Abstract
Electrocorticogram (ECoG) and myometrial electromyogram (EMG) were recorded continuously in chronically instrumented late gestation sheep fetuses (n = 9) to analyse: (1) 24 h ECoG patterns, (2) relationships between ECoG and myometrial contractility, and (3) 24 h ECoG patterns at the spontaneous onset of labour.
Spontaneous onset of labour was determined from the myometrial EMG. ECoG signals were purified by a denoising procedure of wavelet decomposition. High-voltage slow ECoG activity (HV) and low-voltage fast ECoG activity (LV) were determined mathematically, and HV-LV cycle periodicity was calculated by periodogram analysis.
Twenty-four hour rhythms were present in fetal ECoG HV-LV cycles in the 3–5 days prior to spontaneous onset of labour (P < 0.01). Cycle frequency was lower at 08.00–12.00 h and higher at 16.00–20.00 h (lights on at 07.00 h, lights off at 21.00 h). LV duration was longer at 20.00–24.00 h, and HV was shorter at 16.00–20.00 h. No relationship was found between ECoG cycles and myometrial contracture cycles. Twenty-four hour ECoG rhythms disappeared 1 day before the spontaneous onset of labour.
ECoG patterns changed 7 to 4 h prior to spontaneous onset of labour, percentage of time spent and duration of HV ECoG increased, and percentage of time spent in LV decreased significantly. ECoG HV-LV cyclicity was reduced 4–5 h prior to spontaneous onset of labour, indicating that the altered fetal hormonal and blood gas environment around the spontaneous onset of labour alter fetal neural function.
Since Halberg (1969) first used the term ‘chronobiology,’ a variety of 24 h rhythm studies have been reported during pregnancy in both maternal and fetal physiology. Several studies have demonstrated 24 h rhythms in fetal plasma cortisol (Nathanielsz et al. 1977; McMillen et al. 1987), melatonin (Houghton et al. 1993), prolactin (McMillen et al. 1987), and vasopressin (Stark & Daniel, 1989) in late gestation sheep. Rhythms have also been demonstrated in fetal heart rate (Dalton et al. 1977; Visser et al. 1982; Fletcher et al. 1996), blood pressure (Brace & Moore, 1992), fetal breathing movements (Boddy et al. 1973; Dawes & Robinson, 1976), and urine production (Brace & Moore, 1992).
Although a considerable number of studies have evaluated electrocorticogram (ECoG) activity and the development of sleep cycles after birth (Ellingson, 1972), similar longitudinal studies are lacking in fetal life. ECoG activity is a well-studied characteristic of fetal neurophysiological function. However, little is known about 24 h variation in ECoG patterns in late gestation (Dawes & Robinson, 1976). Characteristic ECoG patterns involving alteration of low-voltage fast activity (LV) and high-voltage slow activity (HV) (Jost et al. 1972; Dawes & Robinson, 1976; Clewlow et al. 1983) have been reported. In the present study, we analysed fetal ECoG patterns in late gestation sheep, applying an index of cyclicity as well as measurement of the percentage of time spent in LV and HV states. Marked changes occur in neuroactive hormone concentrations in fetal sheep in the final days of gestation, and at the time of spontaneous onset of labour (Magyar et al. 1980; Challis & Lye, 1994). Since these hormonal changes are likely to alter function of the fetal central nervous system, we analysed fetal ECoG patterns in relation to the onset of spontaneous term labour.
METHODS
Care of animals and surgery
Nine Rambouillet-Columbia cross-bred ewes (weighing 50–60 kg) bred on a single occasion only, and carrying a fetus of known gestational age, were used. All procedures were approved by the Cornell University Animal Care and Use Committee. All facilities were approved by the American Association for the Accreditation of Laboratory Animal Care. From 7 days before surgery, ewes were housed in a metabolic stall with free access to food and water in a room with controlled light-dark cycles (light on at 07.00 h, and off at 21.00 h). All animals were surgically instrumented at 114- 124 days of gestational age (dGA). Ewes received 1 g of ampicillin sodium (Polycillin-N, Bristol Laboratories, Syracuse, NY, USA), ketamine (5–10 mg kg−1) and 1 mg of glycopyrollate immediately before surgery. Anaesthesia was induced by 4 % halothane for 5 min, followed by intubation. Surgery was performed under 1.5 % halothane general anaesthesia using techniques that have been described in detail elsewhere (Nathanielsz et al. 1980; Robinson et al. 1995; Derks et al. 1997).
In brief, polyvinyl catheters were inserted into a maternal carotid artery and jugular vein and advanced into the arch of the aorta and superior vena cava, respectively. The uterus was exposed through a mid-line abdominal incision. Hysterotomy was performed and fetuses were instrumented with polyvinyl vascular catheters inserted via the carotid artery and jugular vein and directed towards the fetal heart. An amniotic cavity catheter was also placed. Two pairs of multistranded stainless-steel wire (catalogue no. AS 632; Cooner Sales Co., Chastsworth, CA, USA) electrodes were placed into the myometrium to record myometrial activity. ECoG electrodes were placed bilaterally through the frontal bone into dura using stainless-steel wires terminating in metal pins or screws. Following surgical preparation of the ewe and fetus, all fetal catheters and leads were grouped to exit the lateral abdominal wall of the ewe at a single point. Surgical closure was accomplished in layers.
