Abstract
Mechanisms of activity-dependent increases in cerebral blood flow (CBF) were examined in rat cerebellar cortex using the laser Doppler flow technique and extracellular recordings of single unit activity and field potentials.
Stimulation of the monosynaptic climbing fibre system evoked long-lasting complex spikes in Purkinje cells, and extracellular field potentials with a characteristic profile that indicated contributions from both passive and active membrane mechanisms. The concomitant CBF increases were reproducible at fairly short intervals, and suggest that both synaptic activity and spikes may contribute to increased CBF.
Stimulation of the disynaptic parallel fibre system inhibited the spiking activity in Purkinje cells, while the postsynaptic activity increased as indicated by the simultaneously recorded field potential. Nevertheless, CBF always increased. The inhibition of spike firing activity was partly dependent on GABAergic transmission, but may also relate to the intrinsic membrane properties of Purkinje cells.
The CBF increases evoked by parallel or climbing fibre stimulation were highly correlated to the sum of neural activities, i.e. the negativity of field potentials multiplied by the stimulus frequency. This suggests a robust link between extracellular current flow and activity-dependent increases in CBF.
AMPA receptor blockade attenuated CBF increases and field potential amplitudes, while NMDA receptor antagonism did not. This is consistent with the idea that the CBF responses are of neuronal origin.
This study has shown that activity-dependent CBF increases evoked by stimulation of cerebellar parallel fibres are dependent on synaptic excitation, including excitation of inhibitory interneurones, whereas the net activity of Purkinje cells, the principal neurones of the cerebellar cortex, is unimportant for the vascular response. For the climbing fibre system, not only synaptic activity but also the generation of complex spikes from Purkinje cells contribute to the increases in CBF. The strong correlation between CBF and field potential amplitudes suggests that extracellular ion fluxes contribute to the coupling of brain activity to blood flow.
Increases in regional cerebral blood flow (CBF) are commonly used to localize neuronal activity in humans and animals (Raichle, 1987; Villringer & Dirnagl, 1995). The mechanisms that couple CBF and neuronal activity are still only incompletely understood, but the issue is of great interest since in an increasing number of studies in humans the vascular responses are used to map neuronal activity. It is implicit in most papers that regional increases of CBF (‘brain activation’) are associated with increases of net spike activity in the neurones of the active region, even though a reorganization of the activity without increases in net spike activity is more likely to occur in some neuronal networks, in humans as in other species (Creutzfeldt, 1975). Only very few papers have addressed this issue directly by simultaneous recording of CBF and neuronal activity. Therefore, it is unclear what type of nerve cell activity, synaptic excitation, spikes, or both, will trigger increased CBF. Creutzfeldt (1975) estimated the energy consumption used for action potentials of cortical neurones on the assumption of energy demands comparable to those of unmyelinated nerve fibres. The calculations indicated that only a few per cent of the cortical energy consumption could be accounted for by spike activity of cortical nerve cells. This suggested that the re-establishment of intracellular ion concentrations following synaptic activity was the major energy-consuming process of the active cortex, and the main factor determining activity-dependent increases of CBF. However, it must be noted that neurones in the central nervous system do have different electrophysiological properties due to different membrane properties, and that even within the same cell, membrane properties may vary in different parts (Midtgaard, 1994). This complexity and anisotropy at the cellular level is the background for a diversity of spike types being produced, dependent on the distribution and strength of the afferent input system that is stimulated.
The main objective of this study was to examine in more detail the electrophysiological basis of activity-dependent increases of CBF. We chose the rat cerebellar cortex for our investigation since this preparation has well-defined neuronal circuits from which it is possible to record physiological variables involving one, or only a few synapses (Eccles et al. 1967). The cerebellum cannot generate epileptic activity, which is common after stimulation of the cerebral cortex. Therefore, it is possible to carry out experiments in the cerebellum that cannot be performed in other grey matter regions of the central nervous system. The basic circuitry of the cerebellar cortex is organized around the Purkinje cell — the principal neurone of the cerebellar cortex — from which the final and only output originates (Eccles et al. 1967). The activity of Purkinje cells in turn is influenced by two excitatory afferent inputs: parallel and climbing fibres that excite different parts of the cell with important consequences for the evoked activity. Parallel fibre stimulation results in small monosynaptic excitatory postsynaptic potentials (EPSP) and disynaptic inhibitory postsynaptic potentials (IPSP), which modulate the spontaneous firing of simple spikes in Purkinje cells (Eccles et al. 1967). The climbing fibres, which originate from the inferior olive nucleus and make synaptic contact with the proximal dendrites of Purkinje cells (Eccles et al. 1967), evoke large EPSPs, which trigger a large action potential in Purkinje cells followed by a high-frequency burst of action potentials, so-called complex spikes (Eccles et al. 1966a). Thus, stimulation of the two afferent fibre systems allowed us to examine the coupling of neuronal activity and CBF secondary to activity in a monosynaptic or disynaptic pathway. This we related to the spike activity of the Purkinje cells, and the extracellular field potentials elicited by stimulation of the two fibre systems. The measurements were supplemented by appropriate pharmacological studies to document the local, neural origin of the vascular responses. Using this established knowledge the activity-dependent increases in CBF could be assigned to the physiological properties of the two neuronal circuits stimulated.
METHODS
Animal preparation
Forty-six male Wistar rats (300–380 g; Panum Institute, Copenhagen) were anaesthetized with halothane (vapourizer, Fluotec 3, CYPRANE, UK; 3.5% induction, 1.5% during surgery) in 30% O2−70% N2O. The rats were primarily maintained on halothane (0.9%) in 30% O2−70% N2O, but in four rats in which we examined climbing fibre responses, anaesthesia was maintained by α-chloralose (initial dose, 60 mg kg−1i.v.; supplemental doses, 15–30 mg kg−1i.v.; Sigma) together with 30% O2−70% N2O. The results from this small group of rats were the same as for halothane-anaesthetized rats. Therefore, results from the two groups were pooled.
Muscle relaxation was induced by a bolus injection of 7–8 mg suxamethonium chloride i.p. (Hospital Pharmacy, Denmark) followed by continuous infusion through an intraperitoneal catheter at a rate of 2.5 mg h−1 during the experiment. Additional doses of α-chloralose or halothane were given upon pilo-erection or if the arterial blood pressure and heart rate increased by more than 10% during the experiment. Lidocaine (5 mg ml−1s.c.) was used at the operation wounds and at the contact spots (2%, gel) for the ear pins. The trachea was cannulated and the animals were ventilated with a volume respirator throughout the experiments to maintain the arterial blood gasses at Pa,CO2 = 37 mmHg, Pa,O2 = 125 mmHg and pH = 7.35–7.40 (measured by ABL30, Radiometer, Denmark). Catheters were inserted in the femoral artery, for recording of arterial blood pressure and for taking blood into Clinitubes (75–90 μl; Radiometer, Denmark), and in the femoral vein, for the slow infusion of saline and intravenous administration of drugs. The temperature was monitored with a rectal probe and maintained at 37°C with a heating pad. Rats were placed in a head holder, and the cranial bone and the dura were carefully removed over the cerebellar cortex. A pool was built around the craniotomy site with 5% agar in Ringer solution for superfusion of the cerebellar surface with artificial cerebrospinal fluid (ACSF; composition (mm): NaCl, 126; KCl, 2.8; NaHCO3, 22.0; CaCl2, 1.45; Na2HPO4, 1.0; and MgCl2, 0.876, pH 7.4) at 37°C, aerated with 95% air-5% CO2.
