Abstract
The contribution of intracellular pH (pHi) to the failure of Ca2+ release and inhibition of contractile proteins observed during fatigue was assessed in single intact mouse muscle fibres at 22 °C. Fatigue was induced by repeated tetani at intensities designed to induce different levels of intracellular acidosis. Force and either intracellular free Ca2+ concentration ([Ca2+]i; measured using indo-1) or pHi (measured using SNARF-1) were recorded in fibres fatigued at two different intensities.
Intensity was varied by the repetition rate of tetani and quantified by the duty cycle (the fraction of time when the muscle was tetanized). Stimulation at the low intensity (duty cycle ∼0.1) reduced force to 30 % of initial values in 206 ± 21 s (60 ± 7 tetani); at the high intensity (duty cycle ∼0.3) force was reduced to 30 % in 42 ± 7 s (43 ± 7 tetani) (P < 0.05; n = 14).
When force was reduced to 30 % of initial values, tetanic [Ca2+]i had fallen from 648 ± 87 to 336 ± 64 nm (48 % decrease) at the low intensity but had only fallen from 722 ± 84 to 468 ± 60 nm (35 % decrease) at the higher intensity (P < 0.05 low vs. high intensity; n = 7).
Fatigue resulted in reductions in Ca2+ sensitivity of the contractile proteins which were greater at the high intensity (pre-fatigue [Ca2+]i required for 50 % of maximum force (Ca50) = 354 ± 23 nm; post-fatigue Ca50 = 421 ± 48 nm and 524 ± 43 nm for low and high intensities, respectively). Reductions in maximum Ca2+-activated force (Fmax) were similar at the two intensities (pre-fatigue Fmax = 328 ± 22 μN; post-fatigue Fmax = 271 ± 20 and 265 ± 19 μN for low and high intensities, respectively).
Resting pHi was 7.15 ± 0.05. During fatigue at the low intensity, pHi was reduced by 0.12 ± 0.02 pH units and at the high intensity pHi was reduced by 0.34 ± 0.07 pH units (P < 0.05; n = 5).
Our results indicate that the more rapid fall in force at a high intensity is due to a reduction in Ca2+ sensitivity of the contractile proteins, probably related to the greater acidosis. Our data also indicate that the failure of Ca2+ release and reduced maximum Ca2+-activated force observed during fatigue are not due to reductions in intracellular pH.
During repeated tetanic contractions there is a reduction in force output known as muscle fatigue. The decrease in force during fatigue is due to at least three mechanisms: (i) decrease in Ca2+ released from the sarcoplasmic reticulum (SR); (ii) reduced Ca2+ sensitivity of the myofibrillar proteins; and (iii) reduced maximum Ca2+-activated force (see Fitts, 1994; Allen et al. 1995a for recent reviews). The alterations in contractile protein function appear to be due primarily to the accumulation of metabolites in the myoplasm. Studies with skinned single fibres have shown that increases in hydrogen ions (H+) and inorganic phosphate (Pi) can account for the reductions in Ca2+ sensitivity and maximum Ca2+-activated force observed during fatigue (Metzger & Moss, 1987; Godt & Nosek, 1989). The underlying cause(s) for the reduction in Ca2+ release are not fully understood, but there appear to be both metabolic (Westerblad & Allen, 1992; Owen et al. 1996; Allen et al. 1997) and non-metabolic (Fryer et al. 1995; Chin & Allen, 1996) factors involved. In particular, the role of intracellular acidosis in Ca2+ release failure and muscle fatigue is still controversial (Allen et al. 1995b).
Changes in muscle pH have been suggested to play a critical role in fatigue (Fitts, 1994). Numerous studies show strong correlations between muscle [H+] and force during exercise and recovery (Hermansen & Osnes, 1972; Kushmerick & Meyer, 1985; Thompson et al. 1992). The close association between force and [H+] along with evidence that acidosis inhibits the opening of isolated SR Ca2+ release channels and reduces ryanodine binding (Ma et al. 1988; Favero et al. 1995) make it tempting to postulate a causal relationship. However, experiments with intact single fibres have shown that decreases in force and intracellular free Ca2+ concentration ([Ca2+]i) occur in the absence of changes in pH during fatigue (Westerblad & Allen, 1992). Conversely, changes in muscle pH induced by CO2 exposure reduce force but increase [Ca2+]i indicating that Ca2+ release failure is not the cause of the fall in force during acidosis (Westerblad & Allen, 1993). Furthermore, in skinned fibres with intact t-tubular SR coupling, acidosis has very little effect on depolarization-induced Ca2+ release (Lamb et al. 1992). The extent to which changes in muscle pH explain the fall of force during fatigue is therefore still unclear. A further complication is the recent demonstration that acidosis has much less effect on the contractile proteins at near physiological temperatures than at room temperature (Pate et al. 1995; Westerblad et al. 1997).
It is well established that the rate at which muscles fatigue depends on the intensity and duration of contraction and on the fibre type composition of the muscle (Metzger & Moss, 1987; Barclay et al. 1995). One hypothesis to explain the controversy regarding the role of pHi in fatigue is that pHi-dependent mechanisms are intensity and fibre-type specific. For example, H+ ions may only accumulate to levels that impair force production when muscles contract at a high intensity where rates of ATP demand exceed ATP supply and when primarily fast glycolytic fibres are recruited. In mixed muscle preparations these mechanisms are difficult to assess due to alterations in the numbers and types of fibres recruited. We have therefore used single fibres from a fast muscle to control for fibre type variability and assessed the role of intracellular pH at two different intensities. Our observations indicate that fatigue at a high compared with a low intensity exhibits a greater acidosis, which is associated with a larger decrease in Ca2+ sensitivity and a smaller decrease in tetanic [Ca2+]i. These data suggest that in fast fatiguable fibres at room temperature, pH-dependent alterations in contractile protein function play a greater role in fatigue at high intensities and that impairments in excitation-contraction coupling, which appear to be pH independent, predominate during fatigue at low intensities.
