Abstract
4-Aminomethylbenzoic acid, a molecule which mimics the spatial configuration of a dipeptide, competitively inhibits peptide influx in both Xenopus laevis oocytes expressing rabbit PepT1 and through PepT1 in rat renal brush border membrane vesicles.
This molecule is not translocated through PepT1 as measured both by direct HPLC analysis in PepT1-expressing oocytes and indirectly by its failure to trans-stimulate labelled peptide efflux through PepT1 in oocytes and in renal membrane vesicles.
However 4-aminomethylbenzoic acid does reverse trans-stimulation through expressed PepT1 of labelled peptide efflux induced by unlabelled peptide. Quantitatively this reversal is compatible with 4-aminomethylbenzoic acid competitively binding to the external surface of PepT1.
4-Aminomethylbenzoic acid (the first molecule discovered to be a non-translocated competitive inhibitor of proton-coupled oligopeptide transport) and its derivatives may thus be particularly useful as experimental tools.
Since the discovery of the natural plant product phlorizin (Nakazawa, 1922) as a highly specific, non-transported, inhibitor of glucose absorption in kidney and small intestine, it has been of great value in elucidating the many different physiological roles of sodium-coupled glucose transport (for example in lung and choroid plexus, see Hediger & Rhoads, 1994). In the field of peptide transport no such non-transported inhibitor has yet been described, and the absence of such a reagent may explain the relatively slow progress made in investigating the function (Matthews, 1991; Fei et al. 1998) of peptide transporters, e.g. within the central nervous system. We report here that 4-aminomethylbenzoic acid (4-AMBA) is such an inhibitor and suggest that it may form the basis for the design of synthetic high-affinity ligands for the PepT1 family (analogous to phlorizin for the sodium-glucose cotransporter SGLT). This finding is also of interest because of the very close chemical similarity between 4-AMBA, a competitive inhibitor which is not translocated, and 4-aminophenylacetic acid (4-APAA) which recently has been shown to be a substrate for translocation by epithelial peptide transporters (Temple et al. 1998).
METHODS
4-Aminomethylbenzoic acid (4-AMBA), 4-aminophenylacetic acid (4-APAA) and D-Phe-L-Gln were synthesized in-house using standard techniques. 4-AMBA and 4-APAA are also available commercially (Sigma-Aldrich, Poole, UK). [4-3H-d-Phe]-l-Gln was custom synthesized by Zeneca (Northwich, Cheshire, UK). All other reagents were of research grade and obtained as previously described (Temple et al. 1998).
All animal procedures were carried out under Home Office guidelines. Brush border membrane vesicles were obtained from rat renal cortex using standard methods (Biber et al. 1981). Renal tissue was removed from rats under pentobarbitone (Sagatal) anaesthesia (30 mg, i.p.), and the animal killed by cervical dislocation. Flux measurements through PepT1 (Temple & Boyd, 1998) were determined by rapid filtration, under initial rate conditions at 37°C as described previously (Temple et al. 1995, 1996). Xenopus laevis were anaesthetized with 0.1 % w/v MS-222 (Sigma, Poole, UK) prior to ovariectomy. Oocyte expression of rabbit PepT1 cRNA was as previously described (Fei et al. 1994; Meredith et al. 1996) and influx and efflux experiments were performed at room temperature as described in Temple et al. 1998. HPLC analysis of 4-AMBA and 4-APAA was determined using a modified method of Temple et al. 1996. Oocytes were suspended in 3 % v/v perchloric acid (35 μl), centrifuged for 10 min at 14 000 g; 30 μl of 1 m KOH was added to 30 μl of supernatant and samples freeze-thawed and recentrifuged; 20 μl of resulting supernatant was injected onto a 250 mm C18-RP column (Merck). Elution was carried out using methanol-H2O (25 % for 4-AMBA and 2 % for 4-APAA) mobile phase buffered with 21 mm KH2PO4 at a flow rate of 1 ml min−1 and sample detected by UV absorbance at 236 nm (Lister et al. 1995). The level of sensitivity of the assay is some 7-fold lower for 4-AMBA compared with 4-APAA (integrated area under the curve for 1 nmol of 4-AMBA is 453 compared with 3351 for 4-APAA).
