Abstract
Extra- or intracellular recordings were made from seventy-six γ-motoneurones of hindlimb muscles in chloralose anaesthetized cats to re-assess the coupling between secondary muscle spindle afferents (group II muscle afferents) and these neurones. The latencies of a number of responses evoked by group II muscle afferents in γ-motoneurones were shorter than minimal latencies of responses induced disynaptically in other spinal neurones. These latencies are therefore compatible with monosynaptic coupling between muscle spindle secondaries and γ-motoneurones.
Responses fulfilling criteria for monosynaptically evoked responses were seen in about one third of γ-motoneurones with input from the group II muscle afferents tested (in 6 of 18 motoneurones recorded intracellularly and in 26 of 74 motoneurones recorded extracellularly). They were usually evoked from only one of the stimulated nerves, stimulation of group II afferents of other nerves being followed by responses at longer latencies.
Most γ-motoneurones were excited by group II afferents from several muscles, both flexors and extensors. However, a comparison of group II input to γ-motoneurones innervating medial gastrocnemius and four other hindlimb muscles revealed differences in both incidence and sources.
This study extends results of previous studies by providing evidence that some synaptic actions of group II afferents, including afferents from the same muscle, are evoked monosynaptically, and may assist in sustaining the activation of γ-motoneurones by positive feedback.
Muscle stretches and small amplitude vibration, which should be selective or almost selective for group I a afferents, caused only weak facilitation, and in only a small proportion of tonically firing triceps γ-motoneurones, in non-anaesthetized decerebrate or spinal preparations (Fromm & Noth, 1976; Ellaway & Trott, 1978). However, in the same type of preparations, larger ramp and hold stretches, such as would excite secondary as well as primary endings, very effectively enhanced background tonic discharges of γ-motoneurones (Noth, 1981). Both effects were seen in neurones in which electrical stimulation of muscle nerves at group II strength likewise facilitated their tonic discharges. The same phenomena could be also readily demonstrated in anaesthetized preparations in which stretches of the tenuissimus and other limb muscles invariably elicited intrafusal contractions in tenuissimus spindles (Gladden et al. 1995). In addition, when the excitatory effects of muscle stretches on γ-motoneurones were assessed from changes in responses of muscle spindle afferents (Appelberg et al. 1982) it was concluded that they were caused by activation of muscle spindle secondary endings. All these studies showed that γ-motoneurones can be excited by group II muscle afferents from quite distant muscles as well as related muscles. However, two essential questions concerning the group II input to γ-motoneurones have not yet been answered, and have been taken up in the present study.
The first question is whether group II afferents make synaptic contacts with γ-motoneurones, or whether excitation of γ-motoneurones by group II afferents is mediated only via interneurones. Previous electrophysiological analysis of responses of single intra- or extra-cellularly recorded γ-motoneurones did not encourage a search for monosynaptic coupling between group II muscle afferents and γ-motoneurones, since it was concluded that group II afferents excite them via di-, tri- or even polysynaptic pathways (Appelberg et al. 1983b). However, during the course of preliminary experiments we found that the latencies for the activation of a number of γ-motoneurones were shorter than expected for indirectly evoked synaptic actions of group II afferents. This led us to reconsider the possibility of monosynaptic coupling.
The second question addressed in this study is whether group II afferents from a particular muscle provide input to all, or only some populations of γ-motoneurones, including those that innervate the muscle itself. In other words, should synaptic actions of group II afferents be considered only in terms of a fairly diffuse input, or are the pathways organized within a framework of specific patterns related to individual muscles and their function. The evidence appears to suggest that stretching a muscle during a movement will facilitate activation of some γ-motoneurones belonging to the muscle (Noth, 1981; Gladden et al. 1995). If the group II input pathways were orderly rather than diffuse, this ‘homonymous’ group II facilitation might be augmented by excitatory input from group II afferents of other stretched muscles in a combination controlled at the spinal level to be appropriate to the movement. In fact, the terms ‘homonymous’ and ‘autogenetic’ have been applied rather loosely; the previous evidence for homonymous excitation of γ-motoneurones was not definitive, because effects were termed ‘homonymous’ when evoked by group II afferents of three or four muscles stimulated together within the hamstring or triceps surae nerves (Noth & Thilmann, 1980; Appelberg et al. 1983b). The reported effects on γ-motoneurones might therefore have been evoked by afferents of any of these muscles. This was also the case when γ-motoneurones were activated by stretches of the whole triceps surae (Fromm & Noth, 1976; Ellaway & Trott, 1978), or both posterior biceps and semitendinosus (Appelberg et al. 1982) - the facilitation could have been evoked from muscle spindle secondaries of a synergist rather than from the same muscle.
A preliminary report of this work has been published (Gladden et al. 1997).
METHODS
Preparation
Responses of γ-motoneurones analysed in this study were recorded in seven cats anaesthetized with pentobarbital sodium (45 mg kg−1i.p.), supplemented with chloralose (about 5 mg kg−1 h−1i.v.). During recording the animals were paralysed with pancuronium bromide (Pavulon i.v.), with initial doses of 0.4 mg supplemented by similar doses every 2–3 h. They were killed with an overdose of pentobarbital and/or formaline perfusion. Regularly repeated tests were made to ensure that the pupils remained constricted to the same extent throughout the experiments and that the animals did not respond with an increase in either blood pressure or heart rate to any stimuli after they had been paralysed. Control observations made on non-paralysed animals have shown that all those anaesthetized with similar or weaker doses of pentobarbital and chloralose remained deeply anaesthetized over the same period of time. In two cats 4-aminopyridine (4-AP, 0.1–0.2 mg kg−1) was applied at the beginning of the recording in an attempt to increase the effectiveness of synaptic activation of γ-motoneurones (see Jankowska et al. 1982).
The care of the preparation and the general experimental procedures were as described by Jankowska & Riddell (1994). Briefly, the blood pressure was kept above 100 mmHg, and the end tidal CO2 about 4 %, by adjusting the volume of the artificial respiration and by a continuous infusion of a bicarbonate buffer solution. The core temperature was kept at 37–38°C, and the temperature in the hindlimb oil pool between 33 and 36°C by heating lamps. A number of hindlimb nerves were dissected and stimulated either through a tunnel electrode (quadriceps (Q)) or in a mineral oil pool (separate muscle and skin branches of the sciatic nerve: posterior biceps and semitendinosus (PBST); anterior biceps and semimembranosus (ABSM); medial gastrocnemius (MG); lateral gastrocnemius and soleus (LGS); plantaris (Pl); flexor digitorum longus (FDL); deep peroneal (DP) (including anterior tibial (TA), and extensor digitorum longus (EDL)); sural (Sur); and superficial peroneal (SP)). The laminectomy exposed the spinal cord from L4 to the sacral segments, the dura was opened and the L7 and sacral dorsal roots were reflected to provide access to the lateral funiculus at the L7 and S1 levels.
Recording and stimulation
Micropipettes used for extracellular and intracellular recording were filled with 2 m NaCl and 2 m potassium citrate solution, respectively. Responses of γ-motoneurones were recorded with a high frequency cut-off at 10 kHz and digitized using a 20 or 40 μs sampling rate. Five or ten of these responses were averaged on line, in parallel with records of incoming volleys from the surface of the spinal cord, a few millimetres caudal to the recording level. Both the original records and their averages were stored for future analysis. Responses were classified as being present only if they appeared in at least 40 % of trials with the same stimulus parameters. Records of incoming volleys were obtained with one silver ball electrode in contact with the cord dorsum at the level of entry of the dorsal roots, a few millimetres caudal to the site of recording; the reference electrode was in contact with one of the back muscles. The peripheral nerves were stimulated by rectangular current pulses, 0.1 ms in duration and with an intensity expressed in multiples of threshold (T) for the most sensitive nerve fibres in a given nerve.