METHODS
Mice were killed by cervical dislocation and single muscle fibres dissected from the flexor brevis muscle. Fibres from this muscle are predominantly type II X and II B (Allen et al. 1993). Methods for fibre dissection and mounting were similar to those previously used in this laboratory (Westerblad & Allen, 1991). After dissection and transfer to a stimulation chamber fibres were superfused at 22°C with a solution containing (mm): NaCl, 121; KCl, 5.0; CaCl2, 1.8; MgCl2, 0.5; NaH2PO4, 0.4; NaHCO3, 24; and glucose, 5.5. The solution was bubbled with 95 % O2 and 5 % CO2 to give a pH of 7.3. Approximately 0.2 % (v/v) fetal calf serum was added.
The fibres were stimulated with platinum electrodes using pulses of 0.5 ms duration at an intensity of approximately 1.2 × threshold. All tetanic contractions were 0.35 s in duration. Force produced during unfused tetani was assessed by the average output over the final 100 ms.
Intracellular free calcium concentration and pH measurements
In separate experiments, [Ca2+]i was measured with the fluorescent dye indo-1 and intracellular pH (pHi) was measured with SNARF-1. The dyes were introduced into the fibres by micro-injection. For indo-1, fibres were illuminated at 360 nm and the emitted light measured at 400 and 510 nm. For SNARF-1, fibres were illuminated at 540 nm and emitted light collected at 590 and 640 nm. The experimental set-up was similar to that described by Lee et al. (1991). Emitted light was measured by two photomultiplier tubes and passed on to an analog divide circuit which allowed continuous monitoring of the 400/510 ([Ca2+]i) or the 590/640 (pHi) ratio signal. The background fluorescence was subtracted from all measurements. The indo-1 ratio was converted to [Ca2+]i using the in vivo calibration previously described (Westerblad & Allen, 1993) and the steady-state [Ca2+]i for each tetanus was determined by the average output over the last 100 ms. For pHi SNARF-1 was calibrated as previously described (Buckler & Vaughan-Jones, 1990; Westerblad & Allen, 1992).
Experimental protocols
The aim of this study was to examine the intensity dependence of pHi changes and to identify pH-dependent mechanisms of fatigue. We hypothesized that pHi would change to a greater extent at a high compared with a low work intensity and that Ca2+ release failure and alterations in contractile protein function would be exacerbated at the higher intensity. To test these hypotheses we used single muscle fibres in a repeated measures design to control for fibre type differences. Changes in pHi and [Ca2+]i were compared during series of tetani at two different intensities. Intensity was varied by altering the rest interval between tetani. For the low intensity, fatigue was induced using a previously characterized protocol (Allen et al. 1989; Westerblad & Allen, 1991) with 100 Hz tetani repeated every 4 s for 2 min, then the inter-tetanus interval decreased to 3 s for 2 min, then to 2.5 s for 2 min etc., until force reached 30 % of initial values. In a typical fatigue run lasting 3–4 min, the average rate of tetanic contractions was one every 3.5 s. In previous experiments this fatigue protocol resulted in an acidosis of only 0.06 pH units (Westerblad & Allen, 1992). At the higher intensity, fatigue was induced using 100 Hz tetani of the same duration (350 ms) with tetani repeated every 1 s. For both protocols tetani were continued until force was reduced to 30 % of initial values. For experiments measuring [Ca2+]i, both protocols were applied to the same fibre with 1 h of recovery between fatigue bouts. For experiments measuring pHi, fibres were fatigued using both protocols but recovery time was reduced to 20 min to minimize the reduction of SNARF-1 signal over time. In a few experiments pHi was measured at the two intensities in separate fibres. In experiments where both protocols were used the order was randomized with an equal number of fibres given the high and low intensity first.
Assessment of contractile protein function
In order to determine the Ca2+ sensitivity and maximum Ca2+-activated force of the contractile proteins we used a protocol described previously (Westerblad & Allen, 1991). Briefly, before each fatigue bout fibres were activated at a range of frequencies (30, 50, 70, 100 Hz and 100 Hz in the presence of 5 or 10 mm caffeine) at 1 min intervals. Force and [Ca2+]i at each frequency were used to construct force-Ca2+ curves and to determine the Ca2+ sensitivity and maximum Ca2+-activated force of a rested fibre. Force-Ca2+ curves were fitted using a Hill equation:
where F is force, Fmax is the force at saturating [Ca2+]i, N is a constant describing the steepness of the relation and Ca50 represents the [Ca2+]i required for 50 % of maximum force. To assess the changes in Ca2+ sensitivity during fatigue, force and [Ca2+]i from the final phase of fatigue, when both are declining, were used (see Westerblad & Allen, 1991). Force and [Ca2+]i from a 100 Hz tetanus in the presence of caffeine, representing maximum Ca2+-activated force, were measured at the end of the fatigue protocol and added to the force-Ca2+ plot, which could then be fitted by a Hill equation as described above. Initially maximum Ca2+-activated force was assessed in the presence of 5 or 10 mm caffeine delivered during the fatigue run (n = 5). To ensure that the measurement of maximum Ca2+-activated force was not compromised by a depletion of SR Ca2+ during fatigue, in some experiments caffeine was added at the end of the fatigue run and the 100 Hz + caffeine data point obtained after a 15 s pause (n = 6). The results of the force-Ca2+ curve analyses for the two sets of data were similar so the results were combined.
Assessment of low frequency fatigue
We have previously shown that fatigue induced by repeated short tetani using our standard fatigue protocol (i.e. low intensity) results in a prolonged reduction of Ca2+ release and changes in force characteristic of low frequency fatigue. Low frequency fatigue was first defined by Edwards et al. (1977) and refers to a reduction of force at low stimulation frequencies (i.e. 30–50 Hz) which persists 1 h after activity with no reduction at high stimulation frequencies (100 Hz) or in maximum force (100 Hz + caffeine). We have also shown that low frequency fatigue is dependent on the elevation of [Ca2+]i and the duration of this period of elevated [Ca2+]i (i.e. the cumulative [Ca2+]i-time integral) (Chin & Allen, 1996). We were interested in whether fatigue at a higher intensity, which would be shortened in duration, would elevate the cumulative [Ca2+]i-time integral to a level sufficient to induce low frequency fatigue. To assess low frequency fatigue, force and [Ca2+]i were measured at a range of stimulation frequencies (30, 50, 70, 100 Hz and 100 Hz in the presence of 5 or 10 mm caffeine) 5 min before and 60 min after both fatigue protocols (n = 9). Low frequency fatigue was assessed only at the end of the first fatigue run for each fibre.