Recording and stimulation procedures
The experimental set-up is shown in Fig. 1. CBF was continuously monitored by a laser Doppler meter using a probe at a fixed position 0.3–0.5 mm above the pial surface (PF 403; o.d., 450 μm; fibre separation, 150 μm; wavelength, 780 nm; maximal intensity, 1 mW; Periflux 4001 Master, Perimed AB, JärFälla, Sweden). Recording of electrical signals from the cerebellar cortex was performed by single-barrel glass electrodes (pulled from capillary tubes; o.d., 1.8 mm; i.d., 1.2 mm; Modulohm, Denmark) filled with 2 m NaCl. The tip diameter was approximately 2 μm and the electrode impedance varied between 2 and 5 MΩ. The reference electrode consisted of low-impedance Ag-AgCl wire resting in the agar pool. Single unit spike activity was amplified 5000 times by a FET input amplifier filtered at 0.3–3 kHz. Evoked field potentials were amplified 1000 times with a bandwidth of 0.5 Hz and 4 kHz (custom made). On-line and off-line analyses were performed by the Spike2 program with a 1401plus interface (Cambridge Electronic Design). The digital sampling rate was 10 Hz for the CBF trace, 10 kHz for the neuronal signals and 100 Hz for the blood pressure trace. Neuronal signals and blood pressure traces were continuously displayed on a digital storage oscilloscope (Beckman Industrial).
Figure 1. Schematic three-dimensional drawing of experimental set-up, including neurones of interest and position of laser Doppler probe, stimulating and recording electrodes.
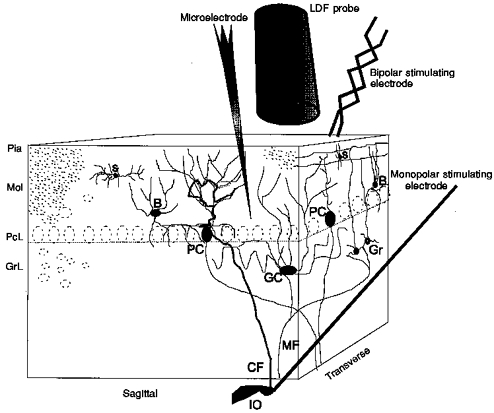
The positions of the three cerebellar layers, molecular (Mol, with a thickness of 400 μm), Purkinje cell (PcL, about 100 μm) and granular (GrL, 400–500 μm), are indicated. The molecular layer contains granule cell axons, called parallel fibres, the dendrites of Purkinje cells, stellate cells (S) and basket cells (B). The granule cell layer contains granule cells (Gr) and Golgi cells (GC). The superficial parallel fibres were stimulated by a bipolar stimulating electrode, while climbing fibres (CF) were stimulated by a monopolar electrode lowered into the caudal part of the inferior olive (IO). Field potentials and single unit spike activity were recorded with a glass microelectrode. CBF was recorded by a laser Doppler flowmetry (LDF) probe located 0.3–0.5 mm above the pial surface (Pia).
Parallel fibre stimulation was performed with two Teflon-insulated and twisted platinum-iridium wires (separation, 100 μm; Advent Research Materials Ltd, UK) placed on the cerebellar surface. The laser Doppler probe and the recording glass electrode were placed lateral to the bipolar stimulating electrode, which is ‘on-beam’ with regard to the point of stimulation and direction of parallel fibres (Akgören et al. 1994, 1996). Climbing fibres were stimulated by a monopolar electrode (SNEX-300, RMI Inc., USA) lowered stereotaxically into the caudal part of the inferior olive. The location of the electrode was verified by the evoked field potentials recorded in the cerebellar cortex. The caudal part of the inferior olive projects via climbing fibres to lobules V and VI in the vermis region (Azizi & Woodward, 1987). Parallel fibres and the inferior olive were stimulated by constant current units (ISO-flex, A.M.P.I., Israel), with 200 μs-long pulses, 2–3 mA or 150–250 μA, respectively. The laser Doppler probe and the glass electrode were positioned along the parallel fibres in the vermis of lobule V or VI (Akgören et al. 1994, 1996, 1997), care being taken to avoid surface vessels when positioning electrodes and probes. All changes of CBF were calculated as a percentage of the baseline value immediately preceding the test as described previously (Fabricius & Lauritzen, 1994). The laser Doppler flowmetry monitor displays blood flow readings in arbitrary units which do not allow for measurement of CBF in terms of absolute values, but the method is valid in determining relative changes of CBF during moderate flow increases (Fabricius & Lauritzen, 1996).
Single unit recordings were obtained from Purkinje cells at a depth of approximately 200–500 μm. Purkinje cells were identified by their ability to fire both simple and complex spikes spontaneously, or the production of a complex spike 5–8 ms after electrical stimulation of the inferior olive.
The experiments were performed after a postoperation recovery period of at least 1 h in order to obtain a stable level of anaesthesia, a stable laser Doppler baseline, a stable heart rate (∼300 beats min−1) and an arterial blood pressure of between 90 and 160 mmHg, not varying more than 10% in the same animal. Arterial blood pressure remained constant during electrical stimulation. Thus, the CBF increases noted were not due to fluctuations in blood pressure. At end of the experiment, rats were killed by intravenous injection of air.
Drugs
The NMDA receptor antagonists 2-amino-7-phosphonoheptanoic acid (APH, Sigma) and (+)-5-methyl-10,11-dihydro-5H-dibenz(a,d)cycloheptene-5,10-imine hydrogen maleate (MK-801, Research Biochemicals International) were dissolved in saline. The AMPA receptor antagonist 1-(4-aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine (GYKI 52466, Sigma) was dissolved in saline. The AMPA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, Research Biochemicals International) was dissolved in ACSF. The two GABAA antagonists picrotoxin (Sigma) and (−)-bicuculline methiodide (Sigma) were dissolved in ACSF. The solutions were ultrasonicated when necessary, and drugs dissolved in ACSF were kept at 37°C while bubbling with 95% air-5% CO2. The pH of the solutions was 7.30–7.40.
