 1982). The secondaries might in addition increase the already high excitability of γ-motoneurones, acting either via interneuronal pathways or directly, as indicated in the companion paper (Gladden et al. 1998).
The present study was undertaken to extend previous observations on the effects of systemically applied noradrenergic agents on γ-motoneurones (Grillner et al. 1967; Bergmans & Grillner, 1968; Grillner, 1969; Bennett et al. 1996) by investigating whether these agents act directly on them. To this end, we analysed the modulatory actions of NA and its two agonists on responses of γ-motoneurones, either evoked by group II muscle afferents or by other neurones, the latter expressed as tonic background activity. Local actions of NA and tizanidine (which are particularly effective in depressing synaptic transmission from group II muscle afferents to other neurones (Bras et al. 1990)) were investigated following their ionophoretic application close to γ-motoneurones. Actions of clonidine were investigated after its systemic application, as in the study of effects of clonidine on responses of muscle spindle afferents (Bennett et al. 1996). In both approaches the effects were estimated using records from single γ-motoneurones. A preliminary report of this work has been published (Jankowska et al. 1997)
METHODS
Preparation
The experiments were performed on six cats. Anaesthesia was induced with pentobarbital sodium (45 mg kg−1i.p.) and maintained with chloralose (about 5–10 mg kg−1 h−1i.v.). The animals were killed with an overdose of pentobarbital and/or formaline perfusion. During recording they were paralysed with pancuronium bromide (Pavulon i.v.), with initial doses of 0.4 mg supplemented with similar doses every 2–3 h and artificially ventilated. Regularly repeated tests were made to ensure that the pupils remained constricted to the same extent throughout the experiments and/or that the animals did not respond with an increase in blood pressure or heart rate to any stimuli after they had been paralysed. In two experiments 4-aminopyridine (4-AP, 0.1–0.2 mg kg−1i.v.) was applied at the beginning of the recording in order to increase the effectiveness of synaptic activation of γ-motoneurones (see Jankowska et al. 1982). For further details of the experimental procedures see Gladden et al. 1998.
Recording and drug application
The recording electrodes (tip size about 2 μm; resistance 1.5–5 MΩ were filled with 2 m NaCl solution. Noradrenaline and tizanidine were ionophoresed from 0.2 m solutions in water at pH 4.5 by a 20 nA positive constant current. Micropipettes containing them (tip size 2–3 μm, resistance 10–20 MΩ) were introduced using a separate micromanipulator as described by Bras et al. (1989b, 1990). Their tips were positioned within 5–10 μm from the tip of the recording electrode. A 10–30 nA retaining current was used before the ejection of the tested compounds, and 20–30 nA during recovery. Series of twenty-five stimuli (2 per second) were applied every 30 s and both single responses evoked by these stimuli and peri-stimulus time histograms and cumulative sums of the same responses were stored on line. The γ-motoneurones were activated antidromically by single 0.1 ms current pulses at 3–13 times the threshold (T) for the most excitable fibres in a nerve. The antidromic responses were evoked for two purposes: first, to identify the neurones as γ-motoneurones, and second, to ensure that the conditions of recording remained unchanged during drug application, even if the cell stopped responding to stimulation of group II afferents. In order to evoke synaptic responses attributable to group II afferents beyond a reasonable doubt, muscle nerves were stimulated at an intensity submaximal for group II afferents (3–5T), since stronger stimuli could also excite group III afferents. However, when single stimuli were used, activation of a number of neurones required 7–10T stimuli. In order to keep the stimulation intensity below threshold for group III afferents, double (≥ 5T stimuli (separated by 2.5 or 3.3 ms) were used instead of single stimuli. Such stimuli were effective for all but one deep peroneal (DP) γ-motoneurone. Responses of the latter required 10T stimuli but their latency (< 3.5 ms) precluded the possibility that they were evoked by group III afferents (see Gladden et al. 1998). The responses to be tested were evoked by even weaker (3–5T) stimuli, which were adjusted to evoke them in only about one-half to two-thirds of the trials; under these conditions it is easier to demonstrate both the depressive and facilitatory effects of the tested drugs. Furthermore, when double stimuli were used, their intensity was adjusted such that the first stimulus was ineffective (as tested when it was applied alone) and the responses were evoked only by the second stimulus.
Sampling of γ-motoneurones
The γ-motoneurones were identified as described in the companion paper (Gladden et al. 1998). No particular γ-motoneurones were selected, although most of them innervated the medial gastrocnemius (MG) and posterior biceps-semitendinosus (PBST) muscles, and effects of the drugs were tested on responses of any γ-motoneurones evoked by group II muscle afferents that were sufficiently regular to allow construction of their peri-stimulus time histograms.
Analysis
Peri-stimulus time histograms and cumulative sums of the action potentials (for brevity referred to as ‘responses’) evoked by twenty-five successive peripheral stimuli were obtained on line, as described by Jankowska et al. (1997). They were used to compare the numbers and latencies of responses evoked before and during ionophoresis of the various compounds, and during the 15–30 min of recovery. The latencies of the responses were measured from incoming volleys (the first positive deflection) induced by group I afferents. Shortest latencies of responses evoked either by single stimuli (even if their intensity exceeded 5T, see above) or by the second of the two stimuli, if the first one was not effective, were considered as minimal latencies. The histograms and cumulative sums were made within time windows of 5–10 ms from the earliest action potentials; practically all action potentials which were originally linked to the stimuli appeared within these windows. As illustrated in Figs 2B and 6A, the cells never responded with more than a single action potential to a stimulus and the intensity of the stimuli was adjusted so that the neurones would respond to ten to twenty out of twenty-five stimuli (14–16 on average). The histograms were constructed using sampling rates of 40, 80 or 160 μs. As a measure of background activity, histograms and cumulative sums of responses appearing during 25–100 ms periods preceding the reflex responses were used. Examples of such histograms and cumulative sums are shown in Fig. 2D and E. The statistical significance of differences in the number of action potentials evoked by twenty-five stimuli before and after application of NA, tizanidine and clonidine was estimated by using Student's t test and Wilcoxon's test for matched pairs (one tailed).