The molecular modelling of 4-AMBA and 4-APAA (on a Power Macintosh G3) was performed using CambridgeSoft Chem3D Pro version 3.5.1 and was controlled by a custom Applescript (K. M. Morgan). This generated random starting points and governed the fine tuning of minimizations in order to obtain global minimum energy conformations.
Kinetic calculations
Inhibition constants (and apparent inhibition constants) for influx were calculated from standard Michaelis-Menten kinetics as described previously (Temple et al. 1995). Efflux rate constants were calculated as described by Temple et al. (1996). Kinetics describing efflux of labelled substrate (S) from oocytes into a medium containing external analogue (substrate, A) and increasing concentration of external unlabelled inhibitor (I) is shown:
| (1) |
where VS, KS, VSA, KSA, KA and KI are also defined (Deves & Boyd, 1989).
RESULTS
Figure 1 shows experiments using both rabbit PepT1 expressed in Xenopus laevis oocytes (Fig. 1A) and native epithelial membranes (rat renal cortex brush border membrane vesicles (BBMV), Fig. 1B). The results in both systems show that 4-aminomethylbenzoic acid (4-AMBA) is a competitive inhibitor of labelled peptide influx. In each case 4-AMBA inhibits in a concentration-dependent fashion with data well described by a single system. Additionally, in the presence of external unlabelled substrate the Ki (inhibition constant) is shifted to the right. Quantitatively these results are compatible with predictions for simple competitive inhibition since the concentrations of unlabelled substrate employed (tracer and 1.0 mm in oocytes; tracer and 0.21 mm in renal vesicles) were chosen on the basis of previous work (Meredith et al. 1998) which showed that for these peptides these values are the approximate Km values in the two systems. For a competitive inhibitor the Michaelis-Menten equation predicts that the Ki observed in the presence of unlabelled substrate at this concentration therefore should be double the Ki observed in its absence. For both assays, within the limits of experimental error, this is what is observed (the Ki in oocytes is increased from 3.1 ± 0.4 to 6.9 ± 1.2 mm while the analogous Ki values in BBMV are 1.8 ± 0.1 and 5.1 ± 1.3 mm). (Note that all affinity constants measured are smaller in renal BBMV (by approximately 2- to 5-fold) when compared with those measured in the Xenopus oocyte expression system. We attribute this relatively small difference to the different experimental conditions between the two assay systems (electrochemical driving force and temperature) and not to the involvement of the higher affinity isoform of the peptide transporter PepT2, which previously we have shown (using D-Phe-L-Gln) not to be functionally present in this preparation (Temple & Boyd, 1998).
Figure 1. Fractional inhibition of labelled D-Phe-L-Gln (0.42 μm) influx.
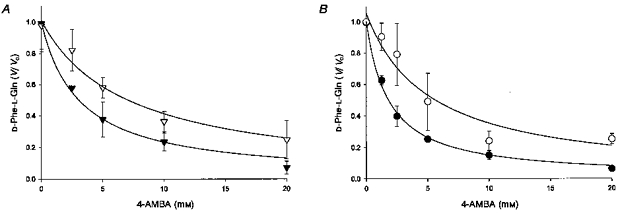
A, fractional inhibition (V/V0) of labelled D-Phe-L-Gln (0.42 μm) influx into PepT1-expressing Xenopus laevis oocytes by 4-AMBA (0–20 mm) when pHo = 5.5. Continuous lines represent the best fit of data to Michaelis-Menten kinetics for a single system in the absence (▾) and presence (▿) of unlabelled D-Phe-l-Gln (1.0 mm). Calculated inhibition constants (Ki) are 3.1 ± 0.4 and 6.9 ± 1.2 mm, respectively. Data has been corrected for non-mediated transport by subtracting D-Phe-L-Gln flux seen in H2O-injected control oocytes. Labelled D-Phe-L-Gln flux in the absence of 4-AMBA has been normalized to 1. Absolute fluxes in the absence and presence of unlabelled D-Phe-L-Gln are 194 ± 36 and 118 ± 18 fmol oocyte−1 h−1, respectively. Data points are means ± s.e.m. (n = 5). B, fractional inhibition of labelled D-Phe-L-Gln (0.34 μm) influx into rat renal BBMV by 4-AMBA (0–20 mm) under equilibrium exchange conditions: pHo = 5.5, pHi = 7.4, 4-AMBAo = 4-AMBAi and membrane potential clamped inside negative. Continuous lines represent the best fit of data to Michaelis-Menten kinetics for a single system in the absence (•) and presence (^) of unlabelled D-Phe-l-Gln (0.21 mm). Calculated inhibition constants (Ki) are 1.8 ± 0.1 and 5.1 ± 1.3 mm, respectively. Data has been corrected for non-mediated transport by subtracting labelled D-Phe-L-Gln flux seen in the presence of a saturating concentration of unlabelled peptide (10 mm). Labelled D-Phe-L-Gln flux in the absence of 4-AMBA (Vo) has been normalized to 1. Absolute fluxes in the absence and presence of unlabelled D-Phe-L-Gln are 854 ± 227 and 464 ± 208 fmol (mg protein)−1 s−1, respectively. Data points are means ± s.e.m. (n = 3–6).
