Abstract
To determine whether Paneth cells exhibit functional expression of cAMP-activated Cl− currents and molecular expression of the cystic fibrosis transmembrane conductance regulator (CFTR), we applied whole-cell patch clamp and single-cell mRNA analysis by reverse transcription (RT) followed by polymerase chain reaction (PCR) amplification to single Paneth cells in crypts isolated from the guinea-pig small intestine.
Prominent activation of Cl− currents was consistently observed after stimulation with dibutyryl cAMP and forskolin or with vasoactive intestinal polypeptide (VIP). The cAMP-activated Cl− current was inhibited by removal of intracellular ATP or administration of an inhibitor of protein kinase A.
Many of the biophysical and pharmacological properties of the currents were phenotypically similar to those of the CFTR Cl− channel, such as the ohmic current-voltage relationship, the anion selectivity with a Type III sequence (Br− > Cl− > I− ≫ F− ≥ gluconate−), I−-induced blockage, insensitivity to a stilbene-derivative Cl− channel blocker, and sensitivity to a carboxylate analogue Cl− channel blocker. The sensitivity of the current to glibenclamide was, however, much weaker than that reported for the CFTR Cl− channel current. In contrast to the time independence of CFTR currents, the inward component of the Paneth cell Cl− currents exhibited inactivation kinetics.
Expression of CFTR mRNA could not be detected by RT-PCR analysis in almost all single Paneth cells, although its expression was consistently detected at the whole-crypt level. The presence of a small number of CFTR-expressing epithelial cells, which were scattered both in villi and crypts but not at the crypt base where Paneth cells were located, was demonstrated by immunocytochemistry.
Taken together, it appears that guinea-pig Paneth cells functionally express cAMP-activated Cl− conductance without relevant evidence for molecular expression of CFTR. Functional expression of VIP receptors in the Paneth cells was also demonstrated.
Paneth cells are granulated secretory epithelial cells located at the base of the crypts of Lieberkühn in the small intestine of most mammalian species. Since Paneth cells release granules, which contain lysozyme, immunoglobulin, phospholipase A2, α1-antitrypsin and α-defensins termed cryptidins, into the lumen of the crypts, Paneth cells are thought to play a key role in host defence (Ouellette, 1997).
In the small intestine the crypt compartment has been postulated to be the site of salt and fluid secretion (Sullivan & Field, 1991), though the evidence is still highly circumstantial. The undifferentiated epithelial cells lining the crypt are thought to be the intestinal Cl−-secreting cells and actually have been shown to depolarize in response to vasoactive intestinal polypeptide (VIP) or forskolin in isolated guinea-pig crypts (Walters et al. 1992). Recently, Paneth cells located at the crypt base adjacent to these undifferentiated intestinal epithelial cells (enterocytes) have been suggested to be involved in small intestinal Cl− secretion by releasing cryptidins which may selectively permeabilize the apical membrane of undifferentiated enterocytes (Lencer et al. 1997). However, no study has been performed to test the possibility that Paneth cells themselves may possess the Cl− conductance.
The cystic fibrosis transmembrane regulator (CFTR) gene codes for a cAMP-activated Cl− channel expressed in the apical membrane of many epithelial cells (Fuller & Benos, 1992). The expression of CFTR mRNA was shown by in situ hybridization in the rat (Trezise & Buchwald, 1991) and human (Strong et al. 1994) small intestine, and the expression of the CFTR protein was shown by immunocytochemistry in the human duodenum (Hoogeveen et al. 1991) and jejunum (Crawford et al. 1991). However, CFTR expression in single Paneth cells has not been intensively examined.
CFTR-like cAMP-activated Cl− currents have consistently been recorded from the human colonic epithelial cell lines T84 (Tabcharani et al. 1990) and Caco-2 (Bear & Reyes, 1992). In contrast, there has been no report of CFTR-like Cl− current recordings from mammalian small intestinal enterocytes, including Paneth cells, although cAMP-independent Cl− currents were recorded from villus enterocytes isolated from guinea-pig small intestine (Sepúlveda et al. 1991) and cAMP-dependent depolarization was observed in the guinea-pig enterocytes located at the upper portion of the isolated crypt (Walters et al. 1992).
The aims of the present study were to record cAMP-activated Cl− currents by whole-cell patch clamp and to assess the molecular expression of CFTR using single-cell reverse transcription-polymerase chain reaction (RT-PCR) and immunocytochemical techniques in guinea-pig Paneth cells in isolated crypts. A preliminary account of part of these data has appeared in abstract form (Tsumura et al. 1997).
methods
Isolation of crypts and villi
Intestinal epithelial fragments were sequentially isolated along the villus crypt axis by a vibration-Ca2+-chelation technique, as described by McNicholas et al. (1994). Briefly, a 15-cm-long segment of proximal small intestine was excised from adult male guinea-pigs under anaesthesia with ether, and the animals were then killed by cervical dislocation before they recovered consciousness. All experiments complied with the Institutional Guidelines for the Care and Use of Laboratory Animals. After being flushed with ice-cold saline (0.9 % NaCl), the segment was everted on a glass spiral and vibrated at 50 Hz at room temperature (23–26°C) in isolation solution which contained (mm): 112 NaCl, 5 KCl, 0.5 dithiothreitol (DTT), 30 Na2EDTA, 15 Hepes and 10 Tris (pH 7.1). This isolation solution was replaced by fresh solution at 30 min intervals, up to six times. Collected fractions were washed with ice-cold storage solution which was composed of (mm): 137.5 sodium gluconate, 4.2 potassium gluconate, 2 MgCl2, 20 mannitol, 2.9 calcium gluconate, 0.5 hydroxybutyrate, 8 Hepes and 6 Na-Hepes (pH 7.4), plus 1 mg ml−1 bovine serum albumin (BSA).
Under a phase-contrast or differential interference microscope, fractions 1–3 were found to contain mainly upper villus fragments, fraction 4 contained fragments consisting of both lower villi and upper or whole crypts, and fractions 5 and 6 contained fragments mainly consisting of lower or whole crypts. These fractions were kept on ice until use. The crypt-rich fractions were used for both electrophysiological and immunocytochemical studies, and other fractions were used for immunocytochemistry. For immunoprecipitation studies, the fractions were combined.
Patch-clamp whole-cell recordings
Isolated crypts were placed in a chamber (0.5 ml) and perfused with extracellular solution. In some experiments crypts were used after being stained with Neutral Red (30 μg ml−1, 30 min). This vital staining did not affect the basal and cAMP-activated currents. In several experiments, to observe the effects of closure of the crypt open end, crypts were used after their open ends were tied off with thread (1 μm in diameter).