Ewes were returned to the same metabolism stalls in the laboratory in which they had been acclimatized before surgery. During the 4 days following surgery, ewes received prophylactic antibiotic treatment of 0.5 g ampicillin sodium (AMP-EQUINE Æ, SmithKline Beecham, West Chester, PA, USA) via the maternal jugular catheter twice daily. Fetuses also received 0.5 g ampicillin via the amniotic catheter twice a day. Phenylbutazone (1 g) (EQUIPHEN Æ, Pharmaceutical Inc., Shirley, NY, USA) was given to the ewe orally twice a day to provide analgesia. Ewes were fed fresh alfalfa daily and water was available ad libitum. All fetuses were allowed to recover for at least 5 days after surgery before any recordings were made.
After surgery, maternal and fetal arterial blood samples (0.5 ml) were taken daily at 10.00 h for measurement of blood gases and pH on a blood gas analyser (ABL500, Radiometer, Copenhagen, Denmark; measurements were corrected to 39°C). Physiological saline containing heparin (10 units ml−1) was continuously infused at a rate of 0.5 ml h−1 to maintain catheter patency. Prior to delivery and at least 10 h following the onset of labour, confirmed by increased, irreversible myometrial electrical activity, the ewe was killed with an overdose of sodium pentobarbitone (Fatal-Plus, Vortech Pharmaceuticals, Dearborn, MI, USA).
Data acquisition and signal processing
Fetal blood pressure, heart rate, ECoG and myometrial electromyogram (EMG) were recorded continuously throughout the study from 5 days after surgery using a data acquisition system (DAS) (Figueroa et al. 1985). Bio-electrical signals were recorded as digital data and stored on a personal computer. In this system, ECoG signals (100 μV full-scale) were collected using an amplifier with 1–30 Hz bandpass filter and full-wave rectified, and myometrial EMG with 3–30 Hz bandpass filter. The signals were sampled at 32 Hz and means of signals obtained over 1 s were stored on computer (Hsu et al. 1989). Data obtained throughout the last 7 days of fetal life were analysed.
Estimation of the timing of onset of spontaneous labour
The time of spontaneous onset of labour was determined retrospectively by calculating frequency power spectra from the fast Fourier transform of myometrial EMG signals (Hsu et al. 1989; Derks et al. 1996). The integrated power in the frequency range of 0.2–0.8 cycles min−1 over 1 h was defined as contraction power for comparative analysis. Baseline contraction power was averaged over the 48 h period during days 5 and 6 before the end of the experiment. The time of onset of spontaneous labour was determined when the contraction power exceeded mean ± 2 s.d. of baseline value (Fig. 1). Contraction power is presented as a percentage of the baseline contraction power (Unno et al. 1997).
Figure 1. Estimation of timing of spontaneous onset of labour by Fourier transform analysis of myometrial EMG (means ± s.e.m.).
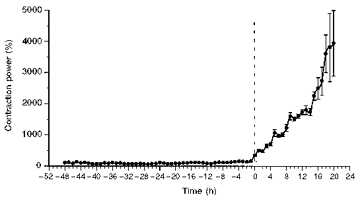
Hourly contraction power (CP) expressed as a percentage of the baseline obtained from the average of 48 h data 5–6 days before the end of experiment. The time of spontaneous onset of labour was determined when the CP value exceeded mean ± 2 s.d. of baseline value.
Processing of ECoG signals
To clarify HV and LV from the ECoG raw data signals, a noise reduction procedure of wavelet packet decomposition using spline wavelet packet was applied. This technique has previously been used in digital signal processing for denoising, data compression and frequency analysis (Graps, 1995; He, 1996). Briefly, the wavelet procedure is to adopt wavelet prototype function, so-called mother wavelets:
where s and 1 are integers that scale and dilate mother function Φ and x is the mother wavelet function. The wave signals were expressed as a divided matrix of scaled and dilated mother wavelet function. The denoising procedure applies a certain threshold to wavelet coefficients. Using this denoising procedure, noise and artefacts were mathematically reduced and continuous ECoG voltage envelope data were calculated. ECoG data analysis was performed on data collected in 3 h windows constantly shifted 1 h.
Calculation of dominant ECoG HV-LV cycle frequency
HV-LV cycles were analysed by means of a periodogram, which evaluated cyclical patterns, frequencies or periodocities by decomposing data into a sum of sine and cosine waves of different amplitude and wavelength (Martin & Brinkmann, 1976).
The value of peak frequency or frequency with maximum power (cycles h−1) was used for analysis. Bartlett's Kolmogorov-Smirnov test was used to prove statistical significance of peak frequency detection from white noise. Uterine contracture cycles were analysed using the same methods as ECoG.
