Abstract
We have studied acid/base transport across the cell membrane of the giant neuropile glial cell in the leech (Hirudo medicinalis) central nervous system induced by changing the external pH (pHo), using double-barrelled, pH-sensitive microelectrodes. In the presence of 5 % CO2 and 24 mm HCO3−, the intracellular pH (pHi) rapidly changes due to a potent, reversible Na+-HCO3− cotransport across the glial membrane. We have now investigated the transport mechanism which leads to pHi changes in the nominal absence of CO2/HCO3−, where the HCO3− concentration is expected to be below 1 mm.
The intracellular pH increased and then decreased when pHo was altered from 7.4 to 7.8 and then 7.0 with a rate of increase of +0.026 ± 0.008 and a rate of decrease of −0.028 ± 0.009 pH units min−1 (± s.d., n = 49), indicating an acid/base flux rate of 0.64 and 0.71 mm min−1 across the glial membrane, respectively.
In the absence of external sodium (Na+replaced by N-methyl-D-glucamine), pHi slowly decreased, and the rate of alkali and acid loading was reduced to 19 and 28 %, respectively, (n = 12). Amiloride (2 mm), which inhibits Na+-H+ exchange, had no effect on the alkali/acid loading (n = 6).
The alkali and acid loading were not impaired after the removal of external chloride (, replaced by gluconate; n = 11), but were significantly reduced by the anion transport inhibitor 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (DIDS, 0.5 mm) to 23 and 16 %, respectively, of the control (P < 0.001; n = 5).
Alkali and acid loading were affected differently by manipulating the availability of residual HCO3−. After adding the membrane-permeable carbonic anhydrase inhibitor ethoxyzolamide (EZA, 2 μm) to the saline, the acid loading, but not the alkali loading, was significantly reduced (by 25 %, P < 0.01), while lowering the residual CO2/HCO3− concentration in the saline by O2 bubbling significantly reduced the alkali loading (by 59 %, P < 0.02), but not the acid loading.
Changing the membrane holding potential in voltage-clamped glial cells or raising the external K+ concentration to 30 mm had no significant effect on acid/base loading.
It is concluded that a residual HCO3− concentration of less than 1 mm in nominally CO2/HCO3−-free salines and HCO3− produced endogenously in the glial cells support alkali and acid loading across the glial cell membrane, presumably by activation of the reversible Na+-HCO3− cotransporter. The results suggest a very high selectivity and affinity of this cotransporter for HCO3−; they imply that HCO3−-dependent processes may not be negligible even in the nominal absence of CO2/HCO3−, when the HCO3− concentration is expected to be in the submillimolar range.
The intracellular pH of glial cells is regulated by four modes of acid/base transport systems, which are Na+-H+ exchange, Na+-dependent and Na+-independent Cl−-HCO3− exchange, and an electrogenic Na+-HCO3− cotransport (cf. Chesler, 1990; Deitmer, 1995; Deitmer & Rose, 1996). The first is a ubiquitous carrier, which contributes to the recovery from an acid load. The Na+-HCO3− cotransport operates in both directions, chiefly depending on the cell membrane potential; it is an alkali loader when directed inwardly during membrane depolarization, and an acid loader when directed outwardly during membrane hyperpolarization (Deitmer, 1991, 1992; Deitmer & Schneider, 1995). The Cl−-HCO3− exchanger is a classical acid loader in most cells (Vaughan-Jones, 1979, 1986), whereas Na+-dependent Cl−-HCO3− exchange is an alkali loader (Thomas, 1977; Boron & Russell, 1983).
The intracellular pH (pHi) of glial cells has been shown to be highly dependent on the external pH (pHo), both in mammalian astrocytes (Mellergard et al. 1993) and in an invertebrate glial cell, the giant glial cell of the leech central nervous system (Deitmer, 1992; Deitmer & Schneider, 1995). In the latter it was shown that the high dependence of pHi on pHo was due to the Na+-HCO3− cotransport, and hence dependent on the presence of CO2/HCO3−. In a saline buffered with 5 % CO2 and 24 mm HCO3−, the pHi changed by 0.8 pH units per unit pHo change, both in voltage clamped and unclamped cells. The rate of pHi change was highly dependent upon HCO3−, indicating that acid/base transport across the glial cell membrane operated at about 10 % of its rate when no CO2/HCO3− was added to the salines (Deitmer, 1992).
These studies also showed, however, that there was still a significant transport of acid/base equivalents induced by a pHo change even in the nominal absence of CO2/HCO3−. This could be due to the influence of external pH on Na+-H+ exchange, the only non-bicarbonate carrier described in these cells. On the other hand, a novel, Cl−-dependent, non-bicarbonate acid loader, presumably a Cl−-OH− exchanger or H+-Cl− cotransporter, has recently been reported in guinea-pig ventricular myocytes (Sun et al. 1996). Such a transporter has not yet been confirmed in any other cell type.
In the present study we have tried to identify the mechanism(s) which lead to the pHo-dependent pHi change in non-bicarbonate-buffered salines. Our results show that neither a Cl−-dependent transporter nor the Na+-H+ exchange contributes to the pHo-dependent pHi changes, but rather a transport process dependent on external Na+ and the residual HCO3− concentration. This suggests that the Na+-HCO3− cotransporter operates at HCO3− concentrations in the submillimolar range to transport base equivalents in both directions across the glial cell membrane.
METHODS
The experiments were performed on the giant glial cells in the neuropile of isolated segmental ganglia of the leech Hirudo medicinalis. The dissection and recording techniques have been described previously (Munsch & Deitmer, 1992, 1994). Briefly, individual ganglia were pinned in a Sylgard-coated Perspex chamber in a modified Leibovitz (L-15) tissue culture medium. The ventral ganglionic capsule was removed mechanically with fine forceps. The ganglia were then incubated for 1 h in 2 mg ml−1 collagenase/dispase containing modified L-15 medium at room temperature (20–25°C). After thoroughly washing the ganglia with enzyme-free medium, neuronal cell bodies overlying the two neuropile glial cells of each segmental ganglion were removed by suction into a glass micropipette, thereby exposing the glial cells. This provided free access for bath solutions, and hence ionic concentrations of the salines, to the exposed face of the glial cell body.