Figure 2. Examples of records from an MG γ-motoneurone and the effects of NA.
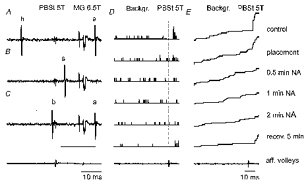
A-C, three sequences (single traces) of extracellular records; a, antidromically evoked responses of the tested γ-motoneurone following 6.5T stimulation of the medial gastrocnemius nerve, preceded by stimulus shock artifacts and antidromic field potentials in the MG motor nucleus; b, spontaneously occurring background discharges; and s, a synaptically evoked response following 5T stimulation (single stimulus) of the PBST nerve. Note that the neurone was antidromically activated in A and C but not in B, where the antidromic response collided with a synaptically evoked spike potential appearing at an interval close to the critical collision interval (indicated by a thin horizontal line in C). D and E, histograms of responses recorded during 25 successive trials and cumulative sums of the same responses. The responses were recorded before (control) and after placement of an NA-containing micropipette and during NA ionophoresis; the duration of ionophoresis is indicated to the right. Vertical lines separate spike potentials preceding and following stimulation of the PBST nerve. The lowermost records in the three columns are from the cord dorsum and show afferent volleys following stimulation of peripheral nerves. Note that the time base in A-C is twice as fast than in D and E. In this and the following figures the negativity in the microelectrode records is downwards and in records from the cord dorsum upwards. The intensity of the stimuli is indicated in multiples of thresholds for the most sensitive fibres in a given nerve.
Figure 6. Effects of clonidine on two γ-motoneurones.
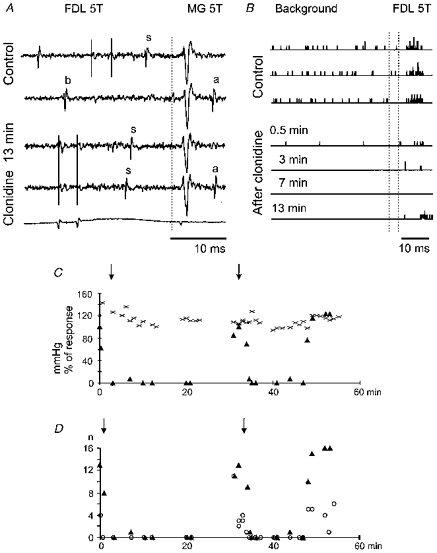
A, examples of responses of one of the two motoneurones tested. The responses were evoked antidromically from the MG nerve and synaptically by the second of the two 5T stimuli applied to the FDL nerve. The afferent volleys induced by them are shown in the bottom record and are indicated by double vertical lines. The single dotted vertical line indicates the time of stimulation of the MG nerve. Note that it was followed by antidromic activation of the neurone (a) only when its synaptic activation (s) or background (b) discharges occurred at an interval longer than the critical collision interval. B, PSTHs of responses of the same neurone, obtained before and after application of clonidine (2.5 μg kg−1). Those to the left of the double vertical lines illustrate changes in the tonic background discharges of the neurone, and those to the right changes in responses evoked by stimulation of the FDL nerve. C and D, plots showing decreases in the number of responses of the other MG motoneurone following two successive applications of clonidine (first 3 μg kg−1; second 1.5 μg kg−1, indicated by arrows). In C the numbers of responses induced by successive series of 30 stimuli applied to the PBST nerve (▴), before (time zero) and at indicated time intervals after the first intravenous injection of clonidine are expressed as a percentage of their control numbers and are plotted together with the mean blood pressure (×) (common ordinate for percentages of the responses and for mmHg). In D the number of these responses actually recorded are plotted together with the number of background discharges (○) occurring during a 40 ms period preceding each stimulus. Note that both applications of clonidine were followed by the disappearance of background discharges as well as of responses evoked by group II afferents, and that the recovery occurred faster after the smaller dose. Note also that these changes were not correlated with changes in the blood pressure. Other indications as in Fig. 2.
RESULTS
Conduction velocity, synaptic connections and spontaneous activity of the tested γ-motoneurones
The reported observations were made on twenty-three γ-motoneurones with conduction velocities of 16–49 m s−1 (15 medial gastrocnemius (MG), 1 lateral gastrocnemius (LG), 5 posterior biceps-semitendinosus (PBST) and 2 deep peroneal (DP)). Effects of NA, tizanidine and clonidine were tested on responses evoked by group II afferents at minimal latencies from group I volleys of 1.4–7 ms. It will be noted that the latencies of some of the tested responses were shorter than the expected minimal latencies of disynaptically evoked responses while latencies of other responses were longer. The shortest ones (boxed in Fig. 1A; tested in 10 motoneurones) are considered to be evoked monosynaptically (cf. the companion paper (Gladden et al. 1998).
