Abstract
The axonal plexus of most hippocampal interneurons is restricted to certain strata within the target region. This lamination suggests a possible functional heterogeneity of inhibitory synapses between different interneurons and CA1 pyramidal cells.
We therefore compared inhibitory postsynaptic potentials (IPSPs) and currents (IPSCs) in CA1 pyramidal cells, which were evoked from two stimulation sites (stratum oriens and stratum radiatum). Stimulation in stratum oriens yielded faster decaying IPSPs and IPSCs than stimulation in stratum radiatum.
IPSP and IPSC kinetics were regulated by GABA uptake in both layers as indicated by the prolongation of the signals under tiagabine, a GAT-1 (neuronal GABA plasma membrane transporter)-specific GABA-uptake blocker. However, the effect of tiagabine was significantly more pronounced following stimulation in stratum radiatum than in stratum oriens (prolongation of IPSC half-decay time by 167 vs. 115%, respectively).
In situ hybridization with antisense mRNA for the GABA-synthesizing enzyme glutamate decarboxylase (GAD65/67) and the GABA transporter GAT-1 showed that the proportion of interneurons expressing GAT-1 was lower in stratum oriens than in stratum radiatum/lacunosum-moleculare.
From these functional and molecular data we conclude that the regulation of IPSP and IPSC kinetics in CA1 pyramidal cells by neuronal GABA uptake differs between layers. Our findings suggest that this laminar difference is caused by a lower expression of GAT-1 in interneurons in stratum oriens than in stratum radiatum/lacunosum-moleculare.
The duration of fast inhibitory postsynaptic potentials (IPSPs) differs between cell types and has important consequences for the cellular and network behaviour of interneurons and principal cells (Whittington et al. 1995). Various mechanisms contribute to the control of the decay time course of IPSPs, including diffusion of GABA from the synaptic cleft, GABA uptake into neurons and glia cells, desensitization and mean open time of postsynaptic GABAA receptors (Roepstorff & Lambert, 1994; Puia et al. 1994; Draguhn & Heinemann, 1996). In CA1 pyramidal cells, IPSPs can be significantly prolonged by pharmacological block of GABA uptake (Dingledine & Korn, 1985; Roepstorff & Lambert, 1992). However, other mechanisms such as postsynaptic receptor kinetics or desensitization also contribute to the decay time course of IPSPs and inhibitory postsynaptic currents (IPSCs) in these cells (Collingridge et al. 1984; Roepstorff & Lambert, 1994; Pearce et al. 1995).
Pearce (1993) demonstrated that fast IPSCs in CA1 pyramidal cells differ kinetically when elicited by stimulation in stratum pyramidale or stratum lacunosum-moleculare. These different synaptic currents are likely to be mediated by two different sets of interneurons with specific postsynaptic GABAA receptor subtypes (Banks et al. 1998). Thus, kinetic determinants of IPSPs and IPSCs from different populations of interneurons can differ within the same postsynaptic target cell.
Here we show another layer-specific difference in inhibitory synaptic function in CA1 pyramidal cells. GABA uptake through GAT-1, the main neuronal GABA plasma membrane transporter (Ribak et al. 1996), plays a larger role for IPSPs elicited in stratum radiatum than in stratum oriens. We compared the electrophysiological results with the expression pattern of GAT-1- and GAD65/67 (glutamate decarboxylase)-specific mRNA and suggest that interneurons in stratum oriens contain less GABA transporter than those in stratum radiatum/lacunosum-moleculare.