Criteria for attributing responses evoked by nerve stimulation to group II afferents
There is an overlap between the thresholds for the most sensitive group II afferents and the least sensitive group I afferents (stimulus intensities within the range of 1.5 to 2.5T; e.g. see Matthews, 1972; Jack, 1978), and similarly for the most sensitive group III afferents and the least sensitive group II afferents (stimulus intensities within the range of about 7 to 12T; e.g. see Eccles & Lundberg, 1959; Ellaway et al. 1982). Therefore afferents cannot be confidently attributed to one group or other using stimulus intensity as the sole criterion. However, differentiation between group II and group I input to γ-motoneurones has been assisted by the fact that single electrical stimuli below threshold for group II afferents do not, as a rule, evoke EPSPs (see Discussion), or spike discharges in anaesthetized, paralysed and considerably denervated preparations such as we have used (Eccles et al. 1960; Grillner, 1969; Kemm & Westbury, 1978; Appelberg et al. 1983a). Natural stimuli which activate both group I and other afferents, and interneuronal pathways with input from these afferents, may nevertheless have excitatory effects on γ-motoneurones in less extensively denervated decerebrate, or spinal lightly- or non-anaesthetized animals (see Fromm & Noth, 1976; Ellaway & Trott, 1976, 1978; Ellaway et al. 1982). With respect to the differentiation between group III and group II effects, we judged that the risk of contaminating synaptic actions evoked by group II afferents with those evoked by group III afferents was insignificant with stimulus intensities between 3–5T. It was considered only for some neurones which were activated by stronger stimuli (6 extracellularly recorded neurones in 2 experiments). In these cases we accepted only those spike potentials, or PSPs that were evoked at latencies precluding a contribution from afferents with conduction velocities of less than 24 m s−1 (cf. Matthews, 1972) i.e. with latencies not exceeding 3.5 ms with respect to the group I afferent volleys from proximal nerves and 5 ms from distal nerves. These were below the lower limits of latencies of responses attributed to group III afferents in other studies (e.g. 4.3 ms for PBST and ABSM, 6 ms for GS, 7 ms for FDL, in the study of Appelberg et al. 1983c).
Sampling of γ-motoneurones
γ-Motoneurones with input from group II muscle afferents were searched for primarily in the MG motor nucleus in the L7 segment but those innervating other muscles (LGS, PBST, DP, FDL) were also investigated. γ-Motoneurones were usually first recorded extracellularly and their antidromic responses were identified by the following criteria: (i) the latencies of the responses were constant when evoked by near-threshold and stronger stimuli, and by the first and later stimuli in a train; (ii) the latencies exceeded the latencies of activation of α-motoneurones by 1.5–9.2 ms and were compatible with conduction velocities of 16–49 m s−1; (iii) the stimulus thresholds exceeded the thresholds of activation for α-motoneurones (2–13 times the thresholds of group I a afferents in a given muscle nerve); (iv) collision could be demonstrated with synaptically or ‘spontaneously’ evoked responses at an appropriate critical interval (twice the latency of the antidromic responses plus about 0.7 ms, as illustrated in Fig. 1).
Figure 1. Examples of collision between antidromically and synaptically evoked responses.
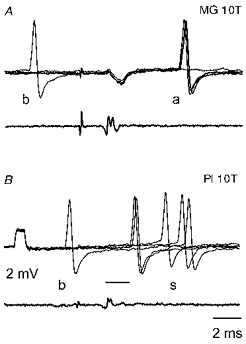
In both pairs of records the top traces are from the same MG γ-motoneurone (just before its penetration) and the bottom traces are from the surface of the spinal cord. A illustrates antidromically evoked responses (labelled a) induced by 10T stimulation of the MG nerve and shows the collision of one of these responses when a background discharge (labelled b) occurred during the critical collision interval. B shows that the appearance of synaptically evoked responses from another muscle nerve (Pl: plantaris, labelled s) was not prevented by a background discharge which occurred at an even shorter interval. The voltage calibration pulse at the beginning of the microelectrode recording in B is 2 mV. In this and in the following figures the negativity in the microelectrode records is downwards and in the cord dorsum records upwards. The intensity of the stimuli is indicated above the records in multiples of thresholds for the most sensitive fibres in the nerve. The thin line in B indicates the minimal latency of group II evoked responses. The first deflection in the cord dorsum record is the stimulus artefact, seen also in the microelectrode records. The second deflection is the afferent volley.
RESULTS
Are any synaptic actions of group II afferents on γ-motoneurones evoked monosynaptically?
When identifying group II-induced responses it has to be kept in mind that the latencies of synaptic actions of group II afferents may overlap with the latencies of antidromic activation of γ-motoneurones (because of the similar ranges of conduction velocities of group II afferents and of γ-motor axons). However, antidromically evoked responses were easily identified by their constant latency, and by collision with background discharges or responses evoked by nerve stimulation that preceded them at a critical collision interval (Fig. 1). The differentiation between monosynaptically and polysynaptically evoked responses following electrical stimulation of muscle nerves, was less straightforward. Firstly, no distinct afferent volleys in group II afferents are usually detectable because they are superimposed on group I volleys recruited at lower stimulus strengths. The latencies of all synaptically evoked responses of γ-motoneurones were therefore measured from afferent volleys in group I afferents. So in order to estimate them with respect to the later arriving group II afferent volleys, a difference in peripheral conduction times of the fastest conducting group I and II afferents had to be subtracted from the measured latencies. These had been previously defined as 0.6–0.7 ms for proximal nerves and as 0.9- 1.2 ms for distal nerves (see Edgley & Jankowska, 1987a; Riddell et al. 1995; J. Riddell & M. Hadian, unpublished observations). The second difficulty was related to the fact that the ranges in conduction velocities of the fastest and slowest group II afferents (at least 24–72 m s−1, see Matthews, 1972) are such that disynaptic actions of the fastest group II afferents might be evoked earlier than monosynaptic actions of the slowest group II afferents. Only latencies shorter than the shortest latencies of responses attributable to disynaptic actions might thus indicate monosynaptic actions of group II afferents on γ-motoneurones.
Two approaches have been used to define the borderlines between those latencies that were compatible with disynaptic or polysynaptic actions of group II afferents on γ-motoneurones, and those that were shorter, and therefore compatible with monosynaptic actions of these afferents. The first was to use the minimal reported latencies of disynaptically evoked EPSPs in α-motoneurones (see Edgley & Jankowska, 1987b; Lundberg et al. 1987a). On this basis the earliest disynaptically evoked EPSPs of group II origin should be evoked at latencies of 2.6 ms from Q, 2.5 ms from PBST and ABSM, 2.4 ms from GS and Pl, 2.5 ms from FDL and 3.0 ms from DP, all with respect to the earliest components of group I volleys. Taking into account some inconsistencies (shorter latencies from GS and Pl than from PBST and ABSM nerves despite the longer conduction distance) and measurement uncertainties, the minimal latencies for disynaptically evoked EPSPs from both proximal and distal nerves can be set at 2.5 ms, and for extracellularly recorded spike potentials at 2.7 ms. Higher values for extracellularly recorded spike potentials reflect the fact that action potentials are generated only when the depolarization of the neurones during the rising phase of the EPSP reaches the threshold level for generating the action potential, usually after at least 0.2 ms from the onset of the EPSP (see Discussion).