Differentiation of HV and LV states
Each ECoG condition (HV or LV) was determined mathematically using the mean voltage after full wave rectification of all ECoG signals obtained in each 3 h window. Voltage above the upper 95 % confidence limit of the mean voltage was classified as HV, below the lower 5 % confidence limit of the mean voltage was considered LV, and within these two limits of the mean was classified as undefined activity (Fig. 2).
Figure 2. Determination of mean ECoG voltage and the 95 % upper confidence limit (UCL) and lower confidence limit (LCL) of the mean to discriminate high voltage (HV) and low voltage (LV).
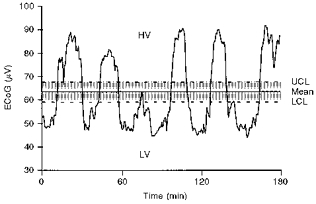
Recording from a fetal sheep at 138 days of gestational age.
Analysis of 24 h rhythms in ECoG and changes at the time of spontaneous onset of labour
Continuous 48 h ECoG recordings obtained from 3–5 days prior to spontaneous onset of labour, and 24 h data recorded during 1 day prior to spontaneous onset of labour, were used for ECoG periodicity analysis. The mean duration of HV and LV and percentages of time spent in HV, LV and undefined activity states were also calculated to analyse 24 h rhythms, as well as changes around the spontaneous onset of labour. The relationship between ECoG cycles and uterine contracture cycles was also analysed by linear correlation, with values obtained every hour.
Data processing and statistical analyses were carried out using Mathematica 2.2.2 with Wavelet Explorer software (Wolfram Research Inc., Champaign, IL, USA), Microsoft Excel (Microsoft Corp., Redmond, WA, USA) and JMP 3.1 software (SAS Institute Inc., Cary, NC, USA).
Multivariate ANOVA was applied for statistical analyses. All data are presented as means ±s.e.m.
RESULTS
Timing of onset of labour by myometrial EMG power analysis
Details of the animals studied are summarized in Table 1. The time of the day at which spontaneous onset of labour occurred in nine ewes was 00.00–04.00 h, n = 0; 04.00- 08.00 h, n = 1; 08.00–12.00 h, n = 1; 12.00–16.00 h, n = 2; 16.00–20.00 h, n = 1; and 20.00–24.00 h, n = 4. Fetal arterial blood gas values throughout the study were within the normal range (Table 1).
Table 1.
Details of animals studied
| Number of animals = 9 | Mean ± s.e.m. |
|---|---|
| Age at surgery | 116.8 ± 1.5 dGA |
| Age at data collection for data on the day before delivery | 138.0 ± 0.7 dGA |
| Age at 48 h of recording used for 24 h rhythm analysis (mid-period) | 140.2 ± 0.5 dGA |
| Age at spontaneous onset of labour (SOL) | 143.7 ± 0.7 dGA |
| Age at end of study | 144.7 ± 0.7 dGA |
| Duration from SOL to end of study | 16.9 ± 1.5 h |
| Fetal weight at end of study | 4.1 ± 0.4 kg |
| Mean fetal pH during analysis period (before SOL) | 7.36 ± 0.01 |
| Mean fetal PO2during analysis period (before SOL) | 21.8 ± 0.4 mmHg |
| Mean fetal pH during analysis period (after SOL) | 7.36 ± 0.04 |
| Mean fetal PO2 during analysis period (after SOL) | 18.5 ± 1.5 mmHg |
dGA, days of gestational age.
ECoG signal processing
The denoising procedure using wavelet packet decomposition produced clear ECoG envelope signals (Fig. 3), which enabled precise mathematical definition of the HV and LV.
Figure 3. Denoising ECoG signals by wavelet packet decomposition.
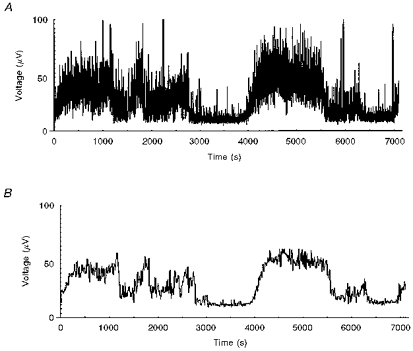
Original data obtained from a data acquisition system (A) and processed data (B). Recording from a fetal sheep at 139 days of gestational age.