Experimental protocol
A schematic drawing of the model is shown in Fig. 1. The Purkinje cells receive excitatory input from one climbing fibre, which makes synaptic contact with the proximal part of the dendrites, and thousands of parallel fibres (Eccles et al. 1967). The parallel fibres run transversely in the molecular layer and make synaptic contacts with the dendrites of Purkinje cells and inhibitory interneurones. Additionally, the cerebellum is under constant inhibitory control by stellate cells and basket cells in the molecular layer, and Golgi cells in the granular cell layer (Eccles et al. 1966b). Climbing and parallel fibres in the cerebellar vermis region release glutamate (Freeman et al. 1983; Vollenweider et al. 1990), which interacts with AMPA receptors in Purkinje cells and a mixture of AMPA and NMDA receptors in interneurones (Garthwaite & Beaumont, 1989; Perkel et al. 1990; Hirano & Kasono, 1993), whereas inhibitory control is provided by GABAergic transmission (Andersen et al. 1965; Bisti et al. 1971). Figure 2 shows the electrical signals recorded from the cerebellar cortex under control conditions and during stimulation.
Figure 2. Neuronal signals recorded from Purkinje cells at a depth of 300 μm with a glass microelectrode.
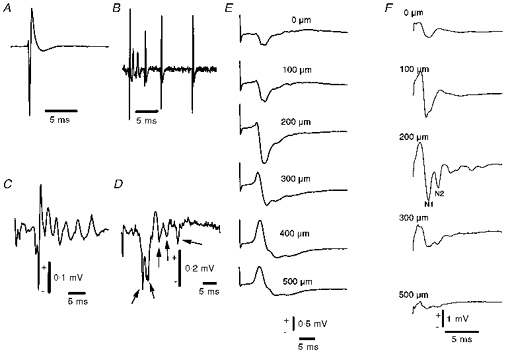
A, spontaneous simple spike (average of 15 spikes). B, spontaneous complex spike, which is characterized by a variable waveform. C, averaged complex spike (average of 10 sweeps) evoked by climbing fibre stimulation. Neuronal signals in A, B and C were recorded with a bandpass filter of 0.3–5 kHz, emphasizing fast components of sodium spikes and the early part of the climbing fibre response. The vertical bar in C indicates 0.1 mV for A, B and C. D, single evoked field potential recorded under the same conditions as in C, but with a filter that allows passage of the slow component of the signal (0.5 Hz to 3 kHz). Sodium spikes are seen as small volleys indicated by arrows. E, laminar analysis of field potentials in response to climbing fibre stimulation. The potential shown is an average of 100 sweeps of the signal shown in D. The depth profile shows a characteristic source-sink profile with a net positive current passing into the cell at the top of the molecular layer giving rise to a negative extracellular potential, with potential reversal at 300–500 μm, corresponding to the Purkinje cell body layer. F, laminar analysis of field potentials in response to parallel fibre stimulation. The parallel fibre response consisted of a presynaptic component (N1) due to action potentials in the fibres, and a postsynaptic component (N2) due to activation of AMPA receptors. The parallel fibre response was largest close to the cerebellar surface and decreased as a function of depth.
In the first part of the study we examined the relationship between Purkinje cell spike activity and CBF increases during electrical stimulation of parallel fibres (21 recordings, n = 8 rats). A population study was carried out with respect to the spontaneous spike activity of Purkinje cells that were ‘on-beam’, i.e. along the electrically activated band of parallel fibres, before, during and following stimulation. Stimulation inhibited spontaneous spike firing activity. The mechanism of inhibition was examined by using topical application of either picrotoxin (4.1 mm, n = 2 rats) or bicuculline methiodide (0.5 mm, n = 4 rats), which have been shown to block postsynaptic GABAA receptors at these doses (Dupont et al. 1979).
We calculated the summed field potential (ΣFP) for each stimulus rate as the product of the synaptic field potential amplitude (in mV) and the stimulus rate (in Hz). The relationship between ΣFP values and amplitudes of CBF increases were examined during both parallel (n = 4 rats) and climbing fibre stimulation (n = 5 rats), as a function of stimulus frequency. Parallel fibres were stimulated at 2–30 Hz for 60 s, and climbing fibres at 1–20 Hz for 60 s. Laminar analysis of the field potentials (Fig. 2) served as a guide to identify the depth at which the maximal potential could be recorded. The maximal parallel fibre-evoked field potentials were recorded at 100–200 μm (Fig. 2F). The maximal climbing fibre-evoked field potential was recorded at depth of 300–400 μm (Fig. 2E) (Eccles et al. 1967), i.e. within the sampling range of the laser Doppler probe used (Fabricius et al. 1997).
In some experiments the cerebellar AMPA receptors were blocked, for two reasons. First, to verify that the climbing fibre-evoked CBF response was indeed due to local synaptic neurotransmission, and second, to carry out a correlation analysis of CBF and the summed field potentials during partial blockade of EPSPs. AMPA receptors were blocked by either topical administration of CNQX (500 μm final concentration on brain surface, n = 4 rats), or intravenous administration of GYKI 52466 (20 mg kg−1, followed by continuous infusion of 4 mg kg−1 min−1, n = 5 rats), which have been shown to block AMPA responses at these doses (Akgören et al. 1996; Mathiesen et al. 1998). The fast onset and relatively fast recovery from postsynaptic inhibition by CNQX and GYKI 52466 made these compounds particularly useful. NMDA receptor blockade by APH (10 mg kg−1i.v., n = 5 rats) or MK-801 (2 mg kg−1i.v., n = 5 rats) has been shown to be effective in vivo (Mathiesen et al. 1998) and was chosen as a negative control and to examine whether NMDA receptor-mediated excitation of interneurones contributes to CBF (Perkel et al. 1990; Hirano & Kasono, 1993).
Statistics
We used Student's paired or non-paired t tests for analysis of results. Linear regression analysis (y=a+bx) was used for estimation of the correlation between field potential amplitude/area and CBF increase. Sigmoidal curve fitting was done using the Boltzmann equation:
(Origin 5.0, Microcal, USA).
The field potentials are shown as averaged traces for each stimulus train; exceptions are noted in the text. The field potential amplitudes were calculated from the averaged field potential traces using Data Reader (Origin 5.0). Values were considered statistically significant at P < 0.05. Values in the text, table and figures are means ± 1 standard error of the mean (s.e.m.), and n indicates the number of rats.