The second approach was to make estimates of the minimal latencies of disynaptically evoked synaptic actions of group II afferents based on the following known delays in intraspinal pathways from these afferents: conduction time along intraspinal collaterals of group II afferents (0.5–1.2 ms, depending on their length; Fu & Schomburg, 1974; Lundberg et al. 1987a), a synaptic delay at the level of an interposed interneurone (0.3 ms), delay between the onset of the EPSP and the action potential in the interneurone (0.2–0.4 ms), conduction time along the interneurone axon and its target neurones (0.3–1.4 ms depending on the distance, see Cavallari et al. 1987), and a synaptic delay in synapses between the interneurone axon terminals and γ-motoneurones (0.3 ms). The minimal values of these central delays total 1.6 ms from the arrival of group II afferent volleys. For estimates with respect to group I volleys these minimal values must thus be added to the difference in conduction times between the fastest group I and II afferents in the various nerves. The resulting estimates of these latencies are 2.2–2.3 ms for EPSPs evoked from proximal nerves (Q, PBST, SMAB) and 2.5–2.8 ms for those evoked from distal nerves (GS, Pl, FDL, DP). Minimal latencies of extracellularly recorded spikes will be 0.2 ms longer because of the time taken to reach threshold for spike generation, i.e. 2.4–2.5 ms and 2.7–3.0 ms.
The theoretically calculated estimates are close to those based on actually measured latencies for synaptic actions of group II afferents from distal nerves but are somewhat shorter for those from proximal nerves. We therefore used these theoretical estimates to separate latencies of synaptic actions compatible, or not compatible, with disynaptic actions of group II afferents: 2.2 and 2.5 ms for intracellularly recorded EPSPs evoked by stimulation of proximal and distal nerves, and 2.4 and 2.7 ms for extracellularly recorded action potentials.
An additional complication in estimating minimal latencies of extracellularly recorded responses evoked by group II afferents was that they varied, depending on the parameters of the stimuli. The second of a pair of stimuli often evoked these responses at a shorter latency than a single stimulus of a similar intensity; stronger stimuli evoked them sometimes at an even shorter latency, as illustrated in Fig. 2C and D. Different intensities of single and double stimuli were therefore used to induce the shortest latency responses, and only their minimal values were used as a measure of coupling between group II afferents and γ-motoneurones. These are plotted separately for extracellularly and intracellularly recorded neurones in Fig. 3. In view of differences between minimal latencies of disynaptically evoked actions of group II afferents from proximal and distal muscles, these data have been further subdivided, depending on the origin of the afferents.
Figure 2. Examples of records used to determine the shortest latency of the responses.
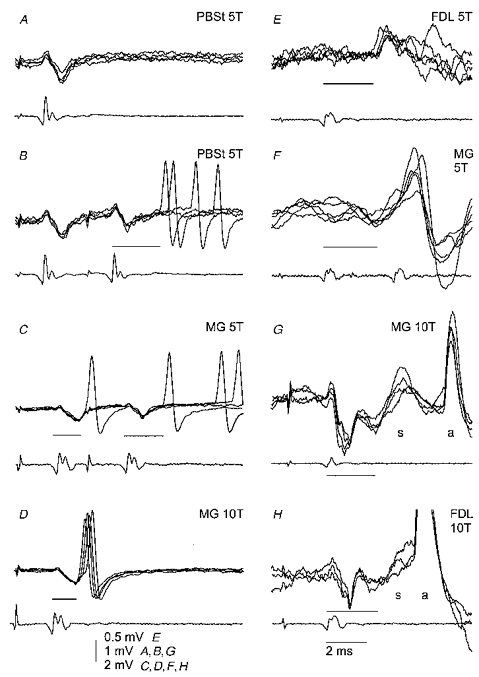
In each pair of records the top ones are 3 or 5 superimposed records from γ-motoneurones (just before penetration in A-D, intracellular in E-H), and the bottom records are from the surface of the spinal cord. In A and B, records from an MG motoneurone illustrate the situation when responses evoked by the second stimulus were used for measurements since there was no response to one stimulus. In C and D, records from another MG motoneurone (the same cell as in Fig. 1) illustrate that with an increase in stimulus intensity (from 5 to 10T) responses to the first stimulus appeared more regularly, and their latencies became shorter. In E and F, records from two MG motoneurones illustrate the occurrence of both early and later postsynaptic potentials evoked by the stimulated afferents. In G and H, records from two motoneurones (MG and FDL motoneurones) illustrate EPSPs (labelled s) evoked in homonymous γ-motoneurones by stimuli suprathreshold for their antidromic activation and therefore followed by blocked antidromic spikes (labelled a). The minimal latencies of the responses in each panel are indicated by thin lines. Other indications are as in Fig. 1.
Figure 3. The shortest latencies of responses evoked by group II afferents in γ-motoneurones.
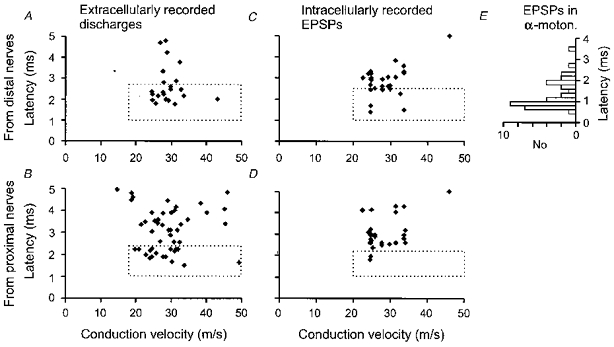
A and B, minimal central latencies of extracellularly recorded responses (ordinate) in neurones with different conduction velocities (abscissa). The latencies were measured from group I afferent volleys. C and D, as in A and B but for EPSPs evoked by group II afferents in intracellularly recorded γ-motoneurones. The dotted rectangles encompass latencies that were shorter than the estimated minimal latencies of disynaptically evoked responses (< 2.7 ms in A, < 2.4 ms in B, < 2.5 ms in C and < 2.2 ms in D). E, for comparison, the histogram of latencies of EPSPs evoked in α-motoneurones following nerve impulses in single group II afferents of triceps surae recorded close to their entry to the spinal cord, re-plotted from Fig. 1 of Stauffer et al. (1976) using 0.2 ms bins. Zero latency in E corresponds to 0.9 ms on the ordinates in A and B, to account for the 0.9 ms later arrival of incoming volleys in triceps surae group II afferents relative to group I afferents. Note that the majority of latencies of EPSPs evoked in α-motoneurones overlapped with latencies of EPSPs classified as evoked monosynaptically in γ-motoneurones.
Figure 3 shows that the latencies of some responses evoked by stimulation of group II afferents of either proximal or distal muscle nerves are shorter than the minimal expected latencies of disynaptically evoked responses. These responses can hardly have been evoked disynaptically and therefore must have been evoked monosynaptically. We have no means of estimating how many responses with longer latencies might likewise be evoked monosynaptically, but an observation reported in the next paragraph indicates that this might be the case for at least some of them.
The long duration of the compound EPSPs evoked from group II afferents and the fractionation of these EPSPs into several components, as illustrated in Fig. 2, made it more difficult than in other neurones to use the presence or absence of temporal facilitation to differentiate polysynaptically from monosynaptically evoked responses. At optimal intervals for temporal facilitation of extracellularly recorded responses (2.5 or 3.3 ms between successive stimuli), EPSPs evoked by the second stimulus were often superimposed on later components of EPSPs or IPSPs evoked by the first stimulus, and no reliable comparisons could be made of their amplitudes. However, several EPSPs like those in Fig. 4A and B showed a marked increase of synaptic actions evoked by the second stimulus. These records are typical of thirty-four EPSPs which displayed a clear-cut temporal facilitation of synaptic actions of group II afferents. In contrast, similar or smaller amplitudes of the earliest components of other EPSPs evoked by the second stimulus, and only marginal shortening or a similar latency of EPSPs evoked by the second stimulus, suggested lack of temporal facilitation. The records in Fig. 4C and D represent thirteen EPSPs in which no obvious indication of temporal facilitation was seen. Six of these were evoked at latencies longer than the calculated minimal latencies of disynaptic actions of the fastest conducting group II afferents. The tests for temporal facilitation indicate thus that group II afferents may activate some γ-motoneurones both monosynaptically and polysynaptically, and other neurones only polysynaptically.
Figure 4. Examples of EPSPs evoked by double stimuli.