Figure 2 shows directly that 4-AMBA (2 mm) is not transported by PepT1; the very small endogenous signal which is detected at the same elution time as 4-AMBA is found identically both in H2O-injected and in PepT1-expressing oocytes (Fig. 2Ac and d). PepT1-mediated transport therefore is not different from zero (Fig. 2B). As a positive control, Fig. 2Aa also shows that the HPLC assay readily detects this molecule in an oocyte extract spiked with 2 nmol of 4-AMBA. The limit of detection of 4-AMBA is 0.05 nmol; it is noteworthy that even with 15 mm 4-AMBA, no PepT1-mediated transport into oocytes could be detected (data not shown). Furthermore 4-aminophenylacetic acid (4-APAA, shown previously by Temple et al. 1998 to be a substrate for PepT1) was, as expected, readily and reproducibly transported in these experiments, which were run in parallel on cells from identical batches of oocytes as those in which transport of 4-AMBA was not detected (Fig. 2B). When both the limit of detection of 4-AMBA (0.05 nmol) and the measured transport rate of 4-APAA (0.3 nmol oocyte−1 h−1 at a substrate concentration of 2 mm) are taken into account then the ‘permeability’ (Vmax/Km) of 4-APAA may be shown to be at least 44-fold greater than that for 4-AMBA.
Figure 2.
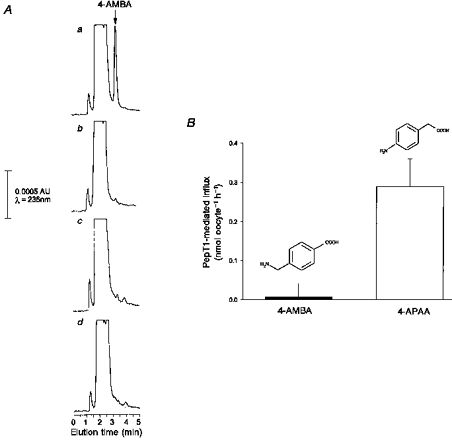
A, representative HPLC traces for (a) oocyte extract spiked with 0.1 mm 4-AMBA (20 μl was injected onto the column and thus the peak is equivalent to 2 nmol), (b) oocyte extract, (c) H2O-injected control oocyte extract after 1 h incubation with 2 mm 4-AMBA and (d) PepT1-injected oocyte extract after 1 h incubation with 2 mm 4-AMBA. HPLC was performed as described in Methods. 4-AMBA was detected by UV absorbance at maximum sensitivity where full scale is equivalent to 0.001 absorbance units (AU). B, PepT1-mediated flux of 4-aminomethylbenzoic acid (4-AMBA, 2 mm) was not different from zero as detected by HPLC; flux of 4-aminophenylacetic acid (4-APAA, 2 mm) is shown for comparison. Fluxes were measured after 1 h incubation at pHo = 5.5 into oocytes injected with PepT1 cRNA and H2O-injected controls. Data shown has been subtracted from that seen in H2O-injected controls. Data points are means ± s.e.m. (n = 5 from a representative oocyte preparation).
In keeping with the result shown in Fig. 2, 4-AMBA does not trans-stimulate labelled peptide efflux from either renal membranes (Fig. 3A) or PepT1-expressing oocytes (Fig. 3B). In contrast, confirming previous results (Temple et al. 1996, 1998), the presence of external unlabelled dipeptide does indeed markedly trans-stimulate in both systems. Taken together the results shown in Figs 2 and 3 show that 4-AMBA, although it is a competitive inhibitor of peptide transport, is not a substrate for translocation by this transporter.