METHODS
Slice preparation and recording
Horizontal hippocampal-entorhinal cortex slices (400 μm) were prepared from adult Wistar rats which had been decapitated under deep ether anaesthesia. Slices were maintained at 34°C in an interface chamber and perfused with artificial cerebrospinal fluid containing (mm): NaCl, 129; NaHCO3, 21; KCl, 3; NaH2PO4, 1.25; CaCl2, 1.6; MgSO4, 1.8; glucose, 10; saturated with 95% O2−5% CO2, pH 7.4. For intracellular recordings, micropipettes (40- 120 MΩ, filled with 2–3 m potassium acetate and 50 mm QX-314 (lidocaine N-ethyl bromide quaternary salt)) and a Neurodata IR 183 (Neurodata Instruments Corp., NY, USA), or a SEC 10L (npi Instruments, Tamm, Germany), amplifier in either bridge or ‘switched’ voltage clamp mode (switching frequency, 22–33 kHz; ¼ duty cycle) were used. Signals were filtered at 3 kHz and sampled at 8 kHz by an ITC-16 interface (HEKA electronic, Lambrecht, Germany) connected to a PC. Monosynaptic IPSPs or IPSCs were evoked in the presence of 6-nitro-7-sulphanoylbenzo(f)quinoxaline-2,3-dione (NBQX; 10 μm), (±)-dl-2-amino-5-phosphonovaleric acid (APV; 30 μm) and CGP 55845A (1–2 μm) using two bipolar stimulation electrodes (interelectrode distance, 50–80 μm) placed in the outer stratum radiatum (just inside the half-distance between stratum pyramidale and the hippocampal fissure) and stratum oriens (in the middle of the alveus). The longitudinal position of both stimulation electrodes was close to the recording site (< 500 μm). The stimulation strength (1–8 V) was constant throughout each experiment and sufficient to yield reliable responses to each pulse. NBQX and tiagabine ([(R)-N-(4,4-bis(3-methyl-2-thienyl)but-3-en-1-yl]nipecotic acid; 50 μm) were kind gifts from Novo Nordisk (Maloev, Denmark). CGP 55845A (1–2 μm) was a kind gift from Ciba-Geigy, Basel, Switzerland.
Data analysis and statistical treatment
Groups of four to fourteen individual consecutive IPSPs or IPSCs were averaged and analysed using WINTIDA software (HEKA electronic). In some recordings, the decay time course could be well fitted by a monoexponential curve. In the remaining records, a biexponentially decaying function was used to measure the time course and the effect of tiagabine (Roepstorff & Lambert, 1994). Charge transfer was calculated from IPSCs as the product of the time constant(s) and the respective amplitude(s). Data are given as means ± standard error of the mean and statistical analysis was performed with the non-parametric Wilcoxon signed-rank test for paired data using SPSS (SPSS GmbH, München, Germany) for windows. P < 0.05 was considered significant.
In situ hybridization
DNA primers were designed based on published cDNA sequences for the neuronal GABA transporter GAT-1 (Guastella et al. 1990; upstream: GCC CCC TCA TCA CCC CTA CAC T; downstream: GCT TGT GGC TTT TCT TTT TCT C) and the isoforms of glutamic decarboxylase GAD65 (Erlander et al. 1991; upstream: CGG GCT CTG GCT TTT GGT CC; downstream: GGT TTG AGA TGA CCA TGC GG) and GAD67 (Michelsen et al. 1991; upstream: TAG AGA CCC CAA GAC CAC CG; downstream: GGG TTG GAG ATG ACC ATC CG). Specific polymerase chain reaction (PCR) products of 625 bp (GAT-1), 1680 bp (GAD65) and 1682 bp (GAD67) were synthesized and cloned into different T-overhang cloning vectors (Invitrogen, Promega, Amersham). Transcripts (sense and antisense probes) were labelled with the digoxigenin RNA Labeling Kit (Boehringer Mannheim) and subsequently partially hydrolysed to reduce their length to about 100 bp. Four adult Wistar rats were perfused with saline under deep anaesthesia (thiobutabarbital, 100 mg kg−1i.p.). The brains were removed, cut into 12 μm sections on a cryotome and fixed in 4% paraformaldehyde using standard pre-treatments to enhance penetration of the probe. Sections containing the hippocampus were then incubated for 16 h at 55°C in hybridization buffer (In situ hybridization buffer, Amersham Life Science) containing 3 μg ml−1 of digoxigenin-labelled RNA probe. The sections were washed following the manufacturer's instructions (supplied with the hybridization buffer) and then processed for immunodetection using a Nucleic Acid Detection Kit (Boehringer Mannheim). The incubation in BCIP/NBT (5-bromo-4-chloro-3-indolyl-phosphate/4-nitroblue tetrazolium chloride) colour solution was performed overnight. The sections were then rinsed in water, air dried and mounted with Entellan (Merck). Cells stained for the respective mRNA were counted by placing an ocular grid alongside CA1 and counting the number of neurons per grid unit length (250 μm) on either side of the pyramidal cell layer (i.e. (1) stratum oriens or (2) stratum radiatum with stratum lacunosum-moleculare up to the fissure).
