Abstract
A novel preparation of the oesophagus with attached vagus nerve from the ferret maintained in vitro was used to study the properties of single vagal afferent nerve fibres with identified receptive fields.
Recordings were made from three types of gastro-oesophageal vagal afferent fibres that were classified on the basis of their sensitivity to mechanical stimulation. There were those responding to mucosal stroking (mucosal receptors), to circular tension (tension receptors) and those responding to mucosal stroking and circular tension, which we have termed tension/mucosal (TM) receptors.
The conduction velocities for mucosal, TM and tension receptor fibres were 6.38 ± 1.22 m s−1 (n = 22), 6.20 ± 1.49 m s−1 (n = 13) and 5.33 ± 0.86 m s−1 (n = 22), respectively.
Receptive fields of afferents showed random topographical distribution by fibre type and conduction velocity. They were found mainly distal but also occasionally proximal to the point of vagal dissection.
Twenty-eight per cent of mucosal, 63 % of TM and 43 % of tension receptors responded to one or more drugs or chemical stimuli applied to the receptive field.
In conclusion, this experimental preparation provides evidence for the existence of three types of oesophageal vagal afferent fibre, namely mucosal, tension and the newly identified tension/mucosal receptors.
Despite the widespread occurrence of diseases involving oesophageal pain, little is actually known about the physiology of oesophageal sensory mechanisms. In the oesophageal wall retrograde tracing studies have yielded abundant anatomical data on the precise terminations of afferent endings. These have been found in both muscular and mucosal layers in animals with striated and smooth muscle oesophagi (Hudson & Cummings, 1985; Clerc & Condamin, 1987). There is some evidence that the epithelium is preferentially innervated by the vagus, whereas the muscle layers are innervated by both vagal and splanchnic fibres (Neuhuber, 1987). Specialization of vagal oesophageal mechanoreceptors has been seen in the distal oesophagus of the cat, such that they form laminar endings within the myenteric ganglia (Christensen, 1984). Epithelial endings have been traced and may have twisted paths within the mucosa, sometimes ending very close to the lumen (Hudson & Cummings, 1985; Clerc & Condamin, 1987).
Much of our knowledge about the electrophysiology of visceral afferents has been gained from whole animal experiments using ‘single fibre’ recording techniques first introduced by Paintal (1953) and Iggo (1955). Whilst in vivo studies of this kind have undoubtedly enhanced our understanding of the function of gastrointestinal sensory receptors, they do have a number of drawbacks. Firstly, the exact location and modality of mechanoreceptive fields of isolated single fibres is not easy to define in vivo. Secondly, when examining the chemosensitivity of these sensory receptors it is not possible to control the effective concentration of exogenous agents at the receptor site whether they are applied intra-arterially or intraluminally. This is a particular problem when the applied agent is itself vasoactive and thereby influences its own distribution. Also, it may be difficult to determine whether drugs evoking a discharge in single afferents are acting directly on the fibre ending or indirectly via changes in gastrointestinal motility or the release of mediators from the vasculature. For this reason, studies of oesophageal vagal afferents are mainly confined to their sensitivity to distension (Clerc & Mei, 1983; Satchell, 1984; Sengupta et al. 1989). Reports of oesophageal vagal afferents which are sensitive to luminally applied chemicals are very limited (Harding & Titchen, 1975; Clerc & Mei, 1983). In order to overcome the problems associated with in vivo preparations and to provide a relatively simple and highly controllable preparation we have modified an in vitro preparation developed by Fox et al. (1993) to allow us to study the properties of vagal afferent fibres innervating the oesophagus. This in vitro approach also allows us to plot accurately the location of the receptive fields along the oesophagus. In addition, the characteristics of the fibre will enable us to determine accurately where the receptive fields are within the oesophageal wall, e.g. in the mucosal or muscle layer of the gut wall.
The main purpose of the present study was to examine the mechanical properties of oesophageal vagal afferent fibres in vitro where stimulation can be localized to the receptive field, and additionally to gain further insights into oesophageal chemosensitivity. We have used a novel preparation of the isolated oesophagus with attached vagal nerves from the ferret, a species used recently for the study of reflux disease (Blackshaw et al. 1998) and oesophageal inflammation (Smid et al. 1998) and in which much is known about the properties of vagal afferents elsewhere in the gastrointestinal tract. A preliminary account of this work has been published in abstract form (Blackshaw & Page, 1995)
METHODS
General
Ferrets (0.4–1.0 kg body weight) were deeply anaesthetized with sodium pentobarbitone (50 mg kg−1i.p.) and the thorax was opened by a mid-line incision. The ferrets were then killed by exsanguination. The stomach, oesophagus with attached vagal nerves, heart and lungs were removed and placed in a modified Krebs solution of the following composition (mm): NaCl, 118.1; KCl, 4.7; NaHCO3, 25.1; Na2PO4, 1.3; MgCl, 1.2; CaCl2, 1.5; citric acid, 1.0; glucose, 11.1; bubbled with 95 % O2-5 % CO2. The temperature was maintained at 4°C during dissection to preserve the tissue and prevent metabolic degradation. The heart, lungs and major blood vessels were removed and the vagus nerve cleared of connective tissue. The diaphragm was also cleared from around the lower oesophageal sphincter. The preparation was then opened out longitudinally along the oesophagus and greater curve of the stomach (2 cm length of stomach) and pinned out flat, mucosa side up, in a Perspex chamber (dimensions 13.0 cm × 4.0 cm × 1.0 cm) and perfused at a rate of 11–12 ml min−1 with Krebs bicarbonate buffer solution maintained at 34°C. The vagus nerve (free length 3.0 cm) was drawn through a small hole into an isolated recording chamber (dimensions 5.0 cm × 5.0 cm × 1.0 cm) where it was bathed in paraffin oil. Under a dissecting microscope a small longitudinal incision was made in the nerve sheath. Using fine forceps nerve fibres were teased back onto a platinum recording electrode. Single units were discriminated on the basis of action potential shape, duration and amplitude using a JRAK window discriminator WD1 (Melbourne, Australia). Figure 1 illustrates the in vitro preparation used. All studies were performed in accordance with the guidelines of the Animal Ethics Committees of the Royal Adelaide Hospital and Institute for Medical and Veterinary Science, Adelaide.