Figure 1. Conduction velocities and latencies of responses of γ-motoneurones evoked by group II afferents tested in this study.
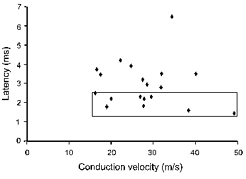
The latencies (ordinate) are with respect to the incoming volleys from group I afferents (see Methods). Those shorter than the expected minimal latencies of responses evoked disynaptically (see the companion paper, Gladden et al. 1998) are boxed. The minimal latencies of disynaptic actions evoked from distal nerves are longer than from proximal nerves but 3 of the 4 γ-motoneurones of the present sample responded to stimulation of the gastrocnemius and soleus (GS) and flexor digitorum longus (FDL) nerve with latencies much below the critical values and within the same range as latencies of responses compatible with monosynaptic actions of group II afferents of quadriceps (Q) and PBST nerves. Only one box was necessary therefore to encompass them. The conduction velocities (abscissa) were calculated by relating the distance between the stimulating and recording electrodes to the latency of antidromic activation of the neurones from which 0.2 ms was subtracted to account for the latent period of inducing action potentials in nerve fibres.
As described in the Methods, all but one of the responses tested were evoked by stimulation of muscle nerves at intensity sub-maximal for group II afferents (3–5T) and lower than the intensity at which the strongest responses of a given neurone were obtained. Low frequency (below 25 Hz) background activity was seen in only about one-half of these γ-motoneurones.
Effects of ionophoretically applied NA and tizanidine on responses evoked by group II afferents before, during and after ionophoresis of NA and tizanidine
During application of NA, group II afferents activated γ-motoneurones much less effectively. The number of action potentials evoked by group II muscle afferents in twenty-five successive trials decreased in all of the motoneurones tested. Figures 2D and and 3A show a moderate decrease in their number already after placement of a NA-containing micropipette, probably due to a small leak of NA. The number of these potentials was decreased further after removal of the retaining negative current and during passage of the positive current, the depression reaching maximum after 1–2 min of NA application. A partial recovery occurred during the following 10–20 min. A similar depression was evoked during application of the α2 NA agonist tizanidine (Fig. 3C). In Fig. 3A–D the data for MG, PBST and DP γ-motoneurones are pooled together but they are subdivided in Fig. 3E and F to allow a comparison of effects of NA and tizanidine on extensor (MG) and flexor (PBST and DP) γ-motoneurones. The comparison shows that the two sub-populations of γ-motoneurones were similarly affected.
Figure 3. Changes in the number of responses of γ-motoneurones during and after NA and tizanidine ionophoresis.
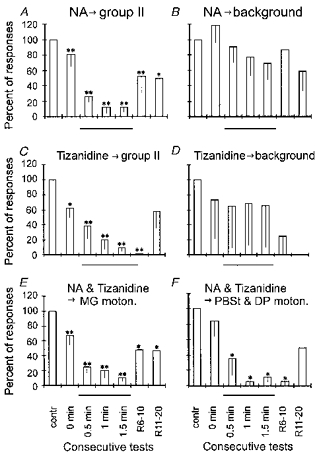
In all plots the numbers of action potentials following stimulation of group II afferents (A, C, E and F) or preceding it (i.e. background discharges: B and D) are compared before, during and after ionophoresis of NA and tizanidine. Responses evoked by group II afferents were sampled within 5–10 ms time windows and the background discharges within 25–100 ms time windows preceding stimulation of peripheral nerves. A and B are for responses of 9 and 7 γ-motoneurones, respectively, tested for the effects of NA. C and D contain similar data for the effects of tizanidine on 8 and 5 γ-motoneurones, respectively. E and F are for 11 MG γ-motoneurones (E) and 6 PBST and DP γ-motoneurones (F) on which effects of either NA or tizanidine were tested. The plots show means and s.e.m. s of the numbers of action potentials recorded during 25 successive trials, expressed as a percentage of the number under control conditions. The comparison involves responses evoked by group II afferents or background discharges appearing after the placement of the drug-containing pipette (0 min, with the retaining current), during ionophoresis (0.5, 1.0 and 1.5 min) and during recovery (R6–10 min, R11–20 min, with the retaining current). Student's t test and Wilcoxon's test for matched pairs (one tailed) were used to evaluate the statistical significance of the differences (*P= 0.01–0.05; **P < 0.01). Thin lines below abscissa indicate the data obtained during ionophoresis.
The decreases in the number of responses evoked by group II muscle afferents were associated with considerable increases in their latencies. The latter are illustrated with PSTHs and cumulative sums from one of the neurones in Fig. 2D and E. In Fig. 4A the increases in the minimal latencies of responses of individual γ-motoneurones (after 0.5 min of NA or tizanidine ionophoresis) are plotted against changes in the number of responses of these neurones. During the recovery the latencies of an increasing number of the responses started to return to their original values (see bottom histograms and cumulative sums in Fig. 2D and E). Similar changes were observed in extensor (GS) and flexor (PBST, DP) γ-motoneurones (Fig. 4B), and in responses evoked by group II afferents from the extensor (Q, GS) and flexor (PBST) muscles (Fig. 4C).
Figure 4. Changes in the latencies and number of responses evoked by group II afferents.