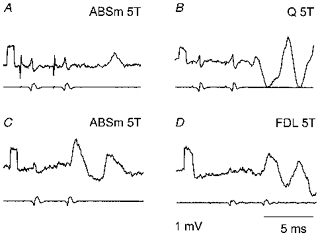
A and B, EPSPs subject to temporal facilitation of effects of two stimuli at a pre-motoneuronal level. Averaged records (n = 5) from two MG γ-motoneurones (upper traces), and records from incoming volleys from the surface of the spinal cord (lower traces). The EPSPs were evoked at latencies of 4.3 ms (A) and 4.15 ms (B) from the 2nd incoming volley. C and D, records from two other MG γ-motoneurones illustrating EPSPs which were apparently not subject to temporal facilitation. They were evoked at latencies of 2.8 and 3.4 ms (C), and of 2.5 and 2.3 ms (D). Voltage calibration pulses at the beginning of the traces: 1 mV. Time calibration in D: 5 ms for all the records. Other indications as in Fig. 1.
Responses evoked at latencies compatible with monosynaptic actions of group II afferents were found in 35 % of γ-motoneurones. The proportions of such short latencies were 22, 32, 19 and 41 % for responses evoked from Q, PBST/ABSM, GS and PL/FDL afferents, respectively. When effects of stimulation of the MG nerve were tested on MG (homonymous) γ-motoneurones, such short latency responses were found in 2 of the 43 extracellularly recorded neurones, and in 3 of the 16 intracellularly recorded neurones. In an additional two MG motoneurones longer latency (3.0 and 3.2 ms) EPSPs might also have been evoked monosynaptically since they were induced at similar latencies and/or amplitudes by the first and second of a pair of stimuli, i.e. without any obvious temporal facilitation. Group II afferents from both homonymous and heteronymous muscles might thus combine in their monosynaptic actions on γ-motoneurones.
Patterns of input from group II afferents
Sources of input from group II afferents to MG γ-motoneurones
Group II afferents of the homonymous muscle were found to activate nearly one-third (13/43) of extracellularly recorded MG γ-motoneurones, and to evoke EPSPs in nearly one-half (7/16) of intracellularly recorded ones. Many MG γ-motoneurones investigated in this study, similarly to those previously investigated by Appelberg et al. (1983b), were also excited by group II afferents from other muscles. Some of the extracellularly recorded γ-motoneurones responded to stimulation of group II afferents of more than one nerve, although about one-fifth did not respond to any stimuli. The ease with which γ-motoneurones were activated in different experiments most likely depended upon the excitability of the preparation. It was not related to their conduction velocity, since cells responding to stimulation of the largest numbers of muscle nerves could have an axon conducting at a low or high velocity. In the most excitable preparations, extracellularly recorded responses of a considerable number (39 %) of MG γ-motoneurones were evoked by stimulation of three to seven nerves; the proportion of intracellularly recorded neurones in which EPSPs were evoked from group II afferents from several nerves was even larger (83 %). The numbers of muscles of origin of group II afferents contributing to excitation of MG γ-motoneurones may in fact exceed the indicated numbers of muscle nerves since most of the nerves tested (Q, PBST, MG, LGS, ABSM, PL, FDL, in some experiments DP) included nerve branches from more than one muscle or muscle head. In addition, considering that a large proportion of neurones tested responded to all nerves tested (17 % of intracellularly recorded neurones), it seems unlikely that we chose the maximum number of nerves that contribute group II excitation to MG γ-motoneurones by chance.
Despite the large number of muscles of origin of group II afferents affecting MG γ-motoneurones, the preferred excitatory input turned out to be from a much more restricted selection of muscles. Afferents of the PBST nerve activated a considerably larger proportion of the least responsive extracellularly recorded MG γ-motoneurones (those responding to stimulation of group II afferents of only 1 or 2 nerves, Fig. 5B) than group II afferents of other nerves. A similar pattern of excitatory input from group II afferents, based on the origin of EPSPs, emerged for intracellularly recorded MG γ-motoneurones (Fig. 5C and D), although all the most excitable neurones (Fig. 5A) were activated by both PBST and FDL nerves. Group II afferents of the PBST muscle appear thus to be the source of the strongest input to MG γ-motoneurones.
Figure 5. Muscles of origin of group II afferents providing excitatory input to MG γ-motoneurones.
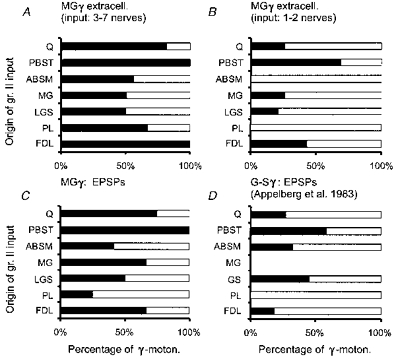
A and B, percentages of extracellularly recorded MG γ-motoneurones responding to stimulation of muscle nerves as indicated, in two populations of γ-motoneurones, those responding to stimulation of 3 or more muscle nerves (17 motoneurones) or of only 1–2 nerves (19 motoneurones). C and D, comparison of the origin of group II EPSPs for the present sample of 14 intracellularly recorded MG γ-motoneurones with data for 36 unidentified triceps surae γ-motoneurones. (D, re-plotted from Fig. 5 in Appelberg et al. 1983b). ▪, activated; □, not activated.
Comparison of sources of group II input to γ-motoneurones of five hindlimb muscles
A comparison of patterns of origin of the most potent excitatory input from group II afferents to various γ-motoneurones indicates that they differ considerably. As shown in Fig. 6, only MG γ-motoneurones were activated by afferents of more than three nerves, and LGS, FDL, PBST and DP γ-motoneurones were preferentially activated by afferents of different nerves: Q and PBST (for LGS γ-motoneurones), PBST and ABSM (for FDL γ-motoneurones), and Q and LGS (for PBST and DP γ-motoneurones). More specific patterns of actions of group II afferents on γ-motoneurones than expected on the basis of previous studies are in particular indicated by a comparison of group II input from two extensors (Q and FDL), or two flexors (PBST and DP) which appear to have largely different target γ-motoneurones. Another difference is suggested by an asymmetry in connections between group II afferents of even closer synergists, since LGS afferents activated both LGS and MG γ-motoneurones, while MG afferents were found to activate only MG γ-motoneurones.
Figure 6. Muscles of origin of group II afferents providing input to different populations of γ-motoneurones.
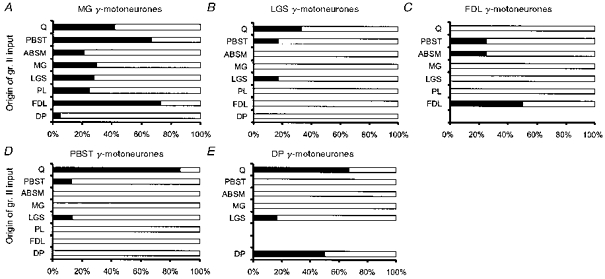
Percentages of 43 MG (A), 6 LGS (B), 3 FDL (C) 15 PBST (D) and 6 DP (E) extracellularly recorded γ-motoneurones responding to stimulation of group II afferents from the nerves indicated on the ordinate. ▪, activation by homonymous afferents or other afferents; □, no activation.