Figure 1. Schematic diagram of the apparatus used for recording single gastro-oesophageal afferent fibres in vitro.
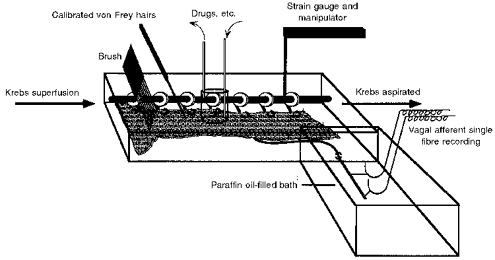
This comprises a Perspex chamber in which the oesophagus and part of the stomach was pinned mucosa uppermost. The vagus nerve was drawn into a second chamber where fibres were teased back onto a recording electrode. Drugs were applied to the afferent fibre receptive field using a small cylinder. Circular tension was applied to the tissue via a pulley system connected to a balance. The pulley system was also used in conjunction with a force transducer to measure muscular activity. The pulley system was always hooked to the edge of the oesophagus adjacent to the receptive field.
Characterization of oesophageal vagal afferent properties
Location of receptive fields along the oesophagus was determined by mechanical stimulation with either a blunt glass rod or a brush. The size of the receptive field was determined by gently probing the receptive field using a 1000 mg von Frey probe and measuring the area where a response was elicited. Mechanical thresholds were determined using calibrated von Frey hairs. Tension response curves were also obtained for each receptive field which were used in combination with von Frey thresholds to determine whether the receptive fields were located in the mucosa or the muscle layer of the oesophageal wall, or both. The tension-response curves were obtained using a pulley system connected to a balance upon which weights were placed (Fig. 1). The pulley system was always hooked to the edge of the oesophagus adjacent to the receptive field under investigation.
In six additional experiments (without electrophysiological recordings) the pulley system was connected to a sensitive force transducer with a preload of 1 g. We were thus able to determine if the chemical stimuli tested on afferent endings (see below) had any effect on muscular activity, and therefore if their effect on afferent endings could be explained by a secondary action via evoked muscle contraction. As contractile responses to the agents were negligible (< 0.1 g in amplitude), often despite ongoing phasic contractile activity of the muscularis mucosae > 0.1 g in amplitude, substance P (10−3m) and acetylcholine (10−2m) were also applied in these additional experiments in order to stimulate more powerfully muscular activity. Muscular responses to these agents were below 0.5 g in amplitude which was below the threshold for tension-sensitive fibres. It was concluded that responses of afferent endings to chemical stimuli were not caused indirectly by induced muscular activity, and no further attempts were made to correlate chemically induced afferent responses with muscular activity.
Of the mechanically sensitive vagal afferent fibres recorded, fifty-seven were characterized as myelinated or unmyelinated according to their conduction velocity calculated from the time and distance between a stimulating (1 Hz, 20 V, 0.1 ms duration) electrode placed over the receptive field and the recording electrodes. Afferent fibres with conduction velocities below 2.0 m s−1 (Cervero & Sharkey, 1988) were classified as C fibres and those less than 25 m s−1 as Aδ fibres. Aβ fibres were not encountered during these experiments. Chemosensitivity of oesophageal vagal afferents was determined after mechanical thresholds had been established. The chemosensitivity of oesophageal fibres was examined by applying chemicals directly onto the mucosal surface overlying the receptive field; agents were introduced to the receptive field by means of a small cylindrical chamber (1 cm diameter) placed around the area of the receptive field (Fig. 1).
A number of agents were tested for their ability to evoke discharges in both myelinated and unmyelinated fibres. Hydrochloric acid (25–150 mm), 5-hydroxytryptamine (0.1 mm; 5-HT), bradykinin (1 μm), prostaglandin E2 (0.1 mm; PGE2), and capsaicin (1 mm) were applied to the receptive fields for periods of up to 7 min. These drugs were selected because of their possible involvement in inflammation (i.e. oesophagitis) or because of their previously documented effect on vagal afferents in other regions of the gastrointestinal tract. In all experiments the mechanical sensitivity of receptive fields was checked between each drug application to ensure continued viability of the unit under investigation. Further application of other drugs did not occur if a certain drug desensitized the receptive field to mechanical stimulation. Unless specified, treatments did not alter mechanical sensitivity. The dose of drug applied to the receptive field was consistent with doses used in pharmacological studies on the gut and electrophysiological studies on afferent fibres (Pike & Kerr, 1987; Belmonte et al. 1991; Mu et al. 1992; Fox et al. 1993). After removal of the drug from around the receptive field the afferent fibres were allowed to return to a normal baseline level of activity. Five minutes of normal baseline activity were maintained before the addition of another drug.