Intracellular pH recording
Double-barrelled, pH-sensitive microelectrodes were prepared as described previously (Deitmer, 1991, 1992). Briefly, two glass capillaries of 1 and 1.5 mm diameter were pulled together and one barrel was silanized, using a drop of 5 % tri-N-butylchlorosilane (Fluka) mixed in 99.9 % pure carbon tetrachloride which was backfilled into the tips. The pipette was then baked on a hot plate at 470°C for 4–5 min. For the pH-sensitive barrel, H+ cocktail (Fluka 95291) was backfilled into the tip of the silanized barrel, which was then filled with 0.1 m sodium citrate, pH 6.0. The reference barrel was filled with 3 m KCl or 2 m potassium acetate (in Cl−-free saline). The tips of the electrodes were bevelled with a jet stream of alumina suspended in water (0.05 μm, Micropolish, Buehler, Lake Bluff, IL, USA). The electrodes were calibrated in leech salines with different pH values (6.6, 7.0, 7.4 and 7.8). The pH-sensitive barrel responded on average with 54 mV for a unit change in pH.
Some experiments were carried out with Na+-sensitive microelectrodes to follow the intracellular Na+ activity during external pH changes. Construction and calibration of these electrodes have been described previously (Deitmer, 1991).
Voltage-clamp recording
Microelectrodes for voltage clamp (made from single-barrelled 1.5 mm glass capillaries, Clark Electromedical GC 150F-15) were filled with 2 m potassium acetate for voltage recording and for current injection. For voltage clamping both microelectrodes were connected to the headstages of an Axoclamp-2A amplifier (Axon Instruments). Membrane currents were recorded by the built-in current measurement circuit of the headstages. The experimental bath was earthed via a Ag/AgCl wire in agar dissolved in normal saline.
For combining the voltage-clamp with the pH-sensitive microelectrode measurements, single-barrelled microelectrodes were used as current electrodes and double-barrelled pH-sensitive microelectrodes (Munsch & Deitmer, 1994) were used for voltage recording and pH measurement. The reference barrel was connected to one headstage of an Axoclamp-2A amplifier and the ion-sensitive barrel to an electrometer input. (For further details see Munsch & Deitmer, 1994; Deitmer & Schneider, 1995.)
During voltage recording in Cl−-free salines, an additional microelectrode in the bath, filled with 2 m potassium acetate, was used as the reference electrode, to avoid artefactual potential shifts in the membrane potential recording (see also Munsch et al. 1995).
All recordings were carried out at room temperature (22–25°C).
Solutions
Modified L-15 medium was prepared by dilution (1 : 3) of original L-15 medium (Gibco, Eggenstein, Germany) with a salt solution of the following composition (mmol l−1): CaCl2, 6.87; MgCl2, 2.51; KCl, 3.32; sodium malate, 20.1; sodium pyruvate, 12.5; Hepes, 15; glucose, 15; gentamicin (10 mg ml−1), 0.3 %; adjusted to pH 7.4 with NaOH.
The standard physiological leech saline for the experiments contained (mmol l−1): NaCl, 85; KCl, 4; CaCl2, 2; MgCl2, 1; Hepes, 10; pH adjusted to 7.4 with 3–5 mm NaOH (total osmolality 195–200 mosmol kg−1). All salines were adjusted to different pH values, using Hepes buffer to avoid side-effects caused by other organic buffers (Schmidt et al. 1996). In some experiments the salines were bubbled with 100 % O2 in semi-closed glass funnels, and the vapour above the salines was led into a 0.5 m KOH solution to reduce the CO2 partial pressure and hence the HCO3− concentration of the salines below ‘nominally free’. The HCO3− and CO2 concentrations were calculated using the modified Henderson-Hasselbalch equation:
| (1) |
and
| (2) |
where pK' is the apparent dissociation constant of carbonic acid, with the value of 6.1 (at 22°C). A solubility coefficient of 0.035 for CO2 in aqueous solutions was taken to convert CO2 percentages to molar CO2 concentrations (Davenport, 1979). While the CO2 concentration of outdoor air is 0.0314 %, equivalent to 8.35 μm, the indoor air of laboratories often has a higher CO2 concentration of about 0.05 %, equivalent to 13.3 μm, due to people's breathing. This would produce HCO3− concentrations of 265, 667 and 106 μm, which were here assumed to be present in the nominally CO2/HCO3− free salines buffered with Hepes to pH 7.4, 7.8 and 7.0, respectively.
In Na+-free saline, Na+ was replaced by N-methyl-D-glucamine (NMDG) and Cl−-free saline was prepared by exchanging the Cl− salts by gluconate salts. Amiloride (2 mm), 4,4′-diisothiocyanatostilbene 2,2′-disulphonic acid (DIDS, 0.5 mm) and ethoxyzolamide (EZA, 2 μm) were added to the salines shortly before use.
Calculation of acid/base transport rates
The maximum rates of pHi change (ΔpHi/dt) were determined from linear fits to the pHi changes during the first 2–3 min after a solution change. Acid/base flux was defined as the net transport of acid/base equivalents across the cell membrane, and expressed as positive values for a pHi increase, and negative values for a pHi decrease. The rate of acid/base flux, JA/B, was calculated as the product of ΔpHi/dt and the mean intrinsic intracellular buffering power βi (25 mm), as determined in nominally CO2/HCO3−-free, Hepes-buffered saline (Deitmer & Schlue, 1987), and given in units of millimolar per minute.