DISCUSSION
Coupling between group II muscle afferents and γ-motoneurones
The results of the present study lead to the conclusion that excitation of γ-motoneurones by group II afferents is mediated not only via interneurones but also through synaptic contacts between these afferents and γ-motoneurones. This conclusion is at variance with that of Appelberg et al. (1983b, p 270), according to whom even the shortest latency actions of group II afferents on γ-motoneurones would be mediated via oligosynaptic pathways. We wish, however, to stress that the latencies of the responses evoked by group II muscle afferents in the majority of γ-motoneurones of our sample, and of that of Appelberg et al. (1983b), fall within the same range, and that the latencies of some extracellularly recorded responses in the present study were shortened by using double, or stronger stimuli. Appelberg et al. (1983b, their Fig. 8 and Table 1) gave the central latencies of responses evoked by group II afferents as 1.6–1.7 ms, calculated with respect to incoming volleys of group II afferents. By adding to these values the minimal differences in the conduction times between group I and group II afferents along peripheral nerves (see Edgley & Jankowska, 1987a; Riddell et al. 1995; J. Riddell & M. Hadian, unpublished observations; and Results) one can reconstruct them with respect to the group I incoming volleys from which they were originally measured, and directly compare them with our data. Their minimal latencies then become 2.5 ms (i.e. 1.6 + 0.9 ms) for GS, and 2.4 ms (i.e. 1.7 + 0.7 ms) for PBST. These revised minimal latencies fall within the range of latencies (1.4–2.7 ms, boxed in Fig. 3) which we consider to be compatible with monosynaptic actions. There is thus no major discrepancy in the experimental data, and what remains to be explained is why we reached a different conclusion. Appelberg et al. (1983b) related the latencies of the responses they recorded in their experiments to the minimal expected latencies of monosynaptically evoked responses - since the recorded latencies turned out to be longer than the minimal theoretical latencies of monosynaptic responses, the responses were classified as being evoked disynaptically. In contrast to Appelberg et al. (1983b), we used as a reference the minimal latencies of disynaptically evoked responses. Since the latencies of responses evoked in a number of γ-motoneurones were shorter than the minimal latencies of disynaptically evoked responses we conclude that these are compatible with latencies of monosynaptically evoked responses. In the following we will evaluate some of the arguments for and against this conclusion in more detail.
Estimates of latencies of disynaptically evoked responses
We made two estimates of the expected minimal latencies of disynaptically evoked actions of group II afferents, firstly for intracellularly recorded EPSPs and secondly for extracellularly recorded action potentials, taking into account an additional delay of 0.2 ms for the induction of action potentials after the onset of EPSPs. The delays with which action potentials are induced in γ-motoneurones may in fact be even longer, taking into account that the rising phases of EPSPs evoked by group II muscle afferents in γ-motoneurones often appear to be slower than in other neurones in which such delays were found, and the times to peak are considerably longer (cf. Fig. 1 in Appelberg et al. 1983b, or in Fig. 2F–H in the present paper with Figs 3, 4, 6 and 8 in Jankowska & Riddell, 1994). When the latencies of extracellularly recorded action potentials were compared with the latencies of EPSPs evoked in the same γ-motoneurones after penetration, there were differences of 0.25–0.8 ms in about two thirds of the neurones, and even up to 2 ms in the remaining ones. For this reason we compared the latencies of action potentials and EPSPs separately with the expected minimal latencies of disynaptically evoked responses. In the data of Appelberg et al. (1983b), the latencies of the intra- and extracellularly recorded responses were pooled together, and cannot therefore be related to the minimal estimates for disynaptically evoked intracellularly or extracellularly recorded responses with more than a 0.2 ms confidence.
Comparison of latencies of responses of γ-motoneurones and of other neurones
There is an overlap between the range of latencies of those synaptic actions of group II muscle afferents that do not exceed the minimal estimates of disynaptically evoked responses, and the range of latencies of population EPSPs (field potentials) evoked by group II muscle afferents in the intermediate zone of caudal lumbar segments, i.e. in the closest neighbourhood of the γ-motoneurones. The latencies for the field potentials were 1.90–3.35 ms for Q, 2.10- 2.95 ms for PBST and ABSM and 2.40–3.35 ms for GS, PL and FDL (J. Riddell & M. Hadian, unpublished observations). The latencies of EPSPs and spike potentials evoked in interneurones from group II afferents of the same muscles varied, depending on their location. Interneurones located in the dorsal horn were activated at shorter latencies than those in the intermediate zone (see Edgley & Jankowska, 1987a) and in segments further away from the level of entry of the afferents. For interneurones located in the intermediate zone of caudal lumbar segments they ranged between about 1.4 and 2.5 ms (J. Riddell & M. Hadian, unpublished observations). Therefore, considering that the pathway to γ-motoneurones is slightly longer, values of 1.4–2.7 ms for latencies in γ-motoneurones seem reasonable.
There is also a good agreement between the latencies of group II actions on MG γ-motoneurones and the latencies of EPSPs evoked by single GS group II afferents in α-motoneurones (Stauffer et al. 1976; Munson et al. 1980), most of which were considered to be evoked monosynaptically. The latencies quoted in these studies were measured from the moment of arrival of nerve impulses in group II afferents at the spinal cord to the detection of EPSPs in α-motoneurones. In order to compare our latencies with these, 0.9 ms must be added, because our latencies were measured from the arrival of impulses in the fastest group I axons - for triceps surae the group II afferents arrive 0.9 ms later. Therefore the latencies for the earliest group of EPSPs evoked in α-motoneurones (0.8–1.7 ms, Fig. 3E) correspond to 1.7–2.6 ms latencies for EPSPs in γ-motoneurones in our experiments. Direct actions of group II afferents should thus contribute to at least the shortest latencies of activation of γ-motoneurones by these afferents.
In our estimates of the number of instances of monosynaptic coupling between group II muscle afferents and γ-motoneurones we are constrained because we are obliged to consider coupling between only the fastest group II afferents and γ-motoneurones. Monosynaptically evoked synaptic actions of the slowest conducting group II afferents may be induced later than disynaptically evoked actions of the fastest conducting ones. Furthermore, synaptic delays in synapses between group II afferents and γ-motoneurones might be longer than the 0.3 ms used in our estimates, since they were found to be 0.61 ± 0.03 and 0.47 ± 0.04 ms (mean ±s.d.) in synapses with α-motoneurones for two sets of group II afferents (Munson et al. 1980). For these reasons it is likely that we have underestimated rather than overestimated the proportions of γ-motoneurones in which synaptic actions of group II afferents might have been evoked monosynaptically. However, even if the proportion of such neurones is relatively large, monosynaptically evoked EPSPs might represent only a small fraction of the excitatory group II input to γ-motoneurones, since short latency components of EPSPs were as a rule followed by later components (see Fig. 2E–H), and/or IPSPs (see Fig. 4B–D). Furthermore, longer latency EPSPs were often evoked in the same γ-motoneurones from other nerves.
Because of the complexity of synaptic actions evoked by group II afferents in γ-motoneurones, the conditions for testing temporal facilitation of EPSPs evoked by these afferents are less favourable than in previously investigated neurones. When the temporal facilitation was marked, it could demonstrate polysynaptic actions. However, when it appeared to be lacking, the comparisons of EPSPs evoked by the first and the second stimulus were not sufficiently reliable to allow definite conclusions. We can therefore only state that we have not found any indication of an increase in the amplitude or of a shortening of the latency of responses evoked by the second stimulus in some γ-motoneurones, and that these observations support the possibility of monosynaptic actions of group II afferents.
Morphological studies
So far there is no morphological evidence of direct contacts between group II muscle afferents and γ-motoneurones since terminal branching of their axon collaterals in motor nuclei (Fyffe, 1979; Hongo, 1992) was detected only close to the cell bodies of large lamina IX neurones (T. Hongo and N. Ishizuka, personal communication). However, contacts between group II afferents and γ-motoneurones would be expected not only in view of the present observations. They could also explain why there are only very small compound monosynaptic EPSPs of group II origin in α-motoneurones (Lundberg et al. 1977, 1987b) despite the large numbers of terminals of group II afferents in motor nuclei (Hongo, 1992). However, such contacts might be expected primarily with γ-motoneurones of homonymous muscles, or with other γ-motoneurones located at the same segmental levels, since group II afferents were found to project to motor nuclei only at the level of location of homonymous motoneurones (Hongo, 1992).
How confidently can synaptic actions of group II afferents on γ-motoneurones be differentiated from those of group I or group III afferents?