RESULTS
Recordings were made from twenty-six CA1 pyramidal cells with a mean resting potential of −51.2 ± 2.1 mV (slightly depolarized due to the presence of QX-314 in the pipette solution) and an input resistance of 94.9 ± 15.7 MΩ. Fast GABAA receptor-mediated IPSPs and IPSCs were isolated in the presence of APV, NBQX and CGP 55845A and elicited for each cell from two different stimulation sites: stratum radiatum or stratum oriens (IPSPR/O, IPSCR/O; Fig. 1). In current clamp recordings, the IPSP amplitude was 8.2 ± 1.9 mV (stimulation in stratum oriens) and 7.6 ± 1.8 mV (stimulation in stratum radiatum). In most cells the decay phase of the IPSPs could be approximated with a single exponential function whereas the sum of two exponential curves was required in four of nineteen cells (stratum oriens) and five of nineteen cells (stratum radiatum). The relaxation of IPSPs was more rapid following stimulation in stratum oriens (half-decay time: 19.9 ± 1.5 ms vs. 25.2 ± 2.5 ms for IPSPR; n = 19; P < 0.01). In voltage clamp recordings, most (8/10) cells displayed biexponentially decaying IPSCs with time constants τfast and τslow of 9.2 ± 1.1 and 15.5 ± 5.5 ms (IPSCO) and 7.9 ± 1.3 and 23.6 ± 4.1 ms (IPSCR), respectively. In stratum oriens, most of the current amplitude was contributed by the fast component (76 ± 8% of total amplitude; mean amplitude, 162 ± 36 pA), whereas in stratum radiatum the slow component was dominant (the fast component contributed 42 ± 12%; total amplitude, 116 ± 19 pA; significantly different from stratum oriens, P < 0.05). The IPSC half-decay times were also different: 10.2 ± 1.3 ms for IPSCO and 13.3 ± 1.4 ms for IPSCR (P < 0.05).
Figure 1. Time course of IPSPs and IPSCs in CA1 pyramidal cells.

A, the time course of IPSPs (left) and IPSCs (right) elicited by stimulation in stratum oriens and radiatum differed in the same postsynaptic cell. Vertical calibration bars apply to potential and current from stratum radiatum; traces from stratum oriens are normalized to traces from stratum radiatum. B, tiagabine (50 μm) prolonged the decay phase of IPSCs after stimulation in stratum radiatum (left) and stratum oriens (right). The effect was more pronounced in IPSCs elicited in stratum radiatum. Amplitudes are normalized to control traces at both sites.
Effects of GABA-uptake blockers
Tiagabine (50 μm), a GABA-uptake blocker specific for the neuronal transporter GAT-1 (Borden et al. 1994), caused a marked prolongation of the IPSPs and IPSCs. The half-decay time of IPSPO increased by 61 ± 12% and that of IPSPR by 87 ± 11%. In voltage clamp recordings, the half-decay prolongation by tiagabine was 115 ± 24% for IPSCO and 167 ± 20% for IPSCR (Figs 1B and 3A). In both recording modes, the effect of tiagabine was larger after stimulation in stratum radiatum than in stratum oriens (P < 0.05; Fig. 3A). This was even more obvious when the increase in charge transfer by IPSCs was calculated: tiagabine increased the charge transfer by IPSCO by 173 ± 44% and by IPSCR by 381 ± 45% (n = 10; P < 0.05; Fig. 3A). Tiagabine preferentially affected the slow time constant: τfast increased in three of eight cells with biexponentially decaying currents, whereas τslow was prolonged in seven of eight (IPSCO) and eight of eight (IPSCR) cells. The IPSC amplitudes (oriens and radiatum) and that of IPSPO were not changed by tiagabine, whereas the amplitude of IPSPR was slightly increased to 121 ± 7% of control (P < 0.01). The effects of tiagabine could only partially be reversed following prolonged washout for more than 1 h.