24 h rhythm analysis
During the period from 3 to 5 days (140.2 ± 0.5 dGA) prior to the spontaneous onset of labour, marked 24 h rhythms in ECoG cycling were observed (P < 0.01). ECoG cycles were less frequent between 08.00 and 12.00 h and more frequent between 16.00 and 20.00 h (Fig. 4Aa) (P < 0.001). In contrast, the ECoG obtained 1 day prior to the spontaneous onset of labour showed no 24 h rhythm in ECoG cycling (Fig. 4Ba). There were no 24 h changes in the time spent in HV and LV in both groups. The mean time spent in HV and LV was around 45 and 40 %, respectively (Fig. 4Ab and Bb). The duration of LV state was longer at 20.00–24.00 h (P < 0.05) and HV state was shorter at 16.00–20.00 h (P < 0.05) (Fig. 4Ac). Comparison between HV and LV duration showed that the LV state was longer (P < 0.01) than the HV state in the evening (16.00–20.00 h and 20.00–24.00 h) in the group of 3–5 days before spontaneous onset of labour (Fig. 4Ac). However, no such differences were found between HV and LV duration of time in the group of 1 day before spontaneous onset of labour (Fig. 4Bc).
Figure 4. Characteristics of ECoG HV-LV 3–5 days prior to spontaneous onset of labour (A) and 1 day before spontaneous onset of labour (B).
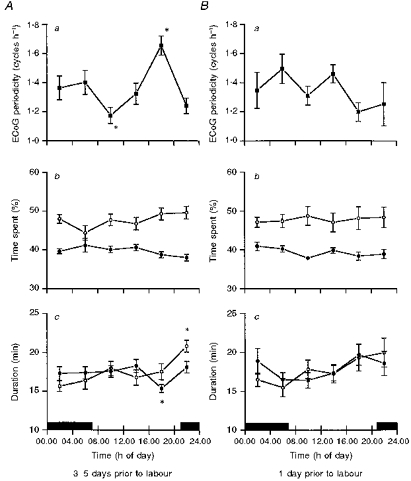
a, ECoG periodicity; b, percentage of time spent in LV (○) and HV (•) (means ± s.e.m. for each 4 h); c, duration of LV and HV epochs. The filled bar represents hours of darkness. Values are given as means ± s.e.m. (n = 9). *P < 0.05.
Relationship between myometrial contracture cycles and ECoG cycles
No 24 h variation was found in myometrial contracture cycles (Fig. 5). No correlation was found between uterine contracture cycles and ECoG cycles (r = 0.016).
Figure 5. Periodogram analysis for myometrial contracture cycles.
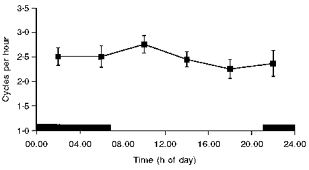
Values are given as means ± s.e.m. (n = 9).
ECoG HV-LV pattern around spontaneous onset of labour
Time spent in HV, LV and undefined ECoG activity did not change until just before spontaneous onset of labour. The percentage of time spent in HV increased at −6 h (P < 0.05), LV was decreased at −5 h (P < 0.05) and undefined activity was increased at −3 h (P < 0.05) when compared with values −48 to −24 h before spontaneous onset of labour. Individual HV and LV duration also changed before spontaneous onset of labour. HV duration was increased at −7 h (P < 0.05) and LV duration was decreased at −5 h (P < 0.05) compared with −48 to −24 h baseline values. ECoG HV-LV cyclicity expressed as 1–2 cycles h−1 periodgram was lower at −4 to 0 h (P < 0.05) before spontaneous onset of labour. However, the periodicity of ECoG increased again at 0 to 4 h of spontaneous labour (P < 0.05) compared with −4 to 0 h and then became increasingly unclear as labour progressed (Fig. 6).
Figure 6. Changes in ECoG pattern around onset of spontaneous term labour (time 0).
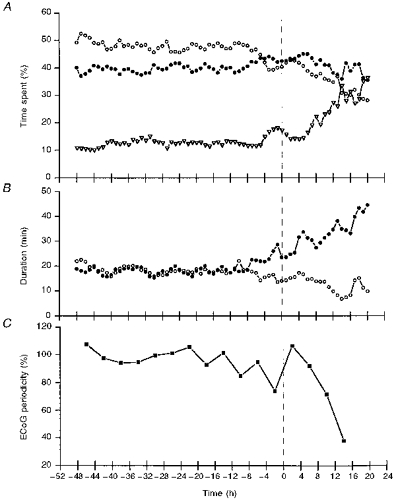
A, percentage of time spent as LV (○), HV (•), or undefined (▿); B, duration of HV (•) and LV (○) calculated hourly; C, ECoG periodicity in the integrated periodogram of 1–2 cycles h−1 range. Data integrated over a 4 h period. Baseline (100 %) obtained at −48 to 24 h before the onset of labour (time 0).