Figure 3. 4-AMBA in contrast to unlabelled dipeptide.
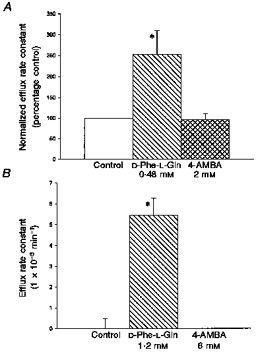
4-AMBA, in contrast to unlabelled dipeptide, does not stimulate efflux of labelled tracer dipeptide from either (A) rat renal BBMV (pHo = pHi = 5.5 and membrane potential is clamped to zero) or (B) PepT1-expressing Xenopus oocytes (pHo = 5.5). Concentration of substrate is 2 × Ki for substrate cis-inhibition of labelled dipeptide influx. Efflux rate constants were calculated as in Methods. A, efflux rate constants are normalized to the control (0.086 s−1). Data points are means ± s.e.m. (triplicate estimates from 4 vesicle preparations) * Data significantly different from control (P < 0.05, unpaired Student's t test). B, no efflux was measurable from H2O-injected control oocytes (data not shown). Data points are means ± s.e.m. (5 oocytes from a representative preparation). * Data significantly different from control (P < 0.001, unpaired Student's t test).
The conclusion that 4-AMBA binds, but does not translocate, is supported by further efflux experiments carried out on PepT1-expressing Xenopus oocytes. Cells were micro-injected with labelled peptide and efflux determined with and without external substrate (0, 1.2 and 20 mm D-Phe-L-Gln) as a function of external 4-AMBA concentration (0–24 mm). The efflux rate constants with no 4-AMBA present (Fig. 4A) are consistent with the predicted effect from Michaelis- Menten kinetics: increasing substrate concentration from 0 to 1.2 mm (i.e. 2 × Ki) D-Phe-l-Gln results in approximately 6 % of the maximal rate of efflux (the maximal rate being that seen with saturating (20 mm) external peptide). The lines fitted to the data in Fig. 4A are the best fits to eqn (1) for a model where there is a translocated substrate (A, in this case D-Phe-l-Gln) and a non-translocated inhibitor (I, 4-AMBA). When there is no A present, increasing concentrations of I have no effect on the efflux rate constant, since under the conditions of the experiment no measurable efflux occurs. In the presence of 1.2 mmA, increasing concentrations of I are able to inhibit the trans-stimulation seen in the absence of I. The ability of I to inhibit the trans-stimulation is reduced when the concentration of A is increased to saturating concentrations (20 mm), as would be predicted for a substrate and inhibitor binding at the same site (i.e. simple competitive binding).
Figure 4.
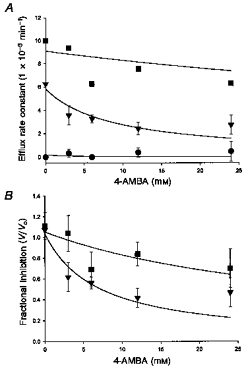
Concentration dependence of 4-AMBA (0–24 mm) inhibition of tracer D-Phe-L-Gln efflux from Xenopus oocytes expressing PepT1; experiments carried out at three different concentrations of external (trans) unlabelled D-Phe-L-Gln; 0 (•), 1.2 mm (▾) and 20 mm (▪). A, continuous lines represent best fit of data to kinetics describing efflux of a labelled substrate into a medium containing unlabelled external substrate and increasing concentration of external unlabelled inhibitor (Deves & Boyd, 1989; see eqn (1) in Methods). Calculated constants are: VS = 44.47 × 10−3 min−1, KS = 0.09 mm, VSA = 195 × 10−3 min−1, KSA = 0.01 mm. Data points are means ± s.e.m. (n = 10 from 2 oocyte preparations). B, data from A above normalized to show fractional inhibition. The intercepts on the y-axis from the curves fitted to the data from Fig. 4A are defined as unity. Continuous lines represent the best fit of the data to Michaelis-Menten kinetics for inhibition of a single system. Calculated apparent half-inhibition constants are 6.8 and 37 mm in the presence of 1.2 and 20 mm unlabelled external D-Phe-L-Gln, respectively.