Figure 3. Effects of tiagabine and relative expression of GAT-1 in stratum oriens and stratum radiatum.
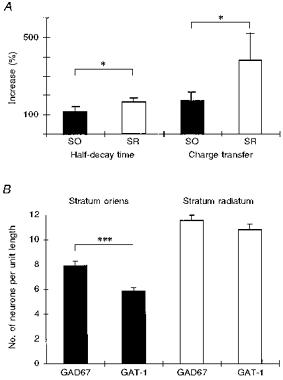
A, increase in half-decay time and net charge transfer following application of tiagabine (50 μm) differed between IPSCs elicited from stratum oriens (SO; ▪) and stratum radiatum (SR; □). *P < 0.05. B, abundance of GAT-1 and GAD67 in putative interneurons in stratum oriens and stratum radiatum. ***P < 0.0001.
In a second series of experiments, we tested the effect of the GABA-uptake blocker β-alanine (200 μm) which predominantly suppresses the molecular GABA transporter subtype GAT-3 (Borden, 1996). This substance did not significantly affect the decay time course regardless of the site of stimulation. The decay time remained 102.1 ± 2.1% of control for IPSPR (n = 7) and 97 ± 8% of control for IPSPO (n = 7). Similarly, no alteration in IPSCs was observed (n = 5) following application of β-alanine.
Expression of GAT-1 and GAD65/67
Our recordings showed that IPSPs and IPSCs elicited in stratum radiatum were more tightly controlled by the GABA transporter GAT-1 than those elicited in stratum oriens. This difference could be explained by different levels of expression of GAT-1 at the two locations. We therefore performed in situ hybridization with mRNA specific for GAT-1 and for the two isoforms of the GABA-synthesizing enzyme glutamate decarboxylase, GAD65 and GAD67 (Fig. 2). The number of stained cells was counted by means of an ocular grid on either side of stratum pyramidale, i.e. in stratum oriens and stratum radiatum together with stratum lacunosum-moleculare. In stratum oriens, about 32% more neurons expressed GAD67 mRNA than GAD65 mRNA (4 animals; n = 30 and 39 slices, respectively; P < 0.0001) whereas in stratum radiatum no difference was found. Therefore, GAD67 was used as a marker for interneurons and the number of GAD67- and GAT-1-expressing cells (41 slices) was compared. In stratum oriens, 7.9 ± 3.3 cells per grid length were positive for GAD67 mRNA and 5.9 ± 2.9 cells for GAT-1 mRNA. This difference was significant (Fig. 3B; P < 0.0001, Wilcoxon test). In contrast, in stratum radiatum, we did not find a significant difference between cells expressing GAD67 and GAT-1 mRNA (11.5 ± 3.9 cells per grid length for GAD67 and 10.8 ± 4.4 cells per grid length for GAT-1; P = 0.07). These results indicate that there is a significant proportion of interneurons in stratum oriens which do not express GAT-1 whereas there is no or little discrepancy in stratum radiatum.
Figure 2. Expression of GAD67 and GAT-1 mRNA in CA1.

A, in situ hybridization for GAD67 mRNA in CA1. Interneurons were mainly visible in stratum oriens, stratum pyramidale and at the border between stratum radiatum and lacunosum-moleculare. B, expression of GAT-1 mRNA in CA1. Note the smaller number of stained cells in stratum oriens compared with A. Calibration bar, 200 μm. Abbreviations: H, hilus; SG, stratum granulare; SM, stratum moleculare; SL-M, stratum lacunosum-moleculare; SR, stratum radiatum; SP, stratum pyramidale (CA1); SO, stratum oriens.