Whole-cell recordings were carried out as previously described (Tsumura et al. 1996), from granular enterocytes (which had been identified as Paneth cells) located at the base of isolated crypts. In contrast to neighbouring distal undifferentiated enterocytes, mouse Paneth cells are known to lack gap junctions (Bjerknes et al. 1985). In fact, in preliminary fluorescence microscope studies in guinea-pig crypts, Lucifer Yellow (100 μm) incorporated in the patch pipette was not found to transfer to adjacent cells from a Paneth cell under whole-cell conditions (n = 4). Thus, Paneth cells are suitable for whole-cell voltage clamp due to negligible electrical cell-to-cell coupling. After compensation for the series resistance (5–25 MΩ) and cell capacitance (4–10 pF), membrane currents were measured using an Axopatch 200A amplifier (Axon Instruments, Foster City, CA, USA). To monitor the time course of current activation and recovery, alternating step pulses (1 s duration) to ±40 mV were routinely applied from a holding potential of 0 mV every 7.5 s. The current-voltage (I-V) curves were obtained by step pulses (0.5 s duration) from −100 to +100 mV in 20 mV increments or by ramp voltage clamp from −100 to +100 mV or −80 to +80 mV for 1.2 s. The amplitude of the instantaneous current was measured at 2.5 ms after the step onset. Junction potentials were measured with a 150 mm KCl bridge and were compensated in studies of anion selectivity. Anion permeability ratios were derived from reversal potentials (Vrev) obtained from I-V plots using the Hodgkin-Katz modification of the Goldman equation. Current signals were filtered and digitized at 1 and 2.5 kHz, respectively. The time course of hyperpolarization-induced inactivation of cAMP-activated Cl− current was well fitted by a double exponential function, and thus the steady-state current level was simultaneously evaluated. pCLAMP software (version 6.0.2; Axon Instruments) was used for command pulse control, data acquisition and analysis.
The bath NaCl or KCl solution contained (mm): 150 NaCl or KCl, 1 CaSO4, 10 mannitol, 15 Hepes and 10 Tris (pH 7.5). To reduce the extracellular Cl− concentration ([Cl−]o), 100 or 135 mm KCl was replaced with 200 or 270 mm mannitol (or 135 mm potassium gluconate). To elevate intracellular [cAMP], both dibutyryl cAMP (db-cAMP) and forskolin were added to the extracellular solution. The pipette (intracellular) solution contained (mm): 150 NaCl (or KCl), 3 MgATP, 5 BAPTA, 15 mannitol, 10 Hepes and 10 Tris (pH 7.4). When stimulated with VIP or secretin added to the bathing solution, GTP (50 μm), GTPγS (100 μm) or GDPβS (100 μm) was added to the pipette solution. To examine anion selectivity, 150 mm KCl in the bath KCl solution was replaced with 150 mm KBr, KI, KF or potassium gluconate. To elevate intracellular [cAMP] in Paneth cells in crypts the open ends of which had been tied off, cAMP stimulants were added to the bathing solution prior to attaching the patch pipette to the cell, because stimulant application by bath perfusion resulted in mechanical movement of the crypts tied off with the thread, thereby interfering with patch-clamp experiments.
Forskolin, db-cAMP, cAMP, porcine VIP, porcine secretin, GTPγS, GDPβS, glibenclamide, 4,4′-diisothiocyanostilbene-2,2′-disulphonic acid (DIDS) and diphenylamine-2-carboxylic acid (DPC) were purchased from Sigma; N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulphonamide (H-89), as well as its derivative N-[2-(N-formyl-p-chlorocinnamylamino)ethyl]-5-isoquinolinesulphonamide (H-85), were from Seikagaku Corporation (Tokyo, Japan). 5-Nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB) was a generous gift from Dr M. Kuno (Shionogi Pharmaceutical Co., Osaka, Japan). The vehicles for stock solutions were ethanol for forskolin and DMSO for H-89 and the Cl− channel blockers. Ethanol and DMSO alone did not affect whole-cell currents at the final concentrations used (less than 0.04 and 0.2 %, respectively).
All patch-clamp studies were performed at 37 ± 1°C. Electrophysiological data are given as means ± s.e.m. of observations for n cells. Statistical differences of the data were evaluated by Student's paired or unpaired t test and considered significant at P < 0.05.
RT-PCR study at the whole-crypt level
Poly(A)+ RNA was extracted from isolated crypts with Magnetic Porous Glass (MPG) Direct mRNA Purification Kit (CPG Inc., NJ, USA). In brief, the cells were lysed in extraction-hybridization buffer (100 mm Tris-HCl, 500 mm LiCl, 10 mm EDTA, 1 % lithium dodecylsulphate (LiDS), 5 mm DTT, pH 8.0) and the poly(A)+ RNA was hybridized with MPG streptavidin plus biotinylated oligo(dT)25. The poly(A)+ RNA was washed three times with 500 μl of washing buffer (10 mm Tris-HCl, 150 mm LiCl, 1 mm EDTA, 0.1 % LiDS, pH 8.0). Then the poly(A)+ RNA was collected magnetically and eluted with 20 μl of 2 mm EDTA solution.
The primers corresponding to the regulatory (R) domain (Diamond et al. 1991) of guinea-pig CFTR were synthesized on an automated DNA synthesizer. To increase the specificity and sensitivity of the PCR for the CFTR gene, we used nested primers. The sequences of the primers were 5′-TGGTCACTTCTAAAATGGAAC-3′ and 5′-GTTATCAGGTTCAACACCGAC-3′ (outer pair, 5′ and 3′, respectively: the amplified fragment corresponds to nucleotides 1937 to 2453 of the human CFTR cDNA sequence) and 5′-CCTGTGGAGAGAAGACTGTCC-3′ and 5′-CACCGACTGTCT CCTACTGC-3′ (inner pair, 5′ and 3′, respectively: the amplified fragment corresponds to nucleotides 2323 to 2439 of the human CFTR cDNA sequence). The expected size of the amplified fragment by the second PCR was 114 bp. As a positive control we amplified a fragment of mRNA of glyceraldehyde 3-phosphate dehydrogenase (G-3-PDH) by single PCR. The sequences of the primers (Clontech, Palo Alto, CA, USA) were 5′-ACCACA GTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGATGTA-3′. The resulting PCR product using these primers was 452 bp. The reverse transcription and PCR were performed as described by Rappolee et al. (1988), using the Superscript Preamplification System for First Strand cDNA Synthesis (Gibco BRL) and Ampli Taq Gold (Perkin Elmer, Norwalk, CT, USA). The PCR programme for the first amplification with the outer primer pair of CFTR was as follows: 12 min preheating for the activation of Ampli Taq Gold at 94°C, followed by thirty-three PCR cycles at 94°C (1 min)/57°C (2 min)/72°C (3 min), and 10 min final elongation at 72°C. Aliquots (2 μl) of this PCR product were subjected to the second-stage amplification with the inner primer pair by thirty-three PCR cycles at 94°C (1 min)/61°C (2 min)/72°C (3 min). The PCR programme for G-3-PDH was the same as that of the first-stage amplification for CFTR. The cDNA amount of the sample was measured by a spectrophotometer (DU640; Beckman, Palo Alto, CA, USA). For each PCR, signal was never detected from reaction buffer alone (without containing sample cDNA).
RT-PCR study at the single Paneth cell level
Using the single-cell RT-PCR technique (Sucher & Deitcher, 1995), analysis of CFTR mRNA was perfomed in single Paneth cells. Glycogen (20 μg ml−1) was added as an mRNA carrier to the pipette solution. To obtain RNase-free conditions, glass micropipettes were heated at 240°C for 4 h, and the pipette solution was treated with diethylpyrocarbonate (DEPC) and autoclaved. After the currents had been recorded, suction was applied to the pipette, thereby collecting the cytosol. Subsequently, the pipette tip was broken into a reaction tube, and approximately 6 μl of KCl pipette solution was ejected by positive pressure into the reaction tube. Latex gloves were worn at all times during the procedure. The RT procedures for single Paneth cell PCR were the same as those for whole crypts. The conditions (such as annealing temperature and number of cycles) of single-cell RT-PCR were optimized by amplifying the diluted whole-crypt cDNA, as recommended by Sucher & Deitcher (1995).