Data recording and analysis
Afferent impulses were amplified with a biological amplifier (JRAK, BA.1) and scaling amplifier (JRAK, SA.1), filtered (JRAK F.1 filter) and monitored using an oscilloscope (Yokogawa, DL 1200A). All data were recorded on magnetic tape and analysed off-line using a personal computer (Apple Macintosh IIfx). Peristimulus-time histograms and discharge traces were displayed using LabView software.
Drugs
Stock solutions of all drugs were kept frozen and diluted to their final concentration in Krebs solution on the day of the experiment. Capsaicin, bradykinin, 5-hydroxytryptamine creatinine sulphate and prostaglandin E2 were all obtained from Sigma.
RESULTS
Mechanical and biophysical properties of gastro-oesophageal vagal afferent fibres
Three types of mechanosensitive fibre were observed using this in vitro preparation (Figs 2 and 3): those responding to circular tension but not to low intensity mucosal stimuli (tension receptors, n = 38), those responding only to mucosal stroking (mucosal receptors, n = 48), and those responding to both mucosal stroking and circular tension, which we have termed tension/mucosal (TM, n = 16) receptors. The group data for responses of each type of fibre to graded tension are illustrated in Fig. 2. An example of each type of fibre is illustrated in Fig. 3. Action potentials evoked in a TM receptor in response to mucosal stroking with a 10 mg von Frey hair and 1 g circular tension are illustrated in Fig. 3B (inset) and demonstrate that the responses to the two different types of stimuli are from the same afferent fibre. Tension receptors often responded to mucosal probing with the 200 mg von Frey hair (Fig. 3C), which was observed to distort the underlying muscular layer.
Figure 2. Tension-response curves of the three types of afferent fibre in this study: mucosal receptors (▵; n = 8), TM receptors (^; n = 16), and tension receptors (□; n = 13).
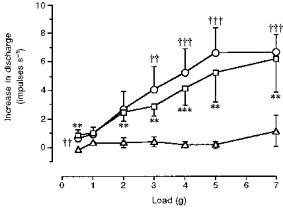
Increase in discharge rate above baseline is shown on the y-axis, and applied force on the x-axis. Only tension and TM receptors showed sigmoidal response curves. Mucosal receptors were insensitive to graded tension. Significant differences between mucosal and tension or TM receptors (analysed by Mann-Whitney U test) are shown nearest each curve (**P < 0.01, ***P < 0.001 tension vs. mucosal receptor responses; ††P < 0.01, †††P < 0.001 TM vs. mucosal receptor responses).
Figure 3. Typical responses of mucosal (A), TM (B) and tension (C) receptors to mucosal stroking with calibrated von Frey hairs (left-hand panels; raw traces) and circular tension (right-hand panels; spike frequency).
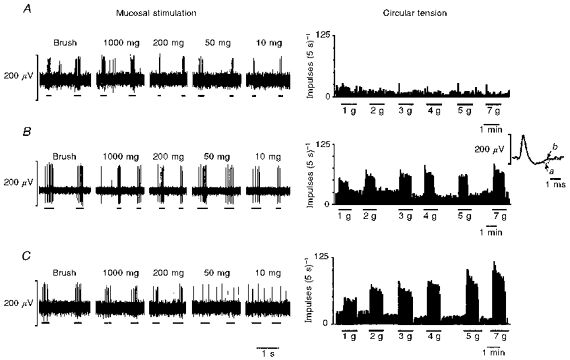
The inset in B illustrates the average spike shape of the TM unit in response to mucosal stroking with a 10 mg calibrated von Frey hair (a) and 1 g circular tension (b), showing that both responses were obtained from the same unit.
The conduction velocities (when tested) for mucosal, TM and tension receptor fibres were 6.38 ± 1.22 m s−1 (0.4–18.75 m s−1; n = 22), 6.20 ± 1.49 m s−1 (0.7–16.7 m s−1; n = 13) and 5.33 ± 0.86 m s−1 (0.75–12.5 m s−1; n = 22), respectively. The conduction velocities did not form a normal Gaussian distribution. There was no significant difference in conduction velocities between the three groups using the non-parametric Kruskal-Wallis test. Thirty-six per cent of mucosal receptors, 31 % of TM receptors and 27 % of tension receptors were classified as C fibres. The remainder of the fibres tested comprised Aδ fibres. Mechanical thresholds to mucosal stroking and circular tension of C fibres and Aδ fibres in each functional group of afferent is illustrated in Fig. 4 (left-hand side). The C fibres and Aδ fibres have broadly similar sensitivity profiles within each group. The distribution of C fibres and Aδ fibres is also illustrated in Fig. 4: both types appeared to have a random distribution over the surface of the oesophagus but predominantly distal to the point of vagal dissection (shown on Fig. 6).
Figure 4. The sensitivity (left-hand histograms) and distribution (right-hand schematics) of mucosal (A), TM (B) and tension (C) with respect to both C and Aδ fibres.