Previous studies failed to detect any synaptic actions of group I afferents on γ-motoneurones following electrical stimulation of peripheral nerves with single stimuli at intensities which did not exceed threshold for group II afferents (Eccles et al. 1960; Grillner, 1969), or revealed them only exceptionally (in less than 5 % of the tested neurones; Kemm & Westbury, 1978; Appelberg et al. 1983a). Of the three EPSPs attributable to group I afferents in the latter study, illustrated in their Fig. 5, one was evoked with a stimulus intensity at which some group II afferents might be affected. The threshold of the second one was not given but its very short latency (0.3 ms) was in keeping with group I rather than group II evoked actions, and both the threshold and the latency of the third EPSP were as expected for those evoked from I a afferents. Since indications for monosynaptically evoked EPSPs of group I origin were found in so few γ-motoneurones one might attribute them to some aberrant connections of group I afferents. However, the latter two EPSPs had larger amplitudes (3–5 mV) than expected for aberrant connections, and their time course resembled typical I a EPSPs evoked by the synchronous actions of a considerable number of I a afferents in homonymous α-motoneurones. One might wonder, therefore, whether the neurones in which they were recorded were not unusually slowly conducting α- or β- rather than γ-motoneurones neurones; the slowing of their conductance velocities could have been caused by damage and regeneration of their axons (see e.g. Waldeck et al. 1995). However, this question cannot be answered a posteriori, and no information was given on whether input from any other nerves to these neurones was more characteristic of γ- or α-motoneurones. If they were exceptionally slowly conducting α- or β-motoneurones, these isolated instances of group I excitatory effects reported by Appelberg et al. (1983b) would not contradict results of previous studies. Our working hypothesis has therefore been that the probability of group I-evoked excitation of γ-motoneurones by single or double electrical stimuli is negligible. Our own data did not reveal any intracellularly or extracellularly recorded responses at latencies compatible with monosynaptically evoked actions of group I afferents such as occurred in the sample of Appelberg et al. (1983b). Furthermore, to activate the γ-motoneurones that were recorded extracellularly in the present sample in 40–60 % of trials required stimulus strengths of at least 3–5T. Despite the overlap in thresholds for group I and group II afferents (within the range of about 1.5–2.5T), the reported effects of stimuli of 5T or less should thus be confidently attributable to group II afferents.
The overlap between the thresholds for group II and group III afferents to electrical stimuli (see e.g. Eccles & Lundberg, 1959; Boyd & Kalu, 1979; Ellaway et al. 1982), and the fact that both groups provide input to γ-motoneurones (see e.g. Appelberg et al. 1983c; Ellaway et al. 1982), required special measures to allow us to differentiate between their effects. These involved a combination of criteria as described in the Methods. The responses of the great majority of γ-motoneurones were evoked by stimuli that did not exceed 5T. Those which required stronger stimuli (responses evoked in just 6 of the extracellularly recorded neurones required 7–10T) were accepted only when their latencies did not exceed 3.5 ms when evoked from proximal muscles, and 5.0 ms from distal muscles. It is thus unlikely that group III afferents contributed to these responses in a decisive way, and certainly not to their earliest components.
Integration of information from group II afferents by γ-motoneurones
The present study provides information about the convergence of group II afferents of a variety of muscles to γ-motoneurones of a single muscle, the medial gastrocnemius, while most previous studies have pooled together information about the input to γ-motoneurones of the several heads of triceps surae. The sample of γ-motoneurones investigated in this study ought to be representative of the whole population of medial gastrocnemius γ-motoneurones because the range of conduction velocities of their axons (19- 49 m s−1) indicates that these cells were not selected by size. The 50–60 % that were excited by homonymous group II afferents were usually also excited by group II afferents from close agonists (LGS), and from three to five of the other muscles tested, at least one from the upper leg (Fig. 4). The pattern of input to MG γ-motoneurones of our sample greatly resembles that for unidentified triceps surae γ-motoneurones, which includes MG γ-motoneurones, in the sample of Appelberg et al. (1983b) after their data were re-plotted (cf. Fig. 4C and D).
The patterns of group II input to other γ-motoneurone pools suggest that they are likewise excited by both homonymous and heteronymous group II afferents, although the sources of the heteronymous input to some of these were much more restricted. Comparison of the muscles of origin of group II input to a number of γ-motoneurone pools (Fig. 5) suggests in addition that each pool might be involved in integrating information from muscle spindles of different combinations of muscles. It is impossible to gauge how far the selectivity of the group II excitatory inputs to the various motoneurone pools recorded in the present experiments reflects the situation in the normal, non-anaesthetized spinal cord. On the one hand the excitability of γ-motoneurones was lowered by anaesthesia and the widespread denervation, and on the other the synchronous volleys recruiting all or most group II afferents would provide an abnormally strong activation. Nevertheless it is interesting to note that there is a striking convergence on the MG γ-motoneurone pool, yet MG group II afferents excite only homonymously, whereas there is far less convergence on LGS γ-motoneurones, and LGS group II afferents excite even antagonist DP and hamstring γ-motoneurones (Fig. 6). The sources of group II excitation for FDL and PBST γ-motoneurones, and the γ-motoneurone pools exited by their group II afferents, also appear to be distinct. One should therefore be cautious in generalizing conclusions on input to γ-motoneurones based on observations from one muscle, and in pooling together observations from their different populations.
Positive feedback to γ-motoneurones
Feedback from homonymous afferents
Excitatory actions of group II afferents of an homonymous muscle were demonstrated here for actions of MG afferents on γ-motoneurones innervating the MG muscle. In the case of other γ-motoneurones in this as well as in most previous studies, they could have been excited by group II afferents of the same muscle or of a close synergist because individual muscle nerves were combined for stimulation. When the nerves to the synergists PB and ST, to LG and S and to TA and EDL were stimulated jointly, the γ-motoneurones whose responses were recorded might have innervated one of them, while they were excited by group II afferents of the other. However, previous observations on γ-motoneurones innervating the tenuissimus muscle showed that they are activated following stretch of this muscle, and demonstrated not only effects of homonymous afferents, but also that in this case the afferents were of muscle spindle origin (Gladden et al. 1995).
Monosynaptic connections between group II afferents and homonymous γ-motoneurones would provide a direct route, in addition to the indirect route via interneuronal pathways, for enhancing excitatory input to γ-motoneurones during muscle contractions, whenever nerve impulses are initiated in the secondaries (see Prochazka, 1996). These monosynaptic connections will thereby assist the co-activation of γ- and α-motoneurones and counteract the unloading of muscle spindles (Lundberg et al. 1987b). Interneurones mediating excitation of γ-motoneurones by group II afferents would most likely facilitate responses of γ-motoneurones of a greater number of muscles since these interneurones project to several motor nuclei in several segments (Bras et al. 1989). A high proportion of direct connections between the group II afferents of a given muscle and γ-motoneurones innervating this muscle might thus strengthen the positive feedback to these particular γ-motoneurones.
Excitation of extensors via group II afferents and γ-motoneurones
The excitation of a considerable proportion of MG, LGS and FDL γ-motoneurones by group II afferents found both in the present study and in previous studies (Ellaway & Trott, 1976; Noth & Thilmann, 1980; Appelberg et al. 1983b) requires a particular comment. It suggests namely that group II afferents might evoke a not-negligible excitation of extensor motoneurones secondarily to activation of γ-motoneurones and muscle spindle primaries, although the dominating pattern of reflex actions of group II afferents is excitation of flexors and inhibition of extensors (Eccles & Lundberg, 1959). The excitation might be particularly marked when γ-motoneurones are most easily activated, e.g. during α-γ- co-activation (see Lundberg et al. 1987b), or on the background of a stronger output from γ-motoneurones following their activation by other neurones. It could therefore be associated with tonic as well as phasic activation of γ-motoneurones, and expressed in both a long-lasting and phasic depolarization of α-motoneurones. So far only phasic activation of extensor motoneurones has been demonstrated in humans either by stretch (Corna et al. 1995) or by electrical stimulation of muscle afferents (Marque et al. 1996).