Immunocytochemistry
Isolated crypts were fixed with 4 % paraformaldehyde for 30–60 min, washed with phosphate-buffered saline (mm: 127 NaCl, 2.7 KCl, 8 Na2HPO4, 1.47 KH2PO4, 0.5 MgCl2, 0.9 CaCl2 and 20 mannitol) supplemented with 1 % BSA, and treated with anti-lysozyme antibody or anti-CFTR antibody (diluted to 10 or 5 μg ml−1, respectively). Mouse polyclonal antibody against rabbit IgG was used as the secondary antibody. Secondary antibody binding was detected with fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG antibody. Rabbit anti-human lysozyme antibody was purchased from Progen Biotechnik GmbH (Heidelberg, Germany). Rabbit anti-CFTR antibodies were raised against the synthetic oligopeptide, DPVERRLS, which corresponds to a part of the R domain of guinea-pig CFTR (corresponding to amino acids 730–737 of human CFTR), and purified by ligand affinity columns using the oligopeptides. Non-immune rabbit serum or purified rabbit IgG (Zymed, San Fransisco, USA) was used as a negative control.
Immunoprecipitation
Isolated intestinal epithelia (fractions 1–6 combined) were solubilized with a buffer containing 0.1 % (w/v) sodium dodecyl sulphate (SDS), 1 % (v/v) Nonidet P-40, 150 mm NaCl, 1 mm EDTA and 10 mm Tris-HCl (pH 7.4) supplemented with 10 μg ml−1 aprotinin (Sigma), sonicated three times for 10 s, and then centrifuged in a microcentrifuge at 4°C for 5 min (15 000 r.p.m.), and the pellet discarded. Solubilized proteins were incubated for 1 h at 4°C with the anti-CFTR antibody (50 μg ml−1) or purified rabbit IgG. Protein A agarose (100 μl ml−1) was then added. Following a 1 h incubation at 4°C, the agarose beads were washed three times with the same buffer used for cell lysates. Immunoprecipitated samples were eluted into SDS-PAGE sample buffer containing 3 % (w/v) SDS, 15 % sucrose and 92.5 mm Tris-HCl (pH 6.9). Samples were heated at 95°C for 5 min and subjected to 10 % SDS-PAGE. Immunoprecipitated CFTR subjected to SDS-PAGE was transferred to nitrocellulose membrane. The signals were detected using the anti-CFTR antibody and ECL kit (Amersham Life Science, Little Chalfont, UK).
RESULTS
Identification of Paneth cells in isolated crypts
A subpopulation of epithelial cells, which contained a large number of granules (indicated by arrowheads in Fig. 1A) in the supranuclear to apical portion, was found at the base of isolated crypts, using a differential interference microscope (Fig. 1A). The granules were selectively stained by Neutral Red (Fig. 1B). Immunocytochemistry showed that these granular cells at the crypt base were reactive to anti-lysozyme antibody (Fig. 1C). Taken together, it was concluded that these cells were Paneth cells and that they could easily be recognized by their location and granular content under a light microscope.
Figure 1. Morphological identification of Paneth cells.
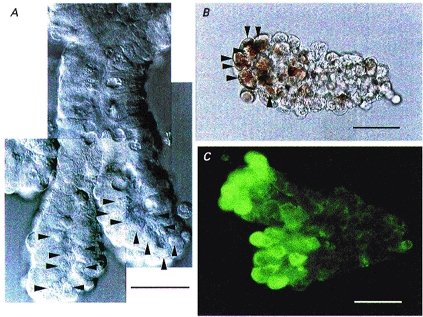
Micrographs of the crypts isolated from the guinea-pig small intestine. A, differential interference micrograph. Numerous granules (arrowheads) could be seen at the supranuclear to apical portion in the enterocytes near the base of crypts. B, light micrograph. Crypt base enterocytes (arrowheads) contained granules preferentially reactive to Neutral Red. C, immunofluorescence micrograph. The crypt base enterocytes were reactive to anti-lysozyme antibody. Scale bars, 30 μm.
cAMP-activated Cl− currents in Paneth cells
When Paneth cells equilibrated with intracellular and extracellular KCl solutions were stimulated with a cAMP-elevating cocktail consisting of forskolin (20 μm) and db-cAMP (500 μm) under whole-cell voltage clamp, prominent current activation was consistently achieved in a reversible manner, as shown in Fig. 2A. The voltage dependence of cAMP-activated currents was investigated by applying step pulses from −100 to +100 mV in 20 mV increments. Both basal currents before stimulation and activated currents after stimulation showed little time or voltage dependence at positive potentials, whereas they exhibited weakly time- and voltage-dependent inactivation at potentials negative to −40 mV (Fig. 2B). The instantaneous peak I-V relationship was almost linear (Fig. 2C). The mean amplitudes of the instantaneous current density recorded at −40 and +40 mV were essentially identical, as summarized in Fig. 2D. Virtually identical cAMP-activated Cl− currents were observed from Paneth cells in the crypt, the open end of which was closed by being tightly tied off with a fine thread, and the current density at −40 mV was −170.7 ± 24.8 pA pF−1 (n = 7), which was not statistically different from that of the control (Fig. 2D).
Figure 2. cAMP-induced activation of whole-cell currents.
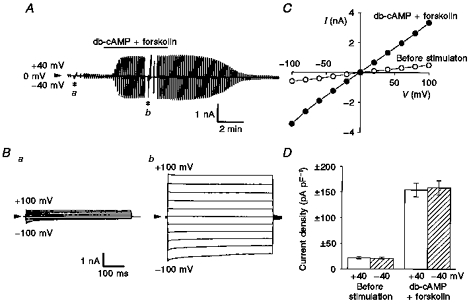
Whole-cell recordings were performed in single Paneth cells equilibrated with KCl solutions. A, representative record before and after stimulation with 500 μm db-cAMP and 20 μm forskolin (indicated by horizontal bar) during application of alternating pulses from 0 to ±40 mV or of step pulses from −100 to +100 mV in 20 mV increments (asterisks). The gain of the chart recorder was changed by one-half when step pulses were applied. In this and subsequent figures, arrowheads indicate the zero-current level. B, expanded traces of the current responses (a and b in A) to step pulses before (a) and after (b) cAMP stimulation. C, peak I-V relationships before (^) and after (•) cAMP stimulation for data shown in B. D, mean peak current density recorded at ±40 mV before and after cAMP stimulation. Error bars represent s.e.m. (n = 39).
The effects of partially replacing the extracellular KCl with mannitol on the I-V curves of cAMP-activated currents were examined by ramp voltage clamp in Paneth cells equilibrated with KCl solutions. As shown in Fig. 3A, the Vrev of the cAMP-activated component obtained after subtraction of basal currents shifted in a positive direction with decreasing extracellular [KCl], indicating that the cAMP-activated component was due to an anionic conductance. The Vrev value shifted by +56.2 mV per 10-fold decrease in [Cl−]o (Fig. 3A, inset), and this was close to the theoretical value for a Cl−-selective electrode (dotted line). In contrast, the Vrev of basal currents largely shifted in a negative direction (−12.5 ± 1.6 mV, n = 3) when the extracellular KCl concentration was decreased (from 150 to 50 mm), indicating that a K+ conductance predominantly contributes to the basal current recorded under these conditions. In fact, the basal current was largely abolished by totally replacing K+ with Na+ (see below).