Two types of stimuli were employed: stroking of the receptive field with calibrated von Frey hairs (10–1000 mg force) or graded tension using balance weights applied via a lever/pulley system (0.5–7.0 g).
Figure 6. Schematic distribution of single mucosal (A, n = 48), TM (B, n = 16) and tension (C, n = 38) receptive fields on the oesophagus and stomach of the ferret.
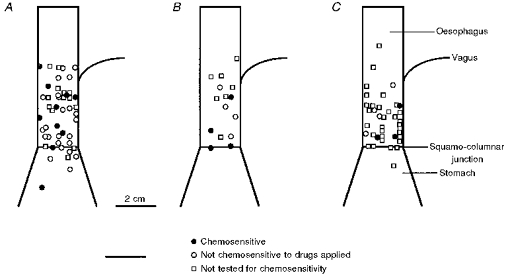
Each point represents the receptive field of a single afferent fibre. •, fibres that responded to one or more chemical stimuli; ^, fibres that did not respond to any of the drugs applied to the receptive field; □, afferents that were not tested for chemosensitivity.
In general, mucosal receptors did not show marked resting activity, although some spontaneous discharge was occasionally evident, as illustrated in Fig. 5. The spontaneous activity of the mucosal receptors had no apparent rhythm (Fig. 5A), whereas some tension and TM receptors fired with regular rhythmic activity (Fig. 5B and C). In some cases tensioning of the tissue when pinned out was found to have induced this. TM receptors were only found in the oesophagus and lower oesophageal sphincter and not in the stomach.
Figure 5. Spontaneous activity of a single mucosal (A), TM (B) and tension (C) receptor.
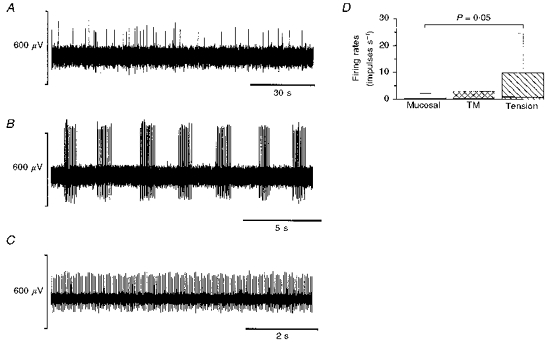
The columns in D denote the interquartile range of the mucosal (n = 28), TM (n = 15) and tension (n = 22) fibre spontaneous firing rates. The bold line within the bar is the median and for mucosal and TM units the value was zero. The bars above the columns indicate the 95 % confidence level. The spontaneous activities of mucosal and tension receptors were significantly different (P = 0.05; using a Mann-Whitney U test).
The receptive fields were 1–3 mm2 in size and there was no difference in the size between the three groups. The distribution of all the mucosal-, TM- and tension-sensitive afferent receptive fields is illustrated in Fig. 6A, B and C, respectively (not all were tested for conduction velocity; see Fig. 4). Receptive fields were randomly distributed across the oesophagus and predominantly distal to the point of vagal dissection. Some units were found proximal to the point of vagal dissection, suggesting that the fibres run down as well as up the oesophagus.
Chemosensitivity of gastro-oesophageal vagal afferents
The chemosensitivity of gastro-oesophageal vagal afferents is illustrated in Table 1 and Fig. 6. A vagal afferent fibre was considered to be chemosensitive if it responded to one or more of the drugs applied to the receptive field. Twenty-eight per cent of mucosal, 63 % of TM and 43 % of tension receptors were chemosensitive; 33 % of mucosal Aδ fibres and 17 % of mucosal C fibres were chemosensitive. Therefore, chemosensitivity of mucosal receptors occurred relatively infrequently. Typical responses of oesophageal mucosal receptors to chemical stimulation are illustrated in Fig. 7.
Table 1.
Summary of the proportion of gastro-oesophageal vagal afferent fibres sensitive to the application of capsaicin (Cap), hydrochloric acid (HCl), 5-hydroxytryptamine (5-HT), bradykinin (BK) and prostaglandin E2 (PGE2) to the mucosal surface directly above the afferent receptive field
| Type | Cap (1 mm) | HCl (150 mm) | 5-HT (0.1 mm) | BK (1 μm) | PGE2 (0.1 mm) |
|---|---|---|---|---|---|
| Mucosal | 6/21 | 3/33 | 3/22 | 2/23 | 3/20 |
| TM | 1/7 | 1/7 | 2/8 | 4/8 | 2/7 |
| Tension | 3/5 | 1/5 | 1/6 | 1/7 | 1/6 |
Numerator corresponds to number of fibres responding, and denominator represents the number of fibres that were tested.
Figure 7. Typical oesophageal mucosal receptor responses to bradykinin (1 μm; A), 5-hydroxytryptamine (0.1 mm; B), prostaglandin E2 (0.1 mm; C), capsaicin (1 mm; D) and hydrochloric acid (150 mm; E).