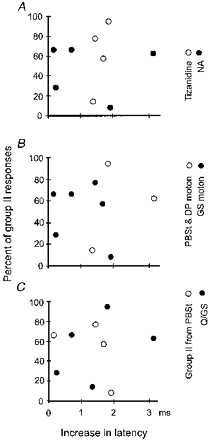
Changes in latencies of responses evoked by group II muscle afferents in 9 γ-motoneurones are plotted against changes in the number of these responses evoked by 25 stimuli. Differences between the minimal latencies of responses evoked after 0.5 min of NA or tizanidine ionophoresis and minimal latencies of control responses are indicated on the abscissa. Decreases in the number of responses are plotted as in Fig. 3. The data are for a smaller number of γ-motoneurones than in Fig. 3A, C, E and F, because responses of 8 out of 17 motoneurones disappeared within 0.5 min of ionophoresis and reappeared only after several minutes of recovery; they could thus be used for plotting changes in the number but not in the latency of the responses. A, data after NA (•) or tizanidine (○) ionophoresis for responses evoked from any of the nerves. B, changes in responses of GS (•) and PBST or DP (○) γ-motoneurones following either NA or tizanidine ionophoresis. C, changes in responses evoked by stimulation of extensor (•) or flexor (○) group II muscle afferents, similarly following either NA or tizanidine ionophoresis.
Tonic discharges before, during and after ionophoresis of NA and tizanidine
No statistically significant differences were found between the number of tonic background discharges of γ-motoneurones before and after 1.5 min of ionophoresis of either NA (Fig. 3B) or tizanidine (Fig. 3D). However, there was a tendency for the background discharges to diminish. It is thus possible that the depressive effects of these noradrenergic agents are not restricted to responses evoked by stimulation of group II afferents, but extend also to the background discharges of γ-motoneurones, although the latter appeared to be only marginally affected. Figure 5 shows in addition that if any changes did occur in the background discharges (○), they did not parallel changes in the number of responses evoked by group II afferents (▪). In some γ-motoneurones these two kinds of response changed in opposite directions (Fig. 5A), in some they tended to change in the same direction (Fig. 5B), while in others only the reflexly evoked responses appeared to be affected (Fig. 5C and D).
Figure 5. Changes in responses evoked by group II afferents and in tonic discharges.
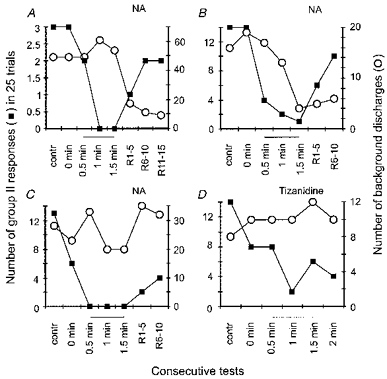
Comparison of the number of responses evoked by stimulation of group II afferents per 25 trials (left ordinate) and of the number of tonic discharges preceding group II-evoked responses during the same trials (right ordinate) of 4 cat γ-motoneurones. The numbers are for periods: before (contr, 0 min) and during (0.5 min, 1.0 min, 1.5 min and 2 min) ionophoresis of NA or tizanidine and during the recovery period (R1–5 min and R6–10 min). Thin lines below abscissa indicate the data obtained during ionophoresis.
Effects of systemically applied clonidine
Systemically applied clonidine depressed both tonic background discharges of γ-motoneurones and their responses to stimulation of group II muscle afferents. The similarity in the effects of clonidine on the two kinds of response is illustrated in Fig. 6B (by comparing the occurrence of responses that preceded or followed stimulation of group II afferents), and in Fig. 6D (where the numbers of such responses are indicated by open circles and filled triangles, respectively). These responses were evoked in two MG γ-motoneurones. The number of both background discharges and responses evoked by stimulation of group II afferents decreased within 0.5 min after injection of 1.5–3 μg kg−1 of clonidine and both responses disappeared thereafter. The degree of their depression was comparable, or possibly the depression of the tonic discharges might have been deeper, as judged by their earlier disappearance (Fig. 6B and D after the first injection) and later recovery (Fig. 6B).
The depression appeared unrelated to changes in blood pressure, as illustrated in Fig. 6C. The decreases in the number of responses seen during the first 1 or 2 min after application of clonidine occurred without any major changes in blood pressure, although the blood pressure dropped thereafter by about 20 mmHg. The recovery was not related to the return of the blood pressure to its original level.
Controls for specificity of effects of NA and tizanidine
Passage of positive current might affect neuronal responses secondarily to either polarization of the neurones or to the ejection of positively charged ions. However, it is more likely to facilitate than depress them since only excitatory effects of H+ have been previously reported (e.g. Krnjevic & Phillis, 1963; see Skydsgaard & Hounsgaard, 1996, for more recent references). The effects of passage of positive current (through pipettes filled with a HCl solution of pH 4.5) were therefore tested on the responses of six γ-motoneurones. Responses evoked from group II afferents in two γ-motoneurones remained unchanged. Tonic discharges of three neurones were not systematically changed (they were the same, larger or smaller). No tonic discharges appeared in two neurones in which they were originally lacking.
The specific depressant effects of the ejection of NA or tizanidine (rather than of H+ ions) on responses evoked by group II afferents were also indicated by the much stronger depression of these responses than of tonic discharges. Only the number of responses evoked by nerve stimulation was significantly decreased and the number of tonic discharges was most often unchanged or increased (see Figs 2 and 5). Furthermore, when the degree of the depression of responses evoked by group II afferents was compared after 1 min passage of 20 nA of positive current (during NA and tizanidine ionophoresis in 6 neurones), and after the subsequent 1 min passage of negative current of the same intensity, it remained unchanged. The effects of 20 nA of positive current alone on the tested neurones are therefore concluded to be negligible.