Figure 3. Anion selectivity of cAMP-activated currents.

I-V curves were measured by ramp voltage clamp (from −80 or −100 to +80 or +100 mV for 1.2 s) in Paneth cells equilibrated with KCl solutions. Extracellular Cl− concentrations and anion species are indicated beside the curves in A and B, respectively. Background basal currents recorded before cAMP stimulation were subtracted from currents recorded after activation. A, effects of changes in extracellular Cl− concentration. Inset, relationship between the reversal potential (ordinate) and [Cl−]o. Each symbol represents the mean ± s.e.m. of 4–12 observations. The dotted line represents the ideal Cl− equilibrium potential. B, effects of anion substitution.
The anion selectivity of the cAMP-activated current was examined in Paneth cells equilibrated with KCl solutions by replacing extracellular Cl− with other anionic species. Representative results are shown in Fig. 3B. The mean Vrev values shifted from −0.5 ± 0.3 mV (n = 27) to −15.6 ± 0.9 mV (n = 6), 6.0 ± 1.5 mV (n = 7), 77.3 ± 7.0 mV (n = 7) and 78.6 ± 6.8 mV (n = 7), when Cl− was totally replaced with Br−, I−, F− and gluconate−, respectively. The relative anion permeability was 1.86 ± 0.07, 1, 0.79 ± 0.04, 0.05 ± 0.01 and 0.04 ± 0.01 for Br−, Cl−, I−, F− and gluconate−, respectively. This sequence corresponds to Eisenman's sequence Type III (Wright & Diamond, 1977). Figure 3B also shows a blocking effect of I−.
Both the amplitude of the cAMP-activated Cl− current and the inactivation kinetics were markedly affected when [Cl−]o was reduced by replacing extracellular KCl with mannitol, as shown in Fig. 4A. Several minutes after reduction of [Cl−]o peak currents were prominently diminished (Fig. 4Ba) despite the presence of a high concentration of intracellular Cl− (150 mm). The time course of the hyperpolarization-induced inactivation, which could be better fitted by a double exponential function than a single exponential function (Fig. 4Ad), became faster when [Cl−]o was decreased (Fig. 4Bb). A plot of the ratio of steady-state to peak currents against [Cl−]o (Fig. 4Bc) showed that the steady-state inactivation was augmented by reduction of the extracellular Cl− level. Essentially identical effects were observed when extracellular KCl was replaced with potassium gluconate. When [Cl−]o was thus reduced to 15 mm using gluconate, the peak current recorded at −80 mV decreased to 58 ± 4 % of that recorded at 150 mm extracellular Cl− () (n = 5), the fast and slow time constants of inactivation decreased to 18.9 ± 2.9 and 230.8 ± 52.7 ms, respectively, and the ratio of steady-state to peak currents decreased to 0.61 ± 0.05. Therefore, it appears that not only channel activity but also inactivation gating are dependent on extracellular [Cl−].
Figure 4. Extracellular [Cl−] dependence of cAMP-activated Cl− currents.
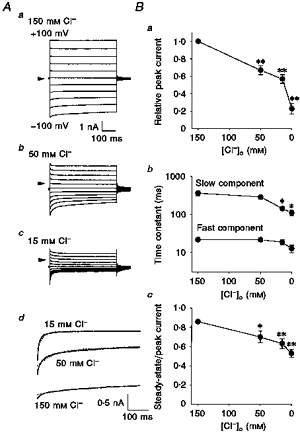
Whole-cell recordings were performed by applying step pulses (from −100 to +100 mV in 20 mV increments) in single Paneth cells equilibrated with KCl solutions. A, representative traces of current responses to step pulses at [Cl−]o of 150 (a), 50 (b) and 15 mm (c), and examples of double (continuous line) and single (dotted line) exponential fits to currents in response to a voltage of −80 mV at 150, 50 and 15 mm (d). B, effects of changes in [Cl−]o on the peak current relative to that recorded at 150 mm [Cl−]o (a), on the time constants of inactivation (b) and on the ratio of steady-state current to peak current (c) at −80 mV. Each symbol represents the mean ± s.e.m. of 5–20 observations. *P < 0.05 and **P < 0.01, significantly different from the value at 150 mm .
When Paneth cells were equilibrated with NaCl solutions, the basal current recorded before stimulation was virtually abolished, as demonstrated in Fig. 5A (see also Figs 6B and 7A), whereas stimulation with a cAMP-elevating cocktail consistently induced current activation (Fig. 5A).
Figure 5. Effects of Cl− channel blockers on cAMP-activated Cl− currents.
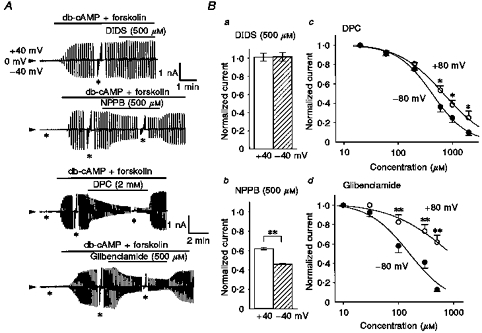
Whole-cell recordings were performed in single Paneth cells equilibrated with NaCl solutions. A, representative records before and after cAMP stimulation (500 μm db-cAMP and 20 μm forskolin) and drug application (indicated by horizontal bars) during application of alternating pulses or step pulses. The gain of the chart recorder was changed by one-half during the step pulses (asterisks). The upper time scale applies to both the top and second traces, and the lower scale to both the third and bottom traces. B, mean values of normalized peak currents in the presence of 500 μm DIDS (a), 500 μm NPPB (b), various concentrations of DPC (c) or glibenclamide (d) against control currents in the absence of drugs at ±40 mV or ±80 mV. Error bars represent s.e.m. (n = 4–8). *P < 0.05 and **P < 0.01, significant difference between data recorded at +40 and −40 mV, or those at +80 and −80 mV. Curves in c and d represent the best fits to the Hill equation. The Hill coefficients for DPC were 1.35 ± 0.13 at −80 mV and 1.03 ± 0.09 at +80 mV and those for glibenclamide were 1.18 ± 0.33 at −80 mV and 0.80 ± 0.23 at +80 mV.
Figure 6. Sensitivity of cAMP-activated Cl− currents to intracellular ATP removal and to extracellular H-89 administration.

Whole-cell recordings were performed in single Paneth cells equilibrated with NaCl solutions. A, representative records before and after cAMP stimulation (500 μm db-cAMP and 20 μm forskolin) using nominally ATP-free pipette solution (a) or after addition (over 30 min) of 10 μm H-89 in the pipette solution (b). Asterisks indicate application of step pulses from −100 to +100 mV in 20 mV increments (see legend to Fig. 2). B, effects of nominally ATP-free pipette solution and H-89 in the intracellular pipette solution on the mean current densities recorded at −40 mV (n = 6–31). Error bars represent s.e.m. **P < 0.01, significantly different from the control value after stimulation.
Figure 7. VIP-induced activation of whole-cell Cl− currents.