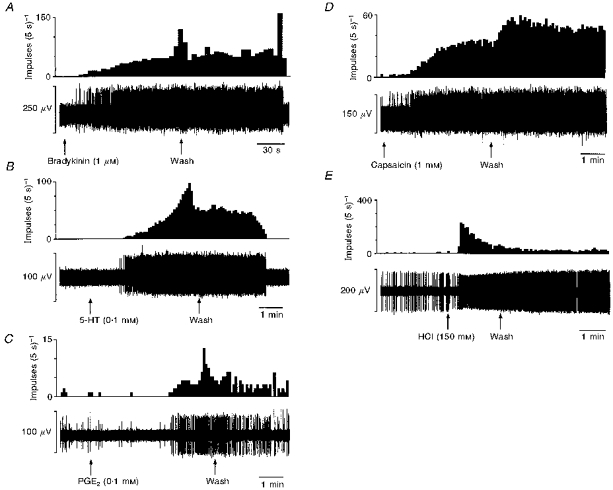
The top trace for each section is spike frequency and the bottom trace is raw recording of electrical activity. The responses in this figure are not all from the same fibre.
With the low yield of chemosensitive vagal afferents it is difficult to quantify population characteristics of time course, reproducibility, desensitization, threshold concentration and rapidity of onset and end of response. Therefore, these details are given in general terms grouping the three types of afferents together. In terms of chemosensitivity there were no obvious differences between the three types of afferents studied, although only one tension receptor showed chemosensitivity to more than one stimulus. Responses of fibres to bradykinin (Fig. 7A) showed a latency in the range of 20–60 s before onset. A further minute elapsed before the maximum discharge rate was reached. If a response to bradykinin occurred this could be repeated upon a second application. The responses stopped abruptly between 1 and 30 min after the initial washout.
Application of 5-HT (Fig. 7B) to the receptive field elicited a response with onset in the range of 1–2 min latency. A maximum firing rate was obtained within the range of 3–4.5 min after the application of 5-HT. The response stopped within 1–4 min of the initial washout. The response obtained to 5-HT was repeatable and also dose dependent over the range 0.1–10 mm.
The latency of response after application of PGE2 (Fig. 7C) ranged from 1 to 4 min, with a peak discharge frequency in the range of 3–5 min after application of PGE2. After the initial washout there was a gradual decrease in firing rate such that it took 1–4 min for discharge to return to basal levels. The latency to onset of responses to capsaicin (Fig. 7D) ranged from 30 s to 2 min, reaching a maximum firing rate within 2–4 min of application. Washout of the capsaicin response occurred after 10–40 min. The response was not always repeatable and desensitization was evident to further application of capsaicin and also to mechanical stimulation. Some units that did not respond to capsaicin were desensitized to mechanical stimulation after application of capsaicin.
In the case of HCl (Fig. 7E) a range of concentrations was applied to the receptive fields (25–150 mm). The afferents responded to 150 mm HCl but not to lower concentrations. On application of HCl to the mucosal surface there was a latency of 20 s-2 min before the onset of the response. The onset was rapid reaching a maximum firing rate within 20 s. The response lasted between 3 and 15 min after washout. On second application the response was either greatly reduced or non-existent. There was also a general desensitization to mechanical stimulation after application of HCl.
The responses of the TM- and tension-sensitive afferents to compounds applied to the receptive field were not secondary to changes in muscular activity as the compounds had no effect on recorded or observed muscular activity at the doses applied to the afferent receptive field (data not shown).
DISCUSSION
The present study provides the first in vitro examination of the general properties of single vagal afferent fibres innervating the gastro-oesophageal region. Recordings were made from Aδ and C fibres which had discrete receptive fields within the gastro-oesophageal wall. Three types of oesophageal vagal afferent fibres were classified according to their responses to mechanical stimulation: first, those responsive to low intensity mucosal stimuli (10 mg) but not to circular tension; second, those responsive to circular tension and to high intensity mucosal stimuli (≥ 200 mg); third, those responsive to low intensity mucosal stimuli (10 mg) and to circular tension. Vagal afferents in the stomach and intestine were previously found to exist in two distinct populations: those exquisitely sensitive to muscular contraction and distension - known as tension receptors (Iggo, 1955; Andrews et al. 1980; Cottrell & Iggo, 1984a; Blackshaw et al. 1987; Blackshaw & Grundy, 1990, 1993b), and those sensitive to mucosal stimuli. There have been many reports on mucosal sensitivity, describing afferent responses to light stroking, inorganic and fatty acids, hypo- and hyperosmolarity, in addition to close systemic stimuli such as 5-hydroxytryptamine, cholecystokinin and bradykinin (e.g. Davison, 1972; Clarke & Davison, 1978; Cottrell & Iggo, 1984b; Blackshaw & Grundy, 1990, 1991, 1993a,b). In addition, retrograde tracing studies have shown terminations of vagal afferent endings in both muscular and mucosal layers of the oesophageal wall (Hudson & Cummings, 1985; Clerc & Condamin, 1987). The first two types of vagal afferent fibre that we encountered (mucosal and tension receptor afferent fibres) correspond to those which have been previously described anatomically in the oesophagus and electrophysiologically in the abdominal viscera. However, the third type of afferent fibre observed that responds to both mucosal stimulation and tension has not been specifically documented. We have used the new term tension/mucosal (TM) receptors to describe these fibres based on their adequate mechanical stimuli. There are two possible options for the position of the TM receptive field: either there are two receptive fields, topographically superimposed one in the mucosa, the other in the muscular layer, or there is one receptive field interposed in the muscularis mucosae. The spontaneous activity of some of these units would suggest the latter as the spasmodic rhythmic activity appears to coincide with the peak contractions of the muscularis mucosae measured in parallel experiments. Davison (1972) also observed a vagal afferent fibre that appeared to be located in the muscularis mucosae of the duodenum. In terms of mechanical sensitivity the oesophageal TM receptors observed in this study closely resembled Davison's fibre.