DISCUSSION
The time course of IPSPs differs markedly between cell types. The present data show such a difference within a single cell, depending on the laminar position of activated interneurons. We found differences between IPSPs and IPSCs stimulated from stratum oriens and stratum radiatum in: (i) the decay time course and (ii) kinetic regulation by GABA uptake. Expression of the GABA transporter GAT-1 in interneurons was also different. Pearce (1993) had already shown a kinetic difference between IPSCs elicited from stratum pyramidale vs. stratum radiatum. More recent data indicate that the respective synapses not only arise from different sets of interneurons but also express different postsynaptic GABAA receptor subtypes (Banks et al. 1998).
Anatomical studies have shown that many interneurons in the hippocampus have clearly stratified axonal arborizations (Freund & Buzsáki, 1996). For example, oriens-lacunosum-moleculare (O-LM) cells have somata in stratum oriens and project to stratum lacunosum-moleculare (Ramón y Cajal, 1893), basket cells predominantly innervate the soma and proximal dendrite (Buhl et al. 1994) and interneurons located in stratum radiatum tend to project to the dendritic trees of pyramidal cells within the same lamina (Freund & Buzsáki, 1996). In this study, we positioned bipolar stimulation electrodes at two different laminar positions. With this technique, it is impossible to know exactly which interneurons have been activated. The dendritic trees and the axonal arborizations of some interneurons cover more than one lamina (Freund & Buzsáki, 1996) and it is possible that our recordings represent a mixture of the activation of somata, dendritic trees and (parts of) axons of different types of interneurons. However, we and others (Pearce, 1993; Banks et al. 1998) found clear differences between IPSPs and IPSCs elicited from different layers. Therefore, it is legitimate to conclude that we preferentially activated different sets of interneurons at the two positions.
We found that the IPSPs and IPSCs elicited by stimulation in stratum radiatum had a slower decay time course than those elicited in stratum oriens. Using slightly different stimulation sites from those of Pearce (1993), our result confirms his earlier finding that the kinetics of IPSCs in CA1 pyramidal cells depends on the laminar position of the stimulated interneurons. Which mechanism underlies the different kinetics? We cannot exclude the possibility that different GABAA receptor subtypes are expressed at the different synapses, as suggested recently (Banks et al. 1998). However, our voltage clamp data show that there is a difference in the relative contribution of the fast decay component. It has been suggested that only the fast component of bi-exponentially decaying IPSCs is determined by kinetic channel properties whereas the slow component depends on the time course of the transmitter in the synaptic cleft (Roepstorff & Lambert, 1994). Thus, the different amplitude ratio suggests that transmitter removal differs between the two sites. This assumption is also corroborated by the finding that tiagabine preferentially affected the slow time constant (see also Roepstorff & Lambert, 1994). We cannot exclude, however, the possibility that spillover of GABA from neighbouring synapses contributes to this slow component when GABA uptake is blocked (Isaacson et al. 1993; Rossi & Hamann, 1998), although the low stimulation strength used in our experiments does not favour this possibility. The different transmitter time course at the two locations may be caused, e.g. by a wider extracellular space at synapses activated by IPSPO and IPSCO. This would facilitate diffusion of the transmitter from the synaptic cleft and make GABA uptake less important at the respective synapses (Draguhn & Heinemann, 1996; Titmus et al. 1996).
Our in situ hybridization experiments suggest a structural explanation for the different efficacy of tiagabine; we found a laminar difference in the proportion of interneurons (marked by the GABA-synthesizing enzyme GAD67) containing GAT-1 (the transporter affected by tiagabine). Cells with somata in stratum oriens express GAT-1 mRNA less frequently than interneurons in stratum radiatum and lacunosum-moleculare. Thus, the latter cells can remove synaptically released GABA from the synaptic cleft more efficiently.