Figure 4B shows data from the same experiment transformed to show the fractional inhibition of efflux seen on increasing the 4-AMBA concentration in the presence of either 1.2 or 20 mm D-Phe-L-Gln. The data are fitted with Michaelis- Menten kinetics for a single system, analogous to that used in Fig. 1. The calculated apparent half-inhibition constants for I (4-AMBA) are 6.8 and 37 mm at analogue A (d-Phe-L-Gln) concentrations of 1.2 and 20 mm, respectively. Further application of the Michaelis-Menten equation then allows an indirect calculation of the substrate Km. Increasing A 16.7-fold (from 1.2 to 20 mm) increases the measured apparent Ki for 4-AMBA 5.4-fold predicting a true Km for D-Phe-L-Gln of 0.4 mm, a value not dissimilar from that determined directly (0.6 mm, Meredith et al. 1998). Thus the findings shown in Fig. 4 provide strong quantitative support for the idea that 4-AMBA binds to, but is not translocated by, PepT1.
DISCUSSION
The cloning from small intestinal mRNA of the gene product (PepT1) responsible for epithelial peptide transport (Fei et al. 1994) marked a watershed in investigation of this process. Although much effort is currently directed towards understanding how this transporter functions (see Daniel, 1997), investigation of its role in normal physiology has long been hampered by the absence of a ‘good’ inhibitor. In this paper we report results suggesting that it will be possible to design a high affinity inhibitor based on peptide mimic chemistry using 4-AMBA as a starting point. It seems likely that the affinity of this molecule for the transporter (4-AMBA having a Ki in the low millimolar range) will be increased by chemical modification, e.g. by incorporating an additional hydrophobic moiety in the region of the aromatic ring (cf. Daniel et al. 1992).
Possible reasons underlying differences in the way in which 4-AMBA and 4-APAA are handled are intriguing. The two molecules (cf. Fig. 2) initially appear structurally similar yet differ sufficiently for the one (4-APAA) both to bind and be translocated rapidly by the transporter whereas the other (4-AMBA) binds with higher affinity yet is not translocated. The small differences in the N (amine) to C (carboxy) spacing (0.643 nm and 0.645 nm for 4-APAA and 4-AMBA, respectively) suggest that this spacing is not an important factor here. However one chemical feature that does distinguish these two molecules is the nature of the N-terminus. As 4-AMBA is not an aromatic amine the N-terminus can be protonated more easily within the physiological range (in contrast to 4-APAA). Thus at pH 5.5 more 4-AMBA will be in the cationic species, whereas 4-APAA is predominantly neutral. It is of note that cationic dipeptides are generally poorer substrates than neutral or anionic ones (Steele et al. 1997). As with the work of Deves & Krupka on the choline transporter (Deves & Krupka, 1979, see Stein, 1990) systematic studies with a series of such analogues should help resolve what are the structural features of PepT substrates that respectively determine binding and translocation. It is interesting that the classical carrier theory developed for analysis of efflux in other well-studied model systems such as erythrocytes is directly applicable to labelled peptide efflux in PepT1-expressing Xenopus oocytes. Specifically the data shown in Fig. 4 has been modelled on the basis of theory for efflux of labelled substrate into a medium containing both unlabelled substrate and a non-transported inhibitor. The excellent fit of the data to the model (Fig. 4B) and the general applicability of the constants derived from the model so that those derived in fitting one set of experimental conditions (1.2 mm external substrate) readily apply to those observed under other conditions (20 mm external substrate) suggest that the applicability of carrier kinetics to this physiologically important cotransporter is robust.
Acknowledgments
We thank the Wellcome Trust for their generous support, M. A. Hediger for the PepT1 clone and G. L. Kellet for reading the manuscript.