RESULTS
Extracellular neuronal signals recorded in rat cerebellar cortex
Purkinje cell spike activity consisted of simple and complex spikes. Simple and complex spikes occurred randomly with a firing rate of about 35 Hz and 1 Hz, respectively, and were best recorded at depths of around 200–400 μm corresponding to the proximal part of the dendrites and cell body of Purkinje cells (Eccles et al. 1966c, 1967). The waveforms (with a low- and high-pass filtering that emphasizes the sodium spike component) of spontaneous simple spikes and evoked complex spikes are shown in Fig. 2. Simple spikes have a characteristic triphasic waveform with a predominant negative component, a positive component and a minor negative component, with an overall duration of 3–4 ms (Fig. 2A). A spontaneous complex spike consists of several spikes with varying amplitudes over time that rarely repeat themselves (Fig. 2B) (Llinás & Sugimori, 1992). Electrical stimulation of the caudal part of the inferior olive evoked complex spikes in Purkinje cells after a delay of 5–7 ms and with an overall duration of between 10 and 20 ms (Fig. 2C), i.e. much longer than the simple spike.
Lowering the high-pass filter from 300 to 0.5 Hz enabled us to record the corresponding evoked field potentials (Fig. 2D–F). Field potentials represent the summation of extracellular current flow produced by activity of ensembles of cells close to the recording electrode. This signal gives information about the synchronized neuronal activity in the stimulated region. The field potential was recorded at a depth of 350 μm in response to climbing fibre stimulation (Fig. 2D). The evoked negativity suggests a powerful depolarization that is followed by a positivity that indicates hyperpolarization of Purkinje cells (Eccles et al. 1966a). The evoked sodium spikes (indicated by arrows in Fig. 2D) have the largest amplitude at 250–350 μm below the surface of the cerebellar cortex, i.e. at the level of the Purkinje cell bodies.
Stimulation of climbing fibres evoked field potentials in the cerebellar cortex with a characteristic depth profile (Fig. 2E). The negative phase of the field potentials increased from the surface to a depth of 300 μm, and potential reversal was observed at a depth around 300–500 μm. The depth profile of the field potential evoked by parallel fibre stimulation as shown in Fig. 2F consisted of a relatively large presynaptic component (the action potential of the parallel fibres with a negativity marked N1), and a smaller postsynaptic part (second negativity, marked N2), which decrease as a function of depth, with maximal amplitude at 200 μm (Eccles et al. 1967).
Relationship between activity-dependent increases of CBF, spike activity and synaptic field potentials
We examined the relationship between simple spikes and CBF increases during stimulation of parallel fibres (n = 8). Using the parallel fibre system it was possible to analyse separately the influence of spike activity in Purkinje cells and slow postsynaptic (EPSP) activity on CBF. The spike activity was inhibited by 84 ± 2% during stimulation in all of twenty-one Purkinje cell recordings (Table 1). In ten recordings in five rats the inhibition outlasted the period of stimulation by up to 110 s (Fig. 3A), and in six recordings in three rats a post-inhibitory rebound excitation was observed (Fig. 3B). In six recordings in four rats spike activity returned to normal after the end of stimulation. In all cases, CBF increased (62 ± 13%). This suggested that increased spike firing rate of Purkinje cells is not required for CBF to increase. Blockade of GABAergic neurotransmission, with bicuculline methiodide (0.5 mm, n = 4) or picrotoxin (4.1 mm, n = 2), attenuated the inhibition of spike activity during parallel fibre activation (P = 0.05, Table 1), but did not affect the CBF increase (Table 1 and Fig. 3C). This suggested that GABAergic activity contributed to the inhibition of Purkinje cell activity, but not to parallel fibre-evoked CBF.
Table 1.
Effects on spike activity and increase in CBF evoked by parallel fibre stimulation at 30 Hz for 30 s
| Change in spike activity | CBF increase | Baseline LDF readings | |
|---|---|---|---|
| Control | −84 ± 2% | 68 ± 9% | 79 ± 11 a.u. |
| Bicuculline methiodide | −26 ± 17% | 62 ± 5% | 100 ± 21 a.u. |
| P value | 0.05 (n = 4) | 0.569 (n = 4) | 0.125 (n = 4) |
LDF (laser Doppler flowmetry) baseline readings are given in arbitrary units (a.u.).
Figure 3. Activity-dependent CBF increases and spike activity in response to parallel fibre stimulation recorded along the activated parallel fibre beam at a stimulation frequency of 30 Hz and stimulus duration of 30 s.
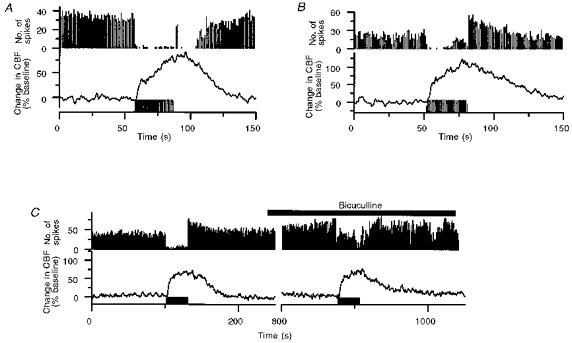
A, Purkinje cell spike firing activity almost vanished after 1–3 s of stimulation, and spontaneous firing did not return to basal levels until 19–25 s after the end of stimulation. CBF increased during stimulation, continued to increase for 5–10 s after the end of stimulation, and reversed to baseline after 40–50 s. B, example of inhibition during stimulation followed by post-inhibitory rebound excitation. The Purkinje cell spike firing rate was inhibited during the first part of stimulation, but gradually returned during the stimulation period. Following the stimulus train the spontaneous activity increased for 25–30 s. CBF started to increase 1–2 s after the start of stimulation, but did not decrease until 1–2 s after the end stimulation. C, bicuculline methiodide (0.5 mm, topical application, horizontal bar) did not affect the evoked CBF increase but attenuated the inhibition of Purkinje cell spike activity during stimulation. Stimulation periods are indicated by horizontal bars below the CBF trace.
Four rats were used to examine the relationship between parallel fibre-evoked summed field potentials and CBF. Typical recordings of field potentials and CBF from one rat during parallel fibre stimulation are shown in Fig. 4. The postsynaptic field potential amplitude was calculated as the difference between the second negativity (N2) and a reference point (marked by the double-headed arrow, Fig. 4B). The field potential amplitude decreased with increasing frequencies (Fig. 4C). The amplitude of this signal was multiplied with the stimulus rate to give the summed field potential (ΣFP), and correlated to the CBF response. There was a sigmoidal correlation between the ΣFP values and CBF increases (an example is shown in Fig. 4D). The relationship was the same for the other three animals.
Figure 4. Frequency-dependent CBF increases in response to parallel fibre stimulation were correlated to the summed field potentials.