From the in situ hybridization data, we could only determine the laminar position of the interneuron somata expressing GAD67 or GAT-1. We may well have elicited IPSPs and IPSCs from dendrites or axons of cells with somata outside the layers where the stimulation electrodes were positioned. In addition, neither the in situ hybridization material nor the stimulation electrode data allow for a clear demarcation of stratum radiatum from stratum lacunosum-moleculare. Indeed, many interneurons are located around the border between stratum radiatum and lacunosum-moleculare (Houser & Esclapez, 1994) and are likely to be activated with a bipolar stimulation electrode placed in the mid to outer radiatum. Therefore, cells were counted throughout both layers up to the hippocampal fissure. Despite these methodological difficulties, the match between our pharmacological data and the lower expression of GAT-1 in stratum oriens interneurons makes it likely that we have indeed preferentially stimulated cells with somata at the respective locations and that the oriens interneurons make functionally distinct inhibitory synapses on CA1 pyramidal cells than interneurons located in stratum radiatum.
In stratum oriens, we found that more cells were positive for GAD67 mRNA expression than for the other isoform, GAD65. Houser & Esclapez (1994) reported a similar distribution of cells stained for either marker in the hippocampus. However, in their study a fraction of GAD65 mRNA-expressing neurons was less intensely stained and might have been under the detection limit in our slices. Based on our material, we decided to use the GAD67 probe as a marker for interneurons. Other methodological limitations of our quantitative histochemical approach (detection limits, no quantification of staining intensity, potential difference between mRNA and protein expression) were equal for the two locations.
It is interesting to note that β-alanine did not have any effect on the decay time course. β-Alanine preferentially blocks the GABA transporter GAT-3 (Borden, 1996) which is expressed in glial cells in the hippocampus (Ribak et al. 1996). Thus, astrocytic GABA uptake seems to have little influence on the transmitter time course in CA1 at the low stimulation intensities used. This is in contrast to IPSCs in juvenile dentate granule cells (Draguhn & Heinemann, 1996) and to the anticonvulsant action of the substance against epileptiform discharges in CA1 in vitro (Pfeiffer et al. 1996). It is possible that glial GABA uptake only becomes important when large amounts of GABA are liberated simultaneously.
In summary, we found two functional differences between inhibitory synapses at different laminar locations on CA1 pyramidal cells: the decay time course of IPSPO and IPSCO is faster than that of IPSPR and IPSCR and GABA uptake contributes more to the decay of inhibitory potentials elicited from stratum radiatum than those elicited from stratum oriens. The structural basis of this laminar difference appears to be a lower expression of GAT-1 in interneurons located in stratum oriens.
Acknowledgments
We thank Dr H. J. Gabriel and Dr H. Siegmund for their technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft, SFB 515 B1 and INK 21/A1-1.
References
- Banks M, Li T-B, Pearce RA. The synaptic basis of GABAA,slow. Journal of Neuroscience. 1998;18:1305–1317. doi: 10.1523/JNEUROSCI.18-04-01305.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden LA. GABA transporter heterogeneity: pharmacology and cellular localization. Neurochemistry International. 1996;29:335–356. doi: 10.1016/0197-0186(95)00158-1. [DOI] [PubMed] [Google Scholar]