DISCUSSION
Dawes & Robinson (1976) described ECoG LV duration as longer at dusk and shorter at dawn in relation to the 24 h variation of fetal breathing movements in the fetal sheep. Ruckenbusch et al. (1977) reported that three out of five fetal sheep in late gestation showed higher percentages of LV ECoG at night-time (01.00–07.00 h) than during the daytime (14.00–20.00 h), and suggest existence of circadian pattern in ECoG. The use of the term circadian suggests that the rhythm is driven by an internal clock whose activity continues in the absence of all external cues. No experiments to date have fulfilled the conditions required to state that these fetal rhythms are circadian. Indeed, since the mother provides clues to the changing day - or her own circadian rhythms in an environment in which she is free of external clues - it is virtually impossible to evaluate fetal function in the absence of external cues. However, our data agree with both the above reports in indicating that 24 h rhythms do occur. There is a distinct 24 h pattern that shows that the duration of LV state is longer from evening to midnight and shorter at midnight to early morning. Our results provide no evidence for a 24 h rhythm in the percentage of time spent at both HV and LV states. These observations are similar to those of Dawes et al. (1976).
Periodogram analysis enables evaluation of the power of cyclicity. The frequency of ECoG cycles depends on the balance of the combined duration of LV and HV in each cycle. Therefore, 24 h rhythms in ECoG LV and HV duration should be clear by analysing their cyclicity. We observed that fetal ECoG periodicity (cycles h−1) showed a distinct 24 h rhythm in the period 3–5 days before the onset of spontaneous term labour. The periodicity disappeared following the onset of labour. The endocrine changes that occur at labour may account for this loss of rhythm.
Our second objective was to relate fetal ECoG patterns to myometrial activity. In pregnant sheep, spontaneous onset of labour has been characterized as an irreversible switch of myometrial EMG activity from low-amplitude, long-lasting (> 4 min), low-frequency (0.5–3 h−1) contractures to high-amplitude, short-lived (< 1 min), high-frequency (12–48 h−1) contractions (Nathanielsz et al. 1977, 1980; Figueroa, 1985). As previously reported, we have utilized integrated power spectral values calculated from myometrial EMG signals as an index of the predominant form of myometrial activity (Hsu et al. 1989; Derks et al. 1996; Unno et al. 1997). We determined time of spontaneous onset of labour as the point when the contraction power exceeded baseline contraction power by 2 s.d.
Several ontogenic studies of fetal ECoG have been reported in fetal sheep (Jost et al. 1972; Dawes & Robinson, 1976; Clewlow et al. 1983; Szeto et al. 1985; Szeto & Hinmann, 1985). However, analysis has either been totally visual or when fast Fourier transform (FFT) or other analytical methods have been used, they have only been employed for short periods of time. Even when FFT has been used, evaluations of LV and HV periods during which FFT was performed have been made visually. To provide more extensive longitudinal data, we analysed a minimum of 5 days continuously.
Uterine contraction activity alters uteroplacental oxygen uptake and fetal behaviour (Jansen et al. 1979; Longo et al. 1986). Contractures are accompanied by small (3–6 mmHg) but significant falls in O2 (Jansen et al. 1979). The fetus can respond to such small falls by increased ACTH secretion (Woudstra et al. 1991). Previous studies showed changes in ECoG state (Nathanielsz et al. 1980), tracheal pressure (Nathanielsz et al. 1980), chest wall diameter (Nathanielsz et al. 1980) and spontaneous motor activity (Robertson et al. 1996) in relation to contractures. Hofmeyr and colleagues (1985) demonstrated a statistically significant relation between contractures and a change to HV in fetal sheep at 121–134 dGA. Following brainstem transection, this association was lost. However, we did not observe a relationship between uterine contracture cycles and ECoG cycles. Therefore, myometrial activity is not a major regulator of the fetal ECoG rhythm, although contractures do have a tendency to switch LV to HV.
Marked hormonal changes occurred around the onset of term labour (Magyar et al. 1980; Challis & Lye, 1994). The most marked are an increase in fetal plasma cortisol (Nathanielsz et al. 1977; Magyar et al. 1980, Challis & Lye, 1994), an increase in both fetal (Nathanielsz et al. 1982) and maternal oestrogen (Challis, 1971), and a decrease in placental progesterone production (Challis & Lye, 1994). Cortisol in fetal sheep begins to increase from 120 to 125 dGA and concentrations are highest during the final 2–3 days of intrauterine life (Nathanielsz et al. 1977; Magyar et al. 1980; Challis & Lye, 1994). Oestrogen rises significantly in the fetus in the final 3 days of gestation (Nathanielsz et al. 1982), but only during the 24 h before spontaneous parturition in the maternal circulation (Challis, 1971). A decrease in placental progesterone production in sheep at term occurs in response to the rise in fetal cortisol (Challis & Lye, 1994). From our analysis, ECoG patterns changed around 4–5 h prior to spontaneous onset of labour when uterine contraction power is still at baseline values. Thus changes in ECoG patterns 4–5 h prior to spontaneous onset of labour are unlikely to be caused by an increase in uterine contractions. We hypothesize that the changes in ECoG pattern such as an increase of HV, decrease in LV, and indistinct ECoG cyclicity, are caused by environmental change of one or more of the above neuroactive hormones.