Statistical analyses
All values are expressed as means ± s.e.m. Student's paired t tests were used to test for statistical significance when measurements were made in the same fibre (pre vs. post fatigue; [Ca2+]i for high vs. low intensity). Unpaired t tests were used to test for significance in pHi experiments. Significance was accepted at P < 0.05.
RESULTS
Changes in muscle force and [Ca2+]i during fatigue at high and low intensities
The underlying mechanisms of fatigue were assessed at two different intensities; at the low intensity tetani were repeated initially every 4 s and at the high intensity tetani were repeated every 1 s. At both intensities tetani were continued until force reached 30 % of initial values. Although the relative decrease in force was similar for both intensities, the time course was dramatically different. Figure 1 shows the time course of fatigue and the force and Ca2+ transients at the beginning and end of fatigue for a representative fibre at both intensities. During both fatigue runs there was a small initial fall in force; thereafter force declined slowly for 1 to 2 min at the low intensity (Fatigue 1) or declined rapidly at the high intensity (Fatigue 2). The average time to fatigue (30 % initial force) was significantly longer for the low compared with the high intensity (206 ± 21 vs. 42 ± 7 s, P < 0.05; n = 14). This represented 60 ± 7 and 43 ± 7 tetani (P < 0.05) for the low and high intensities, respectively.
Figure 1. Force and [Ca2+]i traces from a single fibre fatigued at low and high intensities.
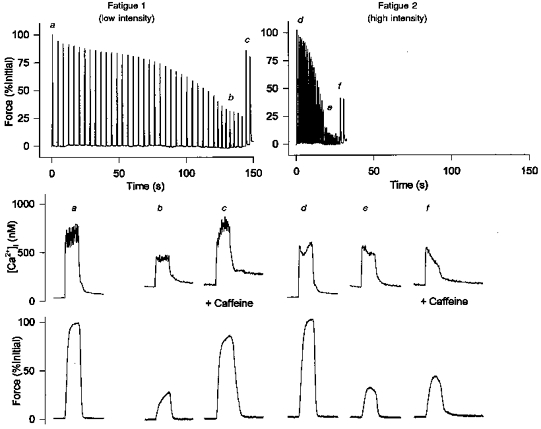
Continuous force records (upper panel) and [Ca2+]i and force transients on an expanded time scale (middle and bottom panels, respectively) are shown during fatigue at low and high intensities. Fatigue 1 was induced by tetani repeated initially every 4 s and increased to a rate of 1/3 s after 2 min (low intensity). Following 60 min of recovery Fatigue 2 was induced by tetani repeated every 1 s (high intensity). Tetani were continued until force reached 30 % of initial values. The fibre was perfused with a 5 mm caffeine solution towards the end of fatigue to assess E-C coupling failure. For Fatigue 1, force and [Ca2+]i, transients are shown for the first tetanus (a), the tetanus at 30 % (b) and a tetanus after exposure to caffeine (c). For Fatigue 2 force and [Ca2+]i, transients represent the first tetanus (d), the tetanus at 30 % (e) and a tetanus after exposure to caffeine (f).
In addition to changes in the rate of fatigue, there were differences in the extent to which [Ca2+]i fell during fatigue at the two intensities. When force was reduced to 30 % of initial values, tetanic [Ca2+]i was higher at the high intensity (Fig. 1b vs. e). For this fibre, [Ca2+]i was reduced at the beginning of Fatigue 2 due to a prolonged reduction of Ca2+ release induced during Fatigue 1 (see last paragraph of Results). The relative reduction in [Ca2+]i for Fatigue 2 was therefore even smaller. The lower starting [Ca2+]i did not alter the overall results since the order of the fatigue protocols was reversed in four of seven fibres. Figure 2 shows the pooled data for seven fibres. When force was reduced to 30 % at the low intensity, [Ca2+]i was reduced from a mean level of 648 ± 87 to 336 ± 64 nm, a decrease of 48 %. At the high intensity [Ca2+]i was reduced from an average of 722 ± 84 to 468 ± 60 nm, a decrease of 35 %. The difference between the low and high intensity (48 % vs. 35 %) was significant. These data suggest that the more rapid fall in force at the higher intensity was due to mechanisms other than Ca2+ release failure.
Figure 2. Average force and [Ca2+]i in response to fatigue at low and high intensities.
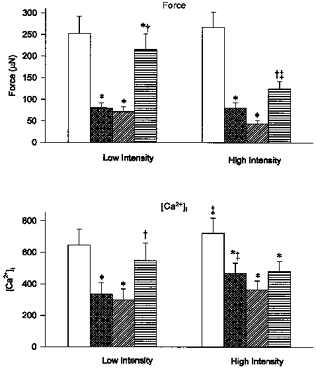
Force (upper panel) and [Ca2+]i, (lower panel) for the first tetanus (pre-fatigue; □), when force reached 30 % (fatigue;  ), minimum force (
), minimum force ( ) and after 5 mm caffeine exposure at the end of fatigue (
) and after 5 mm caffeine exposure at the end of fatigue ( ). Minimum force was defined as the force immediately before a response to caffeine was observed. Changes in force and [Ca2+]i during fatigue at both the low intensity (1/4 s, etc.) and high intensity (1/1 s) are shown. Values represent mean ± s.e.m. (n = 7). *P < 0.05 vs. pre-fatigue; †P < 0.05 vs. fatigue; ‡P < 0.05 vs. low intensity.
). Minimum force was defined as the force immediately before a response to caffeine was observed. Changes in force and [Ca2+]i during fatigue at both the low intensity (1/4 s, etc.) and high intensity (1/1 s) are shown. Values represent mean ± s.e.m. (n = 7). *P < 0.05 vs. pre-fatigue; †P < 0.05 vs. fatigue; ‡P < 0.05 vs. low intensity.