- Borden LA, Dhar TGM, Smith KE, Branchek TA, Weinshank RL, Gluchowski C. Tiagabine, SK&F 89976-A, Cl-966, and NNC-711 are selective for the cloned GABA transporter GAT-1. European Journal of Pharmacology. 1994;269:219–224. doi: 10.1016/0922-4106(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Buhl E, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994;368:823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Gage PW, Robertson B. Inhibitory post-synaptic currents in rat hippocampal CA1 neurons. The Journal of Physiology. 1984;356:551–564. doi: 10.1113/jphysiol.1984.sp015482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Korn SJ. γ-Aminobutyric acid uptake and the termination of inhibitory synaptic potentials in the rat hippocampal slice. The Journal of Physiology. 1985;366:387–409. doi: 10.1113/jphysiol.1985.sp015804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draguhn A, Heinemann U. Different mechanisms regulate IPSC kinetics in early postnatal and juvenile hippocampal granule cells. Journal of Neurophysiology. 1996;76:3983–3993. doi: 10.1152/jn.1996.76.6.3983. [DOI] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. 10.1016/0896-6273(91)90077-D. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsuzsákiaacute;ki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Guastella J, Nelson N, Nelson H, Czyzyk L, Keynan S, Miedel MC, Davidson N, Lester HA, Kanner BI. Cloning and expression of a rat brain GABA transporter. Science. 1990;249:1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- Houser CR, Esclapez M. Localization of mRNAs encoding two forms of glutamic acid decarboxylase in the rat hippocampal formation. Hippocampus. 1994;4:530–545. doi: 10.1002/hipo.450040503. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic action of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. 10.1016/0896-6273(93)90308-E. [DOI] [PubMed] [Google Scholar]
- Michelsen BK, Peterson JS, Boel E, Moeldrup A, Dyrberg T, Madsen D. Cloning, characterization and autoimmune recognition of rat islet glutamic acid decarboxylase in insulin-dependent diabetes mellitus. Proceedings of the National Academy of Sciences of the USA. 1991;88:8754–8758. doi: 10.1073/pnas.88.19.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce RA. Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron. 1993;10:189–200. doi: 10.1016/0896-6273(93)90310-n. 10.1016/0896-6273(93)90310-N. [DOI] [PubMed] [Google Scholar]
- Pearce RA, Grunder SD, Faucher LD. Different mechanisms for use-dependent depression of two GABAA-mediated IPSCs in rat hippocampus. The Journal of Physiology. 1995;484:425–435. doi: 10.1113/jphysiol.1995.sp020675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer M, Draguhn A, Meierkord H, Heinemann U. Effects of γ-aminobutyric acid (GABA) agonists and GABA-uptake inhibitors on pharmacosensitive and pharmacoresistant epileptiform activity in vitro. British Journal of Pharmacology. 1996;119:569–577. doi: 10.1111/j.1476-5381.1996.tb15710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia G, Costa E, Vicini S. Functional diversity of GABA-activated Cl− currents in Purkinje versus granule neurons in rat cerebellar slices. Neuron. 1994;12:117–126. doi: 10.1016/0896-6273(94)90157-0. 10.1016/0896-6273(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Ramamón y cajal S. Estrutura del asta de Ammon y fascia dentata. Anales de la Sociedad Española de Historia Natural. 1893;22 [Google Scholar]
- Ribak CE, Tong WMY, Brecha NC. GABA plasma membrane transporters, GAT-1 and GAT-3, display different distributions in the rat hippocampus. Journal of Comparative Neurology. 1996;367:595–606. doi: 10.1002/(SICI)1096-9861(19960415)367:4<595::AID-CNE9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Roepstorff A, Lambert JDC. Comparison of the effects of the GABA uptake blockers, tiagabine and nipecotic acid, on inhibitory synaptic efficacy in hippocampal CA1 neurones. Neuroscience Letters. 1992;146:131–134. doi: 10.1016/0304-3940(92)90060-k. [DOI] [PubMed] [Google Scholar]
- Roepstorff A, Lambert JDC. Factors contributing to the decay of the stimulus-evoked IPSC in rat hippocampal CA1 neurons. Journal of Neurophysiology. 1994;72:2911–2926. doi: 10.1152/jn.1994.72.6.2911. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M. Spillover-mediated transmission at inhibitory synapses promoted by high-affinity α6 subunit GABAA receptors and glomerular geometry. Neuron. 1998;20:783–795. doi: 10.1016/s0896-6273(00)81016-8. [DOI] [PubMed] [Google Scholar]
- Titmus MJ, Korn H, Faber DS. Diffusion, not uptake, limits glycine concentration in the synaptic cleft. Journal of Neurophysiology. 1996;75:1738–1752. doi: 10.1152/jn.1996.75.4.1738. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JGR. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]