Analysing ECoG visually, Natale et al. (1981) reported that ECoG HV and LV persisted throughout labour and were accompanied by a reduction in fetal movements. Ruckenbusch et al. (1977) also reported an increase in duration of HV and decrease of LV during labour. This increase in HV is probably caused by reduced oxygen supply during labour. As mentioned above, uterine contraction activity reduces oxygen supply and may affect fetal behaviour. Continuous recording of PO2 changes throughout labour (Jansen et al. 1979) shows that well-co-ordinated uterine contractions during labour produced a marked fall in fetal vascular PO2, directly related to the uterine activity. The ECoG voltage amplitude changes in hypoxia are similar to the ECoG changes observed in labour. Boddy et al. (1974) showed a reduction in the percentage of the LV state and Symmes et al. (1970) reported early reduction of higher frequency followed by reduction of overall amplitude in ECoG during fetal hypoxia. Matsuda et al. (1994) reported a decrease in both LV and HV, with an increase in intermediate state at the end of sustained 8 h hypoxia. These findings suggest that the labour-related changes in ECoG pattern before the onset of labour might be an environmental adaptation to prepare for decreasing oxygen availability during delivery.
The present study presents the only longitudinal data precisely related to the onset of labour in this extensively studied model. We observed that as uterine contraction activity increased, the time spent in HV and undefined ECoG activity increased, while LV decreased by almost 50 %. We conclude that although the periodicity decreases, a fundamental basic periodicity of ECoG in the fetus is retained throughout labour. In the first 4 h after the onset of labour, this periodicity increases significantly before falling through labour. We would speculate that the sudden regular stimulation by well-established contractions of labour may result in this increased rhythmicity.
In conclusion, there is a 24 h rhythm in ECoG cyclicity in late gestation fetal sheep, and this rhythm disappears during the day before spontaneous onset of labour. The changes in ECoG patterns appear 4–5 h prior to onset of labour. These results suggest that neuroactive hormonal changes around labour, as well as myometrial contractility changes, alter fetal ECoG activity prior to onset of labour.
Acknowledgments
We would like to thank Dr Xiu-Ying Ding for her surgical assistance and Ms Karen A. Moore for her help with the manuscript.
References
- Boddy K, Dawes GS, Fisher R, Pinter S, Robinson J. Foetal respiratory movements, electrocortical and cardiovascular responses to hypoxaemia and hypercapnoea in sheep. The Journal of Physiology. 1974;243:599–618. doi: 10.1113/jphysiol.1974.sp010768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy K, Dawes GS, Robinson JS. A 24-hour rhythm in the foetus. In: Dawes GS, Cross KW, Comline RS, Nathanielsz PW, editors. Foetal and Neonatal Physiology. Cambridge University Press; 1973. pp. 63–66. [Google Scholar]
- Brace RA, Moore TR. Diurnal rhythms in fetal urine flow, vascular pressures, and heart rate in sheep. American Journal of Physiology. 1992;261:R1015–1021. doi: 10.1152/ajpregu.1991.261.4.R1015. [DOI] [PubMed] [Google Scholar]
- Challis JRG. Sharp increase in free circulating oestrogen immediately before parturition in sheep. Nature. 1971;229:208. doi: 10.1038/229208a0. [DOI] [PubMed] [Google Scholar]
- Challis JRG, Lye SJ. Parturition. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2. New York: Raven Press; 1994. pp. 986–1031. [Google Scholar]
- Clewlow F, Dawes GS, Johnston BM, Walker DW. Changes in breathing, electrocortical and muscle activity in unanaesthetized fetal lambs with age. The Journal of Physiology. 1983;341:463–476. doi: 10.1113/jphysiol.1983.sp014817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KJ, Gawes GS, Patrick JE. Diurnal, respiratory, and other rhythms of heart rate in lambs. American Journal of Obstetrics and Gynecology. 1977;127:414–424. doi: 10.1016/0002-9378(77)90500-2. [DOI] [PubMed] [Google Scholar]
- Dawes GS, Robinson JS. Rhythmic phenomena in prenatal life. Progress in Brain Research. 1976;45:383–389. doi: 10.1016/s0079-6123(08)60999-1. [DOI] [PubMed] [Google Scholar]