Whole-cell recordings were performed in single Paneth cells equilibrated with NaCl solutions. A, VIP-induced Cl− currents. a, representative record before and after stimulation with 250 nm VIP (indicated by the horizontal line) during application of alternating pulses or step pulses (*; i and ii). The gain of the chart recorder was changed by one-half when step pulses were applied. b and c, expanded traces of the current responses (i and ii in a) to step pulses before (i) and after (ii) VIP stimulation. d, peak I-V relationships before (^) and after (•) VIP stimulation for data shown in c. e, concentration-response curve for VIP. Each symbol represents the mean ± s.e.m. of VIP-induced peak current density of 4–7 experiments. The curve represents the best fit to the Hill equation with a Hill coefficient of 0.90 ± 0.07. B, effects of GDPβS, GTPγS and H-89 on VIP-induced Cl− currents. a and b, representative records before and after stimulation with VIP during intracellular loading with 100 μm GDPβS or GTPγS, respectively. Pulse protocol same as that in A. c, mean peak current density recorded at −40 mV before and after stimulation with VIP without (Control, n = 7) and with intracellular loading with H-89 (n = 5), GDPβS (n = 6) or GTPγS (n = 5) for over 30 min. Error bars represent s.e.m. **P < 0.01, significantly different from the control value after stimulation.
A stilbene-derivative Cl− channel blocker, DIDS (500 μm), failed to block the cAMP-activated Cl− current, as shown in Fig. 5A (top trace) and Fig. 5B a. A carboxylate analogue Cl− channel blocker, NPPB (500 μm), rapidly suppressed the cAMP-activated Cl− current in a fairly reversible manner (Fig. 5A, second trace and Bb). The effect was slightly voltage dependent, and the inward current was markedly more diminished than the outward current. Another carboxylate blocker, DPC (2 mm), induced immediate inhibition after administration, in a fully reversible manner (Fig. 5A, third trace). Concentration-inhibition curves (Fig. 5 Bc) clearly show the voltage-dependent inhibition. The concentrations for half-maximal inhibition (IC50) by DPC were 426.0 ± 33.8, 577.0 ± 43.8, 669.4 ± 45.2 and 698.9 ± 50.9 μm at −80, −40, +40 and +80 mV, respectively. Similar blocking effects were also rapidly induced by an antidiabetic sulfonylurea, glibenclamide (500 μm), as shown in Fig. 5A (bottom trace). The concentration dependence and voltage dependence of the glibenclamide effects are summarized in Fig. 5Bd. The IC50 values for the glibenclamide effect were 156.2 ± 44.2, 288.5 ± 54.7, 554.2 ± 105.9 and 896.2 ± 278.0 μm at −80, −40, +40 and +80 mV, respectively.
When Paneth cells were equilibrated with a pipette solution devoid of ATP, cAMP stimulation failed to induce activation of Cl− currents, as shown in Fig. 6Aa. Even in the presence of intracellular ATP, cAMP-induced activation of Cl− currents was largely abolished by pretreatment with an inhibitor of protein kinase A (PKA), H-89 (10 μm), for over 30 min, as shown in Fig. 6Ab. Figure 6B summarizes the effects of nominally ATP-free conditions and of pretreatment with H-89. In contrast, pretreatment with H-85 (10 μm), which is a derivative of, and a negative control for, H-89, did not affect cAMP-induced Cl− current activation (data not shown, n = 3).
VIP-induced activation of Cl− currents in Paneth cells
Since VIP is known to stimulate cAMP production via the VIP receptor coupled to adenylyl cyclase through G proteins in a number of target cells including intestinal cells (Laburthe & Couvineau, 1988), the effect of VIP on Cl− current was examined. Paneth cells equilibrated with NaCl solutions were found to respond to VIP (250 nm) with activation of Cl− currents in a completely reversible manner, as shown in Fig. 7Aa. VIP-induced outward currents showed little time and voltage dependence, whereas the inward currents exhibited weak but distinct inactivation kinetics at large negative potentials (Fig. 7Ac). The fast and slow time constants of inactivation were 20.1 ± 1.5 and 335.5 ± 63.7 ms, respectively, at 150 mm extracellular Cl− and −80 mV, and were not significantly different from those of cAMP-activated Cl− currents (Fig. 4). The instantaneous I-V curve was virtually linear (Fig. 7Ad). When [Cl−]o was reduced from 150 to 50 mm, the Vrev shifted by +29.3 ± 1.5 mV (n = 7), indicating Cl− selectivity (cf. chloride equilibrium potential shift of +27.7 mV). The VIP response was concentration dependent, as shown in Fig. 7Ae, with a half-maximal effective concentration of 1.70 ± 0.12 nm, suggesting an involvement of a high-affinity VIP receptor. Similar Cl− current responses were also induced by stimulation with 1 μm secretin (data not shown, n = 6).
The involvement of G proteins in the VIP response was examined by incorporating GDPβS or GTPγS into the pipette solution. As shown in Fig. 7B, VIP-induced Cl− current activation was largely abolished by 100 μm GDPβS (a and c). However, GDPβS failed to affect Cl− current activation by a cAMP cocktail (data not shown, n = 3). In contrast, 100 μm GTPγS failed to inhibit the VIP response (b and c); however, it inhibited deactivation of the current after washout of VIP (b). Prior treatment with H-89 for over 30 min remarkably suppressed the VIP response (c), suggesting the involvement of cAMP-dependent phosphorylation in the VIP-induced channel activity.
Assessment of CFTR mRNA expression in Paneth cells
To assess the expression of CFTR in Paneth cells, the single-cell RT-PCR technique was adopted. After recording cAMP-activated Cl− currents under the whole-cell mode in Paneth cells in isolated crypts stained with Neutral Red (Fig. 8A), negative pressure was applied to the patch pipette to harvest the cytosol. During suction, the cell was found to shrink, and the cytoplasmic contents including stained granules were actually found to be sucked up into the patch pipette (Fig. 8B). The cytosol thus collected was subjected to RT-PCR with PCR primers for G-3-PDH and CFTR. In all single Paneth cells examined (n = 14), G-3-PDH mRNA was consistently identified (Fig. 8C; Single cell). In contrast, CFTR mRNA could not be identified in thirteen of fourteen Paneth cells (Fig. 8D; Single cell). To increase the copy number of the CFTR gene, the cytoplasmic contents accumulated from three Paneth cells were subjected to RT-PCR studies. Again, however, we failed to detect CFTR mRNA (data not shown, n = 2).
Figure 8. Single-cell RT-PCR analysis for CFTR mRNA expression.

A, micrograph of a crypt stained with Neutral Red during whole-cell recordings in a Paneth cell. Scale bar, 30 μm. B, patch pipette containing the cytoplasmic contents which were sucked up from a Paneth cell after whole-cell recording. Scale bar, 30 μm. C, gel analysis of G-3-PDH-specific PCR product (predicted to be 452 bp) from a whole crypt after dilution (10−5, 10−6 or 10−7 times) or single Paneth cells (a-c). As a negative control, the same procedures were conducted using single-cell cytoplasm without reverse transcriptase (RT-; d and e) or using bath solution from the same experiments (f). A molecular weight marker (φX174 DNA/HaeIII fragment; Gibco BRL) is shown in the left-hand lane (M). D, gel analysis of CFTR-specific PCR product (predicted to be 114 bp) from a whole crypt after dilution (10−4, 10−5 or 10−6 times) or single Paneth cells (a-c).