In Na+-free solutions, in which pHi continuously declined, the rate of alkalinization and acidification at altered pHo was determined by peeling the pHi decline in 0 Na+ at constant pHo off the pHi induced by changing pHo to 7.8 and 7.0. This was done either by recording pHi in Na+-free saline alone for a longer period (≥ 30 min), or by extrapolating the envelope of pHi decline in 0 Na+ over the period of altered pHo; both methods essentially gave the same results. In order to compare the results of ΔpHi/dt, and hence JA/B, over the same pHi range, we pooled the data obtained in the presence and absence of external Na+ into three classes between pHi 6.8 and 7.1 (see Fig. 3).
Figure 3. Sodium-dependent acid/base flux rates.
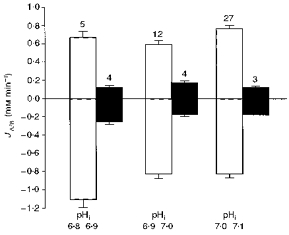
The rate of acid/base flux (JA/B) at different ranges of intracellular pH values in the presence (□, control) and in the absence of external sodium (▪, 0 mm Na+) as measured by changes in the intracellular pH in response to altering the external pH. The figures above the standard deviation bars give the number of experiments.
Statistical analysis was performed with Student's t test or, if appropriate, with a paired t test; and the significance level was set at P < 0.05.
RESULTS
When the pH of a Hepes-buffered, nominally CO2/HCO3−- free saline was changed from 7.4 to 7.8 to 7.0, and then back to 7.4, the pHi followed in the direction of the external pH change (Fig. 1). The pHi increased from 7.0 to 7.3, when the external pH was raised to 7.8, and pHi decreased to near 6.7, when the external pH was lowered to 7.0. Long exposures (> 30 min) to salines of different pH values indicated that the pHi changed by 0.75 pH units per 1 unit change in the external medium. This is close to the values of 0.73 in unclamped cells and 0.8 in voltage-clamped cells found in these cells in 5 % CO2/HCO3−-buffered salines (Deitmer & Schneider, 1995). However, the rate of pHi change induced by altering the external pH was lower by a factor of approximately 10 in the nominal absence of CO2/HCO3−, as compared with that in the presence of 5 % CO2 and 24 mm HCO3− (Deitmer, 1992).
Figure 1. Intracellular pH shifts in the nominal absence of bicarbonate.
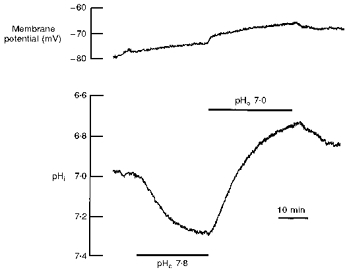
Recording of the membrane potential (upper trace) and intracellular pH (pHi; lower trace) with a double-barrelled, pH-sensitive microelectrode during a change in the external pH (pHo) from 7.4 to 7.8 to 7.0 and back to 7.4 in Hepes-buffered salines in the nominal absence of CO2/HCO3−.
In the present study, using nominally CO2/HCO3−-free salines, pHi changed with a maximum rate of +0.026 ± 0.008 pH units min−1 (±s.d., n = 49) when the external pH was raised from 7.4 to 7.8, and a rate of −0.028 ± 0.009 pH units min−1 (n = 49) when the external pH was lowered from 7.8 to 7.0. Taking the mean intracellular buffering power of 25 mm of these cells into account (Deitmer & Schlue, 1987), these rates indicate a flux of 0.64 and 0.71 acid/base equivalents min−1, respectively, across the glial cell membrane. In the following, the mechanism of these acid/base fluxes was studied using ion substitution experiments and the employment of drugs, which should give some indication of the carrier involved.
The contribution of Na+-dependent carriers
As pointed out in the introduction, pH regulation from an intracellular acidosis is chiefly dependent upon carriers exploiting the Na+ gradient for active extrusion of acid equivalents. Removal of external Na+, therefore, nearly always leads to an intracellular acidification (cf. Thomas, 1984; Deitmer, 1995). In the leech giant glial cell, Na+ removal induces only a slow intracellular acidification in the nominal absence of CO2/HCO3−, but leads to a rapid decrease of pHi in the presence of added CO2/HCO3−, due to the activity of the Na+-HCO3− cotransporter (Deitmer, 1991, 1992).
The change in pHi as produced by altering external pH in nominally CO2/HCO3−-free saline was always significantly larger and faster in the presence than in the absence of external Na+ (Fig. 2). The maximum rate of pHi change decreased to 19 and 28 % in Na+-free saline when the external pH was raised from 7.4 to 7.8 and lowered from 7.8 to 7.0, respectively (P < 0.01, n = 11).
Figure 2. Intracellular pH shifts in the absence of external sodium.
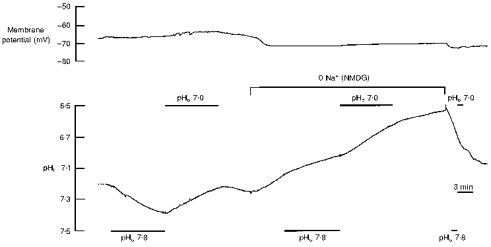
Membrane potential (upper trace) and intracellular pH (pHi, lower trace) during elevating and lowering the external pH in the nominal absence of CO2/HCO3− in the presence, and following the removal of, external Na+ (replaced by NMDG).
However, the change in pHi as induced by different external pH values could not always be followed over the same pHi range in the presence and absence of external Na+ due to the acidification initiated by the removal of external Na+. We therefore measured the rate of acid/base flux, JA/B, over different pHi ranges in the presence and absence of external Na+ (Fig. 3; see Methods for details of data processing). In all three classes of pHi ranges, covering pHi values between 6.8 and 7.1, the rate of acid/base flux was reduced to between 35 and 15 % in Na+-free saline. This indicates that a large fraction of the acid/base fluxes across the glial membrane, as induced by changing the external pH, was due to Na+-dependent acid/base transport.