- Derks JB, Giussani DA, Jenkins SL, Wentworth RA, Visser GHA, Padbury JF, Nathanielsz PW. A comparative study of cardiovascular, endocrine and behavioural effects of betamethasone and dexamethasone administration to fetal sheep. The Journal of Physiology. 1997;499:217–226. doi: 10.1113/jphysiol.1997.sp021922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks JB, Giussani DA, Van dam LM, Jenkins SL, Winter JA, Zhao XF, Hammond GL, Nathanielsz PW. Differential effects of betamethasone and dexamethasone fetal administration of parturition in sheep. Journal of the Society for Gynecologic Investigation. 1996;3:336–341. 10.1016/S1071-5576(96)00024-X. [PubMed] [Google Scholar]
- Ellingson RJ. Development of wakefulness-sleep cycles and associated EEG patterns in mammals. In: Mayer FE, editor. Sleep and Maturing Nervous System. New York: Academic Press; 1972. pp. 165–174. [Google Scholar]
- Figueroa JP, Mahan S, Poor ER, Nathanielsz PW. Characteristics and analysis of uterine electromyographic activity in the pregnant sheep. American Journal of Obstetrics and Gynecology. 1985;151:524–531. doi: 10.1016/0002-9378(85)90282-0. [DOI] [PubMed] [Google Scholar]
- Fletcher KL, Leung K, Myers MM, Stark RI. Diurnal rhythms in cardiorespiratory function of the fetal baboon. Early Human Development. 1996;46:27–42. doi: 10.1016/0378-3782(96)01738-0. 10.1016/0378-3782(96)01738-0. [DOI] [PubMed] [Google Scholar]
- Graps A. An introduction to wavelets. IEEE Computational Science Engineering. 1995;2:60–61. [Google Scholar]
- Halberg F. Chronobiology. In: Hall VE, Giese AC, Sonnenschein R, editors. Annual Review of Physiology. Vol. 31. Palo Alto: Annual Reviews Inc.; 1969. pp. 675–725. [DOI] [PubMed] [Google Scholar]
- He Y. Mathematica Wavelet Explorer. Champaign, IL, USA: Wolfram Research; 1996. [Google Scholar]
- Hofmeyr GJ, Bamford OS, Gianopoulos JG, Parks MJ, Dawes GJ. The partial association of uterine contractions with changes in electrocortical activity, breathing, and Pa,O2 in the fetal lamb: Effects of brainstem section. American Journal of Obstetrics and Gynecology. 1985;152:905–910. doi: 10.1016/s0002-9378(85)80090-9. [DOI] [PubMed] [Google Scholar]
- Houghton DC, Walker DW, Young IR, McMillen IC. Melatonin and the light-dark cycle separately influence daily behavioral and hormonal rhythms in the pregnant ewe and sheep fetus. Endocrinology. 1993;133:90–98. doi: 10.1210/endo.133.1.8319592. 10.1210/en.133.1.90. [DOI] [PubMed] [Google Scholar]
- Hsu HW, Figueroa JP, Honnebier BOM, Wentworth R, Nathanielsz PW. Power spectrum analysis of myometrial electromyogram and intrauterine pressure changes in pregnant Rhesus monkey in late gestation. American Journal of Obstetrics and Gynecology. 1989;161:467–473. doi: 10.1016/0002-9378(89)90543-7. [DOI] [PubMed] [Google Scholar]
- Jansen CA, Krane EJ, Thomas AL, Beck NF, Lowe KC, Joyce P, Parr M, Nathanielsz PW. Continuous variability of fetal PO2 in the chronically catheterized fetal sheep. American Journal of Obstetrics and Gynecology. 1979;134:776–783. doi: 10.1016/0002-9378(79)90947-5. [DOI] [PubMed] [Google Scholar]
- Jost RG, Quilligan EJ, Yeh S, Anderson GG. Intrauterine electroencephalogram of the sheep fetus. American Journal of Obstetrics and Gynecology. 1972;114:535–539. doi: 10.1016/0002-9378(72)90216-5. [DOI] [PubMed] [Google Scholar]
- Longo LD, Dale PS, Gilbert RD. Uteroplacental O2 uptake: continuous measurements during uterine quiescence and contractions. American Journal of Physiology. 1986;250:R1099–1107. doi: 10.1152/ajpregu.1986.250.6.R1099. [DOI] [PubMed] [Google Scholar]
- McMillen IC, Horburn GD, Walker DW. Diurnal variations in plasma concentrations of cortisol, prolactin, growth hormone and glucose in the fetal sheep and pregnant ewe during late gestation. Journal of Endocrinology. 1987;114:65–72. doi: 10.1677/joe.0.1140065. [DOI] [PubMed] [Google Scholar]
- Magyar DM, Fridshal D, Elsner CW, Glatz T, Eliot J, Klein AH, Lowe KC, Buster JE, Nathanielsz PW. Time-trend analysis of plasma cortisol concentrations in the fetal sheep in relation to parturition. Endocrinology. 1980;107:155–159. doi: 10.1210/endo-107-1-155. [DOI] [PubMed] [Google Scholar]
- Martin W, Brinkmann K. Detection and measurement of periodicities in time series. International Journal of Chronobiology. 1976;4:185–196. [Google Scholar]