At the whole-crypt level, even after a 10−5 times dilution, mRNA expression of not only G-3-PDH but also CFTR was consistently observed by RT-PCR analysis (Fig. 8C and D; Whole crypt). However, no signal was detected from the same sample without addition of reverse transcriptase (RT-) and from bath solution which had been collected during the above single-cell RT-PCR experiments (Bath solution). The cDNA content in this sample after 10−5 times dilution was evaluated to be 10.1 pg by spectrophotometry. Since the cDNA content in an ordinary mammalian cell is thought to be around 50 pg (Sucher & Deitcher, 1995), it is unlikely that failure of CFTR mRNA amplification by single-cell RT-PCR was due to insufficient amount of the sample cDNA.
Assessment of CFTR protein expression in Paneth cells
In order to detect the expression of CFTR, protein immunocytochemical studies were performed in isolated villi and crypts using polyclonal antibody against an octapeptide corresponding to the R domain of guinea-pig CFTR. Immunofluorescence signals were consistently detected with anti-CFTR antibodies but not with non-immune rabbit serum or purified rabbit IgG. As shown in Fig. 9A-F, CFTR-positive cells were found to be scattered from the villi to the crypts, and the fluorescence signal was detected throughout the entire cell. This result is in good agreement with the previous finding in human and rat proximal small intestine (Ameen et al. 1995). Distribution of such a unique subset of enterocytes with a high level of intracellular CFTR appeared to be slightly denser at the lower part of villi (Fig. 9D) than at the upper villus (Fig. 9B) and at the crypt (Fig. 9F). At the crypt base, where Paneth cells are located, CFTR-rich cells could not be observed, and the fluorescence signal was not higher than that of the background level observed in the negative control (data not shown).
Figure 9. Immunocytochemistry and immunoprecipitation analysis for CFTR protein expression.

A, C and E, light micrographs of a villus fragment (A), a fragment consisting of a lower villus and upper or whole crypts (C) and a whole-crypt fragment (E) isolated from guinea-pig small intestine. Scale bars, 50 μm. B, D and F, indirect immunofluorescence micrographs of epithelial fragments corresponding to A, C and E, respectively. Immunocytochemistry was performed with anti-CFTR antibody. G, immunoprecipitation of CFTR. Isolated epithelial fragments were solubilized using detergent and CFTR was immunoprecipitated with anti-CFTR antibody. Data represent duplicate experiments.
Immunoprecipitation studies showed that the guinea-pig intestinal epithelium expressed two types of protein, 200 and 135 kDa, which were reactive to the polyclonal anti-CFTR antibody, as demonstrated in Fig. 9G, but not to purified rabbit IgG. Bands at 135 and 200 kDa are close to those previously reported (Gregory et al. 1990; Hoogeveen et al. 1991) and may represent the core-glycosylated form and the fully glycosylated form of CFTR, respectively.
Therefore, it appears that almost all the Paneth cells do not express CFTR, whereas almost all the cells exhibit a cAMP-activated Cl− conductance.
DISCUSSION
Novel type of cAMP-activated Cl− channel in Paneth cells
The molecular identity and functional characteristics of Cl− channels in the mammalian small intestine, which is a site of absorption and secretion of Cl−, are largely unknown. So far only a few patch-clamp studies on small intestinal Cl− channels have been reported. cAMP-insensitive, outwardly rectifying Cl− channels, which exhibit a low field strength anion selectivity, were found in the basolateral membrane of enterocytes isolated from villi of the guinea-pig small intestine (Sepúlveda et al. 1991). In a human small intestinal epithelial cell line, Intestine 407, volume-sensitive outwardly rectifying Cl− channels and Ca2+-activated Cl− channels have consistently been found (Okada, 1997). The electrical coupling via gap junctions between undifferentiated enterocytes has prevented precise voltage-clamp studies of the isolated crypt. Even in the isolated crypt, however, the patch-clamp voltage-clamp study can be applied to Paneth cells which lack gap junctions.
The present patch-clamp study demonstrated, for the first time, that Paneth cells possess cAMP-activated Cl− channels characterized by the following macroscopic properties: (1) ohmic I-V relationship, (2) intracellular ATP requirement, (3) PKA dependence, (4) anion selectivity with a moderate field strength Type III sequence (Br− > Cl− > I− > F−), (5) blockage by I−, (6) insensitivity to DIDS, (7) sensitivity to DPC and NPPB, (8) sensitivity to glibenclamide, (9) weak inactivation at large negative potentials, and (10) extracellular [Cl−] dependence of channel activity and inactivation gating. Most of the above properties (points 1–7) resemble the macroscopic properties of the CFTR Cl− channel (Fuller & Benos, 1992). However, cAMP-activated Cl− currents in Paneth cells exhibited much lower sensitivity to glibenclamide (IC50: 150–260 μm at −80 to −40 mV) than epithelial (IC50: 15 μm at −50 mV; Sheppard & Robinson, 1997) and cardiac (IC50: 30 μm at −10 mV; Tominaga et al. 1995) CFTR Cl− channel currents. Its time- and voltage-dependent inactivation was also distinct from that of CFTR channel current. Moreover, relevant evidence for the molecular expression of CFTR could not be obtained in the present study.
The Paneth cell Cl− currents share [Cl−]o-dependent inactivation kinetics with those of the ClC-0 Cl− channel (Pusch et al. 1995) which was cloned from Torpedo electric organ and shown to have a consensus phosphorylation site for PKA (Jentsch et al. 1990). However, other ClC-0 properties such as outward rectification, anion selectivity of Cl− > Br− > I− and DIDS sensitivity are distinct from those of cAMP-activated Cl− channels expressed in guinea-pig Paneth cells. Some of the properties of the ClC-2G(2α) Cl− channel, which was cloned from a rabbit gastric cDNA library and shown to have potential PKA phosphorylation sites, are similar to those of the Paneth cell Cl− channel, for example, ohmic I-V relationship and PKA-induced activation (Malinowska et al. 1995). However, the anion selectivity (I− > Cl−) of ClC-2G(2α) is distinct from that of the Paneth cell Cl− channel. Moreover, mRNA expression of ClC-0 and ClC-2G(2α) has not been observed in the mammalian small intestine.
PKA-activated Cl− channels in rat and mouse choroid plexus epithelial cells have recently been clearly demonstrated to be distinct from CFTR by the method of RNA in situ hybridization in the normal rat (Kibble et al. 1996) and by employing the cystic fibrosis transgenic mouse (Kibble et al. 1997). The properties of the choroid plexus channel currents differ from those of Paneth cell cAMP-activated Cl− current in their inward rectification, anion selectivity sequence of I− > Cl− > Br− and DIDS sensitivity. PKA-activated Cl− channel currents were also found in isolated Necturus enterocytes (Giraldez et al. 1989). Their outward rectification, low field strength anion selectivity and sensitivity to a stilbene-derivative Cl− channel blocker are at variance with the properties of the Paneth cell PKA-regulated Cl− current.
Prompt changes in the cAMP-activated Cl− current profile were induced by the addition of DPC or glibenclamide to the basolateral bathing solution or by the reduction of basolateral Cl− concentration. These results suggest that the Paneth cell Cl− channel resides on the basolateral membrane but not on the luminal membrane which is not readily exposed to the bathing solution. This inference may also be supported by the fact that closure of the open end of crypt glands did not affect the amplitude of cAMP-activated Cl− current at all. To determine precisely the location of the channel, however, further investigations including single-channel studies are needed.
Taken together, it appears that Paneth cells possess cAMP-activated Cl− channels which are distinct from any known cAMP- or PKA-activated Cl− channels, although the single-channel properties remain to be investigated.