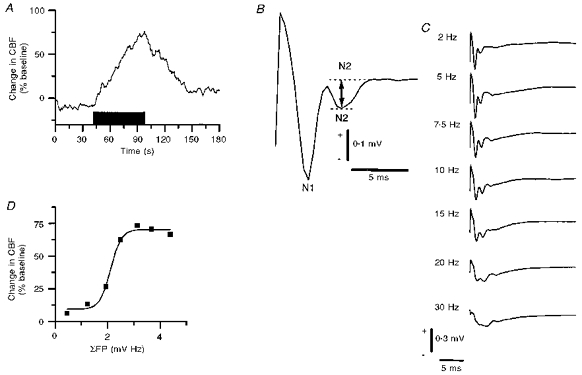
A, typical example of CBF increases evoked by 10 Hz for 60 s. B, enlarged evoked field potential response to indicate how the field potential amplitude was calculated. The first negativity (N1) was associated with the presynaptic action potential, while the second negativity (N2) represents postsynaptic excitation. The amplitude was measured as the voltage difference between the two dashed lines. C, in the frequency range of 2–30 Hz the amplitude of evoked field potentials decreased at high stimulus frequencies due to the short recovery time between stimuli. D, correlation analysis of CBF increases (ordinate) versus summed field potentials (abscissa) from one rat suggested a sigmoidal relationship.
Next, we evoked CBF increases in a frequency-dependent manner using climbing fibre stimulation. An original record of the CBF responses using stimulus rates of 1, 2.5, 5, 10 and 20 Hz for 60 s is shown in Fig. 5A. CBF increased in a frequency-dependent manner and reached a plateau of 141% at a stimulus rate of 10 Hz. Doubling the stimulus frequency did not further increase CBF (1–20 Hz for 60 s, n = 8; Fig. 5B). The evoked field potential amplitude decreased with increasing stimulus rate (Fig. 5C) due to the long recovery period of both pre- and postsynaptic components (Llinás & Volkind, 1973; Takahashi et al. 1995). The calculated ΣFP correlated linearly with the maximal CBF increase as shown in Fig. 5D for one animal (r = 0.985, P = 0.0022). A strong linear correlation was observed between ΣFP and maximal CBF increase for all animals (r = 0.891 ± 0.049, P = 0.007 ± 0.003, n = 5).
Figure 5. Frequency-dependent CBF increases in response to climbing fibre stimulation were directly correlated to the sum of active and passive postsynaptic activity.
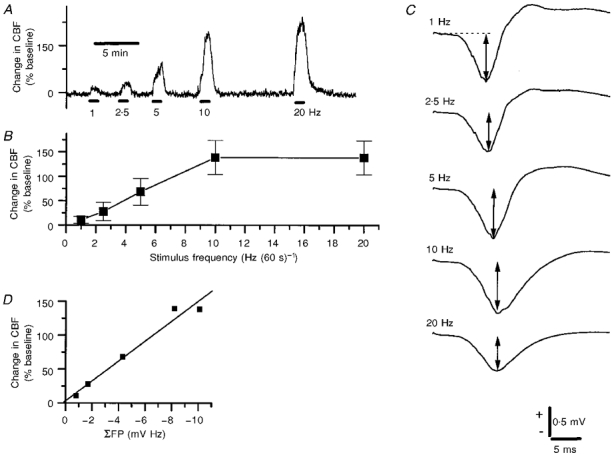
A, typical example of CBF increases evoked by 1, 2.5, 5, 10 and 20 Hz for 60 s (bars indicate time of stimulation). B, CBF (n = 8) increases reached a maximum of around 141% corresponding to a stimulation frequency of 10 Hz. C, profile of evoked field potentials as a function of stimulus frequency. The amplitude of the field potential per stimulus decreased with increasing stimulus rate due to the short recovery time between stimuli. The evoked field potentials were recorded at a depth of 400 μm. D, scatter plot of CBF increases versus summed field potentials, i.e. the product of field potential amplitudes (indicated by arrows in C) and stimulus rate. The relationship was best fitted to a straight line (r =−0.985, P = 0.0022).
This finding was explored further under conditions of reversible AMPA receptor blockade, which inhibited both the CBF increase and the electrophysiological response. In addition, this part of the experiment served to verify that the vascular responses to climbing fibre stimulation were of local neural origin. We used the AMPA receptor antagonists CNQX (Fig. 6), which was applied topically, or GYKI 52466 (Fig. 7) which was administrated intravenously, to block glutamatergic transmission. Topical application of CNQX (500 μm) reversibly inhibited both CBF (Fig. 6A and B) and ΣFP (Fig. 6A and C). There was a direct correlation between the maximal CBF increase and ΣFP as shown in Fig. 6D. The inhibition of CBF increases and field potentials produced by intravenous injection of GYKI 52466 (20 mg kg−1) was fairly short lasting, but the duration of the inhibition could be extended by continuous infusion (4 mg kg−1 min−1, Fig. 7A). The reversible antagonism confirmed the existence of a strong direct correlation between ΣFP and CBF (Fig. 7B; r = 0.956, P < 0.0001). Furthermore, both topical application of CNQX (n = 4) and i.v. administration of GYKI 52466 (n = 6) significantly inhibited CBF increases evoked by climbing fibre stimulation at 10 Hz for 60 s by 73 ± 8 and 84 ± 7% respectively, whereas APH (n = 5) and MK-801 (n = 5), which were used as negative controls (see Methods), had no significant effect on the CBF increase (Fig. 7C). This indicated that 85–95% of the CBF increase elicited by climbing fibres was dependent on postsynaptic activation of AMPA receptors. The results also support the close correlation between ΣFPs, which are dependent on a mixture of passive and active neural events, and CBF.
Figure 6. AMPA receptor blockade inhibited climbing fibre-evoked CBF increases and the major part of the evoked field potential.
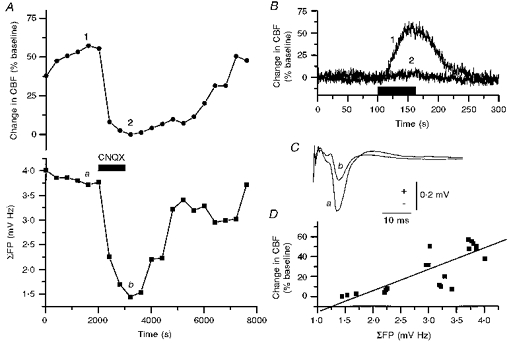
A, data points indicate maximal CBF increase and field potential amplitude evoked during successive periods of climbing fibre stimulation (10 Hz for 60 s) before, during and after topical application of CNQX. Topical application of CNQX (500 μm, horizontal bar) reversibly inhibited climbing fibre-evoked CBF increases (•) and summed field potentials (▪). Original data for CBF increases and field potentials are shown in B and C, respectively. B, CBF trace before (control, 1) and after application of CNQX (2), corresponding to the same numbers in A. C, control trace (a) of field potential, and trace after application of CNQX (b), corresponding to same letters in A. D, correlation analysis of maximal CBF increase versus summed field potentials fitted to a straight line (r = 0.784, P < 0.0001).