Approximately one-third of the vagal afferent fibres whose conduction velocities were measured were classified as C fibres. The remainder comprised Aδ fibres. The conduction velocities seen here are similar to those reported in vivo in species including sheep (Cottrell & Iggo, 1984a), opossum (Sengupta et al. 1989, 1992) and ferret (Andrews & Lang, 1982). This is in contrast with other reports in rat (Clarke & Davison, 1978; Cervero & Sharkey, 1988) and cat (Clerc & Mei, 1983) which have shown that intestinal tension receptors are all C fibres, or that only Aδ fibres are present in sheep (Falempin et al. 1978). There was no difference in this ratio between the three modalities of fibre observed in the present study. The mechanical sensitivity of the C fibres and Aδ fibres was not significantly different within the three groups. Fox et al. (1993) showed that mechanical thresholds of Aδ fibres were significantly lower than those of C fibres. Such a lower threshold of Aδ fibres is evident in observations of tension receptor thresholds (Fig. 4C) in the eight cases reported in the present study, although data are too few to confirm this statistically. The topographical distribution of the Aδ and C fibres we observed was random across the oesophagus but predominantly distal to the point of vagal dissection.
Examination of the chemosensitivity of mucosal, TM and tension receptors showed that a proportion of all types of fibres responded to one or more of the chemical stimuli. Only 28 % of mucosal receptors and 63 % of TM receptors showed chemosensitivity. This was perhaps surprising as the receptive fields of these afferents are probably on or near to the luminal surface of the oesophagus (as evidenced by their fine mechanical sensitivity) and therefore exposed to various chemicals, both in terms of food ingested and also acid and pepsin contained in refluxed gastric juice. Our initial explanation for this infrequent sensitivity of mucosal receptors was the inability of the drugs to reach the receptive field due to mucous secretion or the effective diffusion barrier of the epithelial cells. However, a tension receptor showed similar features of chemosensitivity even though it was probably situated much deeper within the outer striated muscle layers of the oesophagus. Therefore it may be concluded that a proportion of mechanically sensitive mucosal receptors are genuinely insensitive to the chemical stimuli tested in this study. The delay between application of drug to the receptive field and onset of response may be due to mucous secretion and epithelial thickness. The range in latency to onset of response may be due to the varying degrees of mucous secretion between preparations. It is difficult to compare chemosensitivity with that observed in other studies for several reasons; most importantly, there are very few data available in the literature, and in particular there are no studies that have investigated chemosensitivity of oesophageal vagal afferents in vitro. In vivo, many more mucosal afferents appear from preliminary reports to show chemosensitivity (Harding & Titchen, 1975) than in our in vitro preparation. Other studies have focused on the stomach and small intestine, where a greater proportion of mucosal afferents shows chemosensitivity than that observed in the oesophagus in the present study (Davison, 1972; Clarke & Davison 1978; Blackshaw & Grundy, 1990, 1991, 1993a). This is despite the use of a similar range of stimuli. It may be argued teleologically that the mucosal afferents within the oesophagus do not require the same level of chemosensitivity as the afferents within the duodenal wall in order to provide feedback control of digestive function. Alternatively, there may be a population of oesophageal mucosal afferents that are exclusively chemosensitive and show no mechanical sensitivity, which we may have overlooked due to sampling bias. However, recruitment of extra units was never observed after application of drugs to the area around mechanosensitive receptive fields, suggesting that this is not the case.
Chemosensitivity to naturally occurring substances was observed not only in mucosal and TM receptors, where one might expect it, but also in one of the seven tension receptors tested. Its response to chemicals did not appear to be due to induced contractile responses of the musculature because, at the doses used, chemical stimuli induced no measurable motor response. We cannot, however, completely exclude the possibility that there may have been small, very localized responses of the musculature within the small area around the receptive field to which the drugs were applied, which may have escaped detection by our force transducer. In healthy tissue in vivo, exposure of afferent endings in the deeper muscular layers of the oesophagus to refluxed gastric acid would probably be minimal due to the efficient removal of acid diffusing across the mucosa by increases in local blood flow. Chemosensitivity of tension receptors to acid in vivo is therefore only likely to be of significance in pathophysiological states. In oesophagitis, mucosal lesions are often such that the oesophageal muscularis externa and muscularis mucosae are directly exposed to refluxed acid. Chemosensitivity of tension receptors to the inflammatory mediators bradykinin, 5-HT and PGE2 could be physiologically significant under other circumstances. Endogenous release of these substances in response to injury may occur throughout all the layers of the gut wall, and would not require disruption of the epithelial barrier in vivo to allow access to afferent endings.
Direct sensitivity of afferent endings to the chemical stimuli used in this study has been previously documented in other regions of the gastrointestinal tract (see Cervero (1994) for references), where their endogenous release is associated with induction of pain or nausea. Controversy exists, however, as to the capability of drugs such as 5-HT and bradykinin to evoke direct responses in tension receptors. Blackshaw & Grundy (1990, 1991, 1993b), Cottrell & Iggo (1984b) and Sengupta et al. (1992) all found that responses of vagal tension receptors to drugs in vivo were secondary to evoked smooth muscle contraction. The single tension receptor which showed chemosensitivity in the present study did appear to show genuine direct responses, for reasons outlined earlier. Although this was an isolated observation in the face of a lack of chemosensitivity in tension receptors elsewhere in the present study and elsewhere in the literature, it is tempting to speculate that features of our preparation may reveal chemosensitivity in a subset of tension receptors.