We also used Na+-sensitive microelectrodes to measure the intracellular Na+ activity, aNai, in the glial cells during the changes in external pH. From a basal aNai of 4–7 mm (see also Deitmer, 1991; Nett & Deitmer, 1998), aNai increased maximally by 1 ± 0.3 mm when pHo was raised from 7.4 to 7.8, and decreased maximally by 1.3 ± 0.5 mm, when pHo was lowered from 7.8 to 7.0 (n = 4; not shown). These changes of aNai were partly transient, and confirm previous measurements (Deitmer, 1992). They indicate that the pHo-dependent pHi changes were not only greatly dependent on external Na+, but also accompanied by small, but significant, aNai changes.
From our present knowledge, this Na+-dependent acid/base system could either be Na+-H+ exchange, Na+-dependent Cl−-HCO3− exchange or Na+-HCO3− cotransport. The latter two processes at first appeared less likely, because the experiments were carried out in the nominal absence of CO2/HCO3−.
Since Na+-H+ exchange has been shown to be sensitive to amiloride in many cell types (Benos, 1982), including the leech giant glial cell (Deitmer & Schlue, 1987), we looked at the pHi changes induced by different external pH values in the absence and presence of 2 mm amiloride. In six experiments, the mean rate of pHi change during acid and alkali loading was 93 and 104 %, respectively, in the presence of amiloride as compared with the control without amiloride (P > 0.5; see also Fig. 7). This suggests that Na+-H+ exchange was not involved in the acid/base flux induced by altering external pH.
Figure 7. Normalized rates of acid/base fluxes.
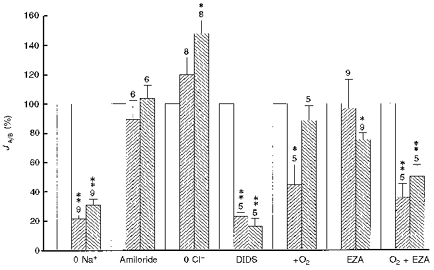
Rates of acid/base fluxes (JA/B) as measured by changes of the intracellular pH induced by altering the external pH from 7.4 to 7.8 and 7.0 in standard saline (□, control, 100 %,) and under various experimental conditions ( , pHi 7.8;
, pHi 7.8;  , pHi 7.0). Figures above bars indicate the number of experiments. * Significantly different from control, P < 0.05; ** highly significantly different from control, P < 0.01.
, pHi 7.0). Figures above bars indicate the number of experiments. * Significantly different from control, P < 0.05; ** highly significantly different from control, P < 0.01.
The contribution of Cl−-dependent carriers
The involvement of Cl− in these acid/base fluxes could originate, at least in part, from a Cl−-HCO3− exchange or a novel Cl−-dependent exchanger as recently described in cardiac myocytes (Sun et al. 1996). The rate of intracellular alkalinization and acidification, as induced by raising and lowering external pH, was therefore also measured in Cl−-free saline. In five experiments the mean maximum rate of pHi change was, compared with Cl−-containing saline (100 %= control), 136 and 197 %, respectively, after replacing Cl− by gluconate in the saline. The 97 % increase in the rate of pHi change in pHo 7.0 was significant (P < 0.01), whereas the rate of pHi change in pHo 7.8 was not significantly different from the control.
Since gluconate chelates Ca2+, and thereby reduces the free Ca2+ concentration in the saline, we repeated the experiments with the added external Ca2+ concentration raised from 2 to 12 mm in the gluconate-based saline; this was found to compensate fully (and perhaps somewhat more) for the loss of free Ca2+ in the Cl−-free gluconate-containing saline as measured with Ca2+-selective electrodes (Munsch et al. 1995). In a set of eight experiments with added Ca2+ (12 mm) and all Cl− removed (Fig. 4A-C), the mean rates of pHi increase upon raising pHo to 7.8, and of pHi decrease upon lowering pHo to 7.0, confirmed the results obtained with 0 Cl− and 2 mm Ca2+. The rate of intracellular acidification in pHo 7.0 was significantly increased by 48 ± 25 % (P < 0.005), whereas the increase in the rate of intracellular alkalinization in pHo 7.8 by 20 ± 33 % was not statistically significant (P > 0.1). Thus, this result suggests that the acid influx, or base efflux, but not the reverse transport, was significantly increased, when the external Cl− had been removed.
Figure 4. Intracellular pH shifts in chloride-free saline.
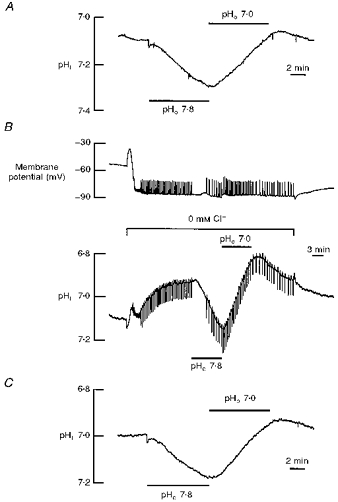
Shifts of intracellular pH (pHi) before (A) and after (B) the removal of external Cl− (replaced by gluconate). The record in C shows the pHi shifts following the re-addition of external Cl− in the same experiment. The downward deflections on the trace in B are due to transient membrane potential depolarizations of the glial cell (membrane potential in B) in Cl−-free saline, which were poorly subtracted from the pHi recording due to the slow time course of the ion-selective microelectrode.
In a Cl−-free saline the intracellular Cl− activity rapidly decreases within 3–5 min in this glial cell (Munsch et al. 1995). Since Cl−-free saline was pre-applied for at least 5–8 min in these experiments, it can be concluded that during the change of external pH to 7.8 and 7.0, the intracellular Cl− concentration was presumably below 1 mm. Hence, although Cl− removal and re-addition may shift pHi on its own, neither extracellular nor intracellular Cl− appears to contribute to the pHo-dependent acid/base fluxes as studied here; on the contrary, the transport rate of acid/base equivalents tends to increase in the absence of extracellular and/or intracellular Cl−.