Serotonin was applied close to two γ-motoneurones from a solution with the same pH and using the same procedure as for ionophoresis of NA and tizanidine. However, its effects were opposite to those of NA and are therefore reported here as increasing the confidence with which the depressant effects reported in the main part of the results are ascribed to specific actions of NA. Figure 7 shows that 5-HT facilitated the appearance of responses evoked by group II afferents as well as of the background discharges preceding the stimuli. The facilitation was potent enough to cause the neurone to respond to one-third or even one-half of the stimuli after 0.5–2.0 min of ionophoresis, although it failed to respond to any stimuli just before, and responded only twice after placement of the 5-HT-containing micropipette. It may be noted that the neurone started to respond at decreasing latencies, and not only after the second but also after the first of the double stimuli. Since the second of the two vertical lines coincides with the second afferent volley, any responses evoked before, or within at least 1 ms after it, would have been evoked by the first stimulus.
Figure 7. Effects of serotonin on an MG γ-motoneurone.
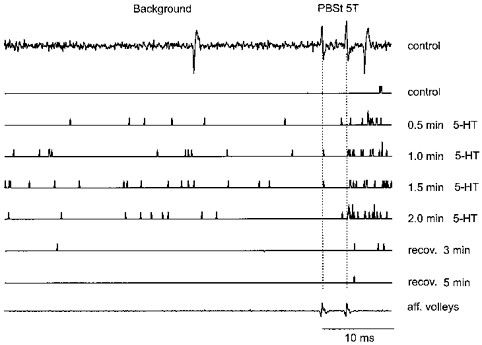
The traces from top to bottom are as follows: top, control - an example of an extracellular record showing, in this case, a single background discharge and a single response evoked by two stimuli applied to the combined posterior biceps and semitendinosus (PBST) nerve at 5T strength; next control and 6 subsequent traces, peri-stimulus time histograms preceding and following stimuli near-threshold for activating the γ-motoneurone; bottom trace, afferent volleys following the 2 stimuli to the PBST nerves. The histograms were obtained just after the 5-HT-containing pipette was inserted close to the tip of the recording electrode (control), during 0.5–2.0 min of 5-HT ionophoresis and after the ionophoresis had ceased (recovery). The two parallel dotted lines coincide with the time of arrival of the two incoming volleys (3.3 ms apart) following stimulation of the PBST nerve. Most of the responses to the right of the second dotted line are considered to be responses evoked by group II afferents. Responses to the left of the first line, or coinciding with it are background discharges but those between the lines are difficult to classify. Other indications as in Fig. 2.
Mechanical factors associated with the placement of a separate drug-containing micropipette had only a negligible effect on many of the previously investigated neurones (Bras et al. 1990; Jankowska et al. 1997). In order to minimize involvement of such mechanical effects, the responses of any tested neurones that changed more than marginally during the approach of the drug-containing pipette were eliminated from further analysis. In order to estimate the importance of mechanical factors, both tonic discharges and responses to stimulation of a peripheral nerve were compared with the control values after the placement of a drug-containing micropipette and after the beginning of ionophoresis. Tonic discharges of the majority of the neurones were either unchanged, or their frequency was increased (the overall changes being not statistically significant, see Figs 3B and F, and 5). Responses evoked by group II afferents in several of the tested neurones also remained practically unchanged. In ten neurones their number was 90–120 % of the control number; however, the number of responses started to decrease markedly, and their latency to increase, only during NA or tizanidine ionophoresis. The depression of responses observed after placement of the drug-containing pipettes in the remaining neurones is therefore interpreted as secondary to a leakage of NA or tizanidine (despite the use of 20 nA of retaining current), rather than to a mechanical depressant effect.
DISCUSSION
At which sites do noradrenergic compounds depress synaptic actions of muscle spindle secondaries on fusimotor neurones?
The noradrenaline precursor l-DOPA and its α2 agonists tizanidine and clonidine were previously shown to depress synaptic actions of group II muscle afferents on α-motoneurones (Andén et al. 1966; Schomburg & Steffens, 1988) and, most likely, β-motoneurones, since β-motoneurones were not differentiated from α-motoneurones, and since α- and β-motoneurones do not seem to differ with respect to their input. The depression of fusimotor drive (Bennett et al. 1996) and of stretch reflexes (Stewart et al. 1991; Nance, 1994; K. Pearson, personal communication) by clonidine might thus be secondary to its effects on β-fusimotor neurones. Since both disynaptically and polysynaptically evoked actions of group II afferents on α- and/or β-motoneurones were depressed, and monosynaptic actions of these afferents are very weak (Lundberg et al. 1977), the depression would be likely to occur primarily at the level of the interposed interneurones. The results of this study show, however, that monoamines may depress synaptic actions of group II muscle afferents not only on these interneurones and β-motoneurones, but also on γ-motoneurones.