Molecular expression of CFTR in the guinea-pig small intestine
Recent studies using cystic fibrosis mouse models made by CFTR gene targeting clearly showed that CFTR is responsible for the small intestinal Cl− secretion (Grubb & Gabriel, 1997). In situ hybridization studies in rat and human small intestines have shown that distribution of CFTR mRNA is greater in the crypt than in the villus (Trezise & Buchwald, 1991; Strong et al. 1994). Uniform expression of CFTR mRNA was suggested in all the crypt enterocytes including Paneth cells in the human duodenum and jejunum (Strong et al. 1994). However, the present single-cell RT-PCR study failed to amplify CFTR mRNA in almost all the guinea-pig Paneth cells.
Immunocytochemical studies have so far provided contradictory information about the distribution of CFTR protein in the mammalian small intestine. Protein expression was found in the crypt of human small intestine by Crawford et al. (1991), and its intense expression was found in the apical region of mouse small intestinal crypts by French et al. (1996). In contrast, the strongest expression was found in the endoplasmic reticulum of goblet cells in human duodenum (Hoogeveen et al. 1991). Recently, Ameen et al. (1995) showed that there are a small number of unique enterocytes expressing a very high level of CFTR protein in the rat and human proximal small intestine. This subpopulation was found to be scattered through villi to crypts but not at the crypt base where Paneth cells reside. The present immunofluorescence study in villi and crypts isolated from guinea-pig small intestine was in good agreement with the findings of Ameen et al. (1995). The role of CFTR in such a unique subset of enterocytes remains to be elucidated. However, it is likely that CFTR protein is not the molecular identity for the cAMP-activated Cl− conductance which was consistently found in guinea-pig Paneth cells.
Physiological significance
Molecular expression of the VIP receptor has been shown in the small intestine of rat (Ishihara et al. 1992) and human (Sreedharan et al. 1995) by Northern blot analysis. Functional expression of VIP receptors coupled to adenylyl cyclase through a Gs protein has also been shown by binding assay and adenylyl cyclase assay in the plasma membrane of crypt enterocytes of human jejunum (Salomon et al. 1993). In the present study, the functional expression of VIP receptors was demonstrated for the first time by patch clamp in Paneth cells of guinea-pig small intestine. The effects of GDPβS and GTPγS indicate an involvement of G proteins in the VIP receptor response. Paneth cells are known to respond to β-adrenergic agonists, which should increase intracellular [cAMP], with lysozyme secretion (Olson & Erlandsen, 1981). Therefore, there is a possibility that a cAMP increase induced by stimulation of VIP receptors is involved not only in activation of Cl− conductance but also in the regulation of exocytosis in Paneth cells.
Paneth cells are thought to play a key role in host defence of the bowel by releasing a number of bioactive contents within the granules into the lumen (Ouellette, 1997). The mechanism of exocytosis in Paneth cells is largely obscure. However, intracellular vesicular transport and cell volume regulation can be expected to occur before, during and after the exocytotic event. In some cell types, it is known that the cAMP-dependent Cl− conductance plays essential roles in exocytotic membrane recycling (Bradbury et al. 1992) and cell volume regulation (Okada, 1997). Thus, it is possible that the cAMP-activated Cl− channel activity in Paneth cells is associated with the exocytotic process. Also, there is a possibility that the Cl− channel mediates net Cl− transport through Paneth cells. However, the exact roles of the cAMP-activated Cl− channel and the VIP receptor expressed in Paneth cells await further investigation.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research and by a grant for Priority Areas of ‘Channel-Transporter Correlation’ (07276 l04) from the Ministry of Education, Science and Culture of Japan. The authors are grateful to A. F. James (King's College, London, UK) for reading the manuscript, to A. Satoh (Asahikawa Medical College, Japan) for the suggestion about Paneth cell identification, to S. Morishima for advice and help, to M. Ohara and A. Hattori for technical assistance and to M. Takazawa for secretarial help.
References
- Ameen NA, Ardito T, Kashgarian M, Marino CR. A unique subset of rat and human intestinal villus cells express the cystic fibrosis transmembrane conductance regulator. Gastroenterology. 1995;108:1016–1023. doi: 10.1016/0016-5085(95)90198-1. [DOI] [PubMed] [Google Scholar]
- Bear CE, Reyes EF. cAMP-activated chloride conductance in the colonic cell line, Caco-2. American Journal of Physiology. 1992;262:C251–256. doi: 10.1152/ajpcell.1992.262.1.C251. [DOI] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H, Erlandsen S. Functional gap junctions in mouse small intestinal crypts. Anatomical Record. 1985;212:364–367. doi: 10.1002/ar.1092120407. [DOI] [PubMed] [Google Scholar]
- Bradbury NA, Jilling T, Berta G, Sorscher EJ, Bridges RJ, Kirk KL. Regulation of plasma membrane recycling by CFTR. Science. 1992;256:530–532. doi: 10.1126/science.1373908. [DOI] [PubMed] [Google Scholar]
- Crawford I, Maloney PC, Zeitlin PL, Guggino WB, Hyde SC, Turley H, Gatter KC, Harris A, Higgins CF. Immunocytochemical localization of the cystic fibrosis gene product CFTR. Proceedings of the National Academy of Sciences of the USA. 1991;88:9262–9266. doi: 10.1073/pnas.88.20.9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond G, Scanlin TF, Zasloff MA, Bevins CL. A cross-species analysis of the cystic fibrosis transmembrane conductance regulator. Potential functional domains and regulatory sites. Journal of Biological Chemistry. 1991;266:22761–22769. [PubMed] [Google Scholar]
- French PJ, van Doorninck JH, Peters RHPC, Verbeek E, Ameen NA, Marino CR, de Jonge HR, Bijman J, Scholte BJ. A ΔF508 mutation in mouse cystic fibrosis transmembrane conductance regulator results in a temperature-sensitive processing defect in vivo. Journal of Clinical Investigation. 1996;98:1304–1312. doi: 10.1172/JCI118917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller CM, Benos DJ. CFTR! American Journal of Physiology. 1992;263:C267–286. doi: 10.1152/ajpcell.1992.263.2.C267. [DOI] [PubMed] [Google Scholar]
- Giraldez F, Murray KJ, Sepepúlvedauacute;lveda FV, Sheppard DN. Characterization of a phosphorylation-activated Cl−-selective channel in isolated Necturus enterocytes. The Journal of Physiology. 1989;416:517–537. doi: 10.1113/jphysiol.1989.sp017775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory RJ, Cheng SH, Rich DP, Marshall J, Paul S, Hehir K, Ostedgaard L, Klinger KW, Welsh MJ, Smith AE. Expression and characterization of the cystic fibrosis transmembrane conductance regulator. Nature. 1990;347:382–386. doi: 10.1038/347382a0. 10.1038/347382a0. [DOI] [PubMed] [Google Scholar]