In previous studies, acute excitation of sensory fibres by capsaicin was found to be either restricted to high-threshold C fibres (Fox et al. 1993) or unrelated to sensory modality or conduction velocity (e.g. Belmonte et al. 1991) as was the case in the present study. Although the chronic degenerative actions of capsaicin are known to be restricted mainly to C fibres, acute excitation by capsaicin is less predictable. Further comparative studies on acute in vitro and in vivo excitatory and desensitizing actions of capsaicin are currently in progress in our laboratory (Page et al. 1998).
There was no significant difference in overall chemosensitivity between Aδ and C fibres, although there was a tendency for more mucosal Aδ fibres to show chemosensitivity than mucosal C fibres. An in vitro study of single vagal afferents innervating guinea-pig airways showed that Aδ fibres never showed chemosensitivity, whereas all C fibres did (Fox et al. 1993). We must therefore conclude that such a distinction between fibre types is neither organ- nor species-specific.
In conclusion, the present study shows that properties of gastro-oesophageal vagal afferents may be studied directly in vitro. The response of tension-sensitive vagal afferent fibres to circular tension in vitro and oesophageal distension in vivo (Clarke & Davison, 1974; Satchell, 1984; Sengupta et al. 1989) is comparable. This suggests that the functional response studies are not distorted by this new in vitro technique. The technique allows direct examination of gastro-oesophageal sensory fibres without the problems associated with whole-animal studies. This preparation has also provided evidence for the existence of three types of oesophageal vagal afferent fibres. Whereas previously there were only thought to be mucosal and tension receptors, we have identified a third group of afferents named TM receptors which are sensitive to both mucosal stroking and circular tension. Future studies are aimed at determining changes in the physiology of these groups of fibres in a model of oesophageal inflammation.
Acknowledgments
We wish to acknowledge the financial support of the National Health and Medical Research Council of Australia and Astra Hässle AB.
References
- Andrews PLR, Grundy D, Scratcherd T. Vagal afferent discharge from mechanoreceptors in different regions of the ferret stomach. The Journal of Physiology. 1980;298:513–524. doi: 10.1113/jphysiol.1980.sp013098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PLR, Lang KM. Vagal afferent discharge from mechanoreceptors in the lower oesophagus of the ferret. The Journal of Physiology. 1982;322:29P. doi: 10.1113/jphysiol.1980.sp013098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte C, Gallar J, Pozo MA, Rebollo I. Excitation by irritant chemical substances of sensory afferent units in the cat's cornea. The Journal of Physiology. 1991;437:709–725. doi: 10.1113/jphysiol.1991.sp018621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. Journal of the Autonomic Nervous System. 1990;31:191–202. doi: 10.1016/0165-1838(90)90185-l. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Bradykinin effects on vagal afferent discharge and motility in the stomach and duodenum. Journal of Gastrointestinal Motility. 1991;3:173. [Google Scholar]
- Blackshaw LA, Grundy D. Effects of 5-hydroxytryptamine on discharge of vagal mucosal afferent fibres from the upper gastrointestinal tract of the ferret. Journal of the Autonomic Nervous System. 1993a;45:41–50. doi: 10.1016/0165-1838(93)90360-7. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Effects of 5-hydroxytryptamine (5HT) on the discharge of vagal mechanoreceptors and motility in the upper gastrointestinal tract of the ferret. Journal of the Autonomic Nervous System. 1993b;45:51–59. doi: 10.1016/0165-1838(93)90361-w. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D, Scratcherd T. Vagal afferent discharge from gastric mechanoreceptors during contraction and relaxation of the ferret corpus. Journal of the Autonomic Nervous System. 1987;18:19–24. doi: 10.1016/0165-1838(87)90130-5. 10.1016/0165-1838(87)90130-5. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Page AJ. In vitro recordings of gastro-oesophageal vagal afferent fibres in the ferret. Neurogastroenterology and Motility. 1995;7:247. [Google Scholar]
- Blackshaw LA, Staunton E, Dent J, Holloway RH, Malbert CH. Mechanisms of gastroesophageal reflux in the ferret. Neurogastroenterology and Motility. 1998;10:49–56. doi: 10.1046/j.1365-2982.1998.00085.x. [DOI] [PubMed] [Google Scholar]
- Cervero F. Sensory innervation of the viscera: peripheral basis of visceral pain. Physiological Reviews. 1994;74:95–138. doi: 10.1152/physrev.1994.74.1.95. [DOI] [PubMed] [Google Scholar]
- Cervero F, Sharkey KA. An electrophysiological and anatomical study of intestinal afferent fibres in the rat. The Journal of Physiology. 1988;401:381–397. doi: 10.1113/jphysiol.1988.sp017168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J. Origin of sensation in the esophagus. American Journal of Physiology. 1984;9:221–225. doi: 10.1152/ajpgi.1984.246.3.G221. [DOI] [PubMed] [Google Scholar]