Locally applied NA and tizanidine strongly depressed activation of γ-motoneurones by group II muscle afferents while they affected their background discharges only weakly and not consistently. After systemic application of clonidine the background discharges were, however, depressed as strongly as responses evoked by group II afferents. Systemically applied l-DOPA was also found to evoke a depression of background discharges (Grillner et al. 1967, 1969). Systemically applied drugs could affect both γ-motoneurones and any neurones presynaptic to them, while drugs ionophoresed close to γ-motoneurones would not be likely to reach neurones located outside the motor nuclei. These observations suggest therefore that the depression of activation of γ-motoneurones by group II afferents may occur at the level of both these motoneurones and of neurones presynaptic to them, while depression of tonic discharges of γ-motoneurones, at least those seen under our experimental conditions, should be primarily due to modulatory actions of monoamines earlier in neuronal pathways. Additional observations on the facilitation of responses of two γ-motoneurones by locally applied 5-HT similarly show that 5-HT may directly facilitate responses of γ-motoneurones, and extend previous observations (Ahlman et al. 1971; Ellaway & Trott, 1975; Myslinski & Anderson, 1978) on the facilitatory effects of the systemically applied 5-HT precursor 5-hydroxy tryptophan.
Interneurones mediating excitation of γ-motoneurones by group II afferents have not yet been identified. If the simplest indirect reflex pathways between group II afferents and γ-motoneurones include only one interneurone, these interneurones would most probably belong to intermediate zone group II interneurones (Edgley & Jankowska, 1987) since only these interneurones appear to project to ipsilateral motor nuclei (Bras et al. 1989a) and be depressed by NA α2 agonists (Bras et al. 1989b, 1990). Polysynaptic actions of group II afferents might nevertheless be mediated by both these and other interneurones. If the same interneurones mediate excitation of α-, β- and γ-motoneurones, any depression of their responses would be reflected in a weakening of reflexes mediated by α- and β-motoneurones. All these actions would therefore be mutually enhancing.
Under the conditions of the present study we had no means of differentiating between static and dynamic γ-motoneurones. However, since group II afferents provide input to both static and dynamic γ-motoneurones (Appelberg et al. 1983), and responses evoked by these afferents were depressed in all of the γ-motoneurones tested, it is unlikely that the depression of synaptic actions of group II afferents is confined to only one of these sub-populations. Tonic discharges of γ-motoneurones in non-anaesthetized preparations have been previously found to be differently affected by systemically applied l-DOPA, both in static and dynamic γ-motoneurones, and in those innervating flexor and extensor muscles (see e.g. Grillner et al. 1967, 1969). In the present study, effects of locally applied NA and tizanidine on responses evoked from group II afferents did not appear to be related to the target muscles of γ-motoneurones, and effects of systemically applied clonidine were similarly strong on all of the neurones analysed. However, our observations should not be considered to be at variance with those previously reported since different interneuronal populations may be responsible for the background discharges of γ-motoneurones under different experimental conditions. In non-anaesthetized preparations monoamines might thus have a differential effect on interneurones that preset activity of either static or dynamic γ-motoneurones, and innervate either flexors or extensors (Grillner et al. 1967, 1969; Hulliger, 1993; Bennett et al. 1996), even if they have similar effects on other interneurones in anaesthetized preparations.
Functional consequences of depression of responses of γ-motoneurones by monoamines
The immediate consequence of depression of synaptic actions of group II afferents on either static or dynamic γ-motoneurones will be a considerable weakening of the positive feedback via these afferents discussed in the previous paper (Gladden et al. 1998). The depression of synaptic actions of group II afferents on γ-motoneurones will be associated with a lowered excitability of these neurones and thereby with a weakening of their responses to any peripheral or central synaptic actions, and the effectiveness with which they adjust the sensitivity of muscle spindles. Weaker responses of muscle spindle primaries and secondaries to muscle stretches will in turn be followed both by weaker responses of β- and γ-motoneurones and by a further reduction in the excitation of γ-motoneurones by muscle stretch. Under normal conditions the setting of muscle spindle responses and of excitability of β- and γ-motoneurones would require a certain degree of depression of synaptic actions of group II afferents. Keeping fusimotor activity at a low level should assist healthy subjects to prevent excessive activation of α-motoneurones by either group I a afferents, or by group II afferents which may have even stronger reflex actions than group I a afferents, both in animals (see e.g. Lundberg et al. 1987a) and in humans (Marque et al. 1996). A phasic depression of the responsiveness of γ-motoneurones might also be useful during certain phases of movements, or types of movements which depend on central commands, rather than on peripheral input. The usefulness of modulation of synaptic actions of group II muscle afferents might also be considered within the framework of adjusting the task-dependent gain of reflexes to muscle stretches (see Prochazka, 1989; Hulliger, 1993; Bennett et al. 1996).
Under conditions of an abnormally high excitability of neurones with input from muscle spindle afferents, e.g. in spastic patients, a depression of the synaptic actions of these afferents would be even more important, and might assist in restoring the optimal mode of operation of α-motoneurones. It might therefore contribute in an essential way to the antispastic effects of clonidine, tizanidine and l-DOPA (see Emre, 1993; Nance, 1994; Corna et al. 1995; Eriksson et al. 1996; B. Bussel, personal communication).