The impairment of excitation-contraction coupling (E-C coupling) was assessed by adding caffeine, a Ca2+ release channel agonist, to the perfusing solution at the end of fatigue. Since the effect of caffeine on force and [Ca2+]i was not immediate, the extent of recovery was determined by comparing the maximum force and [Ca2+]i after addition of caffeine to the level which immediately preceded the caffeine response (defined as the minimum level). During stimulation at the low intensity, caffeine was able to restore force and [Ca2+]i back to 75–85 % of starting levels (Fig. 1C). In contrast, 5 mm caffeine had only a small effect on force and [Ca2+]i during stimulation at the high intensity (Fig. 1f). As illustrated in Fig. 2, in the presence of caffeine force recovered to 70 ± 8 % at the low intensity and to 51 ± 4 % at the high intensity, a difference which was significant. At the low intensity, this recovery of force was associated with a recovery of tetanic [Ca2+]i (from a minimum of 46 ± 11 % to 84 ± 8 % of initial values, P < 0.05). At the high intensity, however, the recovery of [Ca2+]i with caffeine was small and not significant (from a minimum of 50 ± 8 % to 67 ± 9 %). The decrease in caffeine-activated Ca2+ release was not due to a decrease in caffeine sensitivity since similar results were obtained using 10 mm caffeine. These observations suggest there was a decrease in caffeine-activated Ca2+ release as well as E-C coupling failure at the high intensity. The decrease in caffeine-activated Ca2+ release may be due to insufficient re-filling of the SR Ca2+ pool by the SR Ca2+ pumps. To test this interpretation, in five additional experiments, fibres were given a 15 s pause between the last tetanus of fatigue and the tetanus in the presence of caffeine. This resulted in partial recovery of the caffeine-evoked force and [Ca2+]i at the high intensity (force = 72 ± 1 % of initial values and [Ca2+]i = 78 ± 17 % of initial values; data not shown). This rapid reversal of Ca2+ release is consistent with the idea that the impairment of caffeine-activated Ca2+ release is related to refilling of the SR Ca2+ pool. These data are also consistent with previous reports that SR Ca2+ loading but not depolarization-induced Ca2+ release is impaired at an acidic pHi (Lamb et al. 1992) (see Discussion).
Force-[Ca2+]i relationship during fatigue at high and low intensities
The decrease in [Ca2+]i may explain part of the decrease in force during fatigue but another component of force loss is attributable to changes in contractile protein function. We have assessed changes in Ca2+ sensitivity of the contractile proteins and changes in maximum Ca2+-activated force by plotting the force-Ca2+ relationship before and during fatigue. Figure 3 shows force-Ca2+ curves from a fibre that was fatigued at both the high and low intensity. During fatigue at the low intensity there was a rightward shift of the force-Ca2+ curve and a reduction in maximum force (Fmax). During fatigue at the high intensity there was an even greater rightward shift of the force-Ca2+ curve and a similar reduction in Fmax. The mean results for eleven fibres are shown in Table 1. Neither fatigue protocol altered N, suggesting that co-operativity of Ca2+ binding was not altered. Ca2+ sensitivity of the contractile proteins was reduced during low intensity stimulation resulting in a 19 % increase in Ca50. Stimulation at the high intensity reduced Ca2+ sensitivity to a greater extent (48 % increase in Ca50) indicating that contractile protein inhibition was greater with higher intensity stimulation. During stimulation at both high and low intensities, Fmax was reduced to a similar extent (by 17 % and 19 % for low and high intensity, respectively) suggesting that maximum Ca2+-activated force of the contractile proteins was not different between the two intensities.
Figure 3. Force-Ca2+ relationship in response to fatigue at low and high intensities.
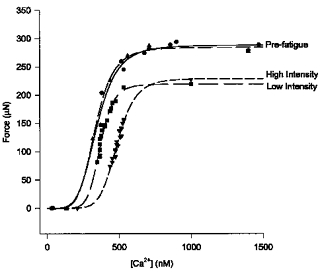
Pre-fatigue force and [Ca2+]i are plotted at 30, 50, 70 and 100 Hz and maximum force obtained at 100 Hz in the presence of 5 mm caffeine. Force and [Ca2+]i are plotted for pre-fatigue (Fatigue 1, •; Fatigue 2, ▴) and during the final phase of fatigue, when force and Ca2+ are decreasing, for the low intensity (▪) and high intensity (▾). Data points representing force and [Ca2+]i after 5 mm caffeine exposure at the end of fatigue are included. All points are fitted to a Hill equation (see Methods). The rightward shift of the force-Ca2+ curve, representing a decrease in Ca2+ sensitivity, is greater at the high vs. low intensity. The plateau, representing maximum Ca2+-activated force, is reduced during fatigue but to a similar extent at the two intensities.
Table 1.
Force-Ca2+ relationship during fatigue with different rest intervals
| N | Ca50 (nm) | Fmax(μN) | |
|---|---|---|---|
| Pre-fatigue | 6.076 ± 1.17 | 354 ± 23 | 328 ± 22 |
| Low intensity | 6.133 ± 0.57 | 421 ± 48* | 271 ± 20 |
| High intensity | 6.426 ± 0.43 | 524 ± 43*† | 265 ± 19* |
Force and [Ca2+]i were measured at 30, 50, 70, 100 Hz and with 100 Hz in the presence of 5 mm (n = 3) or 10 mm (n = 8) caffeine. Values were used to construct force-Ca2+ curves which were fitted using a Hill equation (see Methods). From this, values were obtained for N, which is a constant describing the steepness of the relation, Ca50 which represents the [Ca2+]i required for 50% of maximum force and Fmax, which is the force at saturating [Ca2+]i. The values represent mean ± s.e.m. (n = 11).
P < 0.05vs. Prefatigue
P < 0.05vs. 1/4 s.
Changes in pHi during fatigue at high and low intensities
To assess the importance of pHi during fatigue at different work intensities, pHi was measured in fibres fatigued at the high and low intensities. The resting pHi measured by SNARF-1 was 7.15 ± 0.05. Figure 4 shows the changes in pHi in a representative fibre in response to stimulation at the two intensities and in response to a brief exposure to 30 % CO2. Fatigue at the low intensity resulted in a small and gradual fall in pHi. In five fibres the average change in pHi was 0.12 ± 0.02 pH units, which is similar to previous observations (Westerblad & Allen, 1992). In contrast, fatigue at the high intensity resulted in a large and rapid decrease in pHi which appeared to coincide with the fall in force. In five fibres, pHi was reduced by 0.34 ± 0.07 pH units. The increased rate of acidosis can be explained by increased anaerobic glycolysis and inhibition of the lactate-H+ co-transporter. At the low intensity the pH change was 0.12 pH units (3.43 min)−1 or 0.03 pH units min−1. At the higher intensity the pH change was 0.34 pH units (0.7 min)−1 or 0.49 pH units min−1. At the low intensity energy is initially supplied by phosphocreatine hydrolysis and some anaerobic glycolysis while oxidative phosphorylation is activated. Our data suggest that at this intensity ATP demand is matched by ATP supply without any excess H+ production. As the duty cycle increases, glycolysis is activated to a greater extent but remains at a rate where lactate production can be matched by extrusion via the lactate transporter, thus little acidosis results. However, at the high intensity where tetani start at a rate of 1 s−1, anaerobic glycolysis is activated immediately to supply ATP and it becomes the major source of energy. At this rate lactate and H+ are produced at a faster rate than can be removed by the transporter. The resultant accumulation of H+ and decrease in pHi is greater at the higher intensity and indicates that pHi plays a more important role in fatigue at a higher intensity.