Effects of the anion transport inhibitor DIDS
In order to elucidate the role of anions in the acid/base transport as induced by altering the external pH even further, the pHi changes were measured in the presence of the stilbene DIDS (0.5 mm), which is a widely used anion transport inhibitor, and which has been shown to block Na+-HCO3− cotransport in the leech giant glial cell (Deitmer & Schlue, 1989; Deitmer, 1991; Munsch & Deitmer, 1994). As shown in Fig. 5, DIDS virtually blocked the pHi shifts induced by raising and lowering external pH, and thus rendered the glial pHi nearly insensitive to changes in the external pH. The maximum rates of pHi change, as could be measured from the small pHi changes left in DIDS, were reduced highly significantly to 23 and 16 % (P < 0.0001, n = 5), when the external pH was altered to 7.8 and 7.0, respectively.
Figure 5. Block of pHi shift by DIDS.
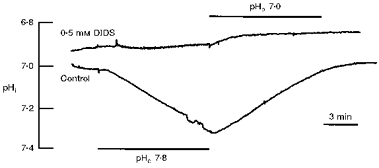
The shift of intracellular pH (pHi) as induced by altering external pH (pHo) is substantially reduced by the anion transport inhibitor DIDS. The control recording was obtained before application of DIDS in the same cell.
Since Cl− could be excluded as one of the major anions involved in the pHo-dependent acid/base transport studied here, we also considered HCO3− as a possible anion, although all these experiments were carried out in the nominal absence of CO2/HCO3−. Nominal absence of CO2/HCO3−, however, does not mean total absence of this buffer from the salines; air CO2 dissolved in the saline in an open system produces about 250–300 μm HCO3− in a saline with a pH of 7.4 (see Methods). This exogenous CO2 is also expected to diffuse into the cells, where it would form HCO3− with the aid of carbonic anhydrase. Furthermore, cellular metabolism also yields some endogenous CO2, which would produce additional intracellular HCO3−.
Reducing residual external CO2 and HCO3−
In a set of experiments we tried to minimize exogenous CO2/HCO3− by reducing the amount of air CO2 dissolved in the salines by bubbling the solutions with 100 % O2 and clearing the vapour of CO2 with KOH (see Methods). In O2-gassed salines, altering the external pH induced a lower rate of pHi change than in ungassed, Hepes-buffered salines (Fig. 6). However, while the mean rate of intracellular acidification at an external pH of 7.0 was not significantly reduced (from 0.018 ± 0.004 to 0.016 ± 0.006 pH units min−1, i.e. by 12 %; P > 0.3; n = 7), the rate of alkalinization at an external pH of 7.8 was significantly decreased by nearly 60 % (P < 0.02; n = 7; see also Fig. 7) from 0.022 ± 0.006 to 0.009 ± 0.005 pH units min−1; acidification and alkalinization correspond to an acid and base flux rate of 0.55 and 0.225 mm min−1, respectively. This suggests that reducing the HCO3− concentration in the salines decreased an inward transport of HCO3−, but not, or only insignificantly, an outward transport of HCO3−.
Figure 6. Effect of lowering residual bicarbonate.
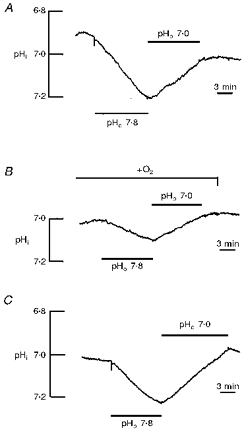
The shifts of intracellular pH (pHi) induced by external pH changes before (A) and after (B) reducing the residual HCO3− concentration by gassing the Hepes-buffered saline with 100 % O2 (see also Methods) are shown. The recording in C shows the pHi shifts again in an ungassed, nominally CO2/HCO3−-free saline in the same experiment.
Affecting residual intracellular HCO3− concentration
Blocking the carbonic anhydrase (CA) with the membrane-permeable CA inhibitor ethoxyzolamide (EZA, 2 μm), the conversion of metabolically produced CO2 into HCO3− in the cells would be slowed considerably (Sapirstein et al. 1984). This would be expected to reduce the intracellular availability of substrate on any HCO3−-dependent carrier, especially at relatively low substrate concentrations (as was presumably the case for intracellular HCO3− with no CO2/HCO3− added to the salines).
In a series of nine experiments, the rate of pHi change induced by altering external pH was measured before and after the addition of EZA, which is an irreversible inhibitor of CA in this preparation (Deitmer & Schlue, 1989). When the external pH was raised to 7.8 in the presence of EZA, the maximum rate of pHi increase was 97 % of that without EZA (P ≥ 0.78). When the external pH was lowered from 7.8 to 7.0, the maximum rate of pHi decrease was significantly reduced to 75 % (P < 0.005) after CA inhibiton by EZA (Fig. 7). This suggests that an outward transport, but not an inward transport, of HCO3− was slowed by blocking the carbonic anhydrase, presumably by reducing the supply of HCO3− from metabolically produced CO2 in the cells.
In the presence of EZA in O2-gassed salines, the acid/base fluxes in both directions were significantly reduced. The fluxes decreased to 36 ± 21 % when pHo was raised from 7.4 to 7.8, and to 50 ± 19 % when pHo was lowered from 7.8 to 7.0 (n = 5; P < 0.01) as compared with those in ungassed salines without EZA (Fig. 7).
The main results of the present study are summarized in Fig. 7. They show that the acid/base transport in nominally CO2/HCO3−-free, Hepes-buffered salines as induced by changing the external pH is dependent on Na+ and HCO3−, but not on Cl−, and is sensitive to the anion transport blocker DIDS, but not to the Na+-H+ exchange inhibitor amiloride. Therefore, we conclude that the Na+-HCO3− cotransport contributes a major fraction to this acid/base transport across the glial membrane. Since the Na+-HCO3− cotransport has been shown to be electrogenic with a stoichiometry of 2 HCO3− : 1 Na+ in this glial cell (Deitmer & Schlue, 1989; Deitmer & Schneider, 1995), we tested whether the acid/base transport studied here was influenced by changes of the membrane potential.