Figure 7. Activity-dependent CBF increases in response to climbing fibre stimulations (10 Hz for 60 s) were dependent on activation of AMPA receptors.
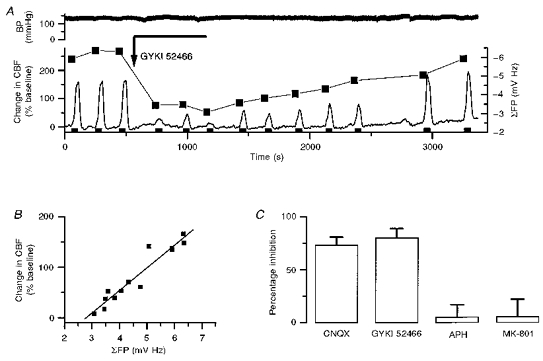
A, example of reversible inhibition of CBF increases and field potential amplitudes by an AMPA receptor antagonist (GYKI 52466; slow bolus of 20 mg kg−1 i.v. (arrow) followed by 10 min continuous infusion of 4 mg kg−1 min−1 (horizontal line)). The figure demonstrates the clear correlation between CBF increases and summed field potentials (▪) during inhibition and recovery from treatment. Blood pressure (BP, upper trace) was unaffected by the slow bolus and subsequent continuous injection of GYKI 52466. The filled bars (bottom) indicate duration of climbing fibre activation. B, analysis of CBF increases versus summed field potential suggested a direct correlation (r =−0.956, P < 0.0001). C, summary of effect of glutamate antagonists on CBF increases after administration of CNQX (500 μm, topical application, n = 4), GYKI 52466 (20 mg kg−1 plus continuous i.v., n = 6), APH (10 mg kg−1 i.v., n = 5) and MK-801 (2 mg kg−1 i.v., n = 5). The AMPA antagonists CNQX and GYKI 52466 significantly inhibited climbing fibre-evoked CBF increases, whereas the NMDA receptor antagonists APH and MK-801 had no effect on climbing fibre-evoked CBF increases.
DISCUSSION
In this study we analysed the relationship between neuronal signals and activity-dependent CBF increases in the rat cerebellum. The data suggest that both simple, passive synaptic mechanisms and complex, synaptic integrative mechanisms involving voltage-gated cation channels increase regional CBF, and that it is impossible on the basis of an increase in regional CBF to decide whether the spike activity in the brain region is inhibited or increased. These observations may be of use in the interpretation of activation studies in which modern brain imaging techniques are used.
Stimulation of both parallel and climbing fibres caused a frequency-dependent and reproducible increase in CBF. We have previously demonstrated that parallel fibre stimulation evokes CBF increases up to a stimulation frequency of about 100 Hz (Akgören et al. 1994, 1996). Simultaneous recordings of the extracellular field potentials revealed that the limitation of the vascular response was related to blockade of parallel fibres due to the short recovery time between stimuli (Akgören et al. 1996). Similar results were obtained in the present study. In response to climbing fibre stimulation the maximal CBF increase was reached at 10 Hz. This corresponds to the highest firing frequency of the inferior olive-climbing fibre system (Llinás & Volkind, 1973), which is limited by the long duration of the complex spike and the following recovery period (Eccles et al. 1967; Takahashi et al. 1995). The data therefore suggested that temporal constraints in the recovery processes of the two nerve networks examined were limiting for the activity-dependent CBF increases.
The effect of a climbing fibre input on Purkinje cell spiking activity is the result of a strong monosynaptic input and the intrinsic membrane properties of the dendritic tree of the Purkinje cells, which include several voltage-gated cation conductances that generate the so-called complex spike (Hounsgaard & Midtgaard, 1989). The success rate for eliciting a complex spike decreases with increasing stimulus rate (Eccles et al. 1967). Furthermore, the shape of complex spikes varies as a function of stimulus frequency, and of the preceding synaptic activity in Purkinje cells evoked by input from the parallel or climbing fibres. The large variability of the complex spike waveform made it impossible to develop an algorithm to count the number of complex spikes and to correlate this to the CBF increase (Campbell & Hesslow, 1986). Consequently, for this afferent system the CBF increase was correlated to the summed field potential (ΣFP) amplitude, which represents extracellular ionic fluxes produced by the combined passive synaptic and active non-synaptic activity.
The effect of a parallel fibre input on Purkinje cell spiking activity is the result of monosynaptic excitation, disynaptic inhibition (Eccles et al. 1966b,c, 1967) and the intrinsic membrane properties of the distal part of the dendritic tree of Purkinje cells — which is mainly dependent on voltage-gated calcium channels (Hounsgaard & Midtgaard, 1989). The net effect of electrical stimulation of parallel fibres was inhibition of Purkinje cell simple spike activity (Eccles et al. 1967), although at the same time, synaptic activity had increased as indicated by the increased field potential amplitude. Blockade of GABAergic transmission during stimulation of parallel fibres attenuated the inhibition of Purkinje cell activity, but the increase of CBF remained constant. This suggests that GABAergic inhibition partly explains the ‘silencing’ of Purkinje cells after excitation of parallel fibres, and confirms that blockade of GABAergic inhibition per se has no influence on activity-dependent CBF increases (Li & Iadecola, 1994; Akgören et al. 1996).
The slow onset and long time course of the vascular response compared with the field potentials (Akgören et al. 1994, 1996) suggested that temporal summation of events with a long time constant determined the development of the CBF increase. Therefore, we correlated the ΣFP values triggered by parallel fibre stimulation and CBF. The results showed a strong correlation between the product of field potential amplitude and stimulus frequency and CBF. Extension of the analysis to the climbing fibre system demonstrated a strong correlation here also between the frequency-dependent increases of CBF and ΣFP amplitudes. Field potentials are generated by the extracellular current flow, secondary to ionic fluxes across the nerve cell membrane (Nicholson & Llinás, 1971; Lopez et al. 1991). In the parallel fibre system, presynaptic action potentials, postsynaptic slow potentials and dendritic calcium spikes all contribute to the field potentials. In the climbing fibre system, passive and active postsynaptic activity, including Purkinje cell spiking, determine the waveform and amplitude of the field potential. However, it is unlikely that presynaptic action potentials in the climbing fibres contribute significantly to the extracellular potentials, because of the slenderness and low density of these structures (Eccles et al. 1967). The conclusion from this part of the study is therefore that the mechanisms underlying the generation of field potentials, i.e. extracellular current flow, whether due to active or passive membrane events are closely related to the increase in CBF.