Figure 4. Force and pHi traces from a single fibre fatigued at low and high intensities.
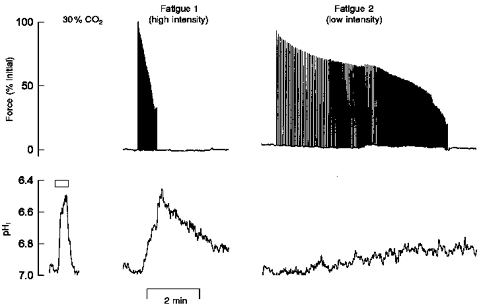
Force (upper panel) and pHi (lower panel) were recorded simultaneously at both the high and low intensity. pHi was measured using SNARF-1. Fatigue 1 was induced by tetani repeated every 1 s (high intensity). Following 20 min of recovery, Fatigue 2 was induced by tetani repeated initially every 4 s then increased to 1/3 s, 1/2.5 s, etc. every 2 min (low intensity). Tetani were continued until force reached 30 % of initial values. Change in pHi induced by 30 % CO2 exposure (∼0.5 pH unit change) is shown relative to the pHi changes at high and low intensities.
Low frequency fatigue following tetani at high and low intensities
We have previously shown that fatigue induced by stimulation at a low intensity results in a prolonged decrease in Ca2+ release and a selective reduction in force at low frequencies known as low frequency fatigue (Westerblad et al. 1993; Chin & Allen, 1996). In the present study we were able to assess whether low frequency fatigue was induced during a shorter fatigue run when force fell more rapidly. Figure 5 shows the changes in force and [Ca2+]i before and 60 min after fatigue at the low (n = 6) and high (n = 7) intensities. Similar to previous observations, force at low frequencies (30 and 50 Hz) was still reduced 60 min after fatigue at the low intensity (55 ± 13 % and 76 ± 11 % initial force for 30 and 50 Hz, respectively). Force was also reduced at 70 Hz (86 ± 8 % of initial values) but had fully recovered at 100 Hz. At the high intensity, force was depressed at all frequencies after 60 min of recovery (reduced to 30 ± 8 %, 50 ± 8 %, 74 ± 7 % and 89 ± 4 % of initial values for 30, 50, 70 and 100 Hz, respectively). The differences between intensities were not significant. Furthermore, maximum Ca2+-activated force (100 Hz + Caff) had fully recovered for both intensities, indicating that contractile protein function was not impaired and that the fibre preparations remained healthy. The reduction of force can be explained by the prolonged reduction of tetanic [Ca2+]i which was still depressed after 60 min of recovery. Similar to previous observations, steady state [Ca2+]i was reduced to a similar extent at all frequencies. On average, [Ca2+]i recovered to 71 ± 2 % and 84 ± 2 % of initial values at the low and high intensities (P < 0.05). These data indicate that fatigue at both intensities resulted in prolonged reductions in Ca2+ release and that the reductions were more pronounced at the high intensity even though fatigue occurred over a much shorter period of time.
Figure 5. Force and [Ca2+]i after 60 min recovery demonstrate low frequency fatigue.
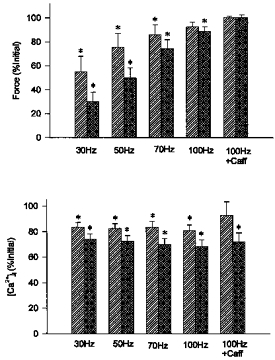
Force (top panel) and [Ca2+]i (bottom panel) after 60 min of recovery from fatigue at low and high intensities ( and
and  , respectively). Reductions in force and [Ca2+]i, shown as % of initial values, were of similar magnitude at both intensities. Values represent mean ± s.e.m. (n = 6–7). * Significant difference (P < 0.05) compared with initial force or [Ca2+]i at the same frequency or condition.
, respectively). Reductions in force and [Ca2+]i, shown as % of initial values, were of similar magnitude at both intensities. Values represent mean ± s.e.m. (n = 6–7). * Significant difference (P < 0.05) compared with initial force or [Ca2+]i at the same frequency or condition.
DISCUSSION
Previous studies have shown that muscle fatigue is due to a combination of decreased release of Ca2+ from the SR, decreased Ca2+ sensitivity of the contractile proteins and reduced maximum Ca2+-activated force (Allen et al. 1989; Westerblad & Allen, 1991). We have examined changes in force, [Ca2+]i and pHi during fatigue at two different intensities to try to elucidate the pH dependence of these mechanisms. Our results indicate that although [Ca2+]i declined at a faster rate, the decrease in tetanic [Ca2+]i that occurred during fatigue was attenuated at a higher intensity when acidosis was more pronounced. Ca2+ release failure therefore does not require acidosis nor does it appear to be greater when acidosis is present. Our data also show that Ca2+ sensitivity of the contractile proteins was reduced to a greater extent during fatigue where a large acidosis was observed. The reduced Ca2+ sensitivity of the contractile proteins is due to both pH-dependent and pH-independent mechanisms and appears to be relatively more important in fatigue at high intensities.