Voltage dependence of the acid/base transport
We used two protocols to test the voltage dependence of the acid/base transport: (1) by inducing the transport at normal membrane potential and after the membrane was depolarized by 30 mm K+ in the saline, and (2) by changing the membrane potential in voltage-clamped glial cells.
If the acid/base transport was due to an electrogenic Na+-HCO3− cotransport, an acceleration of the intracellular alkalinization as induced by changing the external pH to 7.8, and a slowing of the acidification as induced by changing to an external pH of 7.0, would be expected following a membrane depolarization. In our experiments (n = 5), the mean rates of the alkalinization and the acidification amounted to 151 ± 35 and 147 ± 26 %, respectively, in 30 mm K+, in which the membrane depolarized by about 40 mV (to between −30 and −20 mV). Due to the large standard deviation, these changes were not significantly different from the controls in the paired t test (P > 0.1 and P > 0.05, respectively).
In voltage-clamped cells, the acid/base flux was measured at membrane potentials of −65 and −85 mV. The maximum rates of pHi change as induced by altering the external pH to 7.8 and to 7.0 decreased by 1 and 17 % at −85 mV, respectively, without displaying any statistical significance (P > 0.1). Hence, our results were inconclusive with respect to the membrane potential dependence of this acid/base transport.
DISCUSSION
The present results indicate that, despite the nominal absence of CO2/HCO3− in the saline, and hence HCO3− concentrations in the submillimolar range, HCO3− is involved in the acid/base fluxes, which result in the pHi changes induced by altering the external pH. Removal of external Cl− did not block, but even increased, the acid influx/base efflux, while in the absence of external Na+ the acid/base fluxes were greatly reduced. Since these fluxes were also inhibited by DIDS, but not by amiloride, we conclude that they occurred via the Na+-HCO3− cotransporter across the glial cell membrane. This Na+-HCO3− cotransporter is the chief pHi regulator in this glial cell, and carries acid/base equivalents with great efficacy across the cell membrane, when CO2/HCO3− had been added to the saline (Deitmer & Schlue, 1989; Deitmer, 1991, 1992; Deitmer & Schneider, 1995).
Evidence for the involvement of HCO3−
In the nominal absence of CO2/HCO3−, i.e. when the salines were not gassed with CO2 and contained no added HCO3−, and were instead buffered with Hepes alone, HCO3− is produced in a submillimolar concentration by air CO2 dissolving in the solutions. A room CO2 concentration of 0.05 %, or 13.3 μm, in solution, would produce around 265 μm HCO3− in the salines buffered to a pH of 7.4. At a pHi of 7.0, around 106 μm HCO3− would equilibrate in the cells in addition to the endogenously produced CO2/HCO3−. This might add up to a few hundred micromolar HCO3− in the cells, and thus to a similar value as calculated for the extracellular HCO3− concentration (i.e. around 200–300 μm).
In spite of these low HCO3− concentrations both intra- and extracellularly, HCO3− appeared to be involved in the transport of acid/base equivalents across the cell membrane induced by raising or lowering external pH. This conclusion is supported by the following evidence: (1) with the Na+-HCO3− cotransporter these glial cells express a highly efficient carrier to mediate pHi changes (Deitmer, 1991, 1992); (2) amiloride, which inhibits Na+-H+ exchange, the only known HCO3−-independent carrier of acid/base equivalents in these (and most other) cells, had no effect on the pHo-dependent pHi shifts; (3) the anion transport blocker DIDS inhibited these pHi shifts, indicating that anion transport is involved in the underlying acid/base fluxes; (4) reducing the concentration of the residual external HCO3− by oxygenating the nominally CO2/HCO3−-free salines impairs the alkalinization, and reducing the supply of intracellular HCO3− by inhibiting the carbonic anhydrase, impairs the acidification, consistant with an effect on the transport of HCO3− into and out of the cells, respectively.
The dependence of the acid/base fluxes on extracellular Na+ suggests that the carrier transporting the acid/base equivalents across the glial cell membrane is the Na+-HCO3− cotransporter. This is in line with the small, but significant, fall and rise of aNai observed when lowering and raising pHo, respectively. The changes in aNai cannot be used to estimate the Na+-HCO3− cotransporter activity quantitatively, however, because most of the aNai changes recorded are expected to be obscured by the Na+-K+ pump activity.
Although it cannot be excluded that HCO3− ions may pass the cell membrane via Cl− channels, this type of HCO3− transport would not be expected to be Na+ dependent. HCO3− flux through anion channels may therefore contribute to the relatively small fraction of Na+-independent pHi changes observed in our experiments (Figs 2 and 3).
The electrogenic Na+-HCO3− cotransporter was first described in the salamander kidney (Boron & Boulpaep, 1983), and has recently been cloned and expressed in frog oocytes (Romero et al. 1997). This Na+-HCO3− cotransporter is electrogenic not only in epithelial, but also in glial cells (cf. Deitmer & Rose, 1996); in the leech giant glial cell a stoichiometry of 1 Na+ : 2 HCO3− has been found (Deitmer & Schlue, 1989; Deitmer & Schneider, 1995). However, in the present study no evidence was found for a membrane potential dependence of the induced pHi shifts. This may indicate the presence of an additional, electroneutral Na+-HCO3− cotransporter in these cells, a transport mode which has been reported for cardiac muscle (Dart & Vaughan-Jones, 1992). On the other hand, the rates of acid/base flux were one order of magnitude smaller in the nominal absence, as compared with those in the presence, of added CO2/HCO3− (Deitmer, 1992, and this study), which might have obscured a distinct membrane potential dependence of the acid/base transport. In addition, due to the limitations to space-voltage clamping the cell membrane of this large glial cell (diameter 80 μm), the range of membrane holding potentials tested was only 20 mV (for a detailed discussion of the voltage-clamp problems in this cell, see Munsch & Deitmer, 1994). This might be too little for demonstrating a significant influence of membrane voltage on the rate of the induced pHi changes.