- Grubb BR, Gabriel SE. Intestinal physiology and pathology in gene-targeted mouse models of cystic fibrosis. American Journal of Physiology. 1997;273:G258–266. doi: 10.1152/ajpgi.1997.273.2.G258. [DOI] [PubMed] [Google Scholar]
- Hoogeveen AT, Keulemans J, Willemsen R, Scholte BJ, Bijman J, Edixhoven MJ, De jonge HR, Galjaard H. Immunological localization of cystic fibrosis candidate gene products. Experimental Cell Research. 1991;193:435–437. doi: 10.1016/0014-4827(91)90118-e. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Shigemoto R, Mori K, Takahashi K, Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992;8:811–819. doi: 10.1016/0896-6273(92)90101-i. 10.1016/0896-6273(92)90101-I. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ, Steinmeyer K, Schwarz G. Primary structure of Torpedo marmorata chloride channel isolated by expression cloning in Xenopus oocytes. Nature. 1990;348:510–514. doi: 10.1038/348510a0. 10.1038/348510a0. [DOI] [PubMed] [Google Scholar]
- Kibble JD, Garner C, Colledge WH, Brown S, Kajita H, Evans M, Brown PD. Whole-cell Cl− conductances in mouse choroid plexus epithelial cells do not require CFTR expression. American Journal of Physiology. 1997;272:C1899–1907. doi: 10.1152/ajpcell.1997.272.6.C1899. [DOI] [PubMed] [Google Scholar]
- Kibble JD, Trezise AEO, Brown PD. Properties of the cAMP-activated Cl− current in choroid plexus epithelial cells isolated from the rat. The Journal of Physiology. 1996;496:69–80. doi: 10.1113/jphysiol.1996.sp021666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laburthe M, Couvineau A. Molecular analysis of vasoactive intestinal peptide receptors. Annals of the New York Academy of Sciences. 1988;527:296–313. doi: 10.1111/j.1749-6632.1988.tb26988.x. [DOI] [PubMed] [Google Scholar]
- Lencer WI, Cheung G, Strohmeier GR, Currie MG, Ouellette AJ, Selsted ME, Madara JL. Induction of epithelial chloride secretion by channel-forming cryptdins 2 and 3. Proceedings of the National Academy of Sciences of the USA. 1997;94:8585–8589. doi: 10.1073/pnas.94.16.8585. 10.1073/pnas.94.16.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcnicholas CM, Brown CDA, Turnberg LA. Na-K-Cl cotransport in villus and crypt cells from rat duodenum. American Journal of Physiology. 1994;267:G1004–1011. doi: 10.1152/ajpgi.1994.267.6.G1004. [DOI] [PubMed] [Google Scholar]
- Malinowska DH, Kupert EY, Bahinski A, Sherry AM, Cuppoletti J. Cloning, functional expression, and characterization of a PKA-activated gastric Cl− channel. American Journal of Physiology. 1995;268:C191–200. doi: 10.1152/ajpcell.1995.268.1.C191. [DOI] [PubMed] [Google Scholar]
- Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. American Journal of Physiology. 1997;273:C755–789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- Olson RE, Erlandsen SL. Paneth cell function: The effects of cholinergic and adrenergic drugs on lysozyme secretion. Anatomical Record. 1981;199:186A. (abstract) [Google Scholar]
- Ouellette AJ. Paneth cells and innate immunity in the crypt microenvironment. Gastroenterology. 1997;113:1779–1784. doi: 10.1053/gast.1997.v113.pm9352884. [DOI] [PubMed] [Google Scholar]
- Pusch M, Ludewig U, Rehfeldt A, Jentsch TJ. Gating of the voltage-dependent chloride channel ClC-0 by the permeant anion. Nature. 1995;373:527–531. doi: 10.1038/373527a0. 10.1038/373527a0. [DOI] [PubMed] [Google Scholar]
- Rappolee DA, Mark D, Banda MJ, Werb Z. Wound macrophages express TGF-α and other growth factors in vivo: Analysis by mRNA phenotyping. Science. 1988;241:708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Salomon R, Couvineau A, Rouyer-Fessard C, Voisin T, Lavallavalléeeacute;e D, Blais A, Darmoul D, Laburthe M. Characterization of a common VIP-PACAP receptor in human small intestinal epithelium. American Journal of Physiology. 1993;264:E294–300. doi: 10.1152/ajpendo.1993.264.2.E294. [DOI] [PubMed] [Google Scholar]
- Sepepúlveda FV, Fargon F, Mcnaughton PA. K+ and Cl− currents in enterocytes isolated from guinea-pig small intestinal villi. The Journal of Physiology. 1991;434:351–367. doi: 10.1113/jphysiol.1991.sp018474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DN, Robinson KA. Mechanism of glibenclamide inhibition of cystic fibrosis transmembrane conductance regulator Cl− channels expressed in a murine cell line. The Journal of Physiology. 1997;503:333–346. doi: 10.1111/j.1469-7793.1997.333bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedharan SP, Huang J-X, Cheung M-C, Goetzl EJ. Structure, expression, and chromosomal localization of the type I human vasoactive intestinal peptide receptor gene. Proceedings of the National Academy of Sciences of the USA. 1995;92:2939–2943. doi: 10.1073/pnas.92.7.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong TV, Boehm K, Collins FS. Localization of cystic fibrosis transmembrane conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. Journal of Clinical Investigation. 1994;93:347–354. doi: 10.1172/JCI116966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucher NJ, Deitcher DL. PCR and patch-clamp analysis of single neurons. Neuron. 1995;14:1095–1100. doi: 10.1016/0896-6273(95)90257-0. 10.1016/0896-6273(95)90257-0. [DOI] [PubMed] [Google Scholar]
- Sullivan SK, Field M. Ion transport across mammalian small intestine. In: Field M, Frizzell RA, editors. Handbook of Physiology, section 6, The Gastrointestinal System, Intestinal Absorption and Secretion. IV. Bethesda, MD, USA: American Physiological Society; 1991. pp. 287–301. [Google Scholar]
- Tabcharani JA, Low W, Elie D, Hanrahan JW. Low-conductance chloride channel activated by cAMP in the epithelial cell line T84. FEBS Letters. 1990;270:157–164. doi: 10.1016/0014-5793(90)81257-o. 10.1016/0014-5793(90)81257-O. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Horie M, Sasayama S, Okada Y. Glibenclamide, an ATP-sensitive K+ channel blocker, inhibits cardiac cAMP-activated Cl−conductance. Circulation Research. 1995;77:417–423. doi: 10.1161/01.res.77.2.417. [DOI] [PubMed] [Google Scholar]
- Trezise AEO, Buchwald M. In vivo cell-specific expression of the cystic fibrosis transmembrane conductance regulator. Nature. 1991;353:434–437. doi: 10.1038/353434a0. 10.1038/353434a0. [DOI] [PubMed] [Google Scholar]
- Tsumura T, Hazama A, Oiki S, Ueda S, Okada Y. CFTR-type Cl− currents in guinea pig Paneth cells. Japanese Journal of Physiology. 1997;47(suppl.):S123. (abstract) [Google Scholar]
- Tsumura T, Oiki S, Ueda S, Okuma M, Okada Y. Sensitivity of volume-sensitive Cl− conductance in human epithelial cells to extracellular nucleotides. American Journal of Physiology. 1996;271:C1872–1878. doi: 10.1152/ajpcell.1996.271.6.C1872. [DOI] [PubMed] [Google Scholar]
- Walters RJ, O'Brienapos;brien JA, Valverde MA, Sepepúlvedauacute;lveda FV. Membrane conductance and cell volume changes evoked by vasoactive intestinal polypeptide and carbachol in small intestinal crypts. Pflügers Archiv. 1992;421:598–605. doi: 10.1007/BF00375057. [DOI] [PubMed] [Google Scholar]
- Wright E, Diamond JM. Anion selectivity in biological systems. Physiological Reviews. 1977;57:109–156. doi: 10.1152/physrev.1977.57.1.109. [DOI] [PubMed] [Google Scholar]