Role of pHi in Ca2+ release failure during fatigue
The contribution of intracellular acidosis to the failure of E-C coupling during muscle fatigue remains controversial. Observations that acidosis inhibits the opening of SR Ca2+ release channels and reduces ryanodine binding (Ma et al. 1988; Favero et al. 1995) have implicated a role for pHi in the failure of Ca2+ release. If acidosis was responsible for the reduction in Ca2+ release one would expect a greater reduction in tetanic [Ca2+]i during fatigue in which a substantial level of acidosis occurred. However, this was not observed in the present study. Instead [Ca2+]i was 13 % higher at the same relative force output when fatigued at a high intensity (pHi ∼6.8) compared with a low intensity (pHi ∼7.0). These observations are consistent with previous findings that acidosis induced by CO2 exposure in intact single muscle fibres (Allen et al. 1989; Westerblad & Allen, 1993) and in whole frog muscles (Baker et al. 1995) was associated with a 33–37 % increase in tetanic [Ca2+]i. Thus it appears that Ca2+ release evoked by normal E-C coupling mechanisms is not inhibited by a decrease in pHi and that reductions in pHi probably do not contribute to the Ca2+ release failure observed during fatigue.
It is not clear whether the increased tetanic [Ca2+]i during acidosis is caused by increased Ca2+ release, by reduced Ca2+ buffering or by a reduction in the SR Ca2+ pump rate. Studies using isolated SR vesicles indicate that Ca2+ release can be augmented by acidosis (Donoso et al. 1996), but studies with skinned single fibres show that acidosis has little effect on depolarization-induced Ca2+ release (Lamb et al. 1992) and no effect on t-tubule charge movement (Fitts & Balog, 1996). Acidosis can also alter intracellular Ca2+ regulation by increasing the leak of Ca2+ from the SR (Lamb et al. 1992) and slowing the SR Ca2+ pump (MacLennan, 1970). Under acidotic conditions single fibres show a 50–65 % greater rise in resting [Ca2+]i (Westerblad & Allen, 1993; present study) and a slower rate of decline of the Ca2+ tails (Westerblad & Allen, 1993), which are consistent with a pH-induced leak of Ca2+ from the SR and a reduction in Ca2+ uptake. The increased Ca2+ leak and impaired Ca2+ reuptake may be due to direct inhibitory effects of H+ ions on the Ca2+-ATPase or they may be secondary to reduced maximal Ca2+ binding to calsequestrin as pH in the SR lumen is reduced (Donoso et al. 1996). Currently there are no data to indicate whether Ca2+ buffering is altered by changes in sarcoplasmic pH so it is not known whether this mechanism also contributes to the rise in tetanic [Ca2+]i when the muscle becomes acidotic.
An interesting observation from the present study is that reductions in pHi impaired caffeine-activated Ca2+ release. Reductions in caffeine- and Ca2+-activated Ca2+ release have also been shown in isolated SR vesicles (Favero et al. 1995; Kentish & Xiang, 1997) and in skinned fibres (Lamb et al. 1992). There are several possible explanations for the reduction in caffeine-activated Ca2+ release. First, there may be insufficient refilling of the SR Ca2+ pools because of slowing of the SR Ca2+ pump. The rapid recovery of caffeine-activated force and tetanic [Ca2+]i by 15 s following fatigue supports this interpretation. However, if there was inadequate Ca2+ available for release it would suggest there was a decrease in total SR Ca2+ storage and there is reasonable evidence to indicate that this does not happen during fatigue (Gonzalez-Serratos et al. 1978). A second possibility is that the gating mechanisms regulating caffeine and Ca2+-induced Ca2+ release are pH sensitive even though depolarization-induced Ca2+ release is not. There is no direct evidence for differential pH sensitivities of the various gating mechanisms but this may explain the conflicting reports in the literature regarding pH effects on Ca2+ release (Ma et al. 1988; Lamb et al. 1992; Favero et al. 1995). A third possible explanation for the decrease in caffeine-activated Ca2+ release is that Pi becomes elevated in the SR lumen as well as in the cytoplasm and Ca2+ then binds to Pi, forming a Ca2+ orthophosphate precipitate. This mechanism is thought to be involved in the impaired depolarization-induced release of Ca2+ during fatigue (Fryer et al. 1995) and it may also explain the reduced caffeine-activated release.
The relationship between the indo-1 ratio and [Ca2+] is slightly sensitive to pH with a 1 pH unit decrease in pH causing a 25 % reduction in the Ca2+ sensitivity of indo-1 (Westerblad & Allen, 1993). Thus the peak tetanic [Ca2+] at the end of the low intensity fatigue when an acidosis of 0.12 pH units was present would be underestimated by about 3 % (25 % × 0.12) while the peak systolic [Ca2+] at the end of high intensity fatigue would be underestimated by 8.5 % (25 % × 0.34). These small corrections would not affect any of our conclusions.
Role of duty cycle in low frequency fatigue
While the pH-mediated increase in tetanic [Ca2+]i cannot explain the reduction in force during fatigue, it may contribute to the prolonged reduction in Ca2+ release characteristic of low frequency fatigue (Westerblad et al. 1993; Chin & Allen, 1996). In the present study, low frequency fatigue was observed even at the higher intensity when force was reduced to 30 % in less than a minute. The prolonged impairment in Ca2+ release is related to a sustained rise in cytosolic [Ca2+] (Lamb et al. 1995; Chin & Allen, 1996), which occurs both because of the rise in resting [Ca2+]i and the more frequent tetani. Thus, even when muscles fatigue rapidly the cumulative [Ca2+]i-time integral can be elevated above the threshold level required to induce low frequency fatigue (Chin et al. 1997) which suggests that both intensity-dependent and Ca2+-dependent components are of importance in determining whether low frequency fatigue will occur.