In fact, changing the external pH did sometimes alter the membrane potential by a few millivolts even in the nominal absence of CO2/HCO3− (see Fig. 1), which was sensitive to Na+ removal (Fig. 2). This would be in line with electrogenic Na+-HCO3− cotransport. Alkalinization was associated with a small hyperpolarization, and acidification with a small depolarization, consistant with inwardly and outwardly directed electrogenic Na+-HCO3− cotransport, respectively.
Characteristics of this HCO3−-dependent transport
Our results indicate that a HCO3−-dependent transport mechanism mediates the acid/base fluxes across the leech glial cell membrane in the nominal absence of CO2/HCO3−, and suggest that this transport mechanism is a Na+-HCO3− cotransporter. There was no indication that Cl− was involved in the pHo-dependent acid/base fluxes studied here; hence these cells do not appear to express a Cl−-OH− exchanger, recently suggested for mammalian cardiac muscle (Sun et al. 1996). The increased rate of acid influx/base efflux in the absence of Cl− indicates that in millimolar concentrations Cl− may compete with HCO3− in micromolar concentrations at this transporter.
The HCO3−-dependent transport described here appears to be highly selective for, and displays a very high affinity for, HCO3−. Assuming that the endogenously produced CO2 does not lead to accumulation of millimolar concentrations of HCO3− in this exposed glial cell in situ, in which CO2 can move freely out of the cells and into the bath (Deitmer, 1991), our results also suggest a very high affinity of this transporter for HCO3−. Since the glial cell was exposed with one side facing the bath saline, the HCO3− concentration in the immediate environment of at least part of the glial cell membrane was expected to be very similar to the value calculated for the bulk saline in the nominal absence of CO2/HCO3−, i.e. well below 1 mm.
In most studies carried out in nominally CO2/HCO3−-free solutions, the residual HCO3− concentration was presumed to be small and usually negligible. In rat astrocytes, for example, HCO3−-dependent mechanisms were excluded as a contributor to the pHi shifts induced by external pH changes in Hepes-buffered solutions (Mellergard et al. 1993), which may need to be reconsidered. In fact, a Na+- and HCO3−-dependent transport has been considered to contribute to pHi recovery in cultured rat astrocytes even in the nominal absence of CO2/HCO3− (Boron et al. 1990).
The concentration dependence on HCO3− of anion transporters in various cell types has been shown for the millimolar range of HCO3−, e.g. of the Cl−-HCO3− exchanger in red blood cells (Wieth, 1979; Jennings 1992) or the Na+-HCO3− cotransporter in different mammalian epithelial tissue (Jentsch et al. 1985, 1986). Interestingly, the lowest apparent affinity constant for HCO3− of only 2–5 mm has been reported for Na+-dependent HCO3− transport in other invertebrates, i.e. the squid giant axon (Boron, 1985) and barnacle muscle fibres (Boron et al. 1981).
The present study suggests that high-affinity HCO3−-dependent processes cannot be excluded even at submillimolar HCO3− concentrations. The leech glial cell appears to express such a highly selective, high-affinity HCO3− transport, presumably identical with the potent Na+-HCO3− cotransporter, which maintains acid/base transport across the cell membrane in the nominal absence of CO2/HCO3−.
Although the main transporter identified as the mediator between extra- and intracellular pH changes in the present study is likely to be the Na+-HCO3− cotransporter, there remained some pHi shift which was both Na+ and DIDS insensitive. This indicates that there is presumably yet another transport mechanism, which may carry up to 15–30 % of the acid/base equivalents across the glial membrane in the nominal absence of CO2/HCO3−. This corresponds to a rate of 0.1 to 0.2 mm min−1 acid/base fluxes, equivalent to 1–4 % of that measured in the presence of 5 % CO2 and 10–60 mm HCO3− (Deitmer, 1992). This residual transport of acid/base equivalents is likely to be independent of Na+, and might represent H+, OH− and/or HCO3− fluxes through other carriers and/or channels.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft through the Sonderforschungsbereich 246, TP C7.