A large number of nerve cells in the central nervous system generate synaptic activity and spikes involving ionic fluxes that are much longer lasting than those of the classic synaptic potential and action potential. For example, a complex spike is the result of a powerful synaptic depolarization of Purkinje cells that triggers dendritic calcium spikes mediated by P-type channels, calcium-dependent plateau potentials (which produce a slow continuous increase of intracellular calcium), sodium plateau potentials, and spikes at the axon hillock (Hounsgaard & Midtgaard, 1988; Llinás & Sugimori, 1992). Complex spikes are clearly different from classic action potentials in unmyelinated nerve fibres, which served as the basis for the calculations indicating that spikes accounted for a negligible part of brain energy consumption and, in turn, blood flow (Creutzfeld 1975). On this background it was hypothesized that passive synaptic activity determines activity-dependent CBF increases and probably brain energy consumption (Creutzfeld 1975; Raichle, 1987). We would like to modify this hypothesis since, as shown in the present study, both passive and active postsynaptic mechanisms contribute to increases in regional cerebral blood flow, and the relative contribution from either mechanism will depend on the nature of the neuronal circuit stimulated.
Spiking activity in the principal target cell of the activated pathway is, however, not a condition for CBF to increase, since during parallel fibre stimulation, Purkinje cell firing ceased while CBF increased. Therefore, an increase in regional CBF does not necessarily indicate increased firing in the principal nerve cells of that cortical region. Excitation of inhibitory interneurones probably contributes to the CBF response under these conditions, since the flow rise was partially blocked by AMPA receptor antagonists while blockade of GABAergic inhibition had no effect on CBF (Akgören et al. 1996). This is consistent with the observation that during focal epilepsy, increases in glucose metabolism correlate with both excitation due to release of glutamate, and inhibition as a result of GABA release (Bruehl & Witte, 1995). We would like to suggest that in regions with di- or polysynaptic pathways an increase in CBF may represent a decreased level of output from that particular region, and that excitation of inhibitory interneurones contributes to the CBF response. This is in accordance with the view that brain ‘activation’ is associated with a redistribution of spiking activity between ensembles of neurones, rather than an overall increase in spike rate (Creutzfeldt, 1975).
Since the CBF increases evoked by climbing fibre activation were inhibited by AMPA receptor antagonists, the indication was that the relative contribution from postsynaptic structures represented about 85–95% of the total CBF increase. We have previously published similar data for the parallel fibre system (Akgören et al. 1996). The reversible blockade of synaptic transmission by the AMPA receptor antagonists verified the strong and direct correlation between the amplitude of the evoked field potentials and CBF. This in turn supported the idea that the increase in CBF elicited by stimulation of the inferior olive was indeed due to local postsynaptic activity, and not to stimulation of vasomotor brainstem centres that influence the cerebral circulation (Nakai et al. 1983). By contrast, the field potentials and evoked CBF increase were unaffected by NMDA receptor blockade. Thus, the blockade was specific for AMPA receptors.
In conclusion, this is the first study to demonstrate differences in the regulation of activity-dependent CBF increases in mono- and disynaptic pathways at the level of neuronal networks, the strong relationship of CBF to synchronized extracellular current flow, and the contribution of complex spike formations in nerve cells to CBF. We propose that in the cerebellum, evoked CBF increases are independent of the spiking frequency of principal neurones, and may be produced by synaptic excitation of inhibitory interneurones. Therefore it is not possible on the basis of an increase in regional CBF to judge whether the output level of activity of that region is increased or not since this will depend on the connections between the cells in that network.
Acknowledgments
We thank Lillian Groendahl for expert technical assistance. The invaluable help and assistance of the electronic (S. Christoffersen) and mechanical workshop (K. J. Soerensen and co-workers) are much appreciated. This study was supported by Akademiet for de tekniske Videnskaber (EF-0580), The Danish Medical Research Council, The NOVO-Nordisk Foundation, The Danish Medical Association Research Foundation, Broedrene Hartmanns fond, Fonden til Laegevidenskabens Fremme, and the Foundation for Research in Neurology.
References
- Akgören N, Dalgaard P, Lauritzen M. Cerebral blood flow increases evoked by electrical stimulation of rat cerebellar cortex: relation to excitatory synaptic activity and nitric oxide synthesis. Brain Research. 1996;710:204–214. doi: 10.1016/0006-8993(95)01354-7. 10.1016/0006-8993(95)01354-7. [DOI] [PubMed] [Google Scholar]
- Akgören N, Fabricius M, Lauritzen M. Importance of nitric oxide for local increases of blood flow in rat cerebellar cortex during electrical stimulation. Proceedings of the National Academy of Sciences of the USA. 1994;91:5903–5907. doi: 10.1073/pnas.91.13.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgören N, Mathiesen C, Rubin I, Lauritzen M. Laminar analysis of activity-dependent increases of CBF in rat cerebellar cortex: dependence on synaptic strength. American Journal of Physiology. 1997;273:H1166–1176. doi: 10.1152/ajpheart.1997.273.3.H1166. [DOI] [PubMed] [Google Scholar]
- Andersen P, Eccles JC, Voorhoeve PE. Postsynaptic inhibition of cerebellar Purkinje cells. Journal of Neurophysiology. 1965;27:1138–1153. doi: 10.1152/jn.1964.27.6.1138. [DOI] [PubMed] [Google Scholar]
- Azizi SA, Woodward DJ. Inferior olivary nuclear complex of the rat: Morphology and comments on the principles of organization within the olivocerebellar system. Journal of Comparative Neurology. 1987;263:467–484. doi: 10.1002/cne.902630402. [DOI] [PubMed] [Google Scholar]
- Bisti S, Iosif G, Marchesi GF, Strata P. Pharmacological properties of inhibitions in the cerebellar cortex. Experimental Brain Research. 1971;14:24–37. doi: 10.1007/BF00234908. [DOI] [PubMed] [Google Scholar]
- Bruehl C, Witte OW. Cellular activity underlying altered brain metabolism during focal epileptic activity. Annals of Neurology. 1995;38:414–420. doi: 10.1002/ana.410380311. [DOI] [PubMed] [Google Scholar]