Role of pHi in impaired Ca2+ sensitivity during fatigue
A key observation from the present study is that the predominant pH-dependent mechanism of fatigue is a reduction in Ca2+ sensitivity of the contractile proteins. During fatigue reductions in Ca2+ sensitivity are also attributed to elevations in Pi and reduced cellular ATP concentrations (Godt & Nosek, 1989; Parkhouse, 1992). In addition, these metabolic effects may be partly offset by increases in ADP, which increase Ca2+ sensitivity (Godt & Nosek, 1989). The contribution of pHi to alterations in the force-Ca2+ relationship can be predicted from experiments in frog and mouse skeletal muscle (Westerblad & Allen, 1993; Baker et al. 1995). It was previously shown that a 0.5 pH unit change increased Ca50 from 357 to 532 nm, a 1.5-fold increase (0.17 pCa unit) (Westerblad & Allen, 1993). It is expected that a 0.22 pH unit change (0.34–0.12 pH units at the high and low intensities, respectively) would increase Ca50 by 0.22/0.5 × 0.17 pCa units = 0.076 pCa units or a factor of 1.19; this agrees with the observed change in Ca50 of 1.24 (524 nm/421 nm). Thus, the increased acidosis can explain most of the reduction in Ca2+ sensitivity. The molecular mechanism for the pH-induced reduction in Ca2+ sensitivity is not known, but one possibility is that the excess H+ ions compete with Ca2+ and reduce their binding to the Ca2+ binding sites on troponin C (TnC) (Ball et al. 1994). Additionally, there may be changes in interactions between TnC and the other regulatory proteins (troponin I, troponin T and tropomyosin) and/or to altered binding of the troponin complex to the actin filament (Ball et al. 1994; Ding et al. 1996). The precise molecular interactions disrupted by reductions in pHi in skeletal muscle are not currently known.
Role of pHi in impaired maximum Ca2+-activated force
pH-induced decreases in maximum Ca2+-activated force of 10–15 % have been shown in single muscle fibre preparations (Godt & Nosek, 1989; Westerblad & Allen, 1993) as well as in intact whole muscles (Baker et al. 1995). The decrease in maximum Ca2+-activated force during isometric contractions at an acidic pHi has been attributed to a decrease in the mean force produced per cross-bridge (see Westerblad et al. 1997). This is consistent with in vitro studies that show a 50 % decrease in myofibrillar ATPase activity when pHi is reduced from 7.0 to 6.5 (Parkhouse, 1992). While reductions in pHi can decrease maximum force by impairing myosin ATPase activity, the contribution of this mechanism to the reduction in force during fatigue is still questionable. In the present study we observed a 17–19 % decrease in Fmax at both intensities despite the differences in pHi. Also, during repeated tetanic contractions the decline in force during early fatigue (∼10 %) occurs in the first 10–15 tetani. This initial fall in force is due to a reduction in maximum Ca2+-activated force which is not associated with a decline in pHi (Westerblad & Allen, 1992, 1993). Thus, decreases in maximum Ca2+-activated force during repeated tetani and fatigue probably do not result from H+-induced effects on myosin ATPase activity. We cannot, however, rule out the possibility that in late fatigue pHi-induced reductions in myosin ATPase occur but are offset by increases in ADP which increase maximum Ca2+-activated force (Pate & Cooke, 1989).
In summary, the present study shows that in fast fatiguable fibres the predominant mechanism of muscle fatigue and the role of pHi in fatigue are dependent on the work intensity. At lower intensities the predominant mechanism of fatigue is failure of E-C coupling, which is pH independent. In contrast, at higher intensities the reduction in Ca2+ sensitivity of the contractile proteins is largely due to the accompanying acidosis and takes on an increasing role in fatigue. The contribution of acidosis to the reduction in force during fatigue, however, would be smaller at more physiological temperatures.
Acknowledgments
This study was supported by the National Health and Medical Research Council of Australia.
References
- Allen DG, Duty S, Westerblad H. Metabolic changes in muscle during exercise: their effects on muscle function. Proceedings of the Australian Physiological and Pharmacological Society. 1993;24:65–75. [Google Scholar]
- Allen DG, Lännergrenauml;nnergren J, Westerblad H. Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Experimental Physiology. 1995a;80:497–527. doi: 10.1113/expphysiol.1995.sp003864. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lännergrenauml;nnergren J, Westerblad H. The role of ATP in the regulation of intracellular Ca2+ release in single fibres of mouse skeletal muscle. The Journal of Physiology. 1997;498:587–600. doi: 10.1113/jphysiol.1997.sp021885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG, Lee JA, Westerblad H. Intracellular calcium and tension during fatigue in isolated single muscle fibres from Xenopus laevis. The Journal of Physiology. 1989;415:433–458. doi: 10.1113/jphysiol.1989.sp017730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG, Westerblad H, Lännergrenauml;nnergren J. The role of intracellular acidosis in muscle fatigue. Advances in Experimental Medicine and Biology. 1995b;384:57–68. doi: 10.1007/978-1-4899-1016-5_5. [DOI] [PubMed] [Google Scholar]
- Baker AJ, Brandes R, Weiner MW. Effects of intracellular acidosis on Ca2+ activation, contraction, and relaxation of frog skeletal muscle. American Journal of Physiology. 1995;268:C55–63. doi: 10.1152/ajpcell.1995.268.1.C55. [DOI] [PubMed] [Google Scholar]
- Ball KL, Johnson MD, Solaro RJ. Isoform specific interactions of troponin I and troponin C determine pH sensitivity of myofibrillar Ca2+ activation. Biochemistry. 1994;33:8464–8471. doi: 10.1021/bi00194a010. [DOI] [PubMed] [Google Scholar]
- Barclay CJ, Arnold PD, Gibbs CL. Fatigue and heat production in repeated contractions of mouse skeletal muscle. The Journal of Physiology. 1995;488:741–752. doi: 10.1113/jphysiol.1995.sp021005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Application of a new pH-sensitive fluoroprobe (carboxy-SNARF-1) for intracellular pH measurement in small, isolated cells. Pflügers Archiv. 1990;417:234–239. doi: 10.1007/BF00370705. [DOI] [PubMed] [Google Scholar]
- Chin ER, Allen DG. The role of elevations in intracellular [Ca2+] in the development of low frequency fatigue in mouse single muscle fibres. The Journal of Physiology. 1996;491:813–824. doi: 10.1113/jphysiol.1996.sp021259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin ER, Balnave CD, Allen DG. Role of intracellular calcium and metabolites in low-frequency fatigue of mouse skeletal muscle. American Journal of Physiology. 1997;272:C550–559. doi: 10.1152/ajpcell.1997.272.2.C550. [DOI] [PubMed] [Google Scholar]