- Matsuda Y, Patrick J, Carmichael L, Fraher L, Richardson B. Recovery of the ovine fetus from sustained hypoxia: Effects on endocrine, cardiovascular, and biophysical activity. American Journal of Obstetrics and Gynecology. 1994;170:1433–1441. doi: 10.1016/s0002-9378(94)70176-8. [DOI] [PubMed] [Google Scholar]
- Natale R, Clewlow F, Dawes GS. Measurement of forelimb movements in the lamb in utero. American Journal of Obstetrics and Gynecology. 1981;140:545–551. doi: 10.1016/0002-9378(81)90231-3. [DOI] [PubMed] [Google Scholar]
- Nathanielsz PW, Bailey A, Poore ER, Thorburn GD, Harding R. The relationship between myometrial activity and sleep state and breathing in fetal sheep throughout the last third of gestation. American Journal of Obstetrics and Gynecology. 1980;138:653–659. doi: 10.1016/0002-9378(80)90083-6. [DOI] [PubMed] [Google Scholar]
- Nathanielsz PW, Elsner C, Magyar D, Fridshal D, Freeman A, Buster JE. Time trend analysis of plasma unconjugated and sulfoconjugated estrone and 3βΔ5-steroids in fetal and maternal sheep plasma in relation to spontaneous parturition at term. Endocrinology. 1982;110:1402–1407. doi: 10.1210/endo-110-4-1402. [DOI] [PubMed] [Google Scholar]
- Nathanielsz PW, Jack PMB, Krane EJ, Thomas AL, Ratter S, Rees LH. Ciba Foundation Symposium. Vol. 47. Amsterdam: Elsevier; 1977. The role and regulation of corticotropin in the fetal sheep; pp. 73–98. [DOI] [PubMed] [Google Scholar]
- Robertson SS, Jhonson SL, Bacher LF, Wood JR, Wong CH, Robinson SR, Smotherman WP, Nathanielsz PW. Contractile activity of the uterus prior to labor alters the temporal organization of spontaneous motor activity in the fetal sheep. Developmental Psychobiology. 1996;29:667–683. doi: 10.1002/(SICI)1098-2302(199612)29:8<667::AID-DEV3>3.0.CO;2-R. 10.1002/(SICI)1098-2302(199612)29:8<667::AID-DEV3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Wong CH, Robertson SS, Nathanielsz PW, Smotherman WP. Behavioral responses of the chronically instrumented sheep fetus to chemosensory stimuli presented in utero. Behavioral Neuroscience. 1995;109:551–562. 10.1037//0735-7044.109.3.551. [PubMed] [Google Scholar]
- Ruckenbusch Y. Development of sleep and wakefulness in the foetal lamb. Electroencephalography and Clinical Neurophysiology. 1971;32:119–128. doi: 10.1016/0013-4694(72)90134-4. 10.1016/0013-4694(72)90134-4. [DOI] [PubMed] [Google Scholar]
- Ruckenbusch Y, Gaujoux M, Eghbali B. Sleep cycles and kinesis in the foetal lamb. Electroencephalography and Clinical Neurophysiology. 1977;12:226–237. doi: 10.1016/0013-4694(77)90029-3. 10.1016/0013-4694(77)90029-3. [DOI] [PubMed] [Google Scholar]
- Stark RI, Daniel SS. Circadian rhythm of vasopressin levels in cerebrospinal fluid of the fetus: effect of continuous light. Endocrinology. 1989;124:3095–3101. doi: 10.1210/endo-124-6-3095. [DOI] [PubMed] [Google Scholar]
- Symmes D, Prichard JW, Mann LI. Spectral analysis of fetal sheep EEG during hypoxia. Electroencephalography and Clinical Neurophysiology. 1970;29:511–515. doi: 10.1016/0013-4694(70)90067-2. 10.1016/0013-4694(70)90067-2. [DOI] [PubMed] [Google Scholar]
- Szeto HH, Hinmann DJ. Prenatal development of sleep-wake patterns in sheep. Sleep. 1985;8:347–355. doi: 10.1093/sleep/8.4.347. [DOI] [PubMed] [Google Scholar]
- Szeto HH, Vo TDH, Dwyer D, Dogramajian ME, Cox MJ, Senger G. The ontogeny of fetal lamb electrocortical activity: A power spectral analysis. American Journal of Obstetrics and Gynecology. 1985;153:462–466. doi: 10.1016/0002-9378(85)90088-2. [DOI] [PubMed] [Google Scholar]
- Unno N, Jenkins SL, Wu WX, Wong CH, Bennett PR, Nathanielsz PW. Effects of maternal administration of nimesulide, a putative prostaglandin (PG) endoperoxide synthase (PGHS)-2 inhibitor on myometrial (MYO) contraction power in sheep during spontaneous term labor (STL) Journal of the Society for Gynecologic Investigation. 1997;4(suppl.):A336P. [Google Scholar]
- Visser GH, Goodman JDS, Levine DH, Dawes GS. Diurnal and cyclic variations in human fetal heart rate near term. American Journal of Obstetrics and Gynecology. 1982;14:505–544. doi: 10.1016/0002-9378(82)90757-8. [DOI] [PubMed] [Google Scholar]
- Woudstra BR, Kim C, Aarnoudse JG, Nathanielsz PW. Myometrial contracture-related increases in plasma adrenocorticotropin in fetal sheep in the last third of gestation are abolished by maintaining fetal normoxemia. Endocrinology. 1991;129:1709–1713. doi: 10.1210/endo-129-4-1709. [DOI] [PubMed] [Google Scholar]