Positive feedback via static or dynamic γ-motoneurones?
Noth (1981) pointed out that muscle stretch would initiate positive feedback if homonymous spindle secondary afferents were to excite static γ-motoneurones. This is because static γ-motoneurones can activate secondary endings as well as primary sensory endings, while excitatory actions of dynamic γ-motoneurones would mainly affect the primaries. This conclusion was in keeping with observations on stretch-evoked activation of γ-motoneurones made while recording from filaments of peripheral nerves in decerebrate preparations (Fromm & Noth, 1976; Ellaway & Trott, 1978); in such preparations tonically active γ-motoneurones would be more likely to be static than dynamic (see next section).
Appelberg et al. (1983b) emphasized the excitation of dynamic γ-motoneurones by group II afferents, but a proportion of the cells they identified as static were also excited. Neither Noth's (Noth & Thilmann, 1980; Noth, 1981) nor our data fit with a selective, or even preferential excitation of dynamic γ-motoneurones by group II afferents. This is because high proportions of both Noth's and our cell samples were activated, and it is unlikely that these samples were biased in favour of dynamic γ-motoneurones. Furthermore, in the present study, EPSPs were evoked from homonymous group II afferents in at least 63 % of intracellularly recorded MG γ-motoneurones, yet dynamic γ-axons are normally in a minority (see, for example, data for tenuissimus and peroneals in Dickson et al. 1993). Other circumstantial evidence is that stretching the tenuissimus and other hindlimb muscles causes reflex contraction of the static intrafusal fibres (bag2 and chain fibres) in tenuissimus spindles, whereas vibration which would recruit I a afferents had no effect (Gladden et al. 1995). Furthermore, low conduction velocity static γ-axons are more likely to innervate chain fibres (Emonet-Dénand & Gladden, 1993; and F. Emonet-Dénand & M. H. Gladden, unpublished observations) which provide the strongest input to secondary endings (Boyd, 1981), and we found that group II afferents of homonymous muscles provided input to cells with the slowest conducting axons as well as to cells with higher conduction velocities.
How strong is the positive feedback via group II afferents and γ-motoneurones?
Under our experimental conditions the strength of group II input was not very high, because effective activation of γ-motoneurones required near-maximal activation of group II afferents by either single or double stimuli. Furthermore, intracellular records show that the peak amplitudes of EPSPs evoked by these afferents are not very high (Eccles et al. 1960; Appelberg et al. 1983b). However, there is ample evidence of their effectiveness in other preparations (see Introduction). Furthermore, even weak synaptic actions of group II afferents may tip the balance when the background excitation is provided by other kinds of input to γ-motoneurones. As pointed out by Lundberg et al. (1987c), this kind of synaptic action provides ample possibilities for modulation of their effectiveness in both directions, by either enhancing or weakening them.
Some interneurones in the excitatory pathways from group II afferents to γ-motoneurones will be co-excited by I a afferents (see discussion of this point in the companion paper (Jankowska et al. 1998), so that during muscle stretch the two groups of afferents will jointly induce discharges in these interneurones. Under various experimental conditions the contribution of group I afferents to polysynaptically evoked effects of strong stimuli may be either negligible or much weaker, and the same may be true for the contribution of group II afferents to effects of weak stimuli (either electrical, or relatively small muscle stretches or vibration). However, these weak or negligible effects may be enhanced by the use of repetitive stimuli (e.g. by prolonging muscle stretches or vibration, or by applying tests when the background afferent activity is high). Taking into account the shared interneuronal pathways of group I and group II afferents one might in fact question whether it is possible to attribute polysynaptic (but not monosynaptic) actions evoked by these afferents to only one or other of these afferents.
Under conditions of abnormally increased gain, the positive feedback via monosynaptic pathways from group II afferents to γ-motoneurones and via polysynaptic pathways from both group I and II afferents to these neurones might get out of control. A pathological enhancement of reflex actions of not only group II but also of group I a muscle afferents might then occur because stronger actions of γ-motoneurones on muscle spindles would be followed by stronger responses of both primaries and secondaries. Excessive positive feedback might thus contribute to the exaggeration of stretch reflexes under various pathological conditions, e.g. in spastic or parkinsonian patients. Weakening the feedback, by interfering with actions of γ-motoneurones on muscle spindles and/or of group II afferents on γ-motoneurones and spinal interneurones, should then abolish exaggerated stretch reflexes. Such an effect has indeed been reported after a local anaesthetic block sufficient to paralyse both γ-motor axons and at least the smallest group II afferents (see Rushworth, 1960; Dietrichson, 1971). Potent modulatory actions of monoamines in either depressing (NA, tizanidine, clonidine; for references see the companion paper (Jankowska et al. 1998)) or enhancing (5-HT; see Ellaway & Trott, 1975) activation of γ-motoneurones by group II afferents may therefore be one of the important means of pre-setting their mode of operation.
Acknowledgments
The study was supported by grants from the Swedish Medical Research Council (no. 05648) to E.J., from the Wellcome Trust to M.H.G. and from Göteborg University to J.C.B. Our warmest thanks are due to Mrs R. Larsson for her assistance both in the experiments and in the preparation of the illustrations.
References
- Appelberg B, Hulliger M, Johansson H, Sojka P. Fusimotor reflexes in triceps surae elicited by natural stimulation of muscle afferents from the cat ipsilateral hind limb. The Journal of Physiology. 1982;329:211–229. doi: 10.1113/jphysiol.1982.sp014299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B, Hulliger M, Johansson H, Sojka P. Actions on γ-motoneurones elicited by electrical stimulation of group I muscle afferent fibres in the hind limb of the cat. The Journal of Physiology. 1983a;335:237–253. doi: 10.1113/jphysiol.1983.sp014531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B, Hulliger M, Johansson H, Sojka P. Actions on γ-motoneurones elicited by electrical stimulation of group II muscle afferent fibres in the hind limb of the cat. The Journal of Physiology. 1983b;335:255–273. doi: 10.1113/jphysiol.1983.sp014532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B, Hulliger M, Johansson H, Sojka P. Actions on γ-motoneurones elicited by electrical stimulation of group III muscle afferent fibres in the hind limb of the cat. The Journal of Physiology. 1983c;335:275–292. doi: 10.1113/jphysiol.1983.sp014533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd I. The action of the three types of intrafusal fibre in isolated cat muscle spindles on the dynamic and length sensitivities of primary and secondary sensory endings. In: Taylor A, Prochazka A, editors. Muscle Receptors and Movement. London: Macmillan; 1981. pp. 17–32. [Google Scholar]
- Boyd I, Kalu K. Scaling factor relating conduction velocity and diameter for myelinated afferent nerve fibres in the cat hind limb. The Journal of Physiology. 1979;289:277–297. doi: 10.1113/jphysiol.1979.sp012737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bras H, Cavallari P, Jankowska E, Kubin L. Morphology of midlumbar interneurones relaying information from group II muscle afferents in the cat spinal cord. Journal of Comparative Neurology. 1989;290:1–15. doi: 10.1002/cne.902900102. [DOI] [PubMed] [Google Scholar]