- Ding XL, Akella AB, Sonnenblick EH, Rao VG, Gulati J. Molecular basis of depression of Ca2+ sensitivity of tension by acid pH in cardiac muscles of the mouse and the rat. Journal of Cardiac Failure. 1996;2:319–326. doi: 10.1016/s1071-9164(96)80019-4. [DOI] [PubMed] [Google Scholar]
- Donoso P, Beltran M, Hidalgo C. Luminal pH regulates calcium release kinetics in sarcoplasmic reticulum vesicles. Biochemistry. 1996;35:13419–13425. doi: 10.1021/bi9616209. [DOI] [PubMed] [Google Scholar]
- Edwards RHT, Hill DK, Jones DA, Merton PA. Fatigue of long duration in human skeletal muscle after exercise. The Journal of Physiology. 1977;272:769–778. doi: 10.1113/jphysiol.1977.sp012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favero TG, Zable AC, Bowman MB, Thompson A, Abramson JJ. Metabolic end products inhibit sarcoplasmic reticulum Ca2+ release and [3H]ryanodine binding. Journal of Applied Physiology. 1995;78:1665–1672. doi: 10.1152/jappl.1995.78.5.1665. [DOI] [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiological Reviews. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Fitts RH, Balog EM. Effects of intracellular and extracellular ion changes on E-C coupling and skeletal muscle fatigue. Acta Physiologica Scandinavica. 1996;156:169–181. doi: 10.1046/j.1365-201X.1996.191000.x. [DOI] [PubMed] [Google Scholar]
- Fryer MW, Owen VJ, Lamb GD, Stephenson DG. Effects of creatine phosphate and Pi on Ca2+ movements and tension development in rat skinned skeletal muscle fibres. The Journal of Physiology. 1995;482:123–140. doi: 10.1113/jphysiol.1995.sp020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt RE, Nosek TM. Changes in the intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. The Journal of Physiology. 1989;412:155–180. doi: 10.1113/jphysiol.1989.sp017609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Serratos H, Somlyo AV, McClellan G, Shuman H, Borrero LM, Somlyo AP. Composition of vacuoles and sarcoplasmic reticulum in fatigued muscle: electron probe analysis. Proceedings of the National Academy of Sciences of the USA. 1978;75:1329–1333. doi: 10.1073/pnas.75.3.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansen L, Osnes J. Blood and muscle pH after maximal exercise in man. Journal of Applied Physiology. 1972;32:304–308. doi: 10.1152/jappl.1972.32.3.304. [DOI] [PubMed] [Google Scholar]
- Kentish JC, Xiang JZ. Ca2+- and caffeine-induced Ca2+ release from sarcoplasmic reticulum in rat skinned trabeculae: effects of pH and Pi. Cardiovascular Research. 1997;33:314–323. doi: 10.1016/s0008-6363(96)00217-9. [DOI] [PubMed] [Google Scholar]
- Kushmerick MJ, Meyer RA. Chemical changes in rat leg muscle by phosphorus nuclear magnetic resonance. American Journal of Physiology. 1985;248:C542–549. doi: 10.1152/ajpcell.1985.248.5.C542. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Junankar PR, Stephenson DG. Raised intracellular [Ca2+] abolishes excitation-contraction coupling in skeletal muscle fibres of rat and toad. The Journal of Physiology. 1995;489:349–362. doi: 10.1113/jphysiol.1995.sp021056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Recupero E, Stephenson DG. Effect of myoplasmic pH on excitation-contraction coupling in skeletal muscle fibres of the toad. The Journal of Physiology. 1992;448:211–224. doi: 10.1113/jphysiol.1992.sp019037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, Westerblad H, Allen DG. Changes in tetanic and resting [Ca2+]i during fatigue and recovery of single muscle fibres from Xenopus laevis. The Journal of Physiology. 1991;433:307–326. doi: 10.1113/jphysiol.1991.sp018427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Fill M, Knudson CM, Campbell KP, Coronado R. Ryanodine receptor of skeletal muscle is a gap junction-type channel. Science. 1988;242:99–102. doi: 10.1126/science.2459777. [DOI] [PubMed] [Google Scholar]
- MacLennan DH. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. Journal of Biological Chemistry. 1970;245:4508–4518. [PubMed] [Google Scholar]
- Metzger JM, Moss RL. Greater hydrogen ion-induced depression of tension and velocity in skinned single fibres of rat fast than slow muscles. The Journal of Physiology. 1987;393:727–742. doi: 10.1113/jphysiol.1987.sp016850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen VJ, Lamb GD, Stephenson DG. Effect of low [ATP] on depolarization-induced Ca2+ release in skeletal muscle fibres of the toad. The Journal of Physiology. 1996;493:309–315. doi: 10.1113/jphysiol.1996.sp021385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhouse WS. The effects of ATP, inorganic phosphate, protons, and lactate on isolated myofibrillar ATPase activity. Canadian Journal of Physiology and Pharmacology. 1992;70:1175–1181. doi: 10.1139/y92-163. [DOI] [PubMed] [Google Scholar]
- Pate E, Bhimani M, Franks-Skiba K, Cooke R. Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue. The Journal of Physiology. 1995;486:689–694. doi: 10.1113/jphysiol.1995.sp020844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate E, Cooke R. A model of crossbridge action: the effects of ATP, ADP and Pi. Journal of Muscle Research and Cell Motility. 1989;10:181–196. doi: 10.1007/BF01739809. [DOI] [PubMed] [Google Scholar]
- Thompson LV, Balog EM, Fitts RH. Muscle fatigue in frog semitendinosis: role of intracellular pH. American Journal of Physiology. 1992;262:C1507–1512. doi: 10.1152/ajpcell.1992.262.6.C1507. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Changes in myoplasmic calcium concentration during fatigue in single mouse muscle fibres. Journal of General Physiology. 1991;98:615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Changes of intracellular pH due to repetitive stimulation of single fibres from mouse skeletal muscle. The Journal of Physiology. 1992;449:49–71. doi: 10.1113/jphysiol.1992.sp019074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. The influence of intracellular pH on contraction, relaxation and [Ca2+]i in intact single fibres from mouse muscle. The Journal of Physiology. 1993;466:611–628. [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Bruton J D, Lännergrenauml;nnergren J. The effects of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperatures. The Journal of Physiology. 1997;500:193–204. doi: 10.1113/jphysiol.1997.sp022009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Duty S, Allen DG. Intracellular calcium concentration during low-frequency fatigue in isolated single fibers of mouse skeletal muscle. Journal of Applied Physiology. 1993;75:382–388. doi: 10.1152/jappl.1993.75.1.382. [DOI] [PubMed] [Google Scholar]