References
- Benos DJ. Amiloride: a molecular probe of sodium transport in tissues and cells. American Journal of Physiology. 1982;242:C131–145. doi: 10.1152/ajpcell.1982.242.3.C131. [DOI] [PubMed] [Google Scholar]
- Boron WF. Intracellular pH-regulating mechanism of the squid axon. Relation between the external Na+ and HCO3− dependences. Journal of General Physiology. 1985;85:325–345. doi: 10.1085/jgp.85.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron WF, Boulpaep EL. Intracellular pH regulation in the renal proximal tubule of the salamander: basolateral HCO3− transport. Journal of General Physiology. 1983;81:53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron WF, Boyarsky G, Marek KL, Ransom BR. Intracellular pH regulation in astrocytes in the absence of HCO3−. Society for Neuroscience Abstracts. 1990;16:971. [Google Scholar]
- Boron WF, McCormick WC, Roos A. pH regulation in barnacle muscle fibres: dependence on extracellular sodium and bicarbonate. American Journal of Physiology. 1981;240:C80–89. doi: 10.1152/ajpcell.1981.240.1.C80. [DOI] [PubMed] [Google Scholar]
- Boron WF, Russell JM. Stoichiometry and ion dependencies of the intracellular pH-regulating mechanism in squid gian axons. Journal of General Physiology. 1983;81:373–399. doi: 10.1085/jgp.81.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M. The regulation and modulation of pH in the nervous system. Progress in Neurobiology. 1990;34:401–427. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- Dart C, Vaughan-Jones RD. Na+-HCO3− symport in the sheep cardiac Purkinje fibre. The Journal of Physiology. 1992;451:365–385. doi: 10.1113/jphysiol.1992.sp019169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport HW. Säure-Basen-Regulation. Stutgart: Thieme-Verlag; 1979. [Google Scholar]
- Deitmer JW. Electrogenic sodium-dependent bicarbonate secretion by glial cells of the leech central nervous system. Journal of General Physiology. 1991;98:637–655. doi: 10.1085/jgp.98.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer JW. Bicarbonate-dependent changes of intracellular sodium and pH in identified leech glial cells. Pflügers Archiv. 1992;420:584–589. doi: 10.1007/BF00374637. [DOI] [PubMed] [Google Scholar]
- Deitmer JW. pH regulation. In: Kettenmann H, Ransom B, editors. Neuroglial Cells. New York: Oxford University Press; 1995. pp. 230–245. [Google Scholar]
- Deitmer JW, Rose CR. pH regulation and proton signalling by glial cells. Progress in Neurobiology. 1996;48:73–103. doi: 10.1016/0301-0082(95)00039-9. [DOI] [PubMed] [Google Scholar]
- Deitmer JW, Schlue WR. The regulation of intracellular pH by identified glial cells and neurones in the central nervous system of the leech. The Journal of Physiology. 1987;388:261–283. doi: 10.1113/jphysiol.1987.sp016614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer JW, Schlue WR. An inwardly directed, electrogenic sodium bicarbonate co-transport in glial cells of the leech central nervous system. The Journal of Physiology. 1989;411:179–194. doi: 10.1113/jphysiol.1989.sp017567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer JW, Schneider HP. Voltage-dependent clamp of intracellular pH of identified leech glial cells. The Journal of Physiology. 1995;485:157–166. doi: 10.1113/jphysiol.1995.sp020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings ML. Cellular anion transport. In: Seldin DW, Giebisch G, editors. The Kidney: Physiology and Pathophysiology. 2. New York: Raven Press; 1992. pp. 113–145. [Google Scholar]
- Jentsch TJ, Schwartz P, Schill BS, Langner B, Lepple AP, Keller SK, Wiederholt M. Kinetic properties of the sodium bicarbonate (carbonate) symport in monkey kidney epithelial cells (BSC-1) Journal of Biological Chemistry. 1986;261:10673–10679. [PubMed] [Google Scholar]
- Jentsch TJ, Stahlknecht TR, Hollwede H, Fischer DG, Keller SK, Wiederholt M. A bicarbonate-dependent process inhibitable by disulfonic stilbenes and a Na+/H+ exchange mediate 22Na+ uptake into cultured bovine corneal endothelium. Journal of Biological Chemistry. 1985;260:795–801. [PubMed] [Google Scholar]
- Mellergard PE, Ou-Yang YB, Siesjö BK. Intracellular pH regulation in cultured rat astrocytes maintained in CO2/HCO3− containing media. Experimental Brain Research. 1993;95:371–380. doi: 10.1007/BF00227129. [DOI] [PubMed] [Google Scholar]
- Munsch T, Deitmer JW. Calcium transients in identified leech glial cells in situ evoked by high potassium concentration and 5-hydroxytryptamine. Journal of Experimental Biology. 1992;167:251–265. doi: 10.1242/jeb.167.1.251. [DOI] [PubMed] [Google Scholar]
- Munsch T, Deitmer JW. Sodium bicarbonate cotransport current in identified leech glial cells. The Journal of Physiology. 1994;474:43–53. doi: 10.1113/jphysiol.1994.sp020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsch T, Reusch M, Deitmer JW. Intracellular chloride acitivity of leech neurones and glial cells in physiological, low chloride saline. Journal of Comparative Physiology A. 1995;176:273–280. doi: 10.1007/BF00239929. [DOI] [PubMed] [Google Scholar]
- Nett W, Deitmer JW. Intracellular Ca2+ regulation by the leech giant glial cell. The Journal of Physiology. 1998;507:147–162. doi: 10.1111/j.1469-7793.1998.147bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/HCO3− cotransporter. Nature. 1997;387:409–413. doi: 10.1038/387409a0. [DOI] [PubMed] [Google Scholar]
- Sapirstein VS, Strocchi P, Gilbert JM. Properties and function of brain carbonic anhydrase. Annals of the New York Academy of Sciences. 1984;429:481–493. doi: 10.1111/j.1749-6632.1984.tb12375.x. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Mangold C, Deitmer JW. Membrane responses evoked by organic buffers in identified leech neurones. Journal of Experimental Biology. 1996;199:327–335. doi: 10.1242/jeb.199.2.327. [DOI] [PubMed] [Google Scholar]
- Sun B, Leem CH, Vaughan-Jones RD. Novel chloride-dependent acid loader in the guinea-pig ventricular myocyte: part of a dual acid-loading mechanism. The Journal of Physiology. 1996;495:65–82. doi: 10.1113/jphysiol.1996.sp021574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RC. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. The Journal of Physiology. 1977;273:317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RC. Experimental displacement of intracellular pH and the mechanism of its subsequent recovery. The Journal of Physiology. 1984;354:3–22. doi: 10.1113/jphysiol.1984.sp015397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones RD. Regulation of chloride in quiescent sheep heart Purkinje fibres studied using intracellular chloride and pH-sensitive microelectrodes. The Journal of Physiology. 1979;295:111–137. doi: 10.1113/jphysiol.1979.sp012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones RD. An investigation of chloride- bicarbonate exchange in the sheep cardiac Purkinje fibre. The Journal of Physiology. 1986;379:377–406. doi: 10.1113/jphysiol.1986.sp016259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieth JO. Bicarbonate exchange through the red cell membrane determined with [14C] bicarbonate. The Journal of Physiology. 1979;294:521–539. doi: 10.1113/jphysiol.1979.sp012944. [DOI] [PMC free article] [PubMed] [Google Scholar]