- Cavallari P, Edgley SA, Jankowska E. Post-synaptic actions of midlumbar interneurones on motoneurones of hind-limb muscles in the cat. The Journal of Physiology. 1987;389:675–689. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corna S, Grasso M, Nardone A, Schieppati M. Selective depression of medium-latency leg and foot muscle responses to stretch by an α2-agonist in humans. The Journal of Physiology. 1995;484:803–809. doi: 10.1113/jphysiol.1995.sp020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson M, Emonet Denand F, Gladden MH, Petit J, Ward J. Incidence of non-driving excitation of I a afferents during ramp frequency stimulation of static γ-axons in cat hindlimbs. The Journal of Physiology. 1993;460:657–673. doi: 10.1113/jphysiol.1993.sp019492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrichson P. Phasic ankle reflex in spasticity and Parkinsonian rigidity. The role of the fusimotor system. Acta Neurologica Scandinavica. 1971;47:22–51. doi: 10.1111/j.1600-0404.1971.tb07462.x. [DOI] [PubMed] [Google Scholar]
- Eccles J, Eccles R, Iggo A, Lundberg A. Electrophysiological studies on gamma motoneurones. Acta Physiologica Scandinavica. 1960;50:32–40. doi: 10.1111/j.1748-1716.1960.tb02070.x. [DOI] [PubMed] [Google Scholar]
- Eccles R, Lundberg A. Synaptic actions in motoneurones by afferents which may evoke the flexion reflex. Archives Italiennes de Biologie. 1959;97:199–221. [Google Scholar]
- Edgley SA, Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. The Journal of Physiology. 1987a;385:393–413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. The Journal of Physiology. 1987b;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH, Murphy PR, Tripathi A. Closely coupled excitation of γ-motoneurones by group III muscle afferents with low mechanical threshold in the cat. The Journal of Physiology. 1982;331:481–498. doi: 10.1113/jphysiol.1982.sp014385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH, Trott JR. The mode of action of 5-hydroxytryptophan in facilitating a stretch reflex in the spinal cat. Experimental Brain Research. 1975;22:145–162. doi: 10.1007/BF00237685. [DOI] [PubMed] [Google Scholar]
- Ellaway PH, Trott JR. Reflex connections form muscle stretch receptors to their own fusimotor neurones. Progress in Brain Research. 1976;44:113–122. doi: 10.1016/S0079-6123(08)60727-X. [DOI] [PubMed] [Google Scholar]
- Ellaway PH, Trott JR. Autogenetic reflex action on to gamma motoneurones by stretch of triceps surae in the decerebrated cat. The Journal of Physiology. 1978;276:49–66. doi: 10.1113/jphysiol.1978.sp012219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonet-Dénand F, Gladden M. Type of fusimotor excitation induced in individual spindles by their fastest-conducting gamma axons. XXXII Congress of the International Union of Physiological Sciences. 1993:17.6. [Google Scholar]
- Fromm C, Noth J. Reflex responses of gamma motoneurones to vibration of the muscle they innervate. The Journal of Physiology. 1976;256:117–136. doi: 10.1113/jphysiol.1976.sp011315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu TC, Schomburg ED. Electrophysiological investigation of the projection of secondary muscle spindle afferents in the cat spinal cord. Acta Physiologica Scandinavica. 1974;91:314–329. doi: 10.1111/j.1748-1716.1974.tb05687.x. [DOI] [PubMed] [Google Scholar]
- Fyffe R. The morphology of group II muscle afferent fibre collaterals. The Journal of Physiology. 1979;296:39–40P. [PubMed] [Google Scholar]
- Gladden M, Dickson M, Lumsdon T. Reflex activation of γs- and γd-motoneurones observed in isolated muscle spindles of cat hindlimb muscles. In: Taylor A, Gladden MH, Durbaba R, editors. Alpha and Gamma Motor System. New York: Plenum Press; 1995. pp. 129–136. [Google Scholar]
- Gladden M, Jankowska E, Czarkowska-Bauch J. Differences in input from group II muscle afferents to γ-motoneurones of five hindlimb muscles of cats. The Journal of Physiology. 1997;505.P:76–77P. [Google Scholar]
- Grillner S. Supraspinal and segmental control of static and dynamic gamma-motoneurones in the cat. Acta Physiologica Scandinavica. 1969;(suppl. 327):1–34. [PubMed] [Google Scholar]
- Hongo T. Patterns of spinal projection of muscle spindle group II fibres. In: Jami L, Pierrot-Deseilligny E, Zytnicki D, editors. Muscle Afferents and Spinal Control of Movement. Oxford, New York, Seoul, Tokyo: Pergamon Press; 1992. pp. 389–394. [Google Scholar]
- Jack J. Some methods for selective activation of muscle afferent fibres. In: Porter R, editor. Studies in Neurophysiology. Cambridge: Cambridge University Press; 1978. pp. 155–176. [Google Scholar]
- Jankowska E, Lundberg A, Rudomin P, Sykova E. Effects of 4-aminopyridine on synaptic transmission in the cat spinal cord. Brain Research. 1982;240:117–129. doi: 10.1016/0006-8993(82)90649-7. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Gladden MH, Czarkowska-Bauch J. Modulation of responses of feline γ-motoneurones by noradrenaline, tizanidine and clonidine. The Journal of Physiology. 1998;512:521–531. doi: 10.1111/j.1469-7793.1998.521be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Riddell JS. Interneurones in pathways from group II muscle afferents in sacral segments of the feline spinal cord. The Journal of Physiology. 1994;475:455–468. doi: 10.1113/jphysiol.1994.sp020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemm RE, Westbury DR. Some properties of spinal γ-motoneurones in the cat, determined by microelectrode recording. The Journal of Physiology. 1978;282:59–71. doi: 10.1113/jphysiol.1978.sp012448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Comments on reflex actions evoked by electrical stimulation of group II muscle afferents. Brain Research. 1977;122:551–555. doi: 10.1016/0006-8993(77)90466-8. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 1. Distribution and linkage of reflex actions to alpha-motoneurones. Experimental Brain Research. 1987a;65:271–281. doi: 10.1007/BF00236299. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 2. Functional characteristics of reflex pathways to alpha-motoneurones. Experimental Brain Research. 1987b;65:282–293. doi: 10.1007/BF00236300. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 3. Secondary spindle afferents and the FRA: a new hypothesis. Experimental Brain Research. 1987c;65:294–306. doi: 10.1007/BF00236301. [DOI] [PubMed] [Google Scholar]
- Marque P, Pierrot-Deseilligny E, Simonetta-Moreau M. Evidence for excitation of the human lower limb motoneurones by group II muscle afferents. Experimental Brain Research. 1996;109:357–360. doi: 10.1007/BF00231793. [DOI] [PubMed] [Google Scholar]
- Matthews P. Mammalian Muscle Spindles and their Central Action. London: Arnold; 1972. pp. 1–630. [Google Scholar]
- Munson JB, Fleshman JW, Sypert GW. Properties of single-fiber spindle group II EPSPs in triceps surae motoneurons. Journal of Neurophysiology. 1980;44:713–725. doi: 10.1152/jn.1980.44.4.713. [DOI] [PubMed] [Google Scholar]
- Noth J. Autogenetic and antagonistic group II effects on extensor gamma motoneurons of the decerebrate cat. In: Taylor A, Prochazka A, editors. Muscle Receptors and Movement. London: MacMillan; 1981. pp. 207–213. [Google Scholar]
- Noth J, Thilmann A. Autogenetic excitation of extensor gamma-motoneurones by group II muscle afferents in the cat. Neuroscience Letters. 1980;17:23–26. doi: 10.1016/0304-3940(80)90055-5. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Proprioceptive feedback and movement regulation. In: Rowell LB, Sheperd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: American Physiological Society; 1996. pp. 89–127. [Google Scholar]
- Rushworth G. Spasticity and rigidity: an experimental study and review. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:99–117. doi: 10.1136/jnnp.23.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer EK, Watt DG, Taylor A, Reinking RM, Stuart DG. Analysis of muscle receptor connections by spike-triggered averaging. 2. Spindle group II afferents. Journal of Neurophysiology. 1976;39:1393–1402. doi: 10.1152/jn.1976.39.6.1393. [DOI] [PubMed] [Google Scholar]
- Waldeck RF, Murphy EH, Pinter MJ. Properties of motor units after self reinnervation of the cat superior oblique muscle. Journal of Neurophysiology. 1995;74:2309–2318. doi: 10.1152/jn.1995.74.6.2309. [DOI] [PubMed] [Google Scholar]