Monosynaptic actions of muscle spindle secondaries on γ-motoneurones would provide the most specific control of activation of these neurones. Observations reported in the companion paper (Gladden et al. 1998) suggest that group II afferents of homonymous muscles may be responsible for positive feedback to a considerable proportion of γ-motoneurones. However, group II afferents of several muscles might affect individual γ-motoneurones. Di- and trisynaptically evoked group II actions will be induced from an even larger number of muscles since they are mediated by interneurones which are co-excited by afferents of several muscles (Edgley & Jankowska, 1987). It would therefore be useful if direct and indirect synaptic actions of group II afferents were modulated independently, e.g. by sub-populations of NA-releasing neurones, and if actions of these neurones could be restricted to afferents of particular muscles. If not, the function of narrowing the input from group II afferents would have to be played by GABA-mediated presynaptic inhibition (see Jankowska et al. 1997) and by spinal GABAergic interneurones with much more spacially restricted actions.
Acknowledgments
The study was supported by grants from the Swedish Medical Research Council (no. 05648) to E. J., from the Wellcome Trust to M. H. G., and Göteborg University to J. C.-B. Our warmest thanks are due to Mrs R. Larsson for her assistance both in the experiments and in the preparation of the illustrations.
References
- Ahlman H, Grillner S, Udo M. The effect of 5-HTP on the static fusimotor activity and the tonic stretch reflex of an extensor muscle. Brain Research. 1971;27:393–396. doi: 10.1016/0006-8993(71)90269-1. 10.1016/0006-8993(71)90269-1. [DOI] [PubMed] [Google Scholar]
- Andén NE, Jukes MG, Lundberg A, Vyklicky L. The effect of DOPA on the spinal cord. 1. Influence on transmission from primary afferents. Acta Physiologica Scandinavica. 1966;67:373–386. doi: 10.1111/j.1748-1716.1966.tb03324.x. [DOI] [PubMed] [Google Scholar]
- Appelberg B, Hulliger M, Johansson H, Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of group II muscle afferent fibres in the hind limb of the cat. The Journal of Physiology. 1983;335:255–273. doi: 10.1113/jphysiol.1983.sp014532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, De serres SJ, Stein RB. Regulation of soleus muscle spindle sensitivity in decerebrate and spinal cats during postural and locomotor activities. The Journal of Physiology. 1996;495:835–850. doi: 10.1113/jphysiol.1996.sp021636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmans J, Grillner S. Changes in dynamic sensitivity of primary endings of muscle spindle afferents induced by DOPA. Acta Physiologica Scandinavica. 1968;74:629–636. doi: 10.1111/j.1748-1716.1968.tb04273.x. [DOI] [PubMed] [Google Scholar]
- Bras H, Cavallari P, Jankowska E, Kubin L. Morphology of midlumbar interneurones relaying information from group II muscle afferents in the cat spinal cord. Journal of Comparative Neurology. 1989a;290:1–15. doi: 10.1002/cne.902900102. [DOI] [PubMed] [Google Scholar]
- Bras H, Cavallari P, Jankowska E, Mccrea D. Comparison of effects of monoamines on transmission in spinal pathways from group I and II muscle afferents in the cat. Experimental Brain Research. 1989b;76:27–37. doi: 10.1007/BF00253620. [DOI] [PubMed] [Google Scholar]
- Bras H, Jankowska E, Noga B, Skoog B. Comparison of effects of various types of NA and 5-HT agonists on transmission from group II muscle afferents in the cat. European Journal of Neuroscience. 1990;2:1029–1039. doi: 10.1111/j.1460-9568.1990.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Burke D. A reassessment of the muscle spindle contribution to muscle tone in normal and spastic man. In: Feldman R, Young R, Koella W, editors. Disordered Motor Control. Chicago: Year Book Publishers; 1980. pp. 261–278. [Google Scholar]
- Burke D. Critical examination of the case for or against fusimotor involvement in disorders of muscle tone. Advances in Neurology. 1983;39:133–150. [PubMed] [Google Scholar]
- Corna S, Grasso M, Nardone A, Schieppati M. Selective depression of medium-latency leg and foot muscle responses to stretch by an α2-agonist in humans. The Journal of Physiology. 1995;484:803–809. doi: 10.1113/jphysiol.1995.sp020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. The Journal of Physiology. 1987;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH, Trott JR. The mode of action of 5-hydroxytryptophan in facilitating a stretch reflex in the spinal cat. Experimental Brain Research. 1975;22:145–162. doi: 10.1007/BF00237685. [DOI] [PubMed] [Google Scholar]
- Emre M. New developments in the medical treatment of spasticity. In: Thilmann A, Burke D, Rymer Z, editors. Spasticity: Mechanisms and Management. Berlin: Springer Verlag; 1993. pp. 372–384. [Google Scholar]
- Eriksson J, Olausson B, Jankowska E. Antispastic effects of L-dopa. Experimental Brain Research. 1996;111:296–304. doi: 10.1007/BF00227307. [DOI] [PubMed] [Google Scholar]
- Gladden M, Jankowska E, Czarkowska-Bauch J. New observations on coupling between group II muscle afferents and feline γ-motoneurones. The Journal of Physiology. 1998;512:507–520. doi: 10.1111/j.1469-7793.1998.507be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. Supraspinal and segmental control of static and dynamic gamma-motoneurones in the cat. Acta Physiologica Scandinavica. 1969;(suppl. 327):1–34. [PubMed] [Google Scholar]