- Campbell NC, Hesslow G. The secondary spikes of climbing fibre responses recorded from Purkinje cell somata in cat cerebellum. The Journal of Physiology. 1986;377:207–224. doi: 10.1113/jphysiol.1986.sp016182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt OD. Neurophysiological correlates of different functional states of the brain. In: Ingvar DH, Lassen NA, editors. Brain Work. The Coupling of Function, Metabolism and Blood Flow in the Brain. Copenhagen, Denmark: Munksgaard; 1975. pp. 21–46. [Google Scholar]
- Dupont JL, Crepel F, Delhaye-Douchaud N. Influence of bicuculline and picrotoxin on reversal properties of excitatory synaptic potentials in cerebellar Purkinje cells of the rat. Brain Research. 1979;173:577–580. doi: 10.1016/0006-8993(79)90256-7. 10.1016/0006-8993(79)90256-7. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Ito M, Szentágothai J. The Cerebellum as a Neuronal Machine. Berlin, Heidelberg, New York: Springer-Verlag; 1967. p. 335. [Google Scholar]
- Eccles JC, Llinás R, Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. The Journal of Physiology. 1966a;182:268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Llinás R, Sasaki K. The inhibitory interneurones within the cerebellar cortex. Experimental Brain Research. 1966b;1:1–16. doi: 10.1007/BF00235206. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Llinás R, Sasaki K. The mossy fibre-granule cell relay of the cerebellum and its inhibitory control by Golgi cells. Experimental Brain Research. 1966c;1:82–101. doi: 10.1007/BF00235211. [DOI] [PubMed] [Google Scholar]
- Fabricius M, Akgören N, Dirnagl U, Lauritzen M. Laminar analysis of cerebral blood flow in cortex of rats by laser-Doppler flowmetry. A pilot study. Journal of Cerebral Blood Flow and Metabolism. 1997;17:1326–1336. doi: 10.1097/00004647-199712000-00008. [DOI] [PubMed] [Google Scholar]
- Fabricius M, Lauritzen M. Examination of the role of nitric oxide for the hypercapnic rise of cerebral blood flow in rats. American Journal of Physiology. 1994;266:H1457–1464. doi: 10.1152/ajpheart.1994.266.4.H1457. [DOI] [PubMed] [Google Scholar]
- Fabricius M, Lauritzen M. Laser-Doppler evaluation of rat brain microcirculation: Comparison with the [14C]-iodoantipyridine method suggests discordance during cerebral blood flow increases. Journal of Cerebral Blood Flow and Metabolism. 1996;16:156–161. doi: 10.1097/00004647-199601000-00018. [DOI] [PubMed] [Google Scholar]
- Freeman ME, Lane JD, Smith JE. Turnover rates of amino acid neurotransmitters in regions of rat cerebellum. Journal of Neurochemistry. 1983;40:1441–1447. doi: 10.1111/j.1471-4159.1983.tb13588.x. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Beaumont PS. Excitatory amino acid receptors in the parallel fibre pathway in rat cerebellar slices. Neuroscience Letters. 1989;107:151–156. doi: 10.1016/0304-3940(89)90808-2. 10.1016/0304-3940(89)90808-2. [DOI] [PubMed] [Google Scholar]
- Hirano T, Kasono K. Spatial distribution of excitatory and inhibitory synapses on a Purkinje cell in a rat cerebellar culture. Journal of Neurophysiology. 1993;70:1316–1325. doi: 10.1152/jn.1993.70.4.1316. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Midtgaard J. Intrinsic determinants of firing pattern in Purkinje cells of the turtle cerebellum in vitro. The Journal of Physiology. 1988;402:731–749. doi: 10.1113/jphysiol.1988.sp017231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Midtgaard J. Synaptic control of excitability in turtle cerebellar Purkinje cells. The Journal of Physiology. 1989;409:157–170. doi: 10.1113/jphysiol.1989.sp017490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Iadecola C. Nitric oxide and adenosine mediate vasodilation during functional activation in cerebellar cortex. Neuropharmacology. 1994;33:1453–1461. doi: 10.1016/0028-3908(94)90049-3. 10.1016/0028-3908(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Llinás R, Volkind RA. The olivo-cerebellar system: Functional properties as revealed by harmaline-induced tremor. Experimental Brain Research. 1973;18:69–87. doi: 10.1007/BF00236557. [DOI] [PubMed] [Google Scholar]
- Llinás RR, Sugimori M. The electrophysiology of the cerebellar Purkinje cell revisited. In: Llinás RR, Sotelo C, editors. The Cerebellum Revisited. New York: Springer-Verlag; 1992. pp. 167–181. [Google Scholar]
- Lopez L, Chan CY, Okada YC, Nicholson C. Multimodal characterization of population responses evoked by applied electric field in vitro: Extracellular potential, magnetic evoked field, transmembrane potential, and current-source density analysis. Journal of Neuroscience. 1991;11:1998–2010. doi: 10.1523/JNEUROSCI.11-07-01998.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen C, Varming T, Jensen LH. In vivo and in vitro evaluation of AMPA receptor antagonists in rat hippocampal neurones and cultured mouse cortical neurones. European Journal of Pharmacology. 1998;353:159–167. doi: 10.1016/s0014-2999(98)00401-4. 10.1016/S0014-2999(98)00401-4. [DOI] [PubMed] [Google Scholar]
- Midtgaard J. Processing of information from different sources: spatial synaptic integration in the dendrites of vertebrate CNS neurons. Trends in Neurosciences. 1994;17:166–173. doi: 10.1016/0166-2236(94)90095-7. 10.1016/0166-2236(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Nakai M, Iadecola C, Ruggiero DA, Tucker LW, Reis DJ. Electrical stimulation of cerebellar fastigial nucleus increases cerebral cortical blood flow without change in local metabolism: Evidence for an intrinsic system in brain for primary vasodilation. Brain Research. 1983;260:35–49. doi: 10.1016/0006-8993(83)90762-x. 10.1016/0006-8993(83)90762-X. [DOI] [PubMed] [Google Scholar]
- Nicholson C, Llinás R. Field potentials in the alligator cerebellum and theory of their relationship to Purkinje cell dendritic spikes. Journal of Neurophysiology. 1971;34:509–531. doi: 10.1152/jn.1971.34.4.509. [DOI] [PubMed] [Google Scholar]
- Perkel DJ, Hestrin S, Sah P, Nicoll RA. Excitatory synaptic currents in Purkinje cells. Proceedings of the Royal Society B. 1990;241:116–121. doi: 10.1098/rspb.1990.0074. [DOI] [PubMed] [Google Scholar]
- Raichle ME. Handbook of Physiology, section 1, The Nervous System. Bethesda, MD, USA: American Physiological Society; 1987. Circulatory and metabolic correlates of brain function in normal humans; pp. 643–674. part 2. [Google Scholar]
- Takahashi M, Kovalchuk Y, Attwell D. Pre- and postsynaptic determinants of EPSC waveform at cerebellar climbing fiber and parallel fiber to Purkinje cell synapses. Journal of Neuroscience. 1995;15:5693–5702. doi: 10.1523/JNEUROSCI.15-08-05693.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villringer A, Dirnagl U. Coupling of brain activity and cerebral blood flow: Basis of functional neuroimaging. Cerebrovascular and Brain Metabolism Reviews. 1995;7:240–276. [PubMed] [Google Scholar]
- Vollenweider FX, Cuénod M, Do KQ. Effect of climbing fiber deprivation on release of endogenous aspartate, glutamate, and homocysteate in slices of rat cerebellar hemispheres and vermis. Journal of Neurochemistry. 1990;54:1533–1540. doi: 10.1111/j.1471-4159.1990.tb01201.x. [DOI] [PubMed] [Google Scholar]