- Clarke GD, Davison JS. Tension receptors in the oesophagus and stomach of the rat. The Journal of Physiology. 1974;244:41–42P. [PubMed] [Google Scholar]
- Clarke GD, Davison JS. Mucosal receptors in the gastric antrum and small intestine of the rat with afferent fibres in the cervical vagus. The Journal of Physiology. 1978;284:55–67. doi: 10.1113/jphysiol.1978.sp012527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc N, Condamin M. Selective labelling of vagal sensory nerve fibers in the lower esophageal sphincter with anterogradely transported WGA-HRP. Brain Research. 1987;424:216–224. doi: 10.1016/0006-8993(87)91464-8. [DOI] [PubMed] [Google Scholar]
- Clerc N, Mei N. Vagal mechanoreceptors located in the lower oesophageal sphincter of the cat. The Journal of Physiology. 1983;336:487–498. doi: 10.1113/jphysiol.1983.sp014593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell DF, Iggo A. Tension receptors with vagal afferent fibres in the proximal duodenum and pyloric sphincter of sheep. The Journal of Physiology. 1984a;354:457–475. doi: 10.1113/jphysiol.1984.sp015388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell DF, Iggo A. The responses of duodenal tension receptors in sheep to pentagastrin, cholecystokinin and some other drugs. The Journal of Physiology. 1984b;354:477–495. doi: 10.1113/jphysiol.1984.sp015389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison JS. Response of single vagal afferent fibres to mechanical and chemical stimulation of the gastric and duodenal mucosa in cats. Quarterly Journal of Experimental Physiology. 1972;57:405–416. doi: 10.1113/expphysiol.1972.sp002176. [DOI] [PubMed] [Google Scholar]
- Falempin M, Mei N, Rousseau JP. Vagal mechanoreceptors of the inferior thoracic oesophagus, the lower oesophageal sphincter and the stomach in the sheep. Pflügers Archiv. 1978;373:25–30. doi: 10.1007/BF00581145. [DOI] [PubMed] [Google Scholar]
- Fox AJ, Barnes PJ, Urban L, Dray A. An in vitro study of the properties of single vagal afferents innervating guinea-pig airways. The Journal of Physiology. 1993;469:21–35. doi: 10.1113/jphysiol.1993.sp019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding R, Titchen DA. Chemosensitive vagal endings in the oesophagus of the cat. The Journal of Physiology. 1975;247:52–53P. [PubMed] [Google Scholar]
- Hudson LC, Cummings JF. The origins of innervation of the esophagus of the dog. Brain Research. 1985;326:125–136. doi: 10.1016/0006-8993(85)91391-5. [DOI] [PubMed] [Google Scholar]
- Iggo A. Tension receptors in the stomach and urinary bladder. The Journal of Physiology. 1955;128:593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu JZ, Hogan DL, Koss MA, Isenberg JI. Theophylline and prostaglandin E2 on duodenal bicarbonate secretion: role for 5′-cyclic adenosine monophosphate. Gastroenterology. 1992;103:153–159. doi: 10.1016/0016-5085(92)91108-g. [DOI] [PubMed] [Google Scholar]
- Neuhuber WL. Sensory vagal innervation of the rat esophagus and cardia: a light and electron microscopic anterograde tracing study. Journal of the Autonomic Nervous System. 1987;20:243–255. doi: 10.1016/0165-1838(87)90153-6. [DOI] [PubMed] [Google Scholar]
- Page AJ, Partosoedarso ER, Blackshaw LA. Acute capsaicin sensitivity of gastrointestinal vagal afferents. Neurogastroenterology & Motility. 1998;10:89. [Google Scholar]
- Paintal AS. Impulses in vagal afferent fibres from stretch receptors in the stomach and their role in the peripheral mechanism of hunger. Nature. 1953;172:1194–1195. doi: 10.1038/1721194a0. [DOI] [PubMed] [Google Scholar]
- Pike GK, Kerr DIB. The influence of temperature upon depolarizing responses of rat isolated vagal nerve to 5-hydroxytryptamine. Brain Research. 1987;413:388–391. doi: 10.1016/0006-8993(87)91035-3. [DOI] [PubMed] [Google Scholar]
- Satchell PM. Canine oesophageal mechanoreceptors. The Journal of Physiology. 1984;346:287–300. doi: 10.1113/jphysiol.1984.sp015022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta JN, Kauvar D, Goyal RK. Characteristics of vagal esophageal tension-sensitive afferent fibers in the opossum. Journal of Neurophysiology. 1989;61:1001–1010. doi: 10.1152/jn.1989.61.5.1001. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Saha JK, Goyal RK. Differential sensitivity to bradykinin of esophageal distension-sensitive mechanoreceptors in vagal and sympathetic afferents of the opossum. Journal of Neurophysiology. 1992;68:1053–1067. doi: 10.1152/jn.1992.68.4.1053. [DOI] [PubMed] [Google Scholar]
- Smid SD, Page AJ, O'Donnellapos;donnell T, Langman J, Rowland R, Blackshaw LA. Oesophagitis-induced changes in capsaicin-sensitive tachykininergic pathways in the ferret lower oesophageal sphincter. Neurogastroenterology and Motility. 1998 doi: 10.1046/j.1365-2982.1998.00118.x. (in the Press) [DOI] [PubMed] [Google Scholar]