- Grillner S, Hongo T, Lund S. Descending monosynaptic and reflex control of gamma-motoneurones. Acta Physiologica Scandinavica. 1969;75:592–613. doi: 10.1111/j.1748-1716.1969.tb04414.x. [DOI] [PubMed] [Google Scholar]
- Grillner S, Hongo T, Lundberg A. The effect of DOPA on the spinal cord. 7. Reflex activation of static gamma-motoneurones from the flexor reflex afferents. Acta Physiologica Scandinavica. 1967;70:403–411. doi: 10.1111/j.1748-1716.1967.tb03638.x. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Wallin G, Lofstedt L. Muscle spindle responses to stretch in normal and spastic subjects. Scandinavian Journal of Rehabilitation Medicine. 1973;5:156–159. [PubMed] [Google Scholar]
- Hulliger M. Fusimotor control of proprioceptive feedback during locomotion and balancing: can simple lessons be learned for artificial control of gait? Progress in Brain Research. 1993;97:173–180. doi: 10.1016/s0079-6123(08)62275-x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Gladden MH, Czarkowska-Bauch J. Noradrenaline and its α2-agonists counteract activation of γ-motoneurones. The Journal of Physiology. 1997;505.P:77P. [Google Scholar]
- Jankowska E, Hammar I, Djouhri L, Heden C, Szabo lackberg Z, Yin XK. Modulation of responses of four types of feline ascending tract neurons by serotonin and noradrenaline. European Journal of Neuroscience. 1997;9:1375–1387. doi: 10.1111/j.1460-9568.1997.tb01492.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Lundberg A, Rudomin P, Sykova E. Effects of 4-aminopyridine on synaptic transmission in the cat spinal cord. Brain Research. 1982;240:117–129. doi: 10.1016/0006-8993(82)90649-7. 10.1016/0006-8993(82)90649-7. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA. Monosynaptic excitation of motoneurones from secondary endings of muscle spindles. Nature. 1974;252:243–244. doi: 10.1038/252243a0. [DOI] [PubMed] [Google Scholar]
- Krnjevic K, Phillis J. Ionophoretic studies of neurones in the mammalian cerebral cortex. The Journal of Physiology. 1963;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Comments on reflex actions evoked by electrical stimulation of group II muscle afferents. Brain Research. 1977;122:551–555. doi: 10.1016/0006-8993(77)90466-8. 10.1016/0006-8993(77)90466-8. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 1. Distribution and linkage of reflex actions to alpha-motoneurones. Experimental Brain Research. 1987a;65:271–281. doi: 10.1007/BF00236299. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 2. Functional characteristics of reflex pathways to alpha-motoneurones. Experimental Brain Research. 1987b;65:282–293. doi: 10.1007/BF00236300. [DOI] [PubMed] [Google Scholar]
- Marque P, Pierrot-Deseilligny E, Simonetta-Moreau M. Evidence for excitation of the human lower limb motoneurones by group II muscle afferents. Experimental Brain Research. 1996;109:357–360. doi: 10.1007/BF00231793. [DOI] [PubMed] [Google Scholar]
- Munson JB, Sypert GW, Zengel JE, Lofton SA, Fleshman JW. Monosynaptic projections of individual spindle group II afferents to type-identified medial gastrocnemius motoneurons in the cat. Journal of Neurophysiology. 1982;48:1164–1174. doi: 10.1152/jn.1982.48.5.1164. [DOI] [PubMed] [Google Scholar]
- Myslinski NR, Anderson EG. The effect of serotonin precursors on alpha- and gamma-motoneuron activity. Journal of Pharmacological and Experimental Therapy. 1978;204:19–26. [PubMed] [Google Scholar]
- Nance PW. A comparison of clonidine, cyproheptadine and baclofen in spastic spinal cord injured patients. Journal of American Paraplegia Society. 1994;17:150–156. doi: 10.1080/01952307.1994.11735927. [DOI] [PubMed] [Google Scholar]
- Noth J. Trends in the pathophysiology and pharmacotherapy of spasticity. Journal of Neurology. 1991;238:131–139. doi: 10.1007/BF00319679. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Pradat-Diehl P, Robain G. Physiopathology of spasticity. Annuals of Readaptation and Medical Physicotherapy. 1993;36:309–320. [Google Scholar]
- Prochazka A. Sensorimotor gain control: a basic strategy of motor systems? Progress in Neurobiology. 1989;33:281–307. doi: 10.1016/0301-0082(89)90004-x. 10.1016/0301-0082(89)90004-X. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Steffens H. The effect of DOPA and clonidine on reflex pathways from group II muscle afferents to alpha-motoneurones in the cat. Experimental Brain Research. 1988;71:442–446. doi: 10.1007/BF00247505. [DOI] [PubMed] [Google Scholar]
- Skydsgaard M, Hounsgaard J. Multiple actions of iontophoretically applied serotonin on motorneurones in the turtle spinal cord in vitro. Acta Physiologica Scandinavica. 1996;158:301–310. doi: 10.1046/j.1365-201X.1996.558326000.x. 10.1046/j.1365-201X.1996.558326000.x. [DOI] [PubMed] [Google Scholar]
- Stauffer EK, Watt DG, Taylor A, Reinking RM, Stuart DG. Analysis of muscle receptor connections by spike-triggered averaging. 2. Spindle group II afferents. Journal of Neurophysiology. 1976;39:1393–1402. doi: 10.1152/jn.1976.39.6.1393. [DOI] [PubMed] [Google Scholar]
- Stewart JE, Barbeau H, Gauthier S. Modulation of locomotor patterns and spasticity with clonidine in spinal cord injured patients. Canadian Journal of the Neurological Sciences. 1991;18:321–332. doi: 10.1017/s0317167100031887. [DOI] [PubMed] [Google Scholar]