References
- Biber J, Stieger B, Haase W, Murer H. A high yield preparation for rat kidney brush border membranes. Different behaviour of lysosomal markers. Biochimica et Biophysica Acta. 1981;647:169–176. doi: 10.1016/0005-2736(81)90243-1. [DOI] [PubMed] [Google Scholar]
- Daniel H. First insights into the operational mode of epithelial peptide transporters. The Journal of Physiology. 1997;498:561. doi: 10.1113/jphysiol.1997.sp021882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel H, Morse EL, Adibi SA. Determinants of substrate affinity for the oligopeptide/H+ symporter in the renal brush border membrane. Journal of Biological Chemistry. 1992;267:9565–9573. [PubMed] [Google Scholar]
- Deves R, Boyd CAR. The determination of kinetic parameters for carrier-mediated transport of non-labelled substrate analogues: a general method applied to the study of divalent anion transport in placental membrane vesicles. Proceedings of the Royal Society B. 1989;237:85–97. doi: 10.1098/rspb.1989.0038. [DOI] [PubMed] [Google Scholar]
- Deves R, Krupka RM. The binding and translocation steps in transport as related to substrate structure. A study of the choline carrier of erythrocytes. Biochimica et Biophysica Acta. 1979;557:469–485. doi: 10.1016/0005-2736(79)90344-4. [DOI] [PubMed] [Google Scholar]
- Fei YJ, Ganapathy V, Leibach FH. Molecular and structural features of the proton-coupled oligopeptide transporter superfamily. Progress in Nucleic Acid Research. 1998;58:239–261. doi: 10.1016/s0079-6603(08)60038-0. [DOI] [PubMed] [Google Scholar]
- Fei YJ, Kanai Y, Nussberger S, Ganapathy V, Leibach FH, Romero MF, Singh SK, Boron WF, Hediger MA. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature. 1994;368:563–566. doi: 10.1038/368563a0. 10.1038/368563a0. [DOI] [PubMed] [Google Scholar]
- Hediger MA, Rhoads DB. Molecular physiology of sodium-glucose cotransporters. Physiological Reviews. 1994;74:993–1026. doi: 10.1152/physrev.1994.74.4.993. [DOI] [PubMed] [Google Scholar]
- Lister N, Sykes AP, Bailey PD, Boyd CA, Bronk JR. Dipeptide transport and hydrolysis in isolated loops of rat small intestine: effects of stereospecificity. The Journal of Physiology. 1995;484:173–82. doi: 10.1113/jphysiol.1995.sp020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DM. Protein Absorption: Development and Present State of the Subject. New York: Wiley-Liss; 1991. [Google Scholar]
- Meredith D, Temple CS, Boyd CAR. Chemical evidence for the involvement of histidine residues in proton-peptide co-transport by PepT1 expressing Xenopus laevis oocytes. The Journal of Physiology. 1996;493.P:65P. [Google Scholar]
- Meredith D, Temple CS, Boyd CAR. Kinetic evidence that neutral and anionic peptides bind to different conformations of the recombinant epithelial peptide transporter PepT1. The Journal of Physiology. 1998;506.P:131P. doi: 10.1111/j.1469-7793.1998.629bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa F. The influence of phlorhizin on intestinal absorption. Tohoku Journal of Experimental Medicine. 1922;3:288–295. [Google Scholar]
- Steel A, Nussberger S, Romero MF, Boron WF, Boyd CAR, Hediger MA. Stoichiometry and pH dependence of the rabbit proton-dependent oligopeptide transporter PepT1. The Journal of Physiology. 1997;498:563–569. doi: 10.1113/jphysiol.1997.sp021883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein WD. Channel, Carriers and Pumps: an Introduction to Membrane Transport. San Diego: Academic Press Inc.; 1990. [Google Scholar]
- Temple CS, Bailey PD, Bronk JR, Boyd CAR. A model for the kinetics of neutral and anionic dipeptide-proton cotransport by the apical membrane of rat kidney cortex. The Journal of Physiology. 1996;494:795–808. doi: 10.1113/jphysiol.1996.sp021533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple CS, Boyd CAR. Proton-coupled oligopeptide transport by rat renal cortical brush border membrane vesicles: a functional analysis using ACE inhibitors to determine the isoform of the transporter. Biochimica et Biophysica Acta. 1998;1373:277–281. doi: 10.1016/s0005-2736(98)00093-5. [DOI] [PubMed] [Google Scholar]
- Temple CS, Bronk JR, Bailey PD, Boyd CAR. Substrate-charge dependence of stoichiometry shows membrane potential is the driving force for proton-peptide cotransport in rat renal cortex. Pflügers Archiv. 1995;430:825–829. doi: 10.1007/BF00386182. [DOI] [PubMed] [Google Scholar]
- Temple CS, Stewart AK, Meredith D, Lister NA, Morgan KM, Collier ID, Vaughan jones RD, Boyd CAR, Bailey PD, Bronk JR. Peptide mimics as substrates for the intestinal peptide transporter. Journal of Biological Chemistry. 1998;273:20–22. doi: 10.1074/jbc.273.1.20. [DOI] [PubMed] [Google Scholar]


